Abstract
In rat ventricular cardiomyocytes β2-adrenoceptors (AR) couple to Gs- and Gi-protein, and evidence has accumulated that β2-AR agonists can differentially activate either Gs- or Gs- and Gi-protein.
In this study, in isolated adult rat ventricular cardiomyocytes, we assessed the effects of pertussis toxin (PTX) on β2-AR agonist (terbutaline (TER), salbutamol (SAL) and fenoterol (FEN)) evoked inhibition of phenylephrine (PE)-induced increase in the rate of protein synthesis (assessed as [3H]phenylalanine incorporation) to find out which β2-AR agonist activates selectively Gs- or Gs- and Gi-protein.
PE (1 μM) increased the rate of protein synthesis from 100% to 130±2% (n=34). FEN, TER and SAL (1 nM–10 μM) inhibited PE-induced increase in the rate of protein synthesis concentration-dependently. FEN inhibited PE effects almost completely (from 132±3 to 101±1%), whereas TER and SAL caused only partial inhibition (from 131±2 to 114±2 and 129±1 to 111±2%, respectively).
Pretreatment of cardiomyocytes with PTX (250 ng ml−1 for 16 h at 37°C) did not affect FEN effects, but converted TER- and SAL-evoked partial inhibition into complete inhibition.
Inhibitory effects of the three β2-AR agonists were markedly attenuated by β1-AR selective antagonist CGP 20712A (CGP) (300 nM); in contrast, β2-AR selective antagonist ICI 118,551 (55 nM) inhibited the inhibitory effects of the three β2-AR agonists only in PTX-pretreated cardiomyocytes,with β1-AR blocked by CGP.
We conclude that, in adult rat ventricular cardiomyocytes, FEN activates selectively the Gs protein-pathway, while TER and SAL activate the Gs- and Gi-protein pathways. Part of the effects of these three β2-AR agonists appears to be mediated by β1-AR.
Keywords: Protein synthesis, adult rat cardiomyocytes, β2-adrenoceptors, β2-adrenoceptor-Gi-/GS-protein pathway, terbutaline, salbutamol, fenoterol
Introduction
It is now generally accepted that in the heart of various species, including humans, β1- and β2-adrenoceptors (AR) coexist (Brodde & Michel, 1999). Both β-AR subtypes couple to the Gs-protein-adenylyl cyclase pathway and mediate increase in heart rate and contractility. However, evidence has accumulated that, at least in the rat heart, stimulation of β1-AR causes not only positive ino- and chronotropic effects, but can also evoke apoptosis of the cardiomyocytes (Communal et al., 1999; Zaugg et al., 2000; Zhu et al., 2001; Shizukuda & Buttrick, 2002).
Moreover, β2-AR in the rat heart have been shown to couple not only to the Gs-protein but also to the Gi-protein (Xiao et al., 1995), thereby inducing antiapoptosis (Communal et al., 1999; Zaugg et al., 2000; Zhu et al., 2001; Shizukuda & Buttrick, 2002).
Recently, Xiao et al. (2003) showed, in isolated ventricular cardiomyocytes from SHR rats with heart failure, that the contractile response to several β2-AR agonists, including terbutaline (TER), salbutamol (SAL), zinterol and procaterol, could be markedly enhanced when the cardiomyocytes were pretreated with pertussis toxin (PTX), thereby inactivating the Gi-protein. Interestingly, however, the contractile response to another β2-AR agonist, fenoterol (FEN), was not affected by PTX treatment indicating that FEN might activate solely the β2-AR–Gs-protein pathway.
We have recently shown that, in adult rat ventricular cardiomyocytes, stimulation of α1-AR causes increase in the rate of protein synthesis (Schäfer et al., 2001; Pönicke et al., 2003). This could be inhibited by isoprenaline via β1-AR stimulation and involves cAMP because the isoprenaline effect could be mimicked by dibutyryl cyclic AMP (Schäfer et al., 2001).
