Abstract
Tumor necrosis factor (TNF)-α is known to induce the expression of CCL11 and CCR3 via the activation of NF-κB. CCL11 (eotaxin), the C–C chemokine, is a potent chemoattractant for eosinophils and Th2 lymphocytes, and CCR3 is the receptor for CCL11.
In order to determine the effects of rosmarinic acid on the TNF-α-induced upregulation of CCL11 and CCR3 in human dermal fibroblasts, we performed an enzyme-linked immunosorbent assay for CCL11 and a Western blot assay for CCR3. The TNF-α-induced expression of CCL11 and CCR3 genes was attenuated by rosmarinic acid.
In our NF-κB luciferase reporter system, TNF-α-induced NF-κB activation was observed to be reduced by rosmarinic acid. In accordance with this result, rosmarinic acid also inhibited TNF-α-induced phosphorylation and degradation of IκB-α, as well as nuclear translocation of NF-κB heterodimer induced by TNF-α. This suggests that rosmarinic acid downregulates the expression of CCL11 and CCR3 via the inhibition of NF-κB activation signaling.
Using the NF-κB luciferase reporter system, Western blot analysis, and IKK-β activity assay, we determined that rosmarinic acid inhibits IKK-β activity in NF-κB signaling, which upregulates the expression of CCL11 and CCR3. Additionally, TNF-α-induced secretion of soluble intercellular adhesion molecule-1 and soluble vascular cell adhesion molecule-1 molecules was found to be attenuated by rosmarinic acid.
Our results show that rosmarinic acid inhibits the expression of CCL11 and CCR3 by suppressing the IKK-β activity in NF-κB activation signaling. Further, these results suggest that rosmarinic acid might inhibit the expression of NF-κB promoter-related genes.
Keywords: CCL11, IKK-β, nuclear factor-κB, IκB-α, TNF-α
Introduction
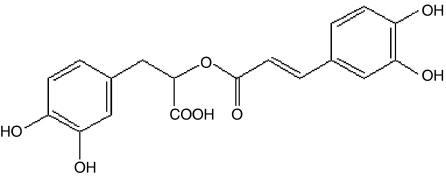
α-o-Caffeoyl-3,4-dihydroxyphenyl lactic acid (rosmarinic acid; Figure 1) is a naturally occurring hydroxylated compound. It is widely distributed in the Labitae herbs, which include rosemary, sweet basil, and perilla (Osakabe et al., 2002; del Bano et al., 2003; Kintzios et al., 2003). Rosmarinic acid has broad range of applications, from food preservatives to cosmetics; it also has medicinal use, by virtue of its antimicrobial and antioxidant activities (Van Kessel & Kalter, 1986; Al-Sereiti et al., 1999; Zheng & Wang, 2001). In addition, it has been reported that rosmarinic acid has the ability to block complement fixation and inhibit lipoxygenase and cyclooxygenase activity (Kimura et al., 1987; Sahu et al., 1999; Kelm et al., 2000).
Figure 1.
Structure of rosmarinic acid (α-o-caffeoyl-3,4-dihydroxyphenyl lactic acid).
The chemokines are a large family of small proteins involved in the activation and recruitment of specific cell populations during the course of disease (Lukacs et al., 1999). CCL11, a CC chemokine, is a potent chemoattractant and an activator of eosinophils (Ponath et al., 1996), basophils (Uguccioni et al., 1997; Yamada et al., 1997), and Th2 lymphocytes (Gerber et al., 1997; Sallusto et al., 1997). CCL11 expression was found to be restricted to a few cell types, including eosinophils, bronchial epithelial cells, and dermal fibroblast cells (Gutierrez-Ramos et al., 1999; Huber et al., 2000). In asthmatics, the expression of CCL11 has been found to be enhanced in these types of cells, and increased expression is associated with disease severity (Mattoli et al., 1997; Ying et al., 1997). Additionally, CCL11 expression in epithelial cells was found to be increased in atopic dermatitis (Yawalkar et al., 1999), as well as in other inflammatory conditions (Garcia-Zepeda et al., 1996). The expression of CCL11 is induced by two potent activators, interleukin-4, which is produced by Th2 cells, mast cells, and basophils, and TNF-α, which is produced by monocytes and macrophages (Atasoy et al., 2003). In contrast to most other eosinophil chemoattractants of the CC-chemokine family that generally act on several receptors, CCL11 only signals through one specific chemokine receptor, namely the G protein-coupled receptor, CCR3 (Baggiolini et al., 1997). CCR3 is prominently expressed on eosinophils, basophils, Th2-type lymphocytes, and fibroblasts (Ying et al., 1997; Huber et al., 2000). Very little is known regarding how CCR3 is regulated at the transcriptional level, although a recent report demonstrated that CCR3 gene expression is induced by TNF-α (Huber et al., 2002).
Recently, the inhibition of T-cell receptor (TCR)-mediated signaling has become viewed as one of the many effects of anti-inflammatory drugs. Despite their different points of action, anti-inflammatory drugs such as tacrolimus, pimecrolimus (Paccani et al., 2002), and hydroxychloroquine (Goldman et al., 2000) have been found to inhibit TCR-induced signaling events, including Ca2+ mobilization, the activation of p38 mitogen-activated protein kinase, and the expression of the CD40 ligand. It was recently determined that rosmarinic acid inhibits the Ca2+-dependent pathways of TCR-mediated signaling by inhibiting PLC-γ1 and Itk activities (Kang et al., 2003). Many reports regarding the anti-inflammatory and immunomodulatory effects of rosmarinic acid led us to study the possibility that rosmarinic acid may exert its inhibitory effect on the expression of CCL11 and CCR3. To this end, using human dermal fibroblast cells, we attempted to determine the effects and mechanisms of action of rosmarinic acid on the TNF-α-induced expression of CCL11 and CCR3. In this report, we demonstrated that rosmarinic acid inhibits the IKK-β downstream signaling pathway in the TNF-α-induced upregulation of CCL11 and CCR3 in human dermal fibroblasts.
