Abstract
The porphyrin precursor 5-aminolevulinic acid (ALA) is being widely used in photodynamic therapy of cancer. Improvement in ALA delivery has been sought through the use of ALA derivatives, in particular the esterification of ALA with aliphatic alcohols, which in certain cases can improve cellular penetration and selectivity.
ALA uptake systems appear to be distinctive for each cell type. The LM3 mammary adenocarcinoma cell line takes ALA up by BETA transporters. In this work, we investigated ALA derivative transport systems through the inhibition of radiolabelled ALA uptake in the LM3 cells. We also performed inhibition studies of γ-aminobutyric acid (GABA) uptake.
The more lipohilic ALA derivatives hexyl-ALA and undecanoyl-ALA inhibit ALA uptake, whereas methyl-ALA, R, S-ALA-2-(hydroxymethyl)tetrahydropyranyl ester and the dendron aminomethane tris methyl 5-ALA does not inhibit ALA uptake. A similar pattern was found for GABA, except that the dendron inhibited GABA uptake. However, hexyl-ALA and undecanoyl-ALA are not taken up by BETA transporters, but by simple diffusion, although they still inhibit ALA uptake by binding to the cell membrane.
These results show that different modifications to the ALA molecule lead to different uptake mechanisms. Whereas ALA is taken up by BETA transporters, none of the ALA derivatives shares the same mechanism. Knowledge of the mechanisms of ALA derivatives entry into the cells is essential to understand and improve ALA-mediated PDT and to the design of new ALA derivatives that may be taken up at a higher rate than ALA.
Keywords: Photodynamic therapy, aminolevulinic acid, ALA esters, dendron, uptake, cells
Introduction
Photodynamic therapy (PDT) of cancer is based on the administration of a photosensitising compound with tumour-localising properties, and subsequent irradiation with light of an appropriate wavelength leading to selective damage to the treated tissue (Dougherty, 1984).
The use of endogenous protoporphyrin IX (PpIX) generated through the heme biosynthetic pathway after the administration of 5-aminolevulinic acid (ALA) has led to many applications in PDT. ALA is frequently administered topically or systemically for PDT of several tumours (Kelty et al., 2002). ALA-induced PpIX accumulation has been shown to be preferentially greater in certain tumoral cells primarily due to the reduced activity of ferrochelatase, the enzyme responsible for the conversion of PpIX into haeme (Van Hillesberg et al., 1992) and a relative enhancement of deaminase activity (Navone et al., 1991).
Clinical advantages of this approach include rapid clearance from tissue resulting in short-lived cutaneous photosensitivity, the choice of either topical or systemic administration of ALA and selective epithelial photosensitisation rendering it suitable for the treatment of early tumours (Kennedy et al., 1990; Jenkins et al., 1998; Krzemien et al., 1999).
ALA has also a great potential as a photodiagnostic or photodetection agent in clinical practice. The use of ALA-induced fluorescence is being exploited for diagnosis of bladder cancer, endometriosis, intraepithelial lesions of the cervix and lung cancer. The technique may also be useful in identifying tumour margins during surgery. This has already been used in breast and brain cancer (Kelty et al., 2002).
Recently, several reports about ALA uptake systems have been published and these systems appear to be distinctive for each cell type. Thus, some authors postulated that ALA is taken up through the di- and tri-peptide transporters PEPT1 and PEPT2 in pancreatoma cells and in yeasts transfected with the intestinal and renal transporters (Döring et al., 1998; Whitaker et al., 2000). Neuman & Brandsch (2003) reported that ALA transport in cholangiocarcinoma cells is mediated by PEPT1 system. Other authors suggested that BETA transporters are involved in ALA transport in colon adenocarcinoma cells (Rud et al., 2000).
In addition, transport of ALA from blood to brain appears to be a diffusional process (Ennis et al., 2003), whereas blood to choroid plexus is carrier mediated via PEPT2 and an organic transporter (Novotny et al., 2000). Renal tubular transport is also mediated by an organic anion transporter (Cheeks & Wedeen, 1986).
We found that in the mammary adenocarcinoma LM3 line, ALA shared with γ-aminobutyric acid (GABA) the same mechanism of transport. In these cells, ALA is taken up by a BETA transporter, probably GAT-2 (Bermúdez Moretti et al., 2002).
The hydrophilic nature of ALA may limit its ability to penetrate through skin or cell membranes, and thereby restricts the use of ALA-PDT to the treatment of superficial diseases. As a result, derivatives of ALA, which are less hydrophilic than the parental compound, are under investigation as possible alternatives to ALA (Kloek & Beijersbergen van Henegouwen, 1996; Gaullier et al., 1997; Casas & Batlle, 2002).
The hexyl ester of ALA improved the detection rates of both flat and papillary lesions in the imaging of bladder tumours (Schmidbauer et al., 2004) and has recently been approved for that purpose in 26 European countries. In addition, the methyl ester of ALA has been approved for certain nonmelanoma skin cancers in the European Union and for actinic keratoses by the FDA of U.S.A. (Gardlo & Ruzicka, 2002).
We recently found that porphyrin synthesis in the adenocarcinoma LM3 cells as a function of prodrug concentration is different for ALA and ALA derivatives, and that the most efficient ester is hexyl-ALA (Perotti et al., 2004). This suggests that ALA and some ALA esters may have different mechanisms of uptake into the cells.
The aim of this work was to study ALA derivative transport systems through the inhibition of the uptake of radiolabelled ALA and GABA in the LM3 mammary adenocarcinoma line, which takes ALA up by BETA transporters. Afterwards, we further characterised the nature of the inhibition by determining whether the derivatives either block or use BETA transporters for translocation to the cytoplasm of the cells.
Knowledge of the mechanisms of ALA derivatives entry into the cells is essential to understand and improve ALA-mediated PDT. Moreover, it is important in the design of new ALA derivatives that may be taken up at a higher rate than ALA and with higher selectivity for tumour cells.
