Abstract
Aminoglutethimide is a clinically available drug that suppresses steroid biosynthesis by inhibiting enzymes such as cytochrome P450scc and aromatase. Because several members of neurosteroids regulate glutamate receptors, we investigated the effect of aminoglutethimide on cell death induced by overactivation of glutamate receptors in CNS neurons.
Long-term pretreatment of organotypic cerebrocortical slice cultures with aminoglutethimide (100–1000 μM) for 6 days or over resulted in concentration-dependent suppression of neuronal cell death induced by NMDA. Aminoglutethimide (1000 μM) also inhibited neurotoxicity of AMPA and kainate, but not of ionomycin or staurosporine.
The protective effect of aminoglutethimide against NMDA cytotoxicity was not mimicked by other steroid synthesis inhibitors including trilostane and exemestane, and was not reversed by concurrent application of steroids such as pregnenolone, estrone, 17β-estradiol and estriol.
In dissociated rat cerebrocortical cell cultures, long-term treatment with aminoglutethimide (10–1000 μM) attenuated NMDA receptor-mediated glutamate cytotoxicity but produced no significant effect on glutamate-induced increases in intracellular Ca2+.
Brief as well as long-term pretreatment with aminoglutethimide (30–1000 μM) prevented NMDA receptor-dependent ischemic neuronal injury in organotypic cerebrocortical slice cultures, which was associated with suppression of glutamate release during the ischemic insult.
These results indicate that aminoglutethimide, irrelevant to its actions on neurosteroid synthesis, protects CNS neurons from excitotoxic and ischemic injuries. Development of aminoglutethimide analogs possessing neuroprotective properties may be of therapeutic value.
Keywords: Cerebral cortex, excitotoxicity, ischemia, neuroactive steroid, neuronal death
Introduction
The term ‘excitotoxicity' corresponds to damage of cells induced by aberrant activation of receptors for glutamate, the major excitatory neurotransmitter in CNS. Overactivation of glutamate receptors typically results from excessive release of glutamate from synaptic terminals and/or glial cells, as observed in seizure-induced neuronal damage and ischemic neuronal injury (Nishizawa, 2001; Holmes, 2002). Reduced capacity for glutamate uptake from the extracellular fluid represents another mechanism of glutamate receptor overactivation, as suggested in pathogenesis of amyotrophic lateral sclerosis (Maragakis & Rothstein, 2004). In addition, neuronal damage by glutamate synergistically with other insults has been implicated in neurodegenerative disorders including Alzheimer's, Parkinson's and Huntington's disease (Meldrum & Garthwaite 1990; Mattson, 2003). In these ways, excitotoxicity plays a fundamental role in various pathological conditions in the brain.
Both neurons and glia can synthesize steroidal compounds called neurosteroids (Zwain & Yen, 1999), which exert various influences on brain function (Mellon & Griffin, 2002; Reddy, 2003). Neurosteroids modulate activities of neurotransmitter receptors including ionotropic glutamate receptors (Wu et al., 1991; Irwin et al., 1994; Weaver et al., 2000), and several members of neurosteroids are shown either to suppress or to exacerbate excitotoxic neuronal injury. For example, dehydroepiandrosterone and its sulfated derivative protect hippocampal neurons from excitotoxic insults by NMDA, AMPA or kainate (Kimonides et al., 1998; Kurata et al., 2004). Pregnanolone sulfate, a metabolite of progesterone, inhibits NMDA-induced membrane current and intracellular Ca2+ increase, and is neuroprotective against NMDA cytotoxicity (Park-Chung et al., 1997; Weaver et al., 1997; Shirakawa et al., 2002). Moreover, Veiga et al. (2003) have demonstrated that pregnenolone and dehydroepiandrosterone, through aromatase-mediated conversion to estradiol, protect hippocampal hilar neurons against kainic acid cytotoxicity. In contrast, pregnenolone sulfate positively modulates NMDA receptor channels and exacerbates NMDA-induced neuronal death (Weaver et al., 1998; Yaghoubi et al., 1998; Shirakawa et al., 2002).
These observations underscore the importance of neurosteroids in regulation of excitotoxicity-mediated neuronal damage. However, the overall influence of biosynthesis of endogenous neurosteroids on excitotoxic injury remains unclear, because some neurosteroids are protective but others are deleterious to neuronal cells. Accordingly, we set out to examine the effect of aminoglutethimide (Figure 1a) on excitotoxicity-related neuronal injury. Aminoglutethimide inhibits cytochrome P450scc with an apparent Ki value of 14 μM (Foster et al., 1983): cytochrome P450scc is an enzyme that catalyzes the first step of neurosteroid synthesis, namely, production of pregnenolone from cholesterol (Salhanick, 1982). Aminoglutethimide also inhibits aromatase, an enzyme involved in production of estrogens, with an apparent Ki value of 0.6 μM (Salhanick, 1982; Foster et al., 1983). On the basis of these actions, clinical use of aminoglutethimide is approved in the United States for the treatment of hormone-dependent malignant tumors (Miller et al., 1987; Alshowaier et al., 1999). Here we report unexpected findings that aminoglutethimide protects cortical neurons from excitotoxic and ischemic injuries, probably through the mechanisms unrelated to steroid biosynthesis.
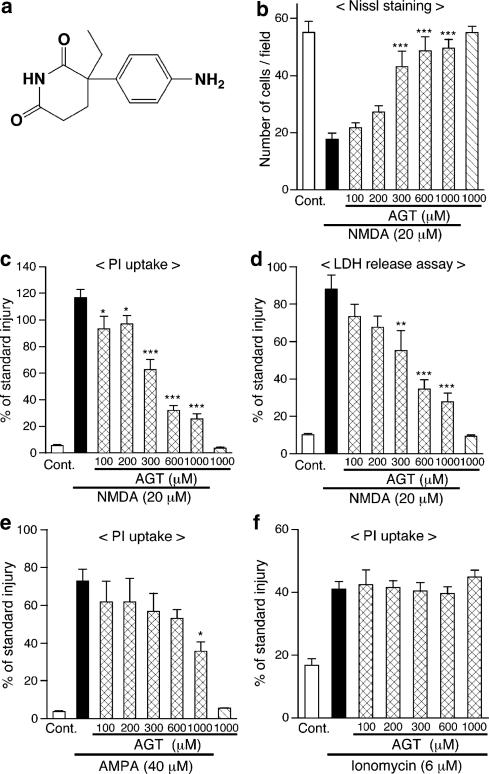
Figure 1.
