Abstract
There are several conditions associated with dysfunction of the lower urinary tract or which result in a reduction in the ability to engage in satisfactory sexual function and result in significant bother to sufferers, partners and/or carers. This review describes some of the animal models that may be used to discover safe and effective medicines with which to treat them. While alpha adrenoceptor antagonists and 5-alpha-reductase inhibitors deliver improvement in symptom relief in benign prostatic hyperplasia sufferers, the availability of efficacious and well-tolerated medicines to treat incontinence is less well served. Stress urinary incontinence (SUI) has no approved medical therapy in the United States and overactive bladder (OAB) therapy is limited to treatment with muscarinic antagonists (anti-muscarinics). SUI and OAB are characterised by high prevalence, a growing ageing population and a strong desire from sufferers and physicians for more effective treatment options. High patient numbers with low presentation rates characterizes sexual dysfunction in men and women. The introduction of Viagra™ in 1998 for treating male erectile dysfunction and the success of the phosphodiesterase type 5 inhibitor class (PDE5 inhibitor) have indicated the willingness of sufferers to seek treatment when an effective alternative to injections and devices is available. The main value of preclinical models in discovering new medicines is to predict clinical outcomes. This translation can be established relatively easily in areas of medicine where there are a large number of drugs with different underlying pharmacological mechanisms in clinical usage. However, apart from, for example, the use of PDE5 inhibitors to treat male erectile dysfunction and the use of anti-muscarinics to treat OAB, this clinical information is limited. Therefore, current confidence in existing preclinical models is based on our understanding of the biochemical, physiological, pathophysiological and psychological mechanisms underlying the conditions in humans and how they are reflected in preclinical models. Confidence in both the models used and the pharmacological data generated is reinforced if different models of related aspects of the same disorder generate confirmatory data. However, these models will only be fully validated in retrospect once the pharmacological agents they have helped identify are tested in humans.
Keywords: Bladder, incontinence, lower urinary tract, sexual function, animal models, drugs, translational medicine
Animal models of lower urinary tract function and dysfunction
The two major functions of the lower urinary tract (LUT) are to store urine and, periodically and at an opportune moment, to empty or expel the stored urine. This involves a complex pattern of efferent (motor) and afferent (sensory) signalling in both the autonomic and somatic nervous systems. The nerves involved form part of a reflex pathway, with an incorporated conscious control component, which can either maintain the bladder in an accommodating state, enabling urine storage at low intravesical pressure, or which can initiate micturition by relaxing the outflow region (urethra) and contracting the bladder (detrusor) smooth muscle. Pathological conditions with regard to LUT function can be broadly classified into those affecting bladder function (including overactive bladder (OAB), urge incontinence, urge without incontinence and frequency) and those affecting urethral function (including stress urinary incontinence (SUI)).
To fully investigate and develop pharmacological therapies for the treatment of urinary incontinence, an understanding of both the normal physiological control of continence and conditions of pathophysiological dysfunction is required. In this regard, it is paramount to utilise animal models that most closely resemble the human LUT, both in terms of anatomical and physiological function. A wide variety of animal species have previously been utilised to investigate LUT function, including non-human primates, dogs, pigs, cats, rabbits, rats, guinea-pigs, mice and hamsters (Harada et al., 1989; Kontani et al., 1992; Yoshimura et al., 1993; Danuser & Thor, 1996; Ghoniem et al., 1996; Watanabe et al., 1997; Giuliani et al., 1998; Yoshimura et al., 1998; Zvara et al., 1998; Doi et al., 1999; Nickel & Venker-van Haagen, 1999; Palea & Pietra, 1999; Pandita et al., 2000; Bae et al., 2001; Calvert et al., 2001; Lecci et al., 2001), the majority of these species sharing a number of common anatomical (DeLancey, 1990; Dwyer & Glenning, 1990; Birder & de Groat, 1993; Fletcher, 1996; Neuhaus et al., 1999; 2001; Dass et al., 2001; Ganzer et al., 2002; 2004; Silva & Karram, 2004), pharmacological (de Groat & Yoshimura, 2001) and neurophysiological (de Groat, 1998; Blok & Holstege, 1999; Blok, 2002) features in comparison to humans. However a number of these species are also known to exhibit specific differences in normal urinary tract structure and function in comparison to humans, which must be considered before extrapolating pharmacological or physiological findings to potential therapeutic treatments in man. With regard to existing knowledge of the pharmacological treatment and understanding of the LUT, a number of extensive reviews are available and recommended to the reader (de Groat & Yoshimura, 2001; Andersson et al., 2002; Moreland et al., 2004).
Models of bladder function
In the bladder, it is known that all species apart from humans and Old World monkeys possess a dual cholinergic (acetylcholine) and purinergic (ATP) excitatory innervation to bladder smooth muscle (Brindley & Craggs, 1976; Craggs & Stephenson, 1985; Craggs et al., 1986; Fujii, 1988; Brading & Mostwin, 1989; Sneddon & Mclees, 1992; Hashitani et al., 2000; Vial & Evans, 2000; de Groat & Yoshimura, 2001; Pessina et al., 2001), thought to be due to the evolutionary requirement of certain species to mark or scent their territory with urine. It has been shown however that normal human bladder smooth muscle cells express functional ATP receptors (P2X1) (Inoue & Brading, 1991; Bayliss et al., 1999; Elneil et al., 2001). Interestingly, there is evidence that functional purinergic innervation may be present in bladder smooth muscle from patients exhibiting pathological conditions such as interstitial cystitis or obstruction (Palea et al., 1993; Bayliss et al., 1999; Andersson, 2002), suggesting that the presence of such innervation in animal models does not reduce their potential importance. Interestingly, a significant anatomical difference is known to occur between rats and humans in that the postganglionic parasympathetic cell bodies innervating the rat bladder reside wholly in the pelvic ganglia as opposed to other species where a substantial proportion of these cell bodies are contained within the bladder wall; the potential importance of this in the interpretation of in vivo experiments in the rat has received little interest, although it is known that unlike other species' bladder from obstructed rats do not undergo partial denervation (Gabella & Uvelius, 1990).
Bladder cystometry
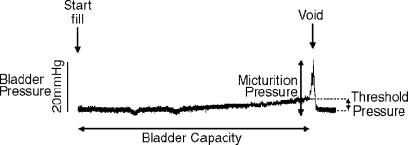
Regardless of the species or model, the most commonly utilised means of exploring bladder function is that of cystometry (Doi et al., 1999; Pandita et al., 2000; Testa et al., 2001; Gu et al., 2002), the slow filling of the bladder while measuring intravesical pressure, via a bladder dome or urethral cannula, until the point of fullness in order to elicit a micturition or voiding response (Figure 1); this can be performed in either anaesthetised or conscious animals with the aid of telemetry, although in conscious animals a measure of intra-abdominal pressure is also advisable to account for transmitted pressure increases from the abdominal cavity. The effect of drugs, nerve ligation or stimulation or addition of intravesical treatments on bladder function can thus be assessed. Similarly, cystometry can be utilised to assess the differences in bladder function between normal and knockout animals (Cockayne et al., 2000; Birder et al., 2002) or between control animals and those in which some form of pathology has been introduced (Pandita et al., 2000). The measurable end points determined in the majority of studies are bladder capacity, threshold pressure, micturition pressure and residual volume; in addition, a measure of bladder wall compliance can be interpreted by the pressure–volume relationship during bladder filling (Figure 1). Multiple studies have suggested the potential for changes in cystometric parameters in animals, reminiscent of those changes seen in humans after application of drugs such as the GABAB receptor agonist baclofen (Giuliani et al., 1992; Igawa et al., 1993; Watanabe et al., 1997) and the TRPV1 activators capsaicin and resiniferatoxin (Ishizuka et al., 1995). Similarly, clinically utilised anti-muscarinics are known to reduce micturition pressure in animal models (Modiri et al., 2002). Such results suggest that the basic control of micturition, as is evident during anaesthetised or conscious cystometry in animals, is indicative of micturition control in humans. This is particularly pertinent when it is considered that cystometry can also be utilised in the clinical investigation of bladder function (Flisser & Blaivas, 2002).
Figure 1.
An example trace of normal bladder cystometry in a urethane-anaesthetised guinea-pig. As saline is infused into the empty bladder (Start Fill) at a constant rate, bladder pressure initially remains low; however, as bladder volume increases, a slow rise in intravesical pressure is evident. Bladder filling continues until a threshold volume (which is equivalent to the bladder capacity) and threshold pressure (equivalent to the difference in pressure between that at the initiation of filling (baseline pressure) and that measured immediately prior to the initiation of the voiding response) are reached to initiate micturition. At this time, urethral pressure decreases and bladder pressure increases (micturition pressure) in order to allow expulsion of bladder contents.
Isovolumetric cystometry
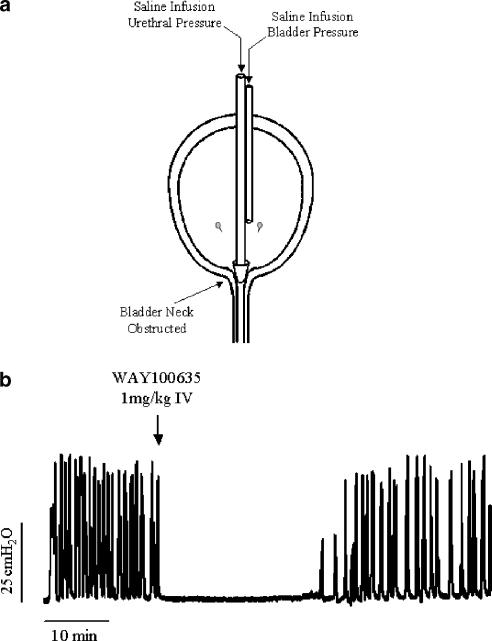
As a variation of normal bladder cystometry, the isovolumetric model examines the behaviour of the urinary bladder after acute and complete ligation or blockade of the bladder neck and urethra, effectively removing the ability of the bladder to empty and so creating an isovolumetric system (Figure 2a). Most commonly utilised in the rat, upon filling the bladder in the isovolumetric state, bladder volume increases until a micturition event is evoked, at which time filling is stopped and the bladder then continues to generate rhythmic isovolumetric contractions of similar amplitude, frequency and duration. The effect of drugs or other stimulations on the amplitude and frequency of rhythmic bladder contractions (RBCs) can then be investigated (Birder & de Groat, 1993; Lecci et al., 1993; Yoshiyama et al., 1993) (Figure 2b). An obvious disadvantage of such a method is the potential damage that may occur to the bladder neck and proximal urethra or their innervation, particularly with ligation. However, methods developed to block the bladder neck, while allowing simultaneous perfusion of the urethra, allow investigation of urethral reflex effects on bladder activity. Owing to the highly non-physiological nature of isovolumetric investigation, interpretation of drug effects need to be considered with respect to what is known about normal bladder function; however, a number of studies have shown effects with drugs known to alter measured parameters during standard cystometry. Both capsaicin and resiniferatoxin, when applied intravesically, have been shown to cause an initial increase in the frequency of RBCs followed by a prolonged inhibition (Cheng et al., 1999; Komiyama et al., 1999), suggesting the importance of afferent TRPV1-expressing nerve fibres in the occurrence of RBCs. Interestingly, experiments investigating the expression of c-fos in L6 spinal cord neurones during normal cystometry showed increased c-fos expression largely in the region of the sacral parasympathetic nucleus, with some staining evident in the dorsal commissure. In comparison, nociceptive stimulation/irritation with intravesical acetic acid markedly increased the number of c-fos-positive cells in the dorsal commissure as did isovolumetric cystometry (Birder & de Groat, 1993). The 5-HT1A antagonist WAY100635 and the ORL-1 agonist nociceptin have both been shown to dose-dependently inhibit RBCs (Lecci et al., 2000; Kakizaki et al., 2001) (Figure 2b) and to increase bladder capacity in standard cystometry (Giuliani et al., 1998; Testa et al., 2001).
Figure 2.
Isovolumetric bladder cystometry. (a) In order to achieve isovolumetric conditions during cystometry, the bladder neck is obstructed either via the external urethral meatus, in which case only bladder pressure can be measured, or via the bladder dome as shown, in which case both bladder and urethral pressure can be measured. (b) Upon filling, bladder volume increases until a micturition reflex is evoked, and when bladder filling is stopped, the bladder continues to exhibit reflex, RBCs. The 5-HT1A antagonist WAY100635 inhibits such reflex contractions.
Irritative cystometry
A further variation of bladder cystometry is to utilise an agent other than saline to infuse into the bladder in order to evoke a painful sensory or irritant response, in particular through C-fibres. Most commonly, acetic acid (up to 1% v v−1) is used as the chemical irritant, although other agents have similar effects. Infusion of acetic acid into the bladder causes an increase in bladder activity, a decrease in bladder capacity or voided volume and a reduction in bladder compliance, while micturition pressure remains normal or is increased. These effects are thought to be due to acetic acid stimulating nociceptive afferent fibres within the bladder wall, potentially mimicking the increased sensory activity, which is thought to occur in OAB and urge (Fowler, 2002). In support of an increased sensory component during irritative cystometry are the findings that increased c-fos expression occurs in rat spinal cord and in regions of the periaqueductal gray with acetic acid in comparison to saline infusion (Birder & de Groat, 1993; Mitsui et al., 2003). Similar studies comparing control rats to those that had previously been exposed to resiniferatoxin in order to desensitise bladder afferent fibres indicate that the increase in c-fos expression that occurs with acetic acid infusion is mediated at least in part by TRPV1-expressing afferent neurones (Avelino et al., 1999). Other investigators have shown that the effects of acetic acid on bladder activity and capacity were absent in resiniferatoxin pretreated rats (Zhang et al., 2003) and in rats where the hypogastric nerves had been transected (Mitsui et al., 2001), again suggesting a strong sensory component in the facilitative effects of acetic acid cystometry on bladder function. Although all available evidence supports the usefulness of irritative cystometry in the identification of compounds or mechanisms important in bladder function and with the potential to treat bladder dysfunction, it should also be considered that acetic acid infusion, particularly at the concentrations commonly utilised, will induce a substantial localised inflammatory response within the bladder wall and cause substantial damage to the bladder urothelium; as such, caution should be exercised when interpreting the effects of compounds known to affect inflammatory mechanisms. In order to reduce the potential impact of inflammation, a milder irritant such as citric acid can also be utilised. Bladder cystometry with citric acid (1–10 mg ml−1, pH 4–4.5) shows similar effects to that with acetic acid, reducing bladder capacity, increasing bladder activity and reducing compliance (Figure 3a); however, histological examination of bladders from control and acetic acid- and citric acid-treated animals showed reduced urothelial damage and inflammation with citric acid (Figure 3b).
Figure 3.
Citric acid bladder cystometry in a urethrane-anaesthetised rat. (a) Infusion of saline into the bladder results in a normal cystometrogram and voiding reflex. Subsequent infusion with citric acid (1 mg ml−1, pH 4.5) leads to shorter filling interval and hence bladder capacity, development of bladder hyperactivity and reduced compliance. (b) Sections of bladder wall (haematoxylin and eosin) from animals treated with saline, citric acid and acetic acid. Note damage to the urothelium (arrow) and suburothelial neutrophilia (*) in the acetic acid-exposed bladder. Trace and sections are courtesy of T.J. Kirkup and J. Cheung.
Ice-water test
Clinical investigations have shown that patients suffering from bladder overactivity of neurogenic origin such as spinal cord damage, Parkinson's disease, multiple sclerosis or various cerebrovascular lesions (Balmaseda et al., 1988; Geirsson et al., 1993; 1995; Ishigooka et al., 1997; Ronzoni et al., 1997; Ismael et al., 2000) exhibit an increased sensitivity and response to the fast infusion of ice-cold saline into the bladder. This is characterised by involuntary sustained bladder contractions and voiding at a threshold volume lower than the normal cystometric capacity observed with warm saline infused at a similar rate and is known as the positive ice-water test (+IWT). It is thought that this bladder-to-bladder excitatory response is mediated by a spinal reflex loop involving C-fibre afferents; individuals with normal bladder function do not exhibit a +IWT (Geirsson et al., 1993; Ishigooka et al., 1997). In a study of elderly patients with uninhibited OAB who lacked a neurological diagnosis but in whom a specific, limited but undetected neuropathy may have existed, the majority were found to exhibit a +IWT (Geirsson et al., 1993); a high occurrence of +IWTs has also been shown in patients with bladder overactivity due to obstruction (Chai et al., 1998; Gotoh et al., 1999; Hirayama et al., 2003) and in children younger than 4 years of age (Geirsson et al., 1994), although other studies have not supported such results. Confirming the sensory nature of this response, spinal cord-injured patients who had previously exhibited bladder hyper-reflexia and a +IWT, showed improvement in bladder function and a negative ice-water test (−IWT) after intravesical treatment with capsaicin (Geirsson et al., 1995). A similar cold-induced bladder reflex response has been identified in α-chloralose-anaesthetised cats and guinea-pigs (Fall et al., 1990; Mazieres et al., 1998; Jiang et al., 2002; Gardiner & Westbrook, 2003), but not anaesthetised rats (Cheng et al., 1997). The potential involvement of afferent C-fibres within this reflex in the guinea-pig has been identified by the inhibition of a +IWT in these species by pretreatment with resiniferatoxin (Gardiner & Westbrook, 2003). Although relatively little has been published with regard to animal models of the ice-water test, such models offer the potential for direct comparison to clinical studies and a further measure of sensory control and function within the micturition cycle.
