Abstract
P-selectin is involved, with P-selectin glycoprotein (GP)-ligand-1 (PSGL-1), in platelet/leukocyte interactions during thrombo-inflammatory reactions; it also stabilizes platelet aggregates. Its antagonism accelerates thrombolysis and enhances the anti-aggregatory effects of GPIIb–IIIa inhibitors. This study was designed to investigate the mechanisms of P-selectin-mediated platelet aggregation.
In freshly isolated human platelets, P-selectin translocation after thrombin stimulation increased rapidly to 48, 72, and 86% positive platelets after 60, 120, and 300 s, respectively. Platelet aggregation at 60 s post-stimulation averaged 46.7±1.9% and its extent followed closely the kinetics of P-selectin translocation.
Pre-treatment of platelets with P-selectin antagonists, a recombinant PSGL-1 (rPSGL-Ig) or a blocking monoclonal antibody, significantly delayed platelet aggregation in a dose-dependent manner. At 100 μg ml−1 of rPSGL-Ig, platelet aggregation was completely inhibited up to 60 s post-stimulation and increased thereafter to reach maximal aggregation at 5 min. The second phase of platelet aggregation, in the presence of rPSGL-Ig, was completely prevented by the addition of a GPIIb–IIIa antagonist (Reopro) at 60 s, whereas its addition in the absence of rPSGL-Ig was without any significant effect.
Combination of rPSGL-Ig with Reopro or with an inhibitor of Pi3K (LY294002), which reduces GPIIb–IIIa activation, showed to be more effective in inhibiting platelet aggregation, in comparison to the effects observed individually.
rPSGL-Ig blocks P-selectin, whereas Reopro and LY294002 block GPIIb–IIIa and its activation, respectively, without a major effect on the percentage of platelets expressing P-selectin.
In summary, platelet P-selectin participates with GPIIb–IIIa in the initiation of platelet aggregation. Its inhibition, with rPSGL-Ig, delays the aggregation process and increases the anti-aggregatory potency of Reopro. Thus, combination of P-selectin and GPIIb–IIIa antagonism may constitute a promising therapeutic option in the management of thrombotic disorders.
Keywords: Platelets, aggregation, P-selectin, GPIIb–IIIa, PSGL-1
Introduction
Platelets are involved in the maintenance of hemostasis and actively participate in thrombo-inflammatory reactions. Platelet reactions are governed by specific cell adhesion molecules that mediate homotypic and heterotypic adhesive interactions with other platelets, leukocytes, endothelial cells, and the extracellular matrix (Jang et al., 1994; Nash, 1994).
Platelet adhesion and activation represent the first step in thrombogenesis. It may be induced by several agonists that provoke granule secretion and aggregation. One of the most important and potent agonists, thrombin, activates platelets through proteinase-activated receptors (PARs) (MacFarlane et al., 2001). Activation of PAR1 and PAR4 in human platelets leads to an increase in intracellular calcium and stimulates inside-out signalling events, which in turn lead to P-selectin translocation and to glycoprotein (GP)IIb–IIIa activation, rendering them able to accomplish their functions in inflammation and hemostasis.
Clinically, inhibition of platelet aggregation, by targeting GPIIb–IIIa and its binding to fibrinogen, is very useful for reducing the thrombotic disorders that occur during percutaneous coronary interventions (Marmur & Cavusoglu, 2002). However, the available GPIIb–IIIa antagonists do not directly target platelet activation and secretion pathways. In this regard, thrombin-induced P-selectin translocation from the α-granules to the platelet surface does not seem to be influenced by GPIIb–IIIa antagonism (Caron et al., 2002). When expressed, P-selectin mediates leukocyte recruitment to activated platelets and endothelial cells via its high-affinity counter-receptor P-selectin glycoprotein ligand-1 (PSGL-1) (McEver & Cummings, 1997, Yang et al., 1999), thus facilitating transcellular metabolism and triggering intracellular signaling (Jang et al., 1994; Marcus, 1994; Nash, 1994). The activation of platelets and leukocytes and their adhesion have been reported in unstable angina and after myocardial infarction and coronary angioplasty (Mickelson et al., 1996; Neumann et al., 1996; Ott et al., 1996; Serrano et al., 1997). P-selectin is believed to participate, as well, in platelet aggregation by stabilizing initial GPIIb–IIIa–fibrinogen interactions, thereby allowing the formation of large stable platelet aggregates (Merten & Thiagarajan, 2000; Merten et al., 2000), thus highlighting an interplay between P-selectin and GPIIb–IIIa in the aggregation process (Caron et al., 2002). In addition, it has been shown that the adhesion of leukocytes to platelets, through P-selectin, enhances platelet aggregation by increasing the secretion of thromboxane A2 (TXA2) (Faraday et al., 2001). These findings may have an important clinical implication in the treatment of patients with acute myocardial infarction or after PCI. Indeed, inhibition of platelet–leukocyte binding with anti-P-selectin antibodies or a recombinant soluble form of PSGL-1 (rPSGL-Ig) has been beneficial in many cardiovascular pathologies associated with thrombotic disorders in animal models (Kumar et al., 1999; Bienvenu et al., 2001; Myers et al., 2001; Wang et al., 2001, 2002; Tanguay et al., 2004). In addition, our earlier observations indicate that P-selectin translocation precedes GPIIb–IIIa activation. Altogether, these findings led us to the hypothesis that P-selectin may participate with GPIIb–IIIa in the initiation of platelet aggregation.