Continuous application of aminoglutethimide (AGT) protects neurons in cerebrocortical slice cultures from cytotoxicity of NMDA and AMPA. (a) Chemical structure of AGT. (b–d) AGT was applied to slice cultures at indicated concentrations from 1 DIV, throughout the entire period of cultivation. NMDA (20 μM) was applied for 24 h at 11 DIV in the presence of AGT, and cellular injury at 12 DIV was determined by the number of Nissl-stained surviving neurons (b, n=6–7 slices), intensity of PI fluorescence (c, n=5–7 slices) and amount of LDH released into medium (d, n=4 culture wells). ‘Cont.' means control cultures that did not receive drug treatment. *P<0.05, **P<0.01, ***P<0.001 vs NMDA alone. (e) AGT was applied to slice cultures at indicated concentrations from 1 DIV. AMPA (40 μM) was applied for 24 h at 11 DIV in the presence of AGT, and cellular injury at 12 DIV was determined by intensity of PI fluorescence (n=4–6 slices). *P<0.05 vs AMPA alone. (f) AGT was applied at indicated concentrations from 1 DIV, and ionomycin (6 μM) was applied for 24 h at 11 DIV in the presence of AGT. Cellular injury at 12 DIV was determined by PI fluorescence (n=5–6 slices). In (c–f), each value was normalized with that in slice cultures receiving the standard injury (200 μM NMDA for 24 h) as 100%.
Methods
Drugs and chemicals
Unless otherwise indicated, drugs and chemicals were obtained from Nacalai Tesque (Kyoto, Japan). DL-Aminoglutethimide, (+)-MK-801, ionomycin, estrone, 17β-estradiol, estriol and pregnenolone were obtained from Sigma-Aldrich Chemicals (St Louis, MO, U.S.A.). Trilostane was purchased from Steraloids Inc. (Newport, RI, U.S.A.) and exemestane was from LKT laboratories (St Paul, MN, U.S.A.). (±)-AMPA was from Tocris Cookson (Bristol, U.K.) and staurosporine was from Calbiochem (San Diego, CA, U.S.A.). Propidium iodide (PI) and KT5720 were from Wako Pure Chemicals (Osaka, Japan).
Cortical slice cultures
All experimental procedures were approved by our institutional animal experimentation committee, and animals were treated in accordance with the guidelines of the U.S. National Institutes of Health regarding the care and use of animals for experimental procedures. Organotypic slice cultures were prepared with the procedures described previously (Shirakawa et al., 2002; 2005). Briefly, we anesthetized postnatal day 2 or 3 Wistar rats (from 35 mother rats in total) by chilling them in ice for 2–3 min, decapitated them, and removed the brain from the skull and cut it into two hemispheres. Each hemisphere was cut into coronal slices of 300 μm thickness with a tissue chopper (Narishige, Tokyo, Japan), and six cerebrocortical slices (seven slices in some cases) at the rostro-caudal level containing the caudate putamen were transferred onto a Millicell-CM insert membrane (30 mm in diameter; Millipore, Bedford, MA, U.S.A.) in six-well plates. Culture medium, consisting of 50% minimal essential medium/HEPES (GIBCO, Invitrogen Japan, Tokyo, Japan), 25% Hanks' balanced salt solution (GIBCO) and 25% heat-inactivated horse serum (GIBCO) supplemented with 6.5 mg ml−1 glucose and 2 mM glutamine, 100 U ml−1 penicillin G potassium and 100 μg ml−1 streptomycin sulfate (GIBCO), was supplied at 0.7 ml well−1. Culture medium was replaced with fresh medium on the next day of culture preparation, and thereafter, every 2 days. Slices were cultured in a humidified atmosphere of 5% CO2 and 95% air at 34°C. From 10 days in vitro (DIV; 6 DIV in several experiments), cultures were maintained in serum-free medium consisting of 75% minimal essential medium/HEPES and 25% Hanks' balanced salt solution supplemented with 6.5 mg ml−1 glucose, 2 mM glutamine, 100 U ml−1 penicillin G potassium and 100 μg ml−1 streptomycin sulfate.
Cell death assay in slice cultures
Slice cultures were treated with excitotoxins, ionomycin and staurosporine for 24 h at 11 DIV with serum-free medium whose composition is described above. Chemical ischemia was applied to cultures at 11 DIV by transfer of culture inserts to the plates containing the conditioning solution. The conditioning solution was glucose-free Ringer's buffer (124 mM NaCl, 4.9 mM KCl, 1.3 mM MgSO4, 2 mM CaCl2, 1.2 mM KH2PO4 and 25.6 mM NaHCO3, pH 7.4) supplemented with 3 mM sodium azide and 10 mM 2-deoxyglucose (Katsuki et al., 2005). After ischemic treatment for 30–60 min, culture inserts were transferred to the plates containing serum-free culture medium and maintained for further 24 h.
Cultures were fixed with 4% paraformaldehyde and 4% sucrose in 0.1 M phosphate buffer overnight, rinsed with distilled water twice and exposed to 0.1% toluidine blue solution for 15 min. After rinse in distilled water, specimens were dehydrated by a graded series of ethanol and mounted with ENTELLAN Neu (Merck KGaA, Darmstadt, Germany). Positively stained cells possessing round or oval cell bodies and clear nuclear boundaries were judged as viable neurons. The maximal number of surviving neurons in an area of 80 × 90 μm2 within the parietal cortex of individual slices was counted.
We also measured PI uptake into slices and lactate dehydrogenase (LDH) efflux to the culture medium. PI (5 μg ml−1) was added to serum-free medium from 24 h before and during treatment with excitotoxins, ionomycin and staurosporine. In the case of ischemia, PI (5 μg ml−1) was applied to slice cultures from 24 h before chemical ischemia, and also during 24 h of post-incubation. Slices were observed with an inverted fluorescence microscope with a rhodamine filter set. Fluorescence images were captured through a monochrome chilled CCD camera (C5985, Hamamatsu Photonics, Hamamatsu, Japan), and stored images were analyzed with NIH image 1.62 software. The average signal intensity in an area of 180 × 180 μm2 within the parietal cortex was obtained as the fluorescence value of each slice. To reduce variability among different sets of experiments, we normalized the fluorescent value with reference to that obtained with the ‘standard injury'. The standard injury for slice cultures was induced by 24 h treatment with 200 μM NMDA, which nearly, but not exactly, equaled ‘complete injury'. In each set of experiments, one culture well received the standard injury, and the mean fluorescence value obtained from slices in this culture well was set to 100%. Values obtained from slices in other wells that received various treatments were expressed as percentage of the standard injury. We occasionally encountered slices exhibiting deteriorated appearance or robust PI fluorescence before application of insults. These slices were excluded from cell death analysis.
