Abstract
The neural mechanisms that determine social bladder control are reviewed, with a particular emphasis on the role played by sensation in the process. Much has been learnt about the neural control of the bladder from studying patients with neurological disease and those disorders that are known to disrupt bladder storage are described. Possible approaches to treatment of the resulting incontinence are reviewed and it is acknowledged that in the future, the optimal treatment for incontinence may be determined by its precise underlying pathophysiology in each instance, for example, suprapontine causes requiring different medication to spinal causes. Although the main emphasis of urological research and development so far has been the treatment of incontinence, effective therapy for other bladder disorders such an impaired emptying or bladder pain could have an important impact on the bladder symptoms of many patients.
Keywords: Voluntary control micturition, functional brain imaging, bladder sensation neurogenic bladder dysfunction
Introduction
A focus on the differences between animal and human bladder control is a good starting point from which to present a clinical perspective of the integrated control of the lower urinary tract. Those differences lie mainly with aspects of human behaviour, which have evolved around our social control of micturition and our need to void in an appropriate place, with a degree of privacy. This review therefore starts with an examination of the neural substrate, which enables us to accomplish controlled voiding and then looks at the effect of neurological disease on that process. Mention is made throughout of therapeutic possibilities based on that knowledge.
Micturition as an example of goal orientated behaviour (GOB)
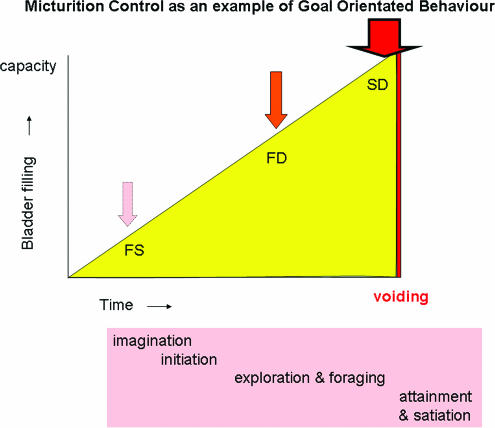
An ethological analysis of an animal's behaviour starts with the basic premise that in response to a specific pattern of stimuli, a central pattern generator produces a fixed behavioural response. This is the basis for GOB, a process that starts with imagination, followed by the initiation of the response, which in turn requires foraging and exploration to reach attainment of the goal and ultimately satiation (Swanson & Mogenson, 1981). In common with other motivated or examples of GOB, it is likely that the neural substrate for goal selection exists in the hypothalamus and activity there together with activity in the cerebral hemispheres mediates planning of behaviour in response to the selected goal. Micturition can be analysed according to the same scheme of GOB (Figure 1).
Figure 1.
An analysis of human bladder control as an example of goal orientated behaviour. FS, first sensation; FD, first desire; SD, strong desire to void.
Among animals there is a hierarchy of behaviours associated with micturition, which involves increasingly sophisticated flexibility, determined by the role of micturition in that animal's social life. Many community living animals void in restricted areas and it seems likely that this ‘social continence' has evolved to maximize the survival of the individuals and their community. However, human behaviour relating to bladder control is fundamentally different from all other animals because, certainly in modern day society, we void in a controlled fashion, preferably in privacy. What other animal experiences embarrassment about their voiding being observed and experiences shame if publicly incontinent?
Figure 1 shows an analysis of human micturition as an example of GOB. Two salient points are evident from this: first, that the process of voiding is initiated by the sensation of bladder fullness and indeed the overall motivation of the behaviour is to attain relief from this increasingly intense sensation of bladder filling, which is achieved by voiding. The second point is that the process of voiding itself lasts for only a very brief period in the total behavioural cycle. This is indeed the case in daily life; if voiding takes between 1 and 2 min and is performed four or five times a day, calculation shows that the bladder is in the storage mode for 99.8% of life.
It is therefore important when examining neural mechanisms for continence in humans to have as central considerations, the neurology of bladder sensation and the process of bladder storage.
Bladder sensation – normal and pathological
As shown in Figure 1, it is the motivation to avoid or be relieved from the unpleasantness and anxiety of extreme bladder filling that drives seeking out the facility to void. Reporting degrees of sensory intensity of bladder fullness are not accessible in animal experiments but are of crucial importance in human studies and clinical practice.
Studies in healthy controls have shown that the first sensation of filling (FSF) occurs when about 40% of capacity is reached, but this sensation is indistinct and it is easily disregarded. The first desire to void (FDV) is reported at approximately 60% of capacity and has been defined by ICS as ‘the feeling, during filling cystometry, that would lead the patient to pass urine at the next convenient moment, but voiding can be delayed if necessary' (Abrams et al., 2002). Whereas at >90% of capacity, strong desire to void (SDV) is reported (Denny-Brown & Robertson, 1933; Wyndaele, 1990), defined by International Continence Society (ICS) as ‘a persistent desire to void without the fear of leakage' (Abrams et al., 2002). Psychological state of mind can effect the perception of bladder fullness. It is well known that anxiety can increase the level of desire to void whereas distraction can also influence awareness of bladder sensation, in much the same way as it can alter awareness of pain (Valet et al., 2004).
Urinary urgency is a sensation of particular clinical importance and its exact definition continues to be the subject of much debate. The ICS has defined it as ‘the complaint of a sudden compelling desire to pass urine which is difficult to defer' (Abrams et al., 2002). It is a sensation that is not experienced in health and appears to occur just prior to the onset of an involuntary detrusor contraction, which may cause leakage, so called ‘urgency incontinence'. When sensed, the subject's physiological response is to contract the pelvic floor and sphincter, which has an inhibitory effect both on bladder sensation and detrusor contraction.
Semantic difficulties have arisen, particularly in translation, in understanding and conveying the meaning of the difference between ‘strong urge to void' and ‘urgency'. However, the two sensations are not on the same continuum since in health a strong urge or desire to void is not associated with the fear of leakage, in contrast to the sensation of urgency that precedes involuntary detrusor contractions. Elimination of urgency is the aim of effective therapy since it is a symptom per se that greatly worries patients but its effective treatment also implies suppression of the underlying abnormal bladder activity, detrusor overactivity (DO).
The storage phase and what we have learnt from functional brain imaging of the neural control of micturition
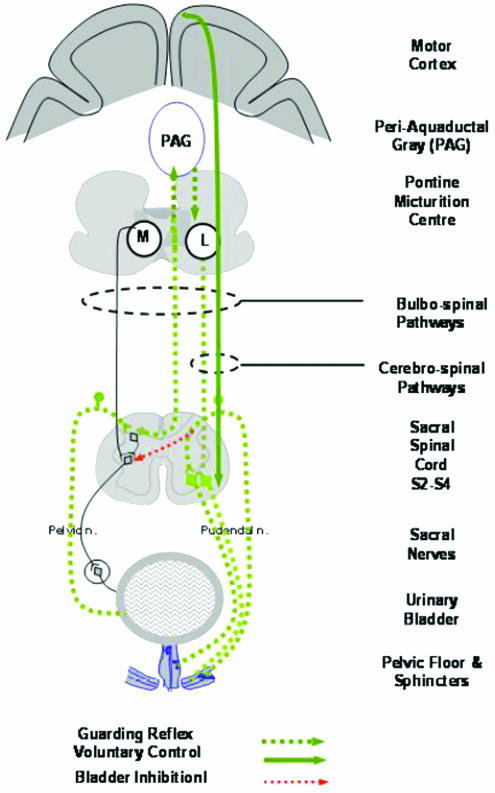
Throughout filling, there is inhibition of the parasympathetic innervation of the detrusor and activation of the smooth and striated urethral sphincter in response to bladder afferent activity conveyed through the pelvic nerves (Figure 2). These reflexes, known collectively as the ‘guarding reflex', are organized in the spinal cord (see de Groat, 1975) and in experimental animals the lumbar sympathetic is known to play a major role in the inhibition of parasympathetic activity at the level of the pelvic plexus (Morrison et al., 2005). In man, the importance of the sympathetic is less obvious since sympatholytics or resection of the sympathetic chain has little effect on bladder storage and some input from the brain stem, the so-called ‘L-region' may be involved particularly in voluntary sphincter control (Griffiths, 2002) (Figure 2). What is clear is that the guarding reflex is driven by afferent input, which through synaptic activity with interneurones either at a local segmental level or via axons to the brain, affects motor pathways. Ascending afferent activity gives rise to conscious perception of sensation.
Figure 2.
Pathways of the guarding reflex by kind permission of Professor Michael Craggs.
