For 40 years, we have known that there is a 2-fold rise in serum GH during the reproductive cycle (Giustina and Veldhuis, 2000; Hull and Harvey, 2002: Frantz and Rabkin, 1965; Faria et al, 1992; Ovesen et al, 1998) and some of these investigators have called this the midcycle GH surge. While it does not have the amplitude of the mid cycle surge of luteinizing hormone (LH), it does provide pulses of GH that are higher both in frequency and in amplitude as the individual approaches ovulation (Frantz and rabkin, 1965; Faria et al, 1992; Ovesen et al, 1998).
The GH surge is also seen in other primate, ovine, and rodent species (Landefeld and Suttie, 1989; Malvern et al, 1995; Scanlan and Sinner, 2002; Copeland et al, 1984; Bethea, 1991). and it can be mimicked by estradiol treatment of the ewe (Malvern et al, 1995; Scanlan and Skinner, 2002) or intact or ovariectomized monkeys (Copeland et al, 1964; Bethea, 1991). Hence, the hypothesis was that estradiol played a primary role in regulating GH, bringing out a female gender-specific secretory pattern during the cycle.
Recent studies of aromatase knockout animals have provided clues about the nature of estrogen’s actions in the pituitary (Yan et al, 2004). These animals (which cannot make estrogen from aromatizable androgens) expressed lower levels of GHRH receptors as well as GH and pit-1 mRNA. Expression of these gene products was rescued by estrogen treatment, in vivo. We recently reported that, in normal cycling female rats (Childs et al, 2000), GH mRNA expression in the pituitary was lowest during a nadir period for serum estrogen and it rose to reach a peak during midcycle, a period of rising estrogen. Whereas the data from these two studies fit the hypothesis that GH expression depends on serum estrogen, they do not tell us where its effects are mediated. In other words, are estrogen’s effects mediated by direct actions on somatotropes, or is the mechanism indirect, via actions on Growth hormone releasing hormone (GHRH) neurons or other hypothalamic regulatory factors.
Proof for direct actions of estrogen on GH cells requires studies of pituitary cells in vitro, as these cells have been removed from sources of hypothalamic stimulation. During the past decade, however, this proof has not been forthcoming for two reasons. First, there has been controversy about the presence or types of estrogen receptors on somatotropes (Kikuta et al, 1993; Friend et al, 1994; Shupnik, 2002; Stefanneau et al, 1994; Zafer et al, 1995; Chaidarun et al, 1997; Chaidarun et al,m 1998; Gittoes et al, 1999; Shupnik et al1998; Hal and McDonnell, 1999). Second, not all in vitro studies have demonstrated an estrogen-mediated increase in GH synthesis or secretion (Webb et al, 1983; Simard et al, 1985; Lam et al, 1996; Tulipano et al, 2004; Fukuta and Martin, 1986; Silverman et al, 1988; Hauspie et al, 2003.). In some studies, estrogen has had no effects or inhibitory effects on expression of GH (Fukuta and Martin, 1986; Silverman et al, 1988; Hauspie et al, 2003.).
Our studies sought to address these questions with new dual labeling techniques that identified both receptor activity and hormone activity in pituitary cells (Childs, 2002; Childs et al, 2004). We also designed the estrogen treatment paradigm to include a broad range of concentrations. to test the hypothesis that estrogen’s actions might be bipotential (Childs et al, 2004)..We hypothesized that the controversy in the literature might be related to the concentration of estrogen used in the previous studies.
