Abstract
The roles of voltage-gated (KV) and large conductance Ca2+-activated K+ (BKCa) channels in regulating basal contractility in myometrial smooth muscle are unresolved. The aim of this study was to determine the effects of inhibition of these channels on spontaneous rhythmic contraction in myometrial strips from four groups of rats: nonpregnant and during early (day 7), mid- (day 14), and late (day 21) pregnancy.
BKCa channels were inhibited using iberiotoxin (1–100 nM), paxilline (1–10 μM) or penitrem A (1–500, or 3000 nM); KV channels were inhibited using tetraethylammonium (TEA; 1–10 mM) and/or 4-aminopyridine (4-AP; 1–5 mM). Contractility was assessed as mean integral tension (MIT). Time/vehicle controls were also performed.
None of the selective BKCa channel inhibitors significantly affected contractility in myometrial strips from either nonpregnant or pregnant animals.
4-AP caused concentration-dependent increases in MIT in myometrium in all four groups. TEA (5 and 10 mM) significantly increased MIT in myometrium from nonpregnant, and mid- and late pregnant rats, but not in myometrium from early pregnant rats. TEA and 4-AP still caused an increase in MIT following treatment with 3000 nM penitrem A or a combination of propranolol, phentolamine, atropine (all 1 μM) and capsaicin (10 μM) in myometrial strips from nonpregnant rats.
These results indicate that whereas BKCa channels play little or no part in controlling basal rhythmicity in rat myometrium, KV channels appear to play a crucial role in this regard, especially during mid- and late pregnancy.
Keywords: Potassium channels, BKCa channels, KV channels, myometrium, rat, pregnancy, smooth muscle
Introduction
The contribution of different types of K+ channels to the regulation of myometrial contractility is incompletely understood. Electrophysiological studies at the whole-cell or single channel level have identified at least four types of K+ currents or channels in human myometrium. These include large conductance Ca2+-activated K+ channels (BKCa) (Anwer et al., 1993) and three types of voltage-gated K+ (KV) currents (Knock et al., 1999). In addition, pharmacological and biochemical evidence for myometrial ATP-activated K+ (KATP) channels has also been presented (Morrison et al., 1993; Curley et al., 2002). Almost nothing is known regarding the contribution of the KV and KATP channels in regulating myometrial contractility, although Hamada et al. (1994) suggested that the β2-adrenoceptor agonist ritodrine activates the latter type of channel in human myometrium. There is, however, substantial evidence that BKCa channels are important in regulating myometrial function. For example, these channels have been shown to be activated by a variety of agents, which inhibit myometrial contractility; these include β-adrenoceptor agonists (Anwer et al., 1992; Hamada et al., 1994), relaxin (Meera et al., 1995), human chorionic gonadotrophin (Carvajal et al., 2003; Doheny et al., 2003) and nitric oxide (Bradley et al., 1998, Okawa et al., 1999). Moreover, Khan and coworkers have presented evidence that BKCa channels in human myometrium from women in active labor lose their Ca2+ sensitivity, and have argued that this transition enhances myometrial contractility by preventing these channels from suppressing membrane excitability as [Ca2+]i rises (Khan et al., 1993; 2001).
The role of BKCa channels in generating basal myometrial rhythmicity has been less well characterized. Anwer et al. (1993) demonstrated that the BKCa channel inhibitors iberiotoxin (IBTX) and tetraethylammonium (TEA) increased the frequency of spontaneous myometrial contractions in myometrial strips from estrogen-primed rats, and also initiated spontaneous contractions in quiescent myometrial strips from term-pregnant women. However, these experiments were only performed on a small number of strips. A number of studies have also subsequently shown that the functional and molecular expression of BKCa channels diminishes in late pregnancy, possibly leading to the membrane depolarisation, which occurs at the same time (Wang et al., 1998; Matharoo-Ball et al., 2003).
Given the relative lack of available information regarding the contributions of both BKCa and KV channels to the regulation of basal myometrial contractility at different stages in pregnancy, we have assessed the effects of various inhibitors of these channels on spontaneous contractions in myometrial strips taken from nonpregnant rats, and also those in early, mid- and late pregnancy. Our results suggest that BKCa channels play little role in regulating these contractions at any time, but inhibition of KV channels causes marked effects on spontaneous activity. This implies that KV channels, as in other types of rhythmically active smooth muscles, are important to the control of frequency and amplitude of spontaneous depolarizations in rat myometrial tissue.
Methods
Tissue isolation and recording of contractility
Female Sprague–Dawley rats (Charles Rivers Laboratories, U.K.; ∼250 g) were killed by cervical dislocation in accordance with U.K. Home Office guidelines. Uterine horns were removed from virgin (nonpregnant) rats, or from pregnant rats at day 7 (early pregnant), day 14 (mid-pregnant) or day 21 (late pregnant), and immediately placed into ice-cold physiological salt solution (PSS (in mM): NaCl 119, NaHCO3 25, glucose 5.5, KH2PO4 1.18. MgSO4 1.17, KCl, 4.7, CaCl2 2.5 and EDTA 0.026). Fetuses, placental tissue and fetal membranes were removed and small tissue strips (approx. 0.5 × 0.5 × 5 mm) were dissected longitudinally with fiber structure and mounted vertically in organs baths, one end of the tissue strip was tied by a thin cotton thread to a fixed support and the other to a tension transducer connected to a MacLab™ recording system. Each organ bath contained 10 ml oxygenated (95% O2, 5% CO2) PSS to maintain the pH at 7.4. The temperature of the baths was maintained at 37°C. Myometrial tissue in vivo experiences different degrees of stretch dependent on pregnancy and gestation. The development of spontaneous contractions in myometrial strips from different gestations in vitro reflects this, as activity is dependent on the initial force applied to resting tissues (unpublished data). In the present study, myometrial strips were stretched by applying 9.81 mN to virgin or early pregnant tissue, 14.72 mN for mid-pregnant tissue and 19.62 mN for late pregnant tissue. Spontaneous contractile activity developed in 90% of preparations (nonpregnant, early pregnant and late pregnant during a 30–90 min equilibration period), and the remainder were considered atypical and discarded. A smaller proportion (∼50%) of tissues from mid-pregnant rats developed sustained spontaneous contractions. The ‘quiescent' tissue strips from mid-pregnant animals were studied separately from those in which spontaneous contractions occurred.
Following the equilibration period, spontaneous contractions (or basal tension in noncontracting strips) were recorded for ∼15 min (control period), followed by the cumulative addition of either paxilline (1 and 10 μM), IBTX (1, 10, 50 and 100 nM), penitrem A (1, 10, 50, 100 and 500 nM), TEA (1, 5 and 10 mM) or 4-aminopyridine (4-AP; 1 and 5 mM). In some experiments, TEA (1, 5 and 10 mM) was added cumulatively in the presence of another K+ channel inhibitor.