Conscious metabowl studies
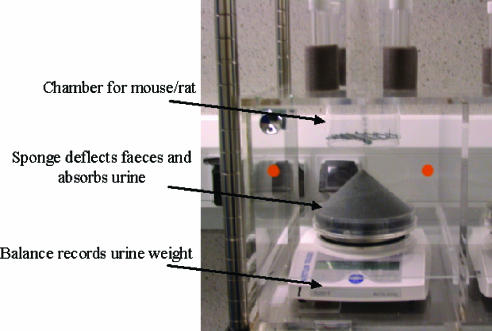
In addition to investigating bladder capacity and activity during cystometric filling via an externalised catheter, measurements of bladder function can also be achieved by investigating normal voided volumes and frequency of voiding during normal filling in conscious animals. This can be carried out by placing animals in individual metabowls with free access to water for 3 h or which have previously been volume loaded with saline. Urine voided by each animal can then be captured on a conical sponge placed within a container beneath each metabowl (Figure 4). The total volume of urine voided within the 3 h period and the volume of urine per void is measured by a balance placed directly beneath the collection container; faecal pellets are either captured on a wire frame above the sponge or deflected by the sponge in order to ensure only urine volume is recorded by the balance. The average volume of urine per void, the total volume voided and the frequency of voiding events can be compared between vehicle- and drug-treated animals, wild-type and knockout mice or animals in which a pathological state has been induced. Changes in these variables, in the absence of changes in the total urine output, are indicative of effects on LUT function. This technique can also be utilised in conjunction with telemetry probes in order to measure bladder pressure and other variables such as body temperature and motor activity; bladder activity recorded during voiding events can also be investigated. Telemetry has previously been used successfully in conscious studies investigating bladder function in multiple species including humans, monkeys and pigs (Thuroff et al., 1981; Miyagawa et al., 1986; Ghoniem et al., 1997; Mills et al., 2000; Fey et al., 2003). Such methods allow various parameters to be monitored continuously in a stress-free and physiologically relevant environment in the absence of anaesthesia, tethering or restraint.
Figure 4.
Metabowl equipment for conscious evaluation of drug effect on normal diuresis filled bladder function and void volume. Different-sized chambers allow for both rat and mouse conscious voided volume experiments and combined with telemetry allow for measurement of bladder pressure and other parameters (courtesy of S. Lewis, Pfizer)
Bladder outlet obstruction
OAB in men is often associated with urethral obstruction due to benign prostatic hyperplasia (BPH). Similar effects have been achieved in multiple animal species by partial obstruction of the urethra using some form of ligature that either immediately occludes the urethra or that increasingly occludes the urethra as the animal grows (Sibley, 1985; 1987; Kato et al., 1988; Pampinella et al., 1997; Bao Jun et al., 2000; Pandita et al., 2000; Calvert et al., 2001; Wolffenbuttel et al., 2001; Das et al., 2002). Such models show many of the structural and physiological bladder wall changes as those seen in human obstruction including increased spontaneous myogenic activity, altered responsiveness to stimuli, patchy denervation of the smooth muscle (although not in the rat; Gabella & Uvelius, 1990), muscle hypertrophy, enlarged sensory neurones and parasympathetic ganglia and increased effectiveness of a spinal micturition pathway (Dupont et al., 1995; Brading, 1997; Turner & Brading, 1997). During conscious or anaesthetised cystometry, obstructed animals have often been shown to exhibit increased bladder capacity, residual volume, threshold pressure and micturition pressure in addition to the occurrence of non-voiding contractions (Sibley, 1985; Mostwin et al., 1991; Igawa et al., 1994; O'Connor et al., 1997). Mechanisms known to directly relax bladder smooth muscle, such as β3 adrenoceptor agonists or potassium channel openers, have been shown to be effective in reducing these non-voiding contractions (Woods et al., 2001; Fabiyi et al., 2003). A major factor thought to underlie neuronal, particularly afferent, plasticity in animal models of obstruction and in the human condition is the increased levels of nerve growth factor (NGF) released within the bladder wall (Steers et al., 1991). Further support for the importance of NGF in animal models is the finding that rats immunised with mouse NGF in order to develop autoantibodies did not develop neural plasticity and urinary frequency in response to obstruction (Steers et al., 1996). Afferent plasticity has also been suggested by the increased effectiveness of tachykinin receptor antagonists in affecting micturition parameters in obstructed as compared to control rats (Ishizuka et al., 1994; Bao Jun et al., 2000). Animal models of obstruction offer a system in which unstable contractions are present and occur in conjunction with chronic changes in both afferent and efferent innervation. However, it should be noted that in this model, such changes are the result of a specific insult that is not thought to occur in the great majority of OAB sufferers.
Spontaneously hypertensive rat
The spontaneously hypertensive rat (SHR) is a genetic model of hypertension, which is also known to exhibit abnormal bladder function; in particular, SHRs have been shown to have reduced bladder capacity and voided volume, increased urinary frequency and increased occurrence of non-voiding contractions in comparison to their genetic control strain the Wistar Kyoto (WKY) rat (Persson et al., 1998; Tang et al., 2002). A number of differences in the in vitro activity of bladder smooth muscle have also been shown between SHR and WKY strains, indicative of fundamental changes in the innervation and physiological response to stimulation of the bladder in SHRs (Tong et al., 1996; Persson et al., 1998; Rajasekaran et al., 2005; Schneider et al., 2005). Although the exact cause of this altered bladder behaviour is not fully understood, a major factor may be the increased NGF levels that have been shown to be produced by bladder smooth muscle from SHRs (Clemow et al., 1998; 1999; 2000; Sherer et al., 2000), a factor that is also thought to at least partly underlie the development of hyperactive voiding in obstructed animals. Interestingly, it has also been shown that bladders from SHRs show increased levels of calcitonin gene-related peptide immunoreactive fibres (presumably afferent) and that neuronal cross-sectional area profiles for bladder afferents in the L6–S1 dorsal root ganglia are significantly larger in SHRs than in WKY animals (Clemow et al., 1997; Jahed & Kawaja, 2001). A similar increase in cross-sectional area was also found for bladder neurones within the major pelvic ganglia in SHRs (Clemow et al., 1997). Both these findings are similar to changes associated with obstruction (see above). Further evidence for a similar phenotype in this species in comparison to the pathology caused by urethral obstruction comes from the finding that α1-adrenoceptors antagonists produce a much more pronounced effect on micturition parameters in both SHRs and obstructed rats in comparison to WKY or control animals (Persson et al., 1998; Gu et al., 2002; Tang et al., 2002). As such, it has been suggested that the SHR represents a model in which changes in neuronal morphology and function similar to those that occur with obstruction are present in the absence of inducing an inflammatory insult in the form of urethral ligation. In particular, there appear to be specific changes in the sensory innervation of the bladder in this species. The potential importance of the afferent innervation in this model can be highlighted by the finding that intrathecal application of antisense oligonucleotide against the tetrodotoxin-resistant sodium channel (Nav1.8) reduces bladder hyperactivity (Lee et al., 2002).
Spinal cord injury
The most commonly utilised and highly informative model of a central lesion with respect to LUT function is that of spinal cord injury (SCI). SCI is known to result in bladder dysfunction, including detrusor hyper-reflexia, in humans (Erickson, 1980; Colachis, 1992; Madersbacher, 1999). Not surprisingly, similar effects can be seen in animal models (primarily cats and rats) of spinal cord lesion (for review, see Yoshimura, 1999; de Groat & Yoshimura, 2005), and such models have contributed to a greater understanding of the spinal control of bladder function. In such models, SCI rostral to the lumbosacral level disrupts voluntary and supraspinal control of voiding and induces a considerable reorganization of the micturition reflex pathway. Following SCI in animals, the urinary bladder is initially areflexic, but can then become hyper-reflexic due to development of a spinal micturition reflex pathway, which occurs through plasticity of neuronal connections within the spinal cord; in addition, detrusor-sphincter dyssynergia also commonly develops (de Groat, 1995; de Groat & Yoshimura, 2005). A major factor involved in the initiation of bladder hyper-reflexia is thought to be additional changes in the afferent C-fibre control of the bladder following recovery from SCI (de Groat et al., 1990; de Groat, 1995). In particular, it is thought that the normally silent C-fibres, which do not typically respond to bladder distension during filling, become predominant in the afferent limb of the reflex (de Groat et al., 1990) due to an increase in excitability, which leads to mechanosensitivity. It has been suggested from studies in rats that one of the underlying causes of this increased excitability is a shift in the expression of Na+ channels from a high-threshold TTX-resistant type to a low-threshold TTX-sensitive type (Yoshimura & de Groat, 1997). A further finding in chronic spinally injured rats is that these C-fibres may, with time, change their phenotype to one resembling Aδ-fibre bladder neurones, which do not respond to capsaicin (Yoshimura et al., 1998). This is not thought to be the case in chronic paraplegic cats (de Groat et al., 1990; Cheng et al., 1999) or in humans (Marianne de Sèze et al., 1998), as capsaicin is effective in affecting micturition parameters. As such, spinal cord-injured animals, particularly cats, offer a reliable model with which to investigate spinal reflex pathways and the control of micturition after SCI and its potential manipulation via electrical stimulation devices in order to treat bladder dysfunction in spinal cord-injured patients (Walter et al., 2005). In addition, such models are also useful to investigate the effect of mechanisms known to act on C-fibre afferents or directly on bladder smooth muscle or its innervation in order to ascertain effects on bladder hyper-reflexia. However, interpretation of results to other forms of bladder overactivity in man needs to take into account the specific nature of the pathology involved.
Models of central lesion or injury
Apart from SCI, a number of central nervous system (CNS) disorders are known to be associated with urinary incontinence in humans, most commonly Parkinson's disease, stroke and multiple sclerosis (Joseph & De, 2001; Sakakibara et al., 2001; Andersson & Pehrson, 2003), all of which are associated with some form of central lesion. As such, it is not surprising that certain animal models in which central lesions are experimentally induced exhibit an abnormal bladder phenotype. In particular, animals with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced lesions and Parkinsonian symptoms exhibit reduced bladder capacity and overactivity during conscious or anaesthetised cystometry (Albanese et al., 1988; Yoshimura et al., 1993; 1998; Dalmose et al., 2004). Similar effects on urinary bladder function can be seen after 6-hydroxydopamine injection into the substantia nigra pars compacta in rats in order to induce Parkinsonian-like symptoms (Kamo et al., 2003). Similar effects are seen in animal models of stroke; in particular, cerebral infarction due to occlusion of the middle cerebral artery in rats leads to significant ischaemia within the putamen and cortex, brain areas known to have important roles in the control of micturition. This has been shown to lead to a reduced bladder capacity, increased frequency and bladder overactivity, both acutely and chronically (Yokoyama et al., 1997; 1998; 2000). Unlike the MPTP-induced bladder hyperactivity model, the effect of drugs has been widely investigated in the cerebral infarcted (CI) rat. It has been shown that the NMDA receptor antagonists dizocilpine or MK-801 and the D2 receptor antagonist sulpiride, alone or in combination, increased bladder capacity in CI but not in control animals, suggesting a role for glutamatergic and dopaminergic stimulation in the development of bladder dysfunction. Similarly, evidence also exists for involvement of nitric oxide synthase and cyclooxygenase-2 in bladder overactivity after cerebral artery occlusion in rats. Drugs that are known to alter bladder function in other animal models have also been shown to affect bladder capacity/overactivity in CI rats, including potassium channel openers, calcium channel antagonists and muscarinic antagonists (Nakamura et al., 1999; Birder et al., 2002; Yokoyama et al., 2005). As can be expected from models of this type, care must be taken in the interpretation of drug action on the micturition response depending on the potential involvement of the drug mechanism directly in lesion-induced effects.
Models of female urethral function
Similar to bladder function models, we must commonly take into account any structural or physiological differences between the various species and man before interpreting results with respect to possible therapeutic potential. Given the preponderance of SUI in females, the majority of investigations into urethral and pelvic floor function with regard to therapeutic potential or animal models of SUI are also carried out in female animals. A number of species have been compared structurally to women including monkey (Ganzer et al., 2004), pig (Dass et al., 2001), dog (Augsburger & Cruzorive, 1995; Nickel & Venker-van Haagen, 1999; Ganzer et al., 2002), cat (Fletcher, 1974; 1996), rabbit (Khanna et al., 1981; Cruz et al., 2002), guinea-pig (Neuhaus et al., 1999) and rat (Poortmans & Wyndaele, 1998; Praud et al., 2003). It is generally accepted that while intraurethral pressure exceeds pressure within the bladder, continence will be maintained. All species utilise both smooth and striated muscles within the urethra to form a urethral sphincter that, in combination with the pelvic floor, is thought to play an important role in promoting continence. With regard to the urethra itself, pressure is generated through the active mechanism of both smooth and striated muscle contraction and the passive mechanism of vascular urothelium that can form an efficient seal. The actual distribution of smooth and striated muscle can differ between species. For instance, in women, striated muscle is thought to occupy greater than two-thirds of the urethral length on the external surface (DeLancey, 1986; Yucel & Baskin, 2004). This is thought to be reasonably similar in the guinea-pig, whereas in the dog striated muscle accounts for approximately 50% of the urethral length and in the pig about 20%. This has obvious implications in the potential importance of differing muscles in maintaining continence in the various species and needs to be considered when investigating changes in urethral function with drug mechanisms before extrapolating to man.
External urethral sphincter electromyographic activity
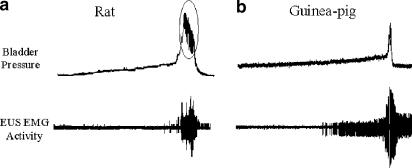
The striated external urethral sphincter (EUS) is thought to provide an important component of the continence mechanism in humans and other species, particularly in times of increased abdominal stress or pressure (Brading, 1999). A widely utilised method for indirectly measuring EUS function is through the measurement of electromyographic (EMG) activity during bladder filling or other manipulation of the urinary tract. Such measurements have highlighted differences in urethral function between certain animals and humans; in particular, many species are thought to utilise their urethral striated sphincter during the micturition process as well as during the storage phase. In rats, the EUS is thought to be active during micturition and to have much less of a role in maintaining continence during anaesthetised cystometry (Conte et al., 1991; Streng et al., 2004). In fact, EMG recording of female rat EUS activity suggests that the EUS becomes more active during micturition than prior to the initiation of the micturition response (Figure 5a) with bursting of activity occurring during voiding. This activity is also reflected in recordings of bladder pressure during the micturition phase whereby oscillations in peak pressure occur with EUS EMG activity. These oscillations are frequently referred to as intraluminal pressure high-frequency oscillations in the literature and are thought to be due to rises in urethral pressure, which may milk urine through the urethra or aid with urine marking of territories (Conte et al., 1991; Van Asselt et al., 1995). Such a milking reflex may occur in other species to differing degrees but is not thought to be present in humans (Brading, 1999) nor in female guinea-pigs (Van Asselt et al., 1995), which show a complete inhibition of EUS EMG activity during voiding (Figure 5b). With regard to the potential of EUS EMG activity to detect drug-induced changes in urethral function, it is interesting to note that duloxetine, which has recently obtained registration for the treatment of SUI in Europe, has been shown to increase EUS EMG activity in response to bladder filling in cats and guinea-pigs (Katofiasc et al., 2002).
Figure 5.
Normal saline cystometry in urethane-anaesthetised female CD rat and Dunkin Hartley guinea-pig. (a) In rat cystometry, bladder filling occurs as in other species; however, little EUS EMG activity does not appear to become active during the filling phase. Upon initiation of micturition, EUS EMG activity increases and high-frequency pressure oscillations become apparent in the bladder pressure trace (ringed). (b) With guinea-pig cystometry, bladder filling occurs as in the rat; however, as bladder volume increases, EUS EMG activity becomes apparent and slowly builds as bladder pressure increases. Upon micturition, EUS EMG activity is abolished and voiding occurs. Post void EUS EMG activity returns to promote urethral closure.
Urethral pressure profilometry
Profilometry is carried out both clinically and preclinically using a number of different methodologies. Preclinical profilometry experiments in animal models tend to utilise perfusion, balloon or micro-tip type pressure transducers with either static (Brune et al., 2001) (i.e. the pressure transducer placed at a single or multiple points along the urethra and kept in place to record resting pressure) or pull-through methodology (Rosin et al., 1980). Urethral function in a number of species has been investigated using pressure profilometry, including rat (Resplande et al., 2002), rabbit (Bodeker et al., 1975), cat (Gookin et al., 1996) and pig (Bridgewater et al., 1993; Greenland et al., 1996). However, the most common utility for this technique has been in female dogs, in terms of drug effects (Brune et al., 2001; Buckner et al., 2002), diagnosis of canine incontinence (Rosin & Barsanti, 1981; Richter & Ling, 1985; Gregory, 1994) and in understanding the physiology of urethral function (Watanachote, 1982; Ali-El-Dein & Ghoneim, 2001). In particular, studies in female dogs utilising pull-through methodology not only provide information with regard to overall urethral pressure but also define which regions produce maximum pressure within the urethra at rest and which regions respond upon drug administration, nerve stimulation or stress-induced pressure rises.
