Abstract
Evidence is increasing for a role of brain 5-hydroxytryptamine (5-HT) systems in schizophrenia. We previously showed that brain 5-HT depletion causes disruption of prepulse inhibition, a measure of sensorimotor gating that is deficient in schizophrenia. Antipsychotic treatment has been reported to reverse these deficits in patients with schizophrenia. The present study was designed to investigate the ability of antipsychotic drugs to reverse prepulse inhibition deficits caused by lesions of the brain 5-HT system in rats.
In male Sprague–Dawley rats, selected parts of the brain 5-HT systems were lesioned by micro-injection of the 5-HT neurotoxin 5,7-dihydroxytryptamine into the dorsal raphe nucleus (DRN) or median raphe nucleus (MRN). The effects of antipsychotic drugs on lesion-induced changes in prepulse inhibition were examined 2 weeks after the surgery.
There was significant disruption of prepulse inhibition in the MRN-lesioned group compared to sham-operated controls. This deficiency in prepulse inhibition was restored by clozapine (1 and 5 mg kg−1) treatment, and by treatment with a relatively high dose of haloperidol (0.25 mg kg−1). There was no significant effect of the DRN lesions on prepulse inhibition compared with sham-operated controls.
These results indicate that 5-HT depletion in MRN-innervated brain structures leads to disruption of prepulse inhibition. Treatment with both antipsychotic drugs, haloperidol and clozapine, significantly increased prepulse inhibition in these animals back to the level seen in sham-operated controls. The present findings highlight the importance of the 5-HT systems in cognitive models of schizophrenia.
Keywords: Prepulse inhibition, haloperidol, clozapine, 5-HT, glutamate, dopamine
Introduction
Schizophrenia is a chronic and severe psychiatric illness characterized by hallucinations, delusions, cognitive impairment and thought disorders (Breier, 1999; Millan, 2000; Rowley et al., 2000). Despite many years of research, mechanisms underlying the development of schizophrenia remain unclear. One established hypothesis of schizophrenia involves overactivity in the mesolimbic/mesocortical dopaminergic systems (Carlsson & Lindqvist, 1963; Spanagel & Weiss, 1999). However, there is emerging evidence to suggest that dysfunction of the dopamine system may be due to pathologic changes in other neurotransmitter systems, particularly 5-hydroxytyptamine (5-HT) (Harrison, 1999; Dean, 2000). Post-mortem studies have shown significant alterations in 5-HT systems in schizophrenia. For example, the density of 5-HT2A receptors was reduced in the frontal cortex of subjects with schizophrenia (Dean & Hayes, 1996), while density of 5-HT1A receptors was increased in this region (Bantick et al., 2001).
In order to characterize the role of brain 5-HT activity in schizophrenia, animal behavioural models are used. Prepulse inhibition of acoustic startle is a model of sensorimotor gating and sensory information processing (Geyer et al., 1990; Swerdlow & Geyer, 1998). In schizophrenia and other neuropsychiatric disorders, there is a deficiency in prepulse inhibition, which may lead to sensory flooding and cognitive fragmentation (Geyer et al., 1990; Swerdlow et al., 1994). In the prepulse inhibition model, a sudden, relatively intense stimulus, such as a loud tone, will induce a startle response. A sensory input will normally initiate a short-term inhibition of responses to a subsequent stimulus. Therefore, when a startle-producing stimulus is preceded by a weak prepulse, the reflex response is significantly reduced, that is, prepulse inhibition (Wiley, 1994; Geyer & Swerdlow, 1998).
Prepulse inhibition is modulated by several brain systems, such as the mesolimbic dopaminergic system (Geyer et al., 1990). In addition, 5-HT in the brain plays an important role in the regulation of prepulse inhibition (Davis et al., 1980; Geyer et al., 1990; Fletcher et al., 2001; Prinssen et al., 2002). Treatment with the serotonin 5-HT1A receptor agonist 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) or serotonin releasers, such as 3,4-methylenedioxymethamphetamine (MDMA) and fenfluramine, disrupted prepulse inhibition in rats (for a review, see Geyer et al., 2001). Most 5-HT projections in the forebrain originate in the dorsal raphe nucleus (DRN) and the median raphe nucleus (MRN) (Azmitia & Whitaker-Azmitia, 1995). The DRN projects to the frontal cortex, ventral hippocampus and striatal regions (Adell & Myers, 1995; McQuade & Sharp, 1997; Mokler et al., 1998), while the MRN projects to the dorsal hippocampus and cingulate cortex (Mokler et al., 1998; Thomas et al., 2000). In a previous study, we showed that 5-HT projections arising from these two distinct raphe nuclei are differentially involved in the regulation of prepulse inhibition (Kusljic et al., 2003). Rats with MRN lesions were found to display marked and significant disruption of prepulse inhibition, whereas disruption of prepulse inhibition in the DRN-lesioned rats was smaller and dependent on prepulse intensity (PP) (Kusljic et al., 2003).
Clinically effective antipsychotic drugs display antagonist activity at dopamine D2-like receptors in the brain (Seeman, 1987; Ellenbroek et al., 1991). Compared to typical antipsychotic drugs, such as haloperidol, atypicals, such as clozapine, display a relatively low potency at dopamine D2-like receptors, but high affinity for a number of 5-HT receptor subtypes, muscarinic (M1–M3), alpha adrenergic and histaminergic H1 receptors (O'Dell et al., 1990; Barnes & Sharp, 1999). In rats, typical and atypical antipsychotic drugs have usually been investigated for their ability to antagonize drug-induced prepulse inhibition deficits. For example, the deficits in prepulse inhibition produced by NMDA receptor antagonists appeared to be more sensitive to clozapine-like atypical antipsychotic drugs than to typical antipsychotic drugs (for a review see (Geyer et al., 2001). Furthermore, atypical antipsychotic drugs seem to be more potent in restoring the sensorimotor gating deficits seen in schizophrenia patients than typical antipsychotic drugs (Geyer et al., 2001; Kumari & Sharma, 2002; Oranje et al., 2002).
The aim of the present study was to investigate the ability of antipsychotic drugs to reverse deficits in prepulse inhibition observed in rats with DRN lesions or MRN lesions. After 5,7-dihydroxytryptamine (5,7-DHT) lesions of the DRN or MRN, rats were tested for prepulse inhibition with or without treatment with the typical antipsychotic, haloperidol, or the atypical antipsychotic drug, clozapine. Haloperidol and clozapine were used as a representative example of clinically effective antipsychotic drugs.