Methods
Cell line and cell culture
LM3 cell line (Werbajh et al., 1998) derived from the murine mammary adenocarcinoma M3 was cultured in minimum essential Eagle's medium, supplemented with 2 mM L-glutamine, 40 μg gentamycin ml−1 and 5% foetal bovine serum, and incubated at 37°C in an atmosphere containing 5% CO2. The number of cells seeded per well and the cell number employed for the normalisations were determined by counting viable cells with the Trypan blue exclusion method.
Chemicals
[4-14C]-ALA hydrochloride and [14C(U)]-GABA were obtained from American Radiolabeled Chemicals Inc. (St Louis, U.S.A.). [1-4-14C]-Succinic acid was obtained from Comisión Nacional de Energía Atómica (Argentina). ALA, GABA, methyl-ALA (Me-ALA), 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazoliumbromide (MTT), L-alanine, glycil–glycine, glycil–glycil–alanine, cefadroxil, muscimol, baclofen and hypotaurine were obtained from Sigma Chemical Co. (St Louis, Mo, U.S.A.). (±) Nipecotic acid and isonipecotic acid were obtained from Aldrich Chemical Co., (Wisconsin, U.S.A.). Other chemicals were of analytical grade.
ALA derivatives were obtained as the hydrochloride salts. Hexylester-ALA (He-ALA) and Und-ALA were synthesised according to the method of Takeya (1992) by reacting ALA with hexanol and undecanol, respectively, in the presence of thionyl chloride. The mixture was stirred at 70°C until ALA. HCl was completely dissolved and the reaction was confirmed by TLC (Cl2CH2 : MeOH mixtures). The excess solvent was evaporated under high vacuum. After the addition of diethylether, the HCl salts of the ALA esters were allowed to crystallise at 4°C. Yields ranged from 60 to 40%.
R, S-ALA-2-(hydroxymethyl)tetrahydropyranyl ester (THP-ALA) was similarly prepared. The crude product was purified by flash column chromatography on silica gel eluting with Cl2CH2 : MeOH mixtures. The yield was about 20%.
The dendron aminomethane tris methyl 5-ALA (3m-ALA), containing three ALA residues, was prepared as the trifluoroacetic acid (TFA) salt. Tris(tert-butyloxycarbonyl-5-ALA) methylamine was synthesised according to previously published literature (Battah et al., 2001) and dissolved in dichloromethane. TFA was added to the solution and the mixture was stirred at room temperature for 4 h. The solvents were evaporated under reduced pressure to yield an oily product dissolved in methanol and diethylether was added under argon until the product was precipitated; the solvents were decanted and the residue was dried in vacuo to give the final product 3m-ALA.
Purities of the synthesised compounds were always higher than 95%, as established by TLC and NMR techniques. ALA derivatives were dissolved in water just before use, employing stock solutions of 12.5 mM. The addition of either ALA or ALA derivatives to the cells did not change the pH of the medium.
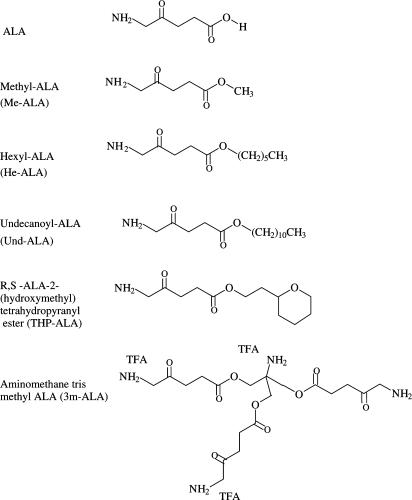
The structures of the ALA derivatives are shown in Figure 1.
Figure 1.
Structures of ALA and ALA derivatives.
ALA and GABA preparations
For cell uptake experiments, unlabelled ALA or GABA were dissolved in 0.1% glucose in phosphate-buffered saline (PBS) and [14C]-ALA or [14C]-GABA were added so that the final solution contained a radioactive content of 0.0185 MBq ml−1 and an ALA or GABA concentration of 0.1 mM.
Cell uptake of ALA and GABA in the presence of putative inhibitors
Uptake measurements were performed in 24-well plates, 72 h after plating, when cells were nearly confluent. Cells were washed twice with 0.5 ml of 0.1% glucose–PBS (glucose was added to the PBS solutions as an energy source for active transport) preheated at 37°C and preincubated 15 min with the putative inhibitors to assure previous binding to their transporters. The final concentrations of the inhibitors were: 2 mM for ALA, GABA, Me-ALA, THP-ALA and L-alanine; 1.2 mM for 3m-ALA, He-ALA and hypotaurine; and 0.45 mM for Und-ALA. The conditions were chosen according to their maximal nontoxic concentrations. Afterwards, 0.1 mM radiolabelled ALA or GABA preheated at 37°C (0.15 μCi) was added. After 30 min of incubation at 37°C, the uptake was stopped by washing the cells four times with 0.5 ml of ice-cold PBS containing either 1 mM ALA or 1 mM GABA to displace unspecific binding. Then, the cells were disrupted in 0.1 mM NaOH and transferred to vials containing scintillation fluid (OptiPhase-Hisafe 3, Perkin-Elmer, England). Radioactive content of the samples was determined in a liquid scintillation counter. The optimal concentration and timing conditions have been established in the previous work (Bermúdez Moretti et al., 2002). As shown previously, the optimal time to perform the uptake experiments is up to 30 min, for which porphyrin synthesis is insignificant.
Succinic acid uptake in cells
A measure of 1 mM succinic acid (0.08 μCi) prepared in 0.1% glucose–PBS preheated at 37°C was added to each well of cells cultured as explained above. After 2, 10, 15 and 30 min of incubation at 37°C, the reaction was stopped by washing four times with cold PBS containing 1 mM succinic acid. For inhibition studies, the cells were preincubated with Und-ALA and He-ALA 15 min before the uptake and during the uptake period. Radioactive content of the samples was determined as explained above.