The present study was designed to assess the interplay between P-selectin and GPIIb–IIIa and the effects of rPSGL-Ig, a P-selectin antagonist, on platelet activation and aggregation. Our results show that, in response to thrombin, platelet P-selectin participates with GPIIb–IIIa in the initiation of platelet aggregation. P-selectin antagonism using rPSGL-Ig delays the aggregation process and increases the effect of Reopro. Thus, rPSGL-Ig could be considered as a novel therapeutic approach for managing thrombotic disorders.
Methods
Platelet preparation
Venous blood (50–100 ml) was obtained from healthy volunteers (free from medication known to interfere with platelet function for at least 10 days before blood sampling) who gave their informed consent in accordance with the policy of the ethic committee of the Montreal Heart Institute. Blood samples were anticoagulated with acid citrate dextrose. As described previously (Bienvenu et al., 2001; Théorêt et al., 2001; Caron et al., 2002), platelets were prepared by centrifugation of whole blood to yield platelet-rich plasma (PRP). The PRP was then centrifuged and the platelet pellet resuspended in Hank's balanced salt solution (HBSS)–HEPES buffer free from Ca2+ and Mg2+ with 0.4 mmol l−1 EDTA and 1 μg ml−1 of prostacyclin (PGI2) (pH 6.5). Any remaining red blood cells were removed by a low centrifugation, and the platelet pellet was resuspended in HBSS–HEPES PGI2 and EDTA free buffer (pH 7.4) with Ca2+ (1.3 mmol l−1 CaCl2) and Mg2+ (0.81 mmol l−1 MgSO4) and adjusted to a physiological concentration of 250 × 106 ml−1 using an automated cell counter (Microdriff16, Beckman Coulter, Inc.). Platelet purity was ascertained by an electronic Coulter counter and by light microscopy examination. No detectable platelet activation was observed after cell preparation, as determined by the low level of P-selectin translocation at baseline and the normal response to thrombin activation.
Platelet aggregation
Optical platelet aggregation was monitored on a four-channel aggregometer (Chronolog) (Caron et al., 2002). In brief, 500 μl (final volume) of the isolated platelet suspension (250 × 106 ml−1) was preincubated at 37°C for 5 min in the presence of increasing concentrations of a specific platelet P-selectin antagonist (rPSGL-Ig, 10–100 μg ml−1, from Wyeth-Genetics Institute, Andover, MA, U.S.A.), a blocking monoclonal antibody (Mab) to P-selectin (P8G6 without sodium azide, 10–40 μg ml−1, from Santa Cruz Biotechnology, Santa Cruz, CA, U.S.A.), or their corresponding isotype-matched control IgG. Samples were then placed in the aggregometer (with a stirring speed of 1000 r.p.m.), and thrombin 0.025 U ml−1 was added. Platelet aggregation was recorded for 5 min, during which the percentage of aggregation was quantified at various time points (15, 30, 60, 120, 180, 240, and 300 s). In other experiments, the effects of a GPIIb–IIIa antagonist (Reopro, 100 nmol l−1, from Centocor, Horsham, PA, U.S.A.) or a phosphoinositide 3 kinase (Pi3K) inhibitor (LY294002, 100 μmol l−1, from Calbiochem, San Diego, CA, U.S.A.) on thrombin-induced platelet aggregation were evaluated in the presence or absence of rPSGL-Ig (100 μg ml−1).
P-selectin translocation and GPIIb–IIIa activation
P-selectin (CD62P) translocation to the platelet membrane from the α-granules and GPIIb–IIIa activation were monitored by flow cytometry (Théorêt et al., 2001; Caron et al., 2002). We used an anti-CD62P phycoerythrin (PE)-conjugated (clone AK6 from Cymbus, Chilworth Southampton, U.K.) and an anti-GPIIb–IIIa fluorescein isothiocyanate (FITC)-conjugated, designed to recognize the active form of GPIIb–IIIa (clone PAC-1, Becton Dickinson, Mississauga, ON, Canada), or their isotype-matched conjugated IgGs. Platelet suspensions (250 × 106 ml−1) were prepared and baseline samples were taken. They were then incubated with saline (as control) or the appropriate drugs for 10 min at room temperature. The kinetics of P-selectin translocation was measured at baseline and over 5 min following the addition of thrombin 0.025 U ml−1. At each time point measured (0, 15, 30, 60, 120, 180, 240, and 300 s), 50 μl of the suspension was taken and fixed in phosphate-buffered saline (PBS) (pH 7.4) containing 1% paraformaldehyde for 1 h at 4°C in the dark. After being washed in PBS–0.1% sodium azide, the cells were labelled with a saturating concentration of the anti-CD62P-PE Mab, or an isotype-matched control, for 30 min at room temperature in the dark. Then platelets were fixed in PBS–1% paraformaldehyde and analyzed by flow cytometry. To determine GPIIb–IIIa activation, the PAC-1 Mab was added in a saturating concentration directly to the platelet suspension before thrombin stimulation; otherwise PAC-1 binding to active GPIIb–IIIa is prevented by bound fibrinogen secreted by the platelets. Samples were then taken at the same time point and fixed for 1 h with PBS–1% paraformaldehyde at 4°C in the dark, before being analyzed by flow cytometry. In additional experiments, the saturation of P-selectin with increasing concentrations of rPSGL-Ig (10–100 μg ml−1) was determined using the Mab AK4 that competes with rPSGL-Ig for P-selectin binding.