The aim of the present study was to find out whether also in this system action of FEN might be different from that of TER and SAL. We hypothesized that if FEN activates only the β2-AR–Gs-protein pathway its inhibition of phenylephrine (PE)-induced increase in cardiomyocyte protein synthesis should be insensitive to PTX treatment, while the inhibitory effects of SAL and TER acting in Gs- and Gi-protein (Xiao et al., 2003) should be enhanced by PTX treatment. Thus, we determined, in isolated adult rat ventricular cardiomyocytes, the effects of PTX treatment on FEN-, TER- and SAL-induced inhibition of PE-evoked increase in the rate of protein synthesis (assessed as incorporation of [3H]phenylalanine).
Methods
Preparation of cardiomyocyte culture of adult rats
Adult rat left ventricular cardiomyocytes were isolated from 12-week-old male Wistar rats exactly as detailed elsewhere (Pönicke et al., 2000; 2003). Freshly isolated cardiomyocytes were gently diluted in sterile culture medium M199, pH 7.4, supplemented with 10% new-born calf serum. The cardiomyocyte suspension was seeded into 12-well plates (16,000 cells per well) which had been coated with 4% fetal calf serum in medium M199 for 24 h at 37°C (in a humidified incubator at 95% air/5% CO2) and incubated for 16 h at 37°C. Thereafter, the cultures were rinsed with serum-free Hank's balanced salt solution to remove damaged, rounded and nonattached cardiomyocytes, and the rod-shaped cells were cultured in serum-free medium M199 supplemented with 2 mM L-carnitine, 5 mM taurine, 5 mM creatine and antibiotics (100 U ml−1 penicillin and 100 μg ml−1 streptomycin). To prevent growth of nonmyocytes, the culture medium was supplemented with 10 μM cytosine-β-D-arabinofuranoside.
[3H]phenylalanine incorporation
Protein synthesis by cardiomyocytes was assessed as incorporation of [3H]phenylalanine into cells exactly as described previously (Pönicke et al., 2000; 2003). Briefly, after addition of [3H]phenylalanine (0.5 μCi ml−1) at 37°C and 1 μM PE, as well as the indicated concentrations of the β2-AR agonists TER, FEN and SAL with or without the various antagonists (added 10 min prior to β2-AR agonists), the cells were incubated overnight (16 h) at 37°C in 95% air/5% CO2. Ascorbate (100 μM) was always present in the medium throughout this incubation period as antioxidant.
In some experiments, 250 ng ml−1 PTX was added to the cardiomyocyte suspension 16 h before the cells were exposed to [3H]phenylalanine (Pönicke et al., 2003).
To terminate the [3H]phenylalanine incorporation, cardiomyocytes were washed with ice-cold 0.9% NaCl-solution to remove attached radioactivity and incubated for 24 h at 4°C with 10% trichloroacetic acid. Acid-insoluble precipitates were washed again with 10% trichloroacetic acid and twice with 0.9% NaCl. The remaining precipitation on the culture dishes was solubilized in 1 N NaOH supplemented with 0.1% sodium dodecyl sulfate at room temperature for 24 h, and incorporation of radioactivity into acid-insoluble cell mass was determined by the use of a liquid scintillation counter (Beckman LS 6000). We have recently shown that under these experimental conditions [3H]phenylalanine incorporation was paralleled by increases in protein mass, cell volume and cross-sectional area of the cells, indicating growth of the cardiomyocytes (Schäfer et al., 2001).
Statistical evaluations
The data given in text and figures are expressed as means±s.e.m. of n experiments. Experimental data for agonist-induced inhibition of 1 μM PE-evoked [3H]phenylalanine incorporation were analyzed by fitting sigmoidal curves to the experimental data using the GraphPad Prism 3.0 program (GraphPad software, San Diego, CA, U.S.A.); the bottom of the curves was fixed to 100% (i.e. complete inhibition of 1 μM PE-evoked [3H]phenylalanine incorporation into the cardiomyocytes), the Hill slopes were fixed as 1.0. Statistical significance of differences was analyzed by paired two-tailed Student's t-test. A P-value <0.05 was considered to be significant. All statistical calculations were performed with the GraphPad Prism 3.0 program.