Time control experiments utilizing the appropriate vehicles (dimethylsulfoxide (DMSO) for IBTX and paxilline, DMSO and water for penitrem A, water for TEA and 4-AP) were performed for each of the protocols described.
Two additional sets of experiments were carried out using myometrial strips from nonpregnant rats. In the first, spontaneous activity was recorded for 15 min followed by the addition of 3000 nM penitrem A to the bath for 15 min. Subsequently, either 10 mM TEA or 5 mM 4-AP were added to the bath and activity was recorded for a further 15 min. In the second, myometrial strips were treated with capsaicin (10 μM) for 20 min to desensitize the tissue to the release of calcitonin gene related peptide, among other peptide mediators that might affect contractility, and prevent tachykinin release (Kakuyama et al., 1998; Vanheel & Van de Voorde, 2001; Szolcsanyi, 2004). Capsaicin was then washed from the bath, and 15 min later, phentolamine, propranolol and atropine (all at 1 μM) were added to inhibit the effects of any release of noradrenaline or acetylcholine. After a further equilibration period of 15 min, 10 mM TEA or 5 mM 4-AP were added to the bath and activity was recorded for 15 min.
Contractility data were recorded using MacLab™ hardware with Chart v4.0 software (Linton, Diss, U.K.). Parameters measured included maximum tension development during each contraction, the contraction integral (total tension developed during each contraction), contraction duration and the contraction interval (time between the start of successive contractions). Mean integral tension (MIT), the sum of the contraction integrals divided by the duration of the period, was used as a measure of mean myometrial activity per unit time. The mean±standard error of the mean (s.e.m.) of the MIT was calculated for the control and experimental periods, and was expressed as a percentage of the value obtained during the control period.
Whole-cell patch-clamp experiments
Myometrial strips from nonpregnant animals were placed in a N-(2-hydroxyethyl)piperazine-N′-(2-ethanesulfonic acid) (HEPES)-buffered PSS containing (in mM): NaCl 130, KCl 5, MgCl2 1.2, CaCl2 1.5, HEPES 10 and glucose 10, with the pH set to 7.4 with NaOH. Strips were minced and incubated at 37°C in a nominally Ca2+-free PSS for 15 min, and then transferred to low Ca2+-free PSS containing 1 mg ml−1 collagenase Type IX (Sigma, Poole, Dorset, U.K.), 1 mg ml−1 papain, 1 mg ml−1 trypsin inhibitor and 125 μM dithithreitol, and incubated for ∼25 min at 37°C. After the enzymatic digestion, tissue pieces were washed in enzyme-free low Ca2+–PSS and gently triturated using a wide-bore glass pipette. The cells were stored in Ca2+-free Ca2+–PSS at 4°C and used within 6–7 h.
Outward (K+) currents were recorded at room temperature (22–23°C) using the conventional whole-cell patch-clamp technique, with an Axopatch 200B amplifier and pCLAMP-9 software (Axon Instruments, CA, U.S.A.). PSS was used as the bath solution, and the pipette solution contained (in mM): KCl 110, MgCl2 2.5, MgATP 1.0, HEPES 10 and EGTA 0.1, with the pH set to 7.2 with NaOH. Data were analyzed off-line using Clamfit 9.0. BKCa currents were assessed in cells held at −60 mV and the pipette potential was ramped from −100 to +100 mV at a frequency of 0.1 Hz in the absence of drug, and then in the presence of 10 or 100 nM IBTX.
Statistical analysis
For spontaneously contracting myometrial strips analysis was conducted in Stata (Versions 8.0 or 8.2, Stata Corp., College Station, TX, U.S.A.) using generalized estimating equations (a form of multiple regression) to correct for repeated measurement on each tissue sample. The fitted model included terms for treatment (inhibitor versus vehicle), drug concentration (on a log scale) and comparisons carried out using an interaction test (Liang & Zeiger, 1986). This adjusted for any effects of time or drug vehicle on contraction parameters (i.e. on the spontaneous activity). Repeated measures ANOVA were used to assess effect of the drugs on quiescent strips (data for mid-pregnant tissue in Figures 5, 6 and 7, all data in Figure 8). Student's t-test was also used when indicated (Figure 9). P-values in the range 0.05–0.001 are taken as increasing evidence against the null hypothesis, but the totality of evidence is regarded as more important than any single test (Sterne & Davey Smith, 2001). Mean data (±s.e.m.) are presented graphically, and mean data with confidence intervals (CI) are discussed in the text. Data from whole-cell patch-clamp experiments were analyzed using Student's paired t-test. N refers to the tissue number from different animals, unless stated.
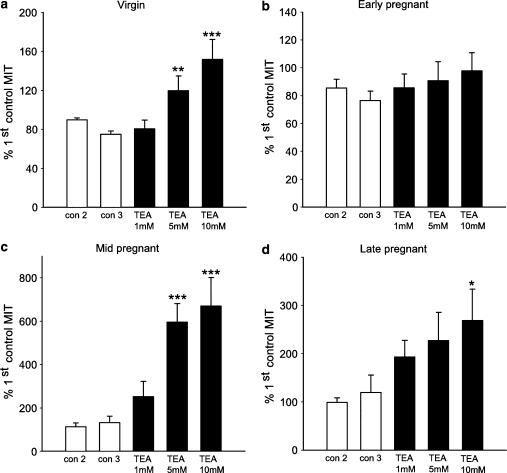
Figure 5.
Effects of TEA on MIT in muscle strips from virgin nonpregnant (a), early pregnant (b), mid-pregnant (c) and late pregnant (d) myometrium. MIT is normalized to the activity observed during an initial 7.5 min control period. The white bars illustrate activity during two preceding control periods, and the black bars show the effects of subsequent cumulative addition of TEA (1, 5 and 10 mM) (n=6, 6, 9 and 8 for virgin, early, mid- and late pregnant strips, each from a different animal). *P<0.05; **P<0.001; ***P<0.001.
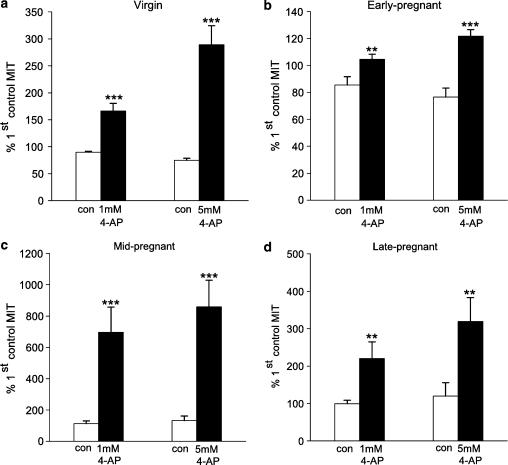
Figure 6.