Porphyrin synthesis in cells after ALA or ALA derivatives uptake at 4°C and 37°C
LM3 cells seeded in 24-well plates were incubated for different time periods (Period I) in the presence of ALA or ALA derivatives at 4°C and 37°C in minimum essential Eagle's medium without serum and kept at the desired temperatures during three washings with PBS. The cells were further incubated at 37°C for 4 h and 30 min (Period II) in minimum essential Eagle's medium without serum. This time period allowed complete conversion of ALA or ALA derivatives into porphyrins. Porphyrins accumulated within the cells were extracted twice with 5% HCl, leaving the cells standing for half an hour in the presence of the acid at 37°C. These conditions proved to be optimal for total porphyrin chemical extraction. The aqueous fraction was used for the fluorimetric detection of porphyrins employing a Shimadzu RF-510 spectrofluorometer. The excitation and emission wavelengths of light used were 406 and 604 nm, respectively. PpIX (Frontier Scientific, Logan, UT, U.S.A.) was used as a reference standard.
Porphyrin synthesis in cells exposed to ALA and BETA substrates and inhibitors
LM3 cells were preincubated 10 min with 2 mM of the BETA transporter substrates or inhibitors hypotaurine, GABA and levulinate. Afterwards, the cells were incubated for 3 h with 0.45 mM ALA or ALA derivatives at 37°C. Porphyrins were extracted with HCl and quantified as described before.
MTT viability assay
Maximal nontoxic concentrations of the inhibitors and GABA-related compounds in LM3 cells were tested by the MTT assay in 24-well plates (Denizot & Lang, 1986). The cells were incubated for 30 min in 1% glucose–PBS with the inhibitors, then the compounds were withdrawn and PBS replaced for complete medium. Immediately after, MTT solution was added to each well in a concentration of 0.5 mg ml−1, and plates were incubated at 37°C for 1 h. The resulting formazan crystals were dissolved by the addition of DMSO and absorbance was read at 560 nm.
Determination of intracellular ALA or ALA derivatives and associated to LM3 membranes
We have previously found that ALA derivatives content can be determined indistinguishably from ALA by the method of Mauzerall & Granick (1956) (Di Venosa et al., 2004). Cells were seeded in 100 mm dishes (1.45 × 106 cells dish−1 at the time of the experiment). After 48 h, medium was removed and cells were exposed to ALA or ALA derivatives at 37°C for different time periods in serum-free medium. Afterwards, cells were washed four times with PBS in 30 s and then 5% trichloroacetic acid was added. After scrapping, the cells were centrifuged and the supernatants were separated from the precipitates to be quantified by the Mauzerall & Granick (1956) method slightly modified as indicated. For intracellular measurements of ALA or ALA derivatives, the supernatants were condensed in the presence of acetyl acetone at pH 4.6 and the resulting pyrroles were quantified by the addition of the Ehrlich's reagent and determination of 555 nm absorbances. In order to quantify the cell membrane associated-ALA or ALA derivatives fractions, the precipitates were also condensed with acetyl acetone at pH 4.6, and quickly centrifuged 5 min at 15,000 × g after the Ehrlich reactive was added. The resulting supernatants were quantified at 555 nm. A set of experiments was also carried out at 4°C and 37°C during Period I (1 h incubation) and at 37°C during Period II (4 h 30 min).
ALA and ALA derivative standards were condensed under similar conditions and thereafter employed for calculations. Under the present conditions, the contribution of the natural pyrrole porphobilinogen to the reaction was negligible.
Statistical analysis
The unpaired t-test was used to establish the significance of differences between groups. Differences were considered statistically significant when P<0.05. Data represent the mean±s.d. of four independent experiments performed in duplicate.
Results
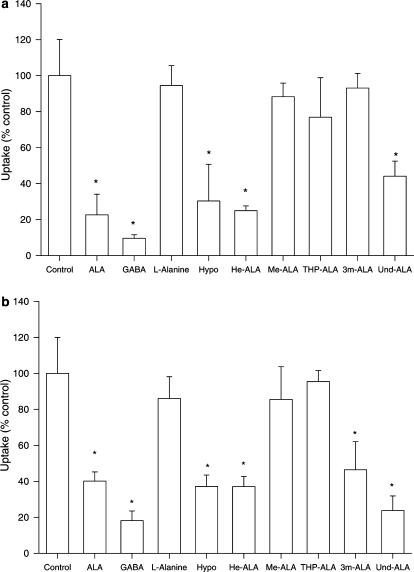
Figure 2 shows the effect of ALA derivatives and other compounds on ALA and GABA uptake in LM3 cells. We can see that the derivatives Und-ALA and He-ALA inhibited ALA uptake, impairing the process by 70–80%, whereas Me-ALA, THP-ALA and 3m-ALA do not interfere in the process (Figure 2a). We have also included hypotaurine and GABA, both substrates of beta transporters (Komura et al., 1996), and L-alanine, an amino acid that is not taken up by that system (Bermúdez Moretti et al., 2002), as positive and negative controls, respectively. Percentages of uptake inhibition remained constant within a 5 to 30 min period.
Figure 2.
Effect of the putative inhibitors of ALA and GABA uptake in LM3 cells. Uptake of ALA at 30 min in the presence of inhibitors was measured as described in the Methods section employing 0.1 mM [14C]-ALA (a) or [14C]-GABA (b). Values are expressed as the percentage of uptake relative to the control uptake without inhibitor. *P<0.05 compared to uptake rates in the absence of inhibitor (control).