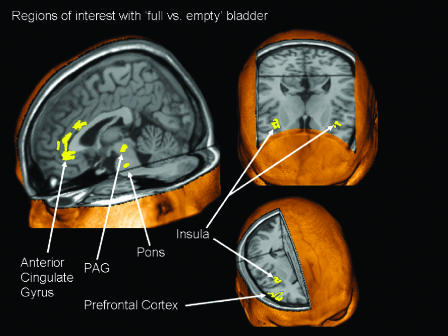
Our understanding of the processing of bladder sensations has been greatly advanced in recent years by the advent of functional brain imaging. These powerful techniques using radioactive isotope concentrations, or more recently magnetic resonance imaging, are allowing us to visualize regions of brain activation during specific tasks performed in the scanner. The requirements of positron emission tomography (PET) are that the subject lies immobile in the scanner ready to perform a simple task. A bolus of radioactive water is injected; the task performed and appropriately timed brain images captured from the scanner. More recently the need to inject a radioactive isotope has been obviated by use of the BOLD (blood oxygenated level dependent) signal, which can be detected using magnetic resonance imaging, the BOLD signal being increased in venous blood from metabolically active brain areas. Unlike structural images, the pictures produced by functional brain imaging result from a comprehensive statistical processing of massive amounts of data, a process that requires the analyst to make a number of decisions that can have considerable bearings on the final result. The fundamental basis of the analysis is to compare activation in different physiological conditions, and thus it is necessary to choose which state to take as ‘baseline'; for functional brain imaging applied to bladder control, a comparison between activation with a full and empty bladder has commonly been used (Figure 3).
Figure 3.
Summary of regions of interest comparing ‘full vs empty' bladder conditions based on the co-ordinates published in five PET studies (Blok et al., 1997; 1998; Nour et al., 2000; Athwal et al., 2001; Matsuura et al., 2002). The figure from Kavia et al. (2005) was prepared using MRIcro (Rorden & Brett, 2000) (with permission from Journal of Comparative Neurology).
The earliest PET studies applied to an analysis of the central control of micturition looked at the process of voiding in first men and then women (Blok et al., 1997; 1998). These studies showed that cortical and brain stem areas are involved in the control of micturition and that the brain stem areas are comparable to those known to exist in the cat. In subjects who were able to void in the scanner (because not all could), a region of activation was seen in the dorsal tegmentum of the pons at a location comparable to that of the pontine micturition centre (PMC) or ‘Barrington's nucleus' in the cat (de Groat, 1975). In those subjects unable to void, a more ventral region of activation was seen, suggesting the existence of a pontine storage centre. The evidence from the animal data for the importance of such a centre is less clear than for the PMC (Griffiths, 2002) since bladder storage is organized in the spinal cord (de Groat, 1975). The PMC is regarded as the final efferent nucleus of the micturition pathway, which projects to the sacral spinal cord. Inhibition of activity of the PMC is required throughout the storage phase until the appropriate moment to void has been attained (Figure 3).
Several functional brain imaging experiments have examined cortical activity during continent bladder filling. A consistent finding of all these studies is an activation of the periaqueductal grey (PAG) (see Kavia et al., 2005, for a review and Figure 3). This is in keeping with experimental studies in the cat, which showed strong afferent connections from the sacral cord to this structure. It has been suggested that the PAG serves as a central relay centre for afferent activity from the pelvic organs and acts as an interface between the afferent and efferent limbs of bladder control circuits, informing the PMC about the degree of bladder fullness (Blok et al., 1995). The extent to which the PMC receives direct afferent input in different species is the subject of continuing debate, but the importance of the afferent relay function of the PAG for micturition in man and cats may reflect the fact that both these species urinate in safe environments, unlike the rat which voids reflexively (Blok & Holstege, 2000). The PAG is known to have multiple connections with higher centres such as the thalamus, insula, cingulate, and prefrontal cortices and it seems likely that it is the influence of these higher centres that determines that the act of micturition occurs at an appropriate time and place.
The insula along with the anterior cingulate gyrus have been shown to be an important region for interoceptive awareness of visceral sensations (Critchley et al., 2004) and were found to be activated during bladder filling (Figure 3) as well as gut distension. The anterior cingulate gyrus is part of the limbic system, which is intimately involved in the control of the autonomic nervous system, as well as important in cognition and task performance (Bush et al., 2000). Classically the anterior cingulate gyrus has been related to affect, but neuroimaging studies show that it is also involved in cognitive processes involving attention and executive control. Autonomic modulation by both the ventral and dorsal regions of the anterior cingulate has also been postulated, with the dorsal region possibly more associated with sympathetic modulation and parasympathetic modulation by the more ventral parts (Critchley et al., 2003; Matthews et al., 2004). Activity in the anterior cingulate was seen in all the various bladder experiments (Figure 3), but the variability of the precise location of activation in the different experiments may be explained by the fact that the anterior cingulate cortex is split functionally into two regions, an anterior/dorsal affective division and a posterior/ventral cognitive division (Bush et al., 2000). In one set of experiments, subjects were asked to rate their level of desire to void, a cognitive task with hence more posterior activation, whereas in others the bladder was simply filled to reported capacity.
The prefrontal cortex is the region of the frontal lobe that is anterior to the primary and association motor cortices. It is thought to be the seat of planning complex cognitive behaviours and in the expression of personality and appropriate social behaviour (Wood & Grafman, 2003). The prefrontal region is also known to have a role in attention mechanisms and response selection mechanisms (Pardo et al., 1991). It has multiple connections with the anterior cingulate gyrus (Carmichael & Price, 1995) and both regions have direct (or indirect) connections with the PAG, hypothalamus and other areas associated with autonomic control. These are the brain regions that are likely to be involved in the conscious and social control of the bladder function. The right inferior frontal gyrus, part of the prefrontal cortex, was seen to be active during the storage and micturition phases of the bladder function in all the studies (Figure 3) and it has been postulated that the task of the prefrontal cortex is to make a decision as to whether or not micturition should take place at a particular place or time.
Micturition
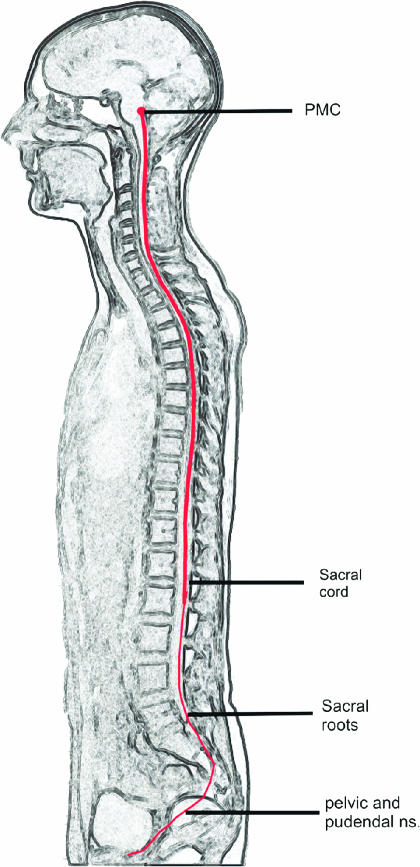
With the decision to void, activation was seen in the prefrontal, insula, hypothalamus and PAG and as already mentioned in the PMC (Blok et al., 1997; 1998). Activation of the PMC is the final brain efferent nucleus and in health results in spinal transmission of activity to sacral segments of the spinal cord (Figure 4).
Figure 4.
The neural pathways involved in the control of the bladder. The pontine micturition centre (PMC) is the final brain efferent nucleus, activation of which results in co-ordinated sphincter relaxation followed by detrusor contraction. Pathways connecting the PMC to peripheral bladder innervation (the pelvic and pudendal nerves) pass down the spinal cord, and through the sacral roots (S2–S4) (with permission from Seandanavian Journal of Urology and Nephrology).
Voiding is achieved by inhibition of the motor outflow to the striated urethral sphincter followed some seconds later by activation of the sacral parasympathetic, which results in contraction of the bladder, an increase in intravesical pressure and the flow of urine. Relaxation of the urethral smooth muscle is mediated by activation of the parasympathetic pathway to the urethra, triggering the release of nitric oxide and by the removal of adrenergic and somatic cholinergic excitatory inputs. Secondary reflexes elicited by flow of urine through the urethra facilitate bladder emptying (Morrison et al., 2005). The reciprocal coordinated inhibition of the sphincter and activation of the detrusor crucially resides in the PMC and with damage to the bulbo-spinal pathways in the spinal cord this is lost, resulting in a simultaneous detrusor and sphincter contraction, the disorder known as ‘detrusor sphincter dyssynergia' (DSD).