Effects of 4-AP on MIT in muscle strips from virgin nonpregnant (a), early pregnant (b), mid-pregnant (c) and late pregnant (d) myometrium. In each case, MIT during 7.5 min applications of first 1 and then 5 mM 4-AP is normalized to the activity observed during a prior 7.5 min control (drug-free) period. The white bars illustrate activity during the second and third 7.5 min periods observed in strips exposed only to drug vehicle (water); again MIT is normalized to a prior (first) control period (controls, n=6, 6, 9 and 8; 4-AP treatment, n=7, 5, 9 and 8, for virgin, early, mid- and late pregnant strips, respectively, each from a different animal). **P<0.01; ***P<0.001.
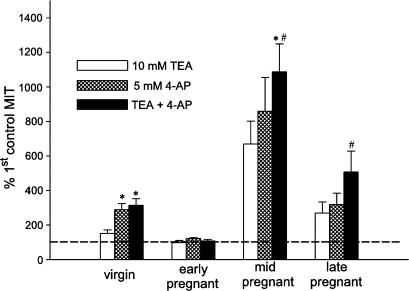
Figure 7.
Comparison of the effects of 10 mM TEA (open bars), 5 mM 4-AP (hatched bars) and their combination (black bars) on MIT in myometrial strips from nonpregnant and pregnant rats. MIT is normalized to the response obtained in the absence of drug, which is represented by the dashed line. Note that the response to 10 mM TEA was significantly higher than the control response in all but the strips from the early pregnant animals (see Figure 4; 10 mM TEA treatment, n=6, 6, 9 and 8; 5 mM 4-AP and combined treatment, n=7, 5, 9 and 8, for virgin, early, mid- and late pregnant strips, respectively, each from a different animal). *Response significantly different from 10 mM TEA, P<0.05. #Response significantly different from 5 mM 4-AP, P<0.05.
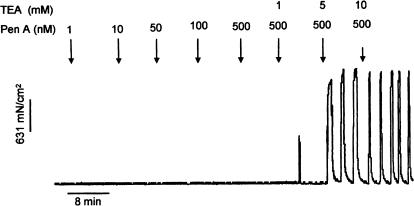
Figure 8.
Experimental protocol and representative traces showing the effect of cumulative addition of a selective BKCa channel inhibitor, in this case penitrem A, followed by application of ascending concentrations of TEA (1, 5 and 10 mM) on activity in quiescent mid-pregnant myometrial strips. Each concentration of drug was applied for 7.5 min.
Figure 9.
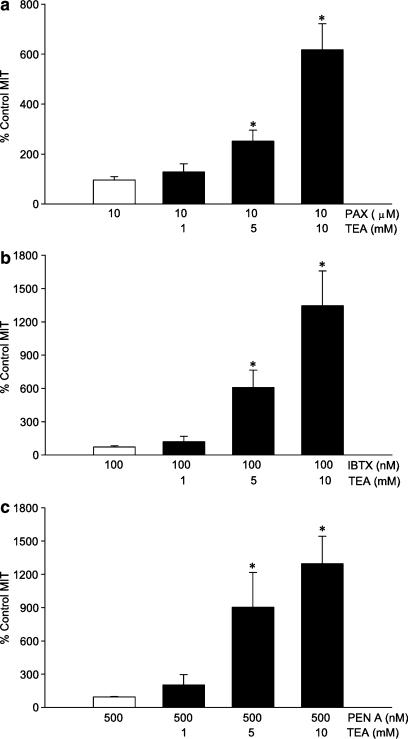
Effects of ascending concentrations of TEA (1, 5 and 10 mM) on MIT in the continuing presence of paxilline (10 μM, panel a; n=4), IBTX (100 nM, panel b; n=7) and penitrem A (500 nM, panel c; n=5) on activity in quiescent mid-pregnant myometrial strips. MIT is normalized to that measured during an initial 7.5 min control period prior to application of the selective BKCa channel inhibitor; this essentially reflected basal tone during these periods, as spontaneous contractions were rare. *P<0.05.
Drugs and chemicals
All K+ channel inhibitors and chemicals used were obtained from Sigma (Poole, Dorset, U.K.). Stock solutions of penitrem A (0.5 mM), IBTX (0.1 mM) and paxilline (10 mM) were made using DMSO, and stock solutions of TEA (1.0 M) and 4-AP (500 mM) were made up in water. The pH of the latter was adjusted to 7.4 using HCl.
Results
Effects of selective inhibitors of BKCa channels
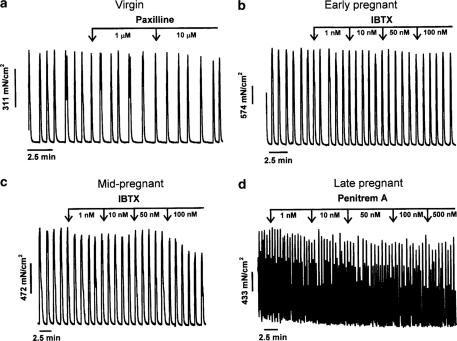
Figure 1 shows representative traces of the effect of cumulative addition of paxilline, IBTX and penitrem A on spontaneous myometrial contractions and MIT; data are illustrated in Figure 2. In no case did any of the three BK channel inhibitors significantly affect MIT (n=4–22 per protocol, NS, P>0.05) compared to time/vehicle controls.
Figure 1.
Experimental protocol and representative traces illustrating the lack of effect of application of progressively higher concentrations of BKCa channel inhibitors on spontaneous myometrial contractions in strips from virgin nonpregnant (a), early pregnant (b), mid-pregnant (c) and late pregnant (d) animals. Each concentration of inhibitor was applied for 5 min.
Figure 2.
