Abstract
Nitric oxide (NO) is known to affect the properties of various proteins via the S-nitrosylation of cysteine residues. This study evaluated the direct effects of the NO donor sodium nitroprusside (SNP) on the pharmacological properties of the AT1 receptor for angiotensin II expressed in HEK-293 cells.
SNP dose-dependently decreased the binding affinity of the AT1 receptor without affecting its total binding capacity. This modulatory effect was reversed within 5 min of removing SNP.
The effect of SNP was not modified in the presence of the G protein uncoupling agent GTPγS or the soluble guanylyl cyclase inhibitor 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one.
The binding properties of a mutant AT1 receptor in which all five cysteine residues within the transmembrane domains had been replaced by serine was not affected by SNP. Systematic analysis of mutant AT1 receptors revealed that cysteine 289 conferred the sensitivity to SNP.
These results suggest that NO decreased the binding affinity of the AT1 receptor by S-nitrosylation of cysteine 289. This modulatory mechanism may be particularly relevant in pathophysiological situations where the beneficial effects of NO oppose the deleterious effects of angiotensin II.
Keywords: AT1 receptor, S-nitrosylation, post-translational modification, redox-related regulation, sodium nitroprusside, reactive cysteine thiols, G-protein coupled receptor
Introduction
The octapeptide hormone angiotensin II (Ang II) produces a wide variety of physiological effects, including vascular contraction, aldosterone secretion, sodium and water retention, neuronal activation, and cardiovascular cell growth and proliferation (for review, see (de Gasparo et al., 2000)). Whereas Ang II can interact with two receptor subtypes (AT1 and AT2), the vast majority of its effects are produced through the activation of the AT1 receptor, which belongs to the G protein-coupled receptor (GPCR) superfamily (Murphy et al., 1991; Sasaki et al., 1991; Kambayashi et al., 1993; Mukoyama et al., 1993; Timmermans et al., 1993). The AT1 receptor functions primarily by coupling to the heterotrimeric G protein (Gq/11), which activates phospholipase C (PLC), which in turn generates the second messengers inositol 1,4,5-trisphosphate (InsP3) and diacylglycerol (Balla et al., 1989; Spat et al., 1991). InsP3 causes the release of Ca2+ from the endoplasmic reticulum while diacylglycerol recruits and activates protein kinase C at the plasma membrane.
The functional properties of the AT1 receptor are regulated by different post-translational modifications such as N-glycosylation (Deslauriers et al., 1999; Jayadev et al., 1999; Lanctot et al., 1999) and phosphorylation (Smith et al., 1998a, 1998b; Thomas et al., 1998; Qian et al., 2001). N-glycosylation is important for the maturation of the AT1 receptor and for targeting it to the cell surface. Serine/threonine phosphorylation of the AT1 receptor participates in the recruitment of arrestin and the internalization of the receptor (Kule et al., 2004), whereas tyrosine phosphorylation of the AT1 receptor reportedly mediates the transactivation of the epidermal growth factor receptor (Seta & Sadoshima, 2003).
The modification of the cysteine thiol by nitric oxide (NO) is another post-translational modification reported to regulate the activity of proteins (Stamler et al., 1992, 1997). This process, which is called S-nitrosylation, is a reversible redox-related post-translational modification that modulates protein functionality. NO is an unstable, gaseous, second messenger that can diffuse across membranes and that is involved in vascular tone, neurotransmission, and immune defense (Lowenstein et al., 1994). It is generated by nitric oxide synthase (NOS) from L-arginine and molecular oxygen (Alderton et al., 2001) and activates soluble guanylyl cyclase, which produces cGMP (Foster et al., 1999). Whereas most of the effects of NO have been attributed to cGMP production, recent evidence suggests that S-nitrosylation is involved in the regulation of several proteins. For example, S-nitrosylation of cysteine 118 of the GTPase p21ras, S-nitrosylation of cysteine 3635 of the ryanodine receptor , and denitrosylation of caspase-3 all lead to the activation of these proteins (Lander et al., 1997; Mannick et al., 1999; Eu et al., 2000; Sun et al., 2001).
Among the 10 cysteines in the sequence of the AT1 receptor, four cysteines on the extracellular loops are involved in the formation of intramolecular disulfide bridges (Yamano et al., 1992; Ohyama et al., 1995) while only one cysteine is located in the cytoplasmic tail of the receptor. The other five cysteines are distributed within the seven transmembrane domains of the receptor at positions 76, 121, 149, 289, and 296. As NO distributes preferentially in membranes (Liu et al., 1998) and because hydrophobic environment promotes S-nitrosylation (Hess et al., 2005), we hypothesized that the five cysteines located in the transmembrane domains are potential targets for S-nitrosylation. To verify this, we treated HEK-293 cells that stably express the AT1 receptor with the NO donor sodium nitroprusside (SNP) and evaluated the pharmacological and functional properties of the AT1 receptor. We also analyzed the susceptibility to S-nitrosylation of a series of AT1 receptor mutants in which the cysteines had been replaced by serines. Our results suggest that S-nitrosylation of cysteine 289 decreases the binding affinity of the AT1 receptor.
Methods
Oligodeoxynucleotide site-directed mutagenesis
Mutant AT1 receptor cDNAs were constructed by oligonucleotide-directed mutagenesis (Expand High Fidelity PCR System; Roche Diagnostics) using the human AT1 receptor as template. Five sets of forward and reverse oligonucleotides were constructed to introduce mutations at Cys76, Cys121, Cys149, Cys289, and Cys296. PCR products were re-inserted into HindIII-XbaI sites of the mammalian expression vector pcDNA3 and mutagenesis was confirmed by automated nucleotide sequencing.
Cell culture and transfections
HEK-293 cells were grown in DMEM supplemented with 10% heat-inactivated FBS, 50 IU ml−1 penicillin and 50 μg ml−1 streptomycin. The day before transfection, 100 mm dishes were seeded with 2.5 × 106 cells. After 24 h, cells were transfected in culture medium with a solution of serum-free DMEM containing 4 μg of DNA and 8 μl of Fugene 6 (for 100 mm dishes) or 8 μg of DNA and 16 μl of Fugene 6 (for 150 mm dishes). Two days after transfection, cells were washed once with phosphate-buffered saline (PBS) and used immediately for SNP treatment and binding experiments.