Leak point pressure
Leak point pressure again has been utilised both clinically (Khullar & Cardozo, 1998) and preclinically in a number of species (Brune et al., 2001; Buckner et al., 2002; Damaser et al., 2003). The methods utilised to induce leak in animal models can vary however, ranging from simply applying pressure to the abdomen with the palm of the hand in dogs (Brune et al., 2001) to placing rats on a tilt table with a pressure clamp (Chermansky et al., 2004) or utilising a blood pressure (BP) cuff (Rawlings et al., 2001). In all cases however, leak point pressure measurements are taken as the peak bladder pressure prior to leak, measured using a bladder cannula, which can also be used to fill the bladder prior to leak point measurement. Leak point pressure assays do have the advantage of being a dynamic test that directly evaluates the ability of the urethra to protect against leakage caused by increases in abdominal pressure and the potential of drugs to improve this ability. In a study comparing the measurement of urethral function using static and pull-through profilometry and leak point pressures in the dog, the authors concluded that all three methodologies provided useful information and were able to predict dose–effect relationships for α1A adrenoceptor agonist-induced effects on urethral function (Brune et al., 2001).
Retrograde perfusion or retro-resistance pressure
Although both urethral pressure profilometry and leak point pressure measurements have been utilised clinically, they require an intraurethral cannula that then alters normal resting anatomy and sensation in the urethra. In addition, as they fail to discriminate the severity of incontinence between patients, a further methodology has been developed that applies an infusion of saline against the closed EUS. Urethral retrograde perfusion or retro-resistance pressure is defined as that pressure required to achieve and maintain an open urethral sphincter. Initial clinical investigations have shown that the technique can reliably differentiate between normal women and women with SUI and between severity of incontinence (Slack et al., 2004). Preclinically, little work has been carried out to determine the usefulness of retrograde perfusion in the measurement of drug effects in animals. Retrograde perfusion pressure measurements were found to be significantly reduced in rats that had undergone transabdominal urethrolysis in comparison to control rats, suggesting the need for further investigation in a number of animal species (Rodriguez et al., 2005).
Models of urethral dysfunction
A number of animal models of urethral dysfunction have been explored in order to induce similar effects as seen in human SUI. These include pudendal nerve crush or transection (Damaser et al., 2003; Hijaz et al., 2005), vaginal distension or birth trauma (Bakircioglu et al., 2001; Kuo, 2002; Resplande et al., 2002; Damaser et al., 2003; Ferguson et al., 2005), electrocauterisation (Chermansky et al., 2004) and urethrolysis (Rodriguez et al., 2005). All these methodologies induce either nerve or muscle/ligament damage or both in order to reduce the function of the urethral continence mechanism. Measurements of urethral competency in these studies strongly suggest that continence mechanisms are impaired. Indeed, studies investigating the effect of sneezing on urethral function in normal rats and rats that have undergone urethral damage suggest that continence is impaired in these animals (Bakircioglu et al., 2001; Adachi et al., 2003; Damaser et al., 2003). Few studies at present have looked at drug-induced changes in urethral function in such models; however, a recent investigation of sneeze-induced incontinence in birth trauma rats has shown increased urethral function and continence after administration of the noradrenaline reuptake inhibitor nisoxetine (Kaiho et al., 2005). Such results provide encouragement for further investigation.
Animal models of sexual behaviour and function: general considerations
In recent years, our understanding of human sexual function and dysfunctions has grown. It is clear that sexual responses are complex, consisting of a number of coordinated psychological and physiological mechanisms. Therefore, different in vivo models exist, focused on the neurobiology, psychophysiology and different functional components of male and female sexual responses (for reviews on the pharmacology and physiology of sexual function, see Andersson, 2001; Munarriz et al., 2002). This section will briefly highlight some of the preclinical models that are commonly used in sexual function research and, where available, highlight clinical data where the animal models have been predictive of pharmacological activity in humans. The roles of hormones in sexual health, while crucial, are outside the scope of this review and will not be discussed.
Reproductive behaviours vary greatly across species. For example, sexually receptive female rats display short episodes of lordosis (see below) interspersed with running or darting away from the male, whereas female hamsters hold the lordosis posture for many minutes without movement. Similarly, males of some species perform multiple intromissions before ejaculation, while others hold an intromission (or lock) for many minutes before ejaculating. Therefore, before investigating the effects of pharmacological agents on reproductive behaviours, it is important that the physical and temporal aspects of reproductive behaviours are established in the species of interest and can be robustly measured.
Studies should be designed to take into account the normal behaviour of the test animal, for example, whether it is diurnal, nocturnal or crepuscular. The laboratory conditions such as the duration of the light cycle can also influence the data generated. This is particularly important to seasonal breeders; for example, reproductive behaviours decline in male hamsters over a number of weeks if they are housed in day lengths shorter than 12.5 h of light per day due to declining testosterone levels brought about by testicular regression. The size and design of the test chamber can also influence the reproductive behaviours displayed. For example, female paced mating behaviour can only be studied when the test arena allows the female to escape from the male. In summary, the test conditions and experimental environment need to be carefully considered, as they can greatly influence the reproductive behaviours displayed (Cherry, 1993; Price, 1993; Meisel & Sachs, 1994).
When trying to understand how these preclinical models translate to humans, it should also be borne in mind that the primary purpose of sexual activity in animals is reproduction, while in humans it is predominantly recreational. The fact that animal sexual behaviours are highly stereotyped and species specific also makes translational interpretation to humans difficult. The translational interpretation of rat reproductive behaviours to man, including behaviours such as mounting, intromission latencies and lordosis quotients (LQ), which have no obvious equivalents in humans, has been and continues to be widely debated and reviewed (Sachs & Barfield, 1976; Dewsbury, 1979; Pfaus et al., 1990; 2003; Meisel & Sachs, 1994; Pfaus, 1996; Agmo et al., 2004).
Models of male sexual function
Erectile function
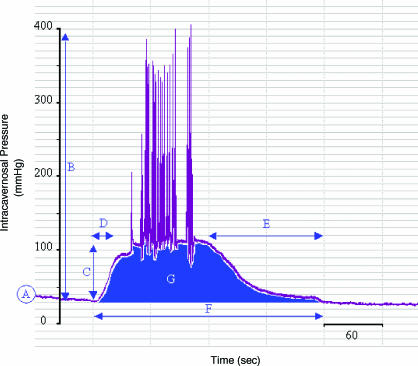
Monitoring intracavernosal pressure (ICP) is the most common method of preclinically monitoring the quality of an erectile response. ICP has been monitored in both conscious and anaesthetised animal models. The development of electronic data capture systems now allows various aspects of the ICP response to be measured (Figure 6). Typically measured endpoints include the basal ICP, peak ICP, plateau ICP, time to erection and detumesence time, duration of response, area under the ICP time response curve and the number of erections observed in a given time period. The aim is to use these end points to quantify the different phases and quality of the ICP response and the effect of the actions of drugs upon them.
Figure 6.
An apomorphine-induced rat penile erection ICP trace. An example of an apomorphine-induced rat penile erection trace recorded using radiotelemetric equipment is shown. Typical measurements include (A) basal ICP, (B) peak ICP, (C) plateau ICP, (D) time to erection, (E) detumescence time, (F) duration of response and (G) area under ICP time response curve.
Anaesthetised animal models of male erectile dysfunction
Nerve stimulation models: In anaesthetised animal models, measurement of ICP is usually performed by the insertion of a hypodermic needle into the body of the corpus cavernosum. The needle is attached to tubing, filled with heparinised saline and connected to a pressure transducer and data capture system. Additionally, systemic BP is monitored and data are usually expressed as a ratio of ICP/BP. This is because ICP is controlled by both penile vascular mechanisms and systemic haemodynamics, and a ratio of ICP over BP provides a measurement reflective of the penile component.
Stimulation of either the cavernous nerve or the pelvic plexus results in an increase in ICP (Rehman et al., 1998). Experiments to investigate the action of novel agents usually involve the selection of submaximal stimulatory frequencies and voltages to evoke an ICP response. The action of a novel agent on this submaximal response is then determined. Studies have been performed in both small and large animals (Carter et al., 1998; Vemulapalli et al., 2001; Ueno et al., 2002). Studies in larger animals such as the dog allow a more detailed investigation of both penile and systemic haemodynamics. Hence, the effects of a novel pharmacological agent on both erectile function and the cardiovascular system may be assessed at the same time in a single experimental animal.
Chemical-induced responses: The action of compounds and neurotransmitters on the penile vasculature can be investigated by infusing agents directly into the corpus cavernosum (Juenemann et al., 1986; Lin & Lin, 1996). Additionally, this technique can be used to stimulate biochemical pathways involved in the control of penile erection, for example, infusion of the nitric oxide donor sodium nitroprusside. The effect of compounds may then be tested on the activated biochemical pathway by monitoring the subsequent downstream functional effects on ICP (Carter et al., 1998).
Compounds and neurotransmitters may also be administered directly to their proposed site of action in the CNS, for example, using stereotaxic procedures to discrete regions of the brain by microinjection or to the spinal cord by intrathecal administration. Additionally, tool compounds and peptides that would not normally cross the blood–brain barrier may be used. These techniques have been successfully used to show that apomorphine has both a central site of action in the paraventricular nucleus (PVN) (Melis et al., 1987) and a spinal pro-erectile site of action (Giuliano et al., 2002). The intrathecal administration of oxytocin helped establish a spinal role for this neurotransmitter in penile erection (Giuliano et al., 2001b).
Electrical stimulation of the central nervous system: Erectile responses may also be induced by stimulation of the CNS. Using stereotaxic procedures, discrete areas of the brain are electrically stimulated and peripheral genital responses (e.g. genital muscle activation or peripheral nerve recordings) are monitored.
The most commonly investigated area of the CNS involved in the modulation of sexual behaviour is the hypothalamus, in particular the medial preoptic area (MPOA) and the PVN. Electrical stimulation of these areas causes an increase in ICP (Giuliano et al., 1996; Chen et al., 1997).
Male rat reproductive behaviours: The rat is the most commonly used animal in male sexual behaviour studies. Male rat reproductive behaviour is a complex series of coordinated motor behaviours and reflexes.
Anogenital investigation of the female rat is usually the first sexual behaviour that male rat displays towards the female. This is followed by a number of mounts and intromissions. A mount is when the male rat mounts the female from the rear and grasps her flanks with his front feet. An intromission is when the male achieves vaginal penetration during a mount. During copulation, male rats mount and intromit a number of times. Following a mount or intromission, the male usually dismounts from the female. At the end of a series (or bout) of intromissions, the male ejaculates. Ejaculation is readily identified by a prolonged intromission, often with deeper thrusting and a slower withdrawing ‘crucifix' posture post-ejaculation. Following ejaculation, the male generally grooms his genitals, ceases sexual interaction with the female for several minutes and issues high ultrasonic vocalisations. Experimental end points such as time of first mount, intromission and ejaculation along with the number of mounts, intromissions and ejaculations in a given period can easily be determined and can be used to help define the copulatory behaviour of the experimental rat.
Conscious models of male erectile dysfunction
Pharmacological agents are commonly tested under the following experimental behavioural paradigms: male animal in isolation and male animal in the presence of but without access to a female animal in behavioural oestrus and during copulation.
Compound-induced erections: Test compound is administered to rats that are not in the presence of female animals and the animal is monitored to see if it induces an erection. These experiments have been used to identify agents that initiate erections, such as apomorphine (Bernabe et al., 1999), melanotan II (MT-II) (Giuliano et al., 2005) and PT-141 (Molinoff et al., 2003). However, care must be taken when designing such experiments and appropriate experimental time-matched controls must be included, as the simple act of handling a rat (such as during dosing) can induce spontaneous erections in the test animal.
Non-contact erections: Non-contact erections are elicited by placing a male animal in the presence of a female animal in oestrus (Sachs et al., 1994). Typically, this is achieved by placing a male animal (usually a rat) in one half of a cage or observation chamber. In the other half is placed a female rat in oestrus (or an ovariectomised female rat placed in behavioural oestrous by the administration of oestrogen and progesterone). A perforated dividing wall separates the two halves of the cage or observation chamber. The dividing wall allows the passage of auditory, visual and pheromonal cues between the two animals. The number of erections or if the animal is telemetered its ICP response is monitored over a given period. As a non-contact evoked erection is dependent on visual, olfactory and auditory cues from the female and not (due to separation by a barrier) due to tactile reflexive mechanisms, the response is believed to be primarily under the influence of forebrain regions of the brain. Male rats with lesions to the MPOA of the hypothalamus show normal non-contact erectile responses and reduced copulatory behaviour (Liu et al., 1997). It has been postulated that the frequency of non-contact erections (alternatively called female-enhanced spontaneous erections) is an indicator of sexual arousal (Sach, 2000).
These studies may be used to identify agents that are facilitators of erectile function. Facilitators are agents that initiate minimal or no erectile responses in their own right, but enhance erectile responses generated by a pro-erectile agent in the presence of a female animal in oestrus (Anderson et al., 1999).
Copulation studies: Copulation studies and the associated end point measures described are routinely used to define the sexual behaviour of a test animal and the effect of a pharmacological agent upon it, or alternatively to phenotypically profile genetically modified animals. These types of studies offer the most comprehensive way of evaluating sexual function in a normal behavioural context. However, as various factors can influence copulatory behaviour, such as motivation, locomotion and the behavioural status/sexual experience of the partner animal, it is difficult to accurately identify the effect of a pharmacological agent on a single physiology such as erection. While end point measures such as the intromission ratio (intromission ratio=number of intromissions/(number of intromissions+number of mounts)) are commonly used as an indicator of erectile function, any observed differences should be followed up with confirmatory experiments using more robust experimental measures such as ICP measurements.
Telemetric recording
ICP may be measured in conscious rats using radiotelemetric techniques (Figure 6) (Bernabe et al., 1999). Following the washout of a test drug, rats may be re-tested with a second dose of the test drug or an alternative agent. Thus, telemetered animals allow the use of statistically robust crossover experimental designs, reducing experimental variability and ultimately the number of test animals. Instrumented rats may be monitored intermittently over many months and therefore the effect of age on the quality of the ICP response (and the development of erectile dysfunction) can be monitored. Additionally, the choice of inbred rat strain to be implanted with the telemetric device (e.g. the diabetic Zucker fa/fa rat) and/or experimental manipulation (e.g. induction of dietary-induced hypercholesterolemia) to mimic the disease situation can further refine the experimental model.
Cavernous nerve injury model
The incidence of erectile dysfunction increases following pelvic surgery, in particular following radical prostatectomy, due to damage of the cavernous nerves. In the cavernous nerve injury model (CNI), the cavernous nerves are either unilaterally or bilaterally sectioned or damaged to impair erectile function. The unilateral CNI model has been used to investigate mechanisms and potential pharmacological and gene therapy treatments associated with nerve regeneration. The bilateral model has been used to investigate the pathological condition and is believed to mimic the condition in humans following radical prostatectomy. It has been used to explore the actions of growth hormones and potential gene therapy approaches (Jung et al., 1998; Bakircioglu et al., 2001; Chen et al., 2005).
Models of ejaculation
Ejaculation is a highly complex, tightly coordinated series of reflexes and mechanisms. Owing to its complexity, ejaculation is very difficult to study experimentally. No preclinical model currently exists in which all components are present and measurable. In recent years, there has been an increase in our knowledge of the neuroanatomical pathways that control ejaculation within the brain (Pfaus & Heeb, 1997; Veening & Coolen, 1998) and the spinal cord (Truitt & Coolen, 2002; Truitt et al., 2003).
Behavioural ejaculation can be identified in experimental animals. The majority of studies on premature or retarded ejaculation have been undertaken in rats with normal sexual behaviour. Recently, it has been shown that within a rat population there exists a subgroup of fast ejaculators and a subgroup of slow ejaculators, each representing approximately 10% of the population at each end of a Gaussian distribution. It has been postulated that these subpopulations are comparable to variations seen in ejaculatory times in the human population (Waldinger et al., 2005a), with the faster ejaculating subset representing a model of premature ejaculation and the slower ejaculating rats representing a model of retarded (an)ejaculation (Waldinger & Olivier, 2005).
A variety of pharmacological agents have been shown to modify sexual behaviour in rats and postulated a role for several neurotransmitters (Bitran & Hull, 1987) and neuropeptides (Argiolas, 1999). 5-HT1A and 5-HT2C receptors are known to control the speed of ejaculation in rats. Activation of 5-HT1A receptors shorten the ejaculation latency time and activation of 5-HT2C receptors delays ejaculation (Ahlenius et al., 1981).
Urogenital reflex as a model of orgasm in both male and female rats
The urogenital reflex (UG reflex) model may be used to study both penile erection and ejaculatory reflexes. In urethane-anaesthetised, acutely spinalised (or brain lesioned) rats, complex coordinated sexual responses may be elicited by urethral distension. In male rats, the elicited response, known as the UG reflex, consists of colonic contractions of the perineal muscles, rhythmic firing of the cavernous nerve, penile erections and ejaculation (Chung et al., 1988; McKenna et al., 1991). The perineal muscles are all activated simultaneously, as occurs in humans at the point of sexual climax (Petersen & Stener, 1970). The paraigoantocellular reticular nucleus in the brainstem is the main source of descending inhibitory control over the spinal sexual reflexes (Marson & Mckenna, 1990). If this region of the brain is lesioned, the inhibitory control is removed and the UG reflex can be evoked in spinally intact animals. The UG reflex may also be evoked in non-spinalised animals by bilateral stimulation of the MPOA (Marson & Mckenna, 1994). This model has predominantly been used to investigate the identification of spinal ejaculatory pattern generators and the supraspinal control of sexual reflexes.
