Abstract
The Post-Genomic age presents many new challenges and opportunities for the improved understanding, diagnosis and treatment of human disease. The long-term goal is to identify molecular correlates of disease processes, and use this information to develop novel and more effective therapeutics. A major hurdle in this regard is ensuring that the molecular targets of interest are indeed relevant to the physiology and/or pathophysiology of the processes being studied, and, moreover, to determine if they are specific to the tissue/organ being investigated. As a first step in this direction, we have reviewed the literature pertaining to bladder and erectile physiology/pharmacology and dysfunction and attempted to summarize some of the critical molecular mechanisms regulating detrusor and corporal myocyte tone. Because of the vast amount of published data, we have limited the scope of this review to consideration of the calcium-mobilizing and calcium-sensitizing pathways in these cells. Despite obvious differences in phenotypic characteristics of the detrusor and corporal myocyte, there are some common molecular changes that may contribute to, for example, the increased myocyte contractility characteristic of bladder and erectile dysfunction (i.e. increased Rho kinase activity and decreased K+ channel function). Of course, there are also some important distinctions in the pathways that modulate contractility in these two cell types (i.e. the contribution of ryanodine-sensitive calcium stores and the nitric oxide/cGMP pathways). This report highlights some of these similarities and distinctions in the hope that it will encourage scientific discourse and research activity in this area, eventually leading to an improved quality of life for those millions of individuals that are afflicted with bladder and erectile dysfunction.
Keywords: Smooth muscle, bladder, penis, detrusor, corpora, molecular mechanisms, erectile dysfunction, overactive bladder
Introduction
The new millennium, the completion of the human genome project and the continuous revolution and evolution of molecular technologies, have created great expectations for their application to the improved understanding, diagnosis and treatment of human diseases. It now seems quite obvious that merely having the genetic or molecular ‘blue print' of a given tissue or cell type is a necessary, but not a sufficient, condition for determining the contribution of molecular differences to differential tissue physiology on the one hand, nor to the illumination of the contributions of molecular alterations to pathophysiological changes in tissue function on the other. In fact, the latter requires detailed understanding of the contribution of the molecular target to the physiological process of interest. More specifically, it is critical to establish verifiable links between changes in the expression or function of a particular gene or protein, and the commensurate changes in organ function.
As with many other organ systems, the application of molecular technologies to diseases of the lower urinary tract holds great promise. The goal of this report is to highlight the utility of increased molecular understanding of myocyte function/dysfunction to the improved pharmacotherapy of bladder and erectile dysfunction. While it is clear that bladder and erectile dysfuction are multifactorial, we will focus this report on the role played by the corporal and detrusor smooth muscle cell. Specifically, we will review the data available in the literature with respect to two main points. First, we will summarize the impact of disease at the molecular level on gene and/or protein expression of critical modulators of corporal and detrusor myocyte function. Secondly, we will describe the use of molecular technologies to alter the expression/function of putative critical modulators of corporal or detrusor smooth muscle cell function (i.e. mouse genetics and gene transfer), and document the corresponding impact on cell, tissue and/or organ function. However, before doing so, a brief review of the physiological contribution of the corporal and detrusor myocyte to organ function is required.
Physiology and pharmacology of erection
Erectile function is intimately linked to corporal tissue physiology. The corporal tissue that forms the paired corporal bodies, in turn, is composed of a series of contiguous, endothelial-lined, sinusoidal spaces in which the primary parenchymal cell type is a specialized vascular smooth muscle cell, referred to as corporal smooth muscle. Penile rigidity sufficient for vaginal penetration requires the careful orchestration of a complex series of neurovascular events. Several recent reviews have been written on this subject (Andersson, 2003; Christ et al., 2004b; Gonzalez-Cadavid et al., 2004), and moreover, the most recent data/information can be found in the proceeding of the Second International Consultation on Erectile Dysfunction (Saenz de Tejada, 2004). Therefore, we will briefly review only those processes central to this report.
Suffice it to say that all penile erections begin in the brain. Stimuli, physical or psychic, are integrated at the level of the limbic system, hypothalamus and brainstem. Activation of these areas of the CNS stimulates the autonomic nervous system of the penis, via the parasympathetic roots from S2-4 and the cavernosal nerves, and initiates an erection. The sympathetic input, also through the cavernous nerves, tends to inhibit erections. This input arises from the T11–L2 levels of the spinal cord. The penis is also well supplied with afferent sensory nerve fibers, which coalesce to form the dorsal penile nerve. These sensory signals reach the spinal cord via the pudendal nerve, eventually making their way to the appropriate centers of the brain. The pudendal nerve, being the prime somatic nerve supplying the penis, also provides control over the extracorporeal striated musculature (bulbocavernosus and ischiocavernosus) (Christ, 1997; Simonsen et al., 2002; Christ et al., 2004b).
However, once the visual or erotic stimuli are processed, penile erection is primarily a vascular event. These vascular events are required first to initiate, and subsequently to maintain, relaxation of corporal smooth muscle cells, increasing blood flow into the penis and resulting in sustained penile erection. The generation of a penile erection or the detumescence of an erect penis requires rapid and coordinated relaxation and contraction of the corporal smooth muscle, respectively. Any aberration of these syncytial actions can manifest as erectile dysfunction, and as described herein, elucidating the molecular correlates of organic erectile dysfunction will greatly assist in better understanding mechanisms of erectile disease, as well as in the identification of novel and more efficacious therapeutic options for restoration of erectile function.
The hemodynamic changes associated with erection occur subsequent to release of neurotransmitters that induce dilation of the penile arteries and relaxation of the corporal smooth muscle cells. The cavernous sinusoids then fill with blood, and the resulting engorgement compresses the subtubical venules against the tunica albuginea, decreasing venous outflow (i.e. veno-occlusive mechanism) with resultant filling of the cavernous tissue to the point of rigidity and the achievement of supradiastolic pressures (Christ et al., 2004b). Detumescence (flaccidity) ensues when the parasympathetic stimulation to the penis ceases, and the downstream mediators have been metabolized or otherwise returned to their normal resting levels. These actions are further augmented by the sympathetic stimulation associated with ejaculation, which contracts the vascular and corporal smooth muscles, decreasing penile blood flow and opening the emissary veins allowing egress of blood from the penis (Christ, 1997; Simonsen et al., 2002; Christ et al., 2004b). Penile flaccidity is maintained via a continuous state of elevated corporal smooth muscle tone.
Molecular and biochemical basis of erection and detumescence/flaccidity
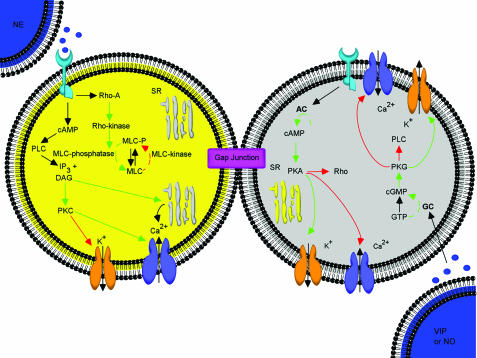
As is clear from the aforementioned discussion, the level of contractility of the corporal myocytes is a critical determinant of erectile function/capacity. In this scenario, the degree of corporal smooth muscle cell tone at any given point in time reflects the complex integration of the effects of a host of neurotransmitters, neuromodulators, hormones (sympathetic, parasympathetic, NANC, etc.). The molecular and biochemical events that coordinate and modulate corporal smooth muscle cell tone (contraction and relaxation) are quite well known at this point. The salient features of this process are depicted in Figure 1, and briefly described below.
Figure 1.
Schematic depiction of the physiological/pharmacological basis for contraction and relaxation of corporal myocytes. Mechanisms are summarized from the text and cited literature throughout this report. Where SR: sarcoplasmic reticulum, PLC: phospholiapse C, DAG: diacylglycerol, PKC: protein kinase C, IP3: inositol trisphosphate, MLC: myosin light chain. Stimulatory pathways are illustrated via the green arrows, while the red arrows represent inhibitory regulation.
Corporal smooth muscle relaxation and erection
Upon visual or tactile stimulation, nitric oxide (NO), a product of NANC neurotransmission, is produced and released by nitrergic nerves as well as the endothelial cells that line the corporal sinusoids. NO is considered to be an important initiator of the erectile process and mediates penile vasodilation largely indirectly, via activation of guanylyl cyclase in the smooth muscle cell, followed by increases in cGMP and activation of protein kinase G (PKG); although some direct effects of NO have also been reported (Saenz de Tejada, 1995). Activation of PKG, in turn, results in phosphorylation of several cellular proteins (e.g. K+ channels, Ca2+ channels and pumps, myosin binding proteins (i.e. altering calcium sensitization), etc.) thereby altering their activity. The end result is decreased intracellular calcium levels and diminished corporal smooth muscle cell tone. cGMP is actively hydrolyzed by phosphodiesterase type 5 to guanosine monophosphate (PDE V) (Christ et al., 2004b; Corbin, 2004; Argiolas et al., 2005; Toda et al., 2005).
In addition to NO, it is clear that other neurotransmitters/neuromodulators also participate in corporal smooth muscle relaxation and erection. Vasoactive intestinal polypeptide (VIP), calcitonin gene-related peptide (CGRP) and the prostaglandins (PGE1 and PGE2) are the most prominent examples. All of these compounds are also thought to induce corporal smooth muscle relaxation via activation of cyclic nucleotides. Specifically, all of these substances activate membrane-bound receptors coupled to Gs proteins, which activate adenylate cyclase, leading to an increase in intracellular cAMP levels, followed by activation of protein kinase A (PKA; see Figure 1). PKA then phosphorylates cellular proteins leading to decreased intracellular calcium levels and smooth muscle relaxation (Lue TF, 2004).
Corporal smooth muscle contraction and detumescence/rigidity
Penile detumescence and flaccidity is achieved or maintained, respectively, by the following sequence of events: activation of α1-adrenoreceptor or ETA receptor subtypes, initiation of a second messenger cascade by activation of the Gq protein, which then leads to activation of phospholipase C (PLC), resulting in cleavage of phosphotidylinositol bisphosphate (PIP2) into inositol trisphosphate (IP3) and diacylglycerol (DAG), respectively. DAG then activates protein kinase C (PKC). PKC phosphorylation of cellular proteins, such as L-type voltage-dependent calcium channels (leading to an increased calcium influx), K+ channels (leading to decreased K+ channel activity), and perhaps inhibition of myosin light chain phosphatase (MLCP) all lead to increased corporal smooth muscle cell tone. In addition, increased IP3 levels induce the release of calcium from intracellular stores (via the IP3 receptor). Activation of the α1-adrenoceptor or ETA receptor subtypes also leads to activation of the Rho A/Rho kinase pathway and inhibition of MLCP (i.e. calcium sensitization; Bivalacqua et al., 2004), and recent evidence suggests that coactivation of these receptor subtypes is synergistic at the level of Rho A/Rho kinase (Wingard et al., 2003). The end result of all of these simultaneously occurring processes is increased corporal smooth muscle cell tone via an increase in intracellular calcium levels and enhanced calcium sensitization. Both continuous transmembrane calcium flux (mediated by L-type voltage-dependent Ca2+ channels) and calcium sensitization are important to the maintenance of penile flaccidity (Christ, 2000a; Andersson, 2001; Christ et al., 2004b; Toda et al., 2005).