Clinical causes of a failure of bladder storage
From the forgoing sections, it will be clear that the process of bladder control is highly complex and depends on the integrity of extensive tracts of the cerebral and extra cerebral nervous system. The roots that innervate the bladder arise from the most distal segments of the spinal cord (S2–S4) and Figure 4 serves by illustration, to highlight the importance of the health of the full-length of the spinal cord for bladder control. The sacral roots pass through the cauda equina and the peripheral innervation of the lower urinary tract; its sympathetic, parasympathetic and somatic innervation reach the detrusor and sphincter via the pelvic and pudendal nerves, respectively.
It is against this background knowledge that the effect of neurological diseases on bladder control will be considered. Neurological causes of clinical significance predominantly involve a failure of the storage phase. Using the scheme of neural control as shown in Figure 4 as the basis of a hierarchical examination of this problem, the different causes are shown in Table 1.
Table 1. Neurological analysis of the causes of a failure of bladder storage.
| 1. Central failure of inhibition of the pontine micturition centre |
| 2. Spinal lesions which permit emergence of a segmental reflex causing DO |
| 3. Abnormal intrinsic bladder reflexes causing DO |
| 4. Genuine stress incontinence |
Central failure of inhibition of the PMC
Central disorders which disrupt the normal inhibition of the PMC during the filling phase will allow bladder emptying at inappropriate times causing incontinence.
Dementia
Numerically the most significant group of patients with incontinence are those with dementia. It is largely such patients whose incontinence has such major social and economic consequences. It seems likely that the structural changes that occur with aging cause a number of abnormal bladder functional parameters, but the continent elderly are able to employ compensatory countermeasures (Resnick, 1995). Incontinence in dementia is due to a multifactorial breakdown in those compensatory mechanisms and so it is unlikely that any specific treatment aimed solely at treating the incontinence in this group will be available in the near future.
Frontal lobe disease
Focal brain disease may affect neural bladder control: that the medial part of the anterior regions of the frontal lobes is critical for bladder control in man was clearly shown by the study of Andrew & Nathan (1964). Their clinico-pathological cases included patients who had had intracranial tumours, damage following rupture of an aneurysm, penetrating brain wounds or frontal lobe leucotomy. Typically, the history was that ‘the feeling of gradual distension of the bladder is lost and the only warning that the patient has that his bladder is full is the sensation associated with the imminence of micturition'. Patients experienced severe urgency, frequency of micturition and urgency incontinence, and furthermore were usually embarrassed by this: only if frontal lobe damage is very extensive do patients become disinhibited and unconcerned about their incontinence. Notably, micturition is normally coordinated.
In addition to the structural detrusor changes that occur with aging, there is a subgroup of elderly patients in whom there is a reduced sensation of bladder filling and urgency incontinence is particularly troublesome. SPECT studies showed this was associated with global underperfusion of the cerebral cortex and more specifically, with underperfusion of the frontal areas of the brain (Griffiths et al., 1994).
Stroke and cerebrovascular disease
Following stroke, a proportion of patients develop urinary incontinence. There may be many different reasons for this including impaired consciousness, impaired ability to communicate, loss of mobility (Brocklehurst et al., 1985) or general loss of decompensatory mechanisms formerly necessary to cope with the abnormal functional behaviour of the aging bladder. Alternatively, there may be an acquired cerebral lesion affecting brain regions involved in bladder control. Various studies have attempted to correlate the site of vascular brain damage with urodynamic changes (Tsuchida et al., 1983; Kuroiwa et al., 1987; Garrett et al., 1989; Khan et al., 1990) without a clear picture emerging. However, it has been established that the commonest cystometric finding is of DO with normally coordinated voiding. Furthermore, it has been shown that anterior brain lesions are much more likely to be associated with incontinence than are posterior, occipital infarcts or haemorrhages (Sakakibara et al., 1996).
Epidemiological studies of urinary incontinence following CVA reach a unanimous and somewhat surprising conclusion. This is that urinary incontinence occurring within the 7 days following a stroke is a specific indicator of poor prognosis (Wade & Langton Hewer, 1985; Barer, 1989). Urinary incontinence appeared to be a more powerful prognostic indicator for poor survival and eventual functional dependence than a history of depressed level of consciousness. The reason for this is not clear, but it may either be that incontinence is the result of a severe general rather than specific loss of function or that those who are incontinent may have suffered more severe loss of morale and self-esteem and are thus less motivated to recover loss of function.
Multiple system atrophy (MSA)
Poor bladder control is a common feature of the neurodegenerative disorder – MSA – a progressive disorder characterized by parkinsonism and autonomic features, but with incontinence occurring early (Beck et al., 1994). Curiously, the neurodegenerative process appears to selectively affect several neural sites that are important for bladder control. Cell loss in the pons is prominent and involves neurons containing corticotrophin-releasing factor, which it has been postulated, may be the cells of the PMC (Benarroch & Schmeichel, 2001), also in the descending sympathetic pathways, the interomediolateral cell column (Oppenheimer, 1980) and in Onuf's nucleus (Sung et al., 1978), the nucleus of anterior horn cells that innervate the sphincter. These deficits may result in DO, poorly sustained detrusor contractions and incomplete bladder emptying, an open bladder neck and weakness of the striated urethral sphincter (Kirby et al., 1986). The extent to which one of these disorders dominates the clinical picture is variable but poor bladder control may be a premonitory symptom of MSA, preceding the onset of other neurological features such as postural hypotension, cerebellar ataxia or parkinsonism (Sakakibara et al., 2000).
Parkinson's disease (PD)
By contrast, poor bladder control is a late feature of idiopathic PD and is associated with increasingly severe disability (Araki and Kuno, 2000). Some authors have suggested that an impaired relaxation or ‘bradykinesia' of the urethral sphincter can result in voiding dysfunction due to bladder outflow obstruction, and hence to DO (Christmas et al., 1988). However, other studies using cystometry have shown that obstructive voiding patterns are not common in PD patients, indicating other mechanisms must play a more significant role (Araki et al., 2000).
The hypothesis which is most widely accepted is that in healthy individuals the basal ganglia have an inhibitory effect on micturition reflex and with cell loss in the substantia nigra, DO develops. In monkeys treated with MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine), an overactive bladder was common, and selective dopamine D1 receptor agonists depressed DO (Yoshimura et al., 1992). In clinical studies, it was demonstrated that the presence of bladder symptom is related to the decrease in the total number of dopaminergic neurones in the striatum and that relative degeneration of the caudate correlates to severity of symptoms (Winge et al., 2004). It is also possible that anti-parkinson's medication may affect bladder function, but studies that have looked at the effect of L-dopa or apomorphine on bladder behaviour have produced conflicting results. However, the discrepancies in the literature may be due to the fact that, as recently shown in advanced PD, L-dopa exacerbates DO in the filling phase, but also improves bladder emptying through increased detrusor contractility, so that the postmicturition residual volumes diminish (Uchiyama et al., 2003). The effects of medication on bladder control have been demonstrated not to be associated with nigrostriatal dopaminergic degeneration but possibly mediated through cortical mechanisms, as the ability to separate and integrate sensory input measured using urodynamics is influenced by medication (Winge et al., 2004).
Possible therapeutic approaches to treat DO of central origin
Since the common cause of DO in the conditions listed above is probably a failure of inhibition of the PMC, a successful therapy would need to have a central action, redressing the balance between storage failure and voluntary micturition. However, complete central inhibition of voiding resulting in urinary retention would be counterproductive.
Spinal lesions that permit emergence of a segmental reflex causing DO
In animal models, acute disconnection of the pathways between the sacral cord and the pontine micturition centre results in urinary retention, which persists until there has been some recovery from ‘spinal shock'. This takes a variable time depending on the species, but the recovery is characterized by the onset of reflex bladder emptying, due to the emergence of a new, segmental spinal reflex. It has been shown in chronic spinal cats that following reorganization of synaptic connections in the spinal cord, previously quiescent C-fibres, form the afferent limb of the reflex, which mediates DO (de Groat et al., 1990). These C-fibres express the TRPV1 (transient potential vanilloid 1) receptor and are sensitive to capsaicin (Maggi & Meli, 1986). Changes in the properties of the C-fibres occur so that they become mechano-sensitive and display increased electrical excitability (Yoshimura, 1999).