Flow cytometry
All samples were analyzed within 6 h on an Altra flow cytometer (Beckman Coulter, Inc.) using single-color immunofluorescence staining with saturating concentrations of fluorescence dye-conjugated monoclonal antibodies. Platelets were identified and gated by their characteristic forward- and side-scatter properties. Antibody binding was determined as the percentage of positive platelets or the mean fluorescence intensity (MFI) over a fluorescence threshold gated over a platelet population stained with the proper isotype-matched control IgG (<2% of positive cells). In all, 20,000 platelets were analyzed from each sample.
Statistics
Results are presented as mean±s.e.m. Student's t-test or a paired t-test was used for dual comparisons. For multiple comparisons, repeated one-way analysis of variance (ANOVA) was used, followed by a Dunnett's test for comparison against control experiments. Values of P<0.05 were considered significant.
Results
Kinetics of thrombi-induced platelet aggregation and P-selectin translocation
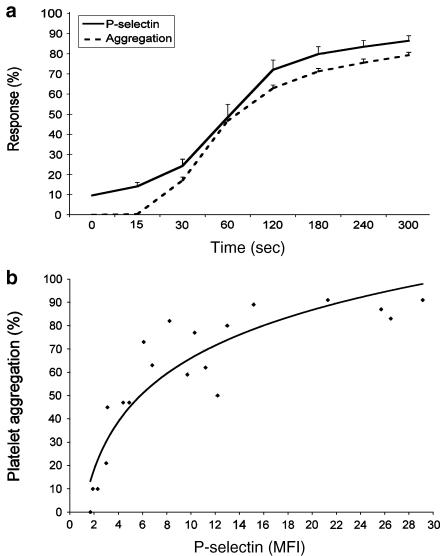
In order to assess the relationship between P-selectin and platelet aggregation following thrombin stimulation, we measured the time course of platelet aggregation and P-selectin translocation. As shown in Figure 1a, the percentage of platelets stained positive for P-selectin increased rapidly after platelet stimulation with thrombin, representing 48.4±6.5, 72.0±4.8, and 86.5±2.5% of positive platelets at 60, 120, and 300 s post-stimulation, respectively. Figure 1 also shows that the kinetic of platelet aggregation follows closely that of P-selectin translocation. This was supported by the correlation made between the MFI of P-selectin translocated to the platelet surface and the aggregation response (Figure 1b). These results point out the possible involvement of P-selectin in the process of platelet aggregation.
Figure 1.
(a) Kinetics of platelet aggregation (dashed line, n=15) and P-selectin redistribution (solid line, n=9) in response to thrombin 0.025 U ml−1. Results are expressed as the percentage of aggregation response or the percentage of positively stained platelets. The correlation between the aggregation response and the MFI of P-selectin on thrombin-stimulated platelets is presented in (b).
Effect of P-selectin antagonism on thrombin-induced platelet aggregation
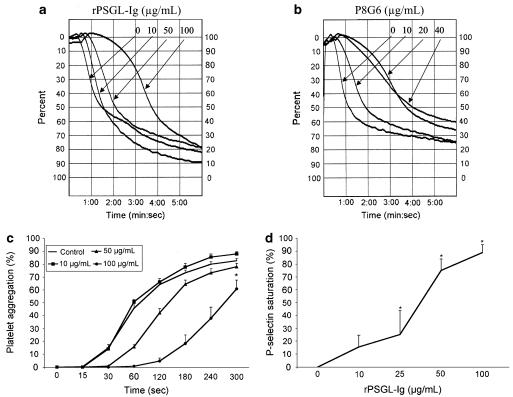
To determine if P-selectin is involved in platelet aggregation, we used rPSGL-Ig, a selective platelet P-selectin antagonist, or a blocking Mab against P-selectin (P8G6), and assessed their effects on the kinetics of platelet aggregation following thrombin stimulation. Representative aggregation responses are presented in Figure 2, which shows that rPSGL-Ig (10–100 μg ml−1, Figure 2a), or P8G6 (10–40 μg ml−1, Figure 2b), but not their isotype-matched IgG (data not shown), dose dependently delayed platelet aggregation. Figure 2c illustrates the mean responses of platelet aggregation obtained with increasing concentrations of rPSGL-Ig. rPSGL-Ig (100 μg ml−1) inhibited platelet aggregation almost completely (≈95% inhibition) in the first 60 s post-stimulation. There was then a slow return to near-normal aggregation levels (60% aggregation) at 300 s. Furthermore, Figure 2d shows that saturation of P-selectin on activated platelets, as assessed by flow cytometry, represents 16, 25, 75, and 90%, with 10, 25, 50, and 100 μg ml−1 rPSGL-Ig, respectively. Note that rPSGL-Ig has no significant effect on the percentage of the activated form of GPIIb–IIIa (PAC-1 labelling) on platelets, but has a minor effect on P-selectin translocation (Figure 3). These results indicate that P-selectin antagonism delays the aggregation process, and that P-selectin may participate with GPIIb–IIIa in the initiation of platelet aggregation.