The first phase of the study involved dual labeling studies for estrogen receptor isoforms and GH antigens (Childs, 2002). In these studies, we showed that somatotropes could express ERα and ERβ. Furthermore, expression of these isoforms by GH cells varied with the estrous cycle in a way that suggested that ERβ might participate in early events (from estrus to metestrus), and ERα expression might be important for somatotropic function during midcycle (proestrus). These data, illustrated in Plate 1, demonstrated that somatotropes did express both receptor isoforms and that regulation of ER expression might play a critical role in the overall expression of GH. Ongoing tests of this hypothesis show differential regulation of GH by agonists of ERα and ERβ. ERβ agonists are inhibitory whereas ERα agonists are stimulatory (Childs et al, unpublished). It is interesting to note that ERβ expression is highest during the fall in pituitary GH mRNA (estrus to metestrus, Childs et al, 2000) and ERα expression is highest during the rise in GH mRNA (Childs et al 2000).
Plate 1.
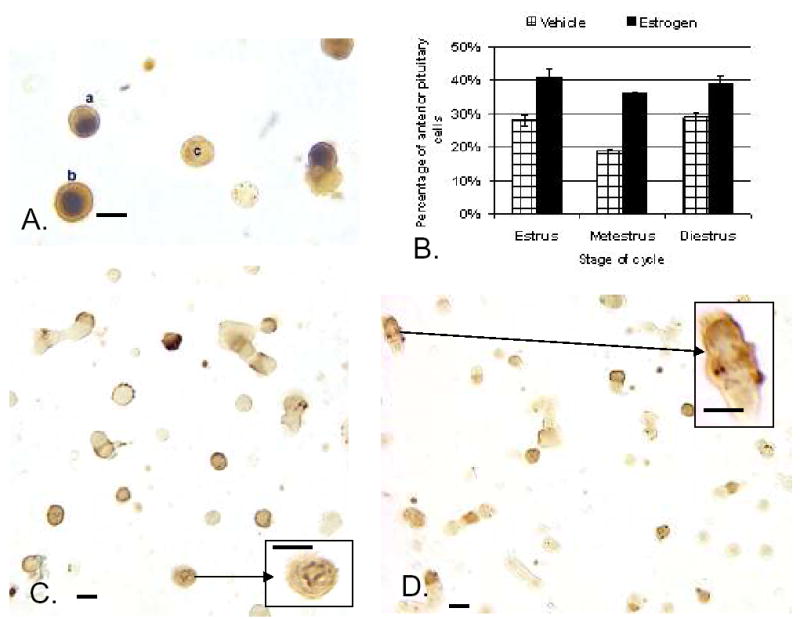
A. Dual labeling for Estrogen Receptor –Alpha isoform (ERα) in the nucleus (black) and growth hormone in the cytoplasm (orange-amber) of freshly dispersed cells from diestrous female rat. Most GH cells in the field express ERα. Cell C does not. B.Exposure to estradiol for 24 h (100 pM) stimulated expression of biotinylated Growth hormone releasing hormone (Bio-GHRH) binding sites, if given to estrous, metestrous, or diestrous female rats. C. Illustration of Bio-GHRH binding (gray-black) and labeling for growth hormone (orange-amber) in vehicle-treated diestrous female rats. D. Increased labeling for both Bio-GHRH (black) and GH (orange) is seen after 24 h in 100 pM estradiol. Insets show a higher magnification of dual labeled GH cells. Bar=15 μm
We then studied effects of exposure to the full dose range of estrogen to learn if its effects varied with the concentration (Childs et al 2004). Pituitaries were taken from normal, cycling female rats during diestrus (which showed, on average, a high expression of ER) and the cells were exposed to 0.001–250 nM water soluble 17β estradiol benzoate (from Sigma) for 24 h. We initially discovered little difference in the enhancing effects of estradiol if given for 15 h – 48 h. So, we settled on 24 h as a time for most of the experiments. Because we used the water soluble form, we could use the defined growth media as our vehicle. After the treatment period, the cells were either fixed for in situ hybridization or immunolabeling, or additional groups were stimulated for 10 min with a biotinylated analog of GHRH to detect any changes in GHRH-receptive somatotropes. This last group of cells was fixed and prepared for affinity cytochemistry for biotinylated GHRH followed by immunolabeling for GH..
