Abstract
The growth hormone secretagogue receptor 1a (GHSR-1a) is a G-protein coupled receptor, involved in the biological actions of ghrelin by triggering inositol phosphates and calcium intracellular second messengers. It has also been reported that ghrelin could activate the 44- and 42-kDa extracellular signal-regulated protein kinases (ERK1/2) in different cell lines, but it is not clear whether this regulation is GHSR-1a dependent or not.
To provide direct evidence for the coupling of GHSR-1a to ERK1/2 activation, this pathway has been studied in a heterologous expression system.
Thus, in Chinese hamster ovary (CHO) cells we showed that ghrelin induced, via the human GHSR-1a, a transient and dose-dep endent activation of ERK1/2 leading to activation of the transcriptional factor Elk1.
We then investigated the precise mechanisms involved in GHSR-1a-mediated ERK1/2 activation using various specific inhibitors and dominant-negative mutants and found that internalization of GHSR-1a was not necessary. Our results also indicate that phospholipase C (PLC) was involved in GHSR-1a-mediated ERK1/2 activation, however, pathways like tyrosine kinases, including Src, and phosphoinositide 3-kinases were not found to be involved. GHSR-1a-mediated ERK1/2 activation was abolished both by a general protein kinase C (PKC) inhibitor, Gö6983, and by PKC depletion using overnight pretreatment with phorbol ester. Moreover, the calcium chelator, BAPTA-AM, and the inhibitor of conventional PKCs, Gö6976, had no effect on the GHSR-1a-mediated ERK1/2 activation, suggesting the involvement of novel PKC isoforms (ɛ, δ), but not conventional or atypical PKCs. Further analyses suggest that PKCɛ is required for the activation of ERK1/2.
Taken together, these data suggest that ghrelin, through GHSR-1a, activates the Elk1 transcriptional factor and ERK1/2 by a PLC- and PKCɛ-dependent pathway.
Keywords: PKC, ghrelin, ERK, hGHSR-1a, Elk1
Introduction
Ghrelin, an acylated 28 amino-acid peptide, is predominantly a gastric hormone but is also produced ubiquitously at a lower level (Kojima et al., 1999; Gnanapavan et al., 2002). Two major physiological functions have been described for this hormone: the ability to induce the release of growth hormone (GH) (Bowers, 2001; Inui, 2001; Nakazato et al., 2001; Cummings et al., 2002) and to stimulate food intake and adiposity both in rodents (Tschop et al., 2000; Wren et al., 2000) and humans (Inui, 2001; Wren et al., 2001). The major circulating form of ghrelin is unacylated (des-acyl ghrelin) but only the acylated form is able to bind its known receptor, the GH secretagogue receptor 1a (GHSR-1a) (Kojima et al., 1999; Hosoda et al., 2000). This Gq/11 protein-coupled receptor (GPCR) is mainly expressed in the pituitary and hypothalamus (Guan et al., 1997; McKee et al., 1997). It was first identified as the receptor for GH secretagogues (GHSs), a group of synthetic molecules endowed with strong GH-releasing activity (Howard et al., 1996). Functionally, activation of GHSR-1a has been shown to trigger both calcium release from intracellular stores through the generation of inositol trisphosphates and calcium entry from the extracellular medium via voltage-operated L-type channels, leading to the release of GH from somatotroph cells or GH-releasing hormone from hypothalamic neurons (Smith et al., 1997; Lall et al., 2004).
In addition to playing an important role in the short-term regulation of peptide secretion, the GHSR-1a could also elicit long-term responses, as other GPCRs do, by stimulating mitogen-activated protein kinases (MAPKs), such as the extracellular signalling-regulated kinase 1 and 2 (ERK1/2), the stress-activated c-jun NH2-terminal kinases (JNK) and p38 kinases (Gutkind, 1998). ERK1/2 are protein-serine/threonine kinases mainly involved in the activation of nuclear transcription factors controlling cell proliferation, cell differentiation and cell death (Gutkind, 2000).
The regulation of ERK1/2 through GPCRs is a complicated process as there is a great heterogeneity in the different mechanisms that have been reported. However, there has been substantial progress in the understanding of cellular events that link the activation of GPCR with ERK1/2 (Lefkowitz et al., 2002). These signalling events can be classified into several distinct pathways including (i) the ras-dependent activation of ERK1/2 via transactivation of receptor tyrosine kinases (RTKs), (ii) the ras-independent ERK1/2 activation via protein kinase C (PKC) that converges with the RTK signalling at the Raf level, (iii) the modulation of ERK1/2 activation via the cAMP/protein kinase A (PKA) pathway, in which the direction of regulation depends on Raf-subtypes, and (iv) the recently discovered β-arrestin-mediated pathway proven in certain classes of GPCRs (Pierce et al., 2001). However, it should be mentioned that these signalling pathways have been deduced from a limited sets of individual receptors or cell types, and more extensive systemic studies are needed for these signalling models to be generalized (Liebmann, 2001).
There is ongoing controversy as to whether the known GHSR-1a receptor is the sole receptor for GHSs or just one member of a group of receptors for such ligands. The existence of different receptors might be endorsed by the differences in the binding activities reported for ghrelin and several other GHSs (Ong et al., 1998; Chen, 2000; Ghe et al., 2002). In addition, different cellular functions involving mitogenic pathways were described for ghrelin in cell lines, which express GHSR-1a and in those that do not express GHSR-1a. Thompson et al. (2004) have shown that both ghrelin and des-acyl ghrelin could promote adipogenesis directly in vivo and suggested a mechanism independent of GHSR-1a. In addition, both ghrelin and des-acyl ghrelin inhibited cell death through activation of the ERK1/2 and the phosphatidylinositol 3-kinase (PI3K)/Akt pathways in H9c2 cardiomyocytes, which do not express GHSR-1a (Baldanzi et al., 2002). Further studies reported an antiproliferative role of ghrelin in the absence of any detectable GHSR-1a on breast (Cassoni et al., 2001), lung (Ghe et al., 2002) and thyroid cell lines (Volante et al., 2003). However, ghrelin was also shown to induce cell proliferation in cell lines expressing the GHSR mRNA. Cell proliferation occurred via the activation of MAPK pathway in the hepatoma HepG2 cell line (Murata et al., 2002) and via a tyrosine kinase-dependent but PKC-independent activation of ERK1/2 in rat adrenal zona glomerulla cell cultures (Andreis et al., 2003). Finally, ghrelin exerts a proliferative effect in cell lines reported to express GHSR-1a mRNA, via activation of the ERK1/2 and the PI3K/Akt pathways in 3T3-L1 adipocytes (Kim et al., 2004) and via a PKC- and TRK-dependent activation of ERK1/2 in the pituitary GH3 cell line (Nanzer et al., 2004). However, in these cell lines, a subtype of the ghrelin receptor distinct from GHSR-1a could also be expressed (Nanzer et al., 2004; Zhang et al., 2004).
Therefore, as the involvement of GHSR-1a in these cellular events and notably in the activation of ERK1/2 is not clear, we developed a cellular model expressing exclusively the GHSR-1a to study the molecular mechanisms by which ghrelin is able to induce ERK activation. We selected Chinese Hamster Ovary (CHO) cells to transiently express the human GHSR-1a (hGHSR-1a). We then (i) pharmacologically characterized this model (ii) determined whether clathrin-mediated endocytosis was required for the activation of ERK1/2 and (iii) identified the putative signalling mediators involved in ERK1/2 activation using various specific inhibitors and dominant-negative mutants.
Methods
Materials
Ham's F-12 medium, fetal bovine serum (FBS), nonessential amino acids and HBSS were all purchased from Invitrogen (Cergy Pontoise, France). Phosphate-buffered saline (PBS), penicillin-streptomycin, L-glutamine, Dulbecco's modified eagle's medium (DMEM) and medium 199 were all from Cambrex (Emerainville, France). Bovine serum albumin (BSA) fraction V, sodium dodecyl sulfate (SDS) and mouse anti-PKCδ came from Euromedex (Souffelweyersheim, France). Human ghrelin, human des-acyl ghrelin and MK0677 were purchased from NeoMPS (Strasbourg, France). Myo-[2-3H]inositol (15 Ci mmol−1) and human 125I-His(9)-ghrelin (2000 Ci mmol−1) were from Amersham (Orsay, France). JMV 1843 (H-Aib-D-Trp-D-gTrp-CHO) and D-Lys(3)-GHRP6 was synthesized in our laboratory. 4-(2-aminoethyl) benzenesulfonyl fluoride hydrochloride (AEBSF) was bought from Fulka Chemie AG (Buchs, Switzerland). MEK1/2 inhibitor Uo126, phospho-p42/p44 MAPK (Thr202/Tyr204) antibody, phospho-p38 MAPK (Thr180/Tyr182) antibody, phospho-SAPK/JNK (Thr183/Tyr185) antibody, anti-rabbit IgG HRP-linked antibody, anti-mouse IgG HRP-linked antibody, lumiGLO reagent and peroxide were all purchased from Cell signalling technology (Ozyme, St Quentin Yvelines, France). The mouse anti-PKCɛ antibody was from Transduction Laboratories (Lexington, KY, U.S.A.). AG1-X8 resin (200–400 mesh, formate form) was purchased from Bio-Rad (Hercules, U.S.A.) and pluronic acid was purchased from Molecular Probes (Paris, France). Phorbol-12-myrsitate-13-acetate (PMA), pertussis toxin (PTX), genistein, tyrphostin 23, concanavalin A (Con A), U-73122 (1-(6(((17β)-3-Methoxyestra-1,3,5 (10)-trien-17-yl)amino))hexyl)-1H-pyrrole-2,5-dione), U-73343 (1-(6(((17β)-3-Methoxyestra-1,3,5 (10)-trien-17-yl)amino))hexyl)-2,5-pyrrolidinedione), rabbit anti-PKCα, poly D-lysine, bacitracin, adenosine, cycloheximide, acetoxymethyl ester of 1, 2-bis(2-aminophenoxy) ethane-N,N,N′,N′-tetraacetic acid (BAPTA-AM) and probenecid were all purchased from Sigma (Lyon, France). 4-amino-5-(4-chlorophenyl)-7-(t-butyl) pyrazol (3, 4-d) pyrimidine (PP2), 4-amino-7-phenylpyrazol (3, 4-D) pyrimidine (PP3), wortmannin, Gö6983 (2-(1-(3-dimethylaminopropyl)-5-methoxyindol-3-yl)-3-(1H-indol-3-yl)maleimide) and Gö6976 (12-(2-cynanoethyl)-6,7,12,13-tetrahydro-13-methy-5-oxo-5H-indol (2, 3-a) pyrrollo (3. 4-c) carbazole) were all purchased from Calbiochem (La Jolla, U.S.A.).
Cell culture and transfection
CHO cells were cultured in DMEM supplemented with 10% (v v−1) FBS, 1% (v v−1) of nonessential amino acids, 2 mM glutamine and streptomycin-penicillin (250 μg ml−1 to 250 IU ml−1), at 37°C in a humidified atmosphere with 5% CO2. For transient transfections, 5 × 106 cells were transfected with 30 μg of DNA/cuvette using Easyject Optima electroporator (Equibio, Kent, U.K.) following the manufacturer's instructions. For co-transfection, the cells were transfected with 10 μg of plasmid encoding hGHSR-1a together with 20 μg of the plasmid of interest (dominant-negative or wild-type dynamins, dominant negative or wild-type β-arrestins, dominant-negative or wild-type PKCs, pcDNA3 or pEGFP-N1). Upon transfection, cells were diluted by addition of DMEM without phenol red, supplemented with 10% FCS, L-glutamine and streptomycin-penicillin. Specific binding of labelled ghrelin under the different experimental co-transfection conditions was assayed as a control for expression level of GHSR-1a.
Plasmids
Wild-type human growth hormone secretagogue receptor 1a (hGHSR-1a), in mammalian expression vector pcDNA3.1 was purchased from Guthrie Reasearch Institute (Sayre, PA, U.S.A.). Vectors pcDNA3 and pEGFP-N1 were from Clontech Laboratories (Palo Alto, CA, U.S.A.). The following plasmids were generous gifts: pEGFP-N1 encoding GFP-tagged human β-arrestin 1 and GFP-tagged human β-arrestin 1319−418 from F. Boulay (CEA, Grenoble, France), the HA epitope-tagged β-arrestin 2 and β-arrestin 2284−409 in pcDNA3 from J.L. Benovic (Thomas Jefferson University, Philadelphia, U.S.A.), pEGFP-N1 encoding GFP-tagged human wild-type PKCα, PKCδ, PKCɛ and pcDNA4 encoding c-myc-tagged rat dominant-negative PKCαK368R, PKCδK379R, PKCɛK436R were all from Dr D. Joubert (IGF, Montpellier, France), and the HA epitope-tagged wild-type and K44A mutant dynamin-1 and 2 in pcDNA3 from Dr Kazuhisa Nakayama (Kyoto University, Japan).