Methods
Animals
Experiments were carried out on 28 male Sprague–Dawley rats (Department of Pathology, University of Melbourne, Australia), weighing 250–300 g at the time of the surgery. The rats were housed under standard conditions in groups of 2–3, with free access to food and water. They were maintained on a 12 : 12 h light/dark cycle (lights on at 0700 h) at a constant temperature of 21°C. The experimental protocol and surgical procedures were approved by the Animal Experimentation Ethics Committee of the University of Melbourne, Australia.
Surgery
Rats were pretreated with 20 mg kg−1 of desipramine, 30 min prior to lesions, to prevent destruction of noradrenergic neurons by 5,7-DHT (Jonsson, 1980), and anaesthetized with sodium pentobarbitone (60 mg/kg intraperitoneally (i.p.), Rhone Merieux, QLD, Australia). The rat was mounted in a Kopf stereotaxic frame (David Kopf Instruments, Tujunga, CA, U.S.A.) with the incisor bar set at −3.3 mm (Paxinos & Watson, 1998). The stereotaxic surgery was performed as described previously (Kusljic et al., 2003). Briefly, a measure of 1 μl of 5 μg μl−1 of 5,7-DHT was microinjected by hand, using a micrometer, over a period of 2 min. With bregma as zero and the stereotaxic arm at 25°, the coordinates were as follows: for the DRN: −8.0 mm posterior, +2.9 mm lateral and −6.8 mm ventral of bregma; for the MRN: −8.0 mm posterior, +3.7 mm lateral and −8.8 mm ventral of bregma. Sham-operated controls, comprising an equal number of DRN or MRN injections, underwent the same surgical procedure and received an equal volume of vehicle solution containing ascorbic acid. The animals were subcutaneously (s.c.) administered with 5 mg kg−1 of carprofen (Heriot AgVet, Rowille, VIC, Australia), a nonsteroidal, anti-inflammatory analgesic, to reduce postoperative inflammation and discomfort. Rats were placed on a heat pad until they recovered from anaesthesia. After surgery, rats were allowed to recover for 2 weeks, during which they were handled and health checks were made 2–3 times a week.
Prepulse inhibition of the acoustic startle reflex and experimental design
Prepulse inhibition experiments were performed starting 2 weeks after the surgery. The study included 10 DRN-lesioned rats, 10 MRN-lesioned rats and eight sham-lesioned rats. To determine the ability of antipsychotic drugs to reverse the disruption of prepulse inhibition caused by 5-hydroxytryptaminergic lesions, rats were pretreated with saline, haloperidol or clozapine (i.p.) 15 min before being placed in the automated chambers. Two doses of haloperidol (0.05, 0.25 mg kg−1) and two doses of clozapine (1, 5 mg kg−1) were used. These doses were chosen on the basis of our preliminary dose–response experiments and literature (Keith et al., 1991; Swerdlow et al., 1991; Swerdlow & Geyer, 1993; Bakshi et al., 1994). Thus, each rat was tested for prepulse inhibition five times with 3–4 days interval, with the sequence of treatments being randomized.
Prepulse inhibition testing was carried out using six automated startle chambers (SR-LAB, San Diego Instruments, San Diego, CA, U.S.A.) consisting of clear Plexiglas cylinders, 9 cm in diameter, resting on a platform inside a ventilated, sound-attenuated and illuminated chamber. A speaker, mounted 24 cm above the cylinder, produced both continuous background noise of 70 dB and acoustic stimuli. Whole-body startle responses of the animal in response to acoustic stimuli caused vibrations of the Plexiglas cylinder, which were then converted to quantitative responses by a piezoelectric accelerometer unit attached underneath the platform. Percentage prepulse inhibition was calculated as 100 × ([response to pulse-alone trials−(response to prepulse+pulse trials)]/(response to pulse-alone trials)) (Geyer & Swerdlow, 1998).
A single prepulse inhibition session lasted for about 45 min and consisted of high- and low-intensity stimulus combinations with a continuous background noise of 70 dB. The session started and ended with a block of 10 pulse-alone trials. These blocks, together with 20 randomly presented pulse-alone trials during the prepulse inhibition protocol, were used to calculate basal startle reactivity and startle habituation. Prepulse inhibition was assessed by random presentation of 115 dB pulses and 10 of each of prepulse-2, -4, -8, -12 and -16 and 10 of ‘no-stim'. For example, prepulse-8 (PP8) is a 20 ms prepulse of 8 dB above the background noise, that is, 78 dB, followed 100 ms later by a 40 ms 115 dB pulse (van den Buuse & Eikelis, 2001; van den Buuse et al., 2004).
Measurement of 5-HT concentrations
At the end of the experiments, rats were killed by decapitation and brains were removed from the skull. Brain structures were dissected on a cold plate and the following tissue samples were weighed and stored until biochemical assays were carried out: frontal cortex, striatum, hypothalamus, dorsal hippocampus, ventral hippocampus and cerebellum (Gispen et al., 1972). The pattern of 5-HT depletion after microinjection of 5,7-DHT was determined using high-performance liquid chromatography (HPLC). HPLC was carried out as described previously (Kusljic et al., 2005). Briefly, the tissue samples were homogenized in 500 μl of 0.1 M perchloric acid by ultrasonication and centrifuged at 15,500 × g for 5 min. A 50 μl aliquot of the supernatant was injected into the HPLC system to determine the content of 5-HT (ng mg−1 tissue of wet weight). Each run was 8 min and the retention time for 5-HT was 2.6 min.
Drugs and solutions
Haloperidol (Serenace®, 5 mg ampoules, Searle Laboratories, Crows Nest, NSW, Australia) was diluted to the required dose in 0.9% saline. Clozapine (Sigma Chemical Co., St Louis, MO, U.S.A.) was dissolved in 0.1 N HCl, diluted with saline and pH adjusted to as close to neutrality as possible. Desipramine HCl (Sigma) was dissolved in distilled water to 20 mg ml−1 and injected i.p. All treatments were administered in an injection volume of 1 ml kg−1 of body weight. The neurotoxin 5,7-DHT (Sigma) was dissolved in 0.1% ascorbic acid (BDH Chemicals, Kilsyth, VIC, Australia) in saline to prevent oxidation of the neurotoxin.
Data analysis
Data were expressed as the mean±the standard error of the mean (s.e.m.). Statistical analysis was performed using the statistical software package SYSTAT 9.0 (SPSS Inc., Chicago, IL, U.S.A.). All data were analysed with analysis of variance (ANOVA), with repeated measures where appropriate. In the prepulse inhibition experiments, factors were group (sham, DRN-lesioned or MRN-lesioned), drug (five treatments) and habituation (four blocks of 10 startle responses) or group (sham, DRN-lesioned or MRN-lesioned), drug (five treatments) and prepulse (five different PPs), where drug, habituation and prepulse were repeated-measures factors. A main effect of prepulse was seen in all experiments and is not reported in detail here, except if an interaction with another statistical factor was found.
