Abstract
In airway smooth muscle (ASM), full and partial muscarinic receptor agonists have been described to have large differences in their ability to induce signal transduction, including Ca2+-mobilization. Despite these differences, partial agonists are capable of inducing a submaximal to maximal ASM contraction.
To further elucidate transductional differences between full and partial muscarinic receptor agonists, we investigated the contribution of Rho-kinase (an important regulator of Ca2+-sensitization) to methacholine-, pilocarpine- and McN-A-343-induced bovine tracheal smooth muscle (BTSM) contraction, using the selective Rho-kinase inhibitor Y-27632. In addition, we measured Ca2+-mobilization and -influx in BTSM cells in response to these agonists in the absence and presence of Y-27632.
Whereas treatment with Y-27632 (1 μM) significantly decreased potency (pEC50) for all agonists, maximal contraction (Emax) was reduced by 23.4±2.8 and 50.4±7.9% for the partial agonists pilocarpine and McN-A-343, respectively, but was unaffected for the full agonist methacholine. However, Emax of methacholine became Rho-kinase dependent after taking away its receptor reserve using the irreversible muscarinic receptor antagonist propylbenzilylcholine mustard.
Pilocarpine and McN-A-343 induced a very small Ca2+-mobilization and -influx as compared to methacholine. In addition, an inverse relationship of these two parameters with the Rho-kinase dependency was observed. Interestingly, no inhibitory effects of Y-27632 were observed on Ca2+-mobilization and-influx for all three agonists, indicating that the effects of Y-27632 on contraction are most likely on the level of Ca2+-sensitization.
In conclusion, in contrast to the full agonist methacholine, the partial muscarinic receptor agonists pilocarpine and McN-A-343 are dependent on Rho-kinase for their maximal contractile effects, presumably as a consequence of differences in transductional reserve, indicating an agonist-dependent role for Rho-kinase in ASM contraction. Moreover, an inverse relationship exists between Rho-kinase dependency and both Ca2+-mobilization and Ca2+-influx for these agonists.
Keywords: Rho-kinase, airway smooth muscle, contraction, Ca2+-mobilization, Ca2+-influx, Ca2+-sensitization, bovine, muscarinic agonists, muscarinic receptors, Y-27632
Introduction
Muscarinic receptor stimulation in airway smooth muscle (ASM) results in the activation of phospholipase C, subsequently followed by the production of sn-1,2-diacylglycerol and inositol1,4,5-trisphosphate (IP3) (Takuwa et al., 1986; Meurs et al., 1989; Yang et al., 1991). In response to IP3, Ca2+ is being mobilized from intracellular stores (Hashimoto et al., 1985), causing a rapid, transient rise in intracellular Ca2+-concentration [Ca2+]i (Yang et al., 1993), which is followed by a sustained influx of extracellular Ca2+. Smooth muscle contraction is then initiated through the formation of Ca2+-calmodulin and subsequent activation of myosin light chain kinase (MLCK), resulting in the phosphorylation of the 20 kDa regulatory myosin light chain (MLC20) (de Lanerolle & Paul, 1991; Pfitzer, 2001). Recently, it has been established that contractile stimuli do not exert their effects only by increasing [Ca2+]i, but also by increasing Ca2+-sensitivity of the smooth muscle. One of the main regulators involved in this so-called Ca2+-sensitization is Rho-kinase, which acts through inhibition of myosin light chain phosphatase, resulting in an enhanced MLC20 phosphorylation and thus an increased level of contraction at a certain [Ca2+]i (Fukata et al., 2001; Somlyo & Somlyo, 2003).
Contraction of ASM preparations by muscarinic receptor agonists is mediated primarily through M3-receptor stimulation. Several studies have demonstrated that the involvement of the M2-receptor in ASM contraction is minor or negligible (Roffel et al., 1988; 1990; Watson et al., 1995). Meurs et al. (1988) compared concentration–response curves (CRCs) for contraction and inositol phosphate accumulation in response to partial and full muscarinic receptor agonists, and demonstrated that a strong linear correlation between these two parameters exists. In addition, they showed a considerable reserve of inositol phosphate production for the full agonists methacholine and oxotremorine, but not for the partial agonist McN-A-343 (Meurs et al., 1988). This transduction reserve is in accordance with studies showing that acetylcholine induces maximal force development by only occupying 4% of the available muscarinic receptors (large receptor reserve), whereas McN-A-343 has to occupy 80% of the receptors (low receptor reserve) to achieve the same degree of force (Gunst et al., 1987; 1989). Furthermore, it has been demonstrated that canine tracheal smooth muscle shortens at a significantly faster rate when contracted with acetylcholine than with McN-A-343 (Gunst et al., 1992). Large differences between full and partial M3-agonists regarding Ca2+-mobilizing capacity have been described, whereas no differences in dependency on Ca2+-influx through voltage-dependent channels appear to be present (al-Hassani et al., 1993). To further elucidate mechanisms underlying the differences between full and partial M3-receptor agonists, we investigated the contribution of Rho-kinase to ASM-contraction, Ca2+-mobilization and Ca2+-influx in response to the full muscarinic agonist methacholine and the partial muscarinic agonists pilocarpine and McN-A-343 (Meurs et al., 1988; Offer et al., 1991). We demonstrate that these agonists are differentially dependent on Ca2+-mobilization/-influx and Rho-kinase for their contractile effects, and that the functional dependency on Ca2+-mobilization and -influx is inversely correlated with Rho-kinase dependency.