SNP treatment
HEK-293 cells stably or transiently expressing the AT1 receptor were washed once with PBS and incubated for 15 min at 37°C in 4.5 ml (for 100 mm dishes) or 13.5 ml (for 150 mm dishes) of treatment buffer (25 mM HEPES, DMEM, pH 7.4). 500 μl (100 mm dishes) or 1.5 ml (150 mm dishes) of treatment buffer containing 10 mM SNP were added to each dish. After appropriate periods of time, treatments were stopped by washing with ice-cold PBS and the dish was immediately frozen in liquid nitrogen.
Ang II binding experiments
Frozen cells were thawed, scraped in ice-cold washing buffer (25 mM Tris-HCl pH 7.4, 100 mM NaCl, 5 mM MgCl2) and broken by five cycles of aspiration-expulsion with a 10 ml serological pipette tightly apposed to the bottom of the dish. Broken cells were centrifuged at 2500 × g for 15 min at 4°C and resuspended in binding buffer (25 mM Tris-HCl pH 7.4, 100 mM NaCl, 5 mM MgCl2, 0.1% BSA, and 0.01% bacitracin). Broken cells were incubated for 1 h at room temperature in binding buffer containing increasing concentrations of 125I-Ang II in a final volume of 0.5 ml. Bound radioactivity was separated from free ligand by filtration through GF/C filters presoaked for 2 h in binding buffer. Nonspecific binding was measured in the presence of 1 μM unlabeled Ang II. Receptor-bound radioactivity was evaluated by γ counting.
Phospholipase C assay
HEK-293 cells stably expressing the AT1 receptor were grown in six-well plates and prelabeled for 20 h in inositol-free DMEM containing 8 μCi ml−1 of myo-[3H]inositol. After two washes with PBS, cells were incubated for 30 min in the stimulation buffer (25 mM HEPES pH 7.4, DMEM, and 0.1 % BSA). Cells were then treated for 30 min with 1 mM SNP at 37°C. Phospholipase C was activated with 100 nM Ang II for 5 min in stimulation buffer at 37°C. Incubations were terminated by the addition of ice-cold perchloric acid (5% v v−1). Cells were scraped and centrifuged at 15,000 × g for 5 min. Water-soluble inositol phosphates were then extracted with an equal volume of the 1 : 1 mixture of 1,1,2-trichlorotrifluoroethane and tri-n-octylamine. The samples were vigorously mixed and centrifuged at 2500 × g for 10 min. The upper phase containing the inositol phosphates was applied to an AG1-X8 resin column. Inositol phosphates were sequentially eluted by addition of ammonium formate/formic acid mixtures of increasing ionic strength (Berridge, 1983).
Materials
The cDNA clone encoding the human AT1 receptor with a N-terminus FLAG epitope was constructed in our laboratory and subcloned in the mammalian expression vector pcDNA3 (Invitrogen, San Diego, CA, U.S.A.). Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum (FBS), penicillin–streptomycin and oligonucleotide primers were purchased from Gibco Life Thechnologies (Gaithersburg, MD, U.S.A.). Fugene 6 was purchased from Roche diagnostics corporation (Indianapolis, IN, U.S.A.). 125I-Ang II (1000 Ci mmol−1) was prepared with Iodogen (Pierce Chemical, Rockford, IL, U.S.A.) as previously described (25) and purified by HPLC on a C-18 column. SNP, GTPγS, BSA and bacitracin were purchased from Sigma (St-Louis, MO, U.S.A.). 1H-[1,2,4]Oxadiazolo[43-a]quinoxalin-1-one (ODQ) was purchased from Calbiochem (San Diego, CA, U.S.A.).
Results
SNP decreases AT1 receptor binding affinity
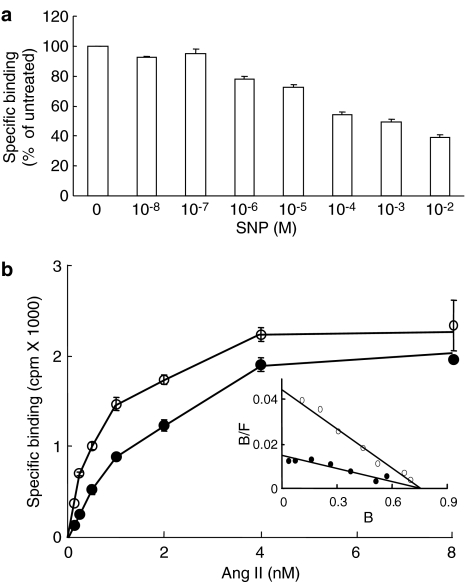
HEK-293 cells that stably express the AT1 receptor were treated with increasing concentrations of SNP and their Ang II binding properties were analyzed. Figure 1a shows that SNP decreased the specific binding of 125I-Ang II in a dose-dependent manner. Binding was decreased by approximately 20% with 1 μM SNP while the maximal inhibitory effect was obtained with 10 mM SNP, which reduced binding by about 60%. The effect of SNP was likely related to its capacity to release NO since other NO donors such as S-nitroso-N-acetylpenicillamine (100 μM) and diethylenetriamine/NO (100 μM) also decreased Ang II binding (data not shown). To better define the effect of SNP, 125I-Ang II saturating binding isotherms were performed with cells that were treated for 30 min with 1 mM SNP. The binding isotherms were compared to those from untreated cells (Figure 1b). Scatchard plot analysis of the binding data revealed that untreated cells had a high-binding affinity (Kd=0.53±0.2 nM) whereas treated cells had a lower-binding affinity (Kd=1.53±0.6 nM). The maximal binding capacities (Bmax of 1.42±0.2 pmol mg−1 of protein) of the treated and untreated cells were not significantly different (Table 1). The SNP treatment thus decreased the binding affinity of the AT1 receptor without affecting its maximal binding capacity.
Figure 1.