Figure 2.
Representative traces illustrating the aggregation response of thrombin-stimulated platelets in the presence of increasing concentrations of rPSGL-Ig (a) or the blocking P-selectin Mab P8G6 (b). (c) The average effect of rPSGL-Ig on the kinetics of thrombin-induced platelet aggregation (n=6–9, *P<0.05, 100 μg ml−1 rPSGL-Ig vs control, repeated ANOVA and Dunnett's test). (d) The percentage of P-selectin saturation in the presence of increasing concentrations of rPSGL-Ig (10–100 μg ml−1) (n=3, *P<0.05 vs 0, ANOVA).
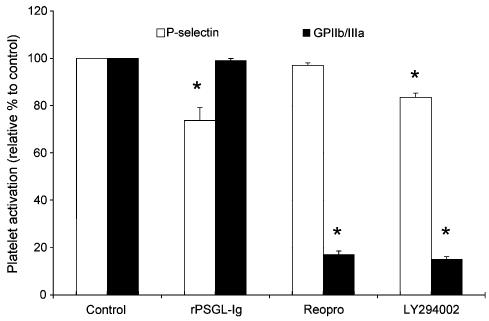
Figure 3.
Effect of a P-selectin antagonist (rPSGL-Ig, 100 μg ml−1), a GPIIb–IIIa antagonist (Reopro, 100 nM), and a Pi3K inhibitor (LY294002, 100 μM) on thrombin-induced platelet P-selectin translocation (n=3–7) and GPIIb–IIIa activation (n=4–7) (*P<0.05 vs control, paired t-test).
Effect of rPSGL-Ig in combination with GPIIb–IIIa inhibitors on platelet aggregation
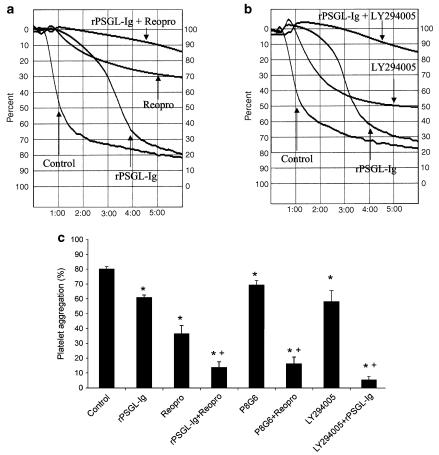
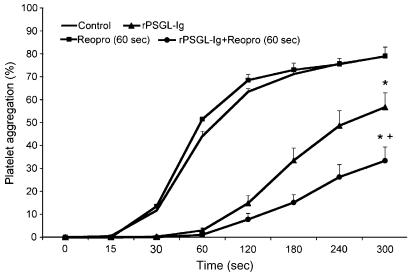
Based on the results showed in Figures 1 and 2, we performed another set of experiments, this time to determine the interplay between P-selectin and GPIIb–IIIa in the regulation of the aggregation process. We used Reopro, which blocks the binding of fibrinogen to GPIIb–IIIa, and a Pi3K inhibitor (LY294002), which prevents GPIIb–IIIa activation by 80% (P<0.05) without a major effect on the percentage of platelets that stained positive for P-selectin (≈15% inhibition) in thrombin-stimulated platelets (Figure 3). Therefore, inhibition of the Pi3K pathway or GPIIb–IIIa blockade in combination with P-selectin antagonism (rPSGL-Ig, or P8G6) allowed us to determine the relative importance of P-selectin and GPIIb–IIIa in the aggregation process. Representative traces of thrombin-induced platelet aggregation with and without rPSGL-Ig, Reopro, and LY294005 are presented in Figures 4a and b, and the mean data of the aggregation response are illustrated in Figure 4c. These figures show that LY294002 significantly reduced thrombin-induced platelet aggregation by about 25%, but its effect was greatly increased in the presence of rPSGL-Ig (94% inhibition) when compared to rPSGL-Ig alone (25% inhibition). Figure 4 also shows that the combination of rPSGL-Ig or the blocking P-selectin Mab (P8G6) with Reopro, co-incubated with platelets before the addition of thrombin, potentiates the effect of each antagonist alone, and significantly reduces platelet aggregation by 84%. In other set of experiments, we tested the effect of adding Reopro 60 s after the onset of the aggregation process (Figure 5). Reopro alone was without any significant effect when added 60 s after the onset of the aggregation process in the absence of rPSGL-Ig. However, the addition of Reopro at 60 s after the onset of aggregation, in the presence of rPSGL-Ig, reversed the second phase of platelet aggregation (60% inhibition) that occurred with rPSGL-Ig alone (25% inhibition). Of note, the addition of rPSGL-Ig at 60 s after the onset of the aggregation was without any significant effect on the aggregation process (data not shown).