Methods
Tissue preparation and organ-culture procedure
Bovine tracheae were obtained from local slaughterhouses and rapidly transported to the laboratory in Krebs–Henseleit (KH) buffer of the following composition (mM): NaCl 117.5, KCl 5.60, MgSO4 1.18, CaCl2 2.50, NaH2PO4 1.28, NaHCO3 25.00 and glucose 5.50, pregassed with 5% CO2 and 95% O2, pH 7.4. After dissection of the smooth muscle layer and careful removal of mucosa and connective tissue, tracheal smooth muscle strips were prepared while incubated in gassed KH-buffer at room temperature. Care was taken to cut tissue strips with macroscopically identical length (1 cm) and width (2 mm). Tissue strips were washed once in sterile Dulbecco's modification of Eagle's medium (DMEM), supplemented with NaHCO3 (10 mM), HEPES (20 mM), sodium pyruvate (1 mM), nonessential amino acid mixture (1:100), gentamicin (45 μg ml−1), penicillin (100 U ml−1), streptomycin (100 μg ml−1) and amphotericin B (1.5 μg ml−1). Next, tissue strips were transferred into suspension culture flasks (containing 2.5 ml medium per tissue strip) and maintained overnight.
Isometric tension measurements
Tissue strips, collected from suspension culture flasks, were washed with several volumes of KH-buffer pregassed with 5% CO2 and 95% O2, pH 7.4, at 37°C. Subsequently, strips were mounted for isometric recording (Grass force–displacement transducer FT03) in 20 ml water-jacked organ baths, containing KH-buffer at 37°C, continuously gassed with 5% CO2 and 95% O2, pH 7.4. During a 90 min equilibration period, with washouts every 30 min, resting tension was gradually adjusted to 3 g. Subsequently, muscle strips were precontracted with 20 and 40 mM isotonic KCl solutions. Following two wash-outs, maximal relaxation was established by the addition of 0.1 μM (−)-isoprenaline. In over 95% of the experiments, no basal myogenic tone was detected. Tension was now re-adjusted to 3 g, immediately followed by two changes of fresh KH-buffer. After another equilibration period of 30 min, cumulative CRCs were constructed to stepwise increasing concentrations of methacholine, pilocarpine or McN-A-343 (1 nM–100 μM). When maximal agonist-induced tension was obtained, the strips were washed several times and maximal relaxation was established using (−)-isoprenaline (10 μM). When used, the Rho-kinase inhibitor Y-27632 (1 μM) was applied to the organ bath 30 min before agonist addition. This concentration has been shown to be effective and selective in smooth muscle (Uehata et al., 1997; Gosens et al., 2004b; Schaafsma et al., 2004). In a separate set of experiments, 100 μM of the alkylating muscarinic receptor antagonist propylbenzilylcholine mustard (PrBCm) was preincubated for 15 min, followed by several washouts, prior to the construction of a cumulative CRC of methacholine.
Isolation of bovine tracheal smooth muscle (BTSM) cells
After the removal of mucosa and connective tissue, tracheal smooth muscle was chopped using a McIlwain tissue chopper, two times at the size of 300 μm and three times at 100 μm. Tissue fragments were washed under sterile conditions (three times) and maintained overnight in DMEM supplemented with NaHCO3 (10 mM), HEPES (20 mM), penicillin (100 U ml−1), streptomycin (100 μg ml−1) and 10% fetal bovine serum (FBS) in an incubator shaker (Innova 4000) at 37°C, 55 r.p.m.