Effect of SNP on the binding properties of AT1 receptor. (a) HEK-293 cells stably expressing the AT1 receptor (50 μg of protein) were treated with increasing concentrations of SNP for 30 min at 37°C. Broken cells were then prepared as described in Methods and incubated with 1 nM 125I-Ang II for 1 h at room temperature as described in Methods. Nonspecific binding was assessed in the presence of 1 μM unlabeled Ang II. (b) HEK-293 cells stably expressing the AT1 receptor (10 μg of protein) were treated (filled symbols) or not (open symbols) with 1 mM SNP for 30 min at 37°C. Broken cells were then incubated for 1 h at room temperature with increasing concentrations of 125I-Ang II. Scatchard analysis of the data is shown in the inset. Nonspecific binding was assessed in the presence of 1 μM unlabeled Ang II. Each data represents the mean of ±s.d. of triplicate values (representative of three independent experiments).
Table 1.
Binding properties of cysteine-substituted hAT1 mutant receptors
|
Receptors |
Kd (nM) |
Bmax (pmole mg−1 prot) |
|
||
|---|---|---|---|---|---|
| Untreated | SNP treated | Untreated | SNP treated | n | |
| hAT1 WT |
0.70±0.2 |
1.50±0.4* |
3.78±0.9 |
3.88±0.8 |
8 |
| hAT1 TM CYS- |
4.03±1.0 |
4.64±1.3 |
0.43±0.2 |
0.43±0.2 |
3 |
| hAT1 C76,121,149S |
0.92±0.3 |
2.12±0.8* |
2.41±0.3 |
2.59±0.5 |
4 |
| hAT1 C296S |
1.38±0.3 |
2.53±0.6* |
1.51±0.4 |
1.39±0.4 |
4 |
| hAT1 C289S |
0.81±0.2 |
0.94±0.2 |
2.53±1.3 |
2.57±1.4 |
6 |
| hAT1 YFFY/A |
1.68±0.5 |
3.05±1.2* |
1.54±0.3 |
1.45±0.3 |
6 |
| hAT1 WTa |
0.53±0.2 |
1.53±0.6* |
1.42±0.2 |
1.40±0.1 |
4 |
| hAT1 N111Ga | 0.70±0.4 | 0.80±0.5 | 1.50±0.8 | 1.44±0.8 | 3 |
HEK-293 cells were transiently or stably atransfected with the appropriate receptor. Binding properties were assayed as described in Methods. Binding parameters (Bmax and Kd) were evaluated by Scatchard analysis of the 125I-Ang II saturation curves. The means±s.d. are shown for n independent experiments. A P-value of <0.05 (*) was considered statistically significant for SNP-treated vs untreated cells (paired Student's t-test).
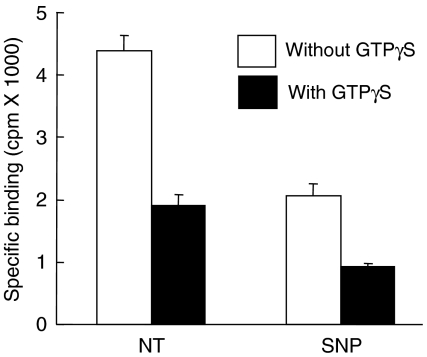
Like other GPCRs, the AT1 receptor can adopt a low-affinity state when it is uncoupled from its cognate G protein (Glossmann et al., 1974; Poitras et al., 1998). To verify whether the low-affinity state induced by an SNP treatment was due to the uncoupling of the AT1 receptor from its G protein, we evaluated the binding properties in the presence of the well-known G protein uncoupling agent GTPγS. Figure 2 shows that in the presence of GTPγS, 125I-Ang II binding to untreated cells was reduced by 59.7±4.4% while 125I-Ang II binding to SNP-treated cells was reduced by 54.8±0.5%. These results show that GTPγS had a strong uncoupling effect on SNP-treated cells, implying that the AT1 receptor was still coupled to its G protein after the SNP treatment and therefore that the mechanism by which SNP decreases the binding affinity of the AT1 receptor is not related to G protein coupling.
Figure 2.
The effect of SNP is not related to AT1 receptor-G protein coupling. HEK-293 cells stably expressing the AT1 receptor were treated for 5 min with 1 mM SNP at 37°C. Broken cells (40 μg of protein) were then prepared as described in Methods and incubated with 1 nM 125I-Ang II for 1 h at room temperature in the absence or presence of 10 μM GTPγS. Nonspecific binding was assessed in the presence of 1 μM unlabeled Ang II. Each data represents the mean±s.d. of triplicate values (representative of three independent experiments).
SNP reduces Ang II binding by a guanylyl cyclase-independent mechanism
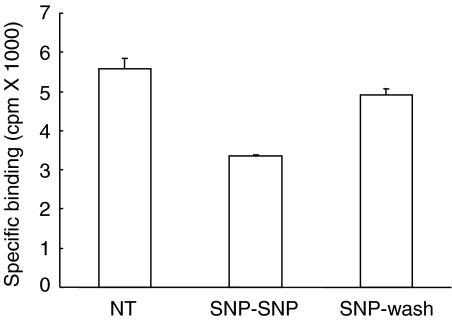
Most of the effects attributed to NO are mediated by the stimulation of NO-sensitive soluble guanylyl cyclase (sGC) and the subsequent intracellular increase in cGMP. To verify whether the modulation of AT1 receptor binding affinity by SNP is a consequence of sGC activation, the SNP treatment was performed in the presence of the selective sGC inhibitor ODQ. Figure 3 shows that the treatment with SNP reduced 125I-Ang II-binding to a level corresponding to 63±5% of control cells. In the presence of ODQ, the SNP treatment reduced 125I-Ang II binding to a level corresponding to 52±3% of control cells, suggesting that the effect of SNP was cGMP-independent. When HEK-293 cells that stably express the AT1 receptor were treated for 5 min with SNP, their 125I-Ang II binding activity decreased to 61±4% compared to that of untreated cells (Figure 4). After washing to remove the SNP, a 5-min incubation in the absence of SNP was sufficient to return the binding activity to 90±8% of untreated cells. This reversible effect of SNP was consistent with the nitrosylation of a sulfhydryl group (Schonhoff et al., 2002).
Figure 3.