The time taken for the C-fibre mediated response to emerge following spinal shock in man is about 6 weeks. Provided some spinal cord function remains intact and bladder sensation is preserved, the DO that develops following a spinal lesions is preceded by the sensation of urgency. Following a complete traumatic spinal cord injury, all bladder sensory function may be lost but with lesser degrees of spinal damage, urgency incontinence is common problem. In a progressive spinal disease, such a multiple sclerosis (MS), there is some correlation between lower limb dysfunction and the severity of bladder dysfunction (Kirchhof & Fowler, 2000). Although when a patient presents with neurogenic bladder complaints, there are usually some other symptoms and signs of neurological disease, the impairment of mobility which reflects the same spinal lesion (Figure 4) may be quite minor, indicating that the neural pathways for bladder control appear to be highly susceptible to spinal disruption. Among patients with neurological disease and urinary incontinence, those with some form of spinal cord dysfunction form the largest group (Fowler, 1999).
Deafferentation by intravesical agents
That the C-fibres were shown to be capsaicin sensitive (de Groat et al., 1990) was the basis for the first use of intravesical capsaicin to treat DO in patients with MS and intractable DO (Fowler et al., 1992). This therapeutic approach was then employed by many other centres worldwide and although a meta-analysis of the published literature (de Seze et al., 1999) almost certainly overestimates the efficacy of intravesical capsaicin instillation (because negative findings were less commonly reported), there is convincing evidence that DO due to a spinal lesion is reduced following intravesical capsaicin. Initially, the capsaicin was dissolved in alcohol and was highly pungent, but the subsequent finding that if it is dissolved in glucidic acid it is less irritant (de Seze et al., 2004) has led to the continued use of these instillations for treatment of a specific group of patients in Bordeaux. Elsewhere, however, with the increasing regulation of nonlicensed agents – the source of capsaicin mostly having been chemical rather than pharmacological suppliers – its use was abandoned in favour of pharmaceutically prepared resiniferatoxin (RTX). RTX is an ultrapotent capsaicinoid with a thousand-fold the selective C-fibre neurotoxicity of capsaicin for comparable pungency obtained from a cactus species of the genus Euphorbia, Euphorbia resinifera (Appendino & Szallasi, 1997). Following early positive reports of its effectiveness in neurogenic and also in fact in idiopathic (see below) DO (Cruz et al., 1997; Lazzeri et al., 1997), large-scale placebo-controlled multicentre clinical trials in Europe and the US were initiated. Unfortunately, these were aborted when it became apparent that contrary to expectation most patients were not responding at all and the problem attributed to thitherto unrecognized difficulties in the preparation and administration of the compound. Although the trial was abandoned, some results however were salvaged (Kim et al., 2003; Brady et al., 2004a, 2004b). In the few responders, it was possible to demonstrate that there had been a reduction of nerve density of the suburothelial innervation, with a parallel reduction in the expression of TRPV1 and P2X receptors (Brady et al., 2004a, 2004b) as well as an effect of the TRPV1 expression by the urothelium (Apostolidis et al., 2005). These findings suggested that the RTX had had a clinical effect by a neurotoxic action on the urothelium and suburothelial innervation.
The response to intravesical vanilloids in some patients with neurogenic detrusor overactivity (NDO) (Fowler, 2000; de Seze et al., 2004) supports the hypothesis that the emergent C-fibre reflex is important in spinal NDO in man. Urgency incontinence in patients with spinal NDO is often regarded as a valuable treatment target in drug development since the pathophysiology of this disorder is better understood than that of idiopathic detrusor overactivity (IDO). Several pharmaceutical companies are now engaged in research with the aim of trying to modify or eliminate the input from the emergent afferent reflex.
Neuromodulation
It has been known for many years that stimulation of pudendal afferents or sacral nerve roots can suppress abnormal or even normal bladder activity and this effect is utilized by sacral neuromodulation. Neuromodulation is the ‘influence of activity in one pathway affecting the pre-existing activity in another' (Craggs & Mcfarlane, 1999) and it is thought that a potential site for the effect of afferent input on the micturition reflex is at the first synapse in the reflex pathway between the bladder primary afferent and the second-order projection neurones in the lateral collateral pathway (Morrison et al., 1995). Sacral neuromodulation using a stimulating electrode inserted through an S3 foramen, connected to a subcutaneously implanted stimulator, stimulating in the region of the presacral pelvic plexus is now a widely used intervention for treatment of intractable idiopathic DO, which has failed to respond to anticholinergic therapy (Schmidt et al., 1999).
In spinal cord injured patients with complete lesions, the roots may be stimulated directly by an implanted device, with anterior root stimulation (a ‘Brindley stimulator') used to achieve bladder emptying and posterior root stimulation (sacral posterior and anterior root stimulator SPARS) to deliver neuromodulation and increase bladder capacity (Kirkham et al., 2002).
DSD in spinal cord disease
Following disconnection from the PMC, in addition to the emergence of the segmental reflex that drives DO, the coordinated reciprocal activity of the sphincter and the detrusor is also lost and DSD occurs. The simultaneous contraction of the sphincter with the detrusor may impede bladder emptying and result in high intravesical pressures, which can endanger the upper urinary tracts. Although it is known that DSD can be abolished by dorsal rhizotomy, this is a destructive procedure, which men following spinal cord injury are usually unwilling to undergo since with it reflex erections are lost. Currently, it is DSD which is hindering attempts to achieve more effective bladder emptying using the SPARS stimulator (Kirkham et al., 2002).
Many patients with spinal lesions need to perform clean intermittent self-catheterization to empty their bladders and some medication which improved striated sphincter relaxation during voiding would have a significant impact in these patients. Nitric oxide donors such as glyceryl trinitrate or isosorbide mononitrate were proposed as agents that might deliver nitric oxide to the sphincter and increase its relaxation (Mamas et al., 2001), and recent studies showed a promising effect of oral administration of nitric oxide donors on bladder outlet obstruction in men with DSD due to spinal lesions (Reitz et al., 2004). Research and development is required to develop a phosphodiesterase inhibitor specific for the striated urethral sphincter.
Abnormal intrinsic bladder reflexes causing bladder overactivity
IDO is the most common type of DO. The cause of this condition remains to be fully elucidated and debate has raged in the past as to whether it is primarily myogenic (Brading, 1997) or neurogenic (de Groat, 1997). The condition has been defined by the ICS as ‘incontinence due to an involuntary detrusor contraction …. when there is no defined cause' (Abrams et al., 2002). Urodynamic studies show the same DO as is seen in neurogenic disorders, but the patient will have no clinical features of a neurological disease. Occasionally, an underlying neurological abnormality is overlooked initially, but in the majority of patients, clinical neurological examination and neurological imaging is normal. There is probably no good animal model of this disorder.
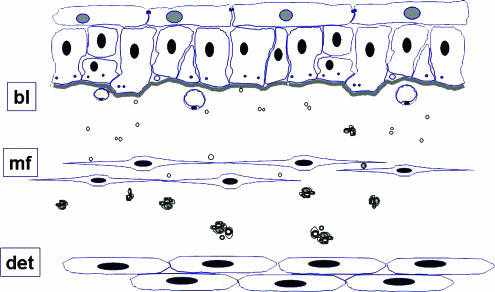
What is becoming increasingly clear is that the urothelium and suburothelium play a major role in initiating detrusor contractions. The urothelium is now recognized as having substantial sensory properties (Birder et al., 1998) and the suburothelium, the lamina propria, is known to be extensively innervated (Dixon & Gilpin, 1987; Wiseman et al., 2002). Naked axons have been seen to pass between the basal processes of the epithelial cells and the basal lamina of the urothelium (Wiseman et al., 2002; Figure 5) and the urothelium has been shown to express a variety of receptors and release acetylcholine, ATP and NO on stretching (Birder et al., 1998). Using transmission electron microscopy a complex sensory network in man has been shown in the suburothelial layer consisting of vesicle-packed naked axons (Wiseman et al., 2002) (Figure 5). The vesicles are clear or larger dense-cored but the contents remain to be identified.
Figure 5.
A schematic diagram showing the main ultrastructural constituent elements of the superficial layers of the human bladder. Flattened cells with pale nuclei, known as ‘umbrella cells' form the innermost layer of the bladder with tight and adhaerens junctions linking them to one another. These are supported by basal epithelial cells which are closely attached to the basal lamina (bl). Naked axons can be seen between the basal processes of the epithelial cells and immediately beneath the basal lamina. In the zone immediately beneath the epithelial basal lamina are fine axons, either naked or in intimate association with flattened cells have the cytological characteristics of myofibroblasts (mf). Also in this layer are fenestrated capillaries orientated towards the urothelial surface. Within the deeper zones of the lamina propria is a diffuse plexus of unmyelinated fibres containing slender axons linking periodic varicosities which enclose numerous small clear and dense cored vesicles. Close to the smooth muscle of the detrusor (det) nerves consist of small myelinated fibres and numerous unmyelinated strands, partially or completed invested by perineurium. From (Fowler et al., 2002) with permission.