Methods
Cell cultures
NIH3T3 mouse fibroblast cells and human dermal fibroblast cells (derived from neonatal foreskin) were obtained from the Amore-Pacific Corporation R&D Center in the Republic of Korea, and were cultured in Dulbecco's-modified Eagle's medium (DMEM) (Hyclone, UT, U.S.A.) containing 10% fetal bovine serum (Invitrogen, CA, U.S.A.), and penicillin–streptomycin at 37°C in a humidified 95% air/5% CO2 atmosphere.
Cytotoxicity assay
Human dermal fibroblast cells were cultured in DMEM (Hyclone) containing 10% fetal bovine serum, and penicillin–streptomycin at 37°C in a humidified 95% air/5% CO2 atmosphere. Cells were seeded on 96-well plates and drug treatment was initiated 24 h after seeding. The general viability of cultured cells was determined by the reduction of WST-8 (2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium, monosodium salt) (Dojindo Laboratories, Japan) to a highly water-soluble formazan dye. This assay was performed after the incubation of human dermal fibroblast cells in the presence or absence of TNF-α, along with indicated concentrations of rosmarinic acid, for 14 h at 37°C in a 5% CO2 atmosphere. To each well, 10 μl of WST-8 solution was added. Cells were then incubated at 37°C for 3 h and the absorbance was measured at 450 nm using a spectrophotometer (Power Wave, Bio-tek Inc., VT, U.S.A.). Data are presented as means±s.d. All values were significant (*P<0.05) compared with values for control. The entire experiment was performed in triplicate and results were confirmed by three independent experiments.
Western blot analysis
Human dermal fibroblast cell lysates were separated by sodium dodecyl sulfate (SDS)–PAGE (16% acrylamide gel) and transferred to Hybond-C membranes. The blots were probed with phospho-IκB-α (1 : 1000), IκB-α (1 : 1000), IKK-β (1 : 1000), NF-κB p65 (1 : 1000), Histone H2B (1 : 1000), Flag (1 : 1000), CCR3 (1 : 500), and β-actin (1 : 1000) antibodies, and the proteins were visualized by the Amersham ECL system and quantified using a densitometer. All results were confirmed by three independent experiments.
Detection of IκB-α phosphorylation and degradation
IκB-α and phosphorylated IκB-α were identified by Western blot analysis using anti-IκB-α or phospho IκB-α antibody, respectively. Human dermal fibroblast cells were incubated in the presence or absence of TNF-α (40 ng ml−1), along with indicated concentrations of rosmarinic acid, for 30 min. Alternatively, human dermal fibroblast cells were transfected with TRAF2, MEKK3, or IKK-β, and were then incubated for 24 h. At that time, the human fibroblast cells were treated with rosmarinic acid for 14 h. The cultured human dermal fibroblast cells were washed with ice-cold phosphate-buffered saline (pH 7.4), and 0.2 ml of lysis buffer (10 mM Tris-HCl (pH 7.4) containing 150 mM NaCl, 2 mM EGTA, 2 mM dithiothreitol, 1 mM sodium orthovanadate, 1 mM phenylmethylsulfhonyl fluoride, 10 μg ml−1 leupeptin, 10 μg ml−1 aprotinin) was then added. The human dermal fibroblast cells were then harvested. After 30 min of centrifugation at 16,000 × g at 4°C, the cell lysates were denatured in boiling water for 5 min. Samples of the supernatant were subjected to SDS–PAGE using 16% polyacrylamide gel. After electrophoresis, the proteins were transferred to Hybond-C membrane at 4°C. In order to detect phosphorylated IκB-α, the sheets were immersed in blocking buffer containing 5% skim milk, 20 mM Tris-buffered saline (pH 7.4), and 0.1% Tween-20 for 3 h at 25°C. The phosphorylation and degradation of IκB-α were detected by protein immunoblotting; this process used a 1 : 1000 dilution of mouse monoclonal antibody (phospho-IκB-α (Ser32/36)) or mouse monoclonal antibody (IκB-α (112B2)). Blots were developed via enhanced chemiluminescence following incubation with HRP-conjugated secondary anti-mouse IgG monoclonal antibody (1 : 2000 dilution) for 1 h at 25°C, according to the manufacturer's instructions. All results were confirmed by three independent experiments.
Subcellular fractionation
The preparation of membrane, cytosolic, and nuclear fractions were performed as described previously (Kabouridis et al., 1997). In brief, 1 × 107 human dermal fibroblast cells were incubated in the presence or absence of TNF-α (40 ng ml−1), along with indicated concentrations of rosmarinic acid for 30 min. The cells were then resuspended in 0.5 ml hypotonic solution (25 mM Tris pH 7.5, 5 mM EGTA, 250 mM sucrose, 25 μg ml−1 aprotinin, 1 mM PMSF, 25 μg ml−1 leupeptin, 5 mM NaF, and 1 mM Na3VO4), and subjected to two successive freeze-thaw cycles. The cell suspension was homogenized on ice using Dounce homogenizer (40 strokes), and the salt concentration was adjusted to 150 mM NaCl. Nuclei were removed by two successive centrifugations at 480 × g for 5 min at 4°C. Soluble and particulate fractions were separated by centrifugation at 100,000 × g for 30 min. Fractionated proteins were resolved by SDS–PAGE and Western blotted with their corresponding antibodies. All results were confirmed by three independent experiments.