Tissue fragments were washed three times in Krebs–Ringer–Henseleit (KRH) buffer containing (mM): NaCl 125.0, KCl 6.0, MgCl2 2.5, CaCl2 1.2, NaH2PO4 1.2, NaHCO3 25.0, glucose 11.0, HEPES 25.0, pH 7.4, supplemented with 2.0 mM (±)-dithiothreitol (DTT) and resuspended in a digestion mixture of collagenase P (0.75 mg ml−1), papain (1 mg ml−1) and trypsin inhibitor (1 mg ml−1) in KHR buffer. The suspension was incubated for 20 min at 37°C (55 r.p.m.) and then gently dispersed with a wide-bored pipette. After another 10 min of incubation at 37°C (70 r.p.m.), the suspension was gently dispersed again and filtered over a 50 μm gauze. The cells were collected by centrifugation (1000 × g, 10 min), washed three times with KHR, pH 7.4, supplemented with 2 mg ml−1 FBS (KRH/FBS), and were allowed to regenerate for 1 h at 37°C (55 r.p.m.). Subsequently, the cells were incubated with the fluorescent dye Fura-2/AM (3 μM) for 30 min at 37°C (55 r.p.m.). The loaded cells were washed three times in KRH/FBS and resuspended to a density of 1 × 106 cells ml−1. The cells were kept at room temperature on a Rock-N-Roller (Breda Scientific, The Netherlands) and used within 2–4 h, during which they remained viable and responsive.
Intracellular Ca2+-measurements
Fura-2 fluorescence of the cells (excitation wavelengths: 340 and 380 nm; emission wavelength: 510 nm) was measured at 37°C with a Perkin-Elmer spectometer (LS-50B). Each cuvet contained 2 ml of magnetically stirred cell suspension. Ca2+-mobilization and -influx induced by methacholine (30 nM–300 μM), pilocarpine (100 nM–300 μM) or McN-A-343 (100 nM–300 μM), added at t=60 s, were measured. When used, Y-27632 (1 μM) was added 30 min prior to agonist addition. The intracellular Ca2+-concentration ([Ca2+]i) was calculated every 0.2 s according to Grynkiewicz et al. (1985). At the end of the experiment, the maximal fluorescence ratio (Rmax) was determined after adding 0.01% of Triton-X-100 as a permeabilizing agent. The minimal fluorescence ratio (Rmin) was determined by addition of 5 mM EGTA to the permeabilized cells.
Data analysis
All data represent means±s.e.m. from n separate experiments. CRCs of contractile responses were analyzed by measuring myogenic tension. No corrections were made for basal tone. Maximal tension (Emax) and pEC50 were calculated from the CRCs. Curves were fitted using the logistic four-parameter model (Sigmaplot 9.0, SPSS Inc.).
Agonist-induced transient rise of [Ca2+]i, representing Ca2+-mobilization from internal stores, was expressed as the maximal increase above basal after addition of the agonist. The plateau level of [Ca2+]i, representing Ca2+-influx, was measured 2 min after agonist addition and expressed as [Ca2+]i above basal. CRCs for Ca2+-mobilization and -influx were expressed as a percentage of the maximal methacholine-induced Ca2+-mobilization.
Statistical significance of differences between data was determined using the two-tailed Student's t-test for paired observations or one-way analysis of variance, where appropriate. Differences were considered to be statistically significant when P<0.05.
Materials
Dulbecco's modification of Eagle's Medium (DMEM) and methacholine hydrochloride were obtained from ICN Biomedicals (Costa Mesa, CA, U.S.A.). Foetal bovine serum, NaHCO3 solution (7.5%), HEPES solution (1 M), sodium pyruvate solution (100 mM), nonessential amino acid mixture, gentamycin solution (10 mg ml−1), penicillin/streptomycin solution (5000 U ml−1; 5000 μg ml−1) and amphotericin B solution (250 μg ml−1) (Fungizone) were obtained from Gibco BRL Life Technologies (Paisley, U.K.). Dithiothreitol (DTT), soybean trypsin inhibitor, Fura-2/AM, pilocarpine hydrochloride and (−)-isoprenaline hydrochloride were from Sigma Chemical Co. (St Louis, MO, U.S.A.). McN-A-343 was purchased from RBI, Natick, MA, U.S.A.
(+)-(R)-trans-4-(1-aminoethyl)-N-(4-pyridyl) cyclohexane carboxamide (Y-27632) was obtained from Tocris Cookson Ltd (Bristol, U.K.). L(+)ascorbic acid was from Merck (Darmstadt, Germany). Propylbenzilylcholine mustard was from NEN products (Boston, MA, U.S.A.). Papain and Collagenase P were from Boehringer (Mannheim, Germany). All other chemicals were of analytical grade.
Results
Effects of Rho-kinase inhibition on methacholine-, pilocarpine- and McN-A-343-induced ASM contraction
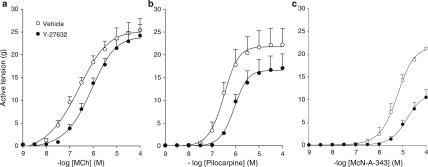
In BTSM strip preparations, pilocarpine and McN-A-343 reached up to 87 and 84%, respectively, of the maximal contraction (Emax) induced by methacholine, indicating that both pilocarpine and McN-A-343 are partial agonists (Figure 1, Table 1).