The effect of the inhibitors on GABA uptake in LM3 cells is almost identical to that obtained with ALA, with the exception of 3m-ALA, which significantly impaired GABA uptake (Figure 2b). Und-ALA, He-ALA and 3m-ALA inhibited GABA uptake, impairing the process by 60–70%, whereas Me-ALA and THP-ALA did not affect the uptake. Hypotaurine and ALA inhibited GABA uptake as expected, and L-alanine did not affect it.
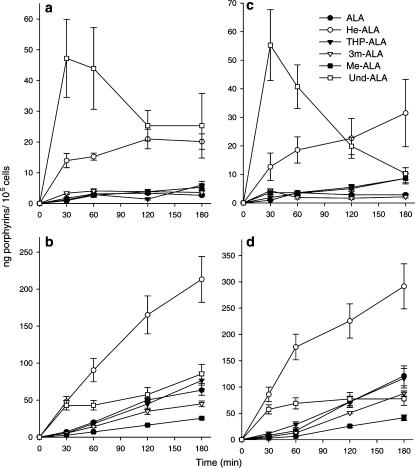
Figure 3 shows the dependence of porphyrin synthesis after ALA or ALA derivative uptake at 4°C and 37°C in order to assess the role of diffusion across the cell membrane. We employed two concentrations, one nonsaturating below the plateau for porphyrin synthesis (0.22 mM in Figures 3a and b) and the other saturating (0.45 mM in Figures 3c and d) (Perotti et al., 2004). We observed that when the uptake is carried out at 4°C at both concentrations, negligible amounts of porphyrins are formed from ALA, Me-ALA, THP-ALA and 3m-ALA, whereas porphyrin synthesis from Und-ALA and He-ALA is significant (Figures 3a and c).
Figure 3.
Porphyrin synthesis in LM3 cells after ALA or ALA derivative uptake at 4°C and 37°C. LM3 cells were incubated for different time periods in the presence of 0.22 mM ALA or ALA derivatives at 4°C (a) and 37°C, (b) and 0.45 mM ALA or ALA derivatives at 4°C (c) and 37°C (d), washed and further incubated at 37°C, so that ALA conversion into porphyrins was complete, for subsequent porphyrin level measurement.
THP-ALA and 3m-ALA at 37°C show similar profiles to ALA, whereas Me-ALA shows the lowest efficiency in terms of porphyrin synthesis (Figures 3b and d).
Porphyrin levels from Und-ALA at 4°C are the highest at short incubation times, and then decrease (Figures 3a and c) due to leakage of porphyrins (data not shown). After uptake at 37°C, porphyrin synthesis is higher for He-ALA within all the concentration range, whereas porphyrin levels from Und-ALA are higher than porphyrins from ALA only during the first 2 h (Figures 3b and d). Measurements of porphyrins released to the medium indicate that this lack of increase of porphyrins from Und-ALA at longer incubation times is due to porphyrin leakage, which is more marked at 4°C (data not shown).
We discarded the hypothesis that Und-ALA binds unspecifically to the membranes during the Period I and is released and converted into porphyrins after warming up during Period II. For this purpose, we quantified directly by the Ehrlich reaction, the amount of Und-ALA bound to the membranes and present in the cytoplasm after a Period I of incubation of 1 h in the presence 0.45 mM Und-ALA at 4°C and 37°C. We found that during Period I of incubation, the amount of Und-ALA bound to the membranes is the same at 4°C and 37°C, whereas the amount of intracellular Und-ALA during Period II correlates well with the amount of porphyrins present at each time point (data not shown).
The fact that the compounds that enter the cells by diffusion, that is He-ALA and Und-ALA, inhibit ALA uptake, which is a process mainly mediated by active transport, seems contradictory. To gain insight into this problem, we assessed porphyrin synthesis from ALA, He-ALA and Und-ALA in LM3 cells (Table 1) in the presence of the substrates of BETA transporters hypotaurine and GABA, and also levulinate, which is an inhibitor but unknown to be effectively transported by the BETA system (Bermúdez Moretti et al., 2002). These compounds impaired 30% porphyrin synthesis from ALA. On the contrary, porphyrin synthesis from He-ALA and Und-ALA are not modified by the presence of the compounds.
Table 1.
Porphyrin synthesis from ALA or ALA derivatives in LM3 cells exposed to BETA substrates and inhibitors
| ng porphyrins per 105 cells | Percentage of control | |
|---|---|---|
| ALA | ||
| Control | 45.2±4.6 | 100 |
| Hypotaurine | 32.1±2.9* | 71.0 |
| GABA | 35.4±3.1* | 78.3 |
| Levulinate | 31.0±2.5* | 68.6 |
| He-ALA | ||
| Control | 48.3±5.3 | 100 |
| Hypotaurine | 47.2±3.4 | 104.4 |
| GABA | 43.0±2.5 | 95.1 |
| Levulinate | 46.1±1.7 | 101.9 |
| Und-ALA | ||
| Control | 36.5±2.9 | 100 |
| Hypotaurine | 40.9±3.6 | 112.0 |
| GABA | 38.3±2.4 | 104.9 |
| Levulinate | 35.3±1.9 | 96.7 |
LM3 cells were preincubated with BETA transporter substrates or inhibitors and afterwards exposed to ALA or ALA derivatievs. Porphyrins synthesised are expressed as ng porphyrin per 105 cells and as the percentage of control in the absence of substrates or inhibitors.
P<0.05 compared to the control.
To test if Und-ALA and He-ALA inhibition of BETA transporters was specific, we performed uptake experiments employing radiolabelled succinate, a molecule that is not taken up by BETA transporters but by a Na+-dependent dicarboxylate transporter (Wright & Wunz, 1987; Moseley et al., 1992). We found that neither He-ALA nor Und-ALA inhibited succinate uptake in LM3 cells (data not shown).