Plasmid construction of the C-terminal tagging hGHSR-1a with GFP (hGHSR-1a-GFP)
The hGHSR-1a cDNA was amplified by polymerase chain reaction using the following sense and antisense oligonucleotide primers: 5′-CTC AAG CTT CGA ATT CTG GCC ACC ATG TGG AAC GCG ACG CCC AGC GAA GAG-3′ (containing the nucleotides ATT, the HindIII restriction site sequence, the kozac sequence and nucleotides 1–27 of the GHS receptor cDNA type 1a) and 5′-CGG TGG ATC CCG GGC TGT ATT AAT ACT AGA TTC TGT CCA GGC CCG-3′ (corresponding to nucleotides 1068–1098, followed by the sequence of the BamHI restriction site, and four additional nucleotides, ACCG. The PCR product (1137 bp) was gel-purified, digested with HindIII and BamHI and was then inserted into the HindIII and BamHI sites of plasmids pEGFP-N1 upstream of the 5′-end of the pEGFP cDNA. The construct was verified by sequencing (Genome express, Meylan, France).
Membrane preparation
After tranfection, cells were cultured in 100-mm dish. Crude membranes were prepared on ice 24 h after transfection. Each 100-mm dish was washed twice with 10 ml PBS and once with 10 ml homogenization buffer (containing 50 mM Tris-HCl (pH 7.4), 5 mM MgCl2, 2.5 mM EDTA, 30 μg ml−1 bacitracin). Homogenization buffer (0.5 ml) was then added to each dish, and cells were removed by scraping and lysed after two freeze–thaw cycles. The homogenate was centrifuged for 20 min at 11,000 × g at 4°C, and the resulting crude membrane pellet (chiefly containing cell membranes and nuclei) was resuspended in homogenization buffer supplemented with 0.06% BSA and stored at −80°C.
Radioligand-binding study
The binding of 125I-labelled ghrelin to crude membranes prepared from transfected cells was performed essentially as described (Guerlavais et al., 2003). Binding reactions were performed at 20°C for 1 h in a total volume of 0.5 ml containing 0.1 ml membrane suspension (10 μg protein), 50 μl competing drug (from 10−12 to 10−3 M), 300 μl homogenization buffer, and 50 μl 125I-labelled ghrelin (2.5 × 10−11 M), for displacement binding studies, or in the presence of 15–700 pM 125I-labelled ghrelin for saturation binding studies. Bound radioligand was separated by filtration through GF/C filters pretreated for 1 h with 0.5% polyethylenimine. After application of the membrane suspension to the filter, the filters were washed three times with 3 ml of ice-cold wash buffer (containing 50 mM Tris-HCl (pH 7.4), 10 mM MgCl2, 2.5 mM EDTA, and 0.015% Triton X-100) and the bound radioactivity on the filters was quantitated by counting. Specific binding (>90% of total) is defined as the difference between total binding and nonspecific binding conducted in the presence of 1 μM unlabelled ghrelin. IC50, Kd and Bmax values were performed using PRISM 3.0 (GraphPad, San Diego, U.S.A.).
Measurement of inositol phosphates production
One day after transfection, cells (105 cells ml−1) plated in 24-multiwell culture plates, were washed with 1 ml of medium 199 containing antibiotics (250 IU ml−1 penicillin and 250 μg ml−1 streptomycin) and cultured for 16 h in 1 ml of the same medium containing 2 μCi of myo-[2-3H]inositol. After this time, the cells were washed in medium 199 with antibiotics and incubated (30 min, 37°C) in the same medium containing 20 mM LiCl. Cells were then washed with 1 ml of IP buffer (containing 135 mM NaCl, 20 mM HEPES, 2 mM CaCl2, 1.2 mM MgSO4, 1 mM EGTA, 10 mM LiCl, 11.1 mM glucose, and 0.5% BSA, pH 7.45) and incubated with or without ghrelin (from 10−13 to 10−6 M) in a final volume of 500 μl of IP buffer. After 1 h incubation at 37°C, the reaction was stopped by removing the incubation medium and adding 1 ml of a mixture of ethanol/HCl (2000 : 1, v v−1). The samples were then applied to the columns containing 1 ml of a 1 : 2 (v v−1) Dowex AG-1-X8 anion exchange resin in distilled water. The columns were washed with 2 × 3 ml of distilled water and 2 × 2 ml of 40 mM ammonium formate. Inositol phosphates were eluted with 2.5 ml of 1 M ammonium formate. The radioactivity of each eluate was counted after addition of 10 ml of Complete Phase Combining System solution. Determinations were made in triplicates. EC50 calculations were performed using PRISM 3.0.
Intracellular Ca2+ measurement
The FlexStation machine (benchtop scanning fluorometer, Molecular Devices, Sunnyvale, CA, U.S.A.) was used to measure agonist-induced calcium mobilization. One day before the assay, hGHSR-1a-expressing CHO cells (105 cells/100 μl medium well−1) were seeded into the black-wall, clear-bottom 96-well plate (Corning 3603) precoated with poly-D-lysine. After 24 h, the medium was discarded, the cells washed and then incubated with 100 μl well−1 of the assay buffer (containing Hanks' balanced salt solution, 0.5% BSA, 20 mM Hepes, 1 mM MgSO4, 3.3 mM Na2CO3, 1.3 mM CaCl2, 2.5 mM probenecid, pH 7.4) containing 1 μM fluo 4-AM (Interchim), 0.06% pluronic acid. The cells were then left to incubate for 60 min at 37°C, 5% CO2. Following this, the cells were washed with 2 × 100 μl of the assay buffer and then 50 μl well−1 of the assay buffer was added. The plate containing the cells was placed into the FlexStation and the assay was started by automatic addition of 50 μl well−1 of the agonist diluted in assay buffer (from 10−12 to 10−2 M). The fluorescence output was measured over 1 min. The basal fluorescence intensity of dye-loaded cells was 500–1000 and the fluorescence peak on maximal response observed with 100 nM ghrelin was 6500–8000 arbitrary units. EC50 calculations were performed using PRISM 3.0.
ERK1/2 phosphorylation
One day after transfection, cells plated in six-well culture plates (3 × 105 cells/well) were starved for 1 h in serum-free Ham's F-12 medium, before treatment at 37°C with selected agents. Cells were then placed on ice, the media was aspirated, and the cells were washed twice with ice-cold PBS and lysed in 60 μl 2X Laemmli sample buffer containing 1 mM AEBSF. The samples were heated to 95°C for 10 min, and centrifuged. Proteins were separed by SDS–PAGE (5% stacking gel, 10% running gel) and electroblotted onto nitrocellulose membrane. The membranes were incubated for 1 h at room temperature in PBS containing 5% nonfat dry milk, followed by overnight incubation at 4°C with antibodies to phospho-ERK1/2 (1 : 2000 dilution). The following day, the membranes were incubated with the horseradish peroxidase-conjugated secondary antibodies for 1 h at room temperature in 5% BSA. Blots were then visualized with the chemiluminescent western blotting kit. Blots were stripped and reprobed with antibodies for total (unphosphorylated+phosphorylated) ERK1/2 to control for protein loading.
125I-ghrelin internalization assay
CHO cells transiently expressing hGHSR-1a were grown in triplicate in six-well culture plates. Twenty-four hours after transfection, the cells were placed on ice, washed twice, and incubated for 3 h at 4°C with 0.3 nM 125I-ghrelin with or without 250 μg ml−1 concanavalin A (Con A) in binding buffer (containing Ham's F-12 medium, 20 mM Hepes, pH 7.4, 1% BSA). In order to block the new receptor synthesis, protein synthesis was inhibited by the addition of 100 μM cycloheximide, which was added in binding buffer and maintained throughout the assay. After 3 h, the media containing labelled ghrelin was removed and the cells were washed twice with ice-cold binding buffer. Fresh binding buffer was added with or without 250 μg ml−1 Con A and cells were incubated at 37°C for 20 min. Incubations were stopped by placing cells on ice and rapidly washing them with ice-cold PBS. The membrane-bound radioligand was stripped by treatment with ice-cold acid wash solution (containing 0.5 M NaCl and 0.2 M acetic acid, pH 2.6) for 15 min. The released radioligand (acid-released) was then collected to estimate the surface-associated 125I-ghrelin. The internalized (acid-resistant) radioligand was estimated after solubilization of the cells with 1 ml of lysis buffer (containing 1% Nonidet P-40, 0.5% Triton X-100 and 1 M NaOH) and washing with an additional 0.5 ml of the same solution. The nonspecific binding was measured in parallel experiments using 1 μM ghrelin. Radioactivity was quantified in a γ-counter. The percentage of internalization of hGHSR-1a was calculated after deduction of the respective nonspecific value: % receptor on cell surface=((c.p.m. acid-released)/(c.p.m. acid-resistant+c.p.m. acid-released)) × 100.
Immunostaining
Cells transiently expressing the hGHSR-1a-GFP were grown on glass coverslips. After stimulation, cells were washed with PBS and fixed with 4% paraformaldehyde for 10 min at room temperature. Cells were then rinsed with PBS, permeabilized by immersing in 100% methanol at −20°C for 10 min, blocked in 3% BSA in PBS, and finally incubated with phospho-p44/p42 MAP kinase antibody diluted (1 : 200 dilution) in the blocking buffer overnight at 4°C. After rinsing with PBS containing 0.1% triton, cells were incubated for 1 h with Cy3-conjugates anti-rabbit IgG secondary antibodies (Jackson Immunoresearch, Cambridgeshire, U.K.) at 1 : 200 dilution in PBS containing 3% BSA for 1 h. Cells were then washed in PBS and mounted with mowiol for fluorescence analysis (Nikon Diaphot 300 microscope).
Elk1 reporter assay
To determine whether Elk1 is activated by ghrelin, the PathDetect Reporting System (Stratagene) was used. A plasmid containing five tandem repeats of the yeast GAL4 binding sites linked to firefly luciferase (pFR-Luc, 5 μg) and another plasmid consisting of the activation domain of Elk1 fused with the DNA-binding domain of the yeast GAL4 (Elk1-GAL4, 5 μg), were co-transfected with 20 μg of hGHSR-1a cDNA into 5 × 106 cells by electroporation. The transfected cells were plated in twelve-well plates (105 cells/well). Sixteen hours after the transfection, the culture medium was changed to serum-free for 24 h. Cells were further cultured in the presence or absence of ghrelin (10−7 M) for an additional 8 h. Cells were then lysed with the reporter lysis buffer (Promega, Charbonniēres, France) and luciferase activity in cleared lysates was measured using a Wallac 1420 Microbeta Jet, upon injection of luciferase detection buffer (containing 15 mM K2HPO4-KH2PO4, 1.05 mM MgCl2, 2.7 mM MgSO4, 0.2 mM EDTA, 0.53 mM ATP, 0.27 mM Coenzyme A and 0.5 mM Luciferin, pH 7.4).
Results
Pharmacological characterisation of the transiently expressed hGHSR-1a in CHO cells
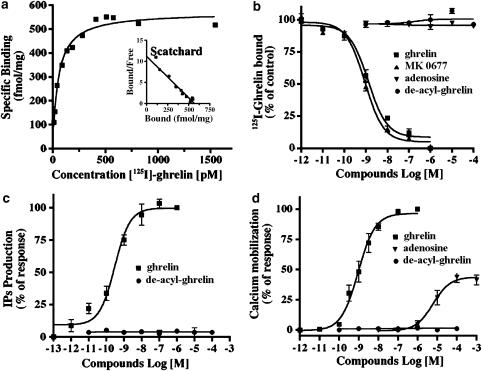
To determine the pharmacological properties of the hGHSR-1a transiently expressed in CHO cells, we performed both 125I-ghrelin binding assays and measures of second messagers using either membrane preparations or direct cell cultures. Saturation experiments showed that hGHSR-1a expressing cells displayed high affinity, saturable and specific binding (dissociation constant Kd=0.1 nM and binding capacity Bmax=580 fmol per mg protein; single class of binding sites; Figure 1a). Competition binding analysis demonstrated high-affinity binding of ghrelin and MK-0677, a synthetic GHS peptidomimetic (IC50=0.7 and 0.9 nM, respectively), while des-acyl ghrelin and adenosine, a nucleotide reported to behave as a partial agonist of the GHSR-1a by binding to a site distinct from the ghrelin binding site (Smith et al., 2000; Tullin et al., 2000) did not bind (Figure 1b). These results are similar to those reported with cells expressing endogenously or heterologously the GHSR-1a (Howard et al., 1996; Camina et al., 2004). In cells transfected with vector alone no specific binding of ghrelin was found (data not shown). The functional analyses of hGHSR-1a were performed using both IP and Ca2+-mobilization assays. As shown in Figure 1c, ghrelin but not des-acyl ghrelin, induced the IPs production in a dose-dependent manner, with an EC50 value of 0.5 nM. Similarly, ghrelin but not des-acyl ghrelin evoked Ca2+-mobilization with an EC50 value of 0.95 nM (Figure 1d). Adenosine behaved as a partial agonist of hGHSR-1a, inducing Ca2+-mobilization with an EC50 value of 9 μM. Cells transfected with vector alone failed to respond to ghrelin and adenosine (concentration tested up to 100 μM; data not shown). Thus, under our experimental conditions, the transiently expressed hGHSR-1a in CHO cells is pharmacologically similar to the endogenous GHSR-1a on native cells.
Figure 1.