Models of female sexual function
Anaesthetised animal models
Nerve stimulation models: Stimulation of the pelvic nerve in anaesthetised female rats (Vachon et al., 2000; Giuliano et al., 2001a) and rabbits (Munarriz et al., 2003) has been shown to increase vaginal blood flow, clitoral ICP, vaginal wall pressure, vaginal length and blood flow, and decreases vaginal luminal pressure. The effect of pharmacological agents on these end points, which are used as an index of sexual arousal, has been investigated (Min et al., 2000). As women have been reported to experience cyclic fluctuations in sexual arousal and desire that coincide with ovulation and female rats display increased behavioural sexual activity, which coincides with the periovulatory period of their oestrus cycle, it is clear that a knowledge of the hormonal status of the animals is key to the interpretation of results. Commonly, ovariectomised animals are used and hormones (oestrogen and progesterone) are administered to establish the level of oestrus required in the experiment.
The development of female models of peripheral arousal has been hindered by the identification of robust end-point measures. Vaginal and clitoral blood flow is commonly measured in anaesthetised animals using laser Doppler flow probes. The identification of robust preclinical and clinical end-point measures of female genital arousal is required.
Chemical-induced models and electrical stimulation of the CNS: The techniques described for males above may also be utilised in female models.
Female rat reproductive behaviours
The motivation or desire for an animal to engage in a sexual encounter and its ability to physically consummate are separate components of reproductive behaviours. Rats display two distinct patterns of behaviour: motivational, proceptive or solicitous behaviours and receptive or consummatory behaviours. These two distinct components of sexual behaviour, motivation and consummation, have been shown in male rats to be mediated by different brain mechanisms (Everitt, 1990).
Female proceptive behaviours include behaviours such as ‘hopping', ‘darting' and ‘ear wiggling' that serve to gain the attention of the male rat and initiate sexual activity. Receptive behaviours include the commonly reported reflex posture of lordosis (see below). Sexually receptive female rats display short episodes of lordosis interspersed with running or darting away from the male. This is known as ‘paced' mating behaviour, which has been shown to be more sexually rewarding than ‘non-paced' mating behaviour (Paredes & Vazquez, 1999).
Experimental procedures have been established for assessing female proceptive behaviours (Beach, 1976). In the past, this has been achieved experimentally by physically restraining the sexually active male in a number of ways (Meyerson & Lindstrom, 1973; Edwards & Pfeifle, 1983); however, more ethological models have now been developed, which allow ‘paced' mating behaviour to be displayed.
Lordosis is the reflex posture that a sexually receptive female rat adopts in response to appropriate tactile stimulation, such as an attempted mount by a male rat or pressure on the back, flanks or anogenital region. The lordosis posture is characterised by pronounced arching of the back, with the head and hindquarters elevated, back feet extended and tail deflected to one side.
Proceptivity model: The proceptivity model consists of a large circular open field with two diametrically opposed chambers on its outer circumference. The sidewall of each chamber faces into the arena and consists of a holed Perspex wall or grill. A single stimulus animal is placed in each outer chamber and a single test animal is placed into the open-field arena. The amount of time the test animal spends actively seeking each stimulus animal, or the amount of time spent in a designated area next to each stimulus animal, is measured. The holed Perspex wall or grill allows visual, olfactory and auditory cues to pass from the stimulus animals into the open-field arena, but prevents direct contact. The sex, hormonal status and sexual experience of the test and stimulus animals can be varied depending on the purpose of the experiment. For example, the effect of a drug on female sexual motivation could be investigated by placing a sexually active male rat in one chamber and a castrated male rat in the other. The drug-treated female rat would then be placed in the open-field arena and monitored. This model has been used to investigate sexual incentive motivation in male (Hetta & Meyerson, 1978) and female (Meyerson & Lindstrom, 1973; Agmo et al., 2004) rats.
Bilevel chambers: Bilevel chambers are behavioural observation chambers that contain two levels connected together by ramps or ladders. These chambers may be used to explore the proceptive behaviours of both male and female animals. Normal female rat reproductive behaviours involve solicitations by the female rat, which results in the male mounting the female and intromission. Following intromission, the female rat will run away and eventually stop and adopt a lordosis posture in order to allow the male to mount and intromit a further time. This process known as pacing will continue until the rat ejaculates. The number of level changes is believed to give an indication of sexual motivation. Copulation studies performed in these chambers have shown that female hormonally primed ovariectomised rats level change significantly more than non-hormonally primed rats (Mendelson & Gorzalka, 1987). Male rats show an increased amount of level changing when in the presence of an oestrus compared to an anoestrus female rat (Mendelson & Pfaus, 1989), and additionally the amount of level changing decreases following ejaculation (van Furth & van Ree, 1996). Pharmacological studies have included the investigation of the mixed MCR3 and MCR4 agonist PT-141 (Pfaus et al., 2004). Currently, there is debate on whether bilevel chambers allow true sexual pacing, as the female rat can only run away and not escape from the male rat (Agmo et al., 2004).
Escape or unilevel pacing chambers: Escape or pacing chambers consist of behavioural observation chambers that are divided into two by a central vertical divider. The bottom of the divider has a number of holes cut into it that are large enough to allow the female rat to pass between the two chambers but small enough to restrict the male rat to one of the chambers. Thus, the female can ‘escape' from the male and by doing so control or ‘pace' copulation (Paredes & Vazquez, 1999). Pharmacological studies have included investigation of PT-141 (Pfaus et al., 2004) and the actions of 5-HT2A and 5-HT2C agonists (Nedergaard et al., 2004) on proceptive sexual behaviours.
Lordosis model: Owing to its ease of identification, lordosis is one of the most widely investigated reflexes in sexual health research (Pfaff & Schwartz-Giblin, 1988). The lordosis reflex is dependent on the presence of oestrogen and progesterone. The administration of oestrogen alone only produces moderate activation of the reflex. However, in ovariectomised animals treated with oestrogen alone, drugs that bind to oxytocin receptors, opioid receptors, adrenoceptors, dopamine receptors or GABA receptors in certain hypothalamic brain regions have been shown to increase lordosis (Kow et al., 1994; Pfaff, 2001).
The most common method of assessing the degree of lordosis is as LQ, in which the frequency of lordosis is scored as a ratio to a fixed number of mounts (usually 10), for example, LQ=(number of lordosis postures adopted/number of attempted mounts) × 100. The lordosis model does not measure proceptive (or desire) behaviours, instead it is a measure of a receptive behaviour and demonstrates the ability to perform the physical motor movements required for copulation.
Pharmacological translation from animals to humans
Several different mechanistic classes demonstrating efficacy in humans suffering from erectile dysfunction or premature ejaculation have also shown some level of efficacy outcome in animal models.
The actions of sildenafil and other phosphodiesterase type 5 (PDE5) inhibitors in preclinical anaesthetised animal models (Carter et al., 1998) and man (Eardley et al., 2002) are well documented and appear to show good correlation. In particular, the anaesthetised dog ICP model is the model of choice for assessing the relative potency of PDE5 inhibitors. Pharmacokinetic and pharmaodynamic modelling has shown that results obtained from the dog model predict results obtained from clinical Rigiscan studies and phase II outpatient studies. Apomorphine, the mixed D1 and D2 receptor agonist, administered subcutaneously induces penile erection in conscious rats (Melis et al., 1987) and man (Lal et al., 1987). Based on microinjection studies, this agent is believed to act in the PVN (Melis & Argiolas, 1997; Sato et al., 1999; Adachi et al., 2003). Intrathecal administration also suggests that it may have a spinal mechanism of action (Giuliano et al., 2002). Conversely, the dopamine antagonist haloperidol has been shown to reduce sexual arousal in rats (Pfaus & Phillips, 1989) and man (Petrie, 1985). Yohimbine is an alpha-2 adrenoceptor antagonist that has demonstrated modest efficacy in animals and humans. Alpha-2 adrenoceptors are located both centrally and peripherally; however, the pro-erectile site of action of yohimbine remains unclear. Melanotan II (MT-II) is a synthetic non-selective analogue of alpha-melanocyte-stimulating hormone (α-MSH). Melanocortins are implicated in the regulation of sexual behaviour, including sexual motivation, penile erection and, in female rats, the secretion of sexual attractants from the preputial gland (Thody et al., 1981). MT-II has been shown to increase ICP in a rabbit model (Vemulapalli et al., 2001). MT-II has been reported to initiate penile erection in normal men (Dorr et al., 1996) and men with psychogenic (Wessells et al., 1998) and organic erectile dysfunction and also increase sexual desire (Wessells et al., 2000). Overall, there is a reasonable correlation between mechanisms that improve sexual function in male animals and humans.
There is currently no regulatory authority-approved treatment for premature ejaculation. Off label pharmacological treatments include the use of serotonin reuptake inhibitors (SSRIs) (e.g. paroxetine, sertraline, fluoxetine), topical local anaesthetics (e.g. lidocaine) and PDE-5 inhibitors. SSRIs are of particular interest, as they have been tested both acutely and chronically in man (Waldinger et al., 2002; 2004; 2005b) and in rat sexual behaviour models (Mos et al., 1999; Waldinger et al., 2002). There appears to be a good translation between the preclinical and clinical studies with respect to the actions of SSRIs. Acute administrations of SSRIs at non-sedative doses have an effect on ejaculatory parameters in rat and man, while chronic administration delays ejaculation further (Cantor et al., 1999). Acute alcohol intoxication has also been shown to delay ejaculation in both men (Malatesta et al., 1979) and rats (Dewsbury, 1967; Pinel et al., 1992).
Conclusions
In recent years, there has been an increase in research activities concerning the LUT and sexual function and, in particular, the discovery and development of new medicines to treat symptoms associated with these conditions. This is due in part to the discovery of pharmacological agents that effectively treat OAB, BPH and male erectile dysfunction and the bothersome symptoms that drive sufferers of these conditions to seek treatment.
Our current understanding of the physiological, pharmacological and psychological mechanisms involved in the LUT and controlling sexual function are based on human and animal studies. Correlations between preclinical models and clinical data are beginning to be established, which will lead to refinement of existing animal models and should increase their ability to predict clinical efficacy in humans. In the last decade, there has been a significant increase in our understanding of the peripheral and central control of the LUT and male erection control. However, our understanding of the peripheral control of female genital blood flow and the role of the CNS in sexual function in both males and females is less well advanced. Further understanding of the translation between animal models of urological function and clinical benefit in humans will need to wait for detailed clinical evaluation of novel mechanisms in clinical trials.
Glossary
- BP
blood pressure
- BPH
benign prostatic hyperplasia
- CI
cerebral infarcted
- CNI
cavernous nerve injury model
- CNS
central nervous system
- EMG
electromyographic
- EUS
external urethral sphincter
- ICP
intracavernosal pressure
- +IWT
positive ice-water test
- −IWT
negative ice-water test
- LQ
lordosis quotient
- LUT
lower urinary tract
- MPOA
medial preoptic area
- OAB
overactive bladder
- PDE5
phosphodiesterase type 5
- PVN
paraventricular nucleus
- RBCs
rhythmic bladder contractions
- NGF
nerve growth factor
- SCI
spinal cord injury
- SHR
spontaneously hypertensive rat
- SSRIs
serotonin reuptake inhibitors
- SUI
stress urinary incontinence
- WKY
Wistar Kyoto
- UG reflex
urogenital reflex
References
- ADACHI H., SATO Y., KATO R., HISASUE S., SUZUIKI K., MASUMORI N., ITOH N., TSUKAMOTO T. Direct evidence of facilitative actions of dopamine in the medial preoptic area on reflexive and noncontact erections in male rats. J. Urol. 2003;169:386–389. doi: 10.1016/S0022-5347(05)64133-X. [DOI] [PubMed] [Google Scholar]
- AGMO A., TURI L.T., ELLINGSEN E., KASPERSEN H. Preclinical models of sexual desire: conceptual and behavioral analyses. Pharmacol. Biochem. Behav. 2004;78:379–404. doi: 10.1016/j.pbb.2004.04.013. [DOI] [PubMed] [Google Scholar]
- AHLENIUS S., LARSSON K., SVENSSON L. Effects of a new type of 5-HT receptor agonist on male rat sexual behaviour. Pharmacol. Biochem. Behav. 1981;15:785–792. doi: 10.1016/0091-3057(81)90023-x. [DOI] [PubMed] [Google Scholar]
- ALBANESE A., JENNER P., MARSDEN C.D., STEPHENSON J.D. Bladder hyperreflexia induced in marmosets by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Neurosci. Lett. 1988;87:46–50. doi: 10.1016/0304-3940(88)90143-7. [DOI] [PubMed] [Google Scholar]
- ALI-EL-DEIN B., GHONEIM M.A. Effects of selective autonomic and pudendal denervation on the urethral function and development of retention in female dogs. J. Urol. 2001;166:1549–1554. [PubMed] [Google Scholar]
- ANDERSSON K.E. Pharmacology of penile erection. Pharmacol. Rev. 2001;53:417–450. [PubMed] [Google Scholar]
- ANDERSSON K.E. Bladder activation: afferent mechanisms. Urology. 2002;59:43–50. doi: 10.1016/s0090-4295(01)01637-5. [DOI] [PubMed] [Google Scholar]