To further analyse the nature of He-ALA and Und-ALA inhibition of BETA transporters, we determined the amount of ALA or ALA derivatives intracellularly taken up by LM3 cells or associated to cell membranes (Table 2). We found that although intracellular He-ALA is 10 times higher compared to Und-ALA, the latter binds much more to the cell membranes. Und-ALA binds to cell membranes at short incubation times and the amount of bound molecules decreases with time, although the unbound ones do not appear to passage to the cytoplasm at long incubation periods. However, the high porphyrin content induced by Und-ALA in these cells (see Figure 3) suggests that the ALA molecules must have been hydrolysed from the membrane-bound Und-ALA and employed straight away in porphyrin synthesis. Both Und-ALA and He-ALA may pass to the cytoplasm after de-esterfication in membrane or after previous release and cytoplasmatic de-esterification.
Table 2.
Intracellular and membrane-associated ALA and ALA derivatives content in LM3 cells
| Intracellular ALA or ALA derivatives (pmol per 105 cells) | ALA or ALA derivatives membrane-associated (pmol per 105 cells) | % ALA or ALA derivatives membrane associated | |
|---|---|---|---|
| Control | 1.62±0.07 | ND | ND |
| ALA (min) | |||
| 30 | 9.78±0.73 | ND | ND |
| He-ALA (min) | |||
| 10 | 30.39±3.18 | 4.38±0.32 | 12.6% |
| 30 | 32.24±4.20 | 1.53±0.11 | 4.5% |
| 180 | 34.19±5.30 | ND | 0% |
| Und-ALA (min) | |||
| 10 | 3.37±3.27 | 25.61±4.25 | 88.4% |
| 30 | 4.21±0.32 | 17.93±2.18 | 80.9% |
| 180 | 3.15±0.28 | 1.15±0.35 | 26.7% |
| Me-ALA (min) | |||
| 30 | 15.61±1.15 | ND | ND |
| THP-ALA (min) | |||
| 30 | 27.32±2.78 | ND | ND |
| 3m-ALA (min) | |||
| 30 | 12.49±1.15 | ND | ND |
LM3 cells were incubated for different time periods with 0.45 mM ALA or ALA derivatives. The amount of intracellular and membrane-associated ALA and ALA derivatives was quantified as explained in Methods section. ND: nondetectable.
Since ALA do not remain bound to the membrane, it is clear that the membrane-associated component is just ALA esters and not hydrolysed ALA.
On the other hand, the proportion of He-ALA bound to the membranes is much lower, and also decreases with time. We have hypothesised that this higher affinity of Und-ALA to cell membranes may be related to a higher affinity for the BETA transporters.
The total amount of ALA or Und-ALA (intracellular and membrane bound) decreases over time when Und-ALA is used and not when He-ALA is used. This is very likely due to leakage of the compound driven by its own detergent-like action, whereas no leakage is shown for porphyrins synthesised from He-ALA, and thus no detergent-like action is probable for He-ALA.
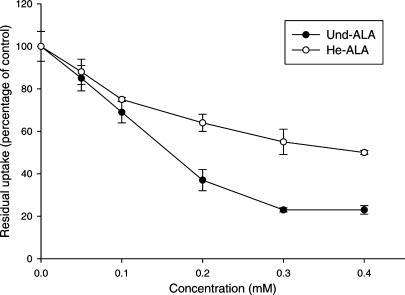
Figure 4 shows the curves of residual uptake of radiolabelled ALA in the presence of different Und-ALA and He-ALA concentrations. Based on the slope of the curves, we can see that Und-ALA displays more affinity for BETA transporters than He-ALA (3.2 times more affinity in the 0.1–0.2 mM range). Since the residual uptake for Und-ALA plateaus at 20%, we could not perform calculations of the inhibitor concentrations required to impair the uptake by 50%. He-ALA concentrations could not be further increased due to toxicity.
Figure 4.
Inhibition of ALA uptake by Und-ALA and He-ALA in LM3 cells. Uptake of ALA at 30 min in the presence of varying concentrations of He-ALA and Und-ALA (0.1–0.4 mM) was measured as described in the Methods section employing 0.1 mM [14C]-ALA. Values are expressed as the percentage of uptake relative to the control uptake without the inhibitor.
To study if Und-ALA specifically interacts with GABA receptors or transporters, we also investigated if several compounds related to GABA interfered with Und-ALA binding to the cell membrane. We employed (R)-nipecotic acid, which is a specific GABA uptake inhibitor in glial cells and isonipecotic acid, which is a specific GABAA receptor agonist (Krogsgaard-Larsen et al., 2000). We also used muscimol, which is an inhibitor of neuronal and glial GABA uptake as well as a very potent GABAA agonist (Krogsgaard-Larsen et al., 2000) and baclofen, which is a GABAB receptor agonist. Employed at concentrations 25 higher than Und-ALA, none of the compounds interfered with Und-ALA binding to the cell membranes or with intracellular uptake (not shown values).
Discussion
In this work, we evaluated the effect of five ALA derivatives on the uptake of ALA in LM3 cells, where ALA is taken up mainly by BETA transport system. We have previously demonstrated that ALA and GABA are taken up by LM3 cells mainly mediated by a BETA transporter mechanism, probably GAT-2. In this work, we found almost an identical profile of inhibition for ALA and GABA uptake, with the exception of 3m-ALA, which only inhibits GABA but not ALA influx. We do not have a possible explanation for this behaviour. However, the inhibitors concentrations could not be further increased due to intrinsic toxicity; therefore, we cannot exclude possible actions of the compounds when employed at higher concentrations.
ALA uptake is impaired by inhibition by the more lipophilic compounds, He-ALA and Und-ALA. However, porphyrin synthesis from Und-ALA and He-ALA in cells is significant when uptake was carried out at 4°C, suggesting that simple diffusion is a mechanism involved.