Functional and binding analysis in CHO cells transiently expressing hGHSR-1a. CHO cells were transiently transfected with the plasmid encoding hGHSR-1a (30 μg/5 × 106 cells). (a) Radioligand binding analysis with 125I-ghrelin by saturation isotherm. Membranes prepared from cells were incubated with increasing amounts of 125I-ghrelin in the presence (nonspecific binding) or absence (total binding) of 0,1 μM unlabelled ghrelin as described under Methods. Specific binding was calculated. Inset, Scatchard analysis of the radioligand binding saturation isotherm. (b) Competitive displacement binding assay of the radiolabelled 125I-ghrelin was determined by incubation of membrane preparations from cells in the presence of increasing amounts of ghrelin, MK-0677, adenosine and des-acyl-ghrelin. (c) Total IPs accumulated in cells incubated with ghrelin and des-acyl-ghrelin was determined as described under Methods and expressed as percentage of the maximal ghrelin effect (100%=3.5±0.5-fold stimulation over basal production). (d) Calcium mobilization in cells stimulated Ghrelin, adenosine and des-acyl-ghrelin was detected by a fluorometric imaging plate reader (FlexStation) assay as described under Methods and expressed as percentage of the maximal ghrelin effect (100%=9.5±1.0-fold stimulation over basal production). Results illustrated in panels a–d are the mean±the standard deviation (s.d.) of three to seven independent experiments, each performed in triplicate.
Ghrelin but not adenosine induces hGHSR-1a-mediated ERK1/2 in CHO cells
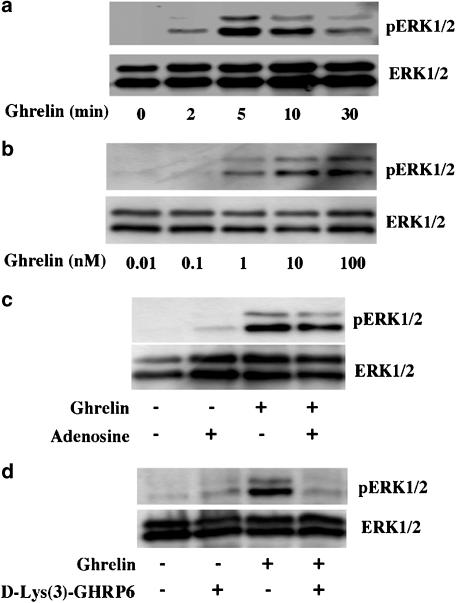
The time course and dose–response relationship of hGHSR-1a-mediated activation of ERK1/2, were studied in CHO cells transiently transfected with the hGHSR-1a. As shown in Figure 2a, the treatment of cells with ghrelin for periods ranging from 0–30 min induced a transient phosphorylation of ERK (pERK1/2). Activation of ERK1/2 occurred as early as 2 min after ghrelin stimulation, peaked between 5 and 10 min, and lasted at least 30 min. The dose dependence of GHSR-1a-mediated ERK1/2 activation was also evaluated. The cells were treated with various concentrations of ghrelin for 5 min corresponding to the incubation time that had previously given maximal ERK1/2 activation. This analysis (Figure 2b) showed that the activation of ERK1/2 was dose-dependent and occurred in response to very low levels of ghrelin (1 nM). In contrast, no ERK1/2 activation was observed with des-acyl ghrelin and in cells transfected with vector alone (data not shown).
Figure 2.
Time course and dose–response relationship of hGHSR-1a-induced activation of ERK1/2 in CHO cells. CHO cells were transiently transfected with the plasmid encoding hGHSR-1a. (a) Time course of hGHSR-1a-mediated activation of ERK1/2. Cells serum-starved for 1 h were then incubated in the presence of 100 nM ghrelin for the indicated times (0–30 min). (b) Dose–response of hGHSR-1a-mediated ERK1/2 activation. Cells serum-starved for 1 h were treated with various concentrations of ghrelin (0.01–100 nM) for 5 min. (c) Effect of adenosine on hGHSR-1a-mediated ERK1/2 activation. Cells serum-starved for 1 h were treated with vehicle (0.1% DMSO) or adenosine (100 μM) for 5 min, before the addition of ghrelin (100 nM, 5 min). (d) Effect of a specific GHSR-1a antagonist, D-Lys(3)-GHRP6, on hGHSR-1a-mediated ERK1/2 activation. Cells serum-starved for 1 h were then pretreated with or without D-Lys(3)-GHRP6 (100 μM, 5 min), before the addition of ghrelin (100 nM, 5 min). Cell extracts were prepared as described under Methods, and analysed on 10% polyacrylamide gels. ERK1/2 phosphorylation (pERK1/2) was measured by immunoblotting with phospho-ERK1/2 antibodies. Blots were stripped and reprobed with ERK1/2 antibody (ERK1/2) as shown in the lower panels to control for protein loading. All blots are representative of three or four experiments with similar results.
Previous studies have demonstrated that the partial agonist adenosine binds to GHSR-1a, a site distinct from the characterized ghrelin-binding pocket. The effect of this non-competitive compound on ERK1/2 activation was therefore tested. Treatment with adenosine (100 μM) for periods ranging from 0–30 min did not induce the phosphorylation of ERK1/2 (data not shown). In addition, pretreatment with adenosine for 5 min, before stimulation with ghrelin for 5 min, did not modulate ghrelin-mediated ERK1/2 activation (Figure 2c).
The effect of a competitive antagonist of ghrelin on ERK1/2 activation was also tested. Treatment with the GHSR-1a antagonist, D-Lys(3)-GHRP-6, did not induce the phosphorylation of ERK1/2, and abolished ghrelin-mediated ERK1/2 activation (Figure 2d). Together, these results suggest that hGHSR-1a-mediated ERK1/2 activation is a specific effect of the competitive agonists of ghrelin.
Localization and transcriptional role of hGHSR-1a-mediated ERK1/2 activation, which is MEK1/2 dependent
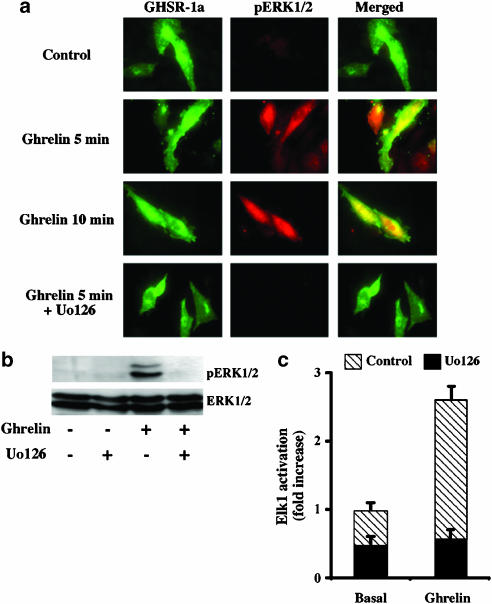
Once activated, ERK1/2 can phosphorylate numerous plasma membrane, cytosolic and cytoskeletal substrates (Pearson et al., 2001). Activated ERK1/2 may also translocate to the nucleus, where it activates transcription by phosphorylating nuclear transcription factors, such as the Elk-1 which is a member of ETS family (Pearson et al., 2001). To gain further insight into the hGHSR-1a-induced ERK1/2 activation, we examined the subcellular localization of pERK1/2 and we investigated whether hGHSR-1a-induced ERK1/2 activation leads to activation of Elk-1.
Under control conditions of no stimulation, no significant cellular staining of pERK1/2 was observed by fluorescence microscopy in cells transiently transfected with the GFP-tagged hGHSR-1a (Figure 3a). Stimulation by ghrelin for 5 or 10 min leads to phosphorylation of ERK1/2 and, cellular staining of pERK1/2 was observed in both the cytoplasm and nucleus of hGHSR-1a-GFP-expressing cells. A similar subcellular accumulation of pERK1/2 was observed in virtually all cells when stimulated with FBS for 10 min under control conditions (data not shown). MEK1/2 is an immediate signalling component that directly controls ERK1/2 activity. As expected, pretreatment of cells with Uo126, a selective MEK1/2 inhibitor, before stimulation with ghrelin for 5 min, fully abolished hGHSR-1a-mediated ERK1/2 activation (Figure 3a and b). This indicates that upstream MEK1/2 activation is a necessary event for hGHSR-1a-mediated ERK1/2 activation. The presence of GFP sequence had no effect on the binding or function of the receptor (data not shown).
Figure 3.
Localization and transcriptional role of hGHSR-1a-mediated ERK1/2 activation, which is MEK1/2 dependent. (a) Effects of the hGHSR-1a activation by ghrelin on the cellular localization of phospho-ERK1/2. CHO cells were transiently transfected with the plasmid encoding the hGHSR-1a-tagged GFP and cells were grown on glass coverslip. Serum-starved cells were then treated with vehicle (control), ghrelin (100 nM, 5 or 10 min), SVF (10%, 10 min), or Uo126 (10 μM, 1 h) before the addition of ghrelin (100 nM, 5 min). Cells were stained with anti-phosphospecific ERK1/2 antibody together with Cy3-conjugated anti-rabbit IgG before examination by fluorescent microscopy as described in Methods. Each image depicts a representative fluorescent microscopic image from one of three separate experiments. (b) Effects of a MEK1/2 inhibitor on hGHSR-1a-mediated activation of ERK1/2. Cells were preincubated for 1 h with vehicle (0.1% DMSO) or the MEK inhibitor Uo126 (10 μM, prepared in DMSO) in serum-free medium, before the addition of ghrelin (100 nM, 5 min). Phosphorylated ERK1/2 (pERK1/2) and total ERK1/2 (ERK1/2) were detected by immunoblotting described in Figure 2. This blot is representative of three experiments with similar results. (c) To analyse Elk1 activation, a reporter gene assay was used as described in Methods. Cells were pretreated with the MEK inhibitor Uo126 (10 μM, prepared in DMSO) or vehicle (0.1% DMSO) for 30 min before stimulating with ghrelin (100 nM) for 8 h. Cells were then lysed and luciferase activity measured. Data are expressed as fold increase versus the basal values, obtained in the absence of ghrelin. The results shown are a representative experiment. Values represent mean±s.d. of triplicate determination. Similar results were obtained from two other experiments.
To determine whether this nuclear accumulation of pERK1/2 could then activate transcription factors, we used a luciferase reporter gene expression assay. In this experiment, activation of the chimeric transcription factor (Elk1-GAL4) can be easily visualized by measuring the corresponding luciferase activity. As shown in Figure 3c, ghrelin stimulation of hGHSR-1a-expressing cells for 8 h led to a 2.5±0.2-fold increase in Elk1-driven luciferase activity. In agreement with the results reported above, in the presence of Uo126, this activation was completely abolished. Taken together, these results indicate that activation of hGHSR-1a by ghrelin leads to activation of the MEK1/2-ERK1/2 pathway and consequently to Elk1.
JMV 1843 induces GHSR-1a-mediated ERK1/2 activation
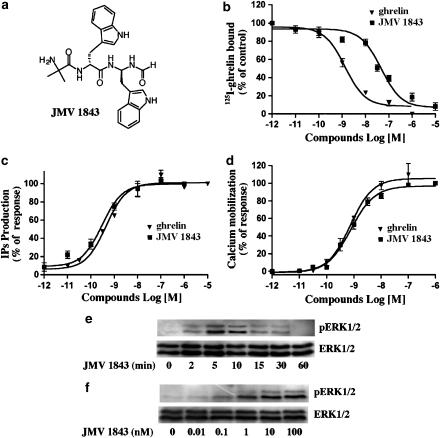
JMV 1843 is the only orally-administered peptidomimetic GHS in clinical development which binds hGHSR-1a (Guerlavais et al., 2003). Recently positive Phase I clinical trial results were reported. Potential indications for JMV 1843 include treatment of GH deficiency disorders in adults and children, as well as metabolic complications associated with critical illnesses, such as AIDS-associated cachexia, cancer, and trauma. As this compound has been previously described as an agonist for the GHSR-1a, we investigated its pharmacological behaviour on our cellular model. As shown in Figure 4, we studied its affinity for hGHSR-1a and its ability to induce IPs production, intracellular Ca2+ mobilization and ERK1/2 activation. In binding, JMV 1843 had 55-fold weaker binding affinity than ghrelin to the hGHSR-1a (IC50=40 nM and IC50=0.7 nM respectively; Figure 4b). In contrast, in the tests of second messenger activation, JMV 1843 and ghrelin demonstrated similar potencies in the sub-nanomolar range (EC50=0.3 nM and EC50=0.8 nM, respectively) (Figure 4c and d). In other words, JMV 1843 demonstrated a higher amplification effect than ghrelin, in that a complete occupancy of its binding sites was not necessary to obtain the maximal biological response. This phenomenon was also observed for three GHSs: NN703 and Ipamorelin (Hansen et al., 1999) and the nonpeptide L-692,429 (Holst et al., 2005). However, the molecular mechanisms behind these observations remain unclear (Kenakin, 2004; Holst et al., 2005). To determine whether this amplification effect of JMV 1843 could also be observed on ERK1/2 activation, cells expressing hGHSR-1a were treated with JMV 1843. As shown in Figure 4e, for periods ranging from 0–60 min, this compound induced a transient phosphorylation of ERK1/2 that occurred as early as 2 min after stimulation and peaked between 5 and 10 min. This activation was dose-dependent and occurred in response to 1 nM JMV 1843 (Figure 4f). These results are similar to values reported with ghrelin. Thus, as observed with the second messager activation, JMV 1843 induced ERK1/2 activation to a similar extent as ghrelin despite its binding affinity for the hGHSR-1a being 100-fold weaker. We then investigated the precise mechanisms involved in hGHSR-1a-mediated ERK1/2 activation with ghrelin and JMV 1843. The results reported below are only given for ghrelin as these were similar to those obtained with JMV 1843.