For HPLC measurements, one-way ANOVA was used, followed by Bonferroni-corrected t-test comparison. A ‘P'-value of <0.05 was considered to be statistically significant.
Results
Effect of raphe lesions on brain 5-HT concentrations
Micro-injection of 5,7-DHT into the DRN or MRN caused a significant reduction in 5-HT concentration in brain regions innervated by these nuclei (Table 1). 5-HT concentrations in the frontal cortex and striatum were significantly different between the groups (F2,25=242.7, P<0.001 and F2,25=531.5, P<0.001, respectively). Post-hoc analysis with Bonferroni-corrected t-test showed significantly lower concentrations of 5-HT in the frontal cortex (71% depletion) and striatum (69% depletion) of DRN-lesioned rats compared to either of the other groups (Table 1). 5-HT concentration of the hypothalamus was also significantly different between the groups (F2,25=304.6, P<0.001), with significantly lower concentrations of 5-HT in both DRN-lesioned (64% depletion) and MRN-lesioned rats (71% depletion) compared to controls (Table 1). 5-HT concentration in the dorsal hippocampus was also significantly reduced (F2,25=267.8, P<0.001) in both DRN- and MRN-lesioned rats. However, depletion in 5-HT concentration was greater in MRN-lesioned rats (73% depletion) than in DRN-lesioned rats (34% depletion). In contrast, significant depletion of 5-HT in the ventral hippocampus (F2,25=221.7, P<0.001) was more pronounced in DRN-lesioned rats (76% depletion) than in MRN-lesioned rats (33% depletion). 5-HT concentrations in cerebellum were significantly reduced (F2,25=128.6, P<0.001) both in DRN-lesioned (37%) and in MRN-lesioned rats (50%) when compared to sham-operated controls.
Table 1.
5-HT content of dissected brain regions of rats with 5,7-DHT induced lesions of the DRN or MRN when compared with sham-operated controls
| Brain region | Sham (n=8) | DRN-lesioned (n=10) | MRN-lesioned (n=10) |
|---|---|---|---|
| Frontal cortex | 0.75±0.03 | 0.22±0.02* | 0.74±0.02 |
| Striatum | 0.85±0.02 | 0.27±0.01* | 0.85±0.01 |
| Hypothalamus | 2.19±0.05 | 0.80±0.04* | 0.63±0.05* |
| Dorsal hippocampus | 1.23±0.02 | 0.82±0.03* | 0.33±0.02* |
| Ventral hippocampus | 1.32±0.04 | 0.32±0.04* | 0.89±0.02* |
| Cerebellum | 0.64±0.02 | 0.40±0.01* | 0.32±0.01* |
Data values represent mean±s.e.m. tissue 5-HT concentration expressed as ng mg−1 of tissue wet weight obtained from six brain areas of sham-operated, DRN-lesioned and MRN-lesioned rats. Differences between the groups were analysed by ANOVA followed by Bonferroni-corrected t-test comparison
P<0.001.
Effect of 5,7-DHT lesions on baseline startle habituation and prepulse inhibition
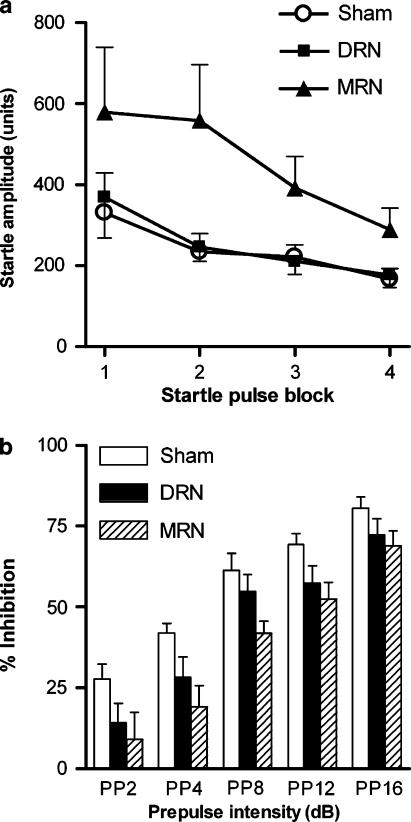
Startle amplitude (four blocks of 10 trials) was different between the groups (Figure 1a). When analyzing combined data after saline treatment from all three groups, ANOVA indicated a strong trend for a main effect of group on startle amplitude (F2,25=3.4, P=0.051). While MRN-lesioned rats tended to display higher startle responses compared to controls (main effect of group F1,16=3.8, P=0.068), there was no effect in DRN-lesioned rats. There was significant habituation of the startle response (main effect of block F3,75=6.9, P<0.001), but the lack of a group × habituation interaction indicated no effect of the lesions on startle habituation (Figure 1a).
Figure 1.
Effect of sham surgery (n=8) or 5,7-DHT lesions of the DRN (n=10) or MRN (n=10) on baseline startle habituation (a) and baseline prepulse inhibition (b). Data are expressed as mean startle amplitudes (arbitrary units)±s.e.m. for each of the four blocks of 10 115-dB pulses and as % inhibition ±s.e.m., respectively. ANOVA indicated a main effect of lesion when comparing prepulse inhibition data from sham-operated and MRN-lesioned rats (P=0.007).
Prepulse inhibition experiments showed that in all groups an increase in PP led to a proportional reduction of the startle response and consequently to a proportional degree of percentage inhibition (Figure 1b). Thus, there was a significant main effect of PP (F4,100=127.9, P<0.001). When comparing all three groups, there was a significant main effect of group (F2,25=3.9, P=0.034) on baseline prepulse inhibition. Further ANOVAs were conducted on data from DRN-lesioned rats and sham-operated controls and on data from MRN-lesioned rats and sham-operated controls. In the MRN-lesioned group, prepulse inhibition was significantly disrupted and this effect was not dependent on the PP (main effect of group F1,16=9.4, P=0.007) (Figure 1b). Further analysis revealed that prepulse inhibition was significantly reduced in MRN-lesioned rats at PP4, PP8 and PP12 (Figure 1b). There were no significant differences between the DRN-lesioned rats and sham-operated controls.