LDH activity in culture medium was measured with Cytotoxicity Detection LDH kit (Kyokuto Pharmaceutical Industrial, Tokyo, Japan). Culture medium (25 μl) was mixed with 25 μl of LDH substrate mixture and 50 μl of 10 mM phosphate-buffered saline in a 96-well plate. After incubation for 1 h at room temperature, the reaction was stopped by addition of 100 μl of 1 M HCl and the absorbance was measured at 570 nm. Each value of absorbance was normalized with that in cultures receiving standard injury (200 μM NMDA for 24 h) as 100%.
Quantification of glutamate released from slice cultures
Amounts of glutamate released during chemical ischemia were measured with Amplex red glutamic acid/glutamate oxidase assay kit (Molecular Probes, Eugene, OR, U.S.A.) as described (Fujimoto et al., 2004). After ischemic treatment, 50 μl of the ischemic conditioning solution in each well was collected and mixed with 50 μl of a reaction buffer containing Amplex red, glutamate oxidase, glutamate pyruvate transaminase, alanine and horseradish peroxidase. After incubation at 37°C, fluorescence of the reaction mixture emitted at 590 nm was measured with a fluorescence microplate reader (FlexStation; Molecular Devices, Sunnyvale, CA, U.S.A.) at an excitation wavelength of 555 nm.
Dissociated cortical culture and cell death assay
Primary cultures of dissociated cortical neurons were prepared from the cerebral cortex of fetal Wistar rats (17–19 days of gestation) derived from 16 mother rats in total, as described previously (Shirakawa et al., 2002). Single cells mechanically dissociated from the whole cerebral cortex were seeded onto 48-well plates coated with polyethylenimine (for cell death assay) or onto glass coverslips coated with polyethylenimine (for Ca2+ measurement) at a density of 4.5 × 105 cells cm−2. Cells were maintained at 37°C in a humidified 5% CO2 atmosphere in Eagle's minimal essential medium (Nissui Pharmaceuticals, Tokyo, Japan) supplemented with glutamine (2 mM), glucose (11 mM in total), NaHCO3 (24 mM), HEPES (10 mM), and 10% heat-inactivated fetal bovine serum (1–7 DIV; JRH Biosciences, Lenexa, KS, U.S.A.) or 10% heat-inactivated horse serum (8–12 DIV; JRH Biosciences). Proliferation of non-neuronal cells was arrested by addition of 10 μM cytosine arabinoside at 6 DIV (Sigma-Aldrich Chemicals).
At 11 DIV, glutamate was added to the medium for 24 h at a final concentration of 300 μM, and cell death was evaluated by LDH release assay as mentioned above for slice culture experiments, except that 15 μl of culture medium was mixed with 30 μl of the LDH substrate mixture and 60 μl of 10 mM phosphate-buffered saline. Cultures treated with 10 mM glutamate for 24 h were used to determine the degree of the standard injury in each set of experiments. Absorbance values were normalized with the absorbance in cultures that received standard injury as 100%.
We also evaluated cell viability by 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyltetrazolium bromide (MTT) assay. Cultured cells were incubated in Eagle's medium containing 0.5 mg ml−1 MTT for 2 h and then solubilized with isopropanol, and the absorbance at 595 nm was measured. Viability was expressed as % of control, by setting the value of control cultures as 100% and the value of cultures receiving the standard injury (10 mM glutamate for 24 h) as 0%.
Measurement of intracellular Ca2+ concentration
Glutamate-induced increases in intracellular Ca2+ concentration ([Ca2+]i) were estimated with a Ca2+-sensitive fluorescent dye, fura-2 acetoxymethyl ester (Dojindo, Kumamoto, Japan), and a fluorescence imaging system (ARGUS-HiSCA, Hamamatsu Photonics, Shizuoka, Japan). Dissociated cortical neurons at 11–12 DIV cultured on a polyethylenimine-coated glass coverslip were incubated in Krebs–Ringer buffer (137 mM NaCl, 5 mM KCl, 1 mM MgCl2, 1.5 mM CaCl2, 10 mM HEPES, 25 mM glucose, pH 7.4) containing 5 μM fura-2 acetoxymethyl ester and 0.01% cremophore EL (Sigma-Aldrich Chemicals) for 30 min at 37°C. After postincubation in fura-2-free Krebs–Ringer buffer for at least 30 min, the coverslip was transferred to a recording chamber settled on the stage of an inverted fluorescence microscope. Fura-2 fluorescence obtained by excitation at wavelengths of 340 and 380 nm was recorded every 3 s at room temperature.
Data presentation and statistical analysis
Data are expressed as mean±s.e.m. Statistical significance of difference was evaluated with one-way ANOVA followed by Student–Newman–Keuls' test or Dunnett's test. Concerning values of glutamate release, two-tail paired t-test was used for evaluation of difference between the group that received ischemia alone and the group that received ischemia plus drug. Probability values less than 5% were considered significant. Values presented in a table and figures concerning Nissl staining (Figures 1b, 2a and 3c) and PI uptake (Table 1, Figures 1c, e, f, 2b, 3a, b, d, 5a and b) in slice cultures as well as LDH release (Figure 4a) and MTT reduction (Figure 4b) in dissociated cultures are from a representative set of experiments. Reproducibility of the results was confirmed in at least two (typically three to four) independent sets of experiments. Values of LDH release in slice cultures (Figure 1d), glutamate-induced [Ca2+]i changes in dissociated cultures (Figure 4d) and glutamate release in slice cultures (Table 1, Figure 5c and d) are combined ones obtained from multiple sets of experiments.
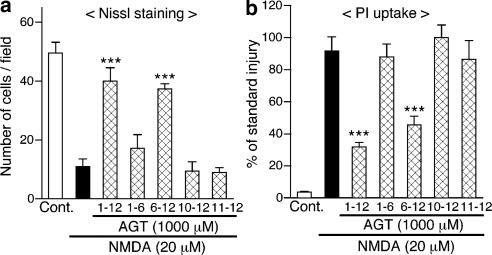
Figure 2.