Drugs
L-[2,3,4,5,6-3H]phenylalanine (spec. activity: 5.03 TBq mmol−1) was purchased from Amersham Bioscience Europe GmbH (Freiburg, Germany). PTX was from Calbiochem (Merck Biosciences GmbH, Schwalbach, Germany). L-PE hydrochloride, L-phenylalanine, cytosine-β-D-arabinofuranoside, sodium dodecyl sulfate, trypsin (crude), L-carnitine, taurine, creatine, ICI 118,551 (ICI), TER sulfate and laminin were purchased from Sigma-Aldrich (Deisenhofen, Germany). FEN hydrobromide were kindly donated by Boehringer Ingelheim (Ingelheim, Germany). SAL sulfate was a generous gift of the Glaxo Group Research Ltd (Greenford, U.K.).
(1-[2-((3-carbamoyl-4-hydroxy)phenoxy)-ethyl-amino]-3-[4-(1-methyl-4-trifluoromethyl-2-imidazolyl)phenoxy]-2-propanol methanesulfonate) (CGP 20712A (CGP)) was kindly donated by Ciba-Geigy (Basel, Switzerland). Hank's balanced salt solution, and culture medium M199 and penicillin–streptomycin were obtained from Life Technologies (Eggenstein, Germany).
All other chemicals were of the highest purity grade commercially available.
Results
Effects of FEN and TER on PE-induced increase in rate of protein synthesis
PE (1 μM) caused, in isolated adult rat cardiomyocytes, an increase in rate of protein synthesis ([3H]phenylalanine incorporation) of about 30% above control. This effect is not affected by the β1-AR antagonist CGP (300 nM), the β2-AR antagonist ICI (55 nM) and by PTX treatment, but is completely blocked by the α1-AR antagonist prazosin (100 nM) (Pönicke et al., 2003).
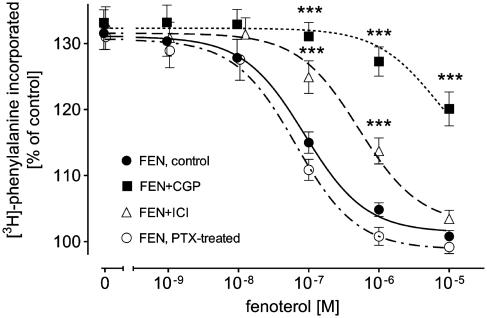
The β2-AR agonist FEN (1 nM–10 μM) concentration-dependently decreased 1 μM PE-induced increase in the rate of protein synthesis; at 10 μM FEN, the rate of protein synthesis was nearly completely suppressed (from 132±3 to 101±1% of control, Figure 1).
Figure 1.
Effects of CGP (300 nM), ICI (55 nM) and PTX pretreatment (250 ng ml−1 for 16 h) on FEN-evoked inhibition of 1 μM PE-induced [3H]phenylalanine incorporation in ventricular cardiomyocytes of 12-week-old male Wistar rats (n=14). Basal [3H]phenylalanine incorporation amounted to 884±97 c.p.m. in control, 914±105 c.p.m. in the presence of CGP, 874±115 c.p.m. in the presence of ICI and 854±137 c.p.m. after PTX pretreatment. Bars represent mean±s.e.m. ***P<0.01 versus FEN.
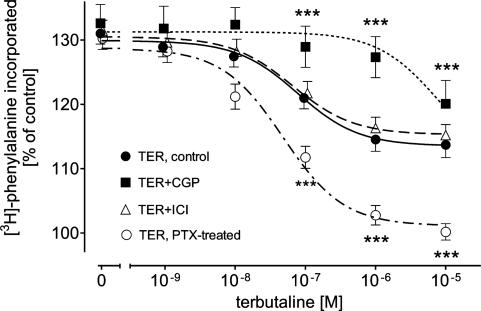
The β2-AR agonist TER (1 nM–10 μM) also concentration-dependently decreased 1 μM PE-induced increase in the rate of protein synthesis; however, in contrast to FEN, the highest concentration of TER (10 μM) caused only partial inhibition of the rate of protein synthesis (from 131±2 to 114±2% of control, Figure 2).
Figure 2.
Effects of 300 nM CGP, 55 nM ICI and PTX pretreatment on TER-evoked inhibition of 1 μM PE-induced [3H]phenylalanine incorporation in ventricular cardiomyocytes of 12-week-old male Wistar rats (n=14). Basal [3H]phenylalanine incorporation amounted to 1008±131 c.p.m. in control, 1021±107 c.p.m. in the presence of CGP, 998±97 c.p.m. in the presence of ICI and 959±117 c.p.m. after PTX pretreatment. Values are means±s.e.m. ***P<0.01 versus TER.