Figure 1.
Effects of Rho-kinase inhibition on contraction induced by full and partial M3-receptor agonists. Methacholine (MCh, a)-, pilocarpine (b)- and McN-A-343 (c)-induced contraction in the absence (open circles) and presence (closed circles) of 1 μM Y-27632 of BTSM strips. Data represent means±s.e.m. of 3–7 experiments, each performed in duplicate.
Table 1.
Effects of Rho-kinase inhibition on contractile properties of bovine tracheal smooth muscle preparations following administration of muscarinic receptor agonists
| Vehicle | Y-27632 | |||
|---|---|---|---|---|
| pEC50 (−log M) | Emax (g) | pEC50 (−log M) | Emax (g) | |
| Methacholine | 6.73±0.15 | 25.4±2.5 | 6.19±0.15** | 24.2±2.4 |
| after PrBCm | 3.59±0.14 | 21.8±1.2 | 2.91±0.17* | 16.3±1.6* |
| Pilocarpine | 6.52±0.08 | 22.2±3.6 | 6.09±0.02* | 17.1±3.1* |
| McN-A-343 | 5.26±0.09 | 21.3±0.2 | 4.93±0.04* | 10.5±1.7* |
Data represent means±s.e.m. of 3–7 experiments, each performed in duplicate.
P<0.05
P<0.01 vs vehicle-treated.
To determine the contribution of Rho-kinase, we used the selective Rho-kinase inhibitor Y-27632 (1 μM). With methacholine, no effect of Rho-kinase inhibition was observed on Emax, but a significant decrease in potency (pEC50) was observed. Interestingly, treatment with Y-27632 significantly decreased Emax of pilocarpine and McN-A-343 by 23.4±2.8 and 50.4±7.9%, respectively, and also significantly decreased potency (Figure 1, Table 1). Remarkably, the effect of Y-27632 in decreasing pEC50 values diminished in the order methacholine>pilocarpine>McN-A-343.
Effects of Rho-kinase inhibition on intracellular Ca2+ homeostasis in response to methacholine, pilocarpine and McN-A-343
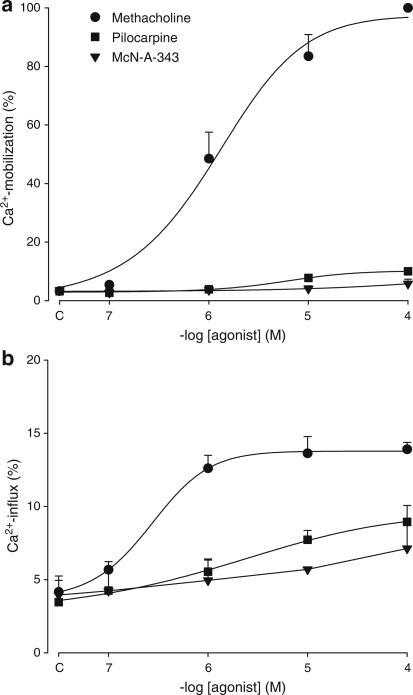
Ca2+-mobilization and -influx were determined in response to several concentrations of methacholine, pilocarpine and McN-A-343 (Figure 2). Both mobilization and influx were calculated as a percentage of the maximal methacholine-induced Ca2+-mobilization, which amounted to 349.7±53.9 nM Ca2+. Mobilization induced by pilocarpine and McN-A-343 (Figure 2a) was significantly lower as compared to that induced by methacholine, and reached up to only 10.0±0.8 and 5.8±1.6%, respectively (P<0.001, both). Ca2+-influx in response to these agonists was also significantly lower (8.9±1.1 and 7.1±1.7%, respectively; Figure 2b) as compared to methacholine-induced influx (13.9±0.5%; P<0.01, both).
Figure 2.
Ca2+-mobilization and -influx in response to full and partial M3-receptor agonists. Methacholine (circles)-, pilocarpine (squares)- and McN-A-343 (triangles)-induced Ca2+-mobilization (a) and -influx (b) in BTSM cells. Data are expressed as percentage of maximal Ca2+-mobilization induced by methacholine and represent means±s.e.m. of three experiments, each performed in duplicate.
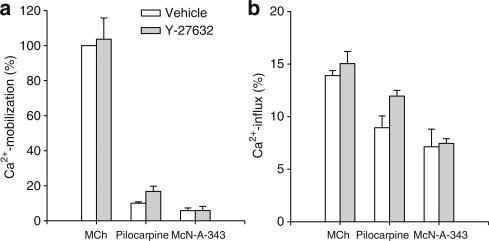
Next, to determine whether the effects of Rho-kinase inhibition on contraction (Figure 1) could be linked to the effects on Ca2+-mobilization or -influx, we measured Ca2+-responses induced by 100 μM methacholine, pilocarpine and McN-A-343 in the presence and absence of 1 μM Y-27632. As shown in Figure 3, Y-27632 had no inhibitory effect on Ca2+-mobilization or -influx induced by either agonist, indicating that the inhibitory effects of Y-27632 on maximal pilocarpine- and McN-A-343-induced contractions are not caused by changes in Ca2+-mobilization or -influx.