Long-term pretreatment is required for aminoglutethimide (AGT) to protect neurons from NMDA cytotoxicity. AGT (1000 μM) was applied to cerebrocortical slice cultures for indicated periods (in DIV). NMDA (20 μM) was applied for 24 h at 11 DIV, and cellular injury at 12 DIV was determined by the number of Nissl-stained surviving neurons (a, n=5–6 slices) and intensity of PI fluorescence (b, n=4–6 slices). In (b) each value was normalized with that in slice cultures receiving the standard injury (200 μM NMDA for 24 h) as 100%. ***P<0.001 vs NMDA alone.
Figure 3.
Neuroprotective effect of aminoglutethimide (AGT) is unrelated to steroid biosynthesis. (a, b) Trilostane and exemestane do not protect cortical neurons from NMDA cytotoxicity. Trilostane (a) or exemestane (b) was continuously applied to cerebrocortical slice cultures at indicated concentrations from 1 DIV. NMDA (20 μM) was applied for 24 h at 11 DIV in the presence of trilostane or exemestane, and cellular injury at 12 DIV was determined by intensity of PI fluorescence (n=4–6 slices). (c, d) Supplementation of various steroids does not reverse the neuroprotective effect of AGT. AGT (1000 μM) was applied to cerebrocortical slice cultures from 6 DIV in serum-free medium. Steroids at 10 μM were applied concomitantly with AGT. NMDA (20 μM) was applied for 24 h at 11 DIV in the presence of AGT and steroids, and cellular injury at 12 DIV was determined by the number of Nissl-stained surviving neurons (c, n=6 slices) and intensity of PI fluorescence (d, n=5–6 slices). In (a, b and d), each value was normalized with that in slice cultures receiving the standard injury (200 μM NMDA for 24 h) as 100%. ***P<0.001 vs NMDA alone.
Table 1.
Chemical ischemia-induced cell injury and glutamate release in cerebrocortical slice cultures
| Treatment | PI fluorescence (% of standard injury) | Glutamate (nM) |
|---|---|---|
| Control | 11.3±1.22 | 217.0±19.2 |
| Chemical ischemia (30 min) | 54.7±7.45*** | 898.8±243.1* |
| Chemical ischemia (45 min) | 94.5±4.67*** | 2373.5±280.2** |
| Chemical ischemia (60 min) | 99.3±4.25*** | 4389.3±613.8** |
Cultures were subjected to chemical ischemia for indicated periods, and the intensity of PI fluorescence after 24 h of postincubation (n=6 slices) and the concentration of glutamate released during ischemia (n=4 culture wells) were measured.
P<0.05
P<0.01
P<0.001 vs control.
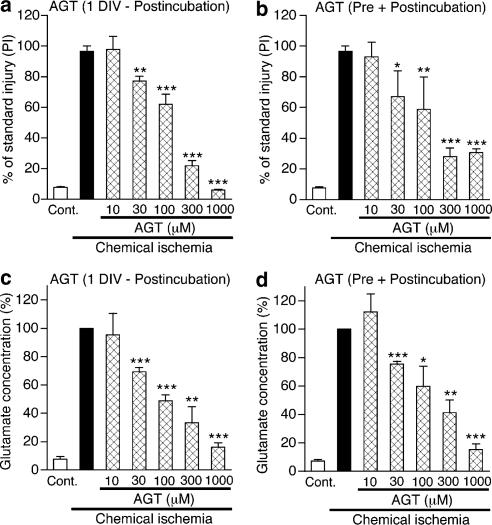
Figure 5.
Aminoglutethimide (AGT) prevents neuronal injury and inhibits glutamate accumulation in cerebrocortical slice cultures that received chemical ischemia. Chemical ischemia was applied to slice cultures for 45 min at 11 DIV. (a, b) Tissue injury was measured by PI fluorescence after 24 h of postincubation. AGT at indicated concentrations was applied to slice cultures from 1 DIV (a) or from 1 h before ischemic treatment (b). Each value was normalized with that in slice cultures receiving the standard injury (200 μM NMDA for 24 h) as 100%. n=6–28 slices. (c, d) Glutamate concentrations in conditioning solution used for 45 min ischemia were measured. AGT at indicated concentrations was applied to slice cultures continuously from 1 DIV (c) or from 1 h before ischemic treatment (d). Values are expressed as percentage of glutamate concentration, with the value in cultures that received chemical ischemia alone at 100%. n=5–13 culture wells. *P<0.05, **P<0.01, ***P<0.001 vs chemical ischemia alone.
Figure 4.
Long-term treatment with aminoglutethimide (AGT) protects dissociated cortical neurons from glutamate cytotoxicity, but has little effect on glutamate-induced increase in [Ca2+]i. (a, b) Cultured cortical neurons were continuously treated with AGT at indicated concentrations from 2 DIV. Glutamate (300 μM) was applied for 24 h at 11 DIV in the presence of AGT, and cell viability at 12 DIV was determined by amount of LDH released into medium (a, n=6 coverslips) and cellular activity of MTT reduction (b, n=6 coverslips). *P<0.05, **P<0.01 vs glutamate alone. Each value of absorbance in LDH release assay was normalized with that in dissociated cultures receiving the standard injury (10 mM glutamate for 24 h) as 100%. The viability in MTT assay was expressed as percentage of control, by setting the value of control cultures as 100% and the value of cultures receiving the standard injury (10 mM glutamate for 24 h) as 0%. (c) Glutamate-induced increase in [Ca2+]i was monitored by fura-2 fluorescence in neurons cultured under control conditions (left) and cultured in the continuous presence of 1000 μM AGT (right). Glutamate (300 μM) was applied during the period indicated by a black bar. Values represent the average value of fluorescence ratio obtained from 63 cells in one coverslip. (d) Summary of data on glutamate-induced [Ca2+]i increase. AGT at indicated concentrations was applied to cortical neurons from 2 DIV. At 11–12 DIV, changes in [Ca2+]i induced by 300 μM glutamate were measured. Values are mean+s.e.m. of the peak amplitude of [Ca2+]i responses obtained from 5–9 coverslips, the average of values of the control group being set at 100%.