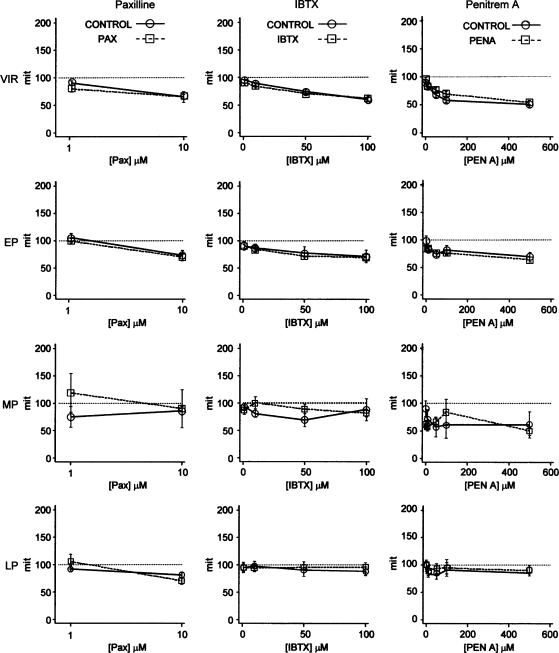
Effects of paxilline (Pax, left panels), iberiotoxin (IBTX, center panels) and penitrem A (Pen A, right panels) on MIT in myometrial strips from virgin nonpregnant (VIR), early pregnant (EP), mid-pregnant (MP) and late pregnant (LP) rats. Each panel illustrates MIT during the period of addition of the drug concentrations shown on the abscissa, normalized to that recorded during a 15 min control period just prior to addition of the first concentration of drug (open circles). Also illustrated is the MIT, expressed in the same way, in strips exposed during the same time intervals to drug vehicle. In no case was MIT significantly affected by BKCa channel inhibitor (n=4–22 strips, each from a different animal), P>0.05.
Analysis of the other parameters of contraction revealed some small, but inconsistent, effects of the three inhibitors on spontaneous contractions. In general, IBTX had little effect on contractility, except for a significant increase in the maximum amplitude of contractions in mid-pregnant tissue (13% per every 10-fold increase in drug concentration, CI: 3–23, P=0.008, n=11) versus controls (n=5). Paxilline did no alter contractility in myometrium from nonpregnant, early or mid-pregnant animals, but decreased contraction integral (31%, CI:−56 to −6, P=0.015), duration (20%, CI: −34 to −3, P=0.018) and contraction interval (19%, CI: −34 to –2, P=0.028) in myometrial strips from late pregnant animals (n=14 versus control n=11). Conversely, penitrem A had a tendency to increase contraction integral but lengthen the interval between contractions in early pregnant (integral 6%, CI: 0.1–12, P=0.05; interval 16%, CI: 5–27, P=0.004, n=8 versus controls n=5) and mid-pregnant (integral 16%, CI: 1–31, P=0.035; interval 19%, CI: 2–36, P=0.025, n=5 versus n=4) tissue compared with time controls. Penitrem A also increased maximum tension (3%, CI: −5 to −1, P=0.004), integral (10%, CI: 5–15, P=0.001) and duration (5%, CI: 2–9) in tissue from nonpregnant (n=19 versus controls, n=14). No single parameter in any of the four groups was affected by more than one of the BKCa channel inhibitors used and none of these effects translated into an alteration in MIT compared to controls at any stage of pregnancy. Additional experiments described below (see Figure 9b) confirmed that penitrem A had no effect on contractility even at a much higher concentration.
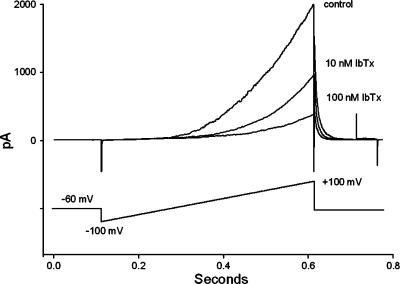
The whole-cell patch-clamp technique was used to confirm that the concentration range of IBTX used in the study inhibited the BKCa current in smooth muscle cells isolated from nonpregnant rat myometrium (Figure 3a). In the presence of 10 and 100 nM IBTX, the current, measured at +100 mV, fell by 39±7% (n=5, P<0.05) and 68±5% (n=7, P<0.05), respectively. In separate experiments, application of 1 mM TEA, which should block the BKCa current completely with relatively little effect on other K+ currents, inhibited the outward current by 71±7% (n=5).
Figure 3.
An example of the effect of IBTX on the outward (K+ current) elicited by voltage ramps in an isolated myometrial smooth muscle cell. Each trace is the average of five to 10 individual current traces recorded under control conditions (upper trace), and when the current had stabilised following addition of 10 nM (middle trace) and then 100 nM IBTX.
Effect of TEA and 4-AP on myometrial contractility
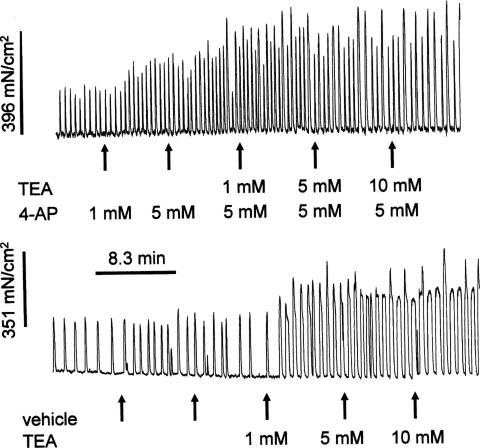
The protocol used to study the effects of TEA and 4-AP on spontaneous contractions is shown in Figure 4 (myometrial tissue from mid-pregnant rats was quiescent before application of the drugs). TEA caused a concentration-dependent increase in MIT in all groups except for tissue from early pregnant animals (Figure 5). However, TEA did increase the maximum amplitude of contraction in myometrium from early pregnant animals, compared to control period 2, by 20% (CI: 1.22–38.91, P=0.037) and 19.59% (CI: 2.78–36.40, P=0.022) at concentrations of 5 and 10 mM, respectively (n=6).
Figure 4.
Experimental protocol and representative traces showing the effect of 4-AP and TEA on myometrial contractions, in this case obtained in strips from late pregnant myometrium. Each concentration of drug was applied for 7.5 min.
Figure 6 summarizes the mean effect of 4-AP (1 and 5 mM) on spontaneous myometrial contractions compared with control strips (treated with the appropriate volume of vehicle, water) in all four groups of animals. 4-AP induced a significant increase in MIT in strips from nonpregnant animals (n=7) from vehicle control (n=6) (1 mM 4-AP: 76.64%, CI: 49.00–104.29; 5 mM 4-AP: 214.28%, CI: 144.86–283.69, P<0.001). 4-AP (n=9) also augmented MIT compared with controls (n=8–9) in tissues from mid-pregnant (1 mM 4-AP: 581.89%, CI: 262.25–901.52; 5 mM 4-AP: 726.76%, CI: 389.35–1064.17, P<0.001) and late pregnant animals (n=8) (1 mM 4-AP: 121%, CI: 31.33–210.67, P=0.008; 5 mM 4-AP: 199.55%, CI: 55.92–343.18, P=0.006). There was a relatively small effect of 4-AP on myometrium from early pregnant animals (n=5) (1 mM 4-AP: 18.97%, CI 4.81–33.13, P=0.009; 5 mM 4-AP: 45.27%, CI: 28.95–61.59, P<0.001) compared with controls (n=6).