Figure 4.
In vitro biological activity of JMV 1843 in CHO cells transiently expressing hGHSR-1a. CHO cells were transiently transfected with the plasmid encoding hGHSR-1a (30 μg/5 × 106 cells). (a) Chemical structure of JMV 1843. (b) Competitive displacement binding assay of the radiolabelled 125I-ghrelin was determined by incubation of membrane preparations from cells in the presence of increasing amounts of ghrelin and JMV 1843. (c) Total IPs accumulated in cells incubated with ghrelin and JMV 1843 was determined as described under Methods and expressed as percentage of the maximal ghrelin effect. (d) Ghrelin and JMV 1843-induced calcium mobilization in cells was detected by a fluorometric imaging plate reader (FlexStation) assay as described under Methods and expressed as percentage of the maximal ghrelin effect. Results illustrated in panels b–d are the mean±the standard deviation (s.d.) of three to seven independent experiments, each performed in triplicate. (e) Time course of hGHSR-1a-mediated activation of ERK1/2. Cells serum-starved for 1 h were then incubated in the presence of 100 nM JMV 1843 for the indicated times (0–60 min). (f) Dose–response of hGHSR-1a-mediated ERK1/2 activation. Cells serum-starved for 1 h were treated with various concentrations of JMV 1843 (0–100 nM) for 5 min. Cell extracts were prepared as described under Methods, and analysed on 10% polyacrylamide gels. ERK1/2 phosphorylation (pERK1/2) was measured by immunoblotting with phospho-ERK1/2 antibodies. Blots were stripped and reprobed with ERK1/2 antibody (ERK1/2) as shown in the lower panels to control for protein loading. Blots are representative of three experiments with similar results.
Clathrin-dependent endocytosis is not a requirement for ERK1/2 activation by hGHSR-1a
Recently, it has been reported that GHSR-1a internalized principally by a clathrin-mediated mechanism (Camina et al., 2004; Holst et al., 2004). On the other hand, it has also been suggested that receptor internalization may be required for the activation of the ERK1/2 cascades (Luttrell et al., 1997; Daaka et al., 1998; Vogler et al., 1999) but this does not appear to be the case for all GPCRs (Budd et al., 1999). To determine whether internalization of hGHSR-1a is required for ERK1/2 activation, we tested whether hGHSR-1a-mediated ERK1/2 activation was sensitive to inhibitors of clathrin-mediated endocytosis. The effects of three mechanistically distinct inhibitors of clathrin-mediated endocytosis, Concanavalin A (Con A), dynamins K44A, and dominant-negative (DN) mutant of β-arrestins, were determined. Con A prevents internalization of membrane-associated receptors by stabilizing the surface integrity due to tetravalent lectin contacts (Toews et al., 1984; Pippig et al., 1995). Dynamins are GTPases that bind to clathrin-coated pits and the point mutant form of dynamins, K44A, is a dominant inhibitory form of the wild type that inhibits vesicular endocytosis (Damke et al., 1994). Similary, DN β-arrestin1318−418 and DN β-arrestin2284−409 are truncated forms of β-arrestin 1 and 2, respectively, that inhibit the association of GPCRs with clathrin-coated pits (Krupnick et al., 1997).
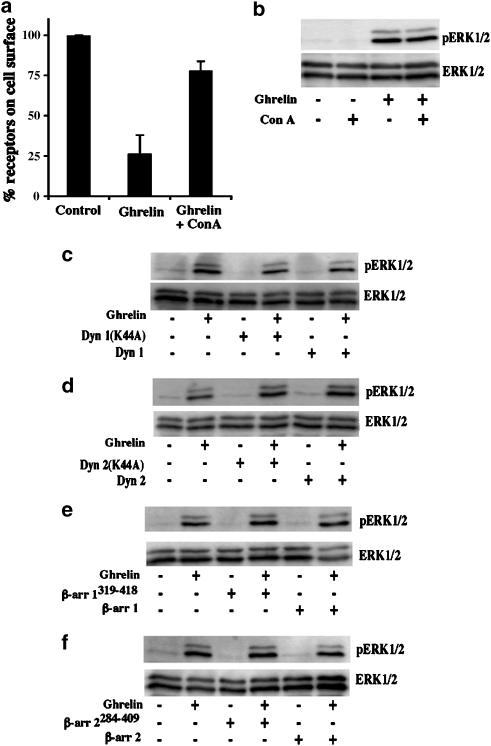
A 125I-labelled ghrelin-induced hGHSR-1a internalization assay revealed that 75±11% of hGHSR-1a was lost from the cell surface, 20 min after 125I-labelled ghrelin stimulation (n=3, Figure 5a). Pretreatment of cells with Con A reduced the amount of ghrelin-induced hGHSR-1a internalization to 21±5% (n=3, Figure 5a). In agreement with two previous studies performed in HEK-293 cells (Camina et al., 2004; Holst et al., 2004), this result shows that the major route of hGHSR-1a endocytosis in transiently transfected CHO cells is via clathrin-coated vesicles. Immunoblotting analysis of whole-cell homogenates from cells exposed to Con A before incubation with ghrelin for 5 min revealed that Con A did not block hGHSR-1a-mediated ERK1/2 activation (Figure 5b). Co-expression of dynamin 1/2 or DN dynamin 1/2 mutants, did not affect hGHSR-1a-induced ERK1/2 activation (Figure 5c and d). Similarly, co-expression of β-arrestin 1/2 or DN β-arrestin 1/2 mutants, did not affect hGHSR-1a-induced ERK1/2 activation (Figure 5e and f). In parallel, Elk1 reporter assays did not show any modification of Elk1 activation induced by hGHSR-1a, in the presence of DN β-arrestins mutants (data not shown). Overall, these results suggest that while hGHSR-1a is internalized via clathrin-coated vesicles, this internalization is not the main determinant for hGHSR-1a-mediated ERK1/2 activation.
Figure 5.
Role of receptor endocytosis in hGHSR-1a-mediated activation of ERK1/2 in CHO cells. (a) Effects of concanavalin A (Con A) on ghrelin-induced receptor internalization. CHO cells were transiently transfected with the plasmid encoding hGHSR-1a (30 μg/5 × 106 cells). One day after transfection, cells were pretreated for 1 h at 37°C with vehicle (0.5% PBS) or an endocytosis inhibitor, Con A (250 μg ml−1, prepared in PBS) in serum-free medium. Following from this, 125I-ghrelin was incubated with the cells for 3 h at 4°C, and cells were then washed at 4°C to remove the unbound radioligand. To induce the internalization, cells treated with vehicle (0.5% PBS) or Con A (250 μg ml−1) were then incubated at 37°C for 20 min. The proportion of hGHSR-1a on the cell surface was calculated as described under Methods. Data shown represent the mean±s.d. of the percentage of radioactivity associated with the starting 125I-ghrelin found (n=3). (b) Effects of Con A on hGHSR-1a-mediated ERK1/2 activation. hGHSR-1a-expressing CHO cells were preteated for 1 h at 37°C with vehicle (0.5% PBS) or Con A (250 μg ml−1, prepared in PBS) in serum-free medium before stimulation with ghrelin (100 nM, 5 min). (c) Role of dynamin I on hGHSR-1a-mediated ERK1/2 activation. CHO cells were transiently transfected with hGHSR-1a (10 μg) together with 20 μg of empty pcDNA3 vector, wild-type dynamin I or DN dynamin I K44A. Cells, which had been serum-starved for 1 h, were stimulated with ghrelin (100 nM, 5 min), before determination of ERK1/2 activity. (d) Role of dynamin II on hGHSR-1a-mediated ERK1/2 activation. CHO cells were transfected and treated as above for dynamin I using wild-type dynamin II or DN dynamin II K44A. (e) Role of β-arrestin 1 on hGHSR-1a-mediated ERK1/2 activation. CHO cells were transfected and treated as above for dynamin I using wild-type β-arrestin 1 or DN β-arrestin 1319−418. (f) Role of β-arrestin 2 on hGHSR-1a-mediated ERK1/2 activation. CHO cells were transfected and treated as above for dynamin I using wild-type β-arrestin 2 or DN β-arrestin 2284−409. Phosphorylated ERK1/2 (pERK1/2) and total ERK1/2 (ERK1/2) were detected by immunoblotting as described in Figure 2. All blots are representative of three experiments with similar results.
The hGHSR-1a-mediated ERK1/2 activation requires Phospholipase C (PLC) but is independent of PTX-sensitive G proteins, tyrosine kinase and the PI3K pathway
The GHSR-1a belongs to the family of receptors operating via the Gq-PLC pathway (Howard et al., 1996). The signalling pathways linking Gq-protein-coupled receptors to ERK1/2 may involve at least three key players: transactivated receptor tyrosine kinases (RTKs), PI3Ks and PLC.
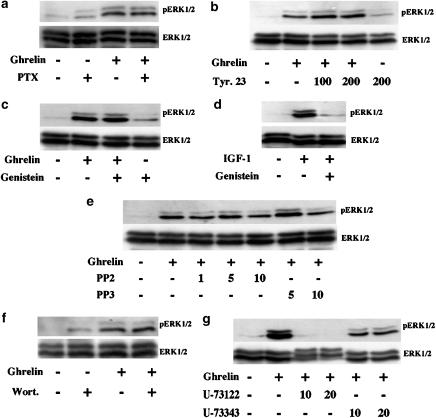
Although GHSR-1a is coupled to Gq proteins for the activation of PLCβ, it is possible that GPCRs could couple to different G proteins for different effector proteins. For instance, the β2-adrenergic receptor, which is coupled to Gs to activate adenylyl cyclase, is coupled to Gi for the activation of ERK1/2 in HEK-293 cells (Daaka et al., 1997). Thus, to exclude the possibility that activation by GHSR-1a involves coupling to PTX-sensitive G proteins, Gi/o, we examined the sensitivity of ghrelin-stimulated ERK1/2 phosphorylation to PTX. PTX is known to catalyse ADP ribosylation of α subunits in the Gi and Go subfamilies of heterotrimeric G proteins, and this covalent modification prevents the Gi/o proteins from interacting with receptors (Gilman, 1987). Overnight treatment with PTX did not abolish the hGHSR-1a-mediated ERK1/2 activation (Figure 6a). This result suggests that Gi/o proteins do not participate in hGHSR-1a-mediated ERK1/2 activation.
Figure 6.
Roles of PTX-sensitive G proteins, tyrosine kinases, PI3-kinases and phospholipase C on hGHSR-1a-mediated activation of ERK1/2. CHO cells were transiently transfected with the plasmid encoding hGHSR-1a (30 μg/5 × 106 cells). (a) Effects of pertussis toxin (PTX) treatment on hGHSR-1a-mediated activation of ERK1/2. Cells were pretreated overnight with or without 100 ng ml−1 PTX. Cells serum-starved for 1 h were then stimulated with ghrelin (100 nM, 5 min). (b) Effects of tyrosine kinases inhibitors on hGHSR-1a-mediated activation of ERK1/2. Cells were preincubated for 1 h with vehicle (0.1% DMSO) or the tyrosine kinase inhibitor tyrphostin 23 (Tyr. 23, 100 μM or 200 μM, prepared in DMSO) in serum-free medium. Cells were then stimulated with ghrelin (100 nM, 5 min). (c) Cells were preincubated for 1 h with vehicle (0.1% DMSO) or the tyrosine kinase inhibitor genistein (50 μM, prepared in DMSO) in serum-free medium. Cells were then stimulated with ghrelin (100 nM, 5 min). (d) Cells were preincubated for 1 h with vehicle (0.1% DMSO) or the tyrosine kinase inhibitor genistein (50 μM, prepared in DMSO) in serum-free medium. Cells were then stimulated with 2 ng ml−1 IGF-1 for 5 min. (e) Cells were preincubated for 1 h with vehicle (0.1% DMSO) or the c-Src kinase family-specific tyrphostin PP2 (1, 5 and 10 μM, prepared in DMSO) or PP3, an inactive homolog to PP2 (5 and 10 μM, prepared in DMSO) in serum-free medium. Cells were then stimulated with ghrelin (100 nM, 5 min). (f) Effects of the PI3Ks inhibitor on hGHSR-1a-mediated ERK1/2 activation. Cells serum-starved for 30 min were then preincubated for 30 min with vehicle (0.1% DMSO) or the PI3Ks inhibitor wortmannin (Wort., 100 μM, prepared in DMSO) in serum-free medium. Cells were then stimulated with ghrelin (100 nM, 5 min). (g) Effects of the PLC inhibitor on hGHSR-1a-mediated activation of ERK1/2. Cells were pretreated for 1 h with vehicle (0.1% DMSO) or the PLC inhibitor U-73122 (10 and 20 μM, prepared in DMSO) or U-73343, an inactive homolog to U-73122 (10 and 20 μM, prepared in DMSO) in serum-free medium. Cells were then stimulated with ghrelin (100 nM, 5 min). Phosphorylated ERK1/2 (pERK1/2) and total ERK1/2 (ERK1/2) were detected by immunoblotting described in Figure 2. All blots are representative of two to four experiments with similar results.