- ANDERSON K.E., GEMALMAZ H., WALDECK K., CHAPMAN T.N., TUTTLE J.B., STEERS W.D. The effect of sildenafil on apomorphine-evoked increasesin intracavernous pressure in the awake rat. J. Urol. 1999;161:1707–1712. [PubMed] [Google Scholar]
- ANDERSSON K.E., HEDLUND P., WEIN A.J., DMOCHOWSKI R.R., STASKIN D.R. Pharmacologic perspective on the physiology of the lower urinary tract. Urology. 2002;60:13–21. doi: 10.1016/s0090-4295(02)01786-7. [DOI] [PubMed] [Google Scholar]
- ANDERSSON K.E., PEHRSON R. CNS involvement in overactive bladder: pathophysiology and opportunities for pharmacological intervention. Drugs. 2003;63:2595–2611. doi: 10.2165/00003495-200363230-00003. [DOI] [PubMed] [Google Scholar]
- ARGIOLAS A. Neuropeptides and sexual behaviour. Neurosci. Biobehav. Rev. 1999;23:1127–1142. doi: 10.1016/s0149-7634(99)00068-8. [DOI] [PubMed] [Google Scholar]
- AUGSBURGER H.R., CRUZORIVE L.M. Stereological analysis of the urethra in sexually intact and spayed female dogs. Acta Anat. 1995;154:135–142. doi: 10.1159/000147760. [DOI] [PubMed] [Google Scholar]
- AVELINO A., CRUZ F., COIMBRA A. Intravesical resiniferatoxin desensitizes rat bladder sensory fibres without causing intense noxious excitation. A c-fos study. Eur. J. Pharmacol. 1999;378:17–22. doi: 10.1016/s0014-2999(99)00451-3. [DOI] [PubMed] [Google Scholar]
- BAE J.H., MOON D.G., LEE J.G. The effects of a selective noradrenaline reuptake inhibitor on the urethra: an in vitro and in vivo study. Br. J. Urol. Int. 2001;88:771–775. doi: 10.1046/j.1464-4096.2001.02389.x. [DOI] [PubMed] [Google Scholar]
- BAKIRCIOGLU M.E., LIN C.S., FAN P., SIEVERT K.D., KAN Y.W., LUE T.F. The effect of adeno-associated virus mediated brain derived neurotrophic factor in an animal model of neurogenic impotence. J. Urol. 2001;165:2103–2109. doi: 10.1097/00005392-200106000-00078. [DOI] [PubMed] [Google Scholar]
- BALMASEDA M.T., JR., REYNOLDS H.T., GORDON C. The value of the ice water test in the management of the neurogenic bladder. Am. J. Phys. Med. Rehabil. 1988;67:225–227. doi: 10.1097/00002060-198810000-00008. [DOI] [PubMed] [Google Scholar]
- BAO JUN G., ISHIZUKA O., IGAWA Y., NISHIZAWA O., ANDERSSON K.E. Role of supraspinal tachykinins for micturition in conscious rats with and without bladder outlet obstruction. Naunyn-Schmiedebergs Arch. Pharmacol. 2000;361:543–548. doi: 10.1007/s002100000228. [DOI] [PubMed] [Google Scholar]
- BAYLISS M., WU C., NEWGREEN D., MUNDY A.R., FRY C.H. A quantitative study of atropine-resistant contractile responses in human detrusor smooth muscle, from stable, unstable and obstructed bladders. J. Urol. 1999;162:1833–1839. [PubMed] [Google Scholar]
- BEACH F.A. Sexual attractivity, proceptivity, and receptivity in female mammals. Horm. Behav. 1976;7:105–138. doi: 10.1016/0018-506x(76)90008-8. [DOI] [PubMed] [Google Scholar]
- BERNABE J., RAMPIN O., SACH B.D., GIULIANO F. Intracavernous pressure during erection in rats: an integrative approach based on telemetric recording. Am. J. Physiol. 1999;276:R441–R449. doi: 10.1152/ajpregu.1999.276.2.R441. [DOI] [PubMed] [Google Scholar]
- BIRDER L.A., DE GROAT W.C. Induction of c-fos expression in spinal neurons by nociceptive and nonnociceptive stimulation of LUT. Am. J. Physiol. 1993;265:R326–R333. doi: 10.1152/ajpregu.1993.265.2.R326. [DOI] [PubMed] [Google Scholar]
- BIRDER L.A., NAKAMURA Y., KISS S., NEALEN M.L., BARRICK S., KANAI A.J., WANG E., RUIZ G., DE GROAT W.C., APODACA G., WATKINS S., CATERINA M.J. Altered urinary bladder function in mice lacking the vanilloid receptor TRPV1. Nat. Neurosci. 2002;5:856–860. doi: 10.1038/nn902. [DOI] [PubMed] [Google Scholar]
- BITRAN D., HULL E.M. Pharmacological analysis of male rat sexual behaviour. Neurosci. Biobehav. Rev. 1987;11:365–389. doi: 10.1016/s0149-7634(87)80008-8. [DOI] [PubMed] [Google Scholar]
- BLOK B.F. Central pathways controlling micturition and urinary continence. Urology. 2002;59:13–17. doi: 10.1016/s0090-4295(01)01633-8. [DOI] [PubMed] [Google Scholar]
- BLOK B.F.M., HOLSTEGE G. The central control of micturition and continence: implications for urology. Br. J. Urol. Int. 1999;83:1–6. doi: 10.1046/j.1464-410x.83.s2.2.x. [DOI] [PubMed] [Google Scholar]
- BODEKER J., VOGT W., KOLLN C.P., NAGEL R. Effects of pressoreceptor stimulation on micturition relfex and urethral pressure profile in rabbits. Invest. Urol. 1975;12:461–467. [PubMed] [Google Scholar]
- BRADING A.F. A myogenic basis for the overactive bladder. Urology. 1997;50:57–67. doi: 10.1016/s0090-4295(97)00591-8. [DOI] [PubMed] [Google Scholar]
- BRADING A.F. The physiology of the mammalian urinary outflow tract. Exp. Physiol. 1999;84:215–221. doi: 10.1111/j.1469-445x.1999.tb00084.x. [DOI] [PubMed] [Google Scholar]
- BRADING A.F., MOSTWIN J.L. Electrical and mechanical responses of guinea-pig bladder muscle to nerve stimulation. Br. J. Pharmacol. 1989;98:1083–1090. doi: 10.1111/j.1476-5381.1989.tb12651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRIDGEWATER M., MACNEIL H.F., BRADING A.F. Regulation of tone in pig urethral smooth muscle. J. Urol. 1993;150:223–228. doi: 10.1016/s0022-5347(17)35451-4. [DOI] [PubMed] [Google Scholar]
- BRINDLEY G.S., CRAGGS M.D. Proceedings: the effect of atropine on the urinary bladder of the baboon and of man. J. Physiol. 1976;256:55P. [PubMed] [Google Scholar]
- BRUNE M.E., O'NEILL A.B., GAUVIN D.M., KATWALA S.P., ALTENBACH R.J., BRIONI J.D., HANCOCK A.A., SULLIVAN J.P. Comparison of [alpha]1-adrenoceptor agonists in canine urethral pressure profilometry and abdominal leak point pressure models. J. Urol. 2001;166:1555–1559. [PubMed] [Google Scholar]
- BUCKNER S.A., MILICIC I., DAZA A.V., MEYER M.D., ALTENBACH R.J., WILLIAMS M., SULLIVAN J.P., BRIONI J.D. ABT-866, a novel [alpha]1A-adrenoceptor agonist with antagonist properties at the [alpha]1B- and [alpha]1D-adrenoceptor subtypes. Eur. J. Pharmacol. 2002;449:159–165. doi: 10.1016/s0014-2999(02)01976-3. [DOI] [PubMed] [Google Scholar]
- CALVERT R.C., THOMPSON C.S., KHAN M.A., MIKHAILIDIS D.P., MORGAN R.J., BURNSTOCK G. Alterations in cholinergic and purinergic signaling in a model of the obstructed bladder. J. Urol. 2001;166:1530–1533. [PubMed] [Google Scholar]
- CANTOR J.M., BINIK Y.M., PFAUS J.G. Chronic fluoxetine inhibits sexual behavior in the male rat: reversal with oxytocin. Psychopharmacology (Berl.) 1999;144:355–362. doi: 10.1007/s002130051018. [DOI] [PubMed] [Google Scholar]
- CARTER A.J., BALLARD S.A., NAYLOR A.M. Effect of the selective phosphodiesterase type 5 inhibitor sildenafil on erectile dysfunction in the anesthetized dog. J. Urol. 1998;160:242–246. [PubMed] [Google Scholar]
- CHAI T.C., GRAY M.L., STEERS W.D. The incidence of a positive ice water test in bladder outlet obstructed patients: evidence for bladder neural plasticity. J. Urol. 1998;160:34–38. [PubMed] [Google Scholar]
- CHEN K.C., MINOR T.X., RAHMAN N.U., HO H.C., NUNES L., LUE T.F. The additive erectile recovery effect of brain-derived neurotrophic factor combined with vascular endothelial growth factor in a rat model of neurogenic impotence. Br. J. Urol. Int. 2005;95:1077–1080. doi: 10.1111/j.1464-410X.2005.05470.x. [DOI] [PubMed] [Google Scholar]
- CHEN K.K., CHAN S.H., CHANG L.S., CHAN J.Y. Participation of paraventricular nucleus of hypothalamus in central regulation of penile erection in the rat. J. Urol. 1997;158:238–244. doi: 10.1097/00005392-199707000-00078. [DOI] [PubMed] [Google Scholar]
- CHENG C.L., CHAI C.Y., DE GROAT W.C. Detrusor-sphincter dyssynergia induced by cold stimulation of the urinary bladder of rats. Am. J. Physiol. 1997;272:R1271–R1282. doi: 10.1152/ajpregu.1997.272.4.R1271. [DOI] [PubMed] [Google Scholar]
- CHENG C.L., LIU J.C., CHANG S.Y., MA C.P., DE GROAT W.C. Effect of capsaicin on the micturition reflex in normal and chronic spinal cord-injured cats. Am. J. Physiol. 1999;277:R786–R794. doi: 10.1152/ajpregu.1999.277.3.R786. [DOI] [PubMed] [Google Scholar]
- CHERMANSKY C.J., CANNON T.W., TORIMOTO K., FRASER M.O., YOSHIMURA N., DE GROAT W.C., CHANCELLOR M.B. A model of intrinsic sphincteric deficiency in the rat: electrocauterization. Neurourol. Urodynam. 2004;23:166–171. doi: 10.1002/nau.10173. [DOI] [PubMed] [Google Scholar]
- CHERRY J.A. Measurement of sexual behaviour: controls for variables. Methods Neurosci. 1993;15:3–15. [Google Scholar]
- CHUNG S.K., MCVARY K.T., MCKENNA K.E. Sexual reflexes in male and female rats. Neurosci. Lett. 1988;94:343–348. doi: 10.1016/0304-3940(88)90042-0. [DOI] [PubMed] [Google Scholar]
- CLEMOW D.B., MCCARTY R., STEERS W.D., TUTTLE J.B. Efferent and afferent neuronal hypertrophy associated with micturition pathways in spontaneously hypertensive rats. Neurourol. Urodynam. 1997;16:293–303. doi: 10.1002/(sici)1520-6777(1997)16:4<293::aid-nau5>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- CLEMOW D.B., SPITSBERGEN J.M., MCCARTY R., STEERS W.D., TUTTLE J.B. Altered NGF regulation may link a genetic predisposition for hypertension with hyperactive voiding. J. Urol. 1999;161:1372–1377. [PubMed] [Google Scholar]
- CLEMOW D.B., STEERS W.D., MCCARTY R., TUTTLE J.B. Altered regulation of bladder nerve growth factor and neurally mediated hyperactive voiding. Am. J. Physiol. 1998;44:R1279–R1286. doi: 10.1152/ajpregu.1998.275.4.R1279. [DOI] [PubMed] [Google Scholar]
- CLEMOW D.B., STEERS W.D., TUTTLE J.B. Stretch-activated signaling of nerve growth factor secretion in bladder and vascular smooth muscle cells from hypertensive and hyperactive rats. J. Cell. Physiol. 2000;183:289–300. doi: 10.1002/(SICI)1097-4652(200006)183:3<289::AID-JCP1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- COCKAYNE D.A., HAMILTON S.G., ZHU Q.M., DUNN P.M., ZHONG Y., NOVAKOVIC S., MALMBERG A.B., CAIN G., BERSON A., KASSOTAKIS L., HEDLEY L., LACHNIT W.G., BURNSTOCK G., MCMAHON S.B., FORD A.P. Urinary bladder hyporeflexia and reduced pain-related behaviour in P2X3-deficient mice. Nature. 2000;407:1011–1015. doi: 10.1038/35039519. [DOI] [PubMed] [Google Scholar]
- COLACHIS S.C., III Autonomic hyperreflexia with spinal cord injury. J. Am. Paraplegia Soc. 1992;15:171–186. doi: 10.1080/01952307.1992.11735871. [DOI] [PubMed] [Google Scholar]
- CONTE B., MAGGI C.A., PARLANI M., LOPEZ G., MANZINI S., GIACHETTI A. Simultaneous recording of vesical and urethral pressure in urethane-anesthetized rats: effect of neuromuscular blocking agents on the activity of the external urethral sphincter. J. Pharmacol. Methods. 1991;26:161–171. doi: 10.1016/0160-5402(91)90041-3. [DOI] [PubMed] [Google Scholar]
- CRAGGS M.D., RUSHTON D.N., STEPHENSON J.D. A putative non-cholinergic mechanism in urinary bladders of New but not Old World primates. J. Urol. 1986;136:1348–1350. doi: 10.1016/s0022-5347(17)45335-3. [DOI] [PubMed] [Google Scholar]
- CRAGGS M.D., STEPHENSON J.D. Bladder electromyograms and function in monkeys after atropine. Br. J. Urol. Int. 1985;57:341–345. doi: 10.1111/j.1464-410x.1985.tb06358.x. [DOI] [PubMed] [Google Scholar]
- CRUZ Y., HUDSON R., PACHECO P., LUCIO R.A., MARTINEZ-GOMEZ M. Anatomical and physiological characteristics of perineal muscles in the female rabbit. Physiol. Behav. 2002;75:33–40. doi: 10.1016/s0031-9384(01)00638-2. [DOI] [PubMed] [Google Scholar]
- DALMOSE A.L., BJARKAM C.R., SORENSEN J.C., DJURHUUS J.C., JORGENSEN T.M. Effects of high frequency deep brain stimulation on urine storage and voiding function in conscious minipigs. Neurourol. Urodynam. 2004;23:265–272. doi: 10.1002/nau.20026. [DOI] [PubMed] [Google Scholar]
- DAMASER M.S., BROXTON-KING C., FERGUSON C., KIM F.J., KERNS J.M. Functional and neuroanatomical effects of vaginal distention and pudendal nerve crush in the female rat. J. Urol. 2003;170:1027–1031. doi: 10.1097/01.ju.0000079492.09716.43. [DOI] [PubMed] [Google Scholar]
- DANUSER H., THOR K.B. Spinal 5-HT2 receptor-mediated facilitation of pudendal nerve reflexes in the anaesthetized cat. Br. J. Pharmacol. 1996;118:150–154. doi: 10.1111/j.1476-5381.1996.tb15378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAS A.K., LEGGETT R.E., WHITBECK C., EAGEN G., LEVIN R.A. Effect of Doxazosin on rat urinary bladder function after partial outlet obstruction. Neurourol. Urodynam. 2002;21:160–166. doi: 10.1002/nau.10045. [DOI] [PubMed] [Google Scholar]
- DASS N., MCMURRAY G., GREENLAND J.E., BRADING A.F. Morphological aspects of the female pig bladder neck and urethra: quantitative analysis using computer assisted 3-dimensional reconstructions. J. Urol. 2001;165:1294–1299. [PubMed] [Google Scholar]
- DE GROAT W.C. Mechanisms underlying the recovery of lower urinary tract function following spinal cord injury. Paraplegia. 1995;33:493–505. doi: 10.1038/sc.1995.109. [DOI] [PubMed] [Google Scholar]
- DE GROAT W.C. Anatomy of the central neural pathways controlling the lower urinary tract. Eur. Urol. 1998;1:2–5. doi: 10.1159/000052265. [DOI] [PubMed] [Google Scholar]
- DE GROAT W.C., KAWATANI M., HISAMITSU T., CHENG C.L., MA C.P., THOR K., STEERS W., ROPPOLO J.R. Mechanisms underlying the recovery of urinary bladder function following spinal cord injury. J. Auton. Nerv. Syst. 1990;30 (Suppl.):S71–S77. doi: 10.1016/0165-1838(90)90105-r. [DOI] [PubMed] [Google Scholar]
- DE GROAT W.C., YOSHIMURA N. Pharmacology of the lower urinary tract. Annu. Rev. Pharmacol. Toxicol. 2001;41:691–721. doi: 10.1146/annurev.pharmtox.41.1.691. [DOI] [PubMed] [Google Scholar]
- DE GROAT W.C., YOSHIMURA N. Mechanisms underlying the recovery of lower urinary tract function following spinal cord injury. Prog. Brain Res. 2005;152:59–84. doi: 10.1016/S0079-6123(05)52005-3. [DOI] [PubMed] [Google Scholar]
- DELANCEY J.O. Correlative study of paraurethral anatomy. Obstet. Gynecol. 1986;68:91–97. [PubMed] [Google Scholar]
- DELANCEY J.O. Functional anatomy of the female lower urinary tract and pelvic floor. Ciba Found. Symp. 1990;151:57–69. doi: 10.1007/978-1-84628-505-9_1. [DOI] [PubMed] [Google Scholar]
- DEWSBURY D.A. Effects of alcohol ingestion on copulatory behavior of male rats. Psychopharmacologia. 1967;11:276–281. doi: 10.1007/BF00405234. [DOI] [PubMed] [Google Scholar]
- DEWSBURY D.A. Factor analysis of measure of copulatory behaviour in three species of muroid rodents. J. Comp. Physiol. Psychol. 1979;93:868–878. [Google Scholar]
- DOI T., KAMO I., IMAI S., OKANISHI S., ISHIMARU T., IKEURA Y., NATSUGARI H. Effects of TAK-637, a tachykinin receptor antagonist, on lower urinary tract function in the guinea pig. Eur. J. Pharmacol. 1999;383:297–303. doi: 10.1016/s0014-2999(99)00657-3. [DOI] [PubMed] [Google Scholar]
- DORR R.T., LINES R., LEVINE N., BROOKS C., XIANG L., HRUBY V.J., HADLEY M.E. Evaluation of melanotan-II, a superpotent cyclic melanotropic peptide in a pilot phase-I clinical study. Life Sci. 1996;58:1777–1784. doi: 10.1016/0024-3205(96)00160-9. [DOI] [PubMed] [Google Scholar]