Figure 7 compares the effect on MIT of 10 mM TEA, 5 mM 4-AP and the combination of TEA and 4-AP at these concentrations. In myometrium from nonpregnant animals, 4-AP (n=7) caused an increase in MIT that was significantly larger than the effect of TEA (n=6) (MIT as % of control period – 5 mM 4-AP: 289.01% CI: 219–358.06 versus 10 mM TEA: 151%, CI: 130.82–172.22, P<0.05). The addition of 10 mM TEA in the presence of 5 mM 4-AP (314.44%, CI: 276.96–351.95, NS) did not further enhance the response. In myometrium from the early pregnant group (n=5), although 4-AP itself significantly increased MIT (Figure 6), the combination of 4-AP and TEA (n=5) did not further increase the response, which was consistent with the observation that TEA itself had no effect (Figure 5). In the mid-pregnant group, the effects of both drugs on myometrial contractility were similarly marked, probably because basal activity was very low in myometrium from this group. Moreover, the combination of TEA and 4-AP caused an increase in MIT that was larger than that caused by either drug by itself (% difference in MIT with 5 mM 4-AP+10 mM TEA versus 10 mM TEA: 416.99%, CI: 9.18–824.81, P<0.05 versus 5 mM 4-AP: 228.41%, CI: 10.80–446.01, n=9, P<0.05). In myometrium from late pregnant animals (n=8), both drugs caused a similar increase in MIT; the effect of the drug combination was larger than that of 4-AP (% increase in MIT: 186.99%, CI: 7.71–366.28, P<0.05) but not of TEA (237.34%, CI: –34.22–508.90, P=0.09).
The effects of TEA (1, 5 and 10 mM) on quiescent myometrial strips from mid-pregnant animals following the cumulative application of paxilline (n=4), IBTX (n=7), or penitrim A (n=5) are illustrated in Figures 8 and 9. In no case did the cumulative addition of selective BKCa channel inhibitors initiate spontaneous activity (MIT in the presence of the lower concentrations of BKCa channel inhibitors is not shown, but was not different from that recorded in parallel time controls, P>0.05). However, TEA applied in the continuing presence of the highest concentration of each BKCa channel antagonist produced a concentration-dependent increase in MIT (Figure 9, P<0.05). This effect of TEA on MIT was similar to time/vehicle controls, which were performed in the absence of paxilline, IBTX or penitrem A (MIT% difference between TEA 10 mM+BKCa inhibitor versus TEA 10 mM+vehicle: for paxilline −294.4%, CI: −600.89–12.1; IBTX 289.21%, CI: –352.75–931.16; penitrem A −106.08, CI: −852.80–640.65, P>0.005). These experiments indicate that the effect of TEA on myometrial contractility was unlikely to have been due to a blockade of BKCa channels.
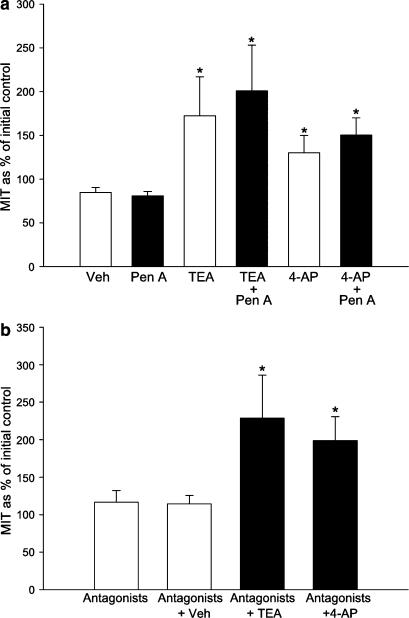
The mean effects of 10 mM TEA or 5 mM 4-AP after preincubation with a much higher concentration of penitrem A (3000 nM) or the appropriate concentration of its vehicle (DMSO) to myometrium from nonpregnant rats are summarized in Figure 10a. Penitrem A (n=21 strips from 16 animals) had no significant effect on contractility over 15 min when compared to the effect of its vehicle (n=18 strips from 15 animals) (MIT Penitrem A 80.74%, CI: 70.2–91.23 versus control 84.66%, 72.80–96.52, P>0.05). Subsequent application of either TEA or 4-AP in the continuing presence of penitrem A (n=4) significantly increased contractility to an extent which was similar to that observed in tissues which had not been treated with penitrem A (Figure 10a).
Figure 10.
Effects of TEA (10 mM) and 4-AP (5 mM) on MIT following treatment with a high concentration of penitrem A (3 μM; a) or a combination of drugs designed to suppress possible effects of transmitter release from nerve endings (b). (a) Penitrem A applied over 15 min (Pen A; n=21 strips from 16 animals) had no significant effect on MIT when compared to the effect of 0.6% DMSO (Veh; n=18 strips from 15 animals). In the presence of penitrem A, both TEA (TEA+Pen A; n=7 strips from seven animals) and 4-AP (4-AP+Pen A; n=10 strips animals from nine animals) caused increases in MIT, which were similar to those recorded in tissues in the presence of TEA (TEA; n=4 strips from four animals) and 4-AP (4-AP; n=4 from four animals) alone, respectively. *MIT during treatment with TEA or 4-AP was significantly different (P<0.05) compared with time/vehicle controls. (b) Following treatment with capsaicin (10 μM) for 20 min, tissues were washed in PSS for 15 min, and then phentolamine, propranolol and atropine (all 1 μM) were added to the bath. MIT during the next 15 min was not significantly different from that recorded prior to capsaicin addition (bar labelled ‘antagonists', n=17 strips from 13 animals). Subsequent addition of either TEA (antagonists+TEA; n=4 strips from n=4 animals) or 4-AP (antagonists+4-AP; n=4 strips from n=4 animals) caused increases in MIT similar to that observed in tissue strips which had not been pretreated (compare with, for example, Figure 10a). *MIT during treatment with TEA or 4-AP was significantly different (P<0.05) compared with time/vehicle controls (antagonists+Veh, n=9 strips from n=4 animals).