Gederaas et al. (2001) found evidence for a partial contribution of a diffusion process in the uptake of Me-ALA in WiDr adenocarcinoma cells. A two-step process was suggested, in which uptake at 4°C is initially high but is significantly lower after 2 h. Moreover, they also found a high initial association of Me-ALA to the plasma membrane and an active transport mechanism. In our cell line, we also found simple diffusion and initial membrane association for He-ALA, and Und-ALA whereas Me-ALA is not taken up at all at 4°C. Gederaas et al. (2001) also found that in contrast to ALA transport, Me-ALA does not seem to be taken up by BETA transporters, but by an active transport mechanism, involving transporters of nonpolar amino acids, and it is likely that more than one transporter is involved.
Employing AMBA, a specific inhibitor of PEPT1, PpIX production from ALA was decreased to a much greater extent than ALA pentyl ester induced PIX in a pancreatoma cell line, suggesting again that ALA and its esters gain entry to the cell via different routes (Whitaker et al., 2000).
The fact that the substrates or inhibitors of BETA transporters, hypotaurine, GABA and levulinate do not impair porphyrin synthesis from He-ALA and Und-ALA confirms the hypothesis that these hydrophobic compounds are not taken up by this mechanism. We also performed uptake experiments employing radiolabelled succinate, a molecule that is not taken up by BETA transporters. We found that neither He-ALA nor Und-ALA inhibit succinate uptake, showing that these derivatives may be directly blocking BETA transporters and not modifying unspecifically the membrane structure. One of the in vivo consequences of blocking BETA transporters by lipophilic derivatives of ALA is that cell uptake of extracellularly hydrolysed ALA will be impaired.
The finding that He-ALA and Und-ALA are partly bound to the cell membrane implies that the compounds, especially Und-ALA, may be either blocking directly BETA transporters or indirectly modifying specifically its structure, thus rendering this transportation system nonfunctional. Und-ALA displays more affinity for the transporter than He-ALA, so that the latter compound may be displaced easily during washings and thus not bind to the cell membrane. Leakage of porphyrins formed from Und-ALA may also be due to the presence of Und-ALA in the membrane, thus changing the porphyrin transport process.
After systemic administration of He-ALA and Und-ALA, a much lower amount reaches a mammary subcutaneous implanted tumour compared to ALA (Perotti et al., 2004), and consequently, a five-fold decrease in porphyrins compared to ALA is observed. It is likely that retention in cell membranes may be contributing to this finding, due to binding to molecules such as BETA transporters, especially GAT-2, which is expressed in peripheral tissues (Borden et al., 1992; Liu et al., 1993).
GABA is an inhibitory neurotransmitter considered important in general anxiety disorders including panic and also in seizure disorders (Baker et al., 2000; Krogsgaard-Larsen et al., 2000). GABA agonists such as baclofen and muscimol have antidepressant action in animal models and in human subjects. In addition, it has been suggested that GABA may be transferred to nerve terminals and/or glial cells to end the synaptic actions of GABA. That is why one strategy for pharmacological intervention with the purpose of stimulating GABA neurotransmission is blockage of glial GABA uptake with nipecotic acid (Krogsgaard-Larsen et al., 2000). However in our study, the glial GABA uptake inhibitors, nipecotic acid and muscimol, did not displace Und-ALA binding to the membranes, suggesting that the ALA derivatives either do not block GABA transporters or that GABA mammary transporter is different from glial. In addition, the GABAA and GABAB receptor agonists isonipecotic acid and baclofen did not either block Und-ALA binding to the cells, suggesting that this compound does not interfere with GABA receptors. Owing to the evidence of neurotoxicity of He-ALA and Und-ALA employed systemically at high doses (Perotti et al., 2002; A. Casas et al., unpublished results), studies with glial and neuronal cells will be carried out in the future to study the action of the lipophilic ALA derivatives on the nervous system.
Analysing the rate of porphyrin synthesis, it is clear that the most efficient compound is the relatively lipophilic He-ALA. This greater efficacy (see Figure 3b and d) is observed under the conditions where ALA and derivatives are withdrawn from the medium and the cells are then allowed to complete total porphyrin synthesis. In addition, He-ALA also improves porphyrin synthesis from ALA employed at low concentrations (0.01–0.15 mM) under classical incubation conditions (Perotti et al., 2004), whereas at plateau concentrations, porphyrin synthesis from He-ALA is comparable to that from ALA. This compound not only freely diffuses into the cell but also binds to the membrane with low affinity. On the contrary, the more lipophilic Und-ALA is also transported by simple diffusion but exhibits binding to the cell membrane with higher affinity, thus impairing to some extent its passage to the cytoplasm and also conferring instability to the membrane, which can contribute to porphyrin and Und-ALA or ALA leakage.
To sum up, the ALA esters THP-ALA and Me-ALA are not taken up by BETA transporters but by another active mechanism. The dendron 3m-ALA containing three ALA residues is probably taken up by BETA transporters but does not displace ALA. The more lipophilic ALA derivatives He-ALA and Und-ALA enter these epithelial cells mainly by simple diffusion. However, they can inhibit BETA transportation by blockage, although they are not taken up by this mechanism. These results show that different modifications to the ALA molecule lead to different uptake mechanisms. Although ALA is taken up by BETA transporters, none of the ALA derivatives shares the same mechanism. In designing new ALA derivatives with increased lipophilicity, ideally they should exhibit simple diffusion into tumour cells with minimal cell membrane retention but not into normal cells. Owing to the high selectivity of He-ALA for tumours, one would expect that tumour and normal cells have different mechanism of ALA derivatives uptake. However, these differences between normal and tumour cells are being currently studied.
Acknowledgments
This research was supported by the Wellcome Trust grant number 067062/Z/029/Z, CONICET (PIP 02283 and 05508/3) and the Science and Technology Argentine Agency (PICT 05-9042). A.B. and A.C. hold the posts of Superior and Assistant Researcher at the CONICET, respectively. We also acknowledge support from the BBSRC.