Effects of ICI and CGP on FEN- or TER-induced inhibition of rate of protein synthesis
We studied the effects of the highly selective β2-AR antagonist ICI (55 nM, i.e. a concentration that occupies more than 95% of β2-AR but less than 5% of β1-AR; Lemoine et al., 1985) on FEN- and TER-induced inhibition of 1 μM PE-induced increase in the rate of protein synthesis. ICI significantly shifted the concentration-inhibition curve of FEN to the right to higher concentrations (Figure 1). In contrast, however, ICI had no significant effect on the concentration–inhibition curve of TER (Figure 2).
On the other hand, the highly selective β1-AR antagonist CGP (300 nM, a concentration that occupies more than 95% of β1-AR but less than 1% of β2-AR; Kaumann & Lemoine, 1987) caused marked shifts to the right of the concentration-inhibition curves of FEN (Figure 1) and TER (Figure 2).
Effects of PTX pretreatment on FEN- or TER-induced inhibition of rate of protein synthesis
We studied the effects of FEN and TER in PTX-pretreated cardiomyocytes. For this purpose cardiomyocytes were treated overnight with PTX (250 ng ml−1) or vehicle at 37°C. We have previously shown that this treatment regimen is sufficient to completely inactivate Gi-protein (Pönicke et al., 2003). PTX pretreatment had no significant effect on FEN-induced inhibition of 1 μM PE-induced increase in the rate of protein synthesis (Figure 1). In contrast, TER-evoked inhibition of rate of protein synthesis was significantly enhanced by the PTX treatment: in PTX-treated cardiomyocytes, TER-induced partial inhibition was converted into a complete one (from 130±2 to 100±1% of control; Figure 2).
Effects of ICI on FEN- and TER-induced inhibition of rate of protein synthesis in PTX treated cardiomyocytes in the presence of CGP
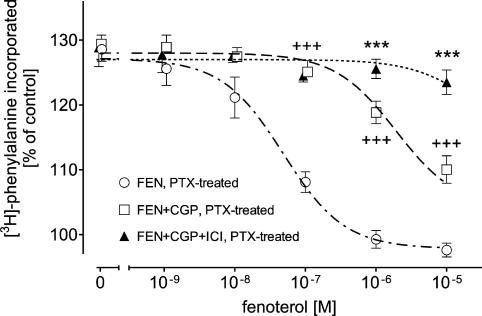
In the next series of experiments, we studied, in PTX-pretreated cardiomyocytes with β1-AR blocked by 300 nM CGP, the effects of ICI (55 nM) on FEN- and TER-induced inhibition of PE-evoked increase in the rate of protein synthesis. In PTX-treated cardiomyocytes, the concentration–response curve for the inhibitory effect of FEN was shifted to the right by CGP (Figure 3), whereby the extent of the rightward shift was comparable with that obtained in not-PTX-treated cardiomyocytes (see Figure 1). Under these experimental conditions, the inhibitory effect of FEN was completely suppressed by ICI (Figure 3).
Figure 3.
Effects of 300 nM CGP or 300 nM CGP plus 55 nM ICI on FEN-evoked inhibition of 1 μM PE-induced [3H]phenylalanine incorporation in PTX-pretreated ventricular cardiomyocytes of 12-week-old male Wistar rats (n=8). Basal [3H]phenylalanine incorporation amounted to 847±86 c.p.m. in PTX-treated control, 871±112 c.p.m. in the presence of CGP and 862±98 c.p.m. in the presence of CGP+ICI. Bars represent mean±s.e.m. +++P<0.01 versus FEN (PTX-treated), ***P<0.01 versus FEN+CGP (PTX-treated).
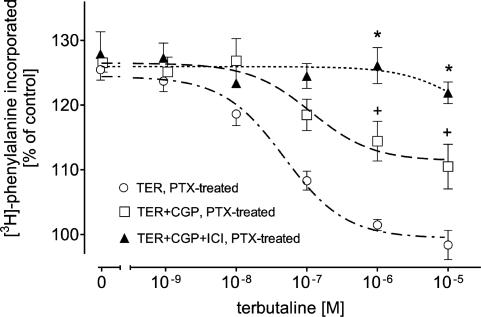
On the other hand, in PTX-treated cardiomyocytes the rightward shift of the concentration–inhibition curve for TER by CGP (Figure 4) was less than that in not-PTX-treated cardiomyocytes (see Figure 2). Similarly to FEN, the inhibitory effect of TER, too, was seen to be completely suppressed by ICI in PTX-treated cardiomyocytes in the presence of CGP (Figure 4).