Luciferase reporter assay
To assay for NF-κB promoter activity, human dermal fibroblast cells were transfected with NF-κB-Luc reporter, or with the indicated genes, including TRAF2, MEKK3, and IKK-β, along with the Renilla luciferase expression vector, driven by the thymidine kinase promoter (Promega, WI, U.S.A.) using Superfect™ reagent (Invitrogen, CA, U.S.A.). After 24 h of incubation, the cells were incubated in the presence or absence of TNF-α (40 ng ml−1), along with indicated concentrations of rosmarinic acid for 14 h. The cells were then harvested and lysed. Supernatants were assayed for luciferase activity. Luciferase activity was determined with a Dual Luciferase Assay system (Promega, WI, U.S.A.) and a LB953 luminometer (Berthold, Germany), and was expressed as a ratio of the NF-κB-dependent firefly luciferase activity divided by the control thymidine kinase Renilla luciferase activity (% control). Results were confirmed by three independent transfections. Data are expressed as the means±s.e.m. *P<0.05, compared with untreated controls. °P<0.05 versus TNF-α (40 ng ml−1) only or transfected controls.
CCL11, soluble vascular cell adhesion molecule-1, and soluble intercellular adhesion molecule-1 assay
The concentrations of CCL11 and soluble vascular cell adhesion molecule-1 (sVCAM-1) in the culture medium were measured with a sandwich immunoassay kit, used according to the manufacture's instructions (R&D Systems). The concentration of soluble intercellular adhesion molecule-1 (sICAM-1) was measured by ELISA using a commercially available kit designed for the quantitative measurement of human sICAM-1 (Endogen, MA, U.S.A.). The standard curve was linearized and subjected to regression analysis. The CCL11 concentration of the unknown samples was extracted using the standard curve. The results were expressed as pg ml−1 of culture medium. Results were confirmed by three independent experiments. Data are expressed as the means±s.e.m. *P<0.05, compared with the untreated control. °P<0.05 versus TNF-α (40 ng ml−1) only.
IκB kinase-β activity assay
A kinase assay based on phosphorylation of substrate was used to investigate IKK-β activity. IKK-β was precipitated from whole-cell extracts with antibodies against IKK-β, and was then treated with 20 μl protein A/G-Sepharose (Pierce, Rockford, IL, U.S.A.). After 2 h, the beads were washed with lysis buffer and assayed in a kinase assay mixture containing 50 μM HEPES (pH 7.4), 20 μM MgCl2, 2 μM DTT, 20 μCi [γ-32P]adenosine triphosphate (ATP), 10 μM unlabeled ATP, and 2 μg substrate glutathione-S-transferase (GST)-IκB-α (i.e., residues 1–54 of IκB-α conjugated with GST). After incubation at 30°C for 30 min, the reaction was terminated by boiling with 5 μl 5 × SDS sample buffer for 5 min. Finally, the protein was resolved on a 10% polyacrylamide gel under reducing conditions, the gel was dried, and the radioactive bands were visualized using a Phosphorimager (Alpha Innotech Corp., San Leandro, CA, U.S.A.). To determine the total amounts of IKK-β in each sample, 30 μg of the whole-cell extract protein was resolved on a 7.5% acrylamide gel and electrotransferred to a nitrocellulose membrane. The membrane was blocked with 5% nonfat milk protein for 1 h and was then incubated with anti-IKK-β for 1 h. The membrane was washed and treated with horseradish peroxidase-conjugated secondary anti-mouse antibody, and the proteins were detected by chemiluminescence analysis (Amersham Biosciences, Bucks, U.K.).
Materials
Polyclonal goat antisera to human CCR3 and rosmarinic acid were obtained from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, U.S.A.) and A.G. Scientific Inc. (CA, U.S.A.) respectively. Antisera to phospho-IκB-α, IκB-α, IKK-β, histone H2B, and NF-κB p65 were purchased from Cell Signaling Technology Inc. (Beverly, MA, U.S.A.). Antibodies against β-actin and Flag M1, TNF-α, pyrollidone dithiocarbamate (PDTC), sodium salicylate (salicylic acid, sodium salt) were obtained from the Sigma Chemical Co. (MI, U.S.A.) Protease inhibitor cocktail was purchased from Roche (Indianapolis, IN, U.S.A.). The chemiluminescence kit was purchased from Amersham Pharmacia Biotech (Buckinghamshire, England). NF-κB-Luc reporter plasmids were purchased from Stratagene. Flag-tagged TRAF2 (Flag-TRAF2) and MEKK3 (Flag-MEKK3) expression vectors were constructed by cloning of a fragment which contained the first methionine codon of TRAF2, MEKK3, into the blunted XhoI site of the expression vector, pcDNA3.1. Flag-tagged, constitutively active IKK-β (S177E, S181E) expression vector was constructed by cloning of a fragment which had been made through site-directed mutagenesis into the blunted XhoI site of the expression vector, pcDNA3.1. Flag-tagged TRAF2, Flag-tagged MEKK3, and constitutive active IKK-β were a gift from Dr Won (Mogam Biotech Res Inst., Republic of Korea).
Statistical evaluation
Results are shown as means±s.e.m. and statistical analysis of the results was performed by Student's t-test for independent samples. Values of P<0.05 were considered to show significant differences between means. From concentration–response data, as in Figures 2a, 3a and b, 9a and b, IC50 values were calculated after regression analysis (DataFit programme).
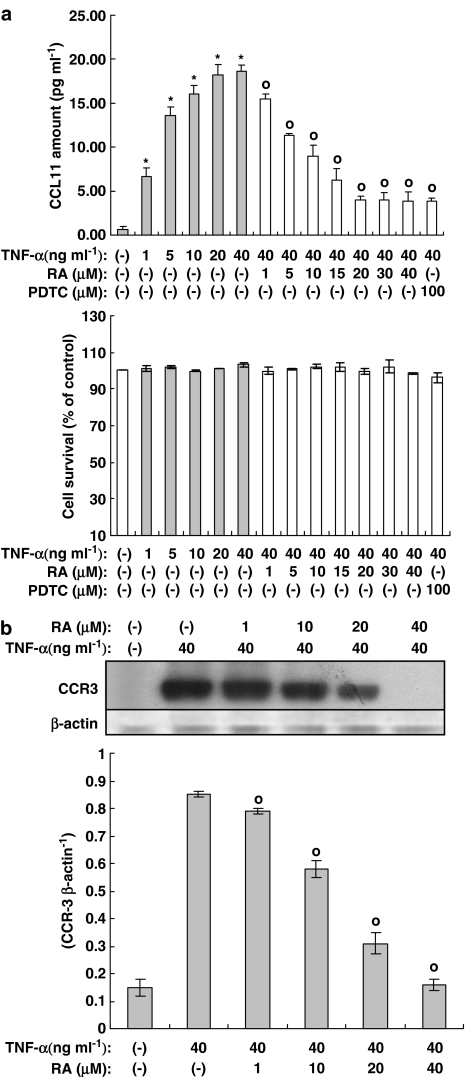
Figure 2.