Gap junctions: the mechanistic basis for coordination of corporal smooth muscle contraction and relaxation
The extant data in both animal models and man reveal a relatively sparse effector innervation of the corporal myocytes, far less than one nerve terminal for each smooth muscle cell. In the light of the fact that corporal myocytes are not electrically excitable (i.e. no action potentials), this raises the question of how a limited supply of autonomic innervation is capable of supporting the rapid and coordinated responses required for penile erection; especially when it involves such a large group of cells enmeshed in a heavily collagenous extracellular matrix. A series of publications has clearly documented that gap junction proteins (primarily connexin43 (Cx43); see Figure 1) provide the anatomic substrate for coordination of cell-to-cell responses among corporal myocytes. While a detailed discussion is beyond the scope of this focused report, suffice it to say that theoretical studies (supported by biological observations) indicate that so long as agonist-mediated changes in intracellular second messenger levels (i.e. calcium, cAMP, cGMP) are on the order of three- to 10-fold over baseline levels, the presence of the intercellular pathway would ensure roughly a doubling of the number of recruited cells (cells not directly activated by a neurotransmitter) over a time frame that is relevant to penile erection/detumescence (Christ et al., 1994; Ramanan et al., 1998). In short, the presence of intercellular communication permits coordinated response generation among the vast array of otherwise largely inexcitable corporal smooth muscle cells (Christ, 2000a, 2000b, 2004b).
Molecular mechanisms and therapies of erectile dysfunction
If there is insufficient/incomplete or unsustained corporal smooth muscle relaxation, inadequate blood flow/pressure will be transmitted to the cavernous sinusoids, resulting in diminished rigidity and erectile dysfunction. Certainly, erectile dysfunction is a multifactorial disease. Nonetheless, researchers have begun to identify molecular alterations that attend or can predispose to erectile failure, and furthermore, have started to leverage this understanding for the development of novel therapies. Some of the better documented targets and therapeutic possibilities are described below.
K+ Channels
In the light of the importance of K+ channels to normal corporal tissue physiology and erectile capacity, perhaps it is not surprising that several studies have indicated that alterations in the expression, regulation or function of K+ channel subtypes may contribute to the etiology of erectile dysfunction (Fan et al., 1995; Christ, 2000b; 2002; Lee, 2000; Archer, 2002; Spektor et al., 2002; Venkateswarlu et al., 2002; Werner et al., 2005). Several K+ channel subtypes have been reported in corporal myocytes, for example, KATP, Kv, and the large conductance, calcium-sensitive K+ channel (i.e. maxi-K or BKCa). The latter, thus far, seems to be the most likely pharmacological/molecular target. As noted above, the intracellular mechanism by which erection/tumescence occurs involves NO-mediated increases in cyclic guanosine cGMP levels and activation of PKG (see Figure 1). PKG, in turn, phosphorylates numerous ion channels and pumps, to elicit a diminution in the free cytosolic calcium levels.
In this regard, experimental evidence in mice lacking the pore forming subunit of the BK channel have documented that loss of this channel leads to a significant decrease in the cavernous-nerve stimulated intracavernous pressure response. Such observations are consistent with impaired rigidity and compromised erectile capacity observed in these animals. Consistent with these findings in mice, recent studies have investigated the use of BK channel openers based on the 3-thio-quinolinone core. In these studies, there was significant functional activity of these novel compounds in rabbit corpus cavernosum tissue strips, confirming the possibility of BK channel openers as a therapy for ED (Hewawasam et al., 2003; Boy et al., 2004). In addition, the decline in erectile capacity observed in rodents with both age (Christ et al., 1998; Melman et al., 2003) and diabetes (Christ et al., 2004a) can be ameliorated following a single intracavernous injection of naked DNA encoding hSlo (again, the pore forming subunit of the human BK channel). This latter observation has led to the initiation of a Phase I clinical trial to examine the efficacy of K+ channel gene therapy for the treatment of erectile dysfunction, and the preliminary findings have been reported (Melman et al., 2005). In addition, there is also evidence from pharmacological studies on isolated human corporal tissue strips that alterations in another K+ channel subtype, namely, the KATP channel (the metabolically-regulated K+ channel), may contribute to the pathophysiology of diabetes-related erectile dysfunction (Venkateswarlu et al., 2002). Thus, there is now compelling evidence from several independent lines of investigation indicating that K+ channel-mediated hyperpolarization of corporal smooth muscle cells may compromised with age, diabetes and/or with vascular disease, contributing to erectile dysfunction. As such, K+ channels are an attractive therapeutic target for improved pharmacotherapy of erectile dysfunction. Nonetheless, it should be pointed out that initial pharmacological treatments targeting K channels have proved somewhat disappointing (see Christ, 2002 for details).
Calcium mobilization, Ca2+ channels and Ca2+ signaling
As with the detrusor myocyte (see below), the free intracellular calcium concentration is an important determinant of corporal myocyte tone. In fact, corporal smooth muscle tone is dependent on the release of stored (i.e. SR stores) intracellular calcium ions (Ca2+) into the cytoplasm in response to the stimuli illustrated in Figure 1, as well as the influx of Ca2+ through specialized membrane channels that are regulated by voltage changes (Christ et al., 2004b). Typical of many smooth muscle cell types, the calcium response in corporal myocytes is biphasic, consisting of a transient peak level that provides the trigger for increased contractility but quickly declines to a level that is near the basal level (i.e. precontractile level). Changes in intracellular calcium levels in corporal myocytes occur as a result of liberation of intracellular calcium stores (i.e. primarily IP3-sensitive SR stores), as well as transmembrane calcium flux through L-type voltage-dependent calcium channels. In this regard, the L-type voltage-dependent calcium channel is apparently the most physiologically relevant calcium channel subtype present in corporal smooth muscle. In fact, the physiological significance of these channels derives from the inward depolarizing Ca2+ currents that they mediate, such that continuous transmembrane calcium influx is an absolute prerequisite to the initiation, maintenance and modulation of corporal smooth muscle cell tone. While the contribution of ryanodine-sensitive SR stores to corporal smooth muscle physiology is as yet undefined, Nelson and co-workers have documented the presence of pronounced BK current transients characteristic of those produced by calcium sparks in other smooth muscle cells (i.e. spontaneous transient outward currents in response to ryanodine receptor (RyR)-mediated calcium release) (Werner et al., 2005).
Gap junctions
Changes in intercellular communication affect coordination and spread of intracellular second messenger signals among corporal myocytes. In this regard, one example of this possibility derives from recent studies in experimentally diabetic rats. Previously, it had bee shown that diabetes related erectile dysfunction has been correlated with diminished synaptophysin reactivity in the corpora of experimentally diabetic rats, a marker of autonomic efferent innervation (Rehman et al., 1997). Such observations might be expected to impact on the coordination of syncytial corporal tissue responses, and thus, in a recent study (Brink, 2006), Brink and co-workers examined the impact of experimental diabetes on Cx43-mediated intercellular communication in short-term cell cultures derived from diabetic rat corporal smooth muscle. These data provided the first direct evidence for a diabetes-related increase in intercellular permselectivity of Cx43-derived gap junction channels. These data indicate that more effective transfer of second messengers such as calcium or IP3, may be enhanced in diabetes, resulting in enhanced corporal smooth muscle cell tone, and thus, promoting erectile dysfunction. Such observations have important implications to the understanding of diabetes-related erectile dysfunction.
NO, G Kinase and PDE V inhibitors
By examining the molecular mechanisms of erectile dysfunction, avenues for therapy become clear. As mentioned elsewhere in this report, the NO/guanylate cyclase/cGMP/G Kinase pathway plays a prominent role in erectile function. Experimental evidence for this at the molecular level derives from a variety of sources. Consistent with this supposition, mice deficient in cGMP-dependent Kinase I were shown to have a very low ability to reproduce, and furthermore, isolated corporal tissue strips from these mice failed to relax upon activation of the NO/cGMP signal transduction pathway (Hedlund et al., 2000). In addition, Chang et al. (2004) have documented a diabetes-related downregulation of PKG-I expression and decreased PKG-I activity in corporal smooth muscle strips that correlated with decreased corporal smooth muscle relaxation due to activation of this pathway.
Further molecular evidence for the importance of this pathway to erectile function/capacity derives from the well-publicized clinical success of phosphosdiesterase V (PDE V) inhibitors such as sildenafil, for the treatment of erectile dysfunction (Carson et al., 2005). Phosphodiesterases metabolize cyclic nucleotides, and thus decrease their physiologic impact. In contrast, inhibition of PDEs increases cyclic nucleotide levels. In particular, PDE V is a prominent PDE present in corporal myocytes, and PDE V inhibition maximizes the impact of NO release on cGMP levels, by preventing the breakdown of cGMP, thus leading to accumulation of cGMP and potentiation of corporal smooth muscle relaxation. More recently, other PDE V inhibitors (vardenafil and tadalafil (Valiquette et al., 2005; Young et al., 2005) have also shown clinical efficacy. Taken together, these observations clearly document the importance and therapeutic potential of manipulation of the cGMP/G Kinase pathway.
Another corollary of the importance of PDEs to erectile function derives from novel recent observations using RNA interference technologies. Specifically, siRNA (small interfering RNA; antisense oligodeoxyribonucleotides ≈20 nucleotides in length that can hybridize to and inactivate mRNA) directed to conserved regions of mammalian PDE V mRNA were shown to cause a decrease in PDE V activity and to prolong cGMP activity in cultured rodent corporal smooth muscle cells. Additionally, injection of a lentiviral vector coding for this siRNA into the rat penis increased the duration of the cavernous nerve stimulated intracavernous pressure response in vivo (Lin et al., 2005). This is simply another independent demonstration of the importance of this pathway to erectile capacity, and shows that inhibiting enzymatic activity produces a result consistent with the pharmacological inhibition seen with the PDE V inhibitors. The implications of such observations to the improved treatment of erectile dysfunction have been discussed in detail elsewhere (see Christ, 2005).
Finally, recent studies have suggested a role for increased NADPH oxidase activity/expression in the etiology of erectile dysfunction related to diabetes and dyslipedemia. The rationale is as follows. Increased NADPH oxidase activity leads to an increase in oxygen free radicals (O2; superoxide), which then react with NO to generate peroxynitrite, thus quenching the NO signal and reducing its bioavailability. Consistent with this supposition, increased superoxide formation was documented in an experimental rabbit model of hypercholesterolemia, in which diminished carbachol-induced relaxation of corporal tissue strips, which was reversed by NADPH oxidase inhibitors. Corresponding to intuition, this same group showed that use of an NO-donating form of sildenafil was more effective than conventional sildenafil in relaxing corporal tissue strips from hypercholesterolemic rats (Shukla et al., 2005). The overall importance of reactive oxygen species (ROS) and the significance of the NO/ROS balance, in the context of corporal smooth muscle cell/collagen ratio, tissue compliance and erectile capacity has been extensively discussed by Gonazalez-Cadavid and Rajfer elsewhere (Gonzalez-Cadavid et al., 2004) and will not be further elaborated upon herein.
Calcium sensitization and Rho-Kinase
There is now little doubt that calcium sensitization makes an important contribution to corporal smooth muscle physiology and perhaps to the etiology of erectile dysfunction as well (Mills et al., 2001; 2002; 2003; Wingard et al., 2003). Recent data in experimental animals point toward altered modulation of calcium sensitivity in diabetes (Bivalacqua et al., 2004), such that an increased activation of the RhoA/Rho kinase pathway leads to downregulation of penile eNOS, and thus erectile impairment. Intracorporal gene transfer of an adeno-associated virus encoding for the dominant-negative RhoA mutant in diabetic rats restored NOS activity, cGMP accumulation and the nerve stimulated intracavernous pressure responses to values similar to that observed in control animals. Similarly, in experimental models of hypertension (Wilkes et al., 2004) or aging-related erectile dysfunction (Rajasekaran et al., 2005), Rho-kinase antagonism and PDE-5 inhibition had a synergistic effect in improving erectile function. As such, it appears that inhibition of the RhoA/Rho kinase pathway might be an attractive molecular target for the treatment of erectile dysfunction.