Analysis of the changes in expression of GH mRNA and proteins was done by new Bioquant Image analysis software including algorithms that allowed us to detect changes in integrated optical density of the label (Childs et al, 2004). This provided information about changes in expression that integrated that due to cell number with that due to the density and area of label in each cell. In addition, we counted the number of labeled cells, expressing the counts as percentages of the total pituitary cell population analyzed.
All analyses showed that estrogen produced bipotential effects on somatotropes. Stimulatory effects were evident in a narrow dose range from 0.01—1 nM (Plate 1.). Higher concentrations either produced no effects, or, depending on the parameter being assayed, reduced the expression of GH. This inhibitory effect became obvious when the percent of cell area containing mRNA was detected following 100 or 250 nM estrogen.
When cells labeled for biotinylated GHRH were counted, there was a significant increase in the percentages of GHRH target cells following exposure to 0.01–1 nM estradiol, the same low dose range that stimulated GH expression. Furthermore 0.1 nM estrogen stimulated more GHRH target cells in populations from estrous, metestrous and diestrous rats, indicating that one of its roles may be to enhance GH receptivity to GHRH (Childs et al, 2004) (Plate 1.).
Many controls over the past 21 years have shown that the dual labeling protocol for biotinylated ligands and antigens is both sensitive and specific and that one can use a second labeling sequence that involves streptavidin as a detection system, with no cross-reactions between the two protocols (Childs et al 1983a, b; Childs et al 1999). The controls for biotinylated GHRH were published in 1999. (Childs et al, 1999) However, in the most recent study (Childs et al, 2004), we added a new protocol to our repertoire to further validate the dual labeling for biotinylated GHRH and GH. We used the new ImmPRESS ® labeling method (Vector Laboratories, Burlingame, CA) for GH, which involves a micropolymer of peroxidase attached to goat anti-rabbit IgG. It labels GH with enhanced sensitivity and does not add any avidin or biotin sequences to the overall mix of reactants. Counts of cells following dual and single labeling proved that this new approach detected the same population detected in previous studies (Childs et al, 1999). This further validated both the labeling and the counts.
The results of these studies showed clearly that estrogen could have direct, enhancing effects on GH cells in a limited dose range (Childs et al, 2004). Relatively low concentrations of estrogen (below 10 nM) stimulated expression of GH mRNA, proteins and also GHRH receptive cells. Thus, the rise in estrogen seen early in the cycle, from metestrus to proestrus, may well stimulate the rise in GH expression by direct actions on somatotropes. In addition, GH cells may become more receptive to GHRH in the presence of rising estrogen.
These data correlate well with those previous in vitro and in vivo studies that used relatively low concentrations of estrogen to show its enhancing effects on somatotropes (Bethea, 1991; Webb et al, 1983; Simard et al, 1986; Lam et al, 1996; Tulipano et al, 2004). However, those studies that used concentrations of 10 nM or greater most often reported no effects, or even inhibitory effects of estrogen (Fukata and Martin, 1986; Silverman et al, 1988; Hauspie et al, 2003). Our dose-response results agree that higher concentrations of estrogen are not stimulatory and in fact, may inhibit certain aspects of GH gene product expression (Childs et al, 2004). This could be translated to an in vivo function related to estrogen negative feedback. It is possible that rising estrogen after ovulation reaches a level that limits GH expression. This would coincide with the fall in expression of GH mRNA seen from estrus to metestrus in our earlier studies (Childs et al, 2000). Thus, the cyclic expression of estrogen in a normal cycle will provide enhanced GH just before ovulation. Then, the postovulatory rise in estrogen may limit expression of GH.
A number of studies have discussed the significance of GH to the reproductive system. GH is well known for its promotion of optimal body composition that would support both reproductive activity and a pregnancy (Giustina and Veldhuis, 2000; Hull and Harvey, 2002). However, its lipolytic effects may reduce key fuel sources and hence a reduction in GH might be needed during the postovulatory period, to promote sufficient fuel in case there of a pregnancy.