Effect of antipsychotic drugs on startle habituation
ANOVA of startle data from all three lesion groups and all treatments revealed significant main effects of group (F2,25=3.9, P=0.033), drug (F4,100=10.9, P<0.001) and block (F3,75=19.3, P<0.001) and an interaction of drug × block (F12,300=1.8, P=0.043).
When comparing habituation data from DRN-lesioned rats and sham-operated controls, there were significant main effects of Drug (F4,64=8.0, P<0.001) and block (F3,48=21.8, P<0.001). Similarly, when comparing habituation data from MRN-lesioned rats and sham-operated controls, there were significant main effects of group (F1,16=5.1, P=0.038), drug (F4,64=6.9, P<0.001) and block (F3,48=9.9, P<0.001).
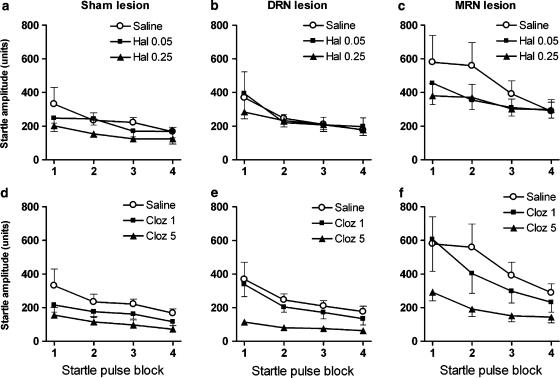
In sham-operated controls, startle amplitude was reduced after treatment with haloperidol or clozapine (Figure 2a and d). ANOVA of combined drug data from this group indicated a significant main effect of drug (F4,28=5.6, P=0.002) and significant habituation (F3,21=9.8, P<0.001). Pairwise analysis of each drug treatment versus saline treatment revealed a significant main effect of 0.25 mg kg−1 of haloperidol (F1,7=9.6, P=0.018) and 5 mg kg−1 of clozapine (F1,7=7.9, P=0.026).
Figure 2.
Effect of antipsychotic drugs on startle amplitude and startle habituation in sham-operated (a and d, n=8), DRN-lesioned (b and e, n=10) and MRN-lesioned rats (c and f, n=10). Rats were treated with haloperidol (Hal 0.05 and Hal 0.25, top panels) or clozapine (Cloz 1 and Cloz 5, bottom panels). Data are expressed as mean startle amplitudes (arbitrary units) ±s.e.m. for each of the four blocks of 10 115-dB pulses.
In DRN-lesioned rats, clozapine treatment reduced startle amplitude (Figure 2e). ANOVA of combined drug data from the DRN-lesioned group revealed a significant main effect of drug (F4,36=4.8, P=0.003) and significant habituation (F3,27=14.2, P<0.001). Further pairwise analysis indicated a significant main effect of 5 mg kg−1 of clozapine (F1,9=15.1, P=0.004) when compared to saline treatment.
In MRN-lesioned rats, startle amplitude was reduced after treatment with haloperidol or clozapine (Figure 2c and f). Again, ANOVA of combined drug data in this group revealed a significant main effect of drug (F4,36=4.5, P=0.005) and a significant habituation (F3,27=6.5, P=0.002). Further pairwise analysis indicated a significant main effect of 0.25 mg kg−1 of haloperidol (F1,9=6.9, P=0.027) and of 5 mg kg−1 of clozapine (F1,9=10.6, P=0.01) when compared to saline treatment.
Effects of antipsychotic drugs on prepulse inhibition
ANOVA of prepulse inhibition data from all three lesion groups and all treatments revealed significant main effects of group (F2,25=3.5, P=0.046), prepulse (F4,100=440.8, P<0.001), and an interaction of group × prepulse (F8,100=2.9, P=0.006) and of drug × prepulse (F16,400=1.7, P=0.039).
When comparing prepulse inhibition data from DRN-lesioned rats and sham-operated controls, there was no significant main effect of group, drug or any of interactions. By contrast, when comparing prepulse inhibition data from MRN-lesioned rats and sham-operated controls for all drug treatments, there was a significant main effect of group (F1,16=10.1, P=0.006) and a group × prepulse interaction (F4,64=5.7, P=0.001).
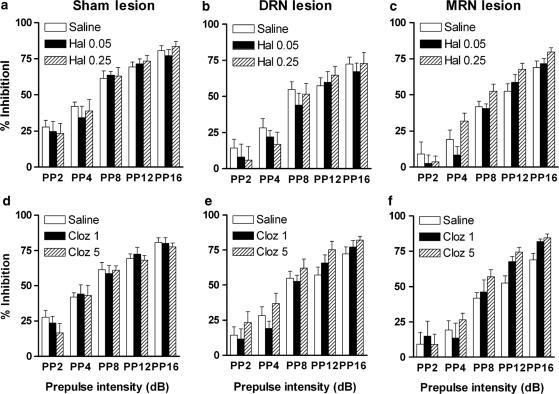
Analysis of prepulse inhibition data in the individual experimental groups showed that, in the sham-operated group, there was no effect of treatment with antipsychotic drugs on prepulse inhibition when compared to saline treatment (Figure 3a and d, Table 2). Similarly, there was no significant effect of treatment with antipsychotic drugs in the DRN-lesioned rats (Figure 3b and e, Table 2). In the MRN-lesioned group (Figure 3c and f, Table 2), there was a significant drug × prepulse interaction after treatment with 0.25 mg kg−1 haloperidol (F4,36=3.1, P=0.028), reflecting higher prepulse inhibition values in these lesioned animals after haloperidol treatment, depending on the PP. However, analysis of prepulse inhibition at individual PPs only reached trend level (PP4: P=0.127, PP8: P=0.145, PP12: P=0.085, PP16: P=0.085).
Figure 3.
Effect of antipsychotic drugs on prepulse inhibition of the acoustic startle. Prepulse inhibition is expressed as % inhibition ±s.e.m. for sham-operated (panels a and d, n=8), DRN-lesioned (panels b and e, n=10) and MRN-lesioned rats (panels c and f, n=10) at different PPs. Animals were treated with haloperidol (Hal 0.05 and Hal 0.25, top panels) or clozapine (Cloz 1 and Cloz 5, bottom panels). ANOVA indicated that treatment with clozapine and 0.25 mg kg−1 haloperidol was able to reverse disruption in prepulse inhibition in MRN-lesioned rats compared to sham-operated controls.
Table 2.