Treatment of tissue from nonpregnant animals with capsaicin (10 μM) led to an inhibition of spontaneous activity, which, however, recovered following washing with fresh PSS. A combination of phentolamine, propranolol and atropine (all at 1 μM) had little effect on MIT compared with the 15 min prior to capsaicin treatment (bar labelled ‘antagonists', n=17 strips from 13 animals (Figure 10b). The addition of 10 mM TEA (bar 4, n=7) or 5 mM 4-AP (bar 5, n=4) 15 min after application of the receptor antagonists significantly increased the MIT to an extent (∼2-fold, P<0.05) similar to that observed in tissues from nonpregnant animals not treated with capasaicin and the receptor antagonists (i.e. Figures 4a, 5a and 10a), whereas addition of an equal volume of vehicle (water, n=9 from four animals) had no effect on MIT.
Discussion
This study shows that three selective inhibitors of BKCa channels, when added in concentrations that markedly suppress single channel or whole-cell BKCa currents (Figure 3; Knaus et al., 1994; Knock et al., 1999) and/or to inhibit the relaxing effects of agents which activate these channels in isolated tissues (Anwer et al., 1992; Adeagbo, 1999; Doheny et al., 2003; Chanrachakul et al., 2004), have no or only minor effects on the spontaneous contractions of rat myometrial strips under basal conditions. On the other hand, inhibition of voltage-gated K+ channels with either 4-AP or TEA enhanced contractility, except in early pregnancy, when TEA had no effect. Spontaneous basal contractile activity was meagre or nonexistent during mid-pregnancy, and was very strongly enhanced by both TEA and 4-AP, suggesting that it is voltage-gated K+ channels, rather than BKCa channels, which contribute to quiescence during this period.
Although a large number of studies support the concept that BKCa channels are important in mediating agonist- or drug-induced suppression of myometrial contractility (Meera et al., 1995; Zhou et al., 2000; Doheny et al., 2003; Chanrachakul et al., 2004), their contribution to the control of basal activity has been less well characterized. Anwer et al. (1993) showed that IBTX initiated spontaneous contractions in quiescent strips from human myometrium, and strongly enhanced the frequency and amplitude of contraction in strips from rat myometrium, whereas Taggart & Wray (1998) showed that IBTX had no obvious effect on frequency, and caused only a small increase in contraction amplitude in rat myometrial strips. Anwer et al. (1993) also showed that when applied to cultured smooth muscle cells isolated from human myometrium, IBTX caused depolarization and a rise in [Ca2+]i. On the other hand, even at a very high concentration (10 mM), TEA did not affect the resting potential in circular muscle strips from rat myometium (Wilde & Marshall, 1988).
In order to assess the role of BKCa channels more thoroughly, we used three potent and selective BKCa channel inhibitors. Since myometrial activity often varies spontaneously, we also carried out experiments using time controls and a statistical analysis designed to distinguish time-dependent changes from those induced by the K+ channel inhibitors. The results were consistent in demonstrating that BKCa channels play no apparent role in controlling basal myometrial rhythmicity in the rat.
Although TEA is widely used as an inhibitor of BKCa channels, it also is known to suppress some types of voltage-gated K+ channel currents at concentrations not much higher than those needed to inhibit BKCa currents. A study by (Wang et al., 1998) demonstrated that in rat myometrium smooth muscle cells, the BKCa current coexists with at least three types of voltage-gated K+ current. These included a 4-AP-sensitive, rapidly inactivating A-type current and two delayed rectifier K+ currents, which could be distinguished on the basis of differing voltage ranges of inactivation. These delayed rectifier currents were present in myometrial smooth muscle cells from both nonpregnant and late pregnant animals, and were suppressed by both TEA (⩾0.5 mM) and 4-AP (⩾0.4 mM). For example, in the cells from nonpregnant myometrium, a current termed INP1 inactivated over a relatively negative potential range, and was inhibited by 73 and 36% by 5 mM 4-AP and 0.5 mM TEA, respectively, whereas a second current, INP2, inactivated over a more positive potential range and was inhibited by 32 and 36% by 4-AP and 0.5 mM TEA, respectively. Two delayed rectifier K+ currents with inactivation properties similar to those observed in rat myometrial cells were also described in human myometrial smooth muscle cells, although these differed from INP1 and INP2 in that the current inactivating at more negative potentials was inhibited by TEA but not 4-AP, while the current inactivating at more positive potentials was inhibited by 4-AP but not TEA (Knock et al., 1999).
In view of the functional evidence for KV channels in both rat and human myometrium, we examined the effects of both TEA and 4-AP on myometrial contraction, and found that both drugs caused concentration-dependent increases in myometrial contractile activity range. A similar effect was observed if TEA was applied in the presence of each of the three BKCa antagonists, or if 4-AP was applied in the presence of penitrem A. Moreover, at a concentration of 1 mM, which should completely block BKCa channels, TEA did not significantly increase MIT, although there was a trend in this direction in most experiments, possibly due to a partial inhibition of the delayed rectifier K+ channels, which would be expected at this concentration (Wang et al., 1998).
4-AP at both 1 and 5 mM also strongly enhanced myometrial contractility in nonpregnant myometrium, as well as in mid- and late pregnant myometrium, providing additional evidence for a role of KV channels in controlling excitability. The effect on myometrial contractility of 4-AP, a broad spectrum inhibitor of KV channels (with the exception of KV2-type channels; Grissmer et al., 1994), or of any other agent that inhibits KV channels with any selectivity, does not appear to have been previously described.
In order to determine whether TEA or 4-AP might be indirectly affecting contractility by depolarizing nerve endings in the myometrial strips and releasing either peptides or autonomic neurotransmitters to act on their smooth receptors, we used capsaicin to desensitize vanilloid receptors and inhibit neurokinin release (Kakuyama et al., 1998; Vanheel & Van de Voorde, 2001; Szolcsanyi, 2004) and inhibitors of both adrenergic and muscarinic receptors to block the potential effects of noradrenaline and acetylcholine release. As shown in Figure 10b, this procedure did not suppress the effects of either TEA or 4-AP, indicating that they were exerting their effects directly on the smooth muscle cells.
It is particularly interesting that in early pregnant myometrium 4-AP significantly increased MIT, albeit to a small extent, whereas TEA did not. Similarly, the effect of 4-AP was larger than that of TEA in nonpregnant myometrium. These results support the concept that TEA and 4-AP were exerting their effects via separate KV channels. In accordance with this possibility, in mid-pregnancy the combination of both drugs caused a greater increase in contractility than did either drug alone.