The lamina propria innervation is currently the subject of intense research using various immunoreactive staining techniques. The unmyelinated nerves have been demonstrated to contain a wide variety of active agents, including CGRP, substance P, VIP, substance Y (Smet et al., 1997) and acetylcholine, and may also release ATP. TRPV1- and P2X3-immunoreactive fine nerve fibres have been found scattered throughout the suburothelium and traversing the muscle layer, with a similar distribution to PGP 9.5-immunoreactive fibres, but less numerous, suggesting localization in subsets of axons (Yiangou et al., 2001). A recent finding of great interest in human bladder biopsies has been the identification of a layer of myofibroblasts in the zone immediately beneath the epithelial basal lamina. These were found lying in parallel to the urothelium and formed a lattice structure, being discoidal in appearance and having attachments to one another at the margins as well as elements of innervation (Figure 5) (Wiseman et al., 2003). It has been postulated that these cells and their innervation collectively have the structure to function as a bladder volume sensing organ. The responsiveness to ATP of cells in the guinea-pig thought to be homologous to these raises the possibility that the urothelium (Wu et al., 2004), suburothelial innervation and myofibroblasts function as a unit to provide sensory information about the state of fullness of the bladder. Communication with the detrusor smooth muscle through intrinsic bladder reflexes may be the basis for autologous bladder activity.
The study which demonstrated intravesical RTX was effective in reducing idiopathic DO was presented as evidence that C-fibres pay an important role in this disorder (Silva et al., 2002). This finding and the well-established fact that the condition responds to antimuscarinics (currently the main therapy) and the injection of botulinum toxin are all consistent with the view that afferent activity is important in the initiation of DO. Andersson has recently pointed out that contrary to the long-held teaching that antimuscarinics were effective in the treatment of urgency incontinence because of their cholinergic blockade of the detrusor innervation, that they are effective in the storage phase and reduce urgency and increase bladder capacity, implying that muscarinic receptors, which have been demonstrated in the urothelium and suburothelial innervation, have a role in the disorder (Andersson, 2004). The urothelium has been shown to release acetylcholine on stretching and the amounts increased in IDO and the aging bladder. Another important function of the urothelium on being stretched is the release of ATP (Ferguson et al., 1997) and the role of the suburothelial purinergic innervation (Burnstock, 2001) is currently being investigated. ATP release has been shown to be increased in aging (Yoshida et al., 2004) and furthermore the sensitivity of the TRPV1 receptor has been shown to be potentiated by ATP (Tominaga et al., 2001).
Detrusor injections of botulinum toxin-A (BTX/A), introduced on the principle that the toxin would block the presynapthic release of acetylcholine (Schurch et al., 2000) have shown an efficacy much greater than that expected from merely paralysing the detrusor muscle. Conclusively demonstrated to be highly effective in treating NDO (Reitz et al., 2003) BTX/A has now been shown to be equally effective in treating IDO (Popat et al., 2005). It appears that BTX/A may have a complex effect on the urothelium and suburothelial innervation (Apostolidis et al., 2006). Our understanding of IDO will be greatly improved when it is understood how botulinum toxin A has such an exceptional effect on the condition.
Painful bladder syndrome
Although it now seems highly likely that IDO is due at least in some part to overactivity of bladder afferents resulting in painless, involuntary detrusor contractions there is another clinical condition also thought to be due to upregulation of afferent activity, the ‘painful bladder syndrome'. This has been defined by the ICS as ‘pain on bladder filling, often accompanied by urinary frequency in the absence of any other demonstrable bladder pathology' (Abrams et al., 2002). In the USA, the condition was formerly referred to as ‘interstitial cystitis' (Gillenwater & Wein, 1988). It is characterized by pain on bladder filling, but not involuntary bladder contractions and incontinence is not a feature of this condition. The cause of this condition remains to be discovered, but there is increasing evidence of urothelial dysfunction (Keay et al., 2004), possibly reducing the protection the suburothelial innervation is usually given, so that the end result is of a neuropathic type pain. The large variety of possible treatments that have been recommended underlies the fact that no single one has been demonstrated yet to be highly effective.
Stress incontinence
Disorders that cause DO and thus incontinence are fundamentally different from those characterized by a weak sphincter or weak pelvic floor, which can also cause incontinence, known as ‘stress urinary incontinence'. This has been defined by ICS as ‘the complaint of involuntary leakage on effort or exertion, or on sneezing or coughing' (Abrams et al., 2002). In this condition, increases in intra-abdominal pressure with exertion such as coughing or exercise are transmitted to the bladder contents causing a rise in intravesicular pressure, which is inadequately counteracted by reflex increase in outflow resistance due to sphincter weakness or poor connective tissue. It seems likely that the stress incontinence that affects middle-aged women is due to a combination of factors including laxity of pelvic floor support structures and an intrinsic weakness of the striated urethral sphincter.
Duloxetine, a norepinephrine and serotonin reuptake inhibitor, which has been shown to significantly increase sphincter muscle activity during bladder filling in the cat acetic acid model of irritated bladder function (Thor & Katofiasc, 1995) has been shown to have a beneficial effect in women with urinary stress incontinence (Dmochowski et al., 2003).
Perspective for the future
Our understanding of the neurology of the bladder has greatly increased in recent years resulting in better and more effective treatments of DO. What has become clear is that the same treatment is unlikely to be effective for all the varied causes of DO and incontinence, and that those various different conditions are going to require different approaches for optimal management. A highly effective oral agent with few significant side effects, which can be used to treat idiopathic DO, would have very considerable potential to improve the quality of life for many patients and hopefully its discovery is not far off.
Although the emphasis of this article has been to review the causes and possible treatments of incontinence, since that is the condition which is seen as having the major personal and economic impact, treatment for other disorders such as incomplete emptying and bladder pain certainly warrant future research.
Glossary
- BOLD signal
blood oxygenated level dependent signal
- BTX/A
botulinum toxin A
- CVA
cerebro vascular accident
- DSD
detrusor sphincter dyssynergia
- DO
detrusor overactivity
- FDV
first desire to void
- FSF
first sensation of filling
- GOB
goal orientated behaviour
- IDO
idiopathic detrusor overactivity
- ICS