- DUPONT M.C., PERSSON K., SPITSBERGEN J., TUTTLE J.B., STEERS W.D. The neuronal response to bladder outlet obstruction, a role for NGF. Adv. Exp. Med. Biol. 1995;385:41–54. doi: 10.1007/978-1-4899-1585-6_6. [DOI] [PubMed] [Google Scholar]
- DWYER P.L., GLENNING P.P. Anatomy and neurology of the lower urinary tract. Curr. Opin. Obstet. Gynecol. 1990;2:573–579. [PubMed] [Google Scholar]
- EARDLEY I., ELLIS P., BOOLELL M., WULFF M. Onset and duration of action of sildenafil for the treatment of erectile dysfunction. Br. J. Clin. Pharmacol. 2002;53:61S–65S. doi: 10.1046/j.0306-5251.2001.00034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDWARDS D.A., PFEIFLE J.K. Hormonal control of receptivity, proceptivity and sexual motivation. Physiol. Behav. 1983;30:437–443. doi: 10.1016/0031-9384(83)90150-6. [DOI] [PubMed] [Google Scholar]
- ELNEIL S., SKEPPER J.N., KIDD E.J., WILLIAMSON J.G., FERGUSON D.R. Distribution of P2X(1) and P2X(3) receptors in the rat and human urinary bladder. Pharmacology. 2001;63:120–128. doi: 10.1159/000056122. [DOI] [PubMed] [Google Scholar]
- ERICKSON R.P. Autonomic hyperreflexia: pathophysiology and medical management. Arch. Phys. Med. Rehabil. 1980;61:431–440. [PubMed] [Google Scholar]
- EVERITT B.J. Sexual motivation: a neural and behavioural analysis of the mechanisms underlying appetitive and copulatory responses of male rats. Neurosci. Biobehav. Rev. 1990;15:217–232. doi: 10.1016/s0149-7634(05)80222-2. [DOI] [PubMed] [Google Scholar]
- FABIYI A.C., GOPALAKRISHNAN M., LYNCH J.J., BRIONI J.D., COGHLAN M.J., BRUNE M.E. In vivo evaluation of the potency and bladder-vascular selectivity of the ATP-sensitive potassium channel openers (−)-cromakalim, ZD6169 and WAY-133537 in rats. Br. J. Urol. Int. 2003;91:284–290. doi: 10.1046/j.1464-410x.2003.03069.x. [DOI] [PubMed] [Google Scholar]
- FALL M., LINDSTROM S., MAZIERES L. A bladder-to-bladder cooling reflex in the cat. J. Physiol. 1990;427:281–300. doi: 10.1113/jphysiol.1990.sp018172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FERGUSON C.L., LIN D.L., RAO S., DAMASER M.S. Short-term functional and neuroregenerative response of the urethra to ovariectomy and vaginal distension in female rats. Int. Urogynecol. J. 2005;16:119–125. doi: 10.1007/s00192-004-1237-6. [DOI] [PubMed] [Google Scholar]
- FEY T.A., GOPALAKRISHNAN M., STRAKE J.G., KING L.L., BRIONI J.D., SULLIVAN J.P., COGHLAN M.J., BRUNE M.E. Effects of ATP-sensitive K+ channel openers and tolterodine on involuntary bladder contractions in a pig model of partial bladder outlet obstruction. Neurourol. Urodyn. 2003;22:147–155. doi: 10.1002/nau.10103. [DOI] [PubMed] [Google Scholar]
- FLETCHER T.F. Anatomy of pelvic viscera. Vet. Clin. N. Am. 1974;4:471–486. doi: 10.1016/s0091-0279(74)50051-6. [DOI] [PubMed] [Google Scholar]
- FLETCHER T.F. Applied anatomy and physiology of the feline lower urinary tract. Vet. Clin. N. Am. 1996;26:181–196. [PubMed] [Google Scholar]
- FLISSER A.J., BLAIVAS J.G. Role of cystometry in evaluating patients with overactive bladder. Urology. 2002;60:33–42. doi: 10.1016/s0090-4295(02)01791-0. [DOI] [PubMed] [Google Scholar]
- FOWLER C.J. Bladder afferents and their role in the overactive bladder. Urology. 2002;59:37–42. doi: 10.1016/s0090-4295(02)01544-3. [DOI] [PubMed] [Google Scholar]
- FUJII K. Evidence for adenosine triphosphate as an excitatory transmitter in guinea-pig, rabbit and pig urinary bladder. J. Physiol. 1988;404:39–52. doi: 10.1113/jphysiol.1988.sp017277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GABELLA G., UVELIUS B. Urinary bladder of rat: fine structure of normal and hypertrophic musculature. Cell Tissue Res. 1990;262:67–69. doi: 10.1007/BF00327747. [DOI] [PubMed] [Google Scholar]
- GANZER R., KOHLER D., NEUHAUS J., DORSCHNER W., STOLZENBURG J.U. Is the rhesus monkey (Macaca mulatta) comparable to humans? Histomorphology of the sphincteric musculature of the lower urinary tract including 3D-reconstruction. Anat. Histol. Embryol. Vet. Med. C. 2004;33:355–361. doi: 10.1111/j.1439-0264.2004.00576.x. [DOI] [PubMed] [Google Scholar]
- GANZER R., NEUHAUS J., DORSCHNER W., STOLZENBURG J.U. Muscle systems of the lower urinary tract of the male rhesus monkey (Macaca mulatta): histomorphology and 3-dimensional reconstruction. J. Urol. 2002;168:1603–1607. doi: 10.1016/S0022-5347(05)64528-4. [DOI] [PubMed] [Google Scholar]
- GARDINER J.C., WESTBROOK S.2003Characterisation of a Cold-induced Micturition Reflex in the Anaesthetised Guinea PigAbstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience; Online, Program No. 189.5. [Google Scholar]
- GEIRSSON G., FALL M., LINDSTROM S. The ice-water test – a simple and valuable supplement to routine cystometry. Br. J. Urol. 1993;71:681–685. doi: 10.1111/j.1464-410x.1993.tb16065.x. [DOI] [PubMed] [Google Scholar]
- GEIRSSON G., FALL M., SULLIVAN L. Clinical and urodynamic effects of intravesical capsaicin treatment in patients with chronic traumatic spinal detrusor hyperreflexia. J. Urol. 1995;154:1825–1829. [PubMed] [Google Scholar]
- GEIRSSON G., LINDSTROM S., FALL M., GLADH G., HERMANSSON G., HJALMAS K. Positive bladder cooling test in neurologically normal young children. J. Urol. 1994;151:446–448. doi: 10.1016/s0022-5347(17)34984-4. [DOI] [PubMed] [Google Scholar]
- GHONIEM G.M., AERTKER M.W., SAKR M.A., SHAABAN A.M., SHOUKRY M.S. A telemetric multichannel computer-based system for monitoring urodynamic parameters in awake rhesus monkeys. J. Urol. 1997;157:704–709. [PubMed] [Google Scholar]
- GHONIEM G.M., SHOUKRY M.S., MONGA M. Effects of anesthesia on urodynamic studies in the primate model. J. Urol. 1996;156:233–236. [PubMed] [Google Scholar]
- GIULIANI S., LECCI A., SANTICIOLI P., DEL BIANCO E., MAGGI C.A. Effect of the GABA(B) antagonist, phaclofen, on baclofen-induced inhibition of micturition reflex in urethane-anesthetized rats. Neuroscience. 1992;48:217–223. doi: 10.1016/0306-4522(92)90350-b. [DOI] [PubMed] [Google Scholar]
- GIULIANI S., LECCI A., TRAMONTANA M., MAGGI C.A. The inhibitory effect of nociceptin on the micturition reflex in anaesthetized rats. Br. J. Pharmacol. 1998;124:1566–1572. doi: 10.1038/sj.bjp.0701983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIULIANO F., ALLARD J., COMPAGNIE S., ALEXANDRE L., DROUPY S., BERNABE J. Vaginal physiological changes in a model of sexual arousal in anesthetized rats. Am. J. Physiol. 2001a;281:R140–R149. doi: 10.1152/ajpregu.2001.281.1.R140. [DOI] [PubMed] [Google Scholar]
- GIULIANO F., ALLARD J., RAMPIN O., DROUPY S., BENOIT G., ALEXANDRE L., BERNABE J. Pro-erectile effect of systemic apomorphine: existence of a spinal site of action. J. Urol. 2002;167:402–406. [PubMed] [Google Scholar]
- GIULIANO F., BERNABE J., MCKENNA K., LONGUEVILLE F., RAMPIN O. Spinal proerectile effect of oxytocin in anesthetized rats. Am. J. Physiol. 2001b;280:R1870–R1877. doi: 10.1152/ajpregu.2001.280.6.R1870. [DOI] [PubMed] [Google Scholar]
- GIULIANO F., RAMPIN O., BROWN K., COURTOIS F., BENOIT G., JARDIN A. Stimulation of the medial preoptic area of the hypothalamus in the rat elicits increases in intracavernous pressure. Neurosci. Lett. 1996;209:1–4. doi: 10.1016/0304-3940(96)12594-5. [DOI] [PubMed] [Google Scholar]
- GIULIANO F., ROSSLER A.-S., CLEMENT P., DROUPY S., ALEXANDRE L., BERNABE J. The use of telemetry technology to test the proerectile effect of melanotan-II (MT-II) in conscious rats. Eur. Urol. 2005;48:145–152. doi: 10.1016/j.eururo.2005.02.023. [DOI] [PubMed] [Google Scholar]
- GOOKIN J.L., STONE E.A., SHARP N.J. Urinary incontinence in dogs and cats. 1. Urethral pressure profilometry. Compendium. Contin. Educ. Pract. Vet. 1996;18:407–411. [Google Scholar]
- GOTOH M., YOSHIKAWA Y., KONDO A.S., KONDO A., ONO Y., OHSHIMA S. Positive bladder cooling reflex in patients with bladder outlet obstruction due to benign prostatic hyperplasia. World J. Urol. 1999;17:126–130. doi: 10.1007/s003450050118. [DOI] [PubMed] [Google Scholar]
- GREENLAND J.E., DASS N., BRADING A.F. Intrinsic urethral closure mechanisms in the female pig. Scand. J. Urol. Nephrol. Suppl. 1996;179:75–80. [PubMed] [Google Scholar]
- GREGORY S.P. Developments in the understanding of the pathophysiology of urethral sphincter mechanism in competence in the bitch. Br. Vet. J. 1994;150:135–150. doi: 10.1016/S0007-1935(05)80222-2. [DOI] [PubMed] [Google Scholar]
- GU B.J., ISHIZUKA O., IGAWA Y., NISHIZAWA O., ANDERSSON K.E. Role of supraspinal alpha 1-adrenoceptors for voiding in conscious rats with and without bladder outlet obstruction. J. Urol. 2002;167:1887–1891. [PubMed] [Google Scholar]
- HARADA T., KUMAZAKI T., KIGURE T., ETORI K., TSUCHIDA S. Effect of adrenergic agents on urethral pressure and urethral compliance measurements in dog proximal urethra. J. Urol. 1989;142:189–192. doi: 10.1016/s0022-5347(17)38708-6. [DOI] [PubMed] [Google Scholar]
- HASHITANI H., BRAMICH N.J., HIRST G.D. Mechanisms of excitatory neuromuscular transmission in the guinea-pig urinary bladder. J. Physiol. 2000;524:565–579. doi: 10.1111/j.1469-7793.2000.t01-2-00565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HETTA J., MEYERSON B.J. Sexual motivation in the male rat. A methodological study of sex-specific orientation and the effects of gonadal hormones. Acta Physiol. Scand. 1978;1:1–67. [PubMed] [Google Scholar]
- HIJAZ A., BENA J., DANESHGARI F. Long-term efficacy of a vaginal sling procedure in a rat model of stress urinary incontinence. J. Urol. 2005;173:1817–1819. doi: 10.1097/01.ju.0000154342.75020.05. [DOI] [PubMed] [Google Scholar]
- HIRAYAMA A., FUJIMOTO K., MATSUMOTO Y., OZONO S., HIRAO Y. Positive response to ice water test associated with high-grade bladder outlet obstruction in patients with benign prostatic hyperplasia. Urology. 2003;62:909–913. doi: 10.1016/s0090-4295(03)00588-0. [DOI] [PubMed] [Google Scholar]
- IGAWA Y., MATTIASSON A., ANDERSSON K.E. Effects of GABA-receptor stimulation and blockade on micturition in normal rats and rats with bladder outflow obstruction. J. Urol. 1993;150:537–542. doi: 10.1016/s0022-5347(17)35542-8. [DOI] [PubMed] [Google Scholar]
- IGAWA Y., MATTIASSON A., ANDERSSON K.E. Micturition and premicturition contractions in unanesthetized rats with bladder outlet obstruction. J. Urol. 1994;151:244–249. doi: 10.1016/s0022-5347(17)34925-x. [DOI] [PubMed] [Google Scholar]
- INOUE R., BRADING A.F. Human, pig and guinea-pig bladder smooth muscle cells generate similar inward currents in response to purinoceptor activation. Br. J. Pharmacol. 1991;103:1840–1846. doi: 10.1111/j.1476-5381.1991.tb12338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISHIGOOKA M., HASHIMOTO T., HAYAMI S., SUZUKI Y., IZUMI T., NAKADA T. Ice water test in patients with overactive bladder due to cerebrovascular accidents and bladder outlet obstruction. Urol. Int. 1997;58:84–87. doi: 10.1159/000282956. [DOI] [PubMed] [Google Scholar]
- ISHIZUKA O., IGAWA Y., LECCI A., MAGGI C.A., MATTIASSON A., ANDERSSON K.E. Role of intrathecal tachykinins for micturition in unanaesthetized rats with and without bladder outlet obstruction. Br. J. Pharmacol. 1994;113:111–116. doi: 10.1111/j.1476-5381.1994.tb16181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISHIZUKA O., MATTIASSON A., ANDERSSON K.E. Urodynamic effects of intravesical resiniferatoxin and capsaicin in conscious rats with and without outflow obstruction. J. Urol. 1995;154:611–616. doi: 10.1097/00005392-199508000-00080. [DOI] [PubMed] [Google Scholar]
- ISMAEL S.S., EPSTEIN T., BAYLE B., DENYS P., AMARENCO G. Bladder cooling reflex in patients with multiple sclerosis. J. Urol. 2000;164:1280–1284. [PubMed] [Google Scholar]
- JAHED A., KAWAJA M.D.2001Localization of Sympathetic and Sensory Axons in the Bladder and Prostatic Urethra of Spontaneously Hypertensive Rats (SHR)Abstract Viewer/Itinerary Planner.. San Diego, CA: Society for Neuroscience; Online, Program No. 254.9. [Google Scholar]
- JIANG C.H., MAZIERES L., LINDSTROM S. Cold- and menthol-sensitive C afferents of cat urinary bladder. J. Physiol. 2002;543:211–220. doi: 10.1113/jphysiol.2002.019042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOSEPH P.A., DE S.M. Sphincter and bladder dysfunction. Rev. Neurol. 2001;157:1051–1059. [PubMed] [Google Scholar]
- JUENEMANN K.P., LUE T.F., FOURNIER G.R., TANAGHO E.A. Hemodynamics of papaverine- and phentolamine-induced penile erection. J. Urol. 1986;136:158–161. doi: 10.1016/s0022-5347(17)44763-x. [DOI] [PubMed] [Google Scholar]
- JUNG G.W., SPENCER E.M., LUE T.F. Growth hormone enhances the regeneration of nitric oxide synthase-containing penile nerves after cavernous nerve neurotomy in rats. J. Urol. 1998;160:1899–1904. [PubMed] [Google Scholar]
- KAIHO Y., KAMO I., SWEENEY D., PRANTIL R., VORP D., DE GROAT W., CHANCELLOR M., YOSHIMURA N. Effects of norepinephrine uptake inhibitor, nisoxetine, on the sneeze induced urethral continence mechanism in rats. Neurourol. Urodyn. 2005;24:569–570. [Google Scholar]
- KAKIZAKI H., YOSHIYAMA M., KOYANAGI T., DE GROAT W.C. Effects of WAY100635, a selective 5-HT1A-receptor antagonist on the micturition-reflex pathway in the rat. Am. J. Physiol. 2001;280:R1407–R1413. doi: 10.1152/ajpregu.2001.280.5.R1407. [DOI] [PubMed] [Google Scholar]
- KAMO I., TORIMOTO K., CHANCELLOR M.B., DE GROAT W.C., YOSHIMURA N. Urethral closure mechanisms under sneeze-induced stress condition in rats: a new animal model for evaluation of stress urinary incontinence. Am. J. Physiol. 2003;285:R356–R365. doi: 10.1152/ajpregu.00010.2003. [DOI] [PubMed] [Google Scholar]
- KATO K., WEIN A.J., KITADA S., HAUGAARD N., LEVIN R.M. The functional effect of mild outlet obstruction on the rabbit urinary bladder. J. Urol. 1988;140:880–884. doi: 10.1016/s0022-5347(17)41849-0. [DOI] [PubMed] [Google Scholar]
- KATOFIASC M.A., NISSEN J., AUDIA J.E., THOR K.B. Comparison of the effects of serotonin selective, norepinephrine selective, and dual serotonin and norepinephrine reuptake inhibitors on lower urinary tract function in cats. Life Sci. 2002;71:1227–1236. doi: 10.1016/s0024-3205(02)01848-9. [DOI] [PubMed] [Google Scholar]
- KHANNA O.P., BARBIERI E.J., ALTAMURA M., MCMICHAEL R. Vesicourethral smooth muscle: function and relation to structure. Urology. 1981;18:211–218. doi: 10.1016/0090-4295(81)90445-3. [DOI] [PubMed] [Google Scholar]
- KHULLAR V., CARDOZO L. The urethra (UPP, MUPP, instability, LPP) Eur. Urol. 1998;34 (Suppl. 1):20–22. doi: 10.1159/000052270. [DOI] [PubMed] [Google Scholar]
- KOMIYAMA I., IGAWA Y., ISHIZUKA O., NISHIZAWA O., ANDERSSON K.E. Effects of intravesical capsaicin and resiniferatoxin on distension-induced bladder contraction in conscious rats with and without chronic spinal cord injury. J. Urol. 1999;161:314–319. [PubMed] [Google Scholar]