Figure 3.
Effects of Rho-kinase inhibition on Ca2+-responses induced by full and partial M3-receptor agonists. Ca2+-mobilization (a) and -influx (b) in response to methacholine (MCh), pilocarpine and McN-A-343 in the absence (white bars) and presence (gray bars) of 1 μM Y-27632 in BTSM cells. Data are expressed as percentage of maximal Ca2+-mobilization induced by methacholine and represent means±s.e.m. of three experiments, each performed in duplicate.
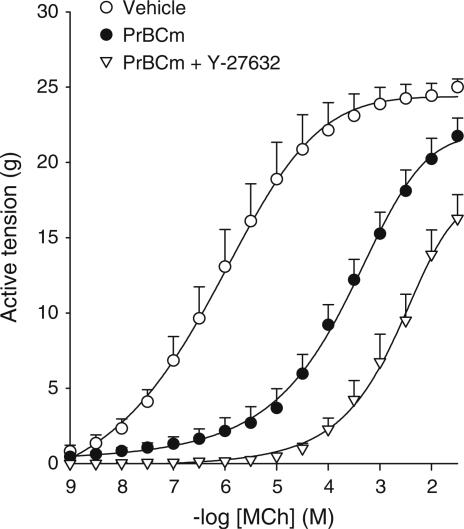
Effects of muscarinic receptor alkylation on the Rho-kinase dependency of the methacholine-induced contraction
To determine whether differences in receptor reserve could be responsible for the difference in Rho-kinase dependency of the contraction induced by full and partial agonists, we removed receptor reserve of the full agonist methacholine by using the alkylating agent propylbenzilylcholine mustard (PrBCm). In contrast to methacholine-induced contraction of untreated strips (Figure 1a), maximal methacholine-induced contraction was Rho-kinase dependent after treatment with PrBCm (24.8±9.2% inhibition of Emax in the presence of Y-27632; P<0.05; Figure 4). These results suggest that the contribution of Rho-kinase to muscarinic agonist-induced contraction is dependent on receptor/transduction reserve. Interestingly, after PrBCm pretreatment, the reduction of the pEC50 value of methacholine by Y-27632 was of similar magnitude as in nonalkylated preparations (Table 1).
Figure 4.
Effects of Rho-kinase inhibition on methacholine-induced contraction after pretreatment with propylbenzilylcholine mustard (PrBCm). Methacholine (MCh)-induced contraction in the absence (closed circles) and presence (triangles) of 1 μM Y-27632 of PrBCm-treated BTSM strips. Data represent means±s.e.m. of 3–4 experiments, each performed in duplicate.
Relationship between Rho-kinase dependency and Ca2+-mobilization and -influx
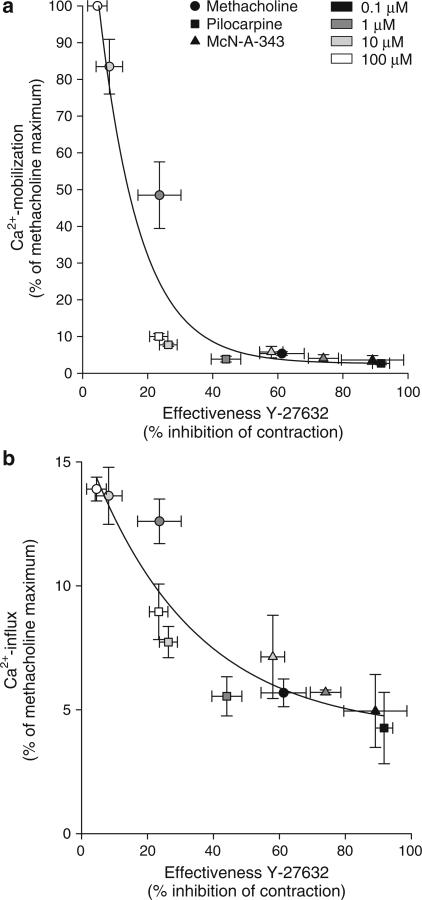
For several agonist concentrations, we have plotted the effectiveness of Y-27632 in decreasing contraction (% inhibition) against the induced Ca2+-mobilization and the Ca2+-influx (Figure 5). An inverse relationship between Rho-kinase dependency and Ca2+-mobilization (Figure 5a) as well as Ca2+-influx (Figure 5b) was found.