Rosmarinic acid inhibits expression of the CCL11 and CCR3 genes induced by TNF-α in human dermal fibroblast cells. Human dermal fibroblast cells were incubated in the presence or absence of TNF-α, along with indicated concentrations of rosmarinic acid for 14 h. (a) The concentration of CCL11 in the culture medium was measured by a sandwich immunoassay kit (upper panel). The cell survival curve is shown in the lower panel. Results were confirmed by three independent experiments. Data are expressed as the means±s.e.m. *P<0.05, compared with untreated controls. °P<0.05 versus TNF-α (40 ng ml−1) only. RA: rosmarinic acid; PDTC: pyrollidone dithiocarbamate (100 μM). (b) The cultured human dermal fibroblast cells were subjected to Western blot analysis using anti-CCR3 and β-actin antibodies. RA: rosmarinic acid. Bands were visualized by an ECL method and quantified using a densitometer. Similar results were obtained in three independent experiments. Data are expressed as means±s.e.m. of three independent experiments (bar graph). °P<0.05 versus TNF-α (40 ng ml−1) only.
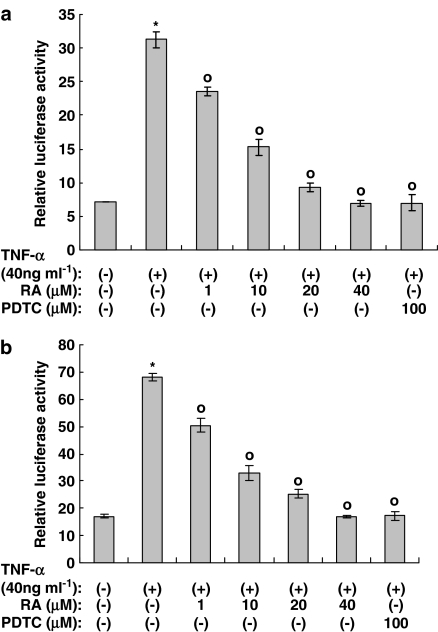
Figure 3.
Rosmarinic acid inhibits NF-κB activation induced by TNF-α in (a) NIH3T3 cells and (b) human dermal fibroblast cells. NIH3T3 cells and human dermal fibroblast cells were transfected with NF-κB-Luc reporters, along with Renilla luciferase expression vector driven by the thymidine kinase promoter using Superfect™ reagent. After 24 h of incubation, cells were treated with rosmarinic acid in the presence or absence of TNF-α (40 ng ml−1) for 14 h. The cells were then harvested and assayed. Renilla luciferase vector was employed as a control for transfection efficiency and the reporter data were processed using the dual luciferase method, as described in Methods. Results were confirmed by three independent transfections. Data are expressed as the means±s.e.m. *P<0.05 compared with untreated controls. °P<0.05 versus TNF-α (40 ng ml−1) only. RA: rosmarinic acid; PDTC: pyrollidone dithiocarbamate (100 μM).
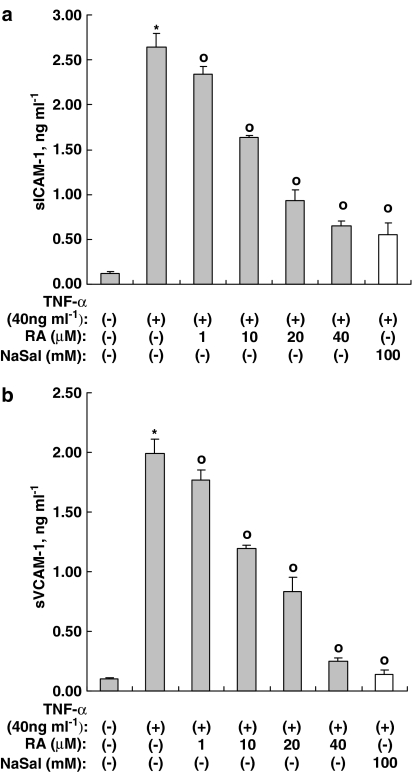
Figure 9.
Rosmarinic acid inhibits the expression of VCAM-1 and ICAM-1 genes induced by TNF-α in human dermal fibroblast cells. Human dermal fibroblast cells were incubated in the presence or absence of TNF-α, along with indicated concentrations of rosmarinic acid, for 14 h. The concentration of sICAM-1 (a) and sVCAM-1 (b) in the culture medium was measured by a sandwich immunoassay kit (upper panel). Results were confirmed by three independent experiments. Data are expressed as the means±s.e.m. *P<0.05, compared with untreated controls. °P<0.05 versus TNF-α (40 ng ml−1) only. RA: rosmarinic acid; NaSal: sodium salicylate (20 mM).