Figure 4.
Effects of 300 nM CGP or 300 nM CGP plus 55 nM ICI on TER-evoked inhibition of 1 μM PE-induced [3H]phenylalanine incorporation in PTX-pretreated ventricular cardiomyocytes of 12-week-old male Wistar rats (n=6). Basal [3H]phenylalanine incorporation amounted to 912±98 c.p.m. in PTX-treated control, 939±111 c.p.m. in the presence of CGP and 928±95 c.p.m. in the presence of CGP+ICI. Bars represent mean±s.e.m. +P<0.05 versus TER (PTX-treated), *P<0.05 versus TER+CGP (PTX-treated).
Effects of SAL on PE-induced increase in the rate of protein synthesis
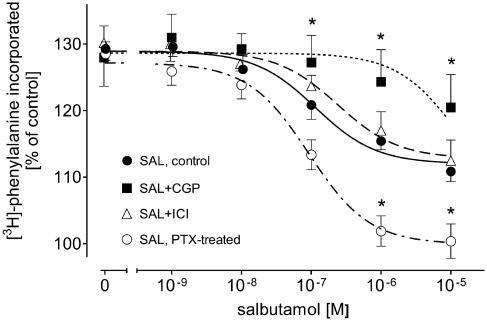
In a final set of experiments, we studied the effects of another well-known β2-AR agonist, SAL, on 1 μM PE-induced increase in the rate of protein synthesis in order to find out whether even under our conditions the pattern of inhibition by SAL resembles that of TER (as described by Xiao et al., 2003). SAL (1 nM–10 μM) concentration-dependently decreased 1 μM PE-induced increase in the rate of protein synthesis; however, similar to TER, the highest concentration of SAL (10 μM) caused only partial inhibition in the rate of protein synthesis (from 129±1 to 111±2% of control; Figure 5). The inhibitory effect of SAL was not at all affected by 55 nM ICI, but markedly attenuated by 300 nM CGP. In PTX-treated cardiomyocytes the SAL-induced partial inhibition of rate of protein synthesis was converted into a complete inhibition (Figure 5). Hence, these data confirm that the pattern of SAL effects on the rate of protein synthesis is very similar to that of TER.
Figure 5.
Effects of 300 nM CGP, 55 nM ICI and PTX pretreatment on SAL-evoked inhibition of 1 μM PE-induced [3H]phenylalanine incorporation in ventricular cardiomyocytes of 12-week-old male Wistar rats (n=7). Basal [3H]phenylalanine incorporation amounted to 784±121 c.p.m. in control, 795±105 c.p.m. in the presence of CGP, 764±137 c.p.m. in the presence of ICI and 752±92 c.p.m. after PTX pretreatment. Bars represent mean±s.e.m. *P<0.05 versus SAL.
Discussion
Xiao et al. (2003) had recently shown, in isolated ventricular cardiomyocytes from SHR rats with heart failure, that the contractile response to several β2-AR agonists, including TER, SAL, zinterol and procaterol, could be markedly enhanced when the cardiomyocytes were pretreated with PTX, thereby inactivating the Gi-protein. On the other hand, the contractile response to another β2-AR agonist, FEN, was not affected by PTX treatment.
In rat heart, increase in contractile force is brought about by the β-AR–Gs-protein pathway, and it had been shown that β2-AR can activate both the rat cardiac Gs- and Gi-protein pathways (Lohse et al., 2003; Pönicke et al., 2003; Xiao et al., 2004). The results from Xiao et al. (2003) indicate that, in the isolated ventricular cardiomyocytes from SHR rats with heart failure, most β2-AR agonists are partial agonists because they stimulate the Gs- and Gi-protein pathways, and they are converted to full agonists after Gi-protein has been inactivated. On the other hand, FEN is a full agonist evoking maximal increases in the force of contraction, which is not affected by PTX treatment. From this, Xiao et al. (2003; 2004) had concluded that FEN activates, in isolated adult rat cardiomyocytes, the β2-AR–Gs-protein pathway selectively.