Gene transfer/therapy
Perhaps some of the best insights into molecular mechanisms of erectile physiology and dysfunction derived from data generated using a plethora of distinct gene transfer approaches. The external location of the penis makes it an ideal organ system for such approaches, and proof-of-concept for the potential utility of gene transfer to the treatment of human erectile dysfunction is currently ongoing in the form of a Phase I clinical trial (Melman et al., 2005). In fact, a variety of molecular targets have been evaluated, ranging from vascular and nerve growth factors, to NOS, superoxide dismutase, RhoA/Rho kinase and ion channels. A detailed description of all of these approaches is outside the scope of this report, but it suffices to say that much has been learned about erectile physiology/dysfunction through the utilization of gene transfer, and the preclinical studies conducted to date are summarized in Table 1, and detailed in several other recent reviews (Christ, 2004; Hafron, 2004; see Kendirci et al., 2005).
Table 1. Gene therapy for erectile dysfunction.
| Growth factors | ||
| VEGF-165 | DNA transfer of VEGF-165 into rat penis for neovascularization | Burchardt et al. (2005) |
| VEGF with AAV-BDNF | VEGF with adeno-associated virus-mediated brain-derived neurotrophic factor for ED treatment after cavernosal nerve injury | Rogers et al. (2003) |
| NT3 gene | Neurotrophin 3 gene, via HSV vector, showed some success with ED due to DM | Bennett et al. (2005) |
| Ion channels | ||
| Maxi-K+ channel | Plasmid vector (hMaxi-K), which contains the hSlo gene, that encodes the alpha subunit of the Maxi K channel | Christ (2002; 2004), Christ et al. (2004a), Melman et al. (2003; 2005) |
| Nitric oxide | ||
| NOS isoforms | Induce eNOS, nNOS, and iNOS to improve erectile function | Gonzalez-Cadavid et al. (2004), Kendirci et al. (2005) |
| Neurotransmitters | ||
| VIP | Some success transfecting penile corpora cavernosa with pcDNA3/vasoactive intestinal polypeptide (VIP) cDNA in streptozotocin (STZ)-diabetic rats | Shen et al. (2005) |
| CGRP | Data suggests that in vivo adenoviral gene transfer of CGRP improves erectile function in the aged rats | Bivalacqua et al. (2001) |
Physiology and pharmacology of micturition
Normal bladder function involves the ability to fill, store, and empty urine. That is, the bladder must be able to fill and store the urine produced by the kidneys at low pressure, while maintaining the ability for rapid, forceful, coordinated contractions that generate sufficient pressure to ensure complete bladder emptying. These functions are obviously critically dependent on the tone of the detrusor myocytes. It follows, therefore, that improved understanding of detrusor myocyte physiology and pathophysiology will provide valuable insight into abnormal bladder function, and provide for the identification of novel therapeutic pathways for the more efficacious treatment of bladder disease (Andersson et al., 2004).
The bladder is a hollow organ, the inner surface is lined by a specialized urothelium, which is covered by lamina propria and smooth muscle cells. The detrusor smooth muscle cells of the bladder are arranged into fascicles, or bundles of smooth muscle cells. Fascicles, in turn, are arranged in groups around the bladder, with the overall pattern being that there are inner and outer longitudinal layers, with a circular middle layer. The muscle bundles may interconnect, and furthermore, the individual smooth muscle cells themselves are interconnected via gap junctions (see below), forming a coordinated syncytial unit (Andersson et al., 2004; Silva et al., 2004).
Bladder function is controlled by autonomic and somatic nerve input. The autonomic input involves adrenergic, cholinergic, and NANC nerve fibers (although in human, the latter is probably only important in disease; see below). These fibers traverse the entire bladder body and divide and intersperse to spread throughout the numerous fascicles of the bladder. The distribution of nerve input is such that most of the adrenergic and cholinergic innervation is located at the base of the bladder in the trigone, with less innervation anteriorly and on the dome of the bladder body. In addition to the prominent adrenergic and cholinergic innervation of the bladder, it is clear from the incomplete of inhibition of detrusor contraction provided by pharmacologic blockade of the adrenergic or cholinergic inputs that there must be another effector of bladder function, namely the nonadrenergic, noncholinergic (NANC) input. This NANC bladder input appears to be based on purinergic neurotransmission, with the neurotransmitter ATP (Burnstock, 1977; McConnell et al., 1982; Ruggieri et al., 1990; Zygmunt et al., 1993).
In order for micturition to occur, the parasympathetic input to the bladder (which initiates smooth muscle contraction) must be activated, while the sympathetic input (which mediates smooth muscle relaxation) must be inhibited. The typical micturition reflex is initiated by activation of afferent nerve fibers in the bladder wall during bladder filling. These afferent nerves, which are myelinated A-delta fibers, carry the signal through the dorsal root ganglion of the spinal cord to the pontine micturition center in the brain stem and the periaqueductal gray (Kavia et al., 2005). This incoming neural signal activates the descending efferent pathways (i.e. the parasympathetic (pelvic) nerves) to initiate detrusor contraction, while simultaneously inhibiting the sympathetic input to the bladder (hypogastric nerves). For a normal micturition to occur, these activities must be coordinated with relaxation of the urethral and striated urethral sphincters (de Groat, 1998).
Gap junctions: another mechanism for coordination of detrusor contraction
Given the fact that detrusor myocytes are electrically excitable, and, moreover, that the innervation density of the bladder wall is quite reasonable, one might wonder whether gap junctional communication would be important at all to normal bladder function. In this regard, light microscopy has not typically documented that junctional plaques (the gross anatomical/structural correlates of intercellular communication) are present between detrusor myocytes (Daniel et al., 1983; Dixon et al., 1983; Elbadawi et al., 1993a, 1993b, 1993c, 1993d), despite the fact that electrophysiological data demonstrate that current injected into one cell flows into neighboring cells (Bramich et al., 1996; Fry et al., 1999; Christ et al., 2003) with a space constant (λ) for decremental current flow of ≈1 mm (compared with a cell length of 150–200 μm; Karicheti et al., 2001; Christ et al., 2003). This five- to 10-fold difference between the space constant for passive current decay and cell length is a clear indication of the presence of an adequate intercellular pathway (Seki et al., 1992; Bramich et al., 1996). Consistent with the electrophysiological observations, recent studies have documented the intercellular diffusion of hydrophilic dyes, such as Lucifer yellow, when injected (dialyzed) into detrusor myocytes in situ. Furthermore, electron microscopy, immunogold labeling and confocal immunofluorescence and Western blot techniques have all confirmed the presence of small junctional plaques comprised of Cx43, and also Cx45, between human detrusor myocytes (Neuhaus et al., 2002; John et al., 2003; Sui et al., 2003). The small size of the junctional plaques observed likely accounts for the difficulty in detecting these classical structures in the first place. In addition, Wang et al. (2001) used double whole-cell patch-clamp recordings together with Northern and Western blot techniques to characterize gap junction channel properties, and to evaluate Cx43 expression in human detrusor cells (Wang et al., 2001). As far as we are aware, the latter are still the only direct electrophysiological recordings of junctional currents between human detrusor myocytes, and these currents were clearly characteristic of Cx43. The presence of Cx43 mRNA and protein in cultured detrusor myocytes, and also in situ was demonstrated. Taken together, these data indicate that gap junctions could indeed have an important role in the initiation, maintenance, and modulation of detrusor tone.
Molecular and biochemical basis of detrusor function
Certainly, the initiation of a normal bladder contraction involves a cascade of events resulting in the rapid and coordinated contraction of its constituent detrusor myocytes. This process is composed of various molecular and biochemical events working in concert to elicit syncytial detrusor smooth muscle contraction. A detailed review of all of these processes is well outside the scope of this report. Nonetheless, below we provide an integrative, albeit brief, global description of some of the major known mechanisms. Out of necessity, we have focused this portion of the report largely on ionic mechanisms (ion channels and calcium mobilization, with a brief review of calcium sensitization). The rationale for this approach is related to our ability to correlate the information below with molecular genetic and/or gene transfer approaches aimed at target validation, which are discussed in the next section. A schematic depiction of those focused pathways of interest is provided in Figures 2 and 3 and described briefly below.
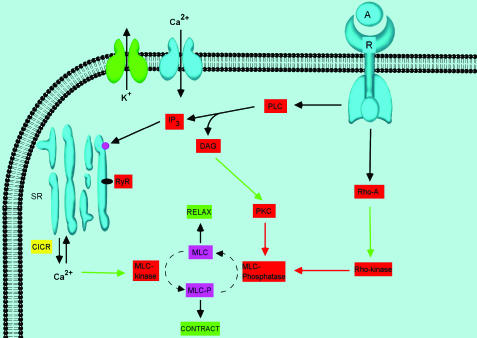
Figure 2.
Schematic depiction of the major regulatory mechanisms that modulate detrusor myocyte tone. Where: denotes a negative or inhibitory effect, although clearly there are several types of K channels known to be present in detrusor myocytes, SR: sarcoplasmic reticulum, RyR: ryanodine receptor, PLC: phospholiapse C, DAG: diacylglycerol, PKC: protein kinase C, IP3: inositol trisphosphate, MLC: myosin light chain, CICR: calcium-induced calcium release, A: agonist, R: receptor. Stimulatory pathways are illustrated via the green arrows, while the red arrows represent inhibitory regulation. Again, mechanisms are summarized from the text and relevant cited literature. Note that nothing is implied by the location and stoichiometry of the depicted potassium and calcium channel, which are merely representative of their presence and physiological/pharmacological importance. A more accurate depiction of their location relative to the SR calcium stores is shown in Figure 3.
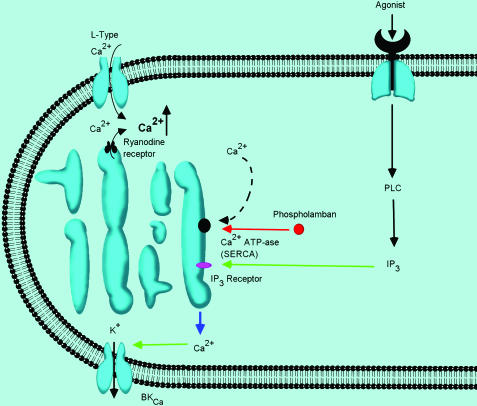
Figure 3.
Detailed depiction of the known calcium mobilizing mechanisms in detrusor myocyte tone. This diagram highlights the multiple points in these processes in which molecular changes have been correlated with alterations in detrusor myocyte function, as summarized in the text.
Detrusor smooth muscle contraction
The muscarinic receptors for the parasympathetic efferent neurons on detrusor myocytes have been described in much detail elsewhere (Wang et al., 1995; Braverman et al., 1998a, 1998b; Andersson, 2004; Andersson et al., 2004). Of the five known muscarinic receptor subtypes (M1–M5), the predominant muscarinic receptor subtype for human and rat detrusor contraction is the M3 receptor subtype (Andersson et al., 2004; Uchiyama et al., 2004). More specifically, the M3 receptor is generally thought to be coupled to Gq/11, leading to activation of phosphoinositide hydrolysis, in turn leading to mobilization of intracellular calcium through liberation of IP3-sensitive intracellular calcium stores (see Figure 2). The presence of M2 receptors has also been documented, and it is believed that they oppose tissue relaxation via two distinct mechanisms. The first is through inhibition of adenylyl cyclase activity, thus functionally antagonizing the effects of sympathetically mediated detrusor relaxation (Hegde et al., 1997; 1999). In addition, Bonev & Nelson. (1993) and Kume et al. (1995a, 1995b) have shown that M2 receptor activation led to inhibition of the KATP and BK channel subtypes in detrusor and airway smooth muscle, thereby resulting in diminished potassium currents and, thus, membrane depolarization and increased detrusor excitability.