Abbreviations
- ALA
5-aminolevulinic acid
- GABA
γ-aminobutyric acid
- He-ALA
hexylester-ALA
- Me-ALA
ALA-methyl ester
- MTT
3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazoliumbromide
- PBS
phosphate-buffered saline
- PDT
photodynamic therapy
- PpIX
protoporphyrin IX
- TFA
trifluoroacetic acid
- THP-ALA
R, S-ALA-2-(hydroxymethyl)tetrahydropyranyl ester
- Und-ALA
undecanoyl-ALA
- 3m-ALA
aminomethane tris methyl 5-ALA
References
- BAKER G., COUTTS R., GREENSHAW A. Neurochemical and metabolic aspects of antidepressants: an overview. J. Psychiatry Neurosci. 2000;25:481–496. [PMC free article] [PubMed] [Google Scholar]
- BATTAH S., CHEE C., NAKANISHI H., GERSCHER S., MACROBERT A., EDWARDS C. Synthesis and biological studies of 5-aminolevulinic acid-containing dendrimers for photodynamic therapy. Biocon. Chem. 2001;12:980–988. doi: 10.1021/bc010027n. [DOI] [PubMed] [Google Scholar]
- BERMÚDEZ MORETTI M., CORREA GARCÍA S., PEROTTI C., BATLLE A., CASAS A. δ-aminolevulinic acid transport in mammary adenocarcinoma cells is mediated by beta transporters. Br. J. Cancer. 2002;87:471–474. doi: 10.1038/sj.bjc.6600481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BORDEN L.A., SMITH K.E., HARTIG P.R., BRANCHEK T.A., WEINSHANK R.L. Molecular heterogeneity of the γ-aminobutyric acid (GABA) transport system. Cloning of two novel high affinity GABA transporters from rat brain. J. Biol. Chem. 1992;267:21098–21104. [PubMed] [Google Scholar]
- CASAS A., BATLLE A. Rational design of 5-aminolevulinic acid derivatives aimed at improving Photodynamic Therapy. Curr. Med. Chem. Anti-cancer Agents. 2002;2:465–475. doi: 10.2174/1568011023353903. [DOI] [PubMed] [Google Scholar]
- CHEEKS C., WEDEEN R. Renal tubular transport of delta-aminolevulinic acid in rats. Proc. Soc. Exp. Biol. Med. 1986;181:596–601. doi: 10.3181/00379727-181-42297. [DOI] [PubMed] [Google Scholar]
- DENIZOT F., LANG R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J. Immunol. Methods. 1986;89:271–277. doi: 10.1016/0022-1759(86)90368-6. [DOI] [PubMed] [Google Scholar]
- DI VENOSA G., FUKUDA H., PEROTTI C., BATLLE A., CASAS A. A simple method for separating ALA from ALA derivatives using ionic exchange chromatography. J. Photochem. Photobiol. B. 2004;75:157–163. doi: 10.1016/j.jphotobiol.2004.07.001. [DOI] [PubMed] [Google Scholar]
- DÖRING F., WALTER J., WILL J., FÖCKING M., BOLL M., AMASHEH S., CLAUSS W., DANIEL H. Delta-aminolevulinic acid transport by intestinal and renal peptide transporters and its physiological and clinical implications. J. Clin. Invest. 1998;101:2761–2767. doi: 10.1172/JCI1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGHERTY T. Photodynamic therapy (PDT) of malignant tumors. Crit. Rev. Oncol. Hematol. 1984;2:83–116. doi: 10.1016/s1040-8428(84)80016-5. [DOI] [PubMed] [Google Scholar]
- ENNIS S., NOVOTNY A., XIANG J., SHAKUI P., MASADA T., STUMMER W., SMITH D., KEEP R. Transport of 5-aminolevulinic acid between blood and brain. Brain Res. 2003;959:226–234. doi: 10.1016/s0006-8993(02)03749-6. [DOI] [PubMed] [Google Scholar]
- GARDLO K., RUZICKA T. Metvix (PhotoCure) Curr. Opin. Invest. Drugs. 2002;3:1672–1678. [PubMed] [Google Scholar]
- GAULLIER J.M., BERG K., PENG Q., ANHOLT H., SELBO P.K., MA L.W., MOAN J. Use of 5-aminolevulinic acid esters to improve photodynamic therapy on cells in culture. Cancer Res. 1997;57:1481–1486. [PubMed] [Google Scholar]
- GEDERAAS O., HOLROYD A., BROWN S., VERNON D., MOAN J., BERG K. 5-Aminolevulinic acid methyl ester transport on amino acid carriers in a human colon adenocarcionama cell lines. Photochem. Photobiol. 2001;73:164–169. doi: 10.1562/0031-8655(2001)073<0164:aameto>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- JENKINS M., BUONACCORSI G., MACROBERT A., BISHOP C., BOWN S., MCEWAN J. Intra-arterial photodynamic therapy using 5-ALA in a swine model. Eur. J. Vasc. Endovasc. Surg. 1998;16:284–291. doi: 10.1016/s1078-5884(98)80047-6. [DOI] [PubMed] [Google Scholar]
- KELTY C., BROWN N., REED M., ACKROYD R. The use of 5-aminolevulinic acid as a phtotosensitiser in photodynamic therapy and photodiagnosis. Photochem. Photobiol. Sci. 2002;1:158–168. doi: 10.1039/b201027p. [DOI] [PubMed] [Google Scholar]
- KENNEDY J., POTTIER R., PROSS D. Photodynamic therapy with endogenous protoporphyrin IX: basic principles and present clinical experience. J. Photochem. Photobiol. B. 1990;6:143–148. doi: 10.1016/1011-1344(90)85083-9. [DOI] [PubMed] [Google Scholar]