We have recently shown that, in isolated ventricular cardiomyocytes from adult rats, the increase in protein synthesis evoked by activation of α1A-AR (Pönicke et al., 2001) can be inhibited by drugs that activate the Gs-protein–adenylyl cyclase pathway and thereby increase intracellular cAMP content: activation of β1-AR inhibited PE-evoked increase in the rate of protein synthesis; this was mediated by cAMP because it could be mimicked by dibutyryl cAMP and could be abolished by the cAMP antagonist Rp-cAMPS (Schäfer et al., 2001). The mechanism underlying this β-AR-mediated inhibition of the α1-AR-induced hypertrophic response is not completely clear.
However, it appears that the β1-AR–Gs- and β2-AR–Gs-protein induced apoptosis counteracts the α1-AR-mediated increase in the rate of protein synthesis. This hypothetical view is supported by our recent findings that, in isolated ventricular cardiomyocytes of adult rats, there was a significant negative correlation between β1- and β2-AR-mediated apoptosis and α1-AR-mediated increase in the rate of protein synthesis (Pönicke et al., 2003).
In the present study, we have used this system to test whether also under these conditions FEN might be a selective activator of the β2-AR–Gs-protein pathway, whereas other β2-AR agonists such as TER or SAL activate both the β2-AR–Gs-protein and the β2-AR–Gi-protein pathways.
As shown in Figures 1, 2 and 5, indeed FEN was a full agonist in inhibiting PE-induced increase in protein synthesis, while TER and SAL were only partial agonists; moreover, FEN effects were not at all affected by PTX pretreatment of the cardiomyocytes, while PTX treatment converted the partial agonist activities of TER and SAL into full agonist activities. These data are compatible with the view that FEN activates only a β-AR–Gs-protein pathway, whereas TER and SAL activate both the β-AR–Gs-protein and the β-AR–Gi-protein pathway. Thus, so far our data are in good agreement with those published by Xiao et al. (2003).
However, when we tried to further classify the β-AR subtype involved in the effects of FEN, SAL and TER, we found that the highly selective β1-AR antagonist CGP, used in a concentration (300 nM) that does not at all act at β2-AR (Kaumann & Lemoine, 1987), caused a marked shift to the right of the concentration–inhibition curves of all the three β2-AR agonists. This effect was only marginally affected when the Gi-protein–adenylyl cyclase pathway was inactivated by PTX. Thus, it appears that, in adult rat cardiomyocytes, part of the action of the β2-AR agonists FEN, TER and SAL is mediated by activation of the β1-AR–Gs-protein pathway. The issue whether or not in isolated ventricular cardiomyocytes from adult rats effects of β2-AR agonists might (at least partly) be mediated by β1-AR is still being controversially discussed, with some authors finding a strong participation of β1-AR (Kuznetsov et al., 1995; Laflamme & Becker, 1998) and others finding no involvement of β1-AR (Xiao et al., 2003; for further references, see Xiao et al., 2004).
In contrast to CGP, in the present study, the highly selective β2-AR antagonist ICI, used at a concentration (55 nM) that does not at all act at β1-AR (Lemoine et al., 1985), caused a significant shift to the right only of the concentration–inhibition curve of FEN, but did not significantly affect the concentration–inhibition curves of TER and SAL. This untypical behavior of ICI might be explained as follows: if the agonist activates the β2-AR–Gs-protein pathway, protein synthesis is inhibited and that is antagonized by ICI, that is the concentration-inhibition curve of that agonist should be shifted to the right by ICI. If the agonist activates in addition to the β2-AR–Gs-protein pathway the β2-AR–Gi-protein pathway, inhibition of protein synthesis is attenuated; now ICI inhibits this attenuating effect of the agonist, that is inhibition of protein synthesis is enhanced and hence the concentration–inhibition curve of that agonist should be shifted to the left by ICI. In the present study, FEN activates – besides β1-AR (see above) – solely the β2-AR–Gs-protein pathway; accordingly, its concentration–inhibition curve should be shifted to the right by ICI, which is indeed the case (see Figure 1). On the other hand, SAL and TER activate – besides β1-AR – the β2-AR–Gs-protein pathway (its inhibition by ICI should lead to a rightward shift of the concentration–inhibition curve) and the β2-AR–Gi-protein pathway (its inhibition by ICI should lead to a leftward shift of the concentration–inhibition curve). The fact that ICI does not significantly affect the concentration–inhibition curves of these two agonists, therefore, implies that activation of β2-AR by both agonists involves the Gs- and Gi-protein pathways to nearly equal amounts.