The effect of SNP is independent of guanylyl cyclase activity. HEK-293 cells stably expressing the AT1 receptor were treated or not for 5 min at 37°C with 1 mM SNP in the absence (NT) or in the presence of 3 μM ODQ. Broken cells (60 μg of protein) were then prepared as described in Methods and incubated with 1 nM 125I-Ang II for 1 h at room temperature. Nonspecific binding was assessed in the presence of 1 μM unlabeled Ang II. Each data represents the mean±s.d. of triplicate values (representative of three independent experiments).
Figure 4.
The effect of SNP is reversible. HEK-293 cells stably expressing the AT1 receptor were pretreated for 5 min at 37°C in the absence (NT) or in the presence of 1 mM SNP (SNP-SNP and SNP-Wash). After the pretreatment, cells were rinsed with PBS and incubated for another 5 min at 37°C in the absence (SNP-Wash) or in the presence of 1 mM SNP (SNP-SNP). Broken cells (60 μg of protein) were then prepared as described in Methods and incubated with 1 nM 125I-Ang II for 1 h at room temperature. Nonspecific binding was assessed in the presence of 1 μM unlabeled Ang II. Each data represents the mean±s.d. of triplicate values (representative of three independent experiments).
Effect of SNP on AT1 receptor signaling
Our results suggest that SNP modified the binding properties of the AT1 receptor. To verify whether SNP could also modify the functional properties of the AT1 receptor, we evaluated Ang II-induced inositol phosphate production in SNP-treated cells. Figure 5 shows a dose–response curve for the production of inositol phosphates induced by Ang II. SNP caused a rightward shift in the dose–response curve, indicating a decrease in the apparent affinity of the receptor. These results are consistent with the decreased affinity observed in the binding studies and suggest that the integrity of the phospholipase C pathway was unaffected by SNP.
Figure 5.
SNP decreases the apparent affinity of the AT1 receptor. HEK-293 cells stably expressing the AT1 receptor were prelabeled for 20 h with 8 μCi ml−1 myo-[3H]inositol. Cells were pre-treated (filled circle) or not (open circle) for 30 min with 1 mM SNP at 37°C and then stimulated for 5 min with increasing concentrations of Ang II. The incubation was stopped with perchloric acid and the inositol phophates were measured as described in Methods. Results represent total inositol phosphates produced over the basal level (10053 c.p.m.) and expressed as the mean±experimental variation of duplicate values. These results are from a typical experiment representative of two independent experiments.
Requirement of a transmembrane cysteine for the effect of SNP
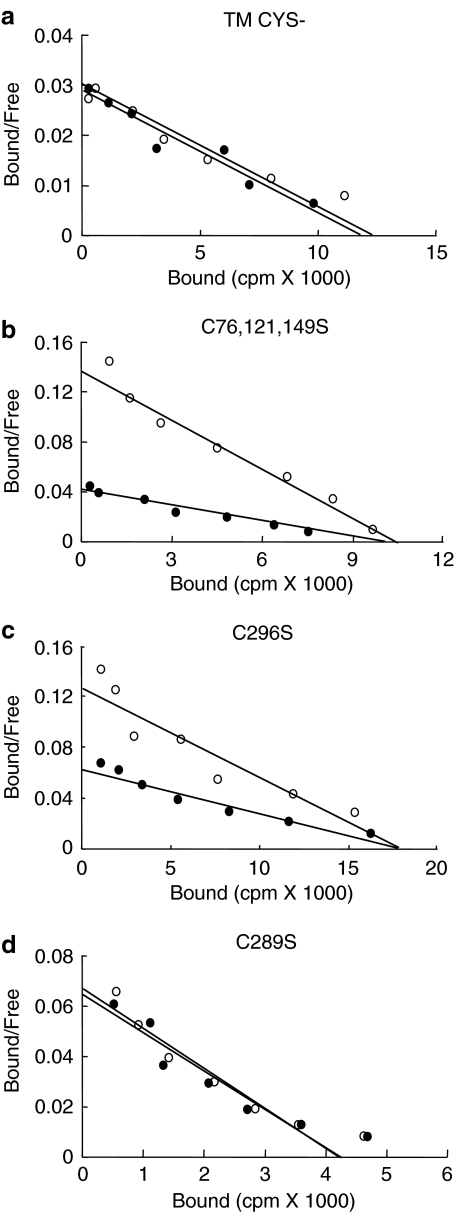
As SNP modulates the pharmacological properties of the AT1 receptor via a readily reversible guanylyl cylclase-independent mechanism, we suspected that it involved S-nitrosylation. NO modifies proteins by the transient S-nitrosylation of free sulfhydryl groups on cysteine residues. Since the cysteine residues in the extracellular segments of the AT1 receptor participate in disulfide bridge formation (Yamano et al., 1992; Ohyama et al., 1995), they cannot be modified by S-nitrosylation. However, the five cysteine residues in the transmembrane domains are potential targets for S-nitrosylation. A mutant AT1 receptor in which all five transmembrane cysteines were replaced by serines (TM CYS-) was constructed and transiently expressed in HEK-293 cells. Figure 6 shows that an SNP treatment did not modify the binding affinity of the mutant receptor, whereas it significantly reduced the binding affinity of the WT-AT1 receptor. These results suggest that at least one cysteine is responsible for the sensitivity of the AT1 receptor to SNP. The SNP treatment also caused a significant decrease in the binding affinity of a mutant AT1 receptor in which cysteines 76, 121, and 149 had been replaced by serines and a mutant receptor in which only cysteine 296 had been replaced by a serine. Interestingly, the substitution of cysteine 289 within the seventh transmembrane domain, conferred to this mutant AT1 receptor a complete insensitivity to SNP (Table 1).
Figure 6.
Cysteine 289 is a major target for SNP. HEK-293 cells transiently expressing mutant AT1 receptors TM CYS- (a), C76,121,149S (b), C296S (c) and C289S (d) were treated (filled symbols) or not (open symbols) for 30 min at 37°C with 1 mM SNP. Broken cells were then prepared as described in Methods, and incubated for 1 h at room temperature with increasing concentrations of 125I-Ang II. Scatchard plots of the binding data are shown. Nonspecific binding was assessed in the presence of 1 μM unlabeled Ang II. Each curve is representative of three independent experiments.