- KLOEK J., BEIJERSBERGEN VAN HENEGOUWEN G.M.J. Prodrugs of 5-aminolevulinic acid for photodynamic therapy. Photochem. Photobiol. 1996;64:994–1000. doi: 10.1111/j.1751-1097.1996.tb01868.x. [DOI] [PubMed] [Google Scholar]
- KOMURA J., TAMAI I., SENMARU M., TERASAKI T., SAI Y., TSUJI A. Sodium and chloride ion-dependent transport of beta-alanine across the blood–brain barrier. J. Neurochem. 1996;67:330–335. doi: 10.1046/j.1471-4159.1996.67010330.x. [DOI] [PubMed] [Google Scholar]
- KROGSGAARD-LARSEN P., FROLUND B., FRYDENVANG K. GABA uptake inhibitors. Design, molecular pharmacology and therapeuctic aspects. Curr. Pharm. Des. 2000;6:1193–1209. doi: 10.2174/1381612003399608. [DOI] [PubMed] [Google Scholar]
- KRZEMIEN A., VAN VUGT D., POTTIER R., DICKSON E., REID R. Evaluation of novel nonlaser light source for endometrial ablation using 5-aminolevulinic acid. Lasers Surg. Med. 1999;25:315–322. doi: 10.1002/(sici)1096-9101(1999)25:4<315::aid-lsm6>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- LIU Q.R., LÓPEZ CORCUERA B., MANDIYAN S., NELSON H., NELSON N. Molecular characterization of four pharmacologically distinct γ-aminobutyric acid transporters in mouse brain. J. Biol. Chem. 1993;268:2106–2112. [PubMed] [Google Scholar]
- MAUZERALL M., GRANICK S. The occurrence and determination of 5-aminolevulinic acid and porphobilinogen in urine. J. Biol. Chem. 1956;219:435–437. [PubMed] [Google Scholar]
- MOSELEY R., JAROSE S., PERMOAD P. Hepatic Na(+)-dicarboxylate cotransport. Identification, characterization and acinar localization. Am. J. Phyisiol. 1992;263 (6 part 1):G871–G879. doi: 10.1152/ajpgi.1992.263.6.G871. [DOI] [PubMed] [Google Scholar]
- NAVONE N., POLO C., FRISARDI A., BATLLE A. Mouse mammary carcinoma PBGase and hydroxymethylbilane synthetase. Comp. Biochem. Physiol. B. 1991;98:67–71. doi: 10.1016/0305-0491(91)90309-2. [DOI] [PubMed] [Google Scholar]
- NEUMAN W., BRANDSCH M. 5-Aminolevulinic acid transport in cancer cells of the human extrahepatic billiary duct. J. Pharm. Exp. Ther. 2003;305:219–224. doi: 10.1124/jpet.102.046573. [DOI] [PubMed] [Google Scholar]
- NOVOTNY A., XIANG J., STUMMER W., TEUSCHER N., SMITH D., KEEP R. Mechanisms of 5-aminolevulinic acid uptake at the choroid plexus. J. Neurochem. 2000;75:321–328. doi: 10.1046/j.1471-4159.2000.0750321.x. [DOI] [PubMed] [Google Scholar]
- PEROTTI C., CASAS A., FUKUDA H., SACCA P., BATLLE A. ALA and ALA hexyl ester induction of porphyrins after their systemic adminsitration to tumour bearing mice. Br. J. Cancer. 2002;87:790–795. doi: 10.1038/sj.bjc.6600559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEROTTI C., FUKUDA H., DI VENOSA G., MACROBERT A., BATLLE A., CASAS A. Porphyrin synthesis from ALA derivatives for photodynamic therapy. In vitro and in vivo studies. Br. J. Cancer. 2004;90:1660–1665. doi: 10.1038/sj.bjc.6601722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUD E., GEDERAAS O., HOGSET A., BERG K. 5-Aminolevulinic acid, but not 5-aminolevulinic acid esters, is transported into adenocarcinoma cells by system BETA transporters. Photochem. Photobiol. 2000;73:164–169. doi: 10.1562/0031-8655(2000)071<0640:aabnaa>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- SCHMIDBAUER J., WITJES F., SCHMELLER N., DONAT R., SUSANI M., MARBERGER M., HEXVIX PCB301/01 STUDY GROUP. Improved detection of urothelial carcinoma in situ with hexaminolevulinate fluorescence cystoscopy. J. Urol. 2004;171:135–138. doi: 10.1097/01.ju.0000100480.70769.0e. [DOI] [PubMed] [Google Scholar]
- TAKEYA H. Preparation of 5-aminolevulinic acid alkyl eters as herbicides. Chem. Abstr. 1992;116:189633m. [Google Scholar]
- VAN HILLESBERG R., VAN DER BERG J., KORT W., TERPSTRA O., WILSON J. Selective accumulation of endogenously produced porphyrins in a liver metastasis model in rats. Gastroenterology. 1992;103:647–651. doi: 10.1016/0016-5085(92)90860-2. [DOI] [PubMed] [Google Scholar]
- WERBAJH S.E., URTREGER A.J., PURICELLI L.I., DE LUSTIG E.S., BAL DE KIER JOFFE E., KORNBLIHTT A.R. Downregulation of fibronectin transcription in highly metastatic adenocarcinoma cells. FEBS Lett. 1998;440:277–281. doi: 10.1016/s0014-5793(98)01473-2. [DOI] [PubMed] [Google Scholar]
- WHITAKER C., BATTAH S., FORSYTH M., EDWARDS C., BOYLE R., MATTHEWS K. Photosensitization of pancreatic tumour cells by 5-aminolevulinic acid esters. Anti-cancer Drugs Design. 2000;15:161–170. [PubMed] [Google Scholar]
- WRIGHT S., WUNZ T. Succinate and citrate transport in renal basolateral and brush-border membranes. Am. J. Physiol. 1987;253:F432–F439. doi: 10.1152/ajprenal.1987.253.3.F432. [DOI] [PubMed] [Google Scholar]