Figure 5.
Relationship between methacholine (circles)-, pilocarpine (squares)- and McN-A-343 (triangles)-induced Ca2+-mobilization (a) and -influx (b) in BTSM cells and Rho-kinase dependency of methacholine-, pilocarpine- and McN-A-343-induced contraction of BTSM strips. Data represent means±s.e.m. of 3–7 experiments, each performed in duplicate.
Discussion
In the present study, we demonstrate that full and partial muscarinic agonists are differentially dependent on Rho-kinase and Ca2+-mobilization for their contractile effects. Moreover, we show that there is an inverse (exponential) relationship between the Rho-kinase dependency of the contraction and the Ca2+-mobilization as well as the Ca2+-influx induced by different concentrations of the agonists. These findings correspond to observations in canine tracheal smooth muscle using acetylcholine and McN-A-343, indicating that differences in Ca2+-kinetics are possibly a consequence of differences in potency of muscarinic agonists to activate the same subcellular pathways, rather than from the activation of distinct subcellular mechanisms (al-Hassani et al., 1993).
A receptor-dependent role of Rho-kinase in agonist-induced ASM contraction appears to exist. Both in guinea-pig (Schaafsma et al., 2005) and human (Gosens et al., 2004a) ASM, it was found that growth factors, which are coupled to receptor tyrosine kinases, induce contractions which are fully Rho-kinase dependent, whereas contractions elicited by histamine, which are mediated through Gq/11-coupled H1 receptors, are not (Gosens et al., 2004a; Schaafsma et al., 2004). In addition, potency and maximal contraction induced by PGF2α are governed by Rho-kinase activity in guinea-pig tracheal smooth muscle to an important extent, whereas histamine-induced contractions are not (Schaafsma et al., 2004). The agonists used in the present study all function through activation of the M3-receptor in order to induce ASM contraction. The observed differential involvement of Rho-kinase by partial and full agonists therefore implies that there is not only a receptor-dependent, but also an agonist-dependent role for the participation of Rho-kinase in ASM contraction.
Rho-kinase inhibition resulted in a decrease in potency of methacholine, pilocarpine and McN-A-343, whereas Emax was affected only for pilocarpine and McN-A-343. Remarkably, the strongest decrease in potency was observed for methacholine and the smallest for McN-A-343; in contrast to the effects on Emax, which were absent for methacholine and most pronounced for McN-A-343. This indicates that methacholine is only dependent on Rho-kinase for its contractile effects in the lower concentration range, without requiring Rho-kinase to achieve its maximal contractile response. In BTSM, there is a considerable reserve of inositol phosphate production (transduction reserve) for methacholine, but not for McN-A-343 (Meurs et al., 1988). If the maximally stimulated M3-receptor generates more Rho-kinase activity than ‘required' for maximal contractile effect, inhibition of this signal transduction will affect the potency, not the efficacy, of the agonist. In other words, methacholine induces the largest IP3-dependent Ca2+-mobilization and -influx, which is probably sufficient to achieve maximal contraction independent of Rho-kinase-mediated Ca2+-sensitization. In contrast, very low levels of Ca2+-mobilization are observed in response to pilocarpine and McN-A-343, presumably as a consequence of a small and neglectable transduction reserve, respectively (Meurs et al., 1988), and therefore these agonists do rely on Rho-kinase for their maximal contractile response. Also, fully in line with this interpretation, maximal methacholine-induced contraction becomes Rho-kinase dependent after pretreatment with the irreversible muscarinic receptor antagonist PrBCm, demonstrating that the contribution of Rho-kinase to muscarinic agonist-induced contraction is dependent on transductional reserve. Remarkably, in the presence of Y-27632, a similar decrease of potency is observed in PrBCm-treated strips as in untreated strips. An additional explanation for the differences between full and partial agonists in the modulation of the maximal contraction might therefore be that partial agonists have different relative activation profiles for Gq- and Gi/o-proteins as compared to full agonists, as demonstrated in Chinese hamster ovary cells (Akam et al., 2001).
The inverse relationship between Rho-kinase dependency and both Ca2+-mobilization and Ca2+-influx, as shown in Figure 5, might suggest that Y-27632 is capable of reducing these Ca2+-responses. However, we demonstrate that Y-27632 does not inhibit Ca2+-mobilization or Ca2+-influx for all three agonists at their maximal concentration. In addition, no effect of Rho-kinase inhibition was observed when a submaximal concentration of methacholine (1 μM) was applied (data not shown), corresponding to findings in guinea-pig trachealis, which showed no effects of Y-27632 (1–10 μM) on methacholine (1 μM)-induced Ca2+-mobilization (Ito et al., 2001). This strongly suggests that the effects of Rho-kinase inhibition are on the level of Ca2+-sensitization rather than on Ca2+-mobilization or -influx.