International Continence Society
- MPTP
(1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine)
- MS
multiple sclerosis
- MSA
multiple system atrophy
- NDO
neurogenic detrusor overactivity
- PD
Parkinson's Disease
- PAG
periaqueductal grey
- PMC
pontine micturition centre
- PET
positron emission tomography
- RTX
resiniferatoxin
- SDV
strong desire to void
- SPARS
sacral posterior and anterior root stimulator
- SPECT
single photon emission computed tomography
- TRPV1
transient potential vanilloid 1
References
- ABRAMS P., CARDOZO L., FALL M., GRIFFITHS D., ROSIER P., ULMSTEN U., VAN KERREBROECK P.E., VICTOR A., WEIN A. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Neurourol. Urodyn. 2002;21:167–178. doi: 10.1002/nau.10052. [DOI] [PubMed] [Google Scholar]
- ANDERSSON K.E. Antimuscarinics for treatment of overactive bladder. Lancet Neurol. 2004;3:47–53. doi: 10.1016/s1474-4422(03)00622-7. [DOI] [PubMed] [Google Scholar]
- ANDREW J., NATHAN P.W. Lesions of the anterior frontal lobes and disturbances of micturition and defaecation. Brain. 1964;87:233–262. doi: 10.1093/brain/87.2.233. [DOI] [PubMed] [Google Scholar]
- APOSTOLIDIS A., BRADY C.M., YIANGOU Y., DAVIS J., FOWLER C.J., ANAND P. Capsaicin receptor TRPV1 in the urothelium of neurogenic human bladders and the effect of intravesical resiniferatoxin. Urology. 2005;65:400–405. doi: 10.1016/j.urology.2004.10.007. [DOI] [PubMed] [Google Scholar]
- APOSTOLIDIS A., DASGUPTA P., FOWLER C.J.2006Hypothesis for the remarkable efficacy of injected botulinum toxin in the treatment of human detrusor overactivity (DO) Eur. Urol.(in press). [DOI] [PubMed]
- APPENDINO G., SZALLASI A. Euphorbium: modern research on its active principle, resiniferatoxin, revives an ancient medicine. Life Sci. 1997;60:681–696. doi: 10.1016/s0024-3205(96)00567-x. [DOI] [PubMed] [Google Scholar]
- ARAKI I., KITAHARA M., OIDA T., KUNO S. Voiding dysfunction and Parkinson's disease: urodynamic abnormalities and urinary symptoms. J. Urol. 2000;164:1640–1643. [PubMed] [Google Scholar]
- ARAKI I., KUNO S. Assessment of voiding dysfunction in Parkinson's disease by the international prostate symptom score. J. Neurol. Neurosurg. Psychiatry. 2000;68:429–433. doi: 10.1136/jnnp.68.4.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ATHWAL B.S., BERKLEY K.J., HUSSAIN I., BRENNAN A., CRAGGS M., SAKAKIBARA R., FRACKOWIAK R.S., FOWLER C.J. Brain responses to changes in bladder volume and urge to void in healthy men. Brain. 2001;124:369–377. doi: 10.1093/brain/124.2.369. [DOI] [PubMed] [Google Scholar]
- BARER D. Continence after stroke: useful predictor or goal therapy. Age Ageing. 1989;18:183–191. doi: 10.1093/ageing/18.3.183. [DOI] [PubMed] [Google Scholar]
- BECK R.O., BETTS C.D., FOWLER C.J. Genito-urinary dysfunction in Multiple System Atrophy: clinical features and treatment in 62 cases. J. Urol. 1994;151:1336–1341. doi: 10.1016/s0022-5347(17)35246-1. [DOI] [PubMed] [Google Scholar]
- BENARROCH E.E., SCHMEICHEL A.M. Depletion of corticotrophin-releasing factor neurons in the pontine micturition area in multiple system atrophy. Ann. Neurol. 2001;50:640–645. doi: 10.1002/ana.1258. [DOI] [PubMed] [Google Scholar]
- BIRDER L.A., APODACA G., DE GROAT W.C., KANAI A.J. Adrenergic- and capsaicin-evoked nitric oxide release from urothelium and afferent nerves in urinary bladder. Am. J. Physiol. 1998;275:F226–F229. doi: 10.1152/ajprenal.1998.275.2.F226. [DOI] [PubMed] [Google Scholar]
- BLOK B., STURMS L., HOLSTEGE G. Brain activation during micturition in women. Brain. 1998;121:2033–2042. doi: 10.1093/brain/121.11.2033. [DOI] [PubMed] [Google Scholar]
- BLOK B., WEER H., HOLSTEGE G. Ultrastructural evidence for a paucity of projections from the lumboscral cord to the pontine micturition centre oe M-region in the cat: a new concept for the organization of the micturition rflex with the periaquductal gray as central relay. J. Comp. Neurol. 1995;359:300–309. doi: 10.1002/cne.903590208. [DOI] [PubMed] [Google Scholar]
- BLOK B., WILLEMSEN T., HOLSTEGE G. A PET study of brain control of micturition in humans. Brain. 1997;120:111–121. doi: 10.1093/brain/120.1.111. [DOI] [PubMed] [Google Scholar]
- BLOK B.F., HOLSTEGE G. The pontine micturition center in rat receives direct lumbosacral input. An ultrastructural study. Neurosci. Lett. 2000;282:29–32. doi: 10.1016/s0304-3940(00)00833-8. [DOI] [PubMed] [Google Scholar]
- BRADING A. A myogenic basis for the overactive bladder. Urology. 1997;50:57–76. doi: 10.1016/s0090-4295(97)00591-8. [DOI] [PubMed] [Google Scholar]
- BRADY C., APOSTOLIDIS A., YIANGOU Y., BAECKER P.A., FORD A.P., FOWLER C.J., ANAND P. P2X3-immunoreactive nerve fibres in neurogenic detrusor overactivity and the effect of intravesical resiniferatoxin (RTX) Eur. Urol. 2004a;46:247–253. doi: 10.1016/j.eururo.2003.12.017. [DOI] [PubMed] [Google Scholar]
- BRADY C.M., APOSTOLIDIS A., HARPER M., YIANGOU Y., BECKETT A., JACQUES T.S., FREEMAN A., SCARAVILLI F., FOWLER C.J., ANAND P. Parallel changes in bladder suburothelial vanilloid receptor TRPV1 (VR1) and pan-neuronal marker PGP9.5 immunoreactivity in patients with neurogenic detrusor overactivity (NDO) following intravesical resiniferatoxin treatment. BJU Int. 2004b;93:770–776. doi: 10.1111/j.1464-410X.2003.04722.x. [DOI] [PubMed] [Google Scholar]
- BROCKLEHURST J., ANDREWS K., RICHARDS B., LAYCOCK P. Incidence and correlates of incontinence in stroke patients. J. Am. Geriatr. Soc. 1985;33:540–542. doi: 10.1111/j.1532-5415.1985.tb04618.x. [DOI] [PubMed] [Google Scholar]
- BURNSTOCK G. Purine-mediated signalling in pain and visceral perception. Trends Pharmacol. Sci. 2001;22:182–188. doi: 10.1016/s0165-6147(00)01643-6. [DOI] [PubMed] [Google Scholar]
- BUSH G., LUU P., POSNER M. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn. Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- CARMICHAEL S.T., PRICE J.L. Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. J. Comp. Neurol. 1995;363:615–641. doi: 10.1002/cne.903630408. [DOI] [PubMed] [Google Scholar]
- CHRISTMAS T.J., KEMPSTER P.A., CHAPPLE C.R., FRANKEL J.P., LEES A.J., STERN G.M. Role of subcutaneous apomorphine in parkinsonian voiding dysfunction. Lancet. 1988;2:1451–1453. doi: 10.1016/s0140-6736(88)90932-4. [DOI] [PubMed] [Google Scholar]
- CRAGGS M., MCFARLANE J. Neuromodulation of the lower urinary tract. Exp. Physiol. 1999;84:149–160. doi: 10.1111/j.1469-445x.1999.tb00080.x. [DOI] [PubMed] [Google Scholar]
- CRITCHLEY H.D., MATHIAS C.J., JOSEPHS O., O'DOHERTY J., ZANINI S., DEWAR B.K., CIPOLOTTI L., SHALLICE T., DOLAN R.J. Human cingulate cortex and autonomic control: converging neuroimaging and clinical evidence. Brain. 2003;126:2139–2152. doi: 10.1093/brain/awg216. [DOI] [PubMed] [Google Scholar]
- CRITCHLEY H.D., WIENS S., ROTSHTEIN P., OHMAN A., DOLAN R.J. Neural systems supporting interoceptive awareness. Nat. Neurosci. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- CRUZ F., GUIMARAES M., SILVA C., REIS M. Suppression of bladder hyperreflexia by intravesical resiniferatoxin. Lancet. 1997;350:640–641. doi: 10.1016/S0140-6736(05)63330-2. [DOI] [PubMed] [Google Scholar]
- DE GROAT W. Nervous control of the urinary bladder of the cat. Brain Res. 1975;87:201–211. doi: 10.1016/0006-8993(75)90417-5. [DOI] [PubMed] [Google Scholar]
- DE GROAT W. A neurologic basis for the overactive bladder. Urology. 1997;50:36–52. doi: 10.1016/s0090-4295(97)00587-6. [DOI] [PubMed] [Google Scholar]