Figure 4.
Representative traces of thrombin-induced platelet aggregation with and without rPSGL-Ig in the presence or absence of Reopro (a), or LY294002 (b). (c) Mean data of the aggregation response in the presence of the different antagonists of P-selectin and GPIIb–IIIa (n=5–6, *P<0.05 vs control, +P<0.05 vs treatment alone, repeated ANOVA and Dunnett's test).
Figure 5.
Platelet aggregation with Reopro added 60 s after the onset of the aggregation process. Thrombin 0.025 U ml−1 (control, straight, n=5), rPSGL-Ig (triangle, n=5), Reopro alone added at 60 s (square, n=3), and rPSGL-Ig with Reopro added at 60 s (circle, n=5) (*P<0.05 vs control and Reopro alone, +P<0.05 vs rPSGL-Ig, Student's t-test, ANOVA).
Discussion
The major findings of the present study are that platelet P-selectin participates with GPIIb–IIIa in the initiation of platelet aggregation. P-selectin antagonism using rPSGL-Ig delays the aggregation process, which is more efficiently inhibited with dual antagonism of P-selectin and GPIIb–IIIa.
In thrombin-stimulated platelets, P-selectin translocation paralleled closely the extent of platelet aggregation, and its inhibition delayed the aggregation process. We believe that the initiation of platelet aggregation is influenced by P-selectin, because the early phase of the aggregation process was abolished by P-selectin antagonism using rPSGL-Ig or a P-selectin-blocking Mab. The effect of rPSGL-Ig was not related to any effects on GPIIb–IIIa activation or P-selectin translocation. Its effect is rather related to its capacity to selectively bind to translocated P-selectin in thrombin-activated platelets and to block its adhesive function. Indeed, we have previously shown (Théorêt et al., 2001) that the binding of rPSGL-Ig to P-selectin on activated platelets correlates with the degree of inhibition of platelet adhesion to neutrophils. Indeed, a high degree of P-selectin saturation (>75%) was required to significantly delay platelet aggregation, suggesting that only a small amount of surface-translocated P-selectin is sufficient to sustain normal platelet aggregation. In this regard, it has been reported that a high degree of platelet GPIIb–IIIa saturation (∼80%) was needed to efficiently prevent thrombosis (Tcheng et al., 1994).
To further support our findings, we demonstrated that the second phase of platelet aggregation seen with rPSGL-Ig treatment alone could be abolished by Reopro, a GPIIb–IIIa antagonist. Reopro blocks GPIIb–IIIa and its binding to fibrinogen during platelet aggregation, without impairing P-selectin translocation. In addition, we used the characteristic of the Pi3K signaling pathway in platelets to reveal that P-selectin is required to induce optimal platelet aggregation. In this regard, GPIIb–IIIa activation was strongly inhibited by LY294002, a Pi3K inhibitor, without a major effect on the percentage of platelets stained positive for P-selectin. Pi3K inhibition was associated with a minor reduction of the initial phase of platelet aggregation, but a full inhibitory response was obtained in conjunction with rPSGL-Ig, as observed previously with Reopro. These findings highlight the importance of P-selectin, in addition to GPIIb–IIIa, in the process of platelet aggregate formation.
P-selectin is a glycoprotein (GP) that contains a lectin domain in its NH2 extremity responsible for ligand recognition. The possible involvement of lectin activity in platelet aggregation, first described by Gartner et al. (1978), showed that thrombin-induced platelet aggregation was inhibited by different types of glycosides. More recently, it has been shown that platelets can roll on activated endothelium expressing P-selectin via the GPIb–IX complex (Romo et al., 1999). In addition, it has been demonstrated that activated platelets can express PSGL-1, the high-affinity ligand for P-selectin (Frenette et al., 2000). Taken together, these findings may well indicate that activated platelets could interact together through P-selectin and PSGL-1, or with nonactivated platelets via P-selectin and GPIb–IX. Accordingly, four candidates may act as ligands for platelet P-selectin: GPIb–IX–V (Romo et al., 1999), PSGL-1 (Frenette et al., 2000), membrane-anchored sulfatides (Merten & Thiagarajan, 2001; Merten et al., 2005), and a novel 28 kDa membranous GP (Li et al., 2002). However, none of these possible ligands have obtained total agreement as to their implication in platelet aggregation. In a previous study (Merten & Thiagarajan, 2000), it was demonstrated that P-selectin stabilizes the initial GPIIb–IIIa–fibrinogen interaction, allowing the formation of large stable platelet aggregates. This mechanism appears to be PSGL-1 and GPIb independent and could possibly occur via an interaction with sulfatides (Merten & Thiagarajan, 2001; Merten et al., 2005). In addition to the stabilizing role of P-selectin in platelet aggregation, our results reveal that P-selectin is involved, as well, in the initiation of platelet aggregation. Indeed, P-selectin is translocated within seconds to the surface of thrombin-activated platelets and may establish the initial contact between activated platelets, thus facilitating the subsequent engagement of activated GPIIb–IIIa and fibrinogen, leading to a full aggregation response, as reported by Merten & Thiagarajan (2000). However, their study on platelet aggregation was performed in PRP and induced with ADP, which is a less potent platelet-degranulating agent than thrombin on washed platelets, as used in the present study. Despite these differences in the experimental conditions, both results highlight an interplay mechanism between P-selectin and GPIIb–IIIa in the regulation of the aggregation process.