In conclusion, this study shows that full and partial muscarinic receptor agonists are differentially dependent on Rho-kinase for their contractile effects, indicating an agonist-dependent role for Rho-kinase in ASM contraction. Furthermore, we demonstrate that there is an inverse relationship between both Ca2+-mobilization and -influx and the functional Rho-kinase dependency, presumably as a consequence of differences in transduction reserve between full and partial M3-receptor agonists. Moreover, Ca2+-mobilization and -influx in response to M3-receptor stimulation seem to be independent of Rho-kinase, suggesting that differences in functional Rho-kinase dependency are on the level of Ca2+-sensitization rather than on Ca2+-mobilization or -influx.
Acknowledgments
This work was financially supported by the Netherlands Asthma Foundation, NAF grant 01.83.
Abbreviations
- ASM
airway smooth muscle
- BTSM
bovine tracheal smooth muscle
- CCRC
cumulative concentration–response curve
- KH
Krebs–Henseleit
- MLC
myosin light chain
- MLCK
myosin light chain kinase
- MLCP
myosin light chain phosphatase
- pEC50
−log10 of the concentration causing 50% of the effect
References
- AKAM E.C., CHALLISS R.A., NAHORSKI S.R. G(q/11) and G(i/o) activation profiles in CHO cells expressing human muscarinic acetylcholine receptors: dependence on agonist as well as receptor-subtype. Br. J. Pharmacol. 2001;132:950–958. doi: 10.1038/sj.bjp.0703892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AL-HASSANI M.H., GARCIA J.G., GUNST S.J. Differences in Ca2+ mobilization by muscarinic agonists in tracheal smooth muscle. Am. J. Physiol. 1993;264:L53–L59. doi: 10.1152/ajplung.1993.264.1.L53. [DOI] [PubMed] [Google Scholar]
- DE LANEROLLE P., PAUL R.J. Myosin phosphorylation/dephosphorylation and regulation of airway smooth muscle contractility. Am. J. Physiol. 1991;261:L1–L14. doi: 10.1152/ajplung.1991.261.2.L1. [DOI] [PubMed] [Google Scholar]
- FUKATA Y., AMANO M., KAIBUCHI K. Rho–Rho-kinase pathway in smooth muscle contraction and cytoskeletal reorganization of non-muscle cells. Trends Pharmacol. Sci. 2001;22:32–39. doi: 10.1016/s0165-6147(00)01596-0. [DOI] [PubMed] [Google Scholar]
- GOSENS R., SCHAAFSMA D., GROOTTE BROMHAAR M.M., VRUGT B., ZAAGSMA J., MEURS H., NELEMANS S.A. Growth factor-induced contraction of human bronchial smooth muscle is Rho-kinase-dependent. Eur. J. Pharmacol. 2004a;494:73–76. doi: 10.1016/j.ejphar.2004.04.035. [DOI] [PubMed] [Google Scholar]
- GOSENS R., SCHAAFSMA D., MEURS H., ZAAGSMA J., NELEMANS S.A. Role of Rho-kinase in maintaining airway smooth muscle contractile phenotype. Eur. J. Pharmacol. 2004b;483:71–78. doi: 10.1016/j.ejphar.2003.10.027. [DOI] [PubMed] [Google Scholar]
- GRYNKIEWICZ G., POENIE M., TSIEN R.Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- GUNST S.J., GERTHOFFER W.T., AL-HASSANI M.H. Ca2+ sensitivity of contractile activation during muscarinic stimulation of tracheal muscle. Am. J. Physiol. 1992;263:C1258–C1265. doi: 10.1152/ajpcell.1992.263.6.C1258. [DOI] [PubMed] [Google Scholar]
- GUNST S.J., STROPP J.Q., FLAVAHAN N.A. Analysis of receptor reserves in canine tracheal smooth muscle. J. Appl. Physiol. 1987;62:1755–1758. doi: 10.1152/jappl.1987.62.4.1755. [DOI] [PubMed] [Google Scholar]
- GUNST S.J., STROPP J.Q., FLAVAHAN N.A. Muscarinic receptor reserve and beta-adrenergic sensitivity in tracheal smooth muscle. J. Appl. Physiol. 1989;67:1294–1298. doi: 10.1152/jappl.1989.67.3.1294. [DOI] [PubMed] [Google Scholar]
- HASHIMOTO T., HIRATA M., ITO Y. A role for inositol 1,4,5-trisphosphate in the initiation of agonist-induced contractions of dog tracheal smooth muscle. Br. J. Pharmacol. 1985;86:191–199. doi: 10.1111/j.1476-5381.1985.tb09449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ITO S., KUME H., HONJO H., KATOH H., KODAMA I., YAMAKI K., HAYASHI H. Possible involvement of Rho kinase in Ca2+ sensitization and mobilization by MCh in tracheal smooth muscle. Am. J. Physiol. Lung Cell Mol. Physiol. 2001;280:L1218–L1224. doi: 10.1152/ajplung.2001.280.6.L1218. [DOI] [PubMed] [Google Scholar]