Results
Rosmarinic acid inhibits the TNF-α-induced expression of CCL11 and CCR3 in human dermal fibroblast cells
TNF-α is known to induce the expression of CCL11 and CCR3 via NF-κB activation. As previously mentioned, rosmarinic acid is a hydroxylated compound (Figure 1) and is known to exhibit anti-inflammatory and immunomodulatory actions. Thus rosmarinic acid may exert an inhibitory effect on the expression of CCL11 and CCR3. As our first step in examining the involvement of rosmarinic acid in the downregulation of CCL11 and CCR3 expression, we performed an enzyme-linked immunosorbent assay assay for CCL11 and a Western blot assay for CCR3. As shown in Figures 2a and b, the TNF-α-induced protein expression of the CCL11 and CCR3 genes were attenuated by rosmarinic acid in a concentration-dependent manner. The IC50 of rosmarinic acid on CCL11 protein expression induced by TNF-α was 9.1±1.5 μM. Additionally, while rosmarinic acid at 40 μM completely inhibited the protein expression of the CCR3 gene, expression of CCL11 protein was not blocked at the same concentration. This result suggests that protein expression of the CCR3 gene is more dependent on the NF-κB promoter than the CCL11 gene. PDTC was employed as a positive control. To assess the possibility that the decreased CCL11 production was due to a cytotoxic effect of rosmarinic acid, we performed an MTT assay in human dermal fibroblast cells. As shown in Figure 2a, rosmarinic acid showed no significant cytotoxic effect at any of the concentrations tested.
Rosmarinic acid inhibits NF-κB activation induced by TNF-α
To investigate whether the inhibitory effect of rosmarinic acid on the TNF-α-induced expression of the CCL11 and CCR3 genes is mediated by the suppression of NF-κB activation, we employed the NF-κB luciferase reporter in both NIH3T3, mouse fibroblast cells, and human dermal fibroblast cells. As shown in Figures 3a and b, NF-κB activation induced by TNF-α was inhibited in a concentration-dependent manner in both NIH3T3 cells and human dermal fibroblast cells, which suggests that rosmarinic acid may act on the NF-κB pathway to inhibit the expression of the CCR3 and CCL11 genes. The IC50 of rosmarinic acid on TNF-α-induced NF-κB activation in NIH3T3 cells and human dermal fibroblast cells was 10.9±1.6 and 9.5±1.5 μM, respectively. PDTC was employed as a positive control.
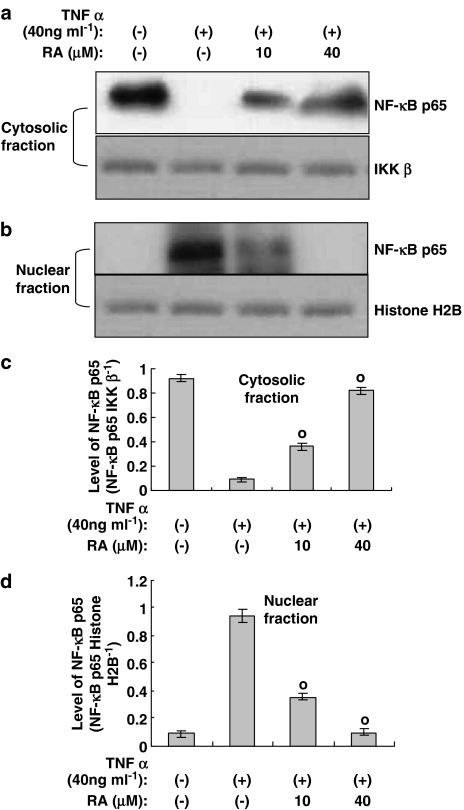
TNF-α-induced nuclear translocation of NF-κB heterodimer is inhibited by rosmarinic acid
In order to examine the effect of rosmarinic acid on nuclear translocation of NF-κB heterodimer induced by TNF-α, we performed a Western blot using nuclear and cytosolic extracts. The cells were stimulated with TNF-α only or with TNF-α plus rosmarinic acid; they were then lysed and separated into nuclear and cytosolic fractions. As controls for cytoplasmic and nuclear fraction, respectively, the levels of IKK-β and histone H2B were analyzed and were found to be consistent throughout the test period (Figure 4). In this experiment, while IKK-β was detected in the cytosolic fraction, but not in the nuclear fraction, histone H2B existed only in the nuclear fraction, and not in the cytosolic fraction (data not shown). This fact allowed us to confirm the purity of the cytosolic and nuclear fractions. The Western blots of NF-κB p65 in the lysates of the nuclei and cytosol revealed the nuclear translocation of NF-κB p65 from the cytosol upon treatment with TNF-α. However, in cases in which cells were incubated in the presence of TNF-α along with rosmarinic acid, nuclear translocation of NF-κB p65 was decreased (Figure 4), thereby indicating that its translocation from the cytosol was inhibited by rosmarinic acid.
Figure 4.
The nuclear translocation of NF-κB heterodimer induced by TNF-α is attenuated by rosmarinic acid. Human dermal fibroblast cells were stimulated using control vehicle, TNF-α (40 ng ml−1), or TNF-α plus rosmarinic acid for 30 min, followed by the isolation of nuclei and cytosol, as described in the Methods. Cytosolic (a) and nuclear (b) fractions were subjected to immunoblotting using anti-NF-κB p65, IKK-β, or histone H2B antibodies. Bands were visualized by an ECL method and quantified using a densitometer (c and d). Similar results were obtained in three independent experiments. Data are expressed as means±s.e.m. of three independent experiments (bar graph). °P<0.05 versus TNF-α (40 ng ml−1) only.