Average prepulse inhibition of rats with 5,7-DHT induced lesions of the DRN or MRN when compared with sham-operated controls
| Treatment | Sham (n=8) | DRN-lesioned (n=10) | MRN-lesioned (n=10) |
|---|---|---|---|
| Saline | 56.3±3.5 | 45.5±4.9 | 38.3±4.4 |
| Haloperidol 0.05 mg kg−1 | 54.3±3.7 | 40.2±5.8 | 36.4±3.5 |
| Haloperidol 0.25 mg kg−1 | 56.5±4.9 | 42.4±7.2 | 47.1±3.3* |
| Clozapine 1 mg kg−1 | 55.8±4.5 | 45.3±4.6 | 44.8±6.0* |
| Clozapine 5 mg kg−1 | 53.3±4.0 | 55.9±4.8 | 50.3±3.8* |
The animals were treated with saline, haloperidol or clozapine.
Data values represent mean±s.e.m. tissue. Average prepulse inhibition was calculated as the mean % inhibition seen at PP2, PP4, PP8, PP12 and PP16 (see Methods). Differences between the groups were analysed by combined and pairwise ANOVA (see text for details).
P<0.05 Group × Prepulse interaction when compared to saline treatment in the same group.
In the MRN-lesioned group, similar to haloperidol treatment, there was a significant drug × prepulse interaction after treatment with 5 mg kg−1 clozapine (F4,36=2.7, P=0.046), reflecting an increase in prepulse inhibition after this treatment, depending on the PP. Analysis of data of individual PPs revealed a strong trend for a significant increase in prepulse inhibition at PP8 (P=0.053) and significant effects of clozapine treatment at PP12 (P=0.011) and PP16 (P=0.042).
Discussion and conclusions
Sensorimotor gating models, such as prepulse inhibition of the acoustic startle response (Geyer & Markou, 1995; Koch, 2000), provide a rare opportunity for cross-species explorations into information processing and attentional deficits in schizophrenia. Deficits in both prepulse inhibition of startle and in startle habituation are characteristics of the illness (Geyer & Braff, 1987). The experiments in the present study were designed to assess the effectiveness of treatment with haloperidol and clozapine in normalizing deficits in sensorimotor gating produced by raphe lesions. We confirmed earlier results (Kusljic et al., 2003) that MRN lesions, caused a disruption of prepulse inhibition. The main finding of this study was that treatment with clozapine and haloperidol increased prepulse inhibition in rats with MRN lesions but not in rats with DRN lesions or controls. Microinjection of 5,7-DHT into the raphe nuclei reduced 5-HT concentrations in anatomically restricted regions, reflecting the distribution of 5-HT terminals arising from the DRN or MRN.
The major pathways thought to be involved in the acoustic startle response are located in the lower brainstem, and the pontine reticular nucleus has been shown to be a critical sensorimotor interface in the pathway that mediates the startle response (Davis et al., 1982; Koch & Schnitzler, 1997). Several studies have indicated that a reduction in brain 5-HT levels is associated with an increased sensitivity to various sensory stimuli (Davis & Sheard, 1974; Davis et al., 1980). Rats with MRN lesions tended to have increased startle responses compared to sham-operated controls. These findings suggest that 5-HT in brain structures innervated by the MRN, such as dorsal hippocampus and cingulate cortex, may be involved in modulation of normal responses to startle stimuli.
In contrast to startle, prepulse inhibition is modulated by a neuronal circuit consisting of cortico-limbic brain structures in which the nucleus accumbens plays an important role (Geyer et al., 1990; Koch & Schnitzler, 1997; Swerdlow & Geyer, 1998). Using a different prepulse inhibition paradigm, robust disruption of prepulse inhibition has also been found in rats with combined 5,7-DHT lesions of the DRN and MRN or after treatment with a tryptophan hydroxylase inhibitor (Fletcher et al., 2001; Prinssen et al., 2002). Our findings confirm that the normal regulation of prepulse inhibition depends on intact 5-HT transmission in brain structures receiving predominant 5-hydroxytryptaminergic input from the MRN, such as dorsal hippocampus and cingulate cortex.
Models of disruption of prepulse inhibition in schizophrenia have been tested with antipsychotic drugs in order to assess the validity of these models for the identification of clinical treatments for schizophrenia. Animal studies have shown that treatment with antipsychotic drugs, such as haloperidol and clozapine, is able to reverse prepulse inhibition deficits induced by excessive central dopaminergic stimulation following apomorphine treatment (Swerdlow et al., 1991; Swerdlow & Geyer, 1993). However, haloperidol failed to restore prepulse inhibition deficits produced by NMDA-receptor antagonists such as phencyclidine and dizocilpine (Keith et al., 1991). In contrast, treatment with atypical antipsychotic drugs, such as clozapine and ziprasidone, was able to antagonize NMDA-receptor antagonist-induced deficits in prepulse inhibition (Bakshi et al., 1994; Bakshi & Geyer, 1995; Mansbach et al., 2001).
In this study, we demonstrated that treatment with both antipsychotic drugs, haloperidol and clozapine, significantly increased prepulse inhibition in rats with lesions of the MRN, effectively bringing prepulse inhibition in these animals back to the level seen in sham-operated controls. Thus, we were able to restore prepulse inhibition deficits produced by a 5-HT depletion in the brain. However, the exact mechanism by which clozapine and haloperidol affect these sensorimotor gating deficits is far from clear. The antipsychotic profile of clozapine appears to reside in its broad pattern of interaction with various dopamine and 5-HT receptor subtypes (Millan, 2000). Meltzer et al. have proposed a model in which affinity for 5-HT2 receptors relative to dopamine D2 receptor affinity is a crucial component differentiating typical from atypical antipsychotic drugs (Meltzer, 1989; Meltzer et al., 1989). However, according to the other school of thought, all antipsychotic drugs, including both typical and atypical, act by blocking dopamine D2 receptors (Kapur & Seeman, 2001). Animal data and in vitro data showed that a rapid dissociation from the D2 receptor at a molecular level, not high affinity at the 5-HT2 receptor, produces the atypical antipsychotic effect (Kapur & Seeman, 2001). These data also matched clinical brain-imaging findings showing that haloperidol remained constantly bound to D2 receptors in humans undergoing two positron emission tomography scans 24 h apart, whereas the occupation of D2 receptors by clozapine has mostly disappeared after 24 h (Seeman, 2002).