In contrast to the muscarinic receptor, the role of NANC innervation in human bladder contractility is ambiguous. Nonetheless, it seems clear that activation of the detrusor smooth muscle requires the participation of at least two transmitters, acetylcholine and ATP (Andersson et al., 2004). ATP, through activation of the P2X receptor (Heppner et al., 2005) is responsible for atropine-resistant, nonadrenergic, noncholinergic, bladder contractions. In animals, the ATP component varies, but can amount to >50% of the total contraction induced by nerve stimulation. In the normal human detrusor, however, the contraction is almost exclusively mediated by muscarinic receptor stimulation. However, it appears that the purinergic component of nerve-mediated detrusor contraction is increased in several types of bladder dysfunction, and this may well contribute to the etiology of detrusor overactivity in humans (Bayliss et al., 1999; O'Reilly et al., 2002). Under such pathophysiological conditions, ATP may be responsible for up to 50% of the nerve-induced contraction of human detrusor in vitro (O'Reilly et al., 2002).
Molecular mechanisms and therapies of bladder dysfunction
K+ Channels
As with many other smooth muscle cell types, K+ channels play a critical role in the modulation of detrusor myocyte tone, and not surprisingly, a number of distinct K channel subtypes have been identified in detrusor myocytes from various species. The central role played by K+ channels in modulating bladder function presumably derives from their functionally antagonistic relationship with transmembrane calcium flux through voltage-dependent calcium channels. Consistent with this supposition, the BKCa channel is arguably the most physiologically relevant modulator of detrusor myocyte excitability. However, in addition to the KCa channel (Sheldon et al., 1997; Siemer et al., 2000; Shieh et al., 2000; Herrera et al., 2001; 2005; Karicheti et al., 2001; Heppner et al., 2003; Meredith et al., 2004; Petkov et al., 2005; Thorneloe et al., 2005), other K+ channel subtypes have also been identified in detrusor myocytes. For example, the Kv 2.1 channel has been documented in rat urinary bladder (Ohya et al., 2000b). Thorneloe et al. (2003) have also identified KV currents in mouse detrusor smooth muscle, and have proposed that heteromultimeric channels composed of Kv2.1, Kv5.1 and Kv6.1 are the relevant molecular species involved (Thorneloe et al., 2003). Intermediate conductance calcium-sensitive K+ channels Ohya et al. (2000a), as well as small conductance calcium sensitive K+ channels (i.e. SK3; Herrera et al. (2000)) have also been identified in detrusor myocytes. Finally, there is also clear evidence for the presence of the metabolically regulated K+ channel subtype, or the KATP channel, in detrusor myocytes (Hu et al., 1997; Wojdan et al., 1999; Buckner et al., 2000; see Shieh et al., 2000; Karicheti et al., 2001).
Consistent with the observations described above, strong evidence for the participation of K+ channels in detrusor myocyte function derives from molecular genetic studies on mice, such as conditional knockouts of the SK3 channel. Studies on isolated detrusor myocytes and strips, as well as in vivo cystometry clearly indicated the importance of SK3 channels to the regulation of detrusor contractility and bladder function. In fact, the data suggested the possibility that activation or increased activity of SK3 channels would result in decreased bladder sensation during filling, which would be of clear benefit for treating bladder overactivity and/or urge incontinence (Herrera et al., 2003). Studies on mice lacking the α- and β1-subunits of the BKCa channel subtype have been similarly illuminating. Cystometric recording in the α-subunit knockout mice revealed demonstrable detrusor overactivity (Meredith et al., 2004; Thorneloe et al., 2005). In addition, enhanced phasic contractile activity (Meredith et al., 2004) and increased sensitivity to nerve stimulation induced contractile responses was observed in isolated detrusor strips derived from these animals (Thorneloe et al., 2005). Interestingly, in detrusor muscle strips from β1-subunit knockout mice (Petkov et al., 2001), enhanced phasic contractile responses were also observed.
The potential therapeutic importance of the BKCa channel to normal detrusor tone has been demonstrated via two other independent lines of investigation. First, recent in vitro findings indicate that hyperpolarization of the detrusor muscle cell membrane via selective activation of the BKCa channel with a new class of BKCa channel openers can elicit relaxation of detrusor muscle strips (Turner et al., 2003). In addition, overexpression of the pore forming subunit of the BKCa using naked DNA gene transfer techniques was shown to improve bladder hyperactivity in rats with detrusor overactivity due to partial urethral outlet obstruction (Christ et al., 2001). More specifically, intravesicular injection of hSlo cDNA (coding for the α-subunit of the human BKCa channel) virtually eliminated the obstruction-associated detrusor overactivity. The implication of these latter findings is that the observed increase in detrusor myocyte excitability associated with outlet obstruction may be partially offset by increased expression of the α-subunit of the BKCa channel. It is important to point out that the ability of gene transfer with BKCa to ameliorate experimental detrusor overactivity is consistent with the opposite phenomenon documented in the work by Nelson and co-workers on mice lacking these channels. Taken together, the existing data strongly support the supposition that alterations in K+ channel subtype expression, regulation or function could have very important implications for normal bladder physiology and the etiology of detrusor dysfunction. Such studies certainly provide for novel therapeutic possibilities.
Calcium mobilization, Ca2+ channels and Ca2+ signaling
A detailed illustrations of calcium signaling pathways that contribute to the modulation of detrusor myocyte tone are highlighted in Figure 3. Similar to the situation described above for the corporal myocyte, changes in the free intracellular calcium concentration are linked to changes in detrusor myocyte tone. However, the mechanism(s) and pathways involved reflect differences that might be expected for a cell that is electrically excitable (i.e. detrusor myocytes are capable of action potentials, whereas corporal myocytes are not). The primary calcium channel subtype present in detrusor smooth muscle is the L-type voltage-dependent calcium channel (Ganitkevich et al., 1992; Wu et al., 2002). Recent studies have demonstrated that Ca2+ influx through voltage-gated Ca2+ channels is the key event in detrusor myocyte depolarization and the subsequent increase in intracellular calcium levels. It has also been shown that that the influx of Ca2+ through L-type Ca2+ channels induces a release of Ca2+ from intracellular (SR) stores (CICR; calcium-induced calcium release; see Figure 3). Taken together, these findings document the nature of initiation and propagation of depolarizing action potentials (both spontaneous and neurogenic) and the induction of contraction in detrusor smooth muscle cells. More specifically, the initiation of the action potential results from Ca2+ entry through L-type Ca2+ channels, while the repolarization is mediated by activation of both KV and BK channels (Heppner et al., 1997; Hashitani et al., 2003; Thorneloe et al., 2003; Petkov et al., 2005) after-hyperpolarization is mediated by the SK3 channel subtype (Herrera et al., 2002; 2003). As noted above (see Figure 2), the IP3-sensitive calcium stores also play an important role in, for example, M3-receptor-mediated contractile responses in detrusor myocytes (Fry et al., 2002). In this regard, while there is some uncertainty regarding the precise nature of the contribution of the L-Type channel to carbachol-induced detrusor contractions (Wu et al., 2002), it also seems clear that regardless of how detrusor myocytes are activated, these channels play a primary role in action potential generation and propagation.
The role of Ca2+ channel subtypes other than the L-type channel to detrusor activation is equivocal. There is some evidence that Ca2+ influx through both T- and L-type Ca2+ channels determine the contractile status of detrusor smooth muscle, and further, that T-type channel activity is important at membrane potentials near the resting level. Evidence for a significant role for T-type channel activity in the resting state (near membrane potential) was seen in recent studies where blockade of the T-type channels had a much larger impact on spontaneous contractility than agonist stimulated responses (Fry et al., 2002; Chow et al., 2003).
The importance of the L-type Ca2+ channel for bladder function is clearly demonstrated in mice deficient in the smooth muscle CaV1.2 calcium channel (SMACKO, smooth muscle alpha1c-subunit calcium channel knockout; Wegener et al., 2004). These mice were found to have a dramatically diminished micturition accompanied by an increased bladder mass. The L-type Ca2+ current, protein expression, and spontaneous contractile activity were all absent in the bladder of SMACKO mice. In addition, K+ and carbachol-induced contractions were reduced 10-fold in detrusor strips from SMACKO mice. Finally, dihydropyridine blockade (i.e. isradipine) inhibited K+ and carbachol-induced contractions of detrusor strips from wild-type mice, but had no effect in preparations from SMACKO mice. Taken together, these data clearly document the important contribution of the L-type Ca2+ channel to detrusor myocyte contractility, and thus, bladder function.
In the light of the aforementioned discussion, it is clear that a delicate balance of mechanisms responsible for maintaining calcium homeostasis/mobilization is critical to normal detrusor function. Therefore, alterations in these processes would be expected to lead to bladder dysfunction. For example, phospholambam is an inhibitor of the SR Ca2+-ATPase (SERCA), and would therefore be expected to be an important modulator of detrusor smooth muscle tone. Consistent with this supposition, mice lacking phospholamban displayed significantly lower values for the maximal amplitude of both the intracellular calcium increase as well as force development, in response to carbachol relative to wild-type mice (Nobe et al., 2001). These findings clearly attest to the importance of SR calcium handling and storage to detrusor contractility.
Recent studies have also demonstrated the importance of the RyR to the regulation of detrusor myocyte tone. Specifically, Jiang et al. (2005) documented that a decrease in RyR expression in a rodent model of outlet obstruction and detrusor overactivity was associated loss of regulation of spontaneous contractile activity (Jiang et al., 2005). In short, abrogation of the negative feedback regulation on the BK channel that is provided by the RyR stores was implicated in the pathogenesis of the increased spontaneous contractile activity observed in bladder strips from rats with detrusor overactivity.
Calcium sensitization and Rho Kinase
Despite the importance of action potentials and associated Ca2+ transients as fundamental mechanisms responsible for generating spontaneous contractions in detrusor smooth muscle (Hashitani et al., 2004), the sensitivity of the contractile proteins for Ca2+, can also be modulated by cyclic nucleotides and Rho kinase, and furthermore, this mechanism appears to play an important role in regulating detrusor myocyte excitability (Andersson et al., 2004). In this regard, while it has been assumed that acetylcholine activation of the detrusor occurs via stimulation of muscarinic M3 receptors, generation of IP3 and intracellular Ca2+ release (Eglen, 1996, see Figure 2), more recent investigations have shown that muscarinic receptor activation is associated with increased Ca2+ sensitivity of the contractile proteins via activation of Rho-kinase and also with Ca2+ influx via voltage-gated dihydropyridine-sensitive Ca2+ channels (Schneider et al., 2004; Wegener et al., 2004). As such, the contractile state of the detrusor myocyte is dependent on the balance between the activities of myosin light chain kinase (MLCK) and MLCP. PKC can also influence the effect of Ca2+ on smooth muscle contractions by phosphorylating CPI-17, which is present in smooth muscle and inhibits MLCP when phosphorylated. Rho-kinase and other kinases have also been reported to phosphorylate the CPI-17 (Andersson & Arner, 2004; Chacko et al., 2004; Takahashi et al., 2004). Consistent with these observations, Bing et al. (2003) showed an overexpression of Rho-kinase (ROCK-b) in rabbit detrusor after partial bladder outlet obstruction, while Rajasekaran et al. (2005) demonstrated that Rho-kinase inhibition suppressed detrusor overactivity in spontaneously hypertensive rats (Bing et al., 2003; Rajasekaran et al., 2005). Chang et al. (2005) also documented an increase in ROCK-b in detrusor from diabetic rabbits.
Gap junctions
Recent observations in human tissue biopsies from patients with neurogenic detrusor overactivity (Haferkamp et al., 2004), as well as in patients with urge symptoms (Neuhaus et al., 2005), clearly demonstrate an increase in the presence of Cx43-derived gap junction channels in detrusor muscle. These human data are consistent with experimental observations in a rodent model of partial urethral outlet obstruction (Christ et al., 2003) that documented a dramatic increase of Cx43 mRNA levels associated with bladder hypertrophy and overactivity (i.e. following 6 weeks of obstruction). A more acute model of obstruction (i.e. hours; Haefliger et al., 2002) was also associated with an increase in Cx43 mRNA expression in detrusor smooth muscle. In addition, in the trigonal smooth muscle, there is evidence for the occurrence of gap junctions (John et al., 2003). Taken together, these data provide good evidence for gap junction coupling between human detrusor cells.