ERK1/2 activation via transactivation of RTKs has been described for certain Gq-protein coupled receptors, for instance, the angiotensin 1 receptor in human mesangial cells (Mondorf et al., 2000) and the bradykinin receptor in COS-7 cells (Gutkind, 1998; Adomeit et al., 1999). To establish whether, in CHO cells, the hGHSR-1a-mediated activation of ERK1/2 is via transactivation of RTKs, we measured the ability of two broad-spectrum tyrosine kinase inhibitors, genistein and tyrphostin 23, to block this ERK1/2 phosphorylation. In addition, IGF-1 was used as a positive control. As shown in Figure 6b and c, pretreatment with genistein or tyrphostin 23 did not affect the hGHSR-1a-mediated ERK1/2 activation, but suppressed IGF-1-stimulated ERK1/2 activity (Figure 6d). Furthermore, the cytoplasmic tyrosine kinase c-Src has often been described to play an essential role upstream of receptor tyrosine kinase activation (Gutkind, 1998; Luttrell et al., 1999). Therefore, we investigated the involvement of Src using PP2, a selective inhibitor of Src tyrosine kinases. PP2 did not affect the hGHSR-1a-mediated ERK1/2 activation more than the inactive analogue PP3 (Figure 6e).
Certain Gq-protein coupled receptors have also been reported to activate ERK1/2 in a PI3K-dependent manner (Seva et al., 1997; Graness et al., 1998). To determine whether PI3Ks play a role in hGHSR-1a-mediated activation of ERK1/2, a specific inhibitor of PI3Ks, wortmannin, was used to pretreat the transfected cells before stimulation with ghrelin. This pretreatment did not alter the ability of ghrelin to stimulate ERK1/2 activity (Figure 6f), suggesting the absence of PI3Ks involvement in ghrelin signalling to ERK1/2, in CHO cells.
Many Gq-protein coupled receptor have been shown to activate ERK via one or more PLC isoforms. The activated PLC hydrolyses phosphatidylinositol 4,5-bisphosphate (PIP2) to diacylglycerol (DAG) and inositol 1,4,5-triphosphate (IP3), which induces the activation of PKCs and the release of Ca2+ from intracellular Ca2+ stores. To test whether PLC activation was required for hGHSR-1a-mediated ERK1/2 phosphorylation, we used the pharmacological PLC inhibitor, U-73122 inhibiting both the hydrolysis of PIP2 to IP3 and the coupling of G-protein-PLC activation (Bleasdale et al., 1990). As shown in Figure 6g, the hGHSR-1a-mediated activation of ERK1/2 was inhibited by pretreatment with U-73122, but not by U-73343, an inactive analogue. Together, these results suggest that the hGHSR-1a-mediated ERK1/2 activation involves PLC but is independent of tyrosine kinases, PI3Ks and PTX-sensitive G proteins pathway.
hGHSR-1a-mediated ERK1/2 activation requires novel PKC isoforms but not calcium
To further dissect the downstream pathways of PLC, the roles of Ca2+ and PKCs in hGHSR-1a-mediated ERK1/2 activation were investigated.
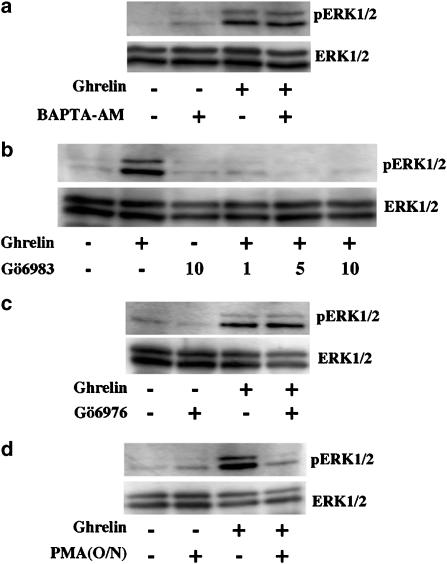
The requirement of intracellular Ca2+ was evaluated using the cell-permeable Ca2+ chelator, BAPTA-AM. As shown in Figure 7a, pretreatment of cells in Ca2+-free medium with BAPTA-AM, before stimulation with ghrelin, caused no effect on hGHSR-1a-stimulated ERK1/2 activity. Using the Ca2+ assay, we confirmed that 30 min pretreatment with BAPTA-AM was sufficient to completely block the increase in intracellular Ca2+ concentration induced by ghrelin, in hGHSR-1a expressing CHO cells (data not shown).
Figure 7.
Role of calcium and protein kinase C on hGHSR-1a-mediated activation of ERK1/2. CHO cells were transiently transfected with the plasmid encoding hGHSR-1a (30 μg/5 × 106 cells). (a) Effects of a calcium sequestrant on hGHSR-1a-mediated ERK1/2 activation. Cells were serum-starved for 30 min, then pretreated for 30 min with vehicle (0.1% DMSO) or the calcium sequestrant BAPTA-AM (50 μM, prepared in DMSO) in medium which was both calcium- and serum-free. Cells were then stimulated with ghrelin (100 nM, 5 min). (b) Effects of the PKC-specific inhibitor, Gö6983, on hGHSR-1a-mediated activation of ERK1/2. Cells serum-starved for 30 min were then pretreated for 30 min with vehicle (0.1% DMSO) or Gö6983 (1–10 μM, prepared in DMSO) in serum-free medium before stimulation with ghrelin (100 nM, 5 min). (c) Effects of the PKC subtype-specific inhibitor, Gö6976 on hGHSR-1a-mediated activation of ERK1/2. Cells serum-starved for 30 min were then pretreated for 30 min with vehicle (0.1% DMSO) or Gö6976 (5 μM, dissolved in DMSO) in serum-free medium before stimulation with ghrelin (100 nM, 5 min). (d) Effects of phorbol ester (PMA)-induced PKC depletion on hGHSR-1a-mediated activation of ERK1/2. Cells were preincubated overnight (O/N) with vehicle (0.1% DMSO) or PMA (1 μM, prepared in DMSO). Cells serum-starved for 1 h were then treated with ghrelin (100 nM, 5 min). Phosphorylated ERK1/2 (pERK1/2) and total ERK1/2 (ERK1/2) were detected by immunoblotting as described in Figure 2. All blots are representative of two to four experiments with similar results.
The involvement of PKC in the hGHSR-1a-mediated ERK1/2 activation was tested using Gö6983, which inhibits all PKC isoforms: the conventional (α, βI, βII, γ) and the novel (η, θ, δ, ɛ) groups as well as the atypical PKC isoforms (ζ, ι/λ) with the exception of PKCμ (Gschwendt et al., 1996). As shown in Figure 7b, pretreatment of cells with Gö6983, before stimulation with ghrelin, abolished the hGHSR-1a-stimulated ERK1/2 activity. To determine which of these PKC groups is involved in the hGHSR-1a-mediated ERK1/2 activation, we first tested the PKC inhibitor Gö6976, which inhibits conventional PKCs and PKCμ (Martiny-Baron et al., 1993). As shown in Figure 7c, pretreatment of cells with Gö6976, before stimulation with ghrelin, did not block the hGHSR-1a-mediated ERK1/2 activation. In connection with the insensivity to the (Ca2+)i chelation (Figure 7a), these two results exclude the involvement of both conventional PKCs which are Ca2+-sensitive enzymes and PKCμ. The involvement of the novel and atypical PKC isoforms was further tested by depletion of endogenous PKCs with overnight phorbol ester (PMA) pretreatment of cells. This treatment affects only PKCs that contain the diacylglycerol/phorbol ester-binding domain C1, such as the PKC isoforms of conventional and novel groups as well as PKCμ (Chen, 1993). It does not affect the atypical PKC isoforms, which do not contain the C1 domain. As shown in Figure 7d, hGHSR-1a-mediated ERK1/2 activation was suppressed by overnight PMA treatment, which excludes the involvement of the atypical PKC group. Together, these data suggest that one or more PKC isoforms from the group of novel PKCs are involved in this hGHSR-1a-mediated ERK1/2 activation.
PKCɛ is involved in the hGHSR-1a-mediated ERK1/2 activation
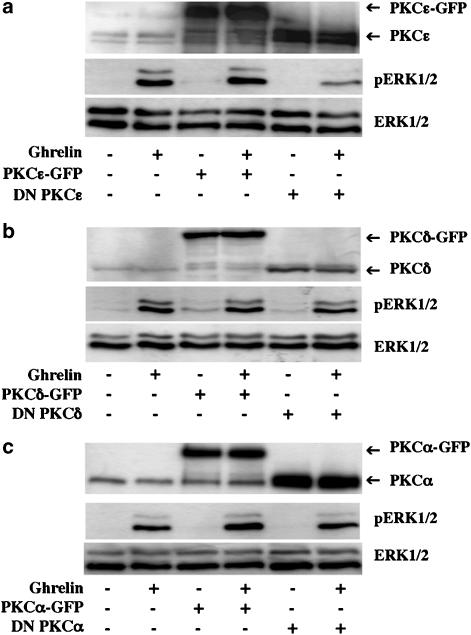
CHO cells are known to constitutively express two novel PKCs, the PKCδ and the PKCɛ (Gschwendt et al., 1996; Shirai et al., 2000). To confirm the involvement of novel PKCs and identify which of these two PKC isoforms is involved in the hGHSR-1a-mediated ERK1/2 activation, we examined the effects on hGHSR-1a-mediated ERK1/2 activation, in cells co-transfected with dominant-negative (DN) forms of these two PKC isozymes (Louis et al., 2005). Our results showed that the expression of DN PKCɛ partially inhibited the hGHSR-1a-mediated ERK1/2 activation, whereas expression of both the corresponding empty vector and the wild-type PKCɛ had no effect (Figure 8a). In contrast, the expression of DN PKCδ, as well as the expression of DN form of the conventional PKCα, which was used as a control, did not block this ERK1/2 activation (Figure 8b and c). These results show that PKCɛ, but not PKCδ is involved in the hGHSR-1a-mediated ERK1/2 activation in CHO cells.
Figure 8.
PKCɛ but not PKCδ is involved in hGHSR-1a-mediated activation of ERK1/2. (a) CHO cells were transiently transfected with plasmid encoding hGHSR-1a (10 μg) together with 20 μg of empty pEGFP-N1 vector, wild-type PKCɛ-GFP or c-myc-tagged DN PKCɛ. Cells, which had been serum-starved for 1 h were stimulated with ghrelin (100 nM, 5 min). (b) CHO cells were transiently transfected with plasmid DNA encoding hGHSR-1a (10 μg) together with 20 μg of empty pEGFP-N1 vector, wild-type PKCδ-GFP or c-myc-tagged DN PKCδ. Cells that had been serum-starved for 1 h were stimulated with ghrelin (100 nM, 5 min). (c) CHO cells were transiently transfected with plasmid DNA encoding hGHSR-1a (10 μg) together with 20 μg of empty pEGFP-N1 vector, wild-type PKCα-GFP or c-myc-tagged DN PKCα. Cells, which had been serum-starved for 1 h, were then stimulated with ghrelin (100 nM, 5 min). Phosphorylated ERK1/2 (pERK1/2) and total ERK1/2 (ERK1/2) were detected by immunoblotting as described in Figure 2. The expression level of the different PKC isoforms was revealed by probing the western blot with anti-PKCα, δ or ɛ antibodies. All blots are representative of three experiments with similar results.
Discussion
Recently, ghrelin-mediated ERK1/2 activation has been reported in several cell lines or primary cell cultures. However, there is controversy about whether the GHSR-1a receptor is the sole receptor for ghrelin or just one of a group of receptors for this ligand. For example, several studies have suggested the presence of a novel, as yet unidentified subtype of the ghrelin receptor, distinct from the GHSR-1a, which is capable of binding both des-acylated and acylated ghrelin forms, in a variety of tissues and cell lines (Cassoni et al., 2001; Baldanzi et al., 2002; Ghe et al., 2002; Bedendi et al., 2003). Moreover, des-acyl-ghrelin has been reported to induce ERK1/2 activation in cell lines, which express GHSR-1a (Nanzer et al., 2004) and in those that do not express GHSR-1a (Baldanzi et al., 2002). In addition, ghrelin-mediated ERK1/2 activation was observed in a cell line where GHSR-1a was not detectable (Baldanzi et al., 2002).
Therefore, as the involvement of GHSR-1a in the activation of ERK1/2 is unclear, we studied whether GHSR-1a mediates ERK1/2 activation using a cellular model expressing exclusively the GHSR-1a and, we have then characterized the signalling machinery involved.
In agreement with several groups (Camina et al., 2004; Van Craenenbroeck et al., 2004), no specific binding of ghrelin was observed on untransfected CHO cells, indicating that these cells are naturally devoid of the endogenous ghrelin receptor. Moreover, CHO cells express endogenously Gq, Gi/o and Gs proteins (Martin et al., 2004), including the Gq/11 protein (Cussac et al., 2002), which has been shown to be coupled to GHSR-1a (Howard et al., 1996). In the present study, we have shown that the transient expression of hGHSR-1a in CHO cells conferred pharmacological properties, measured by radioligand binding, IPs production or Ca2+ mobilization assays, similar to those previously reported in primary cell cultures expressing endogenously the GHSR-1a (Mau et al., 1995; Kojima et al., 1999; Yamazaki et al., 2004).