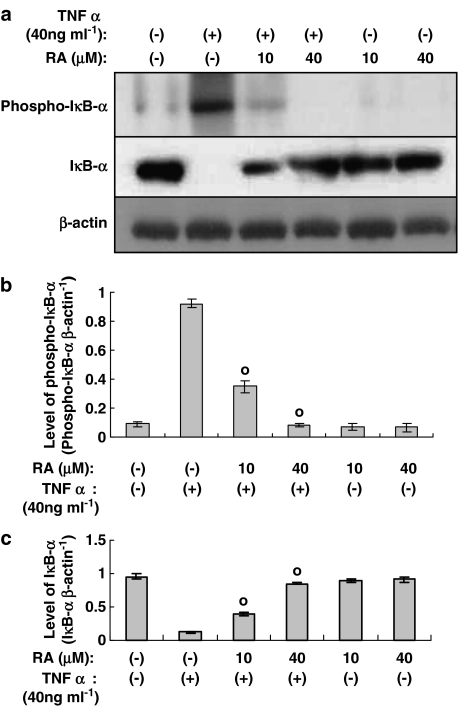
Phosphorylation and degradation of IκB-α induced by TNF-α is attenuated by rosmarinic acid
In previous reports, rosmarinic acid has been found to inhibit activation and nuclear translocation of NF-κB, induced by TNF-α. This result was further confirmed by Western blot analysis, which demonstrated that rosmarinic acid attenuated the phosphorylation and degradation of IκB-α induced by TNF-α. We employed anti-β-actin antibody as a quantitative control (Figure 5a).
Figure 5.
Rosmarinic acid inhibits the phosphorylation and degradation of IκB-α induced by TNF-α in human dermal fibroblast cells. Human dermal fibroblast cells were incubated in the presence or absence of TNF-α, along with the indicated concentrations of rosmarinic acid, for 30 min. The cultured human dermal fibroblast cells were subjected to Western blotting using antiphospho-IκB-α (Ser32/36) Ab or anti-IκB-α Ab (a). RA: rosmarinic acid. Bands were visualized by an ECL method and quantified using a densitometer (b and c). Similar results were obtained in three independent experiments. Data are expressed as means±s.e.m. of three independent experiments (bar graph). °P<0.05 versus TNF-α (40 ng ml−1) only.
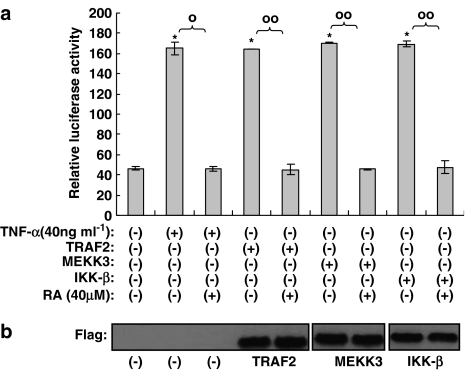
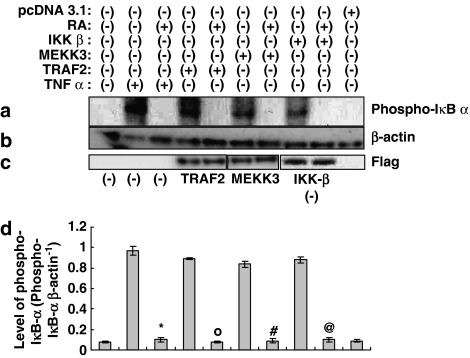
Rosmarinic acid blocks downstream of IKK-β in the NF-κB activation pathway induced by TNF-α
In our experiments, we identified the inhibitory effects of rosmarinic acid on CCL11 and CCR3 gene expression. Two pathways for the induction of NF-κB activity have recently been discovered (Joel & David, 2002). One is a canonical pathway in which TRAF2, MEKK, and IKK-β are involved, and the other is a noncanonical pathway in which NIK (NF-κB-inducing kinase) and IKK-α have been implicated. Activation of NF-κB induced by TNF-α is generally known to be mediated by the canonical pathway. Therefore, in order to characterize the action of rosmarinic acid in NF-κB activation induced by TNF-α, a NF-κB luciferase reporter assay was performed using TRAF2, MEKK3, and IKK-β in the absence of TNF-α. When each molecule was overexpressed by transient transfection into human dermal fibroblast cells, NF-κB luciferase reporter activity was significantly reduced by rosmarinic acid (Figure 6a). This inhibitory effect of rosmarinic acid was further confirmed by a Western blot assay for phosphorylated IκB-α using TRAF2, MEKK3, and IKK-β. As shown in Figure 7a, and also consistent with the results in Figure 6a, rosmarinic acid inhibited IκB-α phosphorylation induced by TRAF2-, MEKK3-, and IKK-β. This suggests that rosmarinic acid operates downstream of IKK-β in NF-κB-activation induced by TNF-α. Western blot analyses in this experiment were performed using anti-β-actin Ab as a quantitative control (Figure 7b).
Figure 6.
A hierarchy of rosmarinic acid that operates in the NF-κB activation pathway. Human dermal fibroblast cells were transfected with NF-κB-Luc reporters or indicated genes, such as TRAF2, MEKK3, and IKK-β, along with Renilla luciferase expression. After 24 h of incubation, the cells were incubated with 40 μM rosmarinic acid for 14 h, after which they were harvested and lysed. Supernatants were assayed for luciferase activity. Luciferase activity was determined using a Dual Luciferase Assay system and a LB953 luminometer; the luciferase activity was expressed as the ratio of the NF-κB-dependent firefly luciferase activity divided by control thymidine kinase Renilla luciferase activity (a). Results were confirmed by three independent transfections. Data are expressed as the means±s.e.m. *P<0.05, compared with the untreated controls. °P<0.05, °°P<0.05 versus TNF-α (40 ng ml−1) only or transfected controls. RA: rosmarinic acid. Western blots using antibody against Flag M1 for Flag-tagged TRAF2, MEKK3, or IKK-β show that each of the genes was transcribed and translated following transfection into cells (b).
Figure 7.
Rosmarinic acid operates downstream of IKK-β in NF-κB activation signaling. Human dermal fibroblast cells were transfected with TRAF2, MEKK3, or IKK-β, and were then incubated for 24 h. At that time, the human fibroblast cells were treated with 40 μM rosmarinic acid for 14 h. The cultured human dermal fibroblast cells were subjected to Western blot analysis using mouse monoclonal antibody (phospho-IκB-α (Ser32/36)) (a) and β-actin antibody (b). Bands were visualized by an ECL method and quantified using a densitometer (d). Similar results were obtained in three independent experiments. Data are expressed as means±s.e.m. of three independent experiments (bar graph). *P<0.05 versus TNF-α-treated control, °P<0.05 versus TRAF2-transfected control, #P<0.05 versus MEKK3-transfected control, @P<0.05 versus IKK-β-transfected control. Western blots using antibody against Flag M1 for Flag-tagged TRAF2, MEKK3, or IKK-β show that each of the genes was transcribed and translated following transfection into cells (c).