The nucleus accumbens is one of the brain sites where excessive dopamine activity disrupts prepulse inhibition (Swerdlow et al., 1990; Koch, 1999). We suggest that sensorimotor gating deficits observed by MRN lesions in the present study occurred as a result of dopamine overactivity in the nucleus accumbens brought about by 5-HT depletion in limbic structures such as the dorsal hippocampus (Kusljic & van den Buuse, 2004). The dopamine receptors critical for modulation of prepulse inhibition appear to be located within both the dorsal striatum and the nucleus accumbens (Swerdlow et al., 1986; 1990). Both of these regions contain dense populations of D2 receptors (Garau et al., 1978). Following intraraphe 5,7-DHT injections, significant increases in 5-HT1B/1D binding were detected in the nucleus accumbens, whereas similar increases in 5-HT2A/2C binding were restricted to the medial striatum (Compan et al., 1998). The ability of clozapine to occupy D2 receptors at the same time as 5-HT2 receptors, and then rapidly dissociate from D2 receptors to allow physiological dopamine neurotransmission, may be responsible for increasing prepulse inhibition in MRN-lesioned rats. In addition, occupancy of dopamine D2 receptors by a relatively high dose of haloperidol is responsible for restoring prepulse inhibition deficits in rats with MRN lesions. Neuroimaging data showed that optimal dopamine D2 receptor occupancy is sufficient to produce the atypical antipsychotic effect (Kapur & Seeman, 2001). If dopamine D2 receptor occupancy is excessive, the atypical profile is lost even in the presence of high 5-HT2 occupancy (Kapur & Seeman, 2001). Therefore, our results suggest that appropriate modulation of the dopamine D2 receptor alone is sufficient to restore sensorimotor gating deficits produced by selective lesions of the brain 5-HT system. In conclusion, these findings highlight the regulatory and behavioural consequences of 5-HT depletion and importance of the dopamine D2 receptor modulation in regulation of sensorimotor gating.
Acknowledgments
This study was supported by the National Health and Medical Research Council of Australia. M. van den Buuse was supported by the Griffith Senior Research Fellowship of the University of Melbourne. We are grateful to Associate Professor Trevor Norman at the Department of Psychiatry, University of Melbourne, who generously provided the resources in his laboratory for HPLC measurements of 5-HT concentrations. The Mental Health Research Institute is a Stanley Research Centre, supported by the Stanley Medical Research Institute.
Abbreviations
- ANOVA
analysis of variance
- 5,7-DHT
5,7-dihydroxytryptamine
- DRN
dorsal raphe nucleus
- MRN
median raphe nucleus
- PP
prepulse intensity
- s.e.m.
standard error of the mean
References
- ADELL A., MYERS R.D. Selective destruction of midbrain raphe nuclei by 5,7-DHT: is brain 5-HT involved in alcohol drinking in Sprague–Dawley rats. Brain Res. 1995;693:70–79. doi: 10.1016/0006-8993(95)00701-q. [DOI] [PubMed] [Google Scholar]
- AZMITIA E.C., WHITAKER-AZMITIA P.M.Anatomy, cell biology, and plasticity of the serotonergic system. Neuropsychopharmacological implications for the actions of psychotropic drugs Psychopharmacology: The Fourth Generation of Progress 1995New York: Raven Press; 443–449.ed. Bloom, F.E. & Kupfer, D.J. pp [Google Scholar]
- BAKSHI V.P., GEYER M.A. Antagonism of phencyclidine-induced deficits in prepulse inhibition by the putative atypical antipsychotic olanzapine. Psychopharmacology (Berlin) 1995;122:198–201. doi: 10.1007/BF02246096. [DOI] [PubMed] [Google Scholar]
- BAKSHI V.P., SWERDLOW N.R., GEYER M.A. Clozapine antagonizes phencyclidine-induced deficits in sensorimotor gating of the startle response. J. Pharmacol. Exp. Ther. 1994;271:787–794. [PubMed] [Google Scholar]
- BANTICK R.A., DEAKIN J.F., GRASBY P.M. The 5-HT1A receptor in schizophrenia: a promising target for novel atypical neuroleptics. J. Psychopharmacol. 2001;15:37–46. doi: 10.1177/026988110101500108. [DOI] [PubMed] [Google Scholar]
- BARNES N.M., SHARP T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- BREIER A. Cognitive deficit in schizophrenia and its neurochemical basis. Br. J. Psychiatry (Suppl) 1999;37:16–18. [PubMed] [Google Scholar]
- CARLSSON A., LINDQVIST M. Effect of chlorpromazine or haloperidol on formation of 3-methoxytyramine and normetanephrine in mouse brain. Acta Pharmacol. Toxicol. 1963;20:140–144. doi: 10.1111/j.1600-0773.1963.tb01730.x. [DOI] [PubMed] [Google Scholar]
- COMPAN V., SEGU L., BUHOT M.C., DASZUTA A. Selective increases in serotonin 5-HT1B/1D and 5-HT2A/2C binding sites in adult rat basal ganglia following lesions of serotonergic neurons. Brain Res. 1998;793:103–111. doi: 10.1016/s0006-8993(98)00168-1. [DOI] [PubMed] [Google Scholar]
- DAVIS M., GENDELMAN D.S., TISCHLER M.D., GENDELMAN P.M. A primary acoustic startle circuit: lesion and stimulation studies. J. Neurosci. 1982;2:791–805. doi: 10.1523/JNEUROSCI.02-06-00791.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS M., SHEARD M.H. Habituation and sensitization of the rat startle response: effects of raphe lesions. Physiol. Behav. 1974;12:425–431. doi: 10.1016/0031-9384(74)90120-6. [DOI] [PubMed] [Google Scholar]
- DAVIS M., STRACHAN D.I., KASS E. Excitatory and inhibitory effects of serotonin on sensorimotor reactivity measured with acoustic startle. Science. 1980;209:521–523. doi: 10.1126/science.7394520. [DOI] [PubMed] [Google Scholar]