Currently, GPIIb–IIIa antagonists are widely used in percutaneous coronary intervention and have been shown to be effective in reducing ischemic events and mortality. Paradoxically, in acute coronary syndromes, the outcome of some GPIIb–IIIa antagonists has been associated with increased ischemic events, mortality, and bleeding problems (Second Symphony Investigators, 2001). In addition, it is still unclear whether GPIIb–IIIa antagonists can elicit intracellular signalling and therefore increase platelet activation (Peter et al., 1998). Therefore, the development of adjunctive treatment aimed at reducing the dosage of GPIIb–IIIa antagonists may constitute a promising avenue in the treatment of occlusive thrombus formation. In this connection, we have already shown that P-selectin could act with GPIIb–IIIa during the aggregation process (Caron et al., 2002). In the present study, the extent of platelet aggregation was gradually delayed by increasing concentrations of P-selectin antagonism. As well, the addition of Reopro to rPSGL-Ig, either before platelet stimulation or 60 s after the onset of aggregation, has been proven to be superior to Reopro or rPSGL-Ig alone in inhibiting platelet aggregation. In our study, Reopro could not impair irreversible platelet aggregation when added 60 s post-thrombin stimulation, unless P-selectin was inhibited. In addition, rPSGL-Ig was unable to destabilize aggregates and prevent irreversible platelet aggregation when added 60 s after the onset of aggregation. This supports the notion that P-selectin may initiate platelet aggregation, whereas GPIIb–IIIa is needed for irreversible aggregation. Taken together, these findings may have an important clinical implication in the treatment of patients undergoing percutaneous coronary intervention. The association of an anti-GPIIb–IIIa treatment with an anti-P-selectin may contribute to reduce the dose of GPIIb–IIIa antagonist needed to inhibit platelet aggregation; and to decrease platelet–leukocyte adhesion, that has been associated with the pathophysiology of acute coronary syndromes (Mickelson et al., 1996).
In conclusion, this study demonstrates that platelet P-selectin participates with GPIIb–IIIa in the initiation of platelet aggregation. Indeed, P-selectin antagonism with rPSGL-Ig delays the aggregation process, and the inhibition of platelet aggregation is best achieved with dual antagonism of GPIIb–IIIa and P-selectin. This may represent a new therapeutic approach in the management of thrombotic disorders.
Acknowledgments
This study was supported by the Canadian Institutes of Health Research and the Heart and Stroke Foundation of Quebec. We thank Dr Anjali Kumar (currently at Critical Therapeutic Inc.) and Dr Robert Schaub from Wyeth-Genetics Institute for providing rPSGL-Ig.
Abbreviations
- ADP
adenosine diphosphate
- ANOVA
analysis of variance
- FITC
fluorescein isothiocyanate
- GP
glycoprotein
- HBSS
Hank's balanced salt solution
- Mab
monoclonal antibody
- MFI
mean fluorescence intensity
- PARs
proteinase-activated receptors
- PBS
phosphate-buffered saline
- PE
phycoerythrin
- PGI2
prostacyclin
- Pi3K
phosphoinositide-3 kinase
- PRP
platelet-rich plasma
- PSGL-1
P-selectin glycoprotein ligand-1
- TRAP
thrombin receptor activating peptide
- TXA2
thromboxane A2
References
- BIENVENU J.G., TANGUAY J.F., THÉORÊT J.F., KUMAR A., SCHAUB R.G., MERHI Y. Recombinant soluble P-selectin glycoprotein ligand-1-Ig reduces restenosis through inhibition of platelet-neutrophil adhesion after double angioplasty in swine. Circulation. 2001;103:1128–1134. doi: 10.1161/01.cir.103.8.1128. [DOI] [PubMed] [Google Scholar]
- CARON A., THÉORÊT J.F., MOUSA S.A., MERHI Y. Anti-platelet effects of GPIIb-IIIa and P-selectin antagonism, platelet activation, and binding to neutrophils. J. Cardiovasc. Pharmacol. 2002;40:296–306. doi: 10.1097/00005344-200208000-00015. [DOI] [PubMed] [Google Scholar]