We also tested the SNP sensitivity of a mutant AT1 receptor (YFFY/A-AT1 receptor) in which the caveolin binding motif located at the end of the seventh transmembrane domain is disrupted. This mutant was shown to have a decreased affinity for Ang II, a slower internalization rate, a decreased level of expression at the plasma membrane and a decreased functionality (Leclerc et al., 2002). Although the YFFY/A mutation modified several pharmacological properties of the AT1 receptor, it did not modify its sensitivity to SNP (Figure 7a). We finally tested another mutant AT1 receptor (N111G-AT1 receptor) in which the crucial asparagine 111, located in third transmembrane domain, was substituted for a glycine. This mutation confers to the AT1 receptor a strong constitutive activity accompanied with a translation of the seventh transmembrane domain away from its ligand binding pocket (Boucard et al., 2003). Interestingly, this mutant was insensitive to SNP treatment (Figure 7b).
Figure 7.
Sensitivity of YFFY/A-AT1 and N111G-AT1 receptors to SNP. HEK-293 cells transiently expressing mutant YFFY/A-AT1 receptor (a) or stably expressing N111G-AT1 receptor (b) were treated (filled symbols) or not (open symbols) for 30 min at 37°C with 1 mM SNP. Broken cells were then prepared as described in Methods, and incubated for 1 h at room temperature with increasing concentrations of 125I-Ang II. Scatchard plots of the binding data are shown. Nonspecific binding was assessed in the presence of 1 μM unlabeled Ang II. Each curve is representative of three independent experiments.
Discussion
This study provides evidence that NO regulates the binding affinity of the AT1 receptor by a direct process of S-nitrosylation. We show that a short (30 min) treatment with SNP reduced the binding affinity of the AT1 receptor on HEK-293 cells without affecting total binding capacity. To our knowledge, this is the first study demonstrating that a treatment with a NO donor modulates the binding affinity of the AT1 receptor. It was previously shown that a lengthy (18–24 h) treatment with an NO donor decreases the expression of the AT1 receptor at the surface of vascular smooth muscle cells (Cahill et al., 1995; Ichiki et al., 1998), while a lengthy treatment with an NO synthase inhibitor (L-NAME) upregulates the expression of the AT1 receptor in adrenal glomerulosa cells (Usui et al., 1998). This effect of NO was attributed to a regulatory mechanism at the transcriptional level. However, the results of the short treatment reported here suggest that NO had a direct effect on the AT1 receptor. NO also decreases the binding affinity of the β2-adrenergic receptor (Adam et al., 1999) and the bradykinin B2 receptor (Miyamoto et al., 1997), two other GPCRs that play important roles in regulating the cardiovascular system. Since NO also promotes the depalmitoylation of the β2-adrenergic receptor, it has been suggested that NO causes the uncoupling of the Gs protein, thus decreasing receptor binding affinity. Likewise, the effect of NO on the bradykinin B2 receptor has been attributed to the inhibition of G proteins of the Gi and Gq family, resulting in a reduction of B2 receptor-G protein coupling. NO donors also directly modulate the signaling partners of other Gi-coupled receptors in various model systems (Kokkola et al., 2005). Our results clearly show that GTPγS-uncoupled AT1 receptors were still sensitive to SNP, which suggests that NO was able to decrease the binding affinity of the AT1 receptor by a process unrelated to G protein coupling.
We show that the production of inositol phosphates induced by a low dose of Ang II was reduced by SNP, whereas the production of inositol phosphates induced by a maximal dose of Ang II was not modified by SNP. These results suggest that NO modulated the apparent affinity of the AT1 receptor. Velardez et al. (2003) also demonstrated that a short (30 min) treatment with NO reduces the production of inositol phosphates in anterior pituitary cells stimulated with a low dose of Ang II (10 nM). Interestingly, their results suggest that NO does not act on phospholipase C protein but rather affects a component of the signaling cascade upstream from phospholipase C. These results are consistent with the notion that NO has a direct effect on the AT1 receptor.
NO acts via two distinct mechanisms: (1) it activates the sGC/cGMP/PKG pathway that leads to protein phosphorylation on PKG consensus sites and (2) it directly modifies proteins by the S-nitrosylation of free cysteine residues (Hanafy et al., 2001). We show that a specific inhibitor of soluble guanylyl cyclase (ODQ) did not modify the effect of SNP on the binding affinity of the AT1 receptor. Similar results were obtained by Velardez et al. (2003), who showed that the effect of NO on Ang II-induced inositol phosphates production is not mimicked by a stable analog of cGMP and is not affected by BAY 41-2272, another specific inhibitor of soluble guanylyl cyclase. These results provide further evidence that NO acts by direct S-nitrosylation of the AT1 receptor.
We show that the effect of SNP on the binding affinity of the AT1 receptor was reversed within 5 min. We identified cysteine 289 as the residue that renders the AT1 receptor sensitive to NO. Interestingly, cysteine 289 is located in the seventh transmembrane domain of AT1 receptor, a region that is critical for ligand binding. In photoaffinity labeling experiments, we previously identified residues 293 and 294 of the AT1 receptor as contact points with the C-terminus of Ang II (Laporte et al., 1999; Perodin et al., 2002). We recently showed that the constitutively active mutant N111G-AT1 receptor adopts a conformation in which the seventh transmembrane domain translates or moves away from the binding pocket (Boucard et al., 2003). Interestingly, the N111G-AT1 receptor is insensitive to SNP treatment. The simple explanation of this result is that S-nitrosylated cysteine 289 in the N111G-AT1 receptor, does not interfere with Ang II binding because the translation of the seventh transmembrane domain leaves enough space within the binding pocket to fully accommodate the ligand. Another conformational change caused by the YFFY/A mutation at the end of the seventh transmembrane domain caused very significant modifications in the pharmacological properties of the AT1 receptor but did not remove its sensitivity to SNP. The exact nature of the conformational change induced by the YFFY/A mutation is not known but clearly it could not compensate for the binding inhibitory effect nitrosylated cysteine 289. Further evidence for the importance of cysteine 289 was provided in a recent study that analyzed the most frequent single nucleotide polymorphisms in the human AT1 receptor gene. Interestingly, the binding affinity of the C289W-AT1 receptor variant is reduced three-fold (Hansen et al., 2004). Except for frog, cysteine 289 is a highly conserved residue among all the different animal species in which the AT1 receptor was analyzed. It indicates that this S-nitrosylation mechanism could be generalized to most animal models used to study the cardiovascular biology of AT1 receptor.