GH has also been shown to directly enhance ovarian pre-ovulatory functions (Giustina and Veldhuis, 2000; Hull and Harvey, 2002).. It facilitates actions of gonadotropins on follicular cells, possibly by increasing gonadotropin receptors. It has been shown to promote the maturation of pre-antral follicles either by itself or with Follicle stimulating hormone (Zhao et al, 2000; Eckery et al, 1993, 1997; Kikuchi et al, 2001). Thus, the GH surge before ovulation could serve to promote follicular development and facilitate LH and FSH actions leading up to ovulation.
In this way GH may facilitate reproductive activity as it promotes metabolic activity and body composition in support of a pregnancy. Estrogen from the growing follicles may feedback to enhance somatotrope expression so GH can support its pre-ovulatory functions. However, higher levels of estrogen after ovulation, from the corpus luteum, may limit GH to maintain energy stores in preparation for a pregnancy. Thus, both the metabolic and reproductive functions may be balanced by estrogen’s bipotential regulatory influcences..
Plate 1.
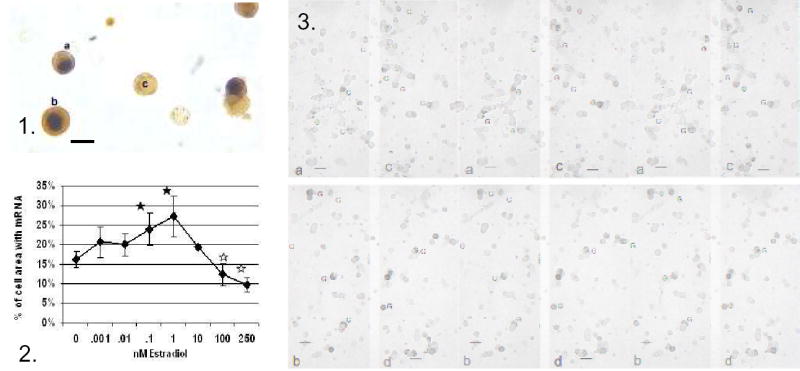
Figure 1.. Dual labeling for Estrogen Receptor –Alpha isoform (ERα) in the nucleus (black) and growth hormone in the cytoplasm (orange-amber) of freshly dispersed cells from diestrous female rat. Most GH cells in the field express ERα. Figure 2. Image analysis showed that 24 h in 0.1–1 nM estradiol produced an increase in average area of label for GH mRNA. Concentrations higher than 10 nM, however produced a decrease in labeling for the mRNA. Figure 3. shows this change by illustrating labeling for GH mRNA in cultures treated with vehicle (a), or 0.1 nM (b), 1 nM (c), or 250 nM estradiol. Bar=15 μm
Acknowledgments
This study was supported by NIH R01 HD 33915-01. The authors would like to acknowledge the Hormone Distribution Program, NIH, and Dr. A. Parlow for the antiserum to rat GH. We also thank Dr. Brian T. Miller, University of Texas Medical Branch, Galveston, TX for the biotinylated analogs of GHRH
References
- Bethea CL. Estrogen action on growth hormone in pituitary cell cultures from adult and juvenile macaques. Endocrinology. 1991;129:2110–2118. doi: 10.1210/endo-129-4-2110. [DOI] [PubMed] [Google Scholar]
- Chaidarun SS, Klibanski A, Alexander JM. Tumor-specific expression of alternatively spliced estrogen receptor messenger ribonucleic acid variants in human pituitary adenomas. J Clin Endocrinol Metab. 1997;82:1058–1065. doi: 10.1210/jcem.82.4.3864. [DOI] [PubMed] [Google Scholar]
- Chaidarun SS, Swearingen B, Alexander JM. Differential expression of estrogen receptor-β (ERβ) in human pituitary tumors: functional interactions with ERα and a tumor-specific splice variant. J Clin Endocrinol Metab. 1998;83:3308–3315. doi: 10.1210/jcem.83.9.5128. [DOI] [PubMed] [Google Scholar]
- Childs GV, Naor Z, Hazum E, Tibolt R, Westlund KM, Hancock MB. Localization of biotinylated gonadotropin releasing hormone on pituitary monolayer cells with avidin–biotin peroxidase complexes. J Histochem Cytochem. 1983a;31:1422–1425. doi: 10.1177/31.12.6195217. [DOI] [PubMed] [Google Scholar]