- DEAN B. Signal transmission, rather than reception, is the underlying neurochemical abnormality in schizophrenia. Aust. NZ J. Psychiatry. 2000;34:560–569. doi: 10.1080/j.1440-1614.2000.00747.x. [DOI] [PubMed] [Google Scholar]
- DEAN B., HAYES W. Decreased frontal cortical serotonin2A receptors in schizophrenia. Schizophrenia Res. 1996;21:133–139. doi: 10.1016/0920-9964(96)00034-5. [DOI] [PubMed] [Google Scholar]
- ELLENBROEK B.A., ARTZ M.T., COOLS A.R. The involvement of dopamine D1 and D2 receptors in the effects of the classical neuroleptic haloperidol and the atypical neuroleptic clozapine. Eur. J. Pharmacol. 1991;196:103–108. doi: 10.1016/0014-2999(91)90414-l. [DOI] [PubMed] [Google Scholar]
- FLETCHER P.J., SELHI Z.F., AZAMPANAH A., SILLS T.L. Reduced brain serotonin activity disrupts prepulse inhibition of the acoustic startle reflex. Effects of 5,7-dihydroxytryptamine and p-chlorophenylalanine. Neuropsychopharmacology. 2001;24:399–409. doi: 10.1016/S0893-133X(00)00215-3. [DOI] [PubMed] [Google Scholar]
- GARAU L., GOVONI S., STEFANINI E., TRABUCCHI M., SPANO P.F. Dopamine receptors: pharmacological and anatomical evidences indicate that two distinct dopamine receptor populations are present in rat striatum. Life Sci. 1978;23:1745–1750. doi: 10.1016/0024-3205(78)90102-9. [DOI] [PubMed] [Google Scholar]
- GEYER M.A., BRAFF D.L. Startle habituation and sensorimotor gating in schizophrenia and related animal models. Schizophr. Bull. 1987;13:643–668. doi: 10.1093/schbul/13.4.643. [DOI] [PubMed] [Google Scholar]
- GEYER M.A., KREBS-THOMSON K., BRAFF D.L., SWERDLOW N.R. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology (Berlin) 2001;156:117–154. doi: 10.1007/s002130100811. [DOI] [PubMed] [Google Scholar]
- GEYER M.A., MARKOU A.Animal models of psychiatric disorders Psychopharmacology: The Fourth Generation of Progress 1995New York: Raven Press; 787–798.ed. Bloom, F. & Kupfer, D. pp [Google Scholar]
- GEYER M.A., SWERDLOW N.R. Measurement of startle response, prepulse inhibition, and habituation. Curr. Protocols Neurosci. 1998;Unit 8.7:8.7.1–8.7.15. doi: 10.1002/0471142301.ns0807s03. [DOI] [PubMed] [Google Scholar]
- GEYER M.A., SWERDLOW N.R., MANSBACH R.S., BRAFF D.L. Startle response models of sensorimotor gating and habituation deficits in schizophrenia. Brain Res. Bull. 1990;25:485–498. doi: 10.1016/0361-9230(90)90241-q. [DOI] [PubMed] [Google Scholar]
- GISPEN W.H., SCHOTMAN P., DE KLOET E.R. Brain RNA and hypophysectomy: a topographical study. Neuroendocrinology. 1972;9:285–296. doi: 10.1159/000122060. [DOI] [PubMed] [Google Scholar]
- HARRISON P.J. The neuropathology of schizophrenia. Brain. 1999;122:593–624. doi: 10.1093/brain/122.4.593. [DOI] [PubMed] [Google Scholar]
- JONSSON G. Chemical neurotoxins as denervation tools in neurobiology. Ann. Rev. Neurosci. 1980;3:169–187. doi: 10.1146/annurev.ne.03.030180.001125. [DOI] [PubMed] [Google Scholar]
- KAPUR S., SEEMAN P. Does fast dissociation from the dopamine d(2) receptor explain the action of atypical antipsychotics? A new hypothesis. Am. J. Psychiatry. 2001;158:360–369. doi: 10.1176/appi.ajp.158.3.360. [DOI] [PubMed] [Google Scholar]
- KEITH V.A., MANSBACH R.S., GEYER M.A. Failure of haloperidol to block the effects of phencyclidine and dizocilpine on prepulse inhibition of startle. Biol. Psychiatry. 1991;30:557–566. doi: 10.1016/0006-3223(91)90025-h. [DOI] [PubMed] [Google Scholar]
- KOCH M. The neurobiology of startle. Prog. Neurobiol. 1999;59:107–128. doi: 10.1016/s0301-0082(98)00098-7. [DOI] [PubMed] [Google Scholar]
- KOCH M. Can animal models help to understand human diseases? Commentary on Swerdlow et al., ‘Animal models of deficient sensorimotor gating: what we know, what we think we know, and what we hope to know soon'. Behav. Pharmacol. 2000;11:205–207. doi: 10.1097/00008877-200006000-00003. [DOI] [PubMed] [Google Scholar]
- KOCH M., SCHNITZLER H.U. The acoustic startle response in rats – circuits mediating evocation, inhibition and potentiation. Behav. Brain Res. 1997;89:35–49. doi: 10.1016/s0166-4328(97)02296-1. [DOI] [PubMed] [Google Scholar]
- KUMARI V., SHARMA T. Effects of typical and atypical antipsychotics on prepulse inhibition in schizophrenia: a critical evaluation of current evidence and directions for future research. Psychopharmacology. 2002;162:97–101. doi: 10.1007/s00213-002-1099-x. [DOI] [PubMed] [Google Scholar]
- KUSLJIC S., BROSDA J., NORMAN T.R., VAN DEN BUUSE M. Brain serotonin depletion by lesions of the median raphe nucleus enhances the psychotomimetic action of phencyclidine, but not dizocilpine (MK-801), in rats. Brain Res. 2005;1049:217–226. doi: 10.1016/j.brainres.2005.05.017. [DOI] [PubMed] [Google Scholar]
- KUSLJIC S., COPOLOV D.L., VAN DEN BUUSE M. Differential role of serotonergic projections arising from the dorsal and median raphe nuclei in locomotor hyperactivity and prepulse inhibition. Neuropsychopharmacology. 2003;28:2138–2147. doi: 10.1038/sj.npp.1300277. [DOI] [PubMed] [Google Scholar]
- KUSLJIC S., VAN DEN BUUSE M. Functional dissociation between serotonergic pathways in dorsal and ventral hippocampus in psychotomimetic drug-induced locomotor hyperactivity and prepulse inhibition in rats. Eur. J. Neurosci. 2004;20:3424–3432. doi: 10.1111/j.1460-9568.2004.03804.x. [DOI] [PubMed] [Google Scholar]
- MANSBACH R.S., CARVER J., ZORN S.H. Blockade of drug-induced deficits in prepulse inhibition of acoustic startle by ziprasidone. Pharmacol. Biochem. Behav. 2001;69:535–542. doi: 10.1016/s0091-3057(01)00561-5. [DOI] [PubMed] [Google Scholar]