Taken together so far, in isolated adult rat ventricular cardiomyocytes inhibition of PE-evoked increase in the rate of protein synthesis by the β2-AR agonists FEN, TER and SAL is (partly) mediated by β1-AR stimulation; in addition, the effects of TER and SAL involve a PTX-sensitive inhibitory component. Hence, the effects of these three β2-AR agonists via the pure β2-AR–Gs-protein pathway can be only demonstrated when β1-AR are blocked and the Gi-protein pathway is inactivated. And this is indeed the case: as shown in Figures 3 and 4, in cardiomyocytes pretreated with PTX, inhibition of protein synthesis by FEN and TER, in the presence of 300 nM CGP, is now completely inhibited by the highly selective β2-AR antagonist ICI.
Conclusion
In adult rat ventricular cardiomyocytes FEN activates only the β2-AR–Gs-protein pathway, while TER and SAL activate the β2-AR–Gs- and β2-AR–Gi-protein pathways. Part of the effects of these three β2-AR agonists in the rat cardiomyocytes appear to be mediated by β1-AR.
Acknowledgments
The skilful technical assistance of I. Adler, A. Hauser, A. Dunemann and M. Niebisch is gratefully acknowledged. We are thankful for generous gifts of CGP to Ciba-Geigy, of fenoterol hydrobromide to Boehringer Ingelheim and for salbutamol sulphate to Glaxo Group Research Ltd. This work was supported by grants of the Deutsche Forschungsgemeinschaft (DFG OS 131/3-3 and BR 526/8-3 to O.-E.B.).
Abbreviations
- AR
adrenoceptors
- CGP
CGP 20712A
- FEN
fenoterol
- ICI
ICI 118,551
- PE
phenylephrine
- PTX
pertussis toxin
- SAL
salbutamol
- TER
terbutaline
References
- BRODDE O.-E., MICHEL M.C. Adrenergic and muscarinic receptors in the human heart. Pharmacol. Rev. 1999;51:651–689. [PubMed] [Google Scholar]
- COMMUNAL C., SINGH K., SAWYER D.B., COLUCCI W.S. Opposing effects of β1- and β2-adrenergic receptors on cardiac myocyte apoptosis: role of a pertussis toxin-sensitive G protein. Circulation. 1999;100:2210–2212. doi: 10.1161/01.cir.100.22.2210. [DOI] [PubMed] [Google Scholar]
- KAUMANN A.J., LEMOINE H. β2-Adrenoceptor-mediated positive inotropic effect of adrenaline in human ventricular myocardium. Naunyn-Schmiedeberg's Arch. Pharmacol. 1987;335:403–411. doi: 10.1007/BF00165555. [DOI] [PubMed] [Google Scholar]
- KUZNETSOV V., PAK E., TOBINSON R.R., STEINBERG S.F. β2-Adrenergic receptor actions in neonatal and adult rat ventricular myocytes. Circ. Res. 1995;76:40–52. doi: 10.1161/01.res.76.1.40. [DOI] [PubMed] [Google Scholar]
- LAFLAMME M.A., BECKER P.L. Do β2-adrenergic receptors modulate Ca2+ in adult rat ventricular myocytes. Am. J. Physiol. 1998;274:H1308–H1314. doi: 10.1152/ajpheart.1998.274.4.H1308. [DOI] [PubMed] [Google Scholar]
- LEMOINE H., EHLE B., KAUMANN A.J. Direct labelling of β2-adrenoceptors. Comparison of binding potency of 3H-ICI 118,551 and blocking potency of ICI 118, 551. Naunyn-Schmiedeberg's Arch. Pharmacol. 1985;331:40–51. doi: 10.1007/BF00498850. [DOI] [PubMed] [Google Scholar]