Summary and conclusion: the absolute need for improved therapy
In summary (see Figure 4), it appears that heightened contractility of both detrusor and corporal smooth muscle cell tone is associated with an apparent INCREASE in Rho kinase expression/activity, and a DECREASE in BKCa expression/activity. By extension, such mechanisms appear to make an important contribution to bladder (i.e. detrusor overactivity) and erectile dysfunction (related to heightened contractility and/or impaired relaxation, and therefore, venoocclusive dysfunction). As such, pharmacological agents that target these pathways may be applicable to both of these common lower urinary tract diseases/disorders. In contrast, and not surprisingly, alterations in the RyR and SERCA appear to be more prevalent in altered detrusor contractility, and therefore, may represent a potentially uro-specific target for pharmacotherapy of bladder dysfunction. Finally, while there is some evidence for the importance of the NO/cGMP/G Kinase pathway to the modulation of detrusor myocyte tone (Deka et al., 2004), the weight of the evidence seems to favor the view that this pathway has little direct impact on detrusor contractility (Andersson et al., 2004); especially when one considers the importance of other pathways (i.e. cAMP). Thus, it would appear that manipulation of this pathway would provide some uro-specific treatment of corporal myocyte tone, relative to detrusor myocyte tone. Certainly, the clinical success of PDEV inhibitors is consistent with this supposition (although indirect effects through bladder afferents cannot be ruled out).
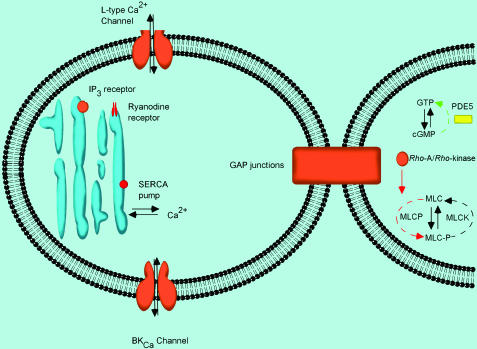
Figure 4.
Illustration of some similarities and distinctions in molecular regulation of detrusor and corporal myocyte tone. In this diagram, targets that are apparently more important to the regulation of the detrusor myocyte are shown in red (RyR receptor and SERCA), while those thought to be more important to the regulation of corporal smooth muscle are shown in yellow (PDEV/cGMP/nitric oxide pathway). If a target is important to both cell types, then it appears orange (BKCa, L-type calcium channel, gap junctions, Rho Kinase). Such observations may point the way to novel therapeutic possibilities.
In conclusion then, despite the tremendous amount of new mechanistic insight, current therapies for both bladder and erectile dysfunction both still have much room for improvement. With respect to the former, current therapies for the past two decades have focused largely on anticholinergic therapy, with only modest success. The rationale for this approach has always been that muscarinic receptors are the primary receptors mediating bladder contractions (Andersson, 2004) Unfortunately, even at high doses, these medications have never been an adequate therapy for detrusor overactivity, and cause many bothersome side effects. The recent development of transdermal methods of administration have limited the side effects of these drugs, but have not adequately improved the efficacy. Thus, the search continues for better agents for the modulation of bladder overactivity (Dmochowski, 2005). With respect to the latter, despite the success of the PDE V inhibitors for the treatment of erectile dysfunction, there are still a significant number of patients that cannot be effectively treated with these drugs (e.g. diabetic patients), and moreover, the PDE V inhibitors have contraindications (e.g. nitrates), and untoward side effects. Again, some pertinent similarities and differences in regulation of calcium mobilization and calcium sensitization in these two phenotypically distinct cell types are highlighted in Figure 4. Undoubtedly, the ultimate clinical utility of these targets will depend on the ability to leverage tissue-specific differences, while also taking into account any disease-related changes in target expression/function. In that regard, both the clinical successes and failures will provide valuable information. Hopefully, continued investigations and integration of molecular, pharmacological and physiologic/pathophysiologic data, as outlined herein, will lead to the identification and development of novel and more efficacious therapies for both of these devastating diseases.
Acknowledgments
This work was supported in part by NIH USPHS grant PO1 DK60037. We are also most grateful to Dr Karl-Erik Andersson for many inspiring discussions during the summer of 2005.
Glossary
- cAMP
cyclic adenosine monophosphate
- cGMP
cyclic guanosine monophosphate
- CGRP
calcitonin gene-related peptide
- CICR
calcium-induced calcium release
- Cx43
connexin43
- DAG
diacylglycerol
- ET
endothelin
- IP3
inositol trisphosphate
- MLCK
myosin light chain kinase
- MLCP
myosin light chain phosphatase
- NADPH
nicotinamide adenine dinucleotide phosphate
- NANC
nonadrenergic noncholinergic
- NO
nitric oxide
- PDE
phosphodiesterase
- PDE V
phosphodiesterase type V
- PGE
prostaglandins E
- PIP2
phosphotidylinositol bisphosphate
- PKA
protein kinase A
- PKC
protein kinase C
- PKG
protein kinase G
- PLC
phospholipase C
- ROS
reactive oxygen species
- SERCA
sarcoplasmic reticulum Ca2+-ATPase
- siRNA
small interfering RNA
- SR
sarcoplasmic reticulum
- RyR
ryanodine receptor
- VIP
vasoactive intestinal polypeptide
References
- ANDERSSON K.E. Neurophysiology/pharmacology of erection. Int. J. Impot. Res. 2001;13 (Suppl 3):S8–S17. doi: 10.1038/sj.ijir.3900718. [DOI] [PubMed] [Google Scholar]
- ANDERSSON K.E.2003Erectile physiological and pathophysiological pathways involved in erectile dysfunction J. Urol. 170S6–S13.discussion S13–S14. [DOI] [PubMed] [Google Scholar]
- ANDERSSON K.E. Detrusor contraction – focus on muscarinic receptors. Scand. J. Urol. Nephrol. Suppl. 2004;215:54–57. doi: 10.1080/03008880410015192. [DOI] [PubMed] [Google Scholar]
- ANDERSSON K.E., ARNER A. Urinary bladder contraction and relaxation: physiology and pathophysiology. Physiol. Rev. 2004;84:935–986. doi: 10.1152/physrev.00038.2003. [DOI] [PubMed] [Google Scholar]
- ARCHER S.L. Potassium channels and erectile dysfunction. Vasc. Pharmacol. 2002;38:61–71. doi: 10.1016/s1537-1891(02)00127-1. [DOI] [PubMed] [Google Scholar]
- ARGIOLAS A., MELIS M.R. Central control of penile erection: role of the paraventricular nucleus of the hypothalamus. Prog. Neurobiol. 2005;76:1–21. doi: 10.1016/j.pneurobio.2005.06.002. [DOI] [PubMed] [Google Scholar]
- BAYLISS M., WU C., NEWGREEN D., MUNDY A.R., FRY C.H. A quantitative study of atropine-resistant contractile responses in human detrusor smooth muscle, from stable, unstable and obstructed bladders. J. Urol. 1999;162:1833–1839. [PubMed] [Google Scholar]
- BENNETT N.E., KIM J.H., WOLFE D.P., SASAKI K., YOSHIMURA N., GOINS W.F, HUANG S., NELSON J.B., DE GROAT W.C., GLORIOSO JC., CHANCELLOR M.B. Improvement in erectile dysfunction after neurotrophic factor gene therapy in diabetic rats. J. Urol. 2005;173:1820–1824. doi: 10.1097/01.ju.0000158056.66236.1f. [DOI] [PubMed] [Google Scholar]
- BING W., CHANG S., HYPOLITE J.A., DISANTO M.E., ZDERIC S.A., ROLF L, WEIN A.J., CHACKO S. Obstruction-induced changes in urinary bladder smooth muscle contractility: a role for Rho kinase. Am. J. Physiol. Renal Physiol. 2003;285:F990–F997. doi: 10.1152/ajprenal.00378.2002. [DOI] [PubMed] [Google Scholar]
- BIVALACQUA T.J., CHAMPION H.C., ABDEL-MAGEED A.B., KADOWITZ P.J., HELLSTROM W.J. Gene transfer of prepro-calcitonin gene-related peptide restores erectile function in the aged rat. Biol. Reprod. 2001;65:1371–1377. doi: 10.1095/biolreprod65.5.1371. [DOI] [PubMed] [Google Scholar]
- BIVALACQUA T.J., CHAMPION H.C., USTA M.F., CELLEK S., CHITALEY K., WEBB R.C, LEWIS R.L., MILLS T.M., HELLSTROM W.J., KADOWITZ P.J. RhoA/Rho-kinase suppresses endothelial nitric oxide synthase in the penis: a mechanism for diabetes-associated erectile dysfunction. Proc. Natl. Acad. Sci. U.S.A. 2004;101:9121–9126. doi: 10.1073/pnas.0400520101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BONEV A.D., NELSON M.T. Muscarinic inhibition of ATP-sensitive K+ channels by protein kinase C in urinary bladder smooth muscle. Am. J. Physiol. 1993;265:C1723–C1728. doi: 10.1152/ajpcell.1993.265.6.C1723. [DOI] [PubMed] [Google Scholar]
- BOY K.M., GUERNON J.M., SIT S.Y., XIE K., HEWAWASAM P., BOISSARD C.G, DWORETZKY S.I., NATALE J., GRIBKOFF V.K., LODGE N., STARRETT J.E., JR 3-Thio-quinolinone maxi-K openers for the treatment of erectile dysfunction. Bioorg. Med. Chem. Lett. 2004;14:5089–5093. doi: 10.1016/j.bmcl.2004.07.080. [DOI] [PubMed] [Google Scholar]
- BRAMICH N.J., BRADING A.F. Electrical properties of smooth muscle in the guinea-pig urinary bladder. J. Physiol. 1996;492 (Part 1):185–198. doi: 10.1113/jphysiol.1996.sp021300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRAVERMAN A.S., KOHN I.J., LUTHIN G.R., RUGGIERI M.R. Prejunctional M1 facilitory and M2 inhibitory muscarinic receptors mediate rat bladder contractility. Am. J. Physiol. 1998a;274:R517–R523. doi: 10.1152/ajpregu.1998.274.2.r517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRAVERMAN A.S., LUTHIN G.R., RUGGIERI M.R. M2 muscarinic receptor contributes to contraction of the denervated rat urinary bladder. Am. J. Physiol. 1998b;275:R1654–R1660. doi: 10.1152/ajpregu.1998.275.5.R1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRINK P.R.V.V., WANG HZ., ZHAO W., DAVIES K., CHRIST GJ.2006Experimental diabetes alters connexin43-derived gap junction permeability in short term cultures of rat corporal vascular smooth muscle cells J. Urol.(in press). [DOI] [PubMed]
- BUCKNER S.A., MILICIC I., DAZA A., DAVIS-TABER R., SCOTT V.E., SULLIVAN J.P., BRIONI J.D. Pharmacological and molecular analysis of ATP-sensitive K(+) channels in the pig and human detrusor. Eur. J. Pharmacol. 2000;400:287–295. doi: 10.1016/s0014-2999(00)00388-5. [DOI] [PubMed] [Google Scholar]
- BURCHARDT M., BURCHARDT T., ANASTASIADIS A.G., BUTTYAN R., DE LA TAILLE A., SHABSIGH A., FRANK J., SHABSIGH R. Application of angiogenic factors for therapy of erectile dysfunction: protein and DNA transfer of VEGF 165 into the rat penis. Urology. 2005;66:665–670. doi: 10.1016/j.urology.2005.03.058. [DOI] [PubMed] [Google Scholar]