Results
Aminoglutethimide protects cortical neurons in slice culture from excitotoxicity
Application of aminoglutethimide to cerebrocortical slice cultures from 1 DIV for 11 days, at concentrations up to 1000 μM, did not cause any changes in the number of Nissl-stained viable cortical neurons, indicating that the drug itself was devoid of cytotoxic activity. NMDA (20 μM) applied to control cultures at 11 DIV for 24 h caused a marked decrease in the number of surviving neurons, as assessed by Nissl staining. This decrease in cell viability was accompanied by robust increases in PI fluorescence of slices and in the amount of LDH released into the culture medium. As shown in Figure 1b, continuous treatment with aminoglutethimide from 1 DIV prevented NMDA cytotoxicity in a concentration-dependent manner. Judged from the number of Nissl-stained cells, significant neuroprotection was obtained with aminoglutethimide at concentrations of 300 μM and higher. The protective effect was confirmed by measurement of PI fluorescence (Figure 1c) and LDH release (Figure 1d).
We next examined whether aminoglutethimide could afford neuroprotection against insults other than NMDA. Long-term treatment with 1000 μM aminoglutethimide inhibited neuronal injury induced by 24 h application of 40 μM AMPA, an excitotoxin acting on non-NMDA subtype of glutamate receptors. The protective effect was confirmed by Nissl staining (data not shown) and PI uptake (Figure 1e). Neuroprotective effect of 1000 μM aminoglutethimide was also observed against cytotoxicity of 40 μM kainate, another non-NMDA receptor agonist (data not shown). On the other hand, neuronal injury induced by 24 h application of a Ca2+ ionophore ionomycin (6 μM) was not affected by aminoglutethimide (Figure 1f). Long-term treatment with aminoglutethimide was also without effect against cell death induced by 24 h application of 6 μM staurosporine (data not shown).
To verify whether or not a prolonged treatment was required for neuroprotection, we varied the periods of application of aminoglutethimide (Figure 2). Treatment with 1000 μM aminoglutethimide for 6 days from 6 DIV suppressed neuronal injury induced by treatment with 20 μM NMDA at 11 DIV. The degree of protection was comparable to that obtained by continuous treatment with 1000 μM aminoglutethimide from 1 DIV. In contrast, treatment with aminoglutethimide during the first half of the cultivation period, that is, from 1 to 6 DIV, did not provide a significant effect. Neither a shorter period of pretreatment from 10 DIV nor a simultaneous treatment at 11 DIV with aminoglutethimide suppressed NMDA cytotoxicity. The results were confirmed by counting of Nissl-stained surviving cells (Figure 2a) and measurement of PI fluorescence (Figure 2b).
Neuroprotective effect of aminoglutethimide is unrelated to inhibition of steroid synthesis
To evaluate whether the neuroprotective effect of aminoglutethimide is mediated by its effect on neurosteroid biosynthesis, we performed additional pharmacological examinations. First, two drugs that inhibit steroidogenic pathways were tested for their potential neuroprotective effects. Trilostane competitively inhibits 3β-hydroxysteroid dehydrogenase, an enzyme involved in the conversion of pregnenolone, 17-hydroxypregnenolone and dehydroepiandrosterone into progesterone, 17-hydroxyprogesterone and Δ4-androsten-3,17-dione, respectively (Robel et al., 1995). The IC50 value of trilostane on the enzyme activity has been reported to be 4.06 μM, when 1 μM pregnenolone is used as a substrate (Coirini et al., 2003). Exemestane is a selective inhibitor of aromatase with a Ki value of 4.3 nM (Di Salle et al., 1994; Miller et al., 2003), therefore mimics part of the properties of aminoglutethimide as an enzyme inhibitor. Trilostane (3–30 μM) and exemestane (1–10 μM) were applied to cortical slice cultures continuously from 1 DIV, and NMDA cytotoxicity was examined at 11 DIV in the presence of these drugs. Neither of these drugs produced a significant effect on cell death induced by 20 μM NMDA, as assessed by Nissl staining (data not shown) and PI fluorescence (Figure 3a and b). We could not test the effect of these drugs at higher concentrations because of their cytotoxicity.
Second, we examined whether the neuroprotective effect of aminoglutethimide against NMDA cytotoxicity could be reversed by application of neurosteroids. Aminoglutethimide was applied to slice cultures from 6 DIV, simultaneously with 10 μM of pregnenolone, estrone, 17β-estradiol or estriol. To avoid any influences caused by steroidal compounds contained in serum, serum-free medium was used throughout the period of drug treatment. In these settings, aminoglutethimide again prevented neuronal death induced by 24 h application of 20 μM NMDA. However, neither of the steroids provided a significant influence on the neuroprotective effect of aminoglutethimide, as assessed by Nissl staining (Figure 3c) and PI fluorescence (Figure 3d). These results do not support the proposal that neuroprotection by aminoglutethimide is mediated by depletion of neurosteroids from the cortical tissue.
Bastida et al. (2001) have reported that aminoglutethimide may act as an inhibitor of protein kinase A. Therefore, we examined the effect of KT5720, a selective protein kinase A inhibitor. However, long-term treatment with 0.1–1 μM KT5720 did not produce a significant effect on NMDA cytotoxicity (data not shown).
Effects of aminoglutethimide on glutamate cytotoxicity and glutamate-induced Ca2+ increase in dissociated cortical neurons
A possible mechanism of neuroprotection by aminoglutethimide is that the drug may somehow inhibit Ca2+ influx through glutamate receptor-associated channels, thereby prevent [Ca2+]i from reaching cytotoxic levels. Accordingly, we examined effect of aminoglutethimide on glutamate-induced increases in [Ca2+]i in cortical neurons. For this purpose, we used dissociated cortical neurons instead of cortical slice cultures, because estimation of [Ca2+]i in slice culture preparations was difficult. Before the experiments on [Ca2+]i, we did cytotoxicity experiments to confirm that aminoglutethimide was also neuroprotective in dissociated cortical cell cultures. Application of 300 μM glutamate for 24 h resulted in neuronal death indicated by an increase in LDH release and a decrease in activity of MTT reduction. Glutamate cytotoxicity under these conditions was abolished by 10 μM MK-801, an NMDA receptor antagonist (data not shown). Continuous treatment of cortical neurons with aminoglutethimide (10–1000 μM) from 2 DIV to the end of the culture period resulted in a concentration-dependent inhibition of glutamate neurotoxicity, as assessed by LDH release assay (Figure 4a) and MTT assay (Figure 4b). The effect of aminoglutethimide was not reversed by concurrent application of 1 μM pregnenolone (data not shown), suggesting again that inhibition of steroidogenesis was not responsible for neuroprotection.