Rosmarinic acid inhibits IKK-β activity in NF-κB activation signaling induced by TNF-α
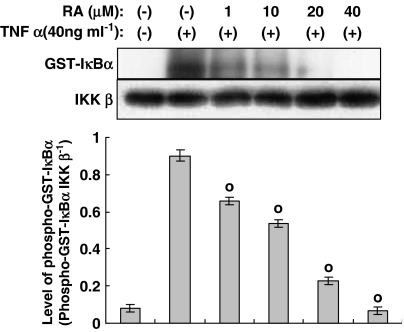
In order to further elucidate the action of rosmarinic acid, we performed an in vitro IKK-β kinase assay using immunoprecipitated IKK-β from human dermal fibroblast cells incubated in the presence or absence of TNF-α (40 ng ml−1), along with the indicated concentrations of rosmarinic acid for 30 min and GST-IκB-α as a substrate. As shown in Figure 8, IKK-β activity was reduced by rosmarinic acid. This result indicates that rosmarinic acid directly inhibits IKK-β kinase activity. Immunoblot analysis of cell extracts from untreated cells and cells treated with rosmarinic acid or TNF-α revealed no significant changes in IKK-β protein levels (Figure 8, bottom row).
Figure 8.
Effect of rosmarinic acid on IκB kinase (IKK)-β activity induced by TNF-α in human dermal fibroblast cells. IKK-β was immunoprecipitated from cells incubated in either the presence or absence of TNF-α (40 ng ml−1), along with the indicated concentrations of rosmarinic acid, for 30 min; the kinase assay was then performed (top row). Western blotting was performed to evaluate the total IKK-β level (bottom row). Bands were visualized by an ECL method and quantified using a densitometer. Similar results were obtained in three independent experiments. Data are expressed as means±s.e.m. of three independent experiments (bar graph). °P<0.05 versus TNF-α (40 ng ml−1) only.
Rosmarinic acid inhibits the TNF-α-induced expression of sVCAM-1 and sICAM-1 in human dermal fibroblast cells
We previously determined that rosmarinic acid inhibits the expression of CCR3 and CCL11 through the inhibition of NF-κB signaling induced by TNF-α. To further confirm this, we studied the effects of rosmarinic acid on the expression of intercellular adhesion molecule-1 (ICAM-1) and VCAM-1, which are encoded by NF-κB target genes. These molecules are known to be involved in the transendothelial migration of leukocytes (Pierce et al., 1996). As shown in Figure 9, the expression of both ICAM-1 and VCAM-1 was reduced by rosmarinic acid. The IC50 of rosmarinic acid on TNF-α-induced expression of sVCAM-1 and sICAM-1 was 14.6±2.4 and 15.8±1.6 μM, respectively. These results indicate that the anti-inflammatory properties of rosmarinic acid can be accounted for by the inhibition of the NF-κB pathway.
Discussion
To our knowledge, this study is the first to investigate the regulatory effects of rosmarinic acid on the TNF-α-induced expression of the CCL11 and CCR3 genes. We discovered that rosmarinic acid inhibits the expression of the CCL11 and CCR3 genes by suppressing NF-κB promoter activity; we also found that rosmarinic acid operates downstream of IKK-β in the TNF-α-induced upregulation of CCL11 and CCR3.
We demonstrated that rosmarinic acid inhibits the TNF-α-induced expression of the CCL11 and CCR3 genes by human dermal fibroblast cells, a cell type that acts as a fundamental component of the inflammatory response (Figure 2). Although rosmarinic acid completely inhibited expression of the CCR3 gene at a concentration of 40 μM, expression of the CCL11 gene was not blocked at this concentration. This result suggests that expression of the CCR3 gene is more dependent on the NF-κB promoter than the CCL11 gene. To be precise, the different inhibitory effects of rosmarinic acid on CCR3 and CCL11 may be attributed to the fact that, while expression of the CCL11 gene is dependent on STAT 6 and NF-κB, expression of the CCR3 gene is only dependent on NF-κB (Matsukura et al., 1999). We used TNF-α in this study because the NF-κB pathway was previously reported to play a key role in the regulation of CCR3 and CCL11 (Huber et al., 2002). To this end, the NF-κB luciferase reporter assay and Western blot analysis for phosphorylated IκB-α or IκB-α were performed in the initial steps of the investigation. Using this approach, we were able to demonstrate that the TNF-α-induced phosphorylation and degradation of IκB-α, and NF-κB activation were inhibited by rosmarinic acid. Nuclear translocation of the NF-κB heterodimer induced by TNF-α was also attenuated by rosmarinic acid. These findings indicated that rosmarinic acid inhibited the activation of NF-κB induced by TNF-α and suggested that rosmarinic acid may inhibit the expression of the CCL11 and CCR3 genes. This is demonstrated in Figure 2a and b.
Here, we show that rosmarinic acid regulates TNF-α-induced expression of the CCL11 and CCR3 genes by inhibiting NF-κB activation. As NF-κB activation induced by TNF-α is known to be mediated by the canonical pathway, we performed a luciferase reporter assay using molecules involved in the NF-κB activation signal, such as TRAF2, MEKK3, and IKK-β. We also performed Western blot analysis for phosphorylated IκB-α Ab in order to clarify the mechanism(s) of action of rosmarinic acid with regard to the NF-κB activation pathway, induced by TNF-α. In this study, we introduced wild-type cDNA for TRAF2 or MEKK3, and the constitutive active form of IKK-β. Several reports have shown that overexpression of the wild-type MEKK3 gene or wild-type TRAF2 gene activates NF-κB signaling (Yang et al., 2001; He et al., 2004; Lee et al., 2005). A report by Mercurio et al. (1997) also showed that overexpression of the constitutively active form of IKK-β activates NF-κB signaling. It is thought that, while the role of TRAF2 and MEKK3 in NF-κB activation is the recruitment of IKK to TNFR 1 by TRAF2 and the activation of IKK by MEKK3, the role of IKK-β is to directly activate IkB-α (Yang et al., 2001).