- BURNSTOCK G. The purinergic nerve hypothesis. Ciba Found. Symp. 1977;48:295–314. doi: 10.1002/9780470720301.ch17. [DOI] [PubMed] [Google Scholar]
- CARSON C.C., LUE T.F. Phosphodiesterase type 5 inhibitors for erectile dysfunction. BJU Int. 2005;96:257–280. doi: 10.1111/j.1464-410X.2005.05614.x. [DOI] [PubMed] [Google Scholar]
- CHACKO S., CHANG S., HYPOLITE J., DISANTO M., WEIN A. Alteration of contractile and regulatory proteins following partial bladder outlet obstruction. Scand. J. Urol. Nephrol. Suppl. 2004;215:26–36. doi: 10.1080/03008880410015147. [DOI] [PubMed] [Google Scholar]
- CHANG S., HYPOLITE J.A., DISANTO M.E., CHANGOLKAR A., WEIN A.J., CHACKO S.2005Increased basal phosphorylation of detrusor smooth muscle myosin in Alloxan-induced diabetic rabbit is mediated by up-regulation of Rho-kinase {beta} and CPI-17 Am. J. Physiol. Renal Physiol[E-pub ahead of print]. [DOI] [PubMed]
- CHANG S., HYPOLITE J.A., VELEZ M., CHANGOLKAR A., WEIN A.J., CHACKO S., DISANTO M.E. Downregulation of cGMP-dependent protein kinase-1 activity in the corpus cavernosum smooth muscle of diabetic rabbits. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004;287:R950–R960. doi: 10.1152/ajpregu.00639.2003. [DOI] [PubMed] [Google Scholar]
- CHOW K.Y., WU C., SUI G.P., FRY C.H. Role of the T-type Ca2+ current on the contractile performance of guinea pig detrusor smooth muscle. Neurourol. Urodyn. 2003;22:77–82. doi: 10.1002/nau.10081. [DOI] [PubMed] [Google Scholar]
- CHRIST G.J. The ‘syncytial tissue triad': a model for understanding how gap junctions participate in the local control of penile erection. World J. Urol. 1997;15:36–44. doi: 10.1007/BF01275155. [DOI] [PubMed] [Google Scholar]
- CHRIST G.J. Gap junctions and ion channels: relevance to erectile dysfunction. Int. J. Impot. Res. 2000a;12 (Suppl 4):S15–S25. doi: 10.1038/sj.ijir.3900573. [DOI] [PubMed] [Google Scholar]
- CHRIST G.J. K+ channels and gap junctions in the modulation of corporal smooth muscle tone. Drug News Perspect. 2000b;13:28–36. [PubMed] [Google Scholar]
- CHRIST G.J. K channels as molecular targets for the treatment of erectile dysfunction. J. Androl. 2002;23:S10–S19. [PubMed] [Google Scholar]
- CHRIST G.J. Gene therapy treatments for erectile and bladder dysfunction. Curr. Urol. Rep. 2004;5:52–60. doi: 10.1007/s11934-004-0012-z. [DOI] [PubMed] [Google Scholar]
- CHRIST G.J. siRNA for erectile dysfunction. J. Urol. 2005;174:819. doi: 10.1097/01.ju.0000174934.17803.5b. [DOI] [PubMed] [Google Scholar]
- CHRIST G.J., BRINK P.R., RAMANAN S.V. Dynamic gap junctional communication: a delimiting model for tissue responses. Biophys. J. 1994;67:1335–1344. doi: 10.1016/S0006-3495(94)80605-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHRIST G.J., DAY N.S., DAY M., ZHAO W., PERSSON K., PANDITA R.K., ANDERSSON K.E. Increased connexin43-mediated intercellular communication in a rat model of bladder overactivity in vivo. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003;284:R1241–R1248. doi: 10.1152/ajpregu.00030.2002. [DOI] [PubMed] [Google Scholar]
- CHRIST G.J., DAY N., SANTIZO C., SATO Y., ZHAO W., SCLAFANI T., BAKAL R., SALMAN M., DAVIES K., MELMAN A. Intracorporal injection of hSlo cDNA restores erectile capacity in STZ-diabetic F-344 rats in vivo. Am. J. Physiol. Heart. Circ. Physiol. 2004a;287:H1544–H1553. doi: 10.1152/ajpheart.00792.2003. [DOI] [PubMed] [Google Scholar]
- CHRIST G.J., DAY N.S., SANTIZO C., ZHAO W., SCLAFANI T., KARICHETI V., VALCIC M., MELMAN A. Bladder instillation of ‘naked' hSlo/pcDNA3 ameliorates detrusor hyperactivity in obstructed rats in vivo. Urology. 2001;57:111. doi: 10.1016/s0090-4295(01)01041-x. [DOI] [PubMed] [Google Scholar]
- CHRIST G.J., LUE T. Physiology and biochemistry of erections. Endocrine. 2004b;23:93–100. doi: 10.1385/ENDO:23:2-3:093. [DOI] [PubMed] [Google Scholar]
- CHRIST G.J., REHMAN J., DAY N., SALKOFF L., VALCIC M., MELMAN A., GELIEBTER J. Intracorporal injection of hSlo cDNA in rats produces physiologically relevant alterations in penile function. Am. J. Physiol. 1998;275:H600–H608. doi: 10.1152/ajpheart.1998.275.2.H600. [DOI] [PubMed] [Google Scholar]
- CORBIN J.D. Mechanisms of action of PDE5 inhibition in erectile dysfunction. Int. J. Impot. Res. 2004;1 (Suppl 1):S4–S7. doi: 10.1038/sj.ijir.3901205. [DOI] [PubMed] [Google Scholar]
- DANIEL E.E., COWAN W., DANIEL V.P. Structural bases for neural and myogenic control of human detrusor muscle. Can. J. Physiol. Pharmacol. 1983;61:1247–1273. doi: 10.1139/y83-183. [DOI] [PubMed] [Google Scholar]
- DE GROAT W.C. Anatomy of the central neural pathways controlling the lower urinary tract. Eur. Urol. 1998;1 (Suppl 1):2–5. doi: 10.1159/000052265. [DOI] [PubMed] [Google Scholar]
- DEKA D.K., BRADING A.F. Nitric oxide activates glibenclamide-sensitive K+ channels in urinary bladder myocytes through a c-GMP-dependent mechanism. Eur. J. Pharmacol. 2004;492:13–19. doi: 10.1016/j.ejphar.2004.03.057. [DOI] [PubMed] [Google Scholar]
- DIXON J.S., GOSLING J.A. Histology and fine structure of the muscularis mucosae of the human urinary bladder. J. Anat. 1983;136:265–271. [PMC free article] [PubMed] [Google Scholar]
- DMOCHOWSKI R. Improving the tolerability of anticholinergic agents in the treatment of overactive bladder. Drug Saf. 2005;28:583–600. doi: 10.2165/00002018-200528070-00003. [DOI] [PubMed] [Google Scholar]
- EGLEN R.M. Muscarinic M2 and M3 receptor function in smooth muscle. Proc. West. Pharmacol. Soc. 1996;39:57–60. [PubMed] [Google Scholar]
- ELBADAWI A., YALLA S.V., RESNICK N.M. Structural basis of geriatric voiding dysfunction. I. Methods of a prospective ultrastructural/urodynamic study and an overview of the findings. J. Urol. 1993a;150:1650–1656. doi: 10.1016/s0022-5347(17)35866-4. [DOI] [PubMed] [Google Scholar]
- ELBADAWI A., YALLA S.V., RESNICK N.M. Structural basis of geriatric voiding dysfunction. II. Aging detrusor: normal versus impaired contractility. J. Urol. 1993b;150:1657–1667. doi: 10.1016/s0022-5347(17)35867-6. [DOI] [PubMed] [Google Scholar]
- ELBADAWI A., YALLA S.V., RESNICK N.M. Structural basis of geriatric voiding dysfunction. III. Detrusor overactivity. J. Urol. 1993c;150:1668–1680. doi: 10.1016/s0022-5347(17)35868-8. [DOI] [PubMed] [Google Scholar]
- ELBADAWI A., YALLA S.V., RESNICK N.M. Structural basis of geriatric voiding dysfunction. IV. Bladder outlet obstruction. J. Urol. 1993d;150:1681–1695. doi: 10.1016/s0022-5347(17)35869-x. [DOI] [PubMed] [Google Scholar]
- FAN S.F., BRINK P.R., MELMAN A., CHRIST G.J. An analysis of the Maxi-K+ (KCa) channel in cultured human corporal smooth muscle cells. J. Urol. 1995;153:818–825. [PubMed] [Google Scholar]
- FRY C.H., COOKLIN M., BIRNS J., MUNDY A.R. Measurement of intercellular electrical coupling in guinea-pig detrusor smooth muscle. J. Urol. 1999;161:660–664. [PubMed] [Google Scholar]
- FRY C.H., SKENNERTON D., WOOD D., WU C. The cellular basis of contraction in human detrusor smooth muscle from patients with stable and unstable bladders. Urology. 2002;59:3–12. doi: 10.1016/s0090-4295(01)01632-6. [DOI] [PubMed] [Google Scholar]
- GANITKEVICH V.Y., ISENBERG G. Contribution of Ca(2+)-induced Ca2+ release to the [Ca2+]i transients in myocytes from guinea-pig urinary bladder. J. Physiol. 1992;458:119–137. doi: 10.1113/jphysiol.1992.sp019409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GONZALEZ-CADAVID N.F., RAJFER J. Molecular pathophysiology and gene therapy of aging-related erectile dysfunction. Exp. Gerontol. 2004;39:1705–1712. doi: 10.1016/j.exger.2004.06.022. [DOI] [PubMed] [Google Scholar]
- HAEFLIGER J.A., TISSIERES P., TAWADROS T., FORMENTON A., BENY J.L., NICOD P., FREY P., MEDA P. Connexins 43 and 26 are differentially increased after rat bladder outlet obstruction. Exp. Cell Res. 2002;274:216–225. doi: 10.1006/excr.2001.5465. [DOI] [PubMed] [Google Scholar]
- HAFERKAMP A., ELBADAWI A. [Ultrastructural changes in the aging bladder] Urologe A. 2004;43:527–534. doi: 10.1007/s00120-004-0566-x. [DOI] [PubMed] [Google Scholar]
- HAFRON J.C.G. Novel therapeutic strategies for the treatment of erectile dysfunction. Drug Discov Today: Therap Strategies. 2004;I:249–257. [Google Scholar]
- HASHITANI H., BRADING A.F. Ionic basis for the regulation of spontaneous excitation in detrusor smooth muscle cells of the guinea-pig urinary bladder. Br. J. Pharmacol. 2003;140:159–169. doi: 10.1038/sj.bjp.0705320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HASHITANI H., BRADING A.F., SUZUKI H. Correlation between spontaneous electrical, calcium and mechanical activity in detrusor smooth muscle of the guinea-pig bladder. Br. J. Pharmacol. 2004;141:183–193. doi: 10.1038/sj.bjp.0705602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEDLUND P., ASZODI A., PFEIFER A., ALM P., HOFMANN F., AHMAD M., FASSLER R., ANDERSSON K.E. Erectile dysfunction in cyclic GMP-dependent kinase I-deficient mice. Proc. Natl. Acad. Sci. U.S.A. 2000;97:2349–2354. doi: 10.1073/pnas.030419997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEGDE S.S., CHOPPIN A., BONHAUS D., BRIAUD S., LOEB M., MOY T.M., LOURY D., EGLEN R.M. Functional role of M2 and M3 muscarinic receptors in the urinary bladder of rats in vitro and in vivo. Br. J. Pharmacol. 1997;120:1409–1418. doi: 10.1038/sj.bjp.0701048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEGDE S.S., EGLEN R.M. Muscarinic receptor subtypes modulating smooth muscle contractility in the urinary bladder. Life Sci. 1999;64:419–428. doi: 10.1016/s0024-3205(98)00581-5. [DOI] [PubMed] [Google Scholar]
- HEPPNER T.J., BONEV A.D., NELSON M.T. Ca(2+)-activated K+ channels regulate action potential repolarization in urinary bladder smooth muscle. Am. J. Physiol. 1997;273:C110–C117. doi: 10.1152/ajpcell.1997.273.1.C110. [DOI] [PubMed] [Google Scholar]
- HEPPNER T.J., BONEV A.D., NELSON M.T. Elementary purinergic Ca2+ transients evoked by nerve stimulation in rat urinary bladder smooth muscle. J. Physiol. 2005;564:201–212. doi: 10.1113/jphysiol.2004.077826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEPPNER T.J., HERRERA G.M., BONEV A.D., HILL-EUBANKS D., NELSON M.T. Ca2+ sparks and K(Ca) channels: novel mechanisms to relax urinary bladder smooth muscle. Adv. Exp. Med. Biol. 2003;539:347–357. doi: 10.1007/978-1-4419-8889-8_26. [DOI] [PubMed] [Google Scholar]