S-nitrosylation reversibly modifies specific cysteine residues in target proteins (Hess et al., 2005). The activity of the ryanodine receptor RyR1, which contains over 50 free cysteine residues, is increased by specific S-nitrosylation of cysteine 3635 (Sun et al., 2001) while the activity of the small G protein p21ras, which contains five free cysteine residues, is increased by specific S-nitrosylation of cysteine 118 (Lander et al., 1997). Our results suggest that the binding affinity of the AT1 receptor is decreased by specific S-nitrosylation of cysteine 289.
In summary, we showed that NO modulates the binding affinity of the AT1 receptor. The effect of NO was reversible, was not related to the G protein coupling state of the receptor, was independent of the sGC/cGMP/PKG pathway, and was a direct consequence of S-nitrosylation of cysteine 289 on the AT1 receptor. This post-translational modification of the AT1 receptor possesses all the characteristics of a regulatory mechanism, including modulation of function, site-specific regulation, and reversibility. S-nitrosylation of the AT1 receptor may be particularly relevant in pathophysiological situations where the beneficial effects of NO oppose the deleterious effects of angiotensin II.
Acknowledgments
This work is part of the Ph.D. thesis of P.C.L. and was supported by grants from the Canadian Institutes of Health Research (CIHR). E.E. is the recipient of a J.C. Edwards Chair in cardiovascular research. R.L. is a Chercheur National of the Fonds de la Recherche en Santé du Québec (FRSQ). P.C.L. is the recipient of a studentship from the Heart and Stroke Foundation of Canada (HSFC). P.M.L. is the recipient of a studentship from FRSQ. M.A.M. is the recipient of a studentship from the Canadian Institutes of Health Research (CIHR).
Abbreviations
- Ang II
angiotensin II
- AT1
angiotensin II type 1 receptor
- GC
guanylyl cyclase
- GPCR
G protein-coupled receptor
- NO
nitric oxide
- ODQ, 1H-[1,2,4]Oxadiazolo[4
3-a]quinoxalin-1-one
- SNP
sodium nitroprusside
References
- ADAM L., BOUVIER M., JONES T.L. Nitric oxide modulates beta(2)-adrenergic receptor palmitoylation and signaling. J. Biol. Chem. 1999;274:26337–26343. doi: 10.1074/jbc.274.37.26337. [DOI] [PubMed] [Google Scholar]
- ALDERTON W.K., COOPER C.E., KNOWLES R.G. Nitric oxide synthases: structure, function and inhibition. Biochem. J. 2001;357:593–615. doi: 10.1042/0264-6021:3570593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BALLA T., HAUSDORFF W.P., BAUKAL A.J., CATT K.J. Inositol polyphosphate production and regulation of cytosolic calcium during the biphasic activation of adrenal glomerulosa cells by angiotensin II. Arch. Biochem. Biophys. 1989;270:398–403. doi: 10.1016/0003-9861(89)90043-x. [DOI] [PubMed] [Google Scholar]
- BERRIDGE M.J. Rapid accumulation of inositol trisphosphate reveals that agonists hydrolyse polyphosphoinositides instead of phosphatidylinositol. Biochem. J. 1983;212:849–858. doi: 10.1042/bj2120849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOUCARD A.A., ROY M., BEAULIEU M.E., LAVIGNE P., ESCHER E., GUILLEMETTE G., LEDUC R. Constitutive activation of the angiotensin II type 1 receptor alters the spatial proximity of transmembrane 7 to the ligand-binding pocket. J. Biol. Chem. 2003;278:36628–36636. doi: 10.1074/jbc.M305952200. [DOI] [PubMed] [Google Scholar]
- CAHILL P.A., REDMOND E.M., FOSTER C., SITZMANN J.V. Nitric oxide regulates angiotensin II receptors in vascular smooth muscle cells. Eur. J. Pharmacol. 1995;288:219–229. doi: 10.1016/0922-4106(95)90197-3. [DOI] [PubMed] [Google Scholar]
- DE GASPARO M., CATT K.J., INAGAMI T., WRIGHT J.W., UNGER T. International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol. Rev. 2000;52:415–472. [PubMed] [Google Scholar]
- DESLAURIERS B., PONCE C., LOMBARD C., LARGUIER R., BONNAFOUS J.C., MARIE J. N-glycosylation requirements for the AT1a angiotensin II receptor delivery to the plasma membrane. Biochem. J. 1999;339 (Part 2):397–405. [PMC free article] [PubMed] [Google Scholar]
- EU J.P., SUN J., XU L., STAMLER J.S., MEISSNER G. The skeletal muscle calcium release channel: coupled O2 sensor and NO signaling functions. Cell. 2000;102:499–509. doi: 10.1016/s0092-8674(00)00054-4. [DOI] [PubMed] [Google Scholar]
- FOSTER D.C., WEDEL B.J., ROBINSON S.W., GARBERS D.L. Mechanisms of regulation and functions of guanylyl cyclases. Rev. Physiol. Biochem. Pharmacol. 1999;135:1–39. doi: 10.1007/BFb0033668. [DOI] [PubMed] [Google Scholar]
- GLOSSMANN H., BAUKAL A., CATT K.J. Angiotensin II receptors in bovine adrenal cortex. Modification of angiotensin II binding by guanyl nucleotides. J. Biol. Chem. 1974;249:664–666. [PubMed] [Google Scholar]