- Childs GV, Naor Z, Hazum E, Tibolt R, Westlund KN, Hancock MB. Cytochemical characterization of pituitary target cells for biotinylated gonadotropin releasing hormone. Peptides. 1983b;4(4):549–555. doi: 10.1016/0196-9781(83)90061-x. [DOI] [PubMed] [Google Scholar]
- Childs GV, Unabia G, Miller BT, Collins TJ. Differential expression of prolactin and gonadotropin antigens by growth hormone releasing hormone (GHRH) target cells from male and female rats. J Endocrin. 1999;162:177–197. doi: 10.1677/joe.0.1620177. [DOI] [PubMed] [Google Scholar]
- Childs GV, Unabia G, Wu P. Differential expression of GH mRNA by growth hormone cells and gonadotropes in male and cycling female rats. Endocrinology. 2000;141:1560–1570. doi: 10.1210/endo.141.4.7429. [DOI] [PubMed] [Google Scholar]
- Childs GV. Development of gonadotropes may involve cyclic transdifferentiation of growth hormone cells. Arch Physiol Biochem. 2002;110:42–49. doi: 10.1076/apab.110.1.42.906. [DOI] [PubMed] [Google Scholar]
- Childs GV, Iruthayanathan M, Akhter N, Unabia G, Whitehead-Johnson B. Bipotential effects of estrogen on growth hormone synthesis and storage, in vitro. Endocrinology. 2004;146:1780–1788. doi: 10.1210/en.2004-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland KC, Johnson DM, Kuehl TJ, Castracane VD. Estrogen stimulates growth hormone and somatomedin-C in castrate and intact female baboons. J Clin Endocrinol Metab. 1984;58:698–703. doi: 10.1210/jcem-58-4-698. [DOI] [PubMed] [Google Scholar]
- Eckery DC, Moeller CL, Nett TM, Sawyer HK. Recombinant bovine somatotropin (rbST, Sometribove) maintains the sensitivity of ovarian follicles to gonadotropins in hypophysectomized ewes. Biol Reprod. 1993:48. Abstract 337. [Google Scholar]
- Eckery DC, Moeller CL, Nett TM, Sawyer HR. Localization and quantification of binding sites for follicle-stimulating hormone, luteinizing hormone, growth hormone, and insulin-like growth factor 1 in sheep ovarian follicles. Biol Rep. 1997;57:507–513. doi: 10.1095/biolreprod57.3.507. [DOI] [PubMed] [Google Scholar]
- Faria ACS, Bekenstein LW, Booth RA, Jr, Vaccaro VA, Asplin CM, Veldhuis JD, Thorner MO, Evans WS. Pulsatile growth hormone release in normal women during the menstrual cycle. Clin Endocrinol (Oxf) 1992;36:591–596. doi: 10.1111/j.1365-2265.1992.tb02270.x. [DOI] [PubMed] [Google Scholar]
- Frantz AG, Rabkin MT. Effects of estrogen and sex difference on secretion of human growth hormone. J, Clin, Endocrinol, Metab. 1965;25:1470–1480. doi: 10.1210/jcem-25-11-1470. [DOI] [PubMed] [Google Scholar]
- Friend KE, Chiou YK, Lopes MB, Laws ER, Jr, Hughes KM, Shupnik MA. Estrogen receptor expression in human pituitary: correlation with immunohistochemistry in normal tissue, and immunohistochemistry and morphology in macroadenomas. J Clin Endocrinol Metab. 1994;78:1497–1504. doi: 10.1210/jcem.78.6.7515390. [DOI] [PubMed] [Google Scholar]