- DE GROAT W., KAWATANI T., HISAMITSU T., CHENG C., MA C., THOR K., STEERS W., ROPPOLO J. Mechanisims underlying the recovery of urinary bladder function following spinal cord injury. J. Auton. Nerv. System. 1990;30:S71–S78. doi: 10.1016/0165-1838(90)90105-r. [DOI] [PubMed] [Google Scholar]
- DE SEZE M., WIART L., DE SEZE M.P., SOYEUR L., DOSQUE J.P., BLAJEZEWSKI S., MOORE N., BROCHET B., MAZAUX J.M., BARAT M., JOSEPH P.A. Intravesical capsaicin versus resiniferatoxin for the treatment of detrusor hyperreflexia in spinal cord injured patients: a double-blind, randomized, controlled study. J. Urol. 2004;171:251–255. doi: 10.1097/01.ju.0000100385.93801.d4. [DOI] [PubMed] [Google Scholar]
- DE SEZE M., WIART L., FERRIERE J., DE SEZE M.P., JOSEPH P., BARAT M. Intravesical instillation of capsaicin in urology: a review of the literature. Eur. Urol. 1999;36:267–277. doi: 10.1159/000020004. [DOI] [PubMed] [Google Scholar]
- DENNY-BROWN D., ROBERTSON E. On the physiology of micturition. Brain. 1933;56:149–190. [Google Scholar]
- DIXON J., GILPIN C. Presumptive sensory axons of the human urinary bladder. A fine ultrastructural study. J. Anat. 1987;151:199–207. [PMC free article] [PubMed] [Google Scholar]
- DMOCHOWSKI R.R., MIKLOS J.R., NORTON P.A., ZINNER N.R., YALCIN I., BUMP R.C. Duloxetine versus placebo for the treatment of North American women with stress urinary incontinence. J. Urol. 2003;170:1259–1263. doi: 10.1097/01.ju.0000080708.87092.cc. [DOI] [PubMed] [Google Scholar]
- FERGUSON D., KENNEDY I., BUTON T. ATP is released from rabbit urinary bladder epithelial cells by hydrostatic pressure changes – a possible sensory mechanism. J. Physiol. 1997;505:503–511. doi: 10.1111/j.1469-7793.1997.503bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOWLER C.J. Neurological disorders of micturition and their treatment. Brain. 1999;122:1213–1231. doi: 10.1093/brain/122.7.1213. [DOI] [PubMed] [Google Scholar]
- FOWLER C.J. Intravesical treatment of overactive bladder. Urology. 2000;55:60–64. doi: 10.1016/s0090-4295(99)00498-7. [DOI] [PubMed] [Google Scholar]
- FOWLER C.J., HARPER M., FRY C.H. Voiding and the sacral reflex arc: lessons from capsaicin instillation. Scand. J. Urol. Nephrol. 2002;210 (Suppl):46–50. doi: 10.1080/003655902320765953. [DOI] [PubMed] [Google Scholar]
- FOWLER C.J., JEWKES D., MCDONALD W.I., LYNN B., DEGROAT W.C. Intravesical capsaicin for neurogenic bladder dysfunction (letter) Lancet. 1992;339:1239. doi: 10.1016/0140-6736(92)91186-c. [DOI] [PubMed] [Google Scholar]
- GARRETT V., SCOTT J., COSTICH J., AUBREY D., GROSS J. Bladder emptying assessment in stroke patients. Arch. Phys. Med. Rehabil. 1989;70:41–43. [PubMed] [Google Scholar]
- GILLENWATER J.Y., WEIN A.J. Summary of the National Institute of Arthritis, Diabetes, Digestive and Kidney Diseases Workshop on Interstitial Cystitis, National Institutes of Health, Bethesda, Maryland, August 28–29, 1987. J. Urol. 1988;140:203–206. doi: 10.1016/s0022-5347(17)41529-1. [DOI] [PubMed] [Google Scholar]
- GRIFFITHS D., MCCRACKEN P., HARRISON G., MOORE K. Urge incontinence in the elderly: the brain factor. Scand. J. Urol. Nephrol. 1994;157:83–88. [PubMed] [Google Scholar]
- GRIFFITHS D.J. The Pontine micturition centres. Scand. J. Urol. Nephrol. 2002;210 (Suppl):21–26. doi: 10.1080/003655902320765926. [DOI] [PubMed] [Google Scholar]
- KAVIA R., DASGUPTA R., FOWLER C.J. Functional imaging and central control of the bladder. J. Comp. Neurol. 2005;493:27–32. doi: 10.1002/cne.20753. [DOI] [PubMed] [Google Scholar]
- KEAY S., ZHANG C.O., CHAI T., WARREN J., KOCH K., GRKOVIC D., COLVILLE H., ALEXANDER R. Antiproliferative factor, heparin-binding epidermal growth factor-like growth factor, and epidermal growth factor in men with interstitial cystitis versus chronic pelvic pain syndrome. Urology. 2004;63:22–26. doi: 10.1016/j.urology.2003.08.024. [DOI] [PubMed] [Google Scholar]
- KHAN Z., STARER P., YANG W.C., BHOLA A. Analysis of voiding disorders in patients with cerebrovascular accidents. Urology. 1990;35:265–270. doi: 10.1016/0090-4295(90)80048-r. [DOI] [PubMed] [Google Scholar]
- KIM J.H., RIVAS D.A., SHENOT P.J., GREEN B., KENNELLY M., ERICKSON J.R., O'LEARY M., YOSHIMURA N., CHANCELLOR M.B. Intravesical resiniferatoxin for refractory detrusor hyperreflexia: a multicenter, blinded, randomized, placebo-controlled trial. J. Spinal Cord Med. 2003;26:358–363. doi: 10.1080/10790268.2003.11753706. [DOI] [PubMed] [Google Scholar]
- KIRBY R.S., FOWLER C.J., GOSLING J., BANNISTER R. Urethro-vesical dysfunction in progressive autonomic failure with multiple system atrophy. J. Neurol. Neurosurg. Psychiatry. 1986;49:554–562. doi: 10.1136/jnnp.49.5.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIRCHHOF K., FOWLER C.J. The value of the Kurtzke Functional Systems Scales in predicting incomplete bladder emptying. Spinal Cord. 2000;38:409–413. doi: 10.1038/sj.sc.3101022. [DOI] [PubMed] [Google Scholar]
- KIRKHAM A.P., KNIGHT S.L., CRAGGS M.D., CASEY A.T., SHAH P.J. Neuromodulation through sacral nerve roots 2 to 4 with a Finetech–Brindley sacral posterior and anterior root stimulator. Spinal Cord. 2002;40:272–281. doi: 10.1038/sj.sc.3101278. [DOI] [PubMed] [Google Scholar]
- KUROIWA Y., TOHGI H., ITOH M. Frequency and urgency of micturition in hemiplegic patients: relationship to hemisphere laterality of lesions. J. Neurol. 1987;234:100–102. doi: 10.1007/BF00314111. [DOI] [PubMed] [Google Scholar]
- LAZZERI M., BENEFORTI P., TURINI D. Urodynamic effects of intravesical resiniferatoxin in humans: preliminary results in stable and unstable detrusor. J. Urol. 1997;158:2093–2096. doi: 10.1016/s0022-5347(01)68164-3. [DOI] [PubMed] [Google Scholar]
- MAGGI C.A., MELI A. The role of neuropeptides in the regulation of the micturition reflex. J. Auton. Pharmacol. 1986;6:133–162. doi: 10.1111/j.1474-8673.1986.tb00640.x. [DOI] [PubMed] [Google Scholar]
- MAMAS M.A., REYNARD J.M., BRADING A.F. Augmentation of nitric oxide to treat detrusor-external sphincter dyssynergia in spinal cord injury. Lancet. 2001;357:1964–1967. doi: 10.1016/S0140-6736(00)05069-8. [DOI] [PubMed] [Google Scholar]
- MATSUURA S., KAKIZAKI H., MITSUI T., SHIGA T., TAMAKI N., KOYANAGI T. Human brain region response to distention or cold stimulation of the bladder: a positron emission tomography study. J. Urol. 2002;168:2035–2039. doi: 10.1016/S0022-5347(05)64290-5. [DOI] [PubMed] [Google Scholar]
- MATTHEWS S.C., PAULUS M.P., SIMMONS A.N., NELESEN R.A., DIMSDALE J.E. Functional subdivisions within anterior cingulate cortex and their relationship to autonomic nervous system function. Neuroimage. 2004;22:1151–1156. doi: 10.1016/j.neuroimage.2004.03.005. [DOI] [PubMed] [Google Scholar]
- MORRISON J., BIRDER L.A., CRAGGS M., DE GROAT W.C., DOWNIE J.W., DRAKE M.J., FOWLER C.J., THOR K.2005Neural control Incontinenceeds. Abrams, P., Cardozo, L., Wein, A.J. & Khoury, S., pp. 373–374.Plymouth: Health Publication Ltd [Google Scholar]
- MORRISON J.F., SATO A., SATO Y., YAMANISHI T. The influence of afferent inputs from skin and viscera on the activity of the bladder and the skeletal muscle surrounding the urethra in the rat. Neurosci. Res. 1995;23:195–205. doi: 10.1016/0168-0102(95)00942-m. [DOI] [PubMed] [Google Scholar]
- NOUR S., SVARER C., KRISTENSEN J.K., PAULSON O.B., LAW I. Cerebral activation during micturition in normal men. Brain. 2000;123 (Part 4):781–789. doi: 10.1093/brain/123.4.781. [DOI] [PubMed] [Google Scholar]