- HERRERA G.M., ETHERTON B., NAUSCH B., NELSON M.T. Negative feedback regulation of nerve-mediated contractions by KCa channels in mouse urinary bladder smooth muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005;289:R402–R409. doi: 10.1152/ajpregu.00488.2004. [DOI] [PubMed] [Google Scholar]
- HERRERA G.M., HEPPNER T.J., NELSON M.T. Regulation of urinary bladder smooth muscle contractions by ryanodine receptors and BK and SK channels. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000;279:R60–R68. doi: 10.1152/ajpregu.2000.279.1.R60. [DOI] [PubMed] [Google Scholar]
- HERRERA G.M., HEPPNER T.J., NELSON M.T. Voltage dependence of the coupling of Ca(2+) sparks to BK(Ca) channels in urinary bladder smooth muscle. Am. J. Physiol. Cell. Physiol. 2001;280:C481–C490. doi: 10.1152/ajpcell.2001.280.3.C481. [DOI] [PubMed] [Google Scholar]
- HERRERA G.M., NELSON M.T. Differential regulation of SK and BK channels by Ca(2+) signals from Ca(2+) channels and ryanodine receptors in guinea-pig urinary bladder myocytes. J. Physiol. 2002;541:483–492. doi: 10.1113/jphysiol.2002.017707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERRERA G.M., POZO M.J., ZVARA P., PETKOV G.V., BOND C.T., ADELMAN J.P., NELSON M.T. Urinary bladder instability induced by selective suppression of the murine small conductance calcium-activated potassium (SK3) channel. J. Physiol. 2003;551:893–903. doi: 10.1113/jphysiol.2003.045914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEWAWASAM P., FAN W., DING M., FLINT K., COOK D., GOGGINS G.D., MYERS R.A., GRIBKOFF V.K., BOISSARD C.G., DWORETZKY S.I., STARRETT J.E., JR, LODGE N.J. 4-Aryl-3-(hydroxyalkyl)quinolin-2-ones: novel maxi-K channel opening relaxants of corporal smooth muscle targeted for erectile dysfunction. J. Med. Chem. 2003;46:2819–2822. doi: 10.1021/jm030005h. [DOI] [PubMed] [Google Scholar]
- HU S., KIM H.S. Modulation of ATP-sensitive and large-conductance Ca++-activated K+ channels by Zeneca ZD6169 in guinea pig bladder smooth muscle cells. J. Pharmacol. Exp. Ther. 1997;280:38–45. [PubMed] [Google Scholar]
- JIANG H.H., SONG B., LU G.S., WEN Q.J., JIN X.Y. Loss of ryanodine receptor calcium-release channel expression associated with overactive urinary bladder smooth muscle contractions in a detrusor instability model. BJU Int. 2005;96:428–433. doi: 10.1111/j.1464-410X.2005.05644.x. [DOI] [PubMed] [Google Scholar]
- JOHN H., WANG X., WEHRLI E., HAURI D., MAAKE C. Evidence of gap junctions in the stable nonobstructed human bladder. J. Urol. 2003;169:745–749. doi: 10.1097/01.ju.0000045140.86986.a7. [DOI] [PubMed] [Google Scholar]
- KARICHETI V., CHRIST G.J. Physiological roles for K+ channels and gap junctions in urogenital smooth muscle: implications for improved understanding of urogenital function, disease and therapy. Curr. Drug Targets. 2001;2:1–20. doi: 10.2174/1389450013348894. [DOI] [PubMed] [Google Scholar]
- KAVIA R.B.C., DASGUPTA R., FOWLER C.J. Functional imaging and the central control of the bladder. J. Comp. Neurol. 2005;493:27–32. doi: 10.1002/cne.20753. [DOI] [PubMed] [Google Scholar]
- KENDIRCI M., GUR S., SIKKA S.C. Gene therapy for erectile dysfunction. Front. Biosci. 2005;10:2758–2769. doi: 10.2741/1733. [DOI] [PubMed] [Google Scholar]
- KUME H., MIKAWA K., TAKAGI K., KOTLIKOFF M.I. Role of G proteins and KCa channels in the muscarinic and beta-adrenergic regulation of airway smooth muscle. Am. J. Physiol. 1995a;268:L221–L229. doi: 10.1152/ajplung.1995.268.2.L221. [DOI] [PubMed] [Google Scholar]
- KUME H., TAKAGI K. Involvement of G proteins between receptors and KCa channels in the regulation of airway tone by the autonomic nervous system] Nihon. Kyobu. Shikkan. Gakkai. Zasshi. 1995b;33 (Suppl 33):116–124. [PubMed] [Google Scholar]
- LEE S.W. Physiological roles and properties of potassium channels in corporal smooth muscle. Drugs Today (Barc.) 2000;36:147–154. doi: 10.1358/dot.2000.36.2-3.568788. [DOI] [PubMed] [Google Scholar]
- LIN G., HAYASHI N., CARRION R., CHANG L.J., LUE T.F., LIN C.S. Improving erectile function by silencing phosphodiesterase-5. J. Urol. 2005;174:1142–1148. doi: 10.1097/01.ju.0000168615.37949.45. [DOI] [PubMed] [Google Scholar]
- LUE TF B.R., ROSEN R, GIULIANO F, KHOURY S, MONTORSI F.2004Sexual medicine: sexual dysfunctions in men and women Second International Consultation on Sexual DysfunctionParis.
- MCCONNELL J., BENSON G.S., WOOD J.G. Autonomic innervation of the urogenital system: adrenergic and cholinergic elements. Brain. Res. Bull. 1982;9:679–694. doi: 10.1016/0361-9230(82)90173-3. [DOI] [PubMed] [Google Scholar]
- MELMAN A., BAR-CHAMA N., MCCULLOUGH A., DAVIES K., CHRIST G. The first human trial for gene transfer therapy for the treatment of erectile dysfunction: preliminary results. Eur. Urol. 2005;48:314–318. doi: 10.1016/j.eururo.2005.05.005. [DOI] [PubMed] [Google Scholar]
- MELMAN A., ZHAO W., DAVIES K.P., BAKAL R., CHRIST G.J. The successful long-term treatment of age related erectile dysfunction with hSlo cDNA in rats in vivo. J. Urol. 2003;170:285–290. doi: 10.1097/01.ju.0000063375.12512.6e. [DOI] [PubMed] [Google Scholar]
- MEREDITH A.L., THORNELOE K.S., WERNER M.E., NELSON M.T., ALDRICH R.W. Overactive bladder and incontinence in the absence of the BK large conductance Ca2+-activated K+ channel. J. Biol. Chem. 2004;279:36746–36752. doi: 10.1074/jbc.M405621200. [DOI] [PubMed] [Google Scholar]
- MILLS T.M., CHITALEY K., WINGARD C.J., LEWIS R.W., WEBB R.C. Effect of Rho-kinase inhibition on vasoconstriction in the penile circulation. J. Appl. Physiol. 2001;91:1269–1273. doi: 10.1152/jappl.2001.91.3.1269. [DOI] [PubMed] [Google Scholar]
- MILLS T.M., LEWIS R.W., WINGARD C.J., CHITALEY K., WEBB R.C. Inhibition of tonic contraction – a novel way to approach erectile dysfunction. J. Androl. 2002;23:S5–S9. [PubMed] [Google Scholar]
- MILLS T.M., LEWIS R.W., WINGARD C.J., LINDER A.E., JIN L., WEBB R.C. Vasoconstriction, RhoA/Rho-kinase and the erectile response. Int. J. Impot. Res. 2003;5 (Suppl 5):S20–S24. doi: 10.1038/sj.ijir.3901068. [DOI] [PubMed] [Google Scholar]
- NEUHAUS J., PFEIFFER F., WOLBURG H., HORN L.C., DORSCHNER W. Alterations in connexin expression in the bladder of patients with urge symptoms. BJU. Int. 2005;96:670–676. doi: 10.1111/j.1464-410X.2005.05703.x. [DOI] [PubMed] [Google Scholar]
- NEUHAUS J., WEIMANN A., STOLZENBURG J.U., WOLBURG H., HORN L.C., DORSCHNER W. Smooth muscle cells from human urinary bladder express connexin 43 in vivo and in vitro. World J. Urol. 2002;20:250–254. doi: 10.1007/s00345-002-0289-9. [DOI] [PubMed] [Google Scholar]
- NOBE K., SUTLIFF R.L., KRANIAS E.G., PAUL R.J. Phospholamban regulation of bladder contractility: evidence from gene-altered mouse models. J. Physiol. 2001;535:867–878. doi: 10.1111/j.1469-7793.2001.00867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'REILLY B.A., KOSAKA A.H., KNIGHT G.F., CHANG T.K., FORD A.P., RYMER J.M., POPERT R., BURNSTOCK G., MCMAHON S.B. P2X receptors and their role in female idiopathic detrusor instability. J. Urol. 2002;167:157–164. [PubMed] [Google Scholar]
- OHYA Y., FUJII K., ETO K., ABE I., FUJISHIMA M. Voltage-dependent Ca2+ channels in resistance arteries from Dahl salt-sensitive rats. Hypertens. Res. 2000b;23:701–707. doi: 10.1291/hypres.23.701. [DOI] [PubMed] [Google Scholar]
- OHYA S., KIMURA S., KITSUKAWA M., MURAKI K., WATANABE M., IMAIZUMI Y. SK4 encodes intermediate conductance Ca2+-activated K+ channels in mouse urinary bladder smooth muscle cells. Jpn. J. Pharmacol. 2000a;84:97–100. doi: 10.1254/jjp.84.97. [DOI] [PubMed] [Google Scholar]
- PETKOV G.V., BONEV A.D., HEPPNER T.J., BRENNER R., ALDRICH R.W., NELSON M.T. Beta1-subunit of the Ca2+-activated K+ channel regulates contractile activity of mouse urinary bladder smooth muscle. J. Physiol. 2001;537:443–452. doi: 10.1111/j.1469-7793.2001.00443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETKOV G.V., NELSON M.T. Differential regulation of Ca2+-activated K+ channels by beta-adrenoceptors in guinea pig urinary bladder smooth muscle. Am. J. Physiol. Cell Physiol. 2005;288:C1255–C1263. doi: 10.1152/ajpcell.00381.2004. [DOI] [PubMed] [Google Scholar]
- RAJASEKARAN M., WHITE S., BAQUIR A., WILKES N. Rho-kinase inhibition improves erectile function in aging male Brown-Norway rats. J. Androl. 2005;26:182–188. doi: 10.1002/j.1939-4640.2005.tb01084.x. [DOI] [PubMed] [Google Scholar]
- RAMANAN S.V., BRINK P.R., CHRIST G.J. Neuronal innervation, intercellular signal transduction and intercellular coupling: a model for syntitial tissue responses in the steady state. J. Theoret. Biol. 1998;193:69–84. doi: 10.1006/jtbi.1998.0687. [DOI] [PubMed] [Google Scholar]
- REHMAN J., CHENVEN E., BRINK P., PETERSON B., WALCOTT B., WEN Y.P., MELMAN A., CHRIST G. Diminished neurogenic but not pharmacological erections in the 2- to 3-month experimentally diabetic F-344 rat. Am. J. Physiol. 1997;272:H1960–H1971. doi: 10.1152/ajpheart.1997.272.4.H1960. [DOI] [PubMed] [Google Scholar]
- ROGERS R.S., GRAZIOTTIN T.M., LIN C.S., KAN Y.W., LUE T.F. Intracavernosal vascular endothelial growth factor (VEGF) injection and adeno-associated virus-mediated VEGF gene therapy prevent and reverse venogenic erectile dysfunction in rats. Int. J. Impot. Res. 2003;15:26–37. doi: 10.1038/sj.ijir.3900943. [DOI] [PubMed] [Google Scholar]
- RUGGIERI M.R., WHITMORE K.E., LEVIN R.M. Bladder purinergic receptors. J. Urol. 1990;144:176–181. doi: 10.1016/s0022-5347(17)39405-3. [DOI] [PubMed] [Google Scholar]
- SAENZ DE TEJADA I. Commentary on mechanisms for the regulation of penile smooth muscle contractility. J. Urol. 1995;153:1762. doi: 10.1097/00005392-199506000-00002. [DOI] [PubMed] [Google Scholar]
- SAENZ DE TEJADA I., CELLEK S., GONZALEZ-CAVADID N.F., HEATON J.P., PICKARD R.S., SIMONSEN V. Physiology of erectile function and pathophysiology of erectile dysfunction. Sex. Med. Sex. Dysfunction Men Women. 2004. pp. 287–344.