- Fukuta J, Martin JB. Influence of sex steroid hormones on rat growth hormone-releasing factor and somatostatin in dispersed pituitary cells. Endocrinology. 1986;119:2256–2261. doi: 10.1210/endo-119-5-2256. [DOI] [PubMed] [Google Scholar]
- Giustina A, Veldhuis JD. Pathophysiology of the neuroregulation of growth hormone secretion in experimental animals and the human. Endocrine Reviews. 2000;19:717–797. doi: 10.1210/edrv.19.6.0353. [DOI] [PubMed] [Google Scholar]
- Gittoes NJ, McCabe CJ, Sheppard MC, Franklyn JA. Pituitary. Vol. 1. 1999. Estrogen receptor beta mRNA expression in normal and adenomatous pituitaries; pp. 99–104. [DOI] [PubMed] [Google Scholar]
- Hall JM, McDonnell DP. The estrogen receptor β-isoform (ERβ) of the human estrogen receptor modulates ERα transcriptional activity and is a key regulator of the cellular response to estrogens and antiestrogens. Endocrinology. 1999;140:5566–5578. doi: 10.1210/endo.140.12.7179. [DOI] [PubMed] [Google Scholar]
- Hauspie A, Seuntjens E, Vankelecom H, Denef C. Stimulation of combinatorial expression of prolactin and glycoprotein hormone α-Subunit genes by gonadotropin-releasing hormone and estradiol-17β in single rat pituitary cells during aggregate cell culture. Endocrinology. 2003;144:388–399. doi: 10.1210/en.2002-220606. [DOI] [PubMed] [Google Scholar]
- Hull KL, Harvey S. GH as a co-gonadotropin: the relevance of correlative changes in GH secretion and reproductive state. J Endocrinology. 2002;172:1–19. doi: 10.1677/joe.0.1720001. [DOI] [PubMed] [Google Scholar]
- Kikuchi N, Andoh K, Abe Y, Yamada K Mizunuma H, Ibuki Y. Inhibitory action of leptin on early follicular growth differs in immature and adult female mice. Biol Rep. 2001;65:66–71. doi: 10.1095/biolreprod65.1.66. [DOI] [PubMed] [Google Scholar]
- Kikuta T, Yamamoto K, Namiki H, Hayashi S. Immunocytochemical localization of estrogen receptor in various anterior pituitary hormone cells of adult male and female rats. Acta Histochem Cytochem. 1993;26:609–614. [Google Scholar]
- Lam KD, Lee MF, Tam SP, Srivastava G. Gene expression of the receptor for growth hormone releasing hormone is physiologically regulated by glucocorticoids and estrogen. Neuroendocrinology. 1996;63:475–480. doi: 10.1159/000127075. [DOI] [PubMed] [Google Scholar]
- Landefeld TD, Suttie JM. Changes in messenger ribonucleic acid concentrations and plasma levels of growth hormone during the ovine estrous cycle and in response to exogenous estradiol. Endocrinology. 1989;125:1474–1478. doi: 10.1210/endo-125-3-1474. [DOI] [PubMed] [Google Scholar]
- Malven PV, Haglof SA, Jiang H. Serum concentrations of luteinizing hormone, growth hormone, and prolactin in untreated and estradiol-treated ovariectomized ewes after immunoneutralization of hypothalamic neuropeptide Y. J Anim Sci. 1995;73:2105–2112. doi: 10.2527/1995.7372105x. [DOI] [PubMed] [Google Scholar]
- Ovesen P, Vahl N, Fisker S, Veldhuis JD, Christiansen JS, Jorgensen JOL. Increased pulsatile, but not basal, growth hormone secretion rates and plasma insulin-like growth factor I levels during the preovulatory interval in normal women. J Clin Endocrinol Metab. 1998;83:1662–1667. doi: 10.1210/jcem.83.5.4761. [DOI] [PubMed] [Google Scholar]