- MCQUADE R., SHARP T. Functional mapping of dorsal and median raphe 5-hydroxytryptamine pathways in forebrain of the rat using microdialysis. J. Neurochem. 1997;69:791–796. doi: 10.1046/j.1471-4159.1997.69020791.x. [DOI] [PubMed] [Google Scholar]
- MELTZER H.Y. Clinical studies on the mechanism of action of clozapine: the dopamine-serotonin hypothesis of schizophrenia. Psychopharmacology (Berlin) 1989;99 (Suppl):S18–S27. doi: 10.1007/BF00442554. [DOI] [PubMed] [Google Scholar]
- MELTZER H.Y., MATSUBARA S., LEE J.C. Classification of typical and atypical antipsychotic drugs on the basis of dopamine D-1, D-2 and serotonin2 pKi values. J. Pharmacol. Exp. Ther. 1989;251:238–246. [PubMed] [Google Scholar]
- MILLAN M.J. Improving the treatment of schizophrenia: focus on serotonin 5-HT1A receptors. J. Pharmacol. Exp. Ther. 2000;295:853–861. [PubMed] [Google Scholar]
- MOKLER D.J., LARIVIERE D., JOHNSON D.W., THERIAULT N.L., BRONZINO J.D., DIXON M., MORGANE P.J. Serotonin neuronal release from dorsal hippocampus following electrical stimulation of the dorsal and median raphe nuclei in conscious rats. Hippocampus. 1998;8:262–273. doi: 10.1002/(SICI)1098-1063(1998)8:3<262::AID-HIPO8>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- O'DELL S.J., LA HOSTE G.J., WIDMARK C.B., SHAPIRO R.M., POTKIN S.G., MARSHALL J.F. Chronic treatment with clozapine or haloperidol differentially regulates dopamine and serotonin receptors in rat brain. Synapse. 1990;6:146–153. doi: 10.1002/syn.890060205. [DOI] [PubMed] [Google Scholar]
- ORANJE B., VAN OEL C.J., GISPEN-DE WIED C.C., VERBATEN M.N., KAHN R.S. Effects of typical and atypical antipsychotics on the prepulse inhibition of the startle reflex in patients with schizophrenia. J. Clin. Psychopharmacol. 2002;22:359–365. doi: 10.1097/00004714-200208000-00005. [DOI] [PubMed] [Google Scholar]
- PAXINOS G., WATSON C. The Rat Brain in Stereotaxic Co-ordinates 1998New York: Academic Press; 4th edn [Google Scholar]
- PRINSSEN E.P., ASSIE M.B., KOEK W., KLEVEN M.S. Depletion of 5-HT disrupts prepulse inhibition in rats. Dependence on the magnitude of depletion, and reversal by a 5-HT precursor. Neuropsychopharmacology. 2002;26:340–347. doi: 10.1016/S0893-133X(01)00348-7. [DOI] [PubMed] [Google Scholar]
- ROWLEY H.L., NEEDHAM P.L., KILPATRICK I.C., HEAL D.J. A comparison of the acute effects of zotepine and other antipsychotics on rat cortical dopamine release, in vivo. Naunyn-Schmiedeberg's Arch. Pharmacol. 2000;361:187–192. doi: 10.1007/s002109900170. [DOI] [PubMed] [Google Scholar]
- SEEMAN P. Dopamine receptors and the dopamine hypothesis of schizophrenia. Synapse. 1987;1:133–152. doi: 10.1002/syn.890010203. [DOI] [PubMed] [Google Scholar]
- SEEMAN P. Atypical antipsychotics: mechanism of action. Can. J. Psychiatry. 2002;47:27–38. [PubMed] [Google Scholar]
- SPANAGEL R., WEISS F. The dopamine hypothesis of reward: past and current status. Trends Neurosci. 1999;22:521–527. doi: 10.1016/s0166-2236(99)01447-2. [DOI] [PubMed] [Google Scholar]
- SWERDLOW N.R., BRAFF D.L., GEYER M.A., KOOB G.F. Central dopamine hyperactivity in rats mimics abnormal acoustic startle response in schizophrenics. Biol. Psychiatry. 1986;21:23–33. doi: 10.1016/0006-3223(86)90005-3. [DOI] [PubMed] [Google Scholar]
- SWERDLOW N.R., BRAFF D.L., MASTEN V.L., GEYER M.A. Schizophrenic-like sensorimotor gating abnormalities in rats following dopamine infusion into the nucleus accumbens. Psychopharmacology. 1990;101:414–420. doi: 10.1007/BF02244063. [DOI] [PubMed] [Google Scholar]
- SWERDLOW N.R., BRAFF D.L., TAAID N., GEYER M.A. Assessing the validity of an animal model of deficient sensorimotor gating in schizophrenic patients. Arch. Gen. Psychiatry. 1994;51:139–154. doi: 10.1001/archpsyc.1994.03950020063007. [DOI] [PubMed] [Google Scholar]
- SWERDLOW N.R., GEYER M.A. Clozapine and haloperidol in an animal model of sensorimotor gating deficits in schizophrenia. Pharmacol. Biochem. Behav. 1993;44:741–744. doi: 10.1016/0091-3057(93)90193-w. [DOI] [PubMed] [Google Scholar]
- SWERDLOW N.R., GEYER M.A. Using an animal model of deficient sensorimotor gating to study the pathophysiology and new treatments of schizophrenia. Schizophr. Bull. 1998;24:285–301. doi: 10.1093/oxfordjournals.schbul.a033326. [DOI] [PubMed] [Google Scholar]
- SWERDLOW N.R., KEITH V.A., BRAFF D.L., GEYER M.A. Effects of spiperone, raclopride, SCH 23390 and clozapine on apomorphine inhibition of sensorimotor gating of the startle response in the rat. J. Pharmacol. Exp. Ther. 1991;256:530–536. [PubMed] [Google Scholar]
- THOMAS H., FINK H., SOHR T.R., VOITS M. Lesion of the median raphe nucleus: a combined behavioral and microdialysis study in rats. Pharmacol. Biochem. Behav. 2000;65:15–21. doi: 10.1016/s0091-3057(99)00119-7. [DOI] [PubMed] [Google Scholar]
- VAN DEN BUUSE M., EIKELIS N. Estrogen increases prepulse inhibition of acoustic startle in rats. Eur. J. Pharmacol. 2001;425:33–41. doi: 10.1016/s0014-2999(01)01139-6. [DOI] [PubMed] [Google Scholar]
- VAN DEN BUUSE M., MORRIS M., CHAVEZ C., MARTIN S., WANG J. Effect of adrenalectomy and corticosterone replacement on prepulse inhibition and locomotor activity in mice. Br. J. Pharmacol. 2004;142:543–550. doi: 10.1038/sj.bjp.0705511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILEY J.L. Clozapine's effects on phencyclidine-induced disruption of prepulse inhibition of the acoustic startle response. Pharmacol. Biochem. Behav. 1994;49:1025–1028. doi: 10.1016/0091-3057(94)90259-3. [DOI] [PubMed] [Google Scholar]