Dissociated cortical neurons that received long-term treatment with 10–1000 μM aminoglutethimide were subjected to measurement of [Ca2+]i. In sham-treated neurons, glutamate (300 μM) induced a robust [Ca2+]i increase that decayed gradually during 9 min of observation period. Neurons treated continuously with aminoglutethimide also responded to glutamate with an increase in [Ca2+]i (Figure 4c). The peak levels of [Ca2+]i increase (Figure 4d) as well as the [Ca2+]i levels at 9 min after the onset of glutamate application (data not shown) did not significantly differ among different treatment groups. Therefore, inhibition of Ca2+ influx is unlikely to mediate the neuroprotective effect of aminoglutethimide.
Aminoglutethimide inhibits glutamate release and neuronal injury induced by ischemia in cortical slice culture
Finally, we examined effects of aminoglutethimide on ischemia-induced neuronal injury. Simulated ischemia was achieved by incubation of cortical slice cultures with glucose-free Ringer's buffer containing sodium azide and 2-deoxyglucose (Katsuki et al., 2005). This treatment resulted in extracellular accumulation of glutamate during the treatment, and neuronal damage at 24 h after the treatment (Table 1). Both consequences were increasingly apparent with longer periods of chemical ischemia. In the following experiments, 45 min was adopted as the period of ischemic treatment. Application of 1 μM MK-801 during and after chemical ischemia almost completely protected cortical neurons (data not shown). Figure 5a shows that continuous treatment of cortical slice cultures with aminoglutethimide from 1 DIV suppressed ischemic neuronal injury. The protective effect of aminoglutethimide was in a concentration-dependent manner, which was significant at or over 30 μM and was almost complete at 1000 μM. Importantly, we found that prolonged treatment was not required for the protective effect. Application of aminoglutethimide from 1 h before chemical ischemia also suppressed neuronal injury (Figure 5b). The range of effective concentrations of aminoglutethimide in this setting (at or over 30 μM) was the same as that in long-term treatment.
Because glutamate cytotoxicity is central to ischemic neuronal injury, we examined whether aminoglutethimide produced any influences on extracellular glutamate accumulation during ischemic insult. We found that long-term treatment of slice cultures with aminoglutethimide (30–1000 μM) from 1 DIV markedly inhibited ischemia-induced glutamate accumulation in a concentration-dependent manner (Figure 5c). The degree of inhibition of glutamate accumulation by various concentrations of aminoglutethimide paralleled the degree of neuroprotection. Moreover, application of aminoglutethimide from 1 h before chemical ischemia was as effective as long-term treatment of the drug in suppressing glutamate accumulation (Figure 5d).
Discussion
The initial purpose of the present study was to verify potential roles of endogenous neurosteroids in regulation of excitotoxic neuronal injury in the cerebral cortex. We supposed that aminoglutethimide would be a useful pharmacological tool to address this issue, because the drug was expected to inhibit biosynthesis of neurosteroids by inhibiting cytochrome P450scc. We found that aminoglutethimide produced potent neuroprotective effects in two paradigms of neuronal injury. First, long-term treatment of cortical slice cultures with aminoglutethimide attenuated NMDA neurotoxicity. The unexpected finding was that supplementation of various steroids did not reverse the neuroprotective effect of aminoglutethimide. In particular, the ineffectiveness of pregnenolone, the immediate product of cytochrome P450scc, indicates that the observed effect of aminoglutethimide is not related to inhibition of neurosteroid biosynthesis. Second, aminoglutethimide prevented ischemic neuronal injury. Although we did not examine the consequences of steroid supplementation in this paradigm, depletion of endogenous neurosteroids is unlikely to mediate neuroprotection by aminoglutethimide, because the drug effectively blocked ischemia-induced accumulation of extracellular glutamate and neuronal injury with only 1 h of pretreatment. Aminoglutethimide has been shown to attenuate excitotoxicity in the retina, but in this case, the effect is mediated by reduction of de novo biosynthesis of pregnenolone sulfate (Guarneri et al., 1998). Steroidogenesis-independent neuroprotective actions of aminoglutethimide are unprecedented findings of the present study.
Several days of treatment were required for the protective actions of aminoglutethimide against NMDA neurotoxicity. In addition, long-term treatment with aminoglutethimide prevented neuronal death induced by excitotoxins including NMDA, AMPA and kainate, but had no effect against other cytotoxic agents such as ionomycin and staurosporine. Hence, a plausible mechanism of neuroprotection was that aminoglutethimide might decrease the number, or suppress the function, of ionotropic glutamate receptors, thereby attenuate excess Ca2+ entry during excitotoxic insults. However, we did not observe a significant effect of aminoglutethimide on glutamate-induced increase in [Ca2+]i. The results indicate that neuroprotective actions of aminoglutethimide may involve some cellular events downstream of glutamate receptor activation. Screening of genes and proteins whose expression levels are modified by long-term treatment with aminoglutethimide may provide useful information concerning the molecular targets mediating neuroprotection.
Our results in the present study do not necessarily exclude the roles of endogenous neurosteroids in regulation of neuronal injury. Substantial lines of evidence suggest that neurosteroids influence excitotoxic consequences in CNS neurons. For example, dehydroepiandrosterone and its sulfate protect hippocampal neurons from excitotoxic insults (Kimonides et al., 1998; Kurata et al., 2004). Pregnenolone sulfate augments, whereas pregnanolone sulfate inhibits, NMDA receptor-mediated Ca2+ influx and resultant cytotoxicity (Weaver et al., 1998; Shirakawa et al., 2002). Because aminoglutethimide is likely to prevent excitotoxic injury through the mechanisms downstream of glutamate receptor activation, the effect of steroid depletion on neuronal death may well be occluded or masked by steroid-independent actions of aminoglutethimide. Alternative methods like specific knockdown of cytochrome P450scc expression may be required to evaluate the net effects of depletion of endogenous neurosteroids.
In contrast to the effect on NMDA cytotoxicity, the neuroprotective effect of aminoglutethimide against ischemic neuronal injury was evident after a brief period of pretreatment. Because the degree of protection paralleled the decrease in the levels of glutamate accumulation during ischemic insult, we suggest that inhibition of aberrant release of glutamate is responsible for the effect of this drug against ischemic neuronal injury. Detailed mechanisms of inhibition of glutamate release by aminoglutethimide remain an open question. Various routes including exocytosis, reversed glutamate transport and efflux through volume-sensitive anion channels have been implicated in glutamate release from neurons and glia during ischemia (Nedergaard et al., 2002). A nearly complete blockade of extracellular glutamate accumulation by 1000 μM aminoglutethimide, in spite of the presence of multiple routes of glutamate release, implies that the drug interferes with cellular events upstream of glutamate release, rather than individual release machineries.