- MEURS H., ROFFEL A.F., POSTEMA J.B., TIMMERMANS A., ELZINGA C.R., KAUFFMAN H.F., ZAAGSMA J. Evidence for a direct relationship between phosphoinositide metabolism and airway smooth muscle contraction induced by muscarinic agonists. Eur. J. Pharmacol. 1988;156:271–274. doi: 10.1016/0014-2999(88)90331-7. [DOI] [PubMed] [Google Scholar]
- MEURS H., TIMMERMANS A., VAN AMSTERDAM R.G., BROUWER F., KAUFFMAN H.F., ZAAGSMA J. Muscarinic receptors in human airway smooth muscle are coupled to phosphoinositide metabolism. Eur. J. Pharmacol. 1989;164:369–371. doi: 10.1016/0014-2999(89)90480-9. [DOI] [PubMed] [Google Scholar]
- OFFER G.J., CHILVERS E.R., NAHORSKI S.R. Beta-adrenoceptor induced inhibition of muscarinic receptor-stimulated phosphoinositide metabolism is agonist specific in bovine tracheal smooth muscle. Eur. J. Pharmacol. 1991;207:243–248. doi: 10.1016/0922-4106(91)90036-h. [DOI] [PubMed] [Google Scholar]
- PFITZER G. Invited review: regulation of myosin phosphorylation in smooth muscle. J. Appl. Physiol. 2001;91:497–503. doi: 10.1152/jappl.2001.91.1.497. [DOI] [PubMed] [Google Scholar]
- ROFFEL A.F., ELZINGA C.R., VAN AMSTERDAM R.G., DE ZEEUW R.A., ZAAGSMA J. Muscarinic M2 receptors in bovine tracheal smooth muscle: discrepancies between binding and function. Eur. J. Pharmacol. 1988;153:73–82. doi: 10.1016/0014-2999(88)90589-4. [DOI] [PubMed] [Google Scholar]
- ROFFEL A.F., ELZINGA C.R., ZAAGSMA J. Muscarinic M3 receptors mediate contraction of human central and peripheral airway smooth muscle. Pulm. Pharmacol. 1990;3:47–51. doi: 10.1016/0952-0600(90)90009-8. [DOI] [PubMed] [Google Scholar]
- SCHAAFSMA D., GOSENS R., BOS I.S., MEURS H., ZAAGSMA J., NELEMANS S.A. Allergic sensitization enhances the contribution of Rho-kinase to airway smooth muscle contraction. Br. J. Pharmacol. 2004;143:477–484. doi: 10.1038/sj.bjp.0705903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHAAFSMA D., GOSENS R., BOS S., MEURS H., ZAAGSMA J., NELEMANS A. Role of contractile prostaglandins and Rho-kinase in growth factor-induced airway smooth muscle contraction. Respir. Res. 2005;6:85. doi: 10.1186/1465-9921-6-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOMLYO A.P., SOMLYO A.V. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol. Rev. 2003;83:1325–1358. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- TAKUWA Y., TAKUWA N., RASMUSSEN H. Carbachol induces a rapid and sustained hydrolysis of polyphosphoinositide in bovine tracheal smooth muscle measurements of the mass of polyphosphoinositides, 1,2-diacylglycerol, and phosphatidic acid. J. Biol. Chem. 1986;261:14670–14675. [PubMed] [Google Scholar]
- UEHATA M., ISHIZAKI T., SATOH H., ONO T., KAWAHARA T., MORISHITA T., TAMAKAWA H., YAMAGAMI K., INUI J., MAEKAWA M., NARUMIYA S. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389:990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- WATSON N., MAGNUSSEN H., RABE K.F. Pharmacological characterization of the muscarinic receptor subtype mediating contraction of human peripheral airways. J. Pharmacol. Exp. Ther. 1995;274:1293–1297. [PubMed] [Google Scholar]
- YANG C.M., CHOU S.P., SUNG T.C. Muscarinic receptor subtypes coupled to generation of different second messengers in isolated tracheal smooth muscle cells. Br. J. Pharmacol. 1991;104:613–618. doi: 10.1111/j.1476-5381.1991.tb12478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANG C.M., YO Y.L., WANG Y.Y. Intracellular calcium in canine cultured tracheal smooth muscle cells is regulated by M3 muscarinic receptors. Br. J. Pharmacol. 1993;110:983–988. doi: 10.1111/j.1476-5381.1993.tb13910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]