- FARADAY N., SCHARPF R.B., DODD-O J.M., MARTINEZ E.A., ROSENFELD B.A., DORMAN T. Leukocytes can enhance platelet-mediated aggregation and thromboxane release via interaction of P-selectin glycoprotein ligand 1 with P-selectin. Anesthesiology. 2001;94:145–151. doi: 10.1097/00000542-200101000-00025. [DOI] [PubMed] [Google Scholar]
- FRENETTE P.S., DENIS C.V., WEISS L., JURK K., SUBBARAO S., KEHREL B., HARTWIG J.H., VESTWEBER D., WAGNER D.D. P-selectin glycoprotein ligand 1 (PSGL-1) is expressed on platelets and can mediate platelet–endothelial interactions in vivo. J. Exp. Med. 2000;191:1413–1422. doi: 10.1084/jem.191.8.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARTNER T.K., WILLIAMS D.C., MINION F.C., PHILLIPS D.R. Thrombin-induced platelet aggregation is mediated by a platelet plasma membrane-bound lectin. Science. 1978;200:1281–1283. doi: 10.1126/science.663608. [DOI] [PubMed] [Google Scholar]
- JANG Y., LINCOFF A.M., PLOW E.F., TOPOL E.J. Cell adhesion molecules in coronary artery disease. J. Am. Coll. Cardiol. 1994;24:1591–1601. doi: 10.1016/0735-1097(94)90162-7. [DOI] [PubMed] [Google Scholar]
- KUMAR A., VILLANI M.P., PATEL U.K., KEITH J.C., JR, SCHAUB R.G. Recombinant soluble form of PSGL-1 accelerates thrombolysis and prevents reocclusion in a porcine model. Circulation. 1999;99:1363–1369. doi: 10.1161/01.cir.99.10.1363. [DOI] [PubMed] [Google Scholar]
- LI L., QIAN K.X., GENG J.G. A 28-kDa glycoprotein functions as a platelet ligand for P-selectin (CD62P) Thromb. Haemost. 2002;87:706–711. [PubMed] [Google Scholar]
- MACFARLANE S.R., SEATTER M.J., KANKE T., HUNTER G.D., PLEVIN R. Proteinase-activated receptors. Pharmacol. Rev. 2001;53:245–282. [PubMed] [Google Scholar]
- MARCUS A.J. Thrombosis and inflammation as multicellular processes: significance of cell–cell interactions. Semin. Hematol. 1994;31:261–269. [PubMed] [Google Scholar]
- MARMUR J.D., CAVUSOGLU E. The use of the glycoprotein IIb/IIIa receptor antagonists during percutaneous coronary intervention. J. Interv. Cardiol. 2002;15:71–84. doi: 10.1111/j.1540-8183.2002.tb01036.x. [DOI] [PubMed] [Google Scholar]
- MCEVER R.P., CUMMINGS R.D. Role of PSGL-1 binding to selectin in leukocyte recruitment. J. Clin. Invest. 1997;100 (Suppl 11):S97–S103. [PubMed] [Google Scholar]
- MERTEN M., BEYTHIEN C., GUTENSOHN K., KUHNL P., MEINERTZ T., THIAGARAJAN P. Sulfatides activate platelets through P-selectin and enhances platelet and platelet-leukocyte aggregation. Arterioscler. Thromb. Vasc. Biol. 2005;25:258–263. doi: 10.1161/01.ATV.0000149675.83552.83. [DOI] [PubMed] [Google Scholar]
- MERTEN M., CHOW T., HELLUMS J.D., THIAGARAJAN P. A new role for P-selectin in shear-induced platelet aggregation. Circulation. 2000;102:2045–2050. doi: 10.1161/01.cir.102.17.2045. [DOI] [PubMed] [Google Scholar]
- MERTEN M., THIAGARAJAN P. P-selectin expression on platelet determines size and stability of platelet aggregates. Circulation. 2000;102:1931–1936. doi: 10.1161/01.cir.102.16.1931. [DOI] [PubMed] [Google Scholar]
- MERTEN M., THIAGARAJAN P. Role for sulfatides in platelet aggregation. Circulation. 2001;104:2955–2960. doi: 10.1161/hc4901.100383. [DOI] [PubMed] [Google Scholar]
- MICKELSON J.K., LAKKIS N.M., VILLARREAL-LEVY G., HUGHES B.J., SMITH C.W. Leukocyte activation with platelet adhesion after coronary angioplasty: a mechanism for recurrent disease. J. Am. Coll. Cardiol. 1996;28:345–353. doi: 10.1016/0735-1097(96)00164-7. [DOI] [PubMed] [Google Scholar]
- MYERS D.D., JR, SCHAUB R., WROBLESKI S.K., LONDY F.J., III, FEX B.A., CHAPMAN A.M., GREENFIELD L.J., WAKEFIELD T.W. P-selectin antagonism causes dose-dependent venous thrombosis inhibition. Thromb. Haemost. 2001;85:423–429. [PubMed] [Google Scholar]