- OPPENHEIMER D.R. Lateral horn cells in progressive autonomic failure. J. Neurol. Sci. 1980;46:393–404. doi: 10.1016/0022-510x(80)90064-7. [DOI] [PubMed] [Google Scholar]
- PARDO J.V., FOX P.T., RAICHLE M.E. Localization of a human system for sustained attention by positron emission tomography. Nature. 1991;349:61–64. doi: 10.1038/349061a0. [DOI] [PubMed] [Google Scholar]
- POPAT R., APOSTOLIDIS A., KALSI V., GONZALES G., FOWLER C.J., DASGUPTA P. A Comparison between the response of patients with idiopathic detrusor overactivity and neurogenic detrusor overactivity to the first intradetrusor injection of botulinum-a toxin. J. Urol. 2005;174:984–988. doi: 10.1097/01.ju.0000169480.43557.31. [DOI] [PubMed] [Google Scholar]
- REITZ A., KNAPP P.A., MUNTENER M., SCHURCH B. Oral nitric oxide donors: a new pharmacological approach to detrusor-sphincter dyssynergia in spinal cord injured patients. Eur. Urol. 2004;45:516–520. doi: 10.1016/j.eururo.2003.11.006. [DOI] [PubMed] [Google Scholar]
- REITZ A., STÖHRER M., KRAMER G., DEL POPOLO G., CHARTIER-KASTLER E.J., PANNECK J., BURGDÖRFER H., GÖCKING K., MADERSBACHER H., SCHUMACHER S., RICHTER R., VON TOBEL J., SCHURCH B. European experience of 200 cases treated with Botulinum-A toxin injections into the detrusor muscle for neurogenic incontinence. Eur. Urol. 2003;2:140. doi: 10.1016/j.eururo.2003.12.004. [DOI] [PubMed] [Google Scholar]
- RESNICK N. Urinary incontinence. Lancet. 1995;346:94–99. doi: 10.1016/s0140-6736(95)92117-6. [DOI] [PubMed] [Google Scholar]
- RORDEN C., BRETT M. Stereotaxic display of brain lesions. Behav. Neurol. 2000;12:191–200. doi: 10.1155/2000/421719. [DOI] [PubMed] [Google Scholar]
- SAKAKIBARA R., HATTORI T., UCHIYAMA T., KITA K., ASAHINA M., SUZUKI A., YAMANISHI T. Urinary dysfunction and orthostatic hypotension in multiple system atrophy: which is the more common and earlier manifestation. J. Neurol. Neurosurg. Psychiatry. 2000;68:65–69. doi: 10.1136/jnnp.68.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAKAKIBARA R., HATTORI T., YASUDA K., YAMANISHI T. Micturitional disturbance after acute hemispheric stroke: analysis of the lesion site by CT and MRI. J. Neurol. Sci. 1996;137:47–56. doi: 10.1016/0022-510x(95)00322-s. [DOI] [PubMed] [Google Scholar]
- SCHMIDT R.A., JONAS U., OLESON K.A., JANKNEGT R.A., HASSOUNA M.M., SIEGEL S., VAN KERREBROECK P.E., GROUP F.T.S.N.S.S. Sacral nerve stimulation for treatment of refractory urinary urge incontinence. J. Urol. 1999;162:352–357. [PubMed] [Google Scholar]
- SCHURCH B., STOHRER M., KRAMER G., SCHMID D.M., GAUL G., HAURI D. Botulinum-A toxin for treating detrusor hyperreflexia in spinal cord injured patients: a new alternative to anticholinergic drugs? Preliminary results. J. Urol. 2000;164:692–697. doi: 10.1097/00005392-200009010-00018. [DOI] [PubMed] [Google Scholar]
- SILVA C., RIBERIO M.J., CRUZ F. The effect of intravesical resiniferatoxin in patients with idipathic detrusor instability suggests that involuntary detrusor contractions are triggered by C-fiber input. J. Urol. 2002;168:575–579. [PubMed] [Google Scholar]
- SMET P., MOORE K., JONAVICIUS J. Distribution and colocalization of calcitonin gene-related peptide, tachykinins and vasoactive intestinal polypeptide in normal and idiopathic unstable human urinary bladder. Lab. Invest. 1997;77:37–49. [PubMed] [Google Scholar]
- SUNG J.H., MASTRI A.R., SEGAL E. Pathology of the Shy-Drager syndrome. J. Neuropathol. Exp. Neurol. 1978;38:253–268. doi: 10.1097/00005072-197907000-00001. [DOI] [PubMed] [Google Scholar]
- SWANSON L.W., MOGENSON G.J. Neural mechanisms for the functional coupling of autonomic, endocrine and somatomotor responses in adaptive behavior. Brain Res. 1981;228:1–34. doi: 10.1016/0165-0173(81)90010-2. [DOI] [PubMed] [Google Scholar]
- THOR K.B., KATOFIASC M.A. Effects of duloxetine, a combined serotonin and norepinephrine reuptake inhibitor, on central neural control of lower urinary tract function in the chloralose-anesthetized female cat. J. Pharmacol. Exp. Ther. 1995;274:1014–1024. [PubMed] [Google Scholar]
- TOMINAGA M., WADA M., MASU M. Potentiation of capsaicin receptor activity by metabotropic ATP receptors as a possible mechanism for ATP-evoked pain and hyperalgesia. Proc. Natl. Acad. Sci. U.S.A. 2001;98:6951–6956. doi: 10.1073/pnas.111025298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TSUCHIDA S., NOTO H., YAMAGUCHI O., ITOH M. Urodynamic studies on hemiplegic patients after cerebrovascular accident. Urology. 1983;21:315–318. doi: 10.1016/0090-4295(83)90099-7. [DOI] [PubMed] [Google Scholar]
- UCHIYAMA T., SAKAKIBARA R., HATTORI T., YAMANISHI T. Short-term effect of a single levodopa dose on micturition disturbance in Parkinson's disease patients with the wearing-off phenomenon. Mov. Disord. 2003;18:573–578. doi: 10.1002/mds.10403. [DOI] [PubMed] [Google Scholar]
- VALET M., SPRENGER T., BOECKER H., WILLOCH F., RUMMENY E., CONRAD B., ERHARD P., TOLLE T.R. Distraction modulates connectivity of the cingulo-frontal cortex and the midbrain during pain – an fMRI analysis. Pain. 2004;109:399–408. doi: 10.1016/j.pain.2004.02.033. [DOI] [PubMed] [Google Scholar]
- WADE D., LANGTON HEWER R. Outlook after an acute stroke: urinary incontinence and loss of consciousness compared in 532 patients. Quart. J. Med. 1985;56:601–608. [PubMed] [Google Scholar]
- WINGE K., WERDELIN L.M., NIELSEN K.K., STIMPEL H. Effects of dopaminergic treatment on bladder function in Parkinson's disease. Neurourol. Urodyn. 2004;23:689–696. doi: 10.1002/nau.20054. [DOI] [PubMed] [Google Scholar]
- WISEMAN O.J., BRADY C.M., HUSSAIN I.F., DASGUPTA P., WATT H., FOWLER C.J., LANDON D.N. The ultrastructure of bladder lamina propria nerves in healthy subjects and patients with detrusor hyperreflexia. J. Urol. 2002;168:2040–2045. doi: 10.1016/S0022-5347(05)64291-7. [DOI] [PubMed] [Google Scholar]
- WISEMAN O.J., FOWLER C.J., LANDON D.N. The role of the human bladder lamina propria myofibroblast. Br. J. Urol. Int. 2003;91:89–93. doi: 10.1046/j.1464-410x.2003.03802.x. [DOI] [PubMed] [Google Scholar]
- WOOD J.N., GRAFMAN J. Human prefrontal cortex: processing and representational perspectives. Nat. Rev. Neurosci. 2003;4:139–147. doi: 10.1038/nrn1033. [DOI] [PubMed] [Google Scholar]
- WU C., SUI G.P., FRY C.H. Purinergic regulation of guinea pig suburothelial myofibroblasts. J. Physiol. 2004;559:231–243. doi: 10.1113/jphysiol.2004.067934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WYNDAELE J. Studies on the different clinical sensations during bladder filling. Neurourol. Urodyn. 1990;9:353. [Google Scholar]
- YIANGOU Y., FACER P., FORDE A., BRADY C., WISEMAN O., FOWLER C.J., ANAND P. Vanilloid receptor VR1 and ATP-gated ion channel P2x3 in human bladder. Br. J. Urol. Int. 2001;87:774–779. doi: 10.1046/j.1464-410x.2001.02190.x. [DOI] [PubMed] [Google Scholar]
- YOSHIDA M., MIYAMAE K., IWASHITA H., OTANI M., INADOME A. Management of detrusor dysfunction in the elderly: changes in acetylcholine and adenosine triphosphate release during aging. Urology. 2004;63:17–23. doi: 10.1016/j.urology.2003.11.003. [DOI] [PubMed] [Google Scholar]
- YOSHIMURA N. Bladder afferent pathway and spinal cord injury: possible mechanisms inducing hyperreflexia of the urinary bladder. Prog. Neurobiol. 1999;57:583–606. doi: 10.1016/s0301-0082(98)00070-7. [DOI] [PubMed] [Google Scholar]
- YOSHIMURA N., SASA M YOSHIDA O, TAKAORI S. Dopamine D1 receptor-mediated inhibition of micturition reflex by central dopamine from the substantia nigra. Neurourol. Urodyn. 1992;11:535–545. [Google Scholar]