In general, neither TEA nor 4-AP affected the contraction interval (data not shown), suggesting that these drugs were not affecting the frequency at which the bursts of action potentials that cause these contractions were being fired. Rather, both agents increased the duration, and particularly the amplitude of contractions, which is consistent with an enhancement of action potential frequency and/or duration, and/or an increase in burst duration. TEA was shown to have all of these effects, and also increased burst frequency, in an electrophysiological study of circular muscle from late pregnant rat myometrium (Wilde & Marshall, 1988). These authors did not observe an effect of 4-AP on the electrical activity in this preparation at concentrations below 10 mM, although above this concentration 4-AP caused a large depolarization. It is worth noting that electrical activity differs somewhat between the circular and longitudinal muscles of the rat myometrium, and that for the current study, myometrial strips were cut in such a way that force was being generated by the longitudinal muscle layer. Both TEA and 4-AP have been shown to increase spiking activity in other rhythmically active smooth muscle tissues, which fire periodic trains of action potentials (e.g. Thornbury et al., 1992; Koh et al., 1999).
To our knowledge, this study provides the first evidence that KV channels make an important contribution to controlling basal myometrial contractility. Although it is impossible to make firm inferences about the roles of particular ion channels in controlling the pattern of excitability based on contractility data, the results at least seem consistent with the concept that TEA- and 4-AP-sensitive KV channels in the rat myometrium are more important in limiting action potential activity once it has been initiated than in controlling the frequency of pacemaker activity. The lack of effect of any of the K+ channel inhibitors tested on contraction, taken with the remarkable insensitivity to these drugs of any of the contractility parameters in early pregnant myometrium, suggests that additional channels are present and contributing to control of the membrane potential and excitability. Recent evidence suggests, for example, that a Ca2+-activated Cl− current is present in pregnant rat myometrial cells, and that inhibitors of these channels reduce the frequency of spontaneous contraction in myometrial strips (Jones et al., 2004). Moreover, twin pore K+ channels have been shown to be present in myometrium (Bai et al., 2005) and other smooth muscle (Gurney et al., 2003), and it will be of interest to determine whether these are also involved in the control of myometrial membrane potential and excitability.
Acknowledgments
This work was supported by Tommy's the baby charity (Reg. Charity No.: 1060508). We thank Mr Paul Seed for statistical assistance, Dr Maarten Raijmakers and Dr Brenda Kelly for help with animal husbandry and Dr Greg Knock for his valuable input into the study.
Abbreviations
- 4-AP
4-aminopyridine
- BKCa
Ca2+-activated K+ channels
- DMSO
dimethylsulfoxide
- HEPES
N-(2-hydroxyethyl)piperazine-N′-2-ethanesulfonic acid
- IBTX
iberiotoxin
- KATP
ATP-activated K+ channels
- Kv
voltage-activated K+ channels
- TEA
tetraethylammonium
References
- ADEAGBO A.S.O. 1-Ethyl-2-benzimidazolinone stimulates endothelial KCa channels and nitric oxide formation in rat mesenteric vessels. Eur. J. Pharmacol. 1999;379:151–159. doi: 10.1016/s0014-2999(99)00489-6. [DOI] [PubMed] [Google Scholar]
- ANWER K., OBERTI C., PEREZ G.J., PEREZ-REYES N., MCDOUGALL J.K., MONGA M., SANBORN B.M., STEFANI E., TORO L. Calcium-activated K+ channels as modulators of human myometrial contractile activity. Am. J. Physiol. 1993;265:C976–C985. doi: 10.1152/ajpcell.1993.265.4.C976. [DOI] [PubMed] [Google Scholar]
- ANWER K., TORO L., OBERTI C., STEFANI E., SANBORN B.M. Ca2+-activated K+ channels in pregnant rat myometrium: modulation by a beta-adrenergic agent. Am. J. Physiol. 1992;263:C1049–C1056. doi: 10.1152/ajpcell.1992.263.5.C1049. [DOI] [PubMed] [Google Scholar]
- BAI X., BUGG G.J., GREENWOOD S.L., GLAZIER J.D., SIBLEY C.P., BAKER P.N., TAGGART M.J., FYFE G.K. Expression of TASK and TREK, two-pore domain K+ channels, in human myometrium. Reproduction. 2005;129:525–530. doi: 10.1530/rep.1.00442. [DOI] [PubMed] [Google Scholar]
- BRADLEY K.K., BUXTON I.L., BARBER J.E., MCGAW T., BRADLEY M.E. Nitric oxide relaxes human myometrium by a cGMP-independent mechanism. Am. J. Physiol. 1998;275:C1668–C1673. doi: 10.1152/ajpcell.1998.275.6.C1668. [DOI] [PubMed] [Google Scholar]
- CARVAJAL J.A., THOMPSON L.P., WEINER C.P. Chorion-induced myometrial relaxation is mediated by large-conductance Ca2+-activated K+ channel opening in the guinea pig. Am. J. Obstet. Gynecol. 2003;188:84–91. doi: 10.1067/mob.2003.102. [DOI] [PubMed] [Google Scholar]
- CHANRACHAKUL B., PIPKIN F.B., KHAN R.N. Contribution of coupling between human myometrial beta2-adrenoreceptor and the BK(Ca) channel to uterine quiescence. Am. J. Physiol. Cell. Physiol. 2004;287:C1747–C1752. doi: 10.1152/ajpcell.00236.2004. [DOI] [PubMed] [Google Scholar]
- CURLEY M., CAIRNS M.T., FRIEL A.M., MCMEEL O.M., MORRISON J.J., SMITH T.J. Expression of mRNA transcripts for ATP-sensitive potassium channels in human myometrium. Mol. Hum. Reprod. 2002;8:941–945. doi: 10.1093/molehr/8.10.941. [DOI] [PubMed] [Google Scholar]
- DOHENY H.C., HOULIHAN D.D., RAVIKUMAR N., SMITH T.J., MORRISON J.J. Human chorionic gonadotrophin relaxation of human pregnant myometrium and activation of the BKCa channel. J. Clin. Endocrinol. Metab. 2003;88:4310–4315. doi: 10.1210/jc.2003-030221. [DOI] [PubMed] [Google Scholar]