- Scanlan N, Skinner DC. Estradiol modulation of growth hormone secretion in the ewe: no growth hormone-releasing hormone neurons and few somatotropes express estradiol receptor. Biol Reprod. 2002;66:1267–1273. doi: 10.1095/biolreprod66.5.1267. [DOI] [PubMed] [Google Scholar]
- Shupnik MA, Pitt LK, Soh AY, Anderson A, Lopes MB, Laws ER. Selective expression of estrogen receptor α and β isoforms in human pituitary tumors. J Clin Endocrinol Metab. 1998;83:3965–3972. doi: 10.1210/jcem.83.11.5236. [DOI] [PubMed] [Google Scholar]
- Shupnik MA. Oestrogen Receptors, Receptor Varients and Oestrogen Actions in the Hypothalamic-Pituitary Axis. J Neuroendocrinology. 2002;14:85–94. doi: 10.1046/j.0007-1331.2001.00744.x. [DOI] [PubMed] [Google Scholar]
- Silverman BL, Kaplan SL, Grumbach MM, Miller WL. Hormonal regulation of growth hormone secretion and messenger ribonucleic acid accumulation in cultured bovine pituitary cells. Endocrinology. 1988;123:1236–1241. doi: 10.1210/endo-122-4-1236. [DOI] [PubMed] [Google Scholar]
- Simard J, Hubert JF, Hosseinzadeh T, Labrie F. Stimulation of growth hormone release and synthesis by estrogens in rat anterior pituitary cells in culture. Endocrinology. 1986;119(5):2004–2011. doi: 10.1210/endo-119-5-2004. [DOI] [PubMed] [Google Scholar]
- Stefanneau L, Kovacs K, Horvath E, Lloyd RV, Buchfelder M, Fahlbusch R, Smyth H. In situ hybridization study of estrogen receptor messenger ribonucleic acid in human adenohypophysial cells and pituitary adenomas. J Clin Endocrinol Metab. 1994;78:83–88. doi: 10.1210/jcem.78.1.8288720. [DOI] [PubMed] [Google Scholar]
- Tulipano G, Bonfanti C, Poiesi C, Burattin A, Turazzi S, Barone G, Cozzi R, Bollati A, Valle D, Giustina A. Effects of the selective estrogen receptor modulator LY117018 on growth hormone secretion: In vitro studies. Metabolism. 2004;53(5):563–570. doi: 10.1016/j.metabol.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Webb CB, Szabo M, Frohman LA. Ectopic growth hormone releasing factor and dibutryl cyclic adenosine monophosphate-stimulated growth hormone release in vitro: effects of corticosterone and estradiol. Endocrinology. 1983;113:1191–1196. doi: 10.1210/endo-113-4-1191. [DOI] [PubMed] [Google Scholar]
- Yan M, Jones MEE, Hernandez M, Liu D, Simpson ER, Chen C. Functional Modification of Pituitary Somatotropes in the Aromatase Knockout Mouse and the Effect of Estrogen Replacement. Endocrinology. 2004;145:604–612. doi: 10.1210/en.2003-0646. [DOI] [PubMed] [Google Scholar]
- Zafer M, Ezzat S, Ramyar L, Pan N, Smyth HS, Asa SL. Cell-specific expression of estrogen receptor in the human pituitary and its adenomas. J Clin Endocrinol Metab. 1995;80:3621–3627. doi: 10.1210/jcem.80.12.8530610. [DOI] [PubMed] [Google Scholar]
- Zhao J, van Tol HT, Taverne MA, van der Weijden GC, Bevers MM, van den Hurk R. The effect of growth hormone on rat pre-antral follicles in vitro. Zygote. 2000;8:275–283. doi: 10.1017/s0967199400001076. [DOI] [PubMed] [Google Scholar]