- SCHNEIDER T., FETSCHER C., KREGE S., MICHEL M.C. Signal transduction underlying carbachol-induced contraction of human urinary bladder. J. Pharmacol. Exp. Ther. 2004;309:1148–1153. doi: 10.1124/jpet.103.063735. [DOI] [PubMed] [Google Scholar]
- SEKI N., KARIM O.M., MOSTWIN J.L. Changes in electrical properties of guinea pig smooth muscle membrane by experimental bladder outflow obstruction. Am. J. Physiol. 1992;262:F885–F891. doi: 10.1152/ajprenal.1992.262.5.F885. [DOI] [PubMed] [Google Scholar]
- SHELDON J.H., NORTON N.W., ARGENTIERI T.M. Inhibition of guinea pig detrusor contraction by NS-1619 is associated with activation of BKCa and inhibition of calcium currents. J. Pharmacol. Exp. Ther. 1997;283:1193–1200. [PubMed] [Google Scholar]
- SHEN Z.J., WANG H., LU Y.L., ZHOU X.L., CHEN S.W., CHEN Z.D. Gene transfer of vasoactive intestinal polypeptide into the penis improves erectile response in the diabetic rat. BJU Int. 2005;95:890–894. doi: 10.1111/j.1464-410X.2005.05422.x. [DOI] [PubMed] [Google Scholar]
- SHIEH C.C., COGHLAN M., SULLIVAN J.P., GOPALAKRISHNAN M. Potassium channels: molecular defects, diseases, and therapeutic opportunities. Pharmacol. Rev. 2000;52:557–594. [PubMed] [Google Scholar]
- SHUKLA N., JONES R., PERSAD R., ANGELINI G.D., JEREMY J.Y. Effect of sildenafil citrate and a nitric oxide donating sildenafil derivative, NCX 911, on cavernosal relaxation and superoxide formation in hypercholesterolaemic rabbits. Eur. J. Pharmacol. 2005;517:224–231. doi: 10.1016/j.ejphar.2005.05.012. [DOI] [PubMed] [Google Scholar]
- SIEMER C., BUSHFIELD M., NEWGREEN D., GRISSMER S. Effects of NS1608 on MaxiK channels in smooth muscle cells from urinary bladder. J. Membr. Biol. 2000;173:57–66. doi: 10.1007/s002320001007. [DOI] [PubMed] [Google Scholar]
- SILVA W.A., KARRAM M.M. Anatomy and physiology of the pelvic floor. Minerva Ginecol. 2004;56:283–302. [PubMed] [Google Scholar]
- SIMONSEN U., GARCIA-SACRISTAN A., PRIETO D. Penile arteries and erection. J. Vasc. Res. 2002;39:283–303. doi: 10.1159/000065541. [DOI] [PubMed] [Google Scholar]
- SPEKTOR M., RODRIGUEZ R., ROSENBAUM R.S., WANG H.Z., MELMAN A., CHRIST G.J. Potassium channels and human corporeal smooth muscle cell tone: further evidence of the physiological relevance of the Maxi-K channel subtype to the regulation of human corporeal smooth muscle tone in vitro. J. Urol. 2002;167:2628–2635. [PubMed] [Google Scholar]
- SUI G.P., COPPEN S.R., DUPONT E., ROTHERY S., GILLESPIE J., NEWGREEN D., SEVERS N.J., FRY C.H. Impedance measurements and connexin expression in human detrusor muscle from stable and unstable bladders. BJU Int. 2003;92:297–305. doi: 10.1046/j.1464-410x.2003.04342.x. [DOI] [PubMed] [Google Scholar]
- TAKAHASHI R., NISHIMURA J., HIRANO K., SEKI N., NAITO S., KANAIDE H. Ca2+ sensitization in contraction of human bladder smooth muscle. J. Urol. 2004;172:748–752. doi: 10.1097/01.ju.0000130419.32165.6b. [DOI] [PubMed] [Google Scholar]
- THORNELOE K.S., MEREDITH A.L., KNORN A.M., ALDRICH R.W., NELSON M.T. Urodynamic properties and neurotransmitter dependence of urinary bladder contractility in the BK channel deletion model of overactive bladder. Am. J. Physiol. Renal Physiol. 2005;289:F604–F610. doi: 10.1152/ajprenal.00060.2005. [DOI] [PubMed] [Google Scholar]
- THORNELOE K.S., NELSON M.T. Properties and molecular basis of the mouse urinary bladder voltage-gated K+ current. J. Physiol. 2003;549:65–74. doi: 10.1113/jphysiol.2003.039859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TODA N., AYAJIKI K., OKAMURA T. Nitric oxide and penile erectile function. Pharmacol. Ther. 2005;106:233–266. doi: 10.1016/j.pharmthera.2004.11.011. [DOI] [PubMed] [Google Scholar]
- TURNER S.C., CARROLL W.A., WHITE T.K., BRUNE M.E., BUCKNER S.A., GOPALAKRISHNAN M., FABIYI A., COGHLAN M.J., SCOTT V.E., CASTLE N.A., DAZA A.V., MILICIC I., SULLIVAN J.P. Structure-activity relationship of a novel class of naphthyl amide KATP channel openers. Bioorg. Med. Chem. Lett. 2003;13:1741–1744. doi: 10.1016/s0960-894x(03)00205-1. [DOI] [PubMed] [Google Scholar]
- UCHIYAMA T., CHESS-WILLIAMS R. Muscarinic receptor subtypes of the bladder and gastrointestinal tract. J. Smooth Muscle Res. 2004;40:237–247. doi: 10.1540/jsmr.40.237. [DOI] [PubMed] [Google Scholar]
- VALIQUETTE L., YOUNG J.M., MONCADA I., PORST H., VEZINA J.G., STANCIL B.N., EDMUNDS K., MONTORSI F. Sustained efficacy and safety of vardenafil for treatment of erectile dysfunction: a randomized, double-blind, placebo-controlled study. Mayo Clin. Proc. 2005;80:1291–1297. doi: 10.4065/80.10.1291. [DOI] [PubMed] [Google Scholar]
- VENKATESWARLU K., GIRALDI A., ZHAO W., WANG H.Z., MELMAN A., SPEKTOR M., CHRIST G.J. Potassium channels and human corporeal smooth muscle cell tone: diabetes and relaxation of human corpus cavernosum smooth muscle by adenosine triphosphate sensitive potassium channel openers. J. Urol. 2002;168:355–361. [PubMed] [Google Scholar]
- WANG H.Z., LEE S.W., DAY N.S., CHRIST G.J. Gap junction channel activity in cultured human bladder smooth muscle cell pairs: gating and unitary conductances. Urology. 2001;57:111. doi: 10.1016/s0090-4295(01)01042-1. [DOI] [PubMed] [Google Scholar]
- WANG P., LUTHIN G.R., RUGGIERI M.R. Muscarinic acetylcholine receptor subtypes mediating urinary bladder contractility and coupling to GTP binding proteins. J. Pharmacol. Exp. Ther. 1995;273:959–966. [PMC free article] [PubMed] [Google Scholar]
- WEGENER J.W., SCHULLA V., LEE T.S., KOLLER A., FEIL S., FEIL R., KLEPPISCH T., KLUGBAUER N., MOOSMANG S., WELLING A., HOFMANN F. An essential role of Cav1.2 L-type calcium channel for urinary bladder function. FASEB J. 2004;18:1159–1161. doi: 10.1096/fj.04-1516fje. [DOI] [PubMed] [Google Scholar]
- WERNER M.E., ZVARA P., MEREDITH A.L., ALDRICH R.W., NELSON M.T. Erectile dysfunction in mice lacking the large-conductance calcium-activated potassium (BK) channel. J. Physiol. 2005;567:545–556. doi: 10.1113/jphysiol.2005.093823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILKES N., WHITE S., STEIN P., BERNIE J., RAJASEKARAN M. Phosphodiesterase-5 inhibition synergizes rho-kinase antagonism and enhances erectile response in male hypertensive rats. Int. J. Impot. Res. 2004;16:187–194. doi: 10.1038/sj.ijir.3901149. [DOI] [PubMed] [Google Scholar]
- WINGARD C.J., HUSAIN S., WILLIAMS J., JAMES S. RhoA-Rho kinase mediates synergistic ET-1 and phenylephrine contraction of rat corpus cavernosum. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003;285:R1145–R1152. doi: 10.1152/ajpregu.00329.2003. [DOI] [PubMed] [Google Scholar]
- WOJDAN A., FREEDEN C., WOODS M., OSHIRO G., SPINELLI W., COLATSKY T.J., SHELDON J.H., NORTON N.W., WARGA D., ANTANE M.M., ANTANE S.A., BUTERA J.A., ARGENTIERI T.M. Comparison of the potassium channel openers, WAY-133537, ZD6169, and celikalim on isolated bladder tissue and in vivo bladder instability in rat. J. Pharmacol. Exp. Ther. 1999;289:1410–1418. [PubMed] [Google Scholar]
- WU C., SUI G., FRY C.H. The role of the L-type Ca(2+) channel in refilling functional intracellular Ca(2+) stores in guinea-pig detrusor smooth muscle. J. Physiol. 2002;538:357–369. doi: 10.1113/jphysiol.2001.013191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOUNG J.M., FELDMAN R.A., AUERBACH S.M., KAUFMAN J.M., GARCIA C.S., SHEN W., MURPHY A.M., BEASLEY M., JR, HAGUE J.A., AHUJA S. Tadalafil improved erectile function at twenty-four and thirty-six hours after dosing in men with erectile dysfunction: US trial. J. Androl. 2005;26:310–318. doi: 10.2164/jandrol.04126. [DOI] [PubMed] [Google Scholar]
- ZYGMUNT P.M., ZYGMUNT P.K., HOGESTATT E.D., ANDERSSON K.E. Effects of omega-conotoxin on adrenergic, cholinergic and NANC neurotransmission in the rabbit urethra and detrusor. Br. J. Pharmacol. 1993;110:1285–1290. doi: 10.1111/j.1476-5381.1993.tb13957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]