In this study, we provided evidence that competitive agonist of ghrelin induced the hGHSR-1a-mediated ERK1/2 activation, in a time-dependent and concentration-dependent manner, which correlated with the affinity of ghrelin for its receptor. Once activated, ERK1/2 may phosphorylate numerous plasma membrane, cytosolic and cytoskeletal substrates (Pearson et al., 2001). In addition, activated ERK1/2 usually translocate to the nucleus where they activate transcription by phosphorylating nuclear transcription factors, such as the ETS family protein Elk-1 and ERF, leading to enhanced transcription of multiple genes (Pearson et al., 2001; Le Gallic et al., 2004). The analysis of Elk-1 activation is classically used to study biological events involving ERK1/2 phosphorylation and PI3K activation, for example in the study of the ghrelin-induced cell proliferation of hepatoma cells (Murata et al., 2002). In the present study, we observed cytoplasmic and nuclear accumulation of phosphorylated ERK1/2 and activation of the Elk1 transcriptional factor. Elk-1 can be activated by several kinases other than ERK1/2, for example, JNK and p38 kinases. Under our experimental conditions, the full inhibiting effect of the selective MEK1/2 inhibitor, as well as the absence of p38 and JNK kinases activation observed by Western blotting using corresponding phospho-antibodies (data not shown), suggested that the activation of Elk-1 by ghrelin was mediated via a MEK1/2-ERK1/2 pathway.
This hGHSR-1a-mediated ERK1/2 activation was also stimulated by compound JMV 1843 the only orally administered peptidomimetic GHS currently in clinical development.
As ghrelin and JMV 1843 were able to induce ERK1/2 activation through hGHSR-1a, we decided to identify the putative signalling mediators involved in ERK1/2 activation using various specific inhibitors and dominant-negative mutants. In the present study, we have shown evidence that the hGHSR-1a-mediated ERK1/2 activation was independent of PTX-sensitive G proteins, tyrosine kinases including Src family kinases, PI3Ks and clathrin-mediated hGHSR-1a internalization in CHO cells.
It has been shown that ghrelin mediated ERK1/2 activation in 3T3-L1 adipocytes via PTX-sensitive proteins, Gi/o (Kim et al., 2004), but the presence of GHSR-1a in these cell lines has been debated (Zhang et al., 2004). According to our model, in CHO cells, the hGHSR-1a-mediated ERK1/2 activation is not attenuated by PTX pretreatment, excluding the involvement of Gi/o proteins in ERK1/2 activation. This result suggests that hGHSR-1a may mediate ERK1/2 activation by Gq in CHO cells, but other PTX-insensitive mechanisms (e.g. via G12/13 proteins or G protein-independent coupling) remain to be explored.
Several lines of evidence suggest a role for tyrosine kinases downstream from Gq-coupled receptors, in ERK1/2 activation. A least three distinct classes of tyrosine kinases may be involved: the Src family non-receptor kinases, the focal adhesion kinase (FAK) family, or the transactived RTKs, including the EGF and PDGF receptors. Moreover, several studies have proposed a central role for Src kinases in linking a variety of GPCRs expressed in different cell backgrounds, to ERK1/2 activation (Gutkind, 1998; Adomeit et al., 1999). For example, Src kinase activity may play a ‘downstream' role from both FAK tyrosine kinases and transactivated RTKs and also, an ‘upstream' role in GPCR-induced RTK transactivation (Eguchi et al., 1998). In the present study, three different inhibitors of tyrosine kinases, genistein, tyrphostin 23 and PP2, which are two broad-spectrum inhibitors of tyrosine kinases and a specific inhibitor of Src tyrosine kinases respectively, did no affect the hGHSR-1a-mediated ERK1/2 activation in CHO cells. This suggests the lack of importance of these kinases in signalling from hGHSR-1a.
PI3Ks are also common factors involved in GPCR-mediated ERK1/2 activation. The PI3Kγ isoform may be stimulated by Gβγ from Gi proteins but also by Gq (Seva et al., 1997; Graness et al., 1998). In addition, the dimeric p85/p110 PI3K isoforms α and or β, which are known as target enzymes of RTKs, may also be activated via GPCRs without prior RTK transactivation (Kurosu et al., 1997). The inability of the specific PI3K inhibitor, wortmannin, to affect the hGHSR-1a-mediated ERK1/2 activation in CHO cells, argues against the involvement of PI3Ks in this ghrelin-stimulated signalling.
Within the last decade, several studies have shown that for some receptors, GPCR internalization is necessary for ERK1/2 activation (Daaka et al., 1998; Vogler et al., 1999; Pierce et al., 2000). However, internalization-independent activation of ERK1/2 has also been documented for a number of receptors (Budd et al., 1999; Li et al., 1999). Recently two different groups have published results showing that GHSR-1a internalises via clathrin-coated pits both in CHO and HEK293 cells (Camina et al., 2004; Holst et al., 2004). We decided to test the relationship between GHSR-1a internalization and ERK1/2 regulation in our model. We used three independent experimental strategies to inhibit recruitment to clathrin-coated pits: DN dynamins, DN β-arrestins and Con A treatment. In all three cases, there was no effect on hGHSR-1a-mediated ERK1/2 activation, suggesting that clathrin-mediated hGHSR-1a internalization was not required for activation of ERK1/2 in CHO cells.
Recently, the roles of β-arrestins, signal-terminating proteins known to be involved in internalization, desensitization and resensitization, have been extended. β-arrestins have been shown to be required for scaffolding complexes containing internalized GPCRs and are involved in the activation and subcellular localization of ERK1/2, leading to the cytosolic retention of pERK1/2 and thus they cannot elicit a transcriptional response (Tohgo et al., 2002; Tohgo et al., 2003). According to our study, β-arrestins neither mediated ERK1/2 phosphorylation, nor affected the Elk1 transcriptional response elicited by ghrelin using a reporter gene assay.
In contrast, we provided several lines of evidence indicating that ERK1/2 activation is related to PLC and novel PKCɛ. Using U-73122, a PLC inhibitor, we found that the PLC pathway was necessary for hGHSR-1a-mediated ERK1/2 activation in CHO cells. PLC activation resulted in the cleavage of PIP2 into DAG, an activator of PKCs, and IP3, a Ca2+-mobilizing second messenger. The free Ca2+ can activate calmodulin (CaM) II, which further phosphorylates and inhibits Ras-GTPase-activating protein, and induces Ras and MAPK activation (Farnsworth et al., 1995; Chen et al., 1998). In addition to its role in CaM kinase II activation, Ca2+ and DAG together can activate either PKC, or can lead to MAPK activation by a specific Ca2+/DAG-GEF and Ras activation (Farnsworth et al., 1995; Chen et al., 1998). However, in the present study, the hGHSR-1a linkage to ERK1/2 was Ca2+-independent. In contrast, we found that the PKC pathway was involved. CHO cells are known to constitutively express two conventional PKCs (α, γ), which are dependent on Ca2+ and are activated by DAG or PMA, two novel PKCs (δ, ɛ) and PKCμ, which are also activated by DAG or PMA but are Ca2+-independent, and one atypical PKC (ζ), which is Ca2+-independent and does not respond to DAG or PMA (Tippmer et al., 1994; Shirai et al., 2000). From the results of our study, the PKCs involved in the hGHSR-1a-mediated ERK1/2 activation are Ca2+-insensitive and insensitive to the conventional PKC inhibitor Gö6976. Moreover, they are sensitive to both PMA and the PKC inhibitor Gö6983. This suggested that only the novel PKC isoforms δ and ɛ could potentially play a critical role. To study which of these two novel PKC isoforms might be involved, we used the co-expression of wild-type or DN forms of PKCδ and PKCɛ. Our results showed that the hGHSR-1a-mediated ERK1/2 activation was only PKCɛ-dependent in CHO cells.
Although it is possible that the signalling pathway may be different in other cellular backgrounds (Dumont et al., 2001), it seems likely that the peripheral and central biological events regulated by the hGHSR-1a, may depend not only on changes in IPs and Ca2+, but also on the regulation of the ERK1/2 pathway. In this regard, it has been shown that ghrelin influences the expression of the transcription factor Pit-1 (responsible for the somatotroph cell-specific expression of the GH gene) via rat GHSR-1a and MEK1/2 (Garcia et al., 2001) but this issue remains controversial (Chan et al., 2004). In summary, our results suggest that in the CHO expression model, the hGHSR-1a controls ERK1/2 activity via a signalling pathway involving the activation of PLC and PKCɛ, and activates the Elk1 transcriptional factor, suggesting the induction of transcriptional events by ghrelin through hGHSR-1a.
Acknowledgments
We are very grateful to Dr D. Joubert and A. Collazos for their generous donations of wild type and dominant-negative PKC mutants (IGF, Montpellier, France). Part of this work was performed thanks to the Pharmacologie & Screening Plateform of the Institut Fédératif de Recherche 3 (IFR3). We thank F. Malhaire and C. Vol (IGF, Montpellier) for their help with the use of the FlexStation. Finally, we also appreciated the technical assistance provided by C. M'Kadmi.
Abbreviations
- BAPTA-AM
acetoxymethyl ester of 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid
- CHO
Chinese hamster ovary
- Con A
concanavalin A
- DMSO
dimethyl sulfoxide
- ERK
extracellular signal-regulated kinase
- FBS
fetal bovine serum
- GFP
green fluorescent protein
- GH
growth hormone
- GPCR
G protein-coupled receptor
- hGHSR-1a
human growth hormone secretagogue receptor 1a
- hGHSR-1a-GFP
GFP-tagged hGHSR-1a
- IGF
insulin-like growth factor
- MAPK
mitogen-activated protein kinase
- PBS
phosphate-buffered saline
- PI3K
phosphatidylinositol 3-kinase
- PKC
protein kinase C
- PLC
phospholipase C
- PMA
phorbol-12-myrsitate-13-acetate
- PTX
pertussis toxin
References
- ADOMEIT A., GRANESS A., GROSS S., SEEDORF K., WETZKER R., LIEBMANN C. Bradykinin B(2) receptor-mediated mitogen-activated protein kinase activation in COS-7 cells requires dual signaling via both protein kinase C pathway and epidermal growth factor receptor transactivation. Mol. Cell. Biol. 1999;19:5289–5297. doi: 10.1128/mcb.19.8.5289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANDREIS P.G., MALENDOWICZ L.K., TREJTER M., NERI G., SPINAZZI R., ROSSI G.P., NUSSDORFER G.G. Ghrelin and growth hormone secretagogue receptor are expressed in the rat adrenal cortex: evidence that ghrelin stimulates the growth, but not the secretory activity of adrenal cells. FEBS Lett. 2003;536:173–179. doi: 10.1016/s0014-5793(03)00051-6. [DOI] [PubMed] [Google Scholar]
- BALDANZI G., FILIGHEDDU N., CUTRUPI S., CATAPANO F., BONISSONI S., FUBINI A., MALAN D., BAJ G., GRANATA R., BROGLIO F., PAPOTTI M., SURICO N., BUSSOLINO F., ISGAARD J., DEGHENGHI R., SINIGAGLIA F., PRAT M., MUCCIOLI G., GHIGO E., GRAZIANI A. Ghrelin and des-acyl ghrelin inhibit cell death in cardiomyocytes and endothelial cells through ERK1/2 and PI 3-kinase/AKT. J. Cell. Biol. 2002;159:1029–1037. doi: 10.1083/jcb.200207165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEDENDI I., ALLOATTI G., MARCANTONI A., MALAN D., CATAPANO F., GHE C., DEGHENGHI R., GHIGO E., MUCCIOLI G. Cardiac effects of ghrelin and its endogenous derivatives des-octanoyl ghrelin and des-Gln14-ghrelin. Eur. J. Pharmacol. 2003;476:87–95. doi: 10.1016/s0014-2999(03)02083-1. [DOI] [PubMed] [Google Scholar]