In conclusion, we report here that aminoglutethimide exerted novel neuroprotective actions against excitotoxic and ischemic injuries. The mechanisms of actions do not involve any known pharmacological properties of this drug and should be addressed in further investigations. Aminoglutethimide has been used for therapies of hormone-dependent malignant tumors in the United States (Miller et al., 1987; Alshowaier et al., 1999). Daily dose of aminoglutethimide is 250–1000 mg, and serum concentration of this drug in patients receiving 1000 mg dosing has been reported to be 9.0 μg ml−1 (38.7 μM; Miller et al., 1987). In addition, Unger et al. (1986) demonstrated accumulation of [14C]-aminoglutethimide in the brain after systemic administration in rats. Development of aminoglutethimide analogs that retain neuroprotective properties but are devoid of actions on steroidogenesis may be useful for therapies against various neurodegenerative disorders.
Acknowledgments
This study was supported in part by Grant-in-aid for Scientific Research from The Ministry of Education, Culture, Sports, Science and Technology, Japan and from Japan Society for the Promotion of Science. H.S. was supported by 21st Century COE Program ‘Knowledge Information Infrastructure for Genome Science'.
Abbreviations
- [Ca2+]i
intracellular Ca2+ concentration
- DIV
days in vitro
- LDH
lactate dehydrogenase
- MTT
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyltetrazolium bromide
- PI
propidium iodide
References
- ALSHOWAIER I.A., EL-YAZIGI A., EZZAT A., ABDEL-WARITH A., NICOLLS P.J. Pharmacokinetics of S- and R-enantiomers of aminoglutethimide following oral administration of racemic drug in breast cancer patients. J. Clin. Pharmacol. 1999;39:1136–1142. [PubMed] [Google Scholar]
- BASTIDA C.M., TEJADA F., CREMADES A., PANAFIEL R. Aminoglutethimide, a steroidogenesis inhibitor, abolishes hormonal induction of ornithine decarboxylase in steroidogenic tissues: evidence for its role as cAMP-dependent protein kinase inhibitor. Biochem. Biophys. Res. Commun. 2001;281:244–248. doi: 10.1006/bbrc.2001.4332. [DOI] [PubMed] [Google Scholar]
- COIRINI H., GOUEZOU M., DELESPIERRE B., LIERE P., PIANOS A., EYCHENNE B., SCHUMACHER M., GUENNOUN R. Characterization and regulation of the 3β-hydroxysteroid dehydrogenase isomerase enzyme in the rat sciatic nerve. J. Neurochem. 2003;84:119–126. doi: 10.1046/j.1471-4159.2003.01512.x. [DOI] [PubMed] [Google Scholar]
- DI SALLE E., BRIATICO G., GIUDICI D., ORNATI G., ZACCHEO T., BUZZETTI F., NESI M., PANZERI A. Novel aromatase and 5α-reductase inhibitors. J. Steroid Biochem. Mol. Biol. 1994;49:289–294. doi: 10.1016/0960-0760(94)90270-4. [DOI] [PubMed] [Google Scholar]
- FOSTER A.B., JARMAN M., LEUNG C.S., ROWLANDS M.G., TAYLOR G.N. Analogues of aminoglutethimide: selective inhibition of cholesterol side-chain cleavage. J. Med. Chem. 1983;26:50–54. doi: 10.1021/jm00355a011. [DOI] [PubMed] [Google Scholar]
- FUJIMOTO S., KATSUKI H., KUME T., KANEKO S., AKAIKE A. Mechanisms of oxygen glucose deprivation-induced glutamate release from cerebrocortical slice cultures. Neurosci. Res. 2004;50:179–187. doi: 10.1016/j.neures.2004.06.013. [DOI] [PubMed] [Google Scholar]
- GUARNERI P., RUSSO D., CASCIO C., DE LEO G., PICCOLI F., GUARNERI R. Induction of neurosteroid synthesis by NMDA receptors in isolated rat retina: a potential early event in excitotoxicity. Eur. J. Neurosci. 1998;10:1752–1763. doi: 10.1046/j.1460-9568.1998.00191.x. [DOI] [PubMed] [Google Scholar]
- HOLMES G.L. Seizure-induced neuronal injury: animal data. Neurology. 2002;59:S3–S6. doi: 10.1212/wnl.59.9_suppl_5.s3. [DOI] [PubMed] [Google Scholar]
- IRWIN R.P., LIN S.-Z., ROGAWSKI M.A., PURDY R.H., PAUL S.M. Steroid potentiation and inhibition of N-methyl-D-aspartate receptor-mediated intracellular Ca2+ responses: structure–activity studies. J. Pharmacol. Exp. Ther. 1994;271:677–682. [PubMed] [Google Scholar]
- KATSUKI H., SHINOHARA A., FUJIMOTO S., KUME T., AKAIKE A. Tetraethylammonium exacerbates ischemic neuronal injury in rat cerebrocortical slice cultures. Eur. J. Pharmacol. 2005;508:85–91. doi: 10.1016/j.ejphar.2004.11.058. [DOI] [PubMed] [Google Scholar]
- KIMONIDES V.G., KHATIBI N.H., SVENDSEN C.N., SOFRONIEW M.V., HERBERT J. Dehydroepiandrosterone (DHEA) and DHEA-sulfate (DHEAS) protect hippocampal neurons against excitatory amino acid-induced neurotoxicity. Proc. Natl. Acad. Sci. U.S.A. 1998;95:1852–1857. doi: 10.1073/pnas.95.4.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KURATA K., TAKEBAYASHI M., MORINOBU S., YAMAWAKI S. Estradiol, dehydroepiandrosterone, and dehydroepiandrosterone sulfate protect against N-methyl-D-aspartate-induced neurotoxicity in rat hippocampal neurons by different mechanisms. J. Pharmacol. Exp. Ther. 2004;311:237–245. doi: 10.1124/jpet.104.067629. [DOI] [PubMed] [Google Scholar]