- HANAFY K.A., KRUMENACKER J.S., MURAD F. NO, nitrotyrosine, and cyclic GMP in signal transduction. Med. Sci. Monit. 2001;7:801–819. [PubMed] [Google Scholar]
- HANSEN J.L., HAUNSO S., BRANN M.R., SHEIKH S.P., WEINER D.M. Loss-of-function polymorphic variants of the human angiotensin II type 1 receptor. Mol. Pharmacol. 2004;65:770–777. doi: 10.1124/mol.65.3.770. [DOI] [PubMed] [Google Scholar]
- HESS D.T., MATSUMOTO A., KIM S.O., MARSHALL H.E., STAMLER J.S. Protein S-nitrosylation: purview and parameters. Nat. Rev. Mol. Cell Biol. 2005;6:150–166. doi: 10.1038/nrm1569. [DOI] [PubMed] [Google Scholar]
- ICHIKI T., USUI M., KATO M., FUNAKOSHI Y., ITO K., EGASHIRA K., TAKESHITA A. Downregulation of angiotensin II type 1 receptor gene transcription by nitric oxide. Hypertension. 1998;31:342–348. doi: 10.1161/01.hyp.31.1.342. [DOI] [PubMed] [Google Scholar]
- JAYADEV S., SMITH R.D., JAGADEESH G., BAUKAL A.J., HUNYADY L., CATT K.J. N-linked glycosylation is required for optimal AT1a angiotensin receptor expression in COS-7 cells. Endocrinology. 1999;140:2010–2017. doi: 10.1210/endo.140.5.6689. [DOI] [PubMed] [Google Scholar]
- KAMBAYASHI Y., BARDHAN S., TAKAHASHI K., TSUZUKI S., INUI H., HAMAKUBO T., INAGAMI T. Molecular cloning of a novel angiotensin II receptor isoform involved in phosphotyrosine phosphatase inhibition. J. Biol. Chem. 1993;268:24543–24546. [PubMed] [Google Scholar]
- KOKKOLA T., SAVINAINEN J.R., MONKKONEN K.S., RETAMAL M.D., LAITINEN J.T. S-nitrosothiols modulate G protein-coupled receptor signaling in a reversible and highly receptor-specific manner. BMC Cell Biol. 2005;6:21. doi: 10.1186/1471-2121-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KULE C.E., KAROOR V., DAY J.N., THOMAS W.G., BAKER K.M., DINH D., ACKER K.A., BOOZ G.W. Agonist-dependent internalization of the angiotensin II type one receptor (AT1): role of C-terminus phosphorylation in recruitment of beta-arrestins. Regul. Pept. 2004;120:141–148. doi: 10.1016/j.regpep.2004.03.001. [DOI] [PubMed] [Google Scholar]
- LANCTOT P.M., LECLERC P.C., ESCHER E., LEDUC R., GUILLEMETTE G. Role of N-glycosylation in the expression and functional properties of human AT1 receptor. Biochemistry. 1999;38:8621–8627. doi: 10.1021/bi9830516. [DOI] [PubMed] [Google Scholar]
- LANDER H.M., HAJJAR D.P., HEMPSTEAD B.L., MIRZA U.A., CHAIT B.T., CAMPBELL S., QUILLIAM L.A. A molecular redox switch on p21(ras). Structural basis for the nitric oxide-p21(ras) interaction. J. Biol. Chem. 1997;272:4323–4326. doi: 10.1074/jbc.272.7.4323. [DOI] [PubMed] [Google Scholar]
- LAPORTE S.A., BOUCARD A.A., SERVANT G., GUILLEMETTE G., LEDUC R., ESCHER E. Determination of peptide contact points in the human angiotensin II type I receptor (AT1) with photosensitive analogs of angiotensin II. Mol. Endocrinol. 1999;13:578–586. doi: 10.1210/mend.13.4.0270. [DOI] [PubMed] [Google Scholar]
- LECLERC P.C., AUGER-MESSIER M., LANCTOT P.M., ESCHER E., LEDUC R., GUILLEMETTE G. A polyaromatic caveolin-binding-like motif in the cytoplasmic tail of the type 1 receptor for angiotensin II plays an important role in receptor trafficking and signaling. Endocrinology. 2002;143:4702–4710. doi: 10.1210/en.2002-220679. [DOI] [PubMed] [Google Scholar]
- LIU X., MILLER M.J., JOSHI M.S., THOMAS D.D., LANCASTER J.R., JR Accelerated reaction of nitric oxide with O2 within the hydrophobic interior of biological membranes. Proc. Natl. Acad. Sci. U.S.A. 1998;95:2175–2179. doi: 10.1073/pnas.95.5.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWENSTEIN C.J., DINERMAN J.L., SNYDER S.H. Nitric oxide: a physiologic messenger. Ann. Intern. Med. 1994;120:227–237. doi: 10.7326/0003-4819-120-3-199402010-00009. [DOI] [PubMed] [Google Scholar]
- MANNICK J.B., HAUSLADEN A., LIU L., HESS D.T., ZENG M., MIAO Q.X., KANE L.S., GOW A.J., STAMLER J.S. Fas-induced caspase denitrosylation. Science. 1999;284:651–654. doi: 10.1126/science.284.5414.651. [DOI] [PubMed] [Google Scholar]
- MIYAMOTO A., LAUFS U., PARDO C., LIAO J.K. Modulation of bradykinin receptor ligand binding affinity and its coupled G-proteins by nitric oxide. J. Biol. Chem. 1997;272:19601–19608. doi: 10.1074/jbc.272.31.19601. [DOI] [PubMed] [Google Scholar]
- MUKOYAMA M., NAKAJIMA M., HORIUCHI M., SASAMURA H., PRATT R.E., DZAU V.J. Expression cloning of type 2 angiotensin II receptor reveals a unique class of seven-transmembrane receptors. J. Biol. Chem. 1993;268:24539–24542. [PubMed] [Google Scholar]
- MURPHY T.J., ALEXANDER R.W., GRIENDLING K.K., RUNGE M.S., BERNSTEIN K.E. Isolation of a cDNA encoding the vascular type-1 angiotensin II receptor. Nature. 1991;351:233–236. doi: 10.1038/351233a0. [DOI] [PubMed] [Google Scholar]
- OHYAMA K., YAMANO Y., SANO T., NAKAGOMI Y., HAMAKUBO T., MORISHIMA I., INAGAMI T. Disulfide bridges in extracellular domains of angiotensin II receptor type IA. Regul. Pept. 1995;57:141–147. doi: 10.1016/0167-0115(95)00030-f. [DOI] [PubMed] [Google Scholar]