- BLEASDALE J.E., THAKUR N.R., GREMBAN R.S., BUNDY G.L., FITZPATRICK F.A., SMITH R.J., BUNTING S. Selective inhibition of receptor-coupled phospholipase C-dependent processes in human platelets and polymorphonuclear neutrophils. J. Pharmacol. Exp. Ther. 1990;255:756–768. [PubMed] [Google Scholar]
- BOWERS C.Y. Unnatural growth hormone-releasing peptide begets natural ghrelin. J. Clin. Endocrinol. Metab. 2001;86:1464–1469. doi: 10.1210/jcem.86.4.7431. [DOI] [PubMed] [Google Scholar]
- BUDD D.C., RAE A., TOBIN A.B. Activation of the mitogen-activated protein kinase pathway by a Gq/11-coupled muscarinic receptor is independent of receptor internalization. J. Biol. Chem. 1999;274:12355–12360. doi: 10.1074/jbc.274.18.12355. [DOI] [PubMed] [Google Scholar]
- CAMINA J.P., CARREIRA M.C., EL MESSARI S., LLORENS-CORTES C., SMITH R.G., CASANUEVA F.F. Desensitization and endocytosis mechanisms of ghrelin-activated growth hormone secretagogue receptor 1a. Endocrinology. 2004;145:930–940. doi: 10.1210/en.2003-0974. [DOI] [PubMed] [Google Scholar]
- CASSONI P., PAPOTTI M., GHE C., CATAPANO F., SAPINO A., GRAZIANI A., DEGHENGHI R., REISSMANN T., GHIGO E., MUCCIOLI G. Identification, characterization, and biological activity of specific receptors for natural (ghrelin) and synthetic growth hormone secretagogues and analogs in human breast carcinomas and cell lines. J. Clin. Endocrinol. Metab. 2001;86:1738–1745. doi: 10.1210/jcem.86.4.7402. [DOI] [PubMed] [Google Scholar]
- CHAN C.B., FUNG C.K., FUNG W., TSE M.C., CHENG C.H. Stimulation of growth hormone secretion from seabream pituitary cells in primary culture by growth hormone secretagogues is independent of growth hormone transcription. Comp. Biochem. Physiol. C. Toxicol. Pharmacol. 2004;139:77–85. doi: 10.1016/j.cca.2004.09.008. [DOI] [PubMed] [Google Scholar]
- CHEN C. Growth hormone secretagogue actions on the pituitary gland: multiple receptors for multiple ligands. Clin. Exp. Pharmacol. Physiol. 2000;27:323–329. doi: 10.1046/j.1440-1681.2000.03258.x. [DOI] [PubMed] [Google Scholar]
- CHEN C.C. Protein kinase C alpha, delta, epsilon and zeta in C6 glioma cells. TPA induces translocation and down-regulation of conventional and new PKC isoforms but not atypical PKC zeta. FEBS Lett. 1993;332:169–173. doi: 10.1016/0014-5793(93)80506-p. [DOI] [PubMed] [Google Scholar]
- CHEN H.J., ROJAS-SOTO M., OGUNI A., KENNEDY M.B. A synaptic Ras-GTPase activating protein (p135 SynGAP) inhibited by CaM kinase II. Neuron. 1998;20:895–904. doi: 10.1016/s0896-6273(00)80471-7. [DOI] [PubMed] [Google Scholar]
- CUMMINGS D.E., CLEMENT K., PURNELL J.Q., VAISSE C., FOSTER K.E., FRAYO R.S., SCHWARTZ M.W., BASDEVANT A., WEIGLE D.S. Elevated plasma ghrelin levels in Prader Willi syndrome. Nat. Med. 2002;8:643–644. doi: 10.1038/nm0702-643. [DOI] [PubMed] [Google Scholar]
- CUSSAC D., NEWMAN-TANCREDI A., DUQUEYROIX D., PASTEAU V., MILLAN M.J. Differential activation of Gq/11 and Gi(3) proteins at 5-hydroxytryptamine(2C) receptors revealed by antibody capture assays: influence of receptor reserve and relationship to agonist-directed trafficking. Mol. Pharmacol. 2002;62:578–589. doi: 10.1124/mol.62.3.578. [DOI] [PubMed] [Google Scholar]
- DAAKA Y., LUTTRELL L.M., LEFKOWITZ R.J. Switching of the coupling of the beta2-adrenergic receptor to different G proteins by protein kinase A. Nature. 1997;390:88–91. doi: 10.1038/36362. [DOI] [PubMed] [Google Scholar]
- DAAKA Y., LUTTRELL L.M., AHN S., DELLA ROCCA G.J., FERGUSON S.S., CARON M.G., LEFKOWITZ R.J. Essential role for G protein-coupled receptor endocytosis in the activation of mitogen-activated protein kinase. J. Biol. Chem. 1998;273:685–688. doi: 10.1074/jbc.273.2.685. [DOI] [PubMed] [Google Scholar]
- DAMKE H., BABA T., WARNOCK D.E., SCHMID S.L. Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J. Cell. Biol. 1994;127:915–934. doi: 10.1083/jcb.127.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUMONT J.E., PECASSE F., MAENHAUT C. Crosstalk and specificity in signalling. Are we crosstalking ourselves into general confusion. Cell Signal. 2001;13:457–463. doi: 10.1016/s0898-6568(01)00168-1. [DOI] [PubMed] [Google Scholar]
- EGUCHI S., NUMAGUCHI K., IWASAKI H., MATSUMOTO T., YAMAKAWA T., UTSUNOMIYA H., MOTLEY E.D., KAWAKATSU H., OWADA K.M., HIRATA Y., MARUMO F., INAGAMI T. Calcium-dependent epidermal growth factor receptor transactivation mediates the angiotensin II-induced mitogen-activated protein kinase activation in vascular smooth muscle cells. J. Biol. Chem. 1998;273:8890–8896. doi: 10.1074/jbc.273.15.8890. [DOI] [PubMed] [Google Scholar]
- FARNSWORTH C.L., FRESHNEY N.W., ROSEN L.B., GHOSH A., GREENBERG M.E., FEIG L.A. Calcium activation of Ras mediated by neuronal exchange factor Ras-GRF. Nature. 1995;376:524–527. doi: 10.1038/376524a0. [DOI] [PubMed] [Google Scholar]
- GARCIA A., ALVAREZ C.V., SMITH R.G., DIEGUEZ C. Regulation of Pit-1 expression by ghrelin and GHRP-6 through the GH secretagogue receptor. Mol. Endocrinol. 2001;15:1484–1495. doi: 10.1210/mend.15.9.0694. [DOI] [PubMed] [Google Scholar]
- GHE C., CASSONI P., CATAPANO F., MARROCCO T., DEGHENGHI R., GHIGO E., MUCCIOLI G., PAPOTTI M. The antiproliferative effect of synthetic peptidyl GH secretagogues in human CALU-1 lung carcinoma cells. Endocrinology. 2002;143:484–491. doi: 10.1210/endo.143.2.8654. [DOI] [PubMed] [Google Scholar]
- GILMAN A.G. G proteins: transducers of receptor-generated signals. Annu. Rev. Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- GNANAPAVAN S., KOLA B., BUSTIN S.A., MORRIS D.G., MCGEE P., FAIRCLOUGH P., BHATTACHARYA S., CARPENTER R., GROSSMAN A.B., KORBONITS M. The tissue distribution of the mRNA of ghrelin and subtypes of its receptor, GHS-R, in humans. J. Clin. Endocrinol. Metab. 2002;87:2988–2991. doi: 10.1210/jcem.87.6.8739. [DOI] [PubMed] [Google Scholar]
- GRANESS A., ADOMEIT A., HEINZE R., WETZKER R., LIEBMANN C. A novel mitogenic signaling pathway of bradykinin in the human colon carcinoma cell line SW-480 involves sequential activation of a Gq/11 protein, phosphatidylinositol 3-kinase beta, and protein kinase Cepsilon. J. Biol. Chem. 1998;273:32016–32022. doi: 10.1074/jbc.273.48.32016. [DOI] [PubMed] [Google Scholar]
- GSCHWENDT M., DIETERICH S., RENNECKE J., KITTSTEIN W., MUELLER H.J., JOHANNES F.J. Inhibition of protein kinase C mu by various inhibitors. Differentiation from protein kinase c isoenzymes. FEBS Lett. 1996;392:77–80. doi: 10.1016/0014-5793(96)00785-5. [DOI] [PubMed] [Google Scholar]
- GUAN X.M., YU H., PALYHA O.C., MCKEE K.K., FEIGHNER S.D., SIRINATHSINGHJI D.J., SMITH R.G., VAN DER PLOEG L.H., HOWARD A.D. Distribution of mRNA encoding the growth hormone secretagogue receptor in brain and peripheral tissues. Brain Res. Mol. Brain Res. 1997;48:23–29. doi: 10.1016/s0169-328x(97)00071-5. [DOI] [PubMed] [Google Scholar]
- GUERLAVAIS V., BOEGLIN D., MOUSSEAUX D., OIRY C., HEITZ A., DEGHENGHI R., LOCATELLI V., TORSELLO A., GHE C., CATAPANO F., MUCCIOLI G., GALLEYRAND J.C., FEHRENTZ J.A., MARTINEZ J. New active series of growth hormone secretagogues. J. Med. Chem. 2003;46:1191–1203. doi: 10.1021/jm020985q. [DOI] [PubMed] [Google Scholar]
- GUTKIND J.S. The pathways connecting G protein-coupled receptors to the nucleus through divergent mitogen-activated protein kinase cascades. J. Biol. Chem. 1998;273:1839–1842. doi: 10.1074/jbc.273.4.1839. [DOI] [PubMed] [Google Scholar]
- GUTKIND J.S. Regulation of mitogen-activated protein kinase signaling networks by G protein-coupled receptors. Sci. STKE. 2000;2000 (40):RE1. doi: 10.1126/stke.2000.40.re1. [DOI] [PubMed] [Google Scholar]
- HANSEN B.S., RAUN K., NIELSEN K.K., JOHANSEN P.B., HANSEN T.K., PESCHKE B., LAU J., ANDERSEN P.H., ANKERSEN M. Pharmacological characterisation of a new oral GH secretagogue, NN703. Eur. J. Endocrinol. 1999;141:180–189. doi: 10.1530/eje.0.1410180. [DOI] [PubMed] [Google Scholar]
- HOLST B., BRANDT E., BACH A., HEDING A., SCHWARTZ T.W. Nonpeptide and peptide growth hormone secretagogues act both as ghrelin receptor agonist and as positive or negative allosteric modulators of ghrelin signaling. Mol. Endocrinol. 2005;9:2400–2411. doi: 10.1210/me.2005-0059. [DOI] [PubMed] [Google Scholar]
- HOLST B., HOLLIDAY N.D., BACH A., ELLING C.E., COX H.M., SCHWARTZ T.W. Common structural basis for constitutive activity of the ghrelin receptor family. J. Biol. Chem. 2004;279:53806–53817. doi: 10.1074/jbc.M407676200. [DOI] [PubMed] [Google Scholar]
- HOSODA H., KOJIMA M., MATSUO H., KANGAWA K. Ghrelin and des-acyl ghrelin: two major forms of rat ghrelin peptide in gastrointestinal tissue. Biochem. Biophys. Res. Commun. 2000;279:909–913. doi: 10.1006/bbrc.2000.4039. [DOI] [PubMed] [Google Scholar]
- HOWARD A.D., FEIGHNER S.D., CULLY D.F., ARENA J.P., LIBERATOR P.A., ROSENBLUM C.I., HAMELIN M., HRENIUK D.L., PALYHA O.C., ANDERSON J., PARESS P.S., DIAZ C., CHOU M., LIU K.K., MCKEE K.K., PONG S.S., CHAUNG L.Y., ELBRECHT A., DASHKEVICZ M., HEAVENS R., RIGBY M., SIRINATHSINGHJI D.J., DEAN D.C., MELILLO D.G., PATCHETT A.A., NARGUND R., GRIFFIN P.R., DEMARTINO J.A., GUPTA S.K., SCHAEFFER J.M., SMITH R.G., VAN DER PLOEG L.H. A receptor in pituitary and hypothalamus that functions in growth hormone release. Science. 1996;273:974–977. doi: 10.1126/science.273.5277.974. [DOI] [PubMed] [Google Scholar]
- INUI A. Ghrelin: an orexigenic and somatotrophic signal from the stomach. Nat. Rev. Neurosci. 2001;2:551–560. doi: 10.1038/35086018. [DOI] [PubMed] [Google Scholar]
- KENAKIN T. G-protein coupled receptors as allosteric machines. Receptors Channels. 2004;10:51–60. doi: 10.1080/10606820490464316. [DOI] [PubMed] [Google Scholar]
- KIM M.S., YOON C.Y., JANG P.G., PARK Y.J., SHIN C.S., PARK H.S., RYU J.W., PAK Y.K., PARK J.Y., LEE K.U., KIM S.Y., LEE H.K., KIM Y.B., PARK K.S. The mitogenic and antiapoptotic actions of ghrelin in 3T3-L1 adipocytes. Mol. Endocrinol. 2004;18:2291–2301. doi: 10.1210/me.2003-0459. [DOI] [PubMed] [Google Scholar]
- KOJIMA M., HOSODA H., DATE Y., NAKAZATO M., MATSUO H., KANGAWA K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- KRUPNICK J.G., GOODMAN O.B., Jr, KEEN J.H., BENOVIC J.L. Arrestin/clathrin interaction. Localization of the clathrin binding domain of nonvisual arrestins to the carboxy terminus. J. Biol. Chem. 1997;272:15011–15016. doi: 10.1074/jbc.272.23.15011. [DOI] [PubMed] [Google Scholar]
- KUROSU H., MAEHAMA T., OKADA T., YAMAMOTO T., HOSHINO S., FUKUI Y., UI M., HAZEKI O., KATADA T. Heterodimeric phosphoinositide 3-kinase consisting of p85 and p110beta is synergistically activated by the betagamma subunits of G proteins and phosphotyrosyl peptide. J. Biol. Chem. 1997;272:24252–24256. doi: 10.1074/jbc.272.39.24252. [DOI] [PubMed] [Google Scholar]