- LOHSE M.J., ENGELHARDT S., ESCHENHAGEN T. What is the role of β-adrenergic signaling in heart failure. Circ. Res. 2003;93:896–906. doi: 10.1161/01.RES.0000102042.83024.CA. [DOI] [PubMed] [Google Scholar]
- PÖNICKE K., GIESSLER C., GRAPOW M., HEINROTH-HOFFMANN I., BECKER K., OSTEN B., BRODDE O.-E. FP-receptor mediated trophic effects of prostanoids in rat ventricular cardiomyocytes. Br. J. Pharmacol. 2000;129:1723–1731. doi: 10.1038/sj.bjp.0703243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PÖNICKE K., HEINROTH-HOFFMANN I., BRODDE O.-E. Role of β1- and β2-adrenoceptors in hypertrophic and apoptotic effects of noradrenaline and adrenaline in adult rat ventricular cardiomyocytes. Naunyn-Schmiedeberg's Arch. Pharmacol. 2003;367:592–599. doi: 10.1007/s00210-003-0754-z. [DOI] [PubMed] [Google Scholar]
- PÖNICKE K., SCHLÜTER K.-D., HEINROTH-HOFFMANN I., SEYFARTH T., GOLDBERG M., OSTEN B.G, PIPER H.M., BRODDE O.-E. Noradrenaline-induced increase in protein synthesis in adult rat cardiomyocytes: involvement of only alpha1A-adrenoceptors. Naunyn-Schmiedeberg's Arch. Pharmacol. 2001;364:444–453. doi: 10.1007/s002100100469. [DOI] [PubMed] [Google Scholar]
- SCHÄFER M., PÖNICKE K., HEINROTH-HOFFMANN I., BRODDE O.-E., PIPER H.M., SCHLÜTER K.-D. Beta-adrenoceptor stimulation attenuates the hypertrophic effect of alpha-adrenoceptor stimulation in adult rat ventricular cardiomyocytes. J. Am. Coll. Cardiol. 2001;37:300–307. doi: 10.1016/s0735-1097(00)01065-2. [DOI] [PubMed] [Google Scholar]
- SHIZUKUDA Y., BUTTRICK P.M. Subtype specific roles of β-adrenergic receptors in apoptosis of adult rat ventricular myocytes. J. Mol. Cell. Cardiol. 2002;34:823–831. doi: 10.1006/jmcc.2002.2020. [DOI] [PubMed] [Google Scholar]
- XIAO R.P., JI X., LAKATTA E.G. Functional coupling of the β2-adrenoceptor to a pertussis toxin-sensitive G protein in cardiac myocytes. Mol. Pharmacol. 1995;47:322–329. [PubMed] [Google Scholar]
- XIAO R.-P., ZHANG S.-J., CHAKIR K., AVDONIN P., ZHU W., BOND R.A., BALKE C.W., LAKATTA E.G., CHENG H. Enhanced Gi signaling selectively negates β2-adrenergic receptor (AR) – but not β1-AR-mediated positive inotropic effect in myocytes from failing rat hearts. Circulation. 2003;108:1633–1639. doi: 10.1161/01.CIR.0000087595.17277.73. [DOI] [PubMed] [Google Scholar]
- XIAO R.-P., ZHU W., ZHENG M., CHAKIR K., BOND R.A., LAKATTA E.G., CHENG H. Subtype-specific β-adrenoceptor signaling pathways in the heart and their potential clinical implications. Trends Pharmacol. Sci. 2004;25:358–365. doi: 10.1016/j.tips.2004.05.007. [DOI] [PubMed] [Google Scholar]
- ZAUGG M., XU W., LUCCHINETTI E., SHAFIQ S.A., JAMALI N.Z., SIDDIQUI M.A.Q. Adrenergic receptor subtypes differentially affect apoptosis in adult rat ventricular myocytes. Circulation. 2000;102:344–350. doi: 10.1161/01.cir.102.3.344. [DOI] [PubMed] [Google Scholar]
- ZHU W.-Z., ZHENG M., KOCH W.J., LEFKOWITZ R.J., KOBILKA B.K., XIAO R.-P. Dual modulation of cell survival and cell death by β2-adrenergic signaling in adult mouse cardiac myocytes. Proc. Natl. Acad. Sci. U.S.A. 2001;98:1607–1612. doi: 10.1073/pnas.98.4.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]