- NASH G.B. Adhesion between neutrophils and platelets: a modulator of thrombotic and inflammatory events. Thromb. Res. 1994;74 (Suppl 1):S3–S11. doi: 10.1016/s0049-3848(10)80002-7. [DOI] [PubMed] [Google Scholar]
- NEUMANN F.J., OTT I., GAWAZ M., PUCHNER G., SCHOMIG A. Neutrophil and platelet activation at balloon-injured coronary artery plaque in patients undergoing angioplasty. J. Am. Coll. Cardiol. 1996;27:819–824. doi: 10.1016/0735-1097(95)00563-3. [DOI] [PubMed] [Google Scholar]
- OTT I., NEUMANN F.J., GAWAZ M., SCHMITT M., SCHOMIG A. Increased neutrophil–platelet adhesion in patients with unstable angina. Circulation. 1996;94:1239–1246. doi: 10.1161/01.cir.94.6.1239. [DOI] [PubMed] [Google Scholar]
- PETER K., SCHWARZ M., YLANNE J., KOHLER B., MOSER M., NORDT T., SALBACH P., KUBLER W., BODE C. Induction of fibrinogen binding and platelet aggregation as a potential intrinsic property of various glycoprotein IIb/IIIa (αIIbβ3) inhibitors. Blood. 1998;92:3240–3249. [PubMed] [Google Scholar]
- ROMO G.M., DONG J.F., SCHADE A.J., GARDINER E.E., KANSAS G.S., LI C.Q., MCINTIRE L.V., BERNDT M.C., LOPEZ J.A. The glycoprotein Ib–IX–V complex is a platelet counterreceptor for P-selectin. J. Exp. Med. 1999;190:803–814. doi: 10.1084/jem.190.6.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Second Symphony Investigators Randomized trial of aspirin, sibrafiban or both for secondary prevention after acute coronary syndromes. Circulation. 2001;103:1727–1733. doi: 10.1161/01.cir.103.13.1727. [DOI] [PubMed] [Google Scholar]
- SERRANO C.V., JR, RAMIRES J.A., VENTURINELLI M., ARIE S., D'AMICO E., ZWEIR J.L., PILEGGI F., DA LUZ P.L. Coronary angioplasty results in leukocyte and platelet activation with adhesion molecule expression. Evidence of inflammatory responses in coronary angioplasty. J. Am. Coll. Cardiol. 1997;29:1276–1283. doi: 10.1016/s0735-1097(97)00070-3. [DOI] [PubMed] [Google Scholar]
- TANGUAY J.F., GEOFFROY P., SIROIS M.G., LIBERSAN D., KUMAR A., SCHAUB R.G., MERHI Y. Prevention of in-stent restenosis via reduction of thrombo-inflammatory reactions with recombinant P-selectin glycoprotein ligand-1. Thromb. Haemost. 2004;91:1186–1193. doi: 10.1160/TH03-11-0701. [DOI] [PubMed] [Google Scholar]
- TCHENG J.E., ELLIS S.G., GEORGE B.S., KEREIAKES D.J., KLEIMAN N.S., TALLEY J.D., WANG A.L., WEISMAN H.F., CALIFF R.M., TOPOL E.J. Pharmacodynamics of chimeric glycoprotein IIb/IIIa integrin antiplatelet antibody Fab 7E3 in high-risk coronary angioplasty. Circulation. 1994;90:1757–1764. doi: 10.1161/01.cir.90.4.1757. [DOI] [PubMed] [Google Scholar]
- THÉORÊT J.F., BIENVENU J.G., KUMAR A., MERHI Y. P-selectin antagonism with recombinant P-selectin glycoprotein ligand-1 (rPSGL-Ig) inhibits circulating activated platelet binding to neutrophils induced by damaged arterial surfaces. J. Pharmacol. Exp. Ther. 2001;298:658–664. [PubMed] [Google Scholar]
- WANG K., ZHOU X., ZHOU Z., TARAKJI K., QIN J.X., SITGES M., SHIOTA T., FORUDI F., SCHAUB R.G., KUMAR A., PENN M.S., TOPOL E.J., LINCOFF A.M. Recombinant soluble P-selectin glycoprotein ligand-Ig (rPSGL-Ig) attenuates infarct size and myeloperoxidase activity in a canine model of ischemia–reperfusion. Thromb. Haemost. 2002;88:149–154. [PubMed] [Google Scholar]
- WANG K., ZHOU Z., ZHOU X., TARAKJI K., TOPOL E.J., LINCOFF A.M. Prevention of intimal hyperplasia with recombinant soluble P-selectin glycoprotein ligand-immunoglobulin in the porcine coronary artery balloon injury model. J. Am. Coll. Cardiol. 2001;38:577–582. doi: 10.1016/s0735-1097(01)01347-x. [DOI] [PubMed] [Google Scholar]
- YANG J., FURIE B.C., FURIE B. The biology of P-selectin glycoprotein ligand-1: its role as a selectin counterreceptor in leukocyte–endothelial and leukocyte platelet interaction. Thromb. Haemost. 1999;81:1–7. [PubMed] [Google Scholar]