- GRISSMER S., NGUYEN A.N., AIYAR J., HANSON D.C., MATHER R.J., GUTMAN G.A., KARMILOWICZ M.J., AUPERIN D.D., CHANDY K.G. Pharmacological characterization of five cloned voltage-gated K+ channels, types Kv1.1, 1.2, 1.3, 1.5, and 3.1, stably expressed in mamMalian cell lines. Mol. Pharmacol. 1994;45:1227–1234. [PubMed] [Google Scholar]
- GURNEY A.M., OSIPENKO O.N., MACMILLAN D., MCFARLANE K.M., TATE R.J., KEMPSALL F.E.J. 2-pore domain K channel, TASK-1 in pulmonary artery smooth muscle cells. Circ. Res. 2003;93:957–964. doi: 10.1161/01.RES.0000099883.68414.61. [DOI] [PubMed] [Google Scholar]
- HAMADA Y., NAKAYA Y., HAMADA S., KAMADA M., AONO T. Activation of K+ channels by ritodrine hydrochloride in uterine smooth muscle cells from pregnant women. Eur. J. Pharmacol. 1994;288:45–51. doi: 10.1016/0922-4106(94)90008-6. [DOI] [PubMed] [Google Scholar]
- JONES K., SHMYGOL A., KUPITTAYANANT S., WRAY S. Electrophysiological characterization and functional importance of calcium-activated chloride channel in rat uterine myocytes. Pflugers Arch. – Eur. J. Physiol. 2004;448:36–43. doi: 10.1007/s00424-003-1224-7. [DOI] [PubMed] [Google Scholar]
- KAKUYAMA M., VALLANCE P., AHLUWALIA A. Endothelium-dependent sensory NANC vasodilatation: involvement of ATP, CGRP and a possible NO store. Br. J. Pharmacol. 1998;123:310–316. doi: 10.1038/sj.bjp.0701610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KHAN R.N., MATHAROO-BALL B., ARULKUMARAN S., ASHFORD M.L. Potassium channels in the human myometrium. Exp. Physiol. 2001;86:255–264. doi: 10.1113/eph8602181. [DOI] [PubMed] [Google Scholar]
- KHAN R.N., SMITH S.K., MORRISON J.J., ASHFORD M.L. Properties of large conductance K+ channels in human myometrium during pregnancy and labour. Proc. R. Soc. Lond. Ser. B. 1993;251:9–15. doi: 10.1098/rspb.1993.0002. [DOI] [PubMed] [Google Scholar]
- KNAUS H.G., MCMANUS O.B., LEE S.H., SCHMALHOFER W.A., GARCIA-CALVO M., HELMS L.M., SANCHEZ M., GIANGIACOMO K., REUBEN J.P., SMITH A.B., JR. Tremorgenic indole alkaloids potently inhibit smooth muscle high-conductance calcium-activated potassium channels. Biochemistry. 1994;33:5819–5828. doi: 10.1021/bi00185a021. [DOI] [PubMed] [Google Scholar]
- KNOCK G.A., SMIRNOV S.V., AARONSON P.I. Voltage-gated K+ currents in freshly isolated myocytes of the pregnant human myometrium. J. Physiol. 1999;518:769–781. doi: 10.1111/j.1469-7793.1999.0769p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOH S.D., WARD S.M., DICK G.M., EPPERSON A., BONNER H.P., SANDERS K.M., HOROWITZ B., KENYON J.L. Contribution of delayed rectifier potassium currents to the electrical activity of murine colonic smooth muscle. J. Physiol. 1999;515:475–487. doi: 10.1111/j.1469-7793.1999.475ac.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIANG K.-Y., ZEIGER S.L. Longitudinal analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- MATHAROO-BALL B., ASHFORD M.L., ARULKUMARAN S., KHAN R.N. Down-regulation of the alpha- and beta-subunits of the calcium-activated potassium channel in human myometrium with parturition. Biol. Reprod. 2003;68:2135–2141. doi: 10.1095/biolreprod.102.010454. [DOI] [PubMed] [Google Scholar]
- MEERA P., ANWER K., MONGA M., OBERTI C., STEFANI E., TORO L., SANBORN B.M. Relaxin stimulates myometrial calcium-activated potassium channel activity via protein kinase A. Am. J. Physiol. 1995;269:C312–C317. doi: 10.1152/ajpcell.1995.269.2.C312. [DOI] [PubMed] [Google Scholar]
- MORRISON J.J., ASHFORD M.L., KHAN R.N., SMITH S.K. The effects of potassium channel openers on isolated pregnant human myometrium before and after the onset of labor: potential for tocolysis. Am. J. Obstet. Gynecol. 1993;169:1277–1285. doi: 10.1016/0002-9378(93)90294-s. [DOI] [PubMed] [Google Scholar]
- OKAWA T., VEDERNIKOV Y.P., SAADE G.R., LONGO M., OLSON G.L., CHWALISZ K., GARFIELD R.E. Roles of potassium channels and nitric oxide in modulation of uterine contractions in rat pregnancy. Am. J. Obstet. Gynecol. 1999;181:649–655. doi: 10.1016/s0002-9378(99)70508-9. [DOI] [PubMed] [Google Scholar]
- STERNE J.A., DAVEY SMITH G. Sifting the Evidence – what's wrong with significance tests. Br. Med. J. 2001;322:226–231. doi: 10.1136/bmj.322.7280.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SZOLCSANYI J. Forty years in capsaicin research for sensory pharmacology and physiology. Neuropeptides. 2004;38:377–384. doi: 10.1016/j.npep.2004.07.005. [DOI] [PubMed] [Google Scholar]
- TAGGART M.J., WRAY S. Contribution of sarcoplasmic reticular calcium to smooth muscle contractile activation: gestational dependence in isolated rat uterus. J. Physiol. 1998;511:133–144. doi: 10.1111/j.1469-7793.1998.133bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THORNBURY K.D., WARD S.M., SANDERS K.M. Outward currents in longitudinal colonic muscle cells contribute to spiking electrical behavior. Am. J. Physiol. 1992;263:C237–C245. doi: 10.1152/ajpcell.1992.263.1.C237. [DOI] [PubMed] [Google Scholar]
- VANHEEL B., VAN DE VOORDE J. Regional differences in anandamide- and methanandamide-induced membrane potential changes in rat mesenteric arteries. J. Pharmacol. Exp. Ther. 2001;296:322–328. [PubMed] [Google Scholar]
- WANG S.Y., YOSHINO M., SUI J.L., WAKUI M., KAO P.N., KAO C.Y. Potassium currents in freshly dissociated uterine myocytes from nonpregnant and late-pregnant rats. J. Gen. Physiol. 1998;112:737–756. doi: 10.1085/jgp.112.6.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILDE D.W., MARSHALL J.M. Effects of tetraethylammonium and 4-aminopyridine on the plateau potential of circular myometrium from the pregnant rat. Biol. Reprod. 1988;38:836–845. doi: 10.1095/biolreprod38.4.836. [DOI] [PubMed] [Google Scholar]
- ZHOU X.B., WANG G.X., RUTH P., HUNEKE B., KORTH M. BKCa channel activation by membrane-associated cGMP kinase may contribute to uterine quiescence in pregnancy. Am. J. Physiol. Cell. Physiol. 2000;279:C1751–C1759. doi: 10.1152/ajpcell.2000.279.6.C1751. [DOI] [PubMed] [Google Scholar]