- MARAGAKIS N.J., ROTHSTEIN J.D. Glutamate transporters: animal models to neurologic disease. Neurobiol. Dis. 2004;15:461–473. doi: 10.1016/j.nbd.2003.12.007. [DOI] [PubMed] [Google Scholar]
- MATTSON M.P. Excitotoxic and excitoprotective mechanisms: abundant targets for the prevention and treatment of neurodegenerative disorders. Neuromolecular Med. 2003;3:65–94. doi: 10.1385/NMM:3:2:65. [DOI] [PubMed] [Google Scholar]
- MELDRUM B., GARTHWAITE J. Excitatory amino acid neurotoxicity and neurodegenerative disease. Trends Pharmacol. Sci. 1990;11:379–387. doi: 10.1016/0165-6147(90)90184-a. [DOI] [PubMed] [Google Scholar]
- MELLON S.H., GRIFFIN L.D. Neurosteroids: biochemistry and clinical significance. Trends Endocrinol. Metab. 2002;13:35–43. doi: 10.1016/s1043-2760(01)00503-3. [DOI] [PubMed] [Google Scholar]
- MILLER A.A., MILLER B.E., HOFFKEN K., SCHMIDT C.G. Clinical pharmacology of aminoglutethimide in patients with metastatic breast cancer. Cancer Chemother. Pharmacol. 1987;20:337–341. doi: 10.1007/BF00262588. [DOI] [PubMed] [Google Scholar]
- MILLER W.R., ANDERSON T.J., EVANS D.B., KRAUSE A., HAMPTON G., DIXON J.M. An integrated view of aromatase and its inhibition. J. Steroid Biochem. Mol. Biol. 2003;86:413–421. doi: 10.1016/s0960-0760(03)00352-2. [DOI] [PubMed] [Google Scholar]
- NEDERGAARD M., TAKANO T., HANSEN A.J. Beyond the role of glutamate as a neurotransmitter. Nat. Rev. Neurosci. 2002;3:748–755. doi: 10.1038/nrn916. [DOI] [PubMed] [Google Scholar]
- NISHIZAWA Y. Glutamate release and neuronal damage in ischemia. Life Sci. 2001;69:369–381. doi: 10.1016/s0024-3205(01)01142-0. [DOI] [PubMed] [Google Scholar]
- PARK-CHUNG M., WU F.-S., PURDY R.H., MALAYEV A.A., GIBBS T.T., FARB D.H. Distinct sites for inverse modulation of N-methyl-D-aspartate receptor by sulfated steroids. Mol. Pharmacol. 1997;52:1113–1123. doi: 10.1124/mol.52.6.1113. [DOI] [PubMed] [Google Scholar]
- REDDY D.S. Pharmacology of endogenous neuroactive steroids. Crit. Rev. Neurobiol. 2003;15:197–234. doi: 10.1615/critrevneurobiol.v15.i34.20. [DOI] [PubMed] [Google Scholar]
- ROBEL P., YOUNG J., CORPECHOT C., MAYO W., PERCHE F., HAUG M., SIMON H., BAULIEU E.E. Biosynthesis and assay of neurosteroids in rats and mice: functional correlates. J. Steroid Biochem. Mol. Biol. 1995;53:355–360. doi: 10.1016/0960-0760(95)00074-a. [DOI] [PubMed] [Google Scholar]
- SALHANICK H.A. Basic studies on aminoglutethimide. Cancer Res. 1982;42:3315s–3321s. [PubMed] [Google Scholar]
- SHIRAKAWA H., KATSUKI H., KUME T., KANEKO S., AKAIKE A. Pregnenolone sulphate attenuates AMPA cytotoxicity on rat cortical neurons. Eur. J. Neurosci. 2005;21:2329–2335. doi: 10.1111/j.1460-9568.2005.04079.x. [DOI] [PubMed] [Google Scholar]
- SHIRAKAWA H., KATSUKI H., KUME T., KANEKO S., ITO J., AKAIKE A. Regulation of N-methyl-D-aspartate cytotoxicity by neuroactive steroids in rat cortical neurons. Eur. J. Pharmacol. 2002;454:165–175. doi: 10.1016/s0014-2999(02)02493-7. [DOI] [PubMed] [Google Scholar]
- UNGER C., EIBL H., VON HEYDEN H.W., KIM D.J., NAGEL G.A. Aminoglutethimide. Penetration of the blood brain barrier. Invest. New Drugs. 1986;4:237–240. doi: 10.1007/BF00179589. [DOI] [PubMed] [Google Scholar]
- VEIGA S., GARCIA-SEGRA L.M., AZCOITIA I. Neuroprotection by the steroids pregnenolone and dehydroepiandrosterone is mediated by the enzyme aromatase. J. Neurobiol. 2003;56:398–406. doi: 10.1002/neu.10249. [DOI] [PubMed] [Google Scholar]
- WEAVER C.E., LAND M.B., PURDY R.H., RICHARDS K.G., GIBBS T.T., FARB D.H. Geometry and charge determine pharmacological effects of steroids on N-methyl-D-aspartate receptor-induced Ca2+ accumulation and cell death. J. Pharmacol. Exp. Ther. 2000;293:747–754. [PubMed] [Google Scholar]
- WEAVER C.E., MAREK P., PARK-CHUNG M., TAM S.W., FARB D.H. Neuroprotective activity of a new class of steroidal inhibitors of the N-methyl-D-aspartate receptor. Proc. Natl. Acad. Sci. U.S.A. 1997;92:10450–10454. doi: 10.1073/pnas.94.19.10450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEAVER C.E., JR, WU F.S., GIBBS T.T., FARB D.H. Pregnenolone sulfate exacerbates NMDA-induced death of hippocampal neurons. Brain Res. 1998;803:129–136. doi: 10.1016/s0006-8993(98)00640-4. [DOI] [PubMed] [Google Scholar]
- WU F.S., GIBBS T.T., FARB D.H. Pregnenolone sulfate: a positive allosteric modulator at the N-methyl-D-aspartate receptor. Mol. Pharmacol. 1991;40:4333–4336. [PubMed] [Google Scholar]
- YAGHOUBI N., MALAYEV A., RUSSEK S.J., GIBBS T.T., FARB D.H. Neurosteroid modulation of recombinant ionotropic glutamate receptors. Brain Res. 1998;803:153–160. doi: 10.1016/s0006-8993(98)00644-1. [DOI] [PubMed] [Google Scholar]
- ZWAIN I.H., YEN S.S. Neurosteroidogenesis in astrocytes, oligodendrocytes, and neurons of cerebral cortex of rat brain. Endocrinology. 1999;140:3843–3852. doi: 10.1210/endo.140.8.6907. [DOI] [PubMed] [Google Scholar]