- LALL S., BALTHASAR N., CARMIGNAC D., MAGOULAS C., SESAY A., HOUSTON P., MATHERS K., ROBINSON I. Physiological studies of transgenic mice overexpressing growth hormone (GH) secretagogue receptor 1A in GH-releasing hormone neurons. Endocrinology. 2004;145:1602–1611. doi: 10.1210/en.2003-1509. [DOI] [PubMed] [Google Scholar]
- LE GALLIC L., VIRGILIO L., COHEN P., BITEAU B., MAVROTHALASSITIS G. ERF nuclear shuttling, a continuous monitor of Erk activity that links it to cell cycle progression. Mol. Cell. Biol. 2004;24:1206–1218. doi: 10.1128/MCB.24.3.1206-1218.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEFKOWITZ R.J., PIERCE K.L., LUTTRELL L.M. Dancing with different partners: protein kinase a phosphorylation of seven membrane-spanning receptors regulates their G protein-coupling specificity. Mol. Pharmacol. 2002;62:971–974. doi: 10.1124/mol.62.5.971. [DOI] [PubMed] [Google Scholar]
- LI J.G., LUO L.Y., KRUPNICK J.G., BENOVIC J.L., LIU-CHEN L.Y. U50,488H-induced internalization of the human kappa opioid receptor involves a beta-arrestin- and dynamin-dependent mechanism. Kappa receptor internalization is not required for mitogen-activated protein kinase activation. J. Biol. Chem. 1999;274:12087–12094. doi: 10.1074/jbc.274.17.12087. [DOI] [PubMed] [Google Scholar]
- LIEBMANN C. Regulation of MAP kinase activity by peptide receptor signalling pathway: paradigms of multiplicity. Cell Signal. 2001;13:777–785. doi: 10.1016/s0898-6568(01)00192-9. [DOI] [PubMed] [Google Scholar]
- LOUIS K., GUERINEAU N., FROMIGUE O., DEFAMIE V., COLLAZOS A., ANGLARD P., SHIPP M.A., AUBERGER P., JOUBERT D., MARI B. Tumor cell-mediated induction of the stromal factor stromelysin-3 requires heterotypic cell contact-dependent activation of specific protein kinase C isoforms. J. Biol. Chem. 2005;280:1272–1283. doi: 10.1074/jbc.M405482200. [DOI] [PubMed] [Google Scholar]
- LUTTRELL L.M., DAAKA Y., LEFKOWITZ R.J. Regulation of tyrosine kinase cascades by G-protein-coupled receptors. Curr. Opin. Cell. Biol. 1999;11:177–183. doi: 10.1016/s0955-0674(99)80023-4. [DOI] [PubMed] [Google Scholar]
- LUTTRELL L.M., DAAKA Y., DELLA ROCCA G.J., LEFKOWITZ R.J. G protein-coupled receptors mediate two functionally distinct pathways of tyrosine phosphorylation in rat 1a fibroblasts. Shc phosphorylation and receptor endocytosis correlate with activation of Erk kinases. J. Biol. Chem. 1997;272:31648–31656. doi: 10.1074/jbc.272.50.31648. [DOI] [PubMed] [Google Scholar]
- MARTIN N.P., WHALEN E.J., ZAMAH M.A., PIERCE K.L., LEFKOWITZ R.J. PKA-mediated phosphorylation of the beta1-adrenergic receptor promotes Gs/Gi switching. Cell. Signal. 2004;16:1397–1403. doi: 10.1016/j.cellsig.2004.05.002. [DOI] [PubMed] [Google Scholar]
- MARTINY-BARON G., KAZANIETZ M.G., MISCHAK H., BLUMBERG P.M., KOCHS G., HUG H., MARME D., SCHACHTELE C. Selective inhibition of protein kinase C isozymes by the indolocarbazole Go 6976. J. Biol. Chem. 1993;268:9194–9197. [PubMed] [Google Scholar]
- MAU S.E., WITT M.R., BJERRUM O.J., SAERMARK T., VILHARDT H. Growth hormone releasing hexapeptide (GHRP-6) activates the inositol (1,4,5)-trisphosphate/diacylglycerol pathway in rat anterior pituitary cells. J. Recept. Signal. Transduct. Res. 1995;15:311–323. doi: 10.3109/10799899509045223. [DOI] [PubMed] [Google Scholar]
- MCKEE K.K., PALYHA O.C., FEIGHNER S.D., HRENIUK D.L., TAN C.P., PHILLIPS M.S., SMITH R.G., VAN DER PLOEG L.H., HOWARD A.D. Molecular analysis of rat pituitary and hypothalamic growth hormone secretagogue receptors. Mol. Endocrinol. 1997;11:415–423. doi: 10.1210/mend.11.4.9908. [DOI] [PubMed] [Google Scholar]
- MONDORF U.F., GEIGER H., HERRERO M., ZEUZEM S., PIIPER A. Involvement of the platelet-derived growth factor receptor in angiotensin II-induced activation of extracellular regulated kinases 1 and 2 in human mesangial cells. FEBS Lett. 2000;472:129–132. doi: 10.1016/s0014-5793(00)01433-2. [DOI] [PubMed] [Google Scholar]
- MURATA M., OKIMURA Y., IIDA K., MATSUMOTO M., SOWA H., KAJI H., KOJIMA M., KANGAWA K., CHIHARA K. Ghrelin modulates the downstream molecules of insulin signaling in hepatoma cells. J. Biol. Chem. 2002;277:5667–5674. doi: 10.1074/jbc.M103898200. [DOI] [PubMed] [Google Scholar]
- NAKAZATO M., MURAKAMI N., DATE Y., KOJIMA M., MATSUO H., KANGAWA K., MATSUKURA S. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–198. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- NANZER A.M., KHALAF S., MOZID A.M., FOWKES R.C., PATEL M.V., BURRIN J.M., GROSSMAN A.B., KORBONITS M. Ghrelin exerts a proliferative effect on a rat pituitary somatotroph cell line via the mitogen-activated protein kinase pathway. Eur. J. Endocrinol. 2004;151:233–240. doi: 10.1530/eje.0.1510233. [DOI] [PubMed] [Google Scholar]
- ONG H., MCNICOLL N., ESCHER E., COLLU R., DEGHENGHI R., LOCATELLI V., GHIGO E., MUCCIOLI G., BOGHEN M., NILSSON M. Identification of a pituitary growth hormone-releasing peptide (GHRP) receptor subtype by photoaffinity labeling. Endocrinology. 1998;139:432–435. doi: 10.1210/endo.139.1.5811. [DOI] [PubMed] [Google Scholar]
- PEARSON G., ROBINSON F., BEERS GIBSON T., XU B.E., KARANDIKAR M., BERMAN K., COBB M.H. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr. Rev. 2001;22:153–183. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- PIERCE K.L., LUTTRELL L.M., LEFKOWITZ R.J. New mechanisms in heptahelical receptor signaling to mitogen activated protein kinase cascades. Oncogene. 2001;20:1532–1539. doi: 10.1038/sj.onc.1204184. [DOI] [PubMed] [Google Scholar]
- PIERCE K.L., MAUDSLEY S., DAAKA Y., LUTTRELL L.M., LEFKOWITZ R.J. Role of endocytosis in the activation of the extracellular signal-regulated kinase cascade by sequestering and nonsequestering G protein-coupled receptors. Proc. Natl. Acad. Sci. U.S.A. 2000;97:1489–1494. doi: 10.1073/pnas.97.4.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIPPIG S., ANDEXINGER S., LOHSE M.J. Sequestration and recycling of beta 2-adrenergic receptors permit receptor resensitization. Mol. Pharmacol. 1995;47:666–676. [PubMed] [Google Scholar]
- SEVA C., KOWALSKI-CHAUVEL A., DAULHAC L., BARTHEZ C., VAYSSE N., PRADAYROL L. Wortmannin-sensitive activation of p70S6-kinase and MAP-kinase by the G protein-coupled receptor, G/CCKB. Biochem. Biophys. Res. Commun. 1997;238:202–206. doi: 10.1006/bbrc.1997.7163. [DOI] [PubMed] [Google Scholar]
- SHIRAI Y., KASHIWAGI K., SAKAI N., SAITO N. Phospholipase A(2) and its products are involved in the purinergic receptor-mediated translocation of protein kinase C in CHO-K1 cells. J. Cell. Sci. 2000;113:1335–1343. doi: 10.1242/jcs.113.8.1335. [DOI] [PubMed] [Google Scholar]
- SMITH R.G., GRIFFIN P.R., XU Y., SMITH A.G., LIU K., CALACAY J., FEIGHNER S.D., PONG C., LEONG D., POMES A., CHENG K., VAN DER PLOEG L.H., HOWARD A.D., SCHAEFFER J., LEONARD R.J. Adenosine: a partial agonist of the growth hormone secretagogue receptor. Biochem. Biophys. Res. Commun. 2000;276:1306–1313. doi: 10.1006/bbrc.2000.3610. [DOI] [PubMed] [Google Scholar]
- SMITH R.G., VAN DER PLOEG L.H., HOWARD A.D., FEIGHNER S.D., CHENG K., HICKEY G.J., WYVRATT M.J., Jr, FISHER M.H., NARGUND R.P., PATCHETT A.A. Peptidomimetic regulation of growth hormone secretion. Endocr. Rev. 1997;18:621–645. doi: 10.1210/edrv.18.5.0316. [DOI] [PubMed] [Google Scholar]
- THOMPSON N.M., GILL D.A., DAVIES R., LOVERIDGE N., HOUSTON P.A., ROBINSON I.C., WELLS T. Ghrelin and des-octanoyl ghrelin promote adipogenesis directly in vivo by a mechanism independent of the type 1a growth hormone secretagogue receptor. Endocrinology. 2004;145:234–242. doi: 10.1210/en.2003-0899. [DOI] [PubMed] [Google Scholar]
- TIPPMER S., QUITTERER U., KOLM V., FAUSSNER A., ROSCHER A., MOSTHAF L., MULLER-ESTERL W., HARING H. Bradykinin induces translocation of the protein kinase C isoforms alpha, epsilon, and zeta. Eur. J. Biochem. 1994;225:297–304. doi: 10.1111/j.1432-1033.1994.00297.x. [DOI] [PubMed] [Google Scholar]
- TOEWS M.L., WALDO G.L., HARDEN T.K., PERKINS J.P. Relationship between an altered membrane form and a low affinity form of the beta-adrenergic receptor occurring during catecholamine-induced desensitization. Evidence for receptor internalization. J. Biol. Chem. 1984;259:11844–11850. [PubMed] [Google Scholar]
- TOHGO A., CHOY E.W., GESTY-PALMER D., PIERCE K.L., LAPORTE S., OAKLEY R.H., CARON M.G., LEFKOWITZ R.J., LUTTRELL L.M. The stability of the G protein-coupled receptor-beta-arrestin interaction determines the mechanism and functional consequence of ERK activation. J. Biol. Chem. 2003;278:6258–6267. doi: 10.1074/jbc.M212231200. [DOI] [PubMed] [Google Scholar]
- TOHGO A., PIERCE K.L., CHOY E.W., LEFKOWITZ R.J., LUTTRELL L.M. β-Arrestin scaffolding of the ERK cascade enhances cytosolic ERK activity but inhibits ERK-mediated transcription following angiotensin AT1a receptor stimulation. J. Biol. Chem. 2002;277:9429–9436. doi: 10.1074/jbc.M106457200. [DOI] [PubMed] [Google Scholar]
- TSCHOP M., SMILEY D.L., HEIMAN M.L. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–913. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- TULLIN S., HANSEN B.S., ANKERSEN M., MOLLER J., VON CAPPELEN K.A., THIM L. Adenosine is an agonist of the growth hormone secretagogue receptor. Endocrinology. 2000;141:3397–3402. doi: 10.1210/endo.141.9.7631. [DOI] [PubMed] [Google Scholar]
- VAN CRAENENBROECK M., GREGOIRE F., DE NEEF P., ROBBERECHT P., PERRET J. Ala-scan of ghrelin (1–14): interaction with the recombinant human ghrelin receptor. Peptides. 2004;25:959–965. doi: 10.1016/j.peptides.2004.03.010. [DOI] [PubMed] [Google Scholar]
- VOGLER O., NOLTE B., VOSS M., SCHMIDT M., JAKOBS K.H., VAN KOPPEN C.J. Regulation of muscarinic acetylcholine receptor sequestration and function by beta-arrestin. J. Biol. Chem. 1999;274:12333–12338. doi: 10.1074/jbc.274.18.12333. [DOI] [PubMed] [Google Scholar]
- VOLANTE M., ALLIA E., FULCHERI E., CASSONI P., GHIGO E., MUCCIOLI G., PAPOTTI M. Ghrelin in fetal thyroid and follicular tumors and cell lines: expression and effects on tumor growth. Am. J. Pathol. 2003;162:645–654. doi: 10.1016/S0002-9440(10)63858-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WREN A.M., SEAL L.J., COHEN M.A., BRYNES A.E., FROST G.S., MURPHY K.G., DHILLO W.S., GHATEI M.A., BLOOM S.R. Ghrelin enhances appetite and increases food intake in humans. J. Clin. Endocrinol. Metab. 2001;86:5992–5995. doi: 10.1210/jcem.86.12.8111. [DOI] [PubMed] [Google Scholar]
- WREN A.M., SMALL C.J., WARD H.L., MURPHY K.G., DAKIN C.L., TAHERI S., KENNEDY A.R., ROBERTS G.H., MORGAN D.G., GHATEI M.A., BLOOM S.R. The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology. 2000;141:4325–4328. doi: 10.1210/endo.141.11.7873. [DOI] [PubMed] [Google Scholar]
- YAMAZAKI M., KOBAYASHI H., TANAKA T., KANGAWA K., INOUE K., SAKAI T. Ghrelin-induced growth hormone release from isolated rat anterior pituitary cells depends on intracellullar and extracellular Ca2+ sources. J. Neuroendocrinol. 2004;16:825–831. doi: 10.1111/j.1365-2826.2004.01237.x. [DOI] [PubMed] [Google Scholar]
- ZHANG W., ZHAO L., LIN T.R., CHAI B., FAN Y., GANTZ I., MULHOLLAND M.W. Inhibition of adipogenesis by ghrelin. Mol. Biol. Cell. 2004;15:2484–2491. doi: 10.1091/mbc.E03-09-0657. [DOI] [PMC free article] [PubMed] [Google Scholar]