- KONTANI H., NAKAGAWA M., SAKAI T. Effects of adrenergic agonists on an experimental urinary incontinence model in anesthetized rabbits. Jap. J. Pharmacol. 1992;58:339–346. doi: 10.1254/jjp.58.339. [DOI] [PubMed] [Google Scholar]
- KOW L.-M., MOBBS C.V., PFAFF D.W. Roles of second-messenger systems and neuronal activity in the regulation of lordosis by neurotransmitters, neuropeptides, and estrogen: a review. Neurosci. Biobehav. Rev. 1994;18:251–268. doi: 10.1016/0149-7634(94)90028-0. [DOI] [PubMed] [Google Scholar]
- KUO H.C. Effects of vaginal trauma and oophorectomy on the continence mechanism in rats. Urol. Int. 2002;69:36–41. doi: 10.1159/000064358. [DOI] [PubMed] [Google Scholar]
- LAL S., LARYEA E., THAVUNDAYIL J.X., NAIR N.P., NEGRETE J., ACKMAN D., BLUNDELL P., GARDINER R.J. Apomorphine-induced penile tumescence in impotent patients – preliminary findings. Prog. Neuropsychopharmacol. Biol. Psychiat. 1987;11:235–242. doi: 10.1016/0278-5846(87)90066-2. [DOI] [PubMed] [Google Scholar]
- LECCI A., CARINI F., TRAMONTANA M., BIRDER L.A., DE GROAT W.C., SANTICIOLI P., GIULIANI S., MAGGI C.A. Urodynamic effects induced by intravesical capsaicin in rats and hamsters. Auton. Neurosci. Basic Clin. 2001;91:37–46. doi: 10.1016/S1566-0702(01)00303-4. [DOI] [PubMed] [Google Scholar]
- LECCI A., GIULIANI S., GARRET C., MAGGI C.A. Evidence for a role of tachykinins as sensory transmitters in the activation of micturition reflex. Neuroscience. 1993;54:827–837. doi: 10.1016/0306-4522(93)90252-b. [DOI] [PubMed] [Google Scholar]
- LECCI A., GIULIANI S., TRAMONTANA M., CRISCUOLI M., MAGGI C.A. Multiple sites of action in the inhibitory effect of nociceptin on the micturition reflex. J. Urol. 2000;163:638–645. [PubMed] [Google Scholar]
- LEE K.-S., DEAN-MCKINNEY T., TUTTLE J.B., STEERS W.D. Intrathecal antisense oligonucleotide against the tetrodotoxin-resistant sodium channel (Nav1.8) reduces bladder hyperactivity in the spontaneously hypertensive rat. J. Urol. 2002;167:38. [Google Scholar]
- LIN Y.M., LIN J.S. The rabbit as an intracavernous injection study model. Urol. Res. 1996;24:27–32. doi: 10.1007/BF00296730. [DOI] [PubMed] [Google Scholar]
- LIU Y.C., SALAMONE J.D., SACHS B.D. Lesions in medial preoptic area and bed nucleus of stria terminalis: differential effects on copulatory behaviour and noncontact erection in male rats. J. Neurosci. 1997;17:5245–5253. doi: 10.1523/JNEUROSCI.17-13-05245.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MADERSBACHER H.G. Neurogenic bladder dysfunction. Curr. Opin. Urol. 1999;9:303–307. doi: 10.1097/00042307-199907000-00005. [DOI] [PubMed] [Google Scholar]
- MALATESTA V.J., POLLACK R.H., WILBANKS W.A., ADAMS H.E. Alcohol effects on the orgasmic-ejaculatory response in human males. J. Sex. Res. 1979;15:101–107. doi: 10.1080/00224497909551027. [DOI] [PubMed] [Google Scholar]
- MARIANNE DE SÈZE L.W., PIERRE-ALAIN J., JEAN-PIERRE D., JEAN-MICHEL M., MICHEL B. Capsaicin and neurogenic detrusor hyperreflexia: a double-blind placebo-controlled study in 20 patients with spinal cord lesions. Neurourol. Urodynam. 1998;17:513–523. doi: 10.1002/(sici)1520-6777(1998)17:5<513::aid-nau7>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- MARSON L., MCKENNA K.E. The identification of a brainstem site controlling spinal sexual reflexes in male rats. Brain Res. 1990;515:303–308. doi: 10.1016/0006-8993(90)90611-e. [DOI] [PubMed] [Google Scholar]
- MARSON L., MCKENNA K.E. Stimulation of the hypothalamus initiates the urethrogenital reflex in male rats. Brain Res. 1994;638:103–108. doi: 10.1016/0006-8993(94)90638-6. [DOI] [PubMed] [Google Scholar]
- MAZIERES L., JIANG C., LINDSTROM S. The C fibre reflex of the cat urinary bladder. J. Physiol. 1998;513:531–541. doi: 10.1111/j.1469-7793.1998.531bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCKENNA K.E., CHUNG S.K., MCVARY K.T. A model for the study of sexual function in anaesthetized male and female rats. Am. J. Physiol. 1991;261:1276–1285. doi: 10.1152/ajpregu.1991.261.5.R1276. [DOI] [PubMed] [Google Scholar]
- MEISEL R.L., SACHS B.D.1994The physiology of male sexual behaviour The Physiology of Reproductioned. Knobil, E. & Neill, J. pp. 3–105.New York: Raven Press [Google Scholar]
- MELIS M.R., ARGIOLAS A. Role of central nitric oxide in the control of penile erection and yawning. Prog. Neuropsychopharmacol. Biol. Psychiat. 1997;21:899–922. doi: 10.1016/s0278-5846(97)00088-2. [DOI] [PubMed] [Google Scholar]
- MELIS M.R., ARGIOLAS A., GESSA G.L. Apomorphine-induced penile erection and yawning: site of action in brain. Brain Res. 1987;415:98–104. doi: 10.1016/0006-8993(87)90272-1. [DOI] [PubMed] [Google Scholar]
- MENDELSON S.D., GORZALKA B.B. An improved chamber for the observation and analysis of the sexual behavior of the female rat. Physiol. Behav. 1987;39:67–71. doi: 10.1016/0031-9384(87)90345-3. [DOI] [PubMed] [Google Scholar]
- MENDELSON S.D., PFAUS J.G. Level searching: a new assay of sexual motivation in the male rat. Physiol. Behav. 1989;45:337–341. doi: 10.1016/0031-9384(89)90136-4. [DOI] [PubMed] [Google Scholar]
- MEYERSON B.J., LINDSTROM L.H. A methodological study applied to the investigation of the effect of estradiol benzoate. Acta Physiol. Scand. Suppl. 1973;389:1–80. [PubMed] [Google Scholar]
- MILLS I.W., NOBLE J.G., BRADING A.F. Radiotelemetered cystometry in pigs: validation and comparison of natural filling versus diuresis cystometry. J. Urol. 2000;164:1745–1750. [PubMed] [Google Scholar]
- MIN K., KIM N.N., MCAULEY I., STANKOWICZ M., GOLDSTEIN I., TRAISH A.M. Sildenafil augments pelvic nerve-mediated female genital sexual arousal in the anesthetized rabbit. Int. J. Impot. Res. 2000;12:S32–S39. doi: 10.1038/sj.ijir.3900610. [DOI] [PubMed] [Google Scholar]
- MITSUI T., KAKIZAKI H., MATSUURA S., AMEDA K., YOSHIOKA M., KOYANAGI T. Afferent fibers of the hypogastric nerves are involved in the facilitating effects of chemical bladder irritation in rats. J. Neurophysiol. 2001;86:2276–2284. doi: 10.1152/jn.2001.86.5.2276. [DOI] [PubMed] [Google Scholar]
- MITSUI T., KAKIZAKI H., MATSUURA S., TANAKA H., YOSHIOKA M., KOYANAGI T. Chemical bladder irritation provokes c-fos expression in the midbrain periaqueductal gray matter of the rat. Brain Res. 2003;967:81–88. doi: 10.1016/s0006-8993(02)04226-9. [DOI] [PubMed] [Google Scholar]
- MIYAGAWA I., NAKAMURA I., UEDA M., NISHIDA H., NAKASHITA E., GOTO H. Telemetric cystometry. Urol. Int. 1986;41:263–265. doi: 10.1159/000281214. [DOI] [PubMed] [Google Scholar]
- MODIRI A.R., ALBERTS P., GILLBERG P.G. Effect of muscarinic antagonists on micturition pressure measured by cystometry in normal, conscious rats. Urology. 2002;59:963–968. doi: 10.1016/s0090-4295(02)01535-2. [DOI] [PubMed] [Google Scholar]
- MOLINOFF P.B., SHADIACK A.M., EARLE D., DIAMOND L.E., QUON C.Y. PT-141: a melanocortin agonist for the treatment of sexual dysfunction. Ann. NY Acad. Sci. 2003;994:96–102. doi: 10.1111/j.1749-6632.2003.tb03167.x. [DOI] [PubMed] [Google Scholar]
- MORELAND R.B., BRIONI J.D., SULLIVAN J.P. Emerging pharmacologic approaches for the treatment of lower urinary tract disorders. J. Pharmacol. Exp. Ther. 2004;308:797–804. doi: 10.1124/jpet.102.034991. [DOI] [PubMed] [Google Scholar]
- MOS J., MOLLET I., TOLBOOM J.T.B.M., WALDINGER M.D., OLIVIER B. A comparison of the effects of different serotonin reuptake blockers on sexual behaviour of the male rat. Eur. Neuropsychopharmacol. 1999;9:123–135. doi: 10.1016/s0924-977x(98)00015-7. [DOI] [PubMed] [Google Scholar]
- MOSTWIN J.L., KARIM O.M., VAN KOEVERINGE G., BROOKS E.L. The guinea pig as a model of gradual urethral obstruction. J. Urol. 1991;145:854–858. doi: 10.1016/s0022-5347(17)38477-x. [DOI] [PubMed] [Google Scholar]
- MUNARRIZ R., KIM N.N., GOLDSTEIN I., TRAISH A.M. Biology of female sexual function. Urol. Clin. N. Am. 2002;29:685–693. doi: 10.1016/s0094-0143(02)00069-1. [DOI] [PubMed] [Google Scholar]
- MUNARRIZ R., KIM S.W., KIM N.N., TRAISH A., GOLDSTEIN I. A review of the physiology and pharmacology of peripheral (vaginal and clitoral) female genital arousal in the animal model. J. Urol. 2003;170:S40–S45. doi: 10.1097/01.ju.0000075352.03144.15. [DOI] [PubMed] [Google Scholar]
- NAKAMURA Y., YOKOYAMA O., KOMATSU K., MITA E., NAMIKI M., KONTANI H. Effects of nifedipine on bladder overactivity in rats with cerebral infarction. J. Urol. 1999;162:1502–1507. [PubMed] [Google Scholar]
- NEDERGAARD P., SANCHEZ C., MELLERUP E. Different roles of 5-HT2A and 5-HT2C receptors in regulation of female rat paced mating behaviour. Behav. Brain Res. 2004;149:151–157. doi: 10.1016/s0166-4328(03)00215-8. [DOI] [PubMed] [Google Scholar]
- NEUHAUS J., DORSCHNER W., MONDRY J., STOLZENBURG J.U. Comparative anatomy of the male guinea-pig and human lower urinary tract: histomorphology and three-dimensional reconstruction. Anat. Histol. Embryol. Vet. Med. C. 2001;30:185–192. [PubMed] [Google Scholar]
- NEUHAUS J., STOLZENBURG J.U., DORSCHNER W. The guinea pig as a model in urological research – an anatomical study. Aktuel. Urol. 1999;30:329–334. [Google Scholar]
- NICKEL R.F., VENKER-VAN HAAGEN A.J. Functional anatomy and neural regulation of the lower urinary tract in female dogs: a review. Vet. Quart. 1999;21:83–85. doi: 10.1080/01652176.1999.9694999. [DOI] [PubMed] [Google Scholar]
- O'CONNOR L.T., JR., VAUGHAN E.D., JR., FELSEN D. In vivo cystometric evaluation of progressive bladder outlet obstruction in rats. J. Urol. 1997;158:631–635. [PubMed] [Google Scholar]
- PALEA S., ARTIBANI W., OSTARDO E., TRIST D.G., PIETRA C. Evidence for purinergic neurotransmission in human urinary bladder affected by interstitial cystitis. J. Urol. 1993;150:2007–2012. doi: 10.1016/s0022-5347(17)35955-4. [DOI] [PubMed] [Google Scholar]
- PALEA S., PIETRA C. Involvement of spinal NK1 and opioids receptors in modulating the inhibitory effect of capsaicin on micturition reflex in the acute spinalized guinea pig. J. Urol. 1999;161:998–1005. [PubMed] [Google Scholar]
- PAMPINELLA F., ROELOFS M., CASTELLUCCI E., PASSERINI-GLAZEL G., PAGANO F., SARTORE S. Time-dependent remodeling of the bladder wall in growing rabbits after partial outlet obstruction. J. Urol. 1997;157:677–682. [PubMed] [Google Scholar]
- PANDITA R.K., FUJIWARA M., ALM P., ANDERSSON K.E. Cystometric evaluation of bladder function in non-anesthetized mice with and without bladder outlet obstruction. J. Urol. 2000;164:1385–1389. [PubMed] [Google Scholar]
- PAREDES R.G., VAZQUEZ B. What do female rats like about sex? Paced mating. Behav. Brain Res. 1999;105:117–127. doi: 10.1016/s0166-4328(99)00087-x. [DOI] [PubMed] [Google Scholar]
- PERSSON K., PANDITA R.K., SPITSBERGEN J.M., STEERS W.D., TUTTLE J.B., ANDERSSON K.E. Spinal and peripheral mechanisms contributing to hyperactive voiding in spontaneously hypertensive rats. Am. J. Physiol. 1998;44:R1366–R1373. doi: 10.1152/ajpregu.1998.275.4.R1366. [DOI] [PubMed] [Google Scholar]
- PESSINA F., MARAZOVA K., KALFIN R., SGARAGLI G., MANGANELLI A., MILENOV K. Mechanical response to electrical field stimulation of rat, guinea-pig, monkey and human detrusor muscle: a comparative study. Naunyn-Schmiedebergs Arch. Pharmacol. 2001;363:543–550. doi: 10.1007/s002100100407. [DOI] [PubMed] [Google Scholar]
- PETERSEN I., STENER I. An electromyographical study of the striated urethreal sphincter, the striated anal sphincter, and the levator ani muscle during ejaculation. Electromyography. 1970;10:23–44. [PubMed] [Google Scholar]
- PETRIE W.M. The Sexual Effects of Antipsychotic, Antidepressant, and Psychomotor Stimulant Drugs. London: John Libbey; 1985. [Google Scholar]
- PFAFF D.W. Drive. Neurobiological and Molecular Mechanisms of Sexual Motivation. Bradford, MA: MIT Press; 2001. [Google Scholar]
- PFAFF D.W., SCHWARTZ-GIBLIN S.1988Cellular mechanisms of female reproductive behaviours The Physiology of Reproductioned. Knobil, E. & Neill, J. pp. 1487–1568.New York: Raven Press [Google Scholar]
- PFAUS J.G. Frank A. Beach award. Homologies of animal and human sexual behaviors. Horm. Behav. 1996;30:187–200. doi: 10.1006/hbeh.1996.0024. [DOI] [PubMed] [Google Scholar]
- PFAUS J.G., HEEB M.M. Implications of immediate-early gene induction in the brain following sexual stimulation of female and male rodents. Brain Res. Bull. 1997;44:397–407. doi: 10.1016/s0361-9230(97)00219-0. [DOI] [PubMed] [Google Scholar]
- PFAUS J.G., KIPPIN T.E., CORIA-AVILA G. What can animal models tell us about human sexual response. Annu. Rev. Sex Res. 2003;14:1–63. [PubMed] [Google Scholar]
- PFAUS J.G., MENDELSON S.D., PHILLIPS A.G. A correlational and factor analysis of anticipatory and consummatory measures of sexual behaviour in the male rat. Psychoneuroendocrinology. 1990;15:329–350. doi: 10.1016/0306-4530(90)90058-h. [DOI] [PubMed] [Google Scholar]
- PFAUS J.G., PHILLIPS A.G. Differential effects of dopamine receptor antagonists on the sexual behavior of male rats. Psychopharmacology (Berl.) 1989;98:363–368. doi: 10.1007/BF00451688. [DOI] [PubMed] [Google Scholar]
- PFAUS J.G., SHADIACK A., VAN SOEST T., TSE M., MOLINOFF P. Selective facilitation of sexual solicitation in the female rat by a melanocortin receptor agonist. Proc. Natl. Acad. Sci. U.S.A. 2004;101:10201–10204. doi: 10.1073/pnas.0400491101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PINEL J.P., PFAUS J.G., CHRISTENSEN B.K. Contingent tolerance to the disruptive effects of alcohol on the copulatory behavior of male rats. Pharmacol. Biochem. Behav. 1992;41:133–137. doi: 10.1016/0091-3057(92)90072-n. [DOI] [PubMed] [Google Scholar]
- POORTMANS A., WYNDAELE J.J. M. levator ani in the rat: does it really lift the anus. Anat. Rec. 1998;251:20–27. doi: 10.1002/(SICI)1097-0185(199805)251:1<20::AID-AR5>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- PRAUD C., SEBE P., MONDET F., SEBILLE A. The striated urethral sphincter in female rats. Anat. Embryol. 2003;207:169–175. doi: 10.1007/s00429-003-0340-7. [DOI] [PubMed] [Google Scholar]
- PRICE E.O. Practical considerations in the measurement of sexual behaviour. Methods Neurosci. 1993;15:16–31. [Google Scholar]
- RAJASEKARAN M., WILKES N., KUNTZ S., E ALBO M. Rho-kinase inhibition suppresses bladder hyperactivity in spontaneously hypertensive rats. Neurourol. Urodynam. 2005;24:295–300. doi: 10.1002/nau.20122. [DOI] [PubMed] [Google Scholar]
- RAWLINGS C., BARSANTI J.A., MAHAFFEY M.B., BEMENT S. Evaluation of colposuspension for treatment of incontinence in spayed female dogs. J. Am. Vet. Med. Assoc. 2001;219:770–775. doi: 10.2460/javma.2001.219.770. [DOI] [PubMed] [Google Scholar]
- REHMAN J., CHRIST G., MELMAN A., FLEISCHMANN J. Intracavernous pressure responses to physical and electrical stimulation of the cavernous nerve in rats. Urology. 1998;51:640–644. doi: 10.1016/s0090-4295(97)00693-6. [DOI] [PubMed] [Google Scholar]