- PERODIN J., DERAET M., AUGER-MESSIER M., BOUCARD A.A., RIHAKOVA L., BEAULIEU M.E., LAVIGNE P., PARENT J.L., GUILLEMETTE G., LEDUC R., ESCHER E. Residues 293 and 294 are ligand contact points of the human angiotensin type 1 receptor. Biochemistry. 2002;41:14348–14356. doi: 10.1021/bi0258602. [DOI] [PubMed] [Google Scholar]
- POITRAS M., SIDIBE A., RICHARD D.E., CHRETIEN L., GUILLEMETTE G. Effect of uncoupling agents on AT1 receptor affinity for antagonist analogs of angiotensin II. Receptors Channels. 1998;6:65–72. [PubMed] [Google Scholar]
- QIAN H., PIPOLO L., THOMAS W.G. Association of beta-Arrestin 1 with the type 1A angiotensin II receptor involves phosphorylation of the receptor carboxyl terminus and correlates with receptor internalization. Mol. Endocrinol. 2001;15:1706–1719. doi: 10.1210/mend.15.10.0714. [DOI] [PubMed] [Google Scholar]
- SASAKI K., YAMANO Y., BARDHAN S., IWAI N., MURRAY J.J., HASEGAWA M., MATSUDA Y., INAGAMI T. Cloning and expression of a complementary DNA encoding a bovine adrenal angiotensin II type-1 receptor. Nature. 1991;351:230–233. doi: 10.1038/351230a0. [DOI] [PubMed] [Google Scholar]
- SCHONHOFF C.M., DAOU M.C., JONES S.N., SCHIFFER C.A., ROSS A.H. Nitric oxide-mediated inhibition of Hdm2-p53 binding. Biochemistry. 2002;41:13570–13574. doi: 10.1021/bi026262q. [DOI] [PubMed] [Google Scholar]
- SETA K., SADOSHIMA J. Phosphorylation of tyrosine 319 of the angiotensin II type 1 receptor mediates angiotensin II-induced trans-activation of the epidermal growth factor receptor. J. Biol. Chem. 2003;278:9019–9026. doi: 10.1074/jbc.M208017200. [DOI] [PubMed] [Google Scholar]
- SMITH R.D., BAUKAL A.J., ZOLYOMI A., GABORIK Z., HUNYADY L., SUN L., ZHANG M., CHEN H.C., CATT K.J. Agonist-induced phosphorylation of the endogenous AT1 angiotensin receptor in bovine adrenal glomerulosa cells. Mol. Endocrinol. 1998a;12:634–644. doi: 10.1210/mend.12.5.0108. [DOI] [PubMed] [Google Scholar]
- SMITH R.D., HUNYADY L., OLIVARES-REYES J.A., MIHALIK B., JAYADEV S., CATT K.J. Agonist-induced phosphorylation of the angiotensin AT1a receptor is localized to a serine/threonine-rich region of its cytoplasmic tail. Mol. Pharmacol. 1998b;54:935–941. doi: 10.1124/mol.54.6.935. [DOI] [PubMed] [Google Scholar]
- SPAT A., ENYEDI P., HAJNOCZKY G., HUNYADY L. Generation and role of calcium signal in adrenal glomerulosa cells. Exp. Physiol. 1991;76:859–885. doi: 10.1113/expphysiol.1991.sp003550. [DOI] [PubMed] [Google Scholar]
- STAMLER J.S., JIA L., EU J.P., MCMAHON T.J., DEMCHENKO I.T., BONAVENTURA J., GERNERT K., PIANTADOSI C.A. Blood flow regulation by S-nitrosohemoglobin in the physiological oxygen gradient. Science. 1997;276:2034–2037. doi: 10.1126/science.276.5321.2034. [DOI] [PubMed] [Google Scholar]
- STAMLER J.S., SIMON D.I., OSBORNE J.A., MULLINS M.E., JARAKI O., MICHEL T., SINGEL D.J., LOSCALZO J. S-nitrosylation of proteins with nitric oxide: synthesis and characterization of biologically active compounds. Proc. Natl. Acad. Sci. U.S.A. 1992;89:444–448. doi: 10.1073/pnas.89.1.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUN J., XIN C., EU J.P., STAMLER J.S., MEISSNER G. Cysteine-3635 is responsible for skeletal muscle ryanodine receptor modulation by NO. Proc. Natl. Acad. Sci. U.S.A. 2001;98:11158–11162. doi: 10.1073/pnas.201289098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THOMAS W.G., MOTEL T.J., KULE C.E., KAROOR V., BAKER K.M. Phosphorylation of the angiotensin II (AT1A) receptor carboxyl terminus: a role in receptor endocytosis. Mol. Endocrinol. 1998;12:1513–1524. doi: 10.1210/mend.12.10.0179. [DOI] [PubMed] [Google Scholar]
- TIMMERMANS P.B., WONG P.C., CHIU A.T., HERBLIN W.F., BENFIELD P., CARINI D.J., LEE R.J., WEXLER R.R., SAYE J.A., SMITH R.D. Angiotensin II receptors and angiotensin II receptor antagonists. Pharmacol. Rev. 1993;45:205–251. [PubMed] [Google Scholar]
- USUI M., ICHIKI T., KATOH M., EGASHIRA K., TAKESHITA A. Regulation of angiotensin II receptor expression by nitric oxide in rat adrenal gland. Hypertension. 1998;32:527–533. doi: 10.1161/01.hyp.32.3.527. [DOI] [PubMed] [Google Scholar]
- VELARDEZ M.O., BENITEZ A.H., CABILLA J.P., BODO C.C., DUVILANSKI B.H. Nitric oxide decreases the production of inositol phosphates stimulated by angiotensin II and thyrotropin-releasing hormone in anterior pituitary cells. Eur. J. Endocrinol. 2003;148:89–97. doi: 10.1530/eje.0.1480089. [DOI] [PubMed] [Google Scholar]
- YAMANO Y., OHYAMA K., CHAKI S., GUO D.F., INAGAMI T. Identification of amino acid residues of rat angiotensin II receptor for ligand binding by site directed mutagenesis. Biochem. Biophys. Res. Commun. 1992;187:1426–1431. doi: 10.1016/0006-291x(92)90461-s. [DOI] [PubMed] [Google Scholar]