- RESPLANDE J., GHOLAMI S.S., GRAZIOTTIN T.M., ROGERS R., LIN C.S., LENG W., LUE T.F. Long-term effect of ovariectomy and simulated birth trauma on the lower urinary tract of female rats. J. Urol. 2002;168:323–330. [PubMed] [Google Scholar]
- RICHTER K.P., LING G.V. Clinical response and urethral pressure profile changes after phenylpropanolamine in dogs with primary sphincter incompetence. J. Am. Vet. Med. Assoc. 1985;187:605–611. [PubMed] [Google Scholar]
- RODRIGUEZ L.V., CHEN S., JACK G.S., DE ALMEIDA F., LEE K.W., ZHANG R. New objective measures to quantify stress urinary incontinence in a novel durable animal model of intrinsic sphincter deficiency. Am. J. Physiol. 2005;288:R1332–R1338. doi: 10.1152/ajpregu.00760.2004. [DOI] [PubMed] [Google Scholar]
- RONZONI G., MENCHINELLI P., MANCA A., DE GIOVANNI L. The ice-water test in the diagnosis and treatment of the neurogenic bladder. Br. J. Urol. 1997;79:698–701. doi: 10.1046/j.1464-410x.1997.00133.x. [DOI] [PubMed] [Google Scholar]
- ROSIN A., ROSIN E., OLIVER J. Canine urethral pressure profile. Am. J. Vet. Res. 1980;41:1113–1116. [PubMed] [Google Scholar]
- ROSIN A.E., BARSANTI J.A. Diagnosis of urinary incontinence in dogs: role of the urethral pressure profile. J. Am. Vet. Med. Assoc. 1981;178:814–822. [PubMed] [Google Scholar]
- SACH B.D. Contextual approaches to the physiology and classification of erectile function, erectile dysfunction, and sexual arousal. Neurosci. Biobehav. Rev. 2000;24:541–560. doi: 10.1016/s0149-7634(00)00022-1. [DOI] [PubMed] [Google Scholar]
- SACHS B.D., AKASOFU K., CITRON J.H., DANIELS S.B., NATOLI J.H. Noncontact stimulation from estrous females evokes penile erection in rats. Physiol. Behav. 1994;55:1073–1079. doi: 10.1016/0031-9384(94)90390-5. [DOI] [PubMed] [Google Scholar]
- SACHS B.D., BARFIELD R.J.1976Functional analysis of masculine copulatory behaviour in the rat Advances in the Study of Behavioured. Rosenblatt, R.A., Hinde, E., Shaw, E. & Beer, C. pp. 91–155.Orlando, FL: Academic Press [Google Scholar]
- SAKAKIBARA R., UCHIYAMA T., YOSHIYAMA M., HATTORI T. Disturbance of micturition in Parkinson's disease. Brain Nerve. 2001;53:1009–1014. [PubMed] [Google Scholar]
- SATO Y., CHRIST G.J., HORITA H., ADACHI H., SUZUKI N., TSUKAMOTO T. The effect of alterations in nitric oxide levels in the paraventricular nucleus on copulatory behaviour and reflexive erections in male rats. J. Urol. 1999;162:2182–2185. doi: 10.1016/S0022-5347(05)68156-6. [DOI] [PubMed] [Google Scholar]
- SCHNEIDER T., HEIN P., BAI J., MICHEL M.C. A role for muscarinic receptors or rho-kinase in hypertension associated rat bladder dysfunction. J. Urol. 2005;173:2178–2181. doi: 10.1097/01.ju.0000158138.07187.f5. [DOI] [PubMed] [Google Scholar]
- SHERER T.B., CLEMOW D.B., TUTTLE J.B. Calcium homeostasis and nerve growth factor secretion from vascular and bladder smooth muscle cells. Cell Tissue Res. 2000;299:201–211. doi: 10.1007/s004419900148. [DOI] [PubMed] [Google Scholar]
- SIBLEY G.N. An experimental model of detrusor instability in the obstructed pig. Br. J. Urol. 1985;57:292–298. doi: 10.1111/j.1464-410x.1985.tb06347.x. [DOI] [PubMed] [Google Scholar]
- SIBLEY G.N. The physiological response of the detrusor muscle to experimental bladder outflow obstruction in the pig. Br. J. Urol. 1987;60:332–336. doi: 10.1111/j.1464-410x.1987.tb04979.x. [DOI] [PubMed] [Google Scholar]
- SILVA W.A., KARRAM M.M. Anatomy and physiology of the pelvic floor. Minerva Ginecol. 2004;56:283–302. [PubMed] [Google Scholar]
- SLACK M., CULLIGAN P., TRACEY M., HUNSICKER K., PATEL B., SUMERAY M. Relationship of urethral retro-resistance pressure to urodynamic measurements and incontinence severity. Neurourol. Urodynam. 2004;23:109–114. doi: 10.1002/nau.20010. [DOI] [PubMed] [Google Scholar]
- SNEDDON P., MCLEES A. Purinergic and cholinergic contractions in adult and neonatal rabbit bladder. Eur. J. Pharmacol. 1992;214:7–12. doi: 10.1016/0014-2999(92)90088-l. [DOI] [PubMed] [Google Scholar]
- STEERS W.D., CREEDON D.J., TUTTLE J.B. Immunity to nerve growth factor prevents afferent plasticity following urinary bladder hypertrophy. J. Urol. 1996;155:379–385. [PubMed] [Google Scholar]
- STEERS W.D., KOLBECK S., CREEDON D., TUTTLE J.B. Nerve growth factor in the urinary bladder of the adult regulates neuronal form and function. J. Clin. Invest. 1991;88:1709–1715. doi: 10.1172/JCI115488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STRENG T., SANTTI R., ANDERSSON K.E., TALO A. The role of the rhabdosphincter in female rat voiding. Br. J. Urol. Int. 2004;94:138–142. doi: 10.1111/j.1464-4096.2004.04875.x. [DOI] [PubMed] [Google Scholar]
- TANG H., CEFALU J., NUNN P., TRYON M., FORD A., BLUE D.2002Hyperactive Voiding in Spontaneously Hypertensive Rat (SHR): A Comparative Study between Conscious Cystometry and Natural Voiding ModelsAbstract Viewer/Itinerary Planner. Orlando, FL: Society for Neuroscience; Online, Program No. 68.7. [Google Scholar]
- TESTA R., GUARNERI L., ANGELICO P., VELASCO C., POGGESI E., CILIA A., LEONARDI A. Effect of different 5-hydroxytryptamine receptor subtype antagonists on the micturition reflex in rats. Br. J. Urol. Int. 2001;87:256–264. doi: 10.1046/j.1464-410x.2001.02038.x. [DOI] [PubMed] [Google Scholar]
- THODY A.J., DONOHOE S.M., SHUSTER S. Alpha-melanocyte stimulating hormone and the release of sex attractant odours in the female rat. Peptides. 1981;2:125–129. doi: 10.1016/s0196-9781(81)80023-x. [DOI] [PubMed] [Google Scholar]
- THUROFF J.W., JONAS U., PETRI E., FROHNEBERG D. Telemetric urodynamic investigations in female incontinence. Prog. Clin. Biol. Res. 1981;78:211–222. [PubMed] [Google Scholar]
- TONG Y., HUNG Y., LIN S., CHENG J. The norepinephrine tissue concentration and neuropeptide Y immunoreactivity in genitourinary organs of the spontaneously hypertensive rat. J. Auton. Nerv. Syst. 1996;56:215–218. doi: 10.1016/0165-1838(95)00088-7. [DOI] [PubMed] [Google Scholar]
- TRUITT W.A., COOLEN L.M. Identification of a potential ejaculation generator in the spinal cord. Science. 297:1566–1599. doi: 10.1126/science.1073885. [DOI] [PubMed] [Google Scholar]
- TRUITT W.A., SHIPLEY M.T., VEENING J.G., COOLEN L.M. Activation of a subset of lumbar spinothalamic neurons after copulatory behaviour in male but not female rats. J. Neurosci. 2003;23:325–331. doi: 10.1523/JNEUROSCI.23-01-00325.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TURNER W.H., BRADING A.F. Smooth muscle of the bladder in the normal and the diseased state: pathophysiology, diagnosis and treatment. Pharmacol. Ther. 1997;75:77–110. doi: 10.1016/s0163-7258(97)00038-7. [DOI] [PubMed] [Google Scholar]
- UENO N., IWAMOTO Y., SEGAWA N., KINOSHITA M., UEDA H., KATSUOKA Y. The effect of sildenafil on electrostimulation-induced erection in the rat model. Int. J. Impot. Res. 2002;14:251–255. doi: 10.1038/sj.ijir.3900860. [DOI] [PubMed] [Google Scholar]
- VACHON P., SIMMERMAN N., ZAHRAN A.R., CARRIER S. Increases in clitoral and vaginal blood flow following clitoral and pelvic plexus nerve stimulations in the female rat. Int. J. Impot. Res. 2000;12:53–57. doi: 10.1038/sj.ijir.3900480. [DOI] [PubMed] [Google Scholar]
- VAN ASSELT E., GROEN J., VAN MASTRIGT R. A comparative study of voiding in rat and guinea pig: simultaneous measurement of flow rate and pressure. Am. J. Physiol. 1995;269:R98–R103. doi: 10.1152/ajpregu.1995.269.1.R98. [DOI] [PubMed] [Google Scholar]
- VAN FURTH W.R., VAN REE J.M. Appetitive sexual behavior in male rats: 1. The role of olfaction in level-changing behavior. Physiol. Behav. 1996;60:999–1005. doi: 10.1016/0031-9384(96)00010-8. [DOI] [PubMed] [Google Scholar]
- VEENING J.G., COOLEN L.M. Neural activation following sexual behavior in the male and female rat brain. Behav. Brain Res. 1998;92:181–193. doi: 10.1016/s0166-4328(97)00190-3. [DOI] [PubMed] [Google Scholar]
- VEMULAPALLI R., KUROWSKI S., SALISBURY B., PARKER E., DAVIS H. Activation of central melanocortin receptors by MT-II increases cavernosal pressure in rabbits by the neuronal release of NO. Br. J. Pharmacol. 2001;134:1705–1710. doi: 10.1038/sj.bjp.0704437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VIAL C., EVANS R.J. P2X receptor expression in mouse urinary bladder and the requirement of P2X(1) receptors for functional P2X receptor responses in the mouse urinary bladder smooth muscle. Br. J. Pharmacol. 2000;131:1489–1495. doi: 10.1038/sj.bjp.0703720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALDINGER M.D., OLIVIER B. Animal models of premature and retarded ejaculation. World J. Urol. 2005;23:115–118. doi: 10.1007/s00345-004-0493-x. [DOI] [PubMed] [Google Scholar]
- WALDINGER M.D., PLAS A.V., PATTIJ T., OORSCHOT R.V., COOLEN L.M., VEENING J.G., OLIVIER B. The selective serotonin re-uptake inhibitors fluvoxamine and paroxetine differ in sexual inhibitory effects after chronic treatment. Psychopharmacology. 2002;160:283–289. doi: 10.1007/s00213-001-0980-3. [DOI] [PubMed] [Google Scholar]
- WALDINGER M.D., QUINN P., DILLEEN M., MUNDAYAT R., SCHWEITZER D.H., BOOLELL M.2005aA multinational population survey of intravaginal ejaculation latency time J. Sex. Med.(in press). [DOI] [PubMed]
- WALDINGER M.D., SCHWEITZER D.H., OLIVIER B. On-demand SSRI treatment of premature ejaculation: pharmacodynamic limitations for relevant ejaculation delay and consequent solutions. J. Sex. Med. 2005b;2:121–131. doi: 10.1111/j.1743-6109.2005.20112.x. [DOI] [PubMed] [Google Scholar]
- WALDINGER M.D., ZWINDERMAN A.H., SCHWEITZER D.H., OLIVIER B. Relevance of methodological design for the interpretation of efficacy of drug treatment of premature ejaculation: a systematic review and meta-analysis. Int. J. Impot. Res. 2004;16:369–381. doi: 10.1038/sj.ijir.3901172. [DOI] [PubMed] [Google Scholar]
- WALTER J.S., WHEELER J.S., FITZGERALD M.P., MCDONNELL A., WURSTER R.D. Chronic instrumentation with model microstimulators in an animal model of the lower urinary tract. J. Spinal Cord Med. 2005;28:114–120. doi: 10.1080/10790268.2005.11753808. [DOI] [PubMed] [Google Scholar]
- WATANABE T., PERKASH I., CONSTANTINOU C.E. Modulation of detrusor contraction strength and micturition characteristics by intrathecal baclofen in anesthetized rats. J. Urol. 1997;157:2361–2365. [PubMed] [Google Scholar]
- WATANACHOTE D. Urethral responses during selective electrostimulation of lumbosacral spinal nerve roots in the female spinal dog. Tohoku J. Exp. Med. 1982;138:87–102. doi: 10.1620/tjem.138.87. [DOI] [PubMed] [Google Scholar]
- WESSELLS H., FUCIARELLI K., HANSEN J., HADLEY M.E., HRUBY V.J., DORR R., LEVINE N. Synthetic melanotropic peptide initiates erections in men with psychogenic erectile dysfunction: double-blind, placebo controlled crossover study. J. Urol. 1998;160:389–393. [PubMed] [Google Scholar]
- WESSELLS H., GRALNEK D., DORR R., HRUBY V.J., HADLEY M.E., LEVINE N. Effect of an alpha-melanocyte stimulating hormone analog on penile erection and sexual desire in men with organic erectile dysfunction. Urology. 2000;56:641–646. doi: 10.1016/s0090-4295(00)00680-4. [DOI] [PubMed] [Google Scholar]
- WOLFFENBUTTEL K.P., KOK D.J., MINEKUS J.P.J., VAN K.G.A., VAN M.R., NIJMAN J.M. Urodynamic follow-up of experimental urethral obstruction in individual guinea pigs. Neurourol. Urodynam. 2001;20:699–713. doi: 10.1002/nau.1021. [DOI] [PubMed] [Google Scholar]
- WOODS M., CARSON N., NORTON N.W., SHELDON J.H., ARGENTIERI T.M. Efficacy of the beta 3-adrenergic receptor agonist CL-316243 on experimental bladder hyperreflexia and detrusor instability in the rat. J. Urol. 2001;166:1142–1147. [PubMed] [Google Scholar]
- YOKOYAMA O., ISHIURA Y., KOMATSU K., MITA E., NAKAMURA Y., KUNIMI K., MORIKAWA K., NAMIKI M. Effects of MK-801 on bladder overactivity in rats with cerebral infarction. J. Urol. 1998;159:571–576. doi: 10.1016/s0022-5347(01)63986-7. [DOI] [PubMed] [Google Scholar]
- YOKOYAMA O., YOSHIYAMA M., NAMIKI M., DE GROAT W.C. Influence of anesthesia on bladder hyperactivity induced by middle cerebral artery occlusion in the rat. Am. J. Physiol. 1997;273:R1900–R1907. doi: 10.1152/ajpregu.1997.273.6.R1900. [DOI] [PubMed] [Google Scholar]
- YOKOYAMA O., YOSHIYAMA M., NAMIKI M., DE GROAT W.C. Role of the forebrain in bladder overactivity following cerebral infarction in the rat. Exp. Neurol. 2000;163:469–476. doi: 10.1006/exnr.2000.7391. [DOI] [PubMed] [Google Scholar]
- YOKOYAMA O., YUSUP A., MIWA Y., OYAMA N., AOKI Y., AKINO H. Effects of tolterodine on an overactive bladder depend on suppression of C-fiber bladder afferent activity in rats. J. Urol. 2005;174:2032–2036. doi: 10.1097/01.ju.0000176793.50410.9e. [DOI] [PubMed] [Google Scholar]
- YOSHIMURA N. Bladder afferent pathway and spinal cord injury: possible mechanisms inducing hyperreflexia of the urinary bladder. Prog. Neurobiol. 1999;57:583–606. doi: 10.1016/s0301-0082(98)00070-7. [DOI] [PubMed] [Google Scholar]
- YOSHIMURA N., DE GROAT W.C. Plasticity of Na+ channels in afferent neurones innervating rat urinary bladder following spinal cord injury. J. Physiol. 1997;503:269–276. doi: 10.1111/j.1469-7793.1997.269bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOSHIMURA N., ERDMAN S.L., SNIDER M.W., DE GROAT W.C. Effects of spinal cord injury on neurofilament immunoreactivity and capsaicin sensitivity in rat dorsal root ganglion neurons innervating the urinary bladder. Neuroscience. 1998;83:633–643. doi: 10.1016/s0306-4522(97)00376-x. [DOI] [PubMed] [Google Scholar]
- YOSHIMURA N., MIZUTA E., KUNO S., SASA M., YOSHIDA O. The dopamine D1 receptor agonist SKF 38393 suppresses detrusor hyperreflexia in the monkey with parkinsonism induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) Neuropharmacology. 1993;32:315–321. doi: 10.1016/0028-3908(93)90151-r. [DOI] [PubMed] [Google Scholar]
- YOSHIYAMA M., ROPPOLO J.R., THOR K.B., DE GROAT W.C. Effects of LY274614, a competitive NMDA receptor antagonist, on the micturition reflex in the urethane-anaesthetized rat. Br. J. Pharmacol. 1993;110:77–86. doi: 10.1111/j.1476-5381.1993.tb13774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YUCEL S., BASKIN L.S. An anatomical description of the male and female urethral sphincter complex. J. Urol. 2004;171:1890–1897. doi: 10.1097/01.ju.0000124106.16505.df. [DOI] [PubMed] [Google Scholar]
- ZHANG X.Y., IGAWA Y., ISHIZUKA O., NISHIZAWA O., ANDERSSON K.E. Effects of resiniferatoxin desensitization of capsaicin-sensitive afferents on detrusor over-activity induced by intravesical capsaicin, acetic acid or ATP in conscious rats. Naunyn-Schmiedebergs Arch. Pharmacol. 2003;367:473–479. doi: 10.1007/s00210-003-0748-x. [DOI] [PubMed] [Google Scholar]
- ZVARA P., SAHI S., HASSOUNA M.M. An animal model for the neuromodulation of neurogenic bladder dysfunction. Br. J. Urol. 1998;82:267–271. doi: 10.1046/j.1464-410x.1998.00676.x. [DOI] [PubMed] [Google Scholar]