When each molecule was overexpressed by transient transfection in human dermal fibroblast cells, NF-κB luciferase reporter activity was significantly reduced by treatment with rosmarinic acid. The phosphorylation level of IκB-α induced by all of the tested genes was also noticeably attenuated by rosmarinic acid (Figures 6 and 7). This indicates that rosmarinic acid operates downstream of IKK-β in the TNF-α-induced activation of NF-κB. This finding was strengthened by an examination of the in vitro IKK-β kinase activity (Figure 8), which indicated that IKK-β activity induced by TNF-α is inhibited by rosmarinic acid.
Recent experiments based on the use of conditional IKK-β loss-of-function mutations indicate that IKK-β activity is required for the inactivation of a severe inflammatory reaction that leads to multi-organ failure in response to ischemia–reperfusion (Chen et al., 2003). IKK-β activity is also required to prevent a considerable number of different cell types from undergoing apoptosis (Karin & Lin, 2002). These results indicate that rosmarinic acid, an IKK-β inhibitor, could be useful for the treatment of inflammatory diseases (Barnes & Karin, 1997; Ghosh et al., 1998; Neurath et al., 1998). However, as an IKK-β inhibitor, an expected side effect inherent to rosmarinic acid would be increased susceptibility to the induction of programmed cell death. Until now, several compounds have been identified as IKK-β inhibitors. Among these are aspirin, salicylates, sulindac, sulfasalazine, thalidomide, cyclopentenone prostaglandins, quinazoline analogue, β-carbolin analogue, and imidazoquinoxaline derivatives (Karin et al., 2004). These compounds have been reported as nanomolar- or micromolar-range inhibitors of IKK-β kinase activity, and have demonstrated inhibitory activity in functional cell-based assays. The compounds have also shown efficacy in experimental models. Like these compounds, rosmarinic acid has an IC50 of ∼12 μM against the IκB-α kinase activity of IKK-β, which suggests the potential use of rosmarinic acid in the treatment of inflammatory diseases. However, until the safety and efficacy profiles of rosmarinic acid are determined, it is not clear whether this compound could be used in the treatment of inflammatory disorders.
One such inflammatory disorder is atopic dermatitis, a common pruritic inflammatory skin disease, which often begins in infancy and frequently affects individuals with a personal or family history of atopic disease (Beltrani, 1996). Increased serum immunoglobulin E levels and blood eosinophilia are also often associated with this condition. Although the pathogenesis of atopic dermatitis is still poorly understood, there are reports suggesting that chemokines are involved in the modulation of tissue inflammation (Schroder et al., 1996; Baggiolini et al., 1997). In a recent report, the transcriptional and translational expression levels of CCL11 and CCR3 were significantly increased in lesional skin from atopic dermatitis, but not in nonatopic controls. (Yawalkar et al., 1999). Therefore, the inhibitory effect of rosmarinic acid on the TNF-α-induced expression of the CCL11 and CCR3 genes and the antibacterial effect of rosmarinic acid against Staphylococcus aureus, a pathogen commonly associated with atopic dermatitis (data not shown), suggest that rosmarinic acid may hold potential as a treatment for this inflammatory disorder.
Acknowledgments
This work was supported by a grant from the Korean Ministry of Commerce, Industry, and Energy (IH-9-12-10018068).
Abbreviations
- IKK-β
IκB kinase β
- MEKK3
MAPK kinase kinase 3
- NF-κB
nuclear factor-κB
- TNF-α
tumor necrosis factor α
- TRAF2
TNF-receptor-associated factor 2
References
- AL-SEREITI M.R., ABU-AMER K.M., SEN P. Pharmacology of rosemary (Rosmarinus officinalis Linn.) and its therapeutic potentials. Ind. J. Expo. Biol. 1999;37:124–130. [PubMed] [Google Scholar]
- ATASOY U., CURRY S.L., LOPEZ DE SILANES I. Regulation of eotaxin gene expression by TNF-alpha and IL-4 through mRNA stabilization: involvement of the RNA-binding protein HuR. J. Immunol. 2003;171:4369–4378. doi: 10.4049/jimmunol.171.8.4369. [DOI] [PubMed] [Google Scholar]
- BAGGIOLINI M., DEWALD B., MOSER B. Human chemokines: an update. Annu. Rev. Immunol. 1997;15:675–705. doi: 10.1146/annurev.immunol.15.1.675. [DOI] [PubMed] [Google Scholar]
- BARNES P.J., KARIN M. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N. Engl. J. Med. 1997;336:1066–1071. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- BELTRANI V.S.The clinical manifestation of atopic dermatitis Atopic Dermatitis. From Pathogenesis to Treatment 1996Heidelberg: Springer-Verlag; 1–35.ed. Leung, D.Y.M., pp [Google Scholar]
- CHEN L.W., EGAN L., LI Z.W., GRETEN F.R., KAGNOFF M.F., KARIN M. The two faces of IKK and NF-kappaB inhibition: prevention of systemic inflammation but increased local injury following intestinal ischemia–reperfusion. Nat. Med. 2003;9:575–581. doi: 10.1038/nm849. [DOI] [PubMed] [Google Scholar]
- DEL BANO M.J., LORENTE J., CASTILLO J. Phenolic diterpenes, flavones, and rosmarinic acid distribution during the development of leaves, flowers, stems, and roots of Rosmarinus officinalis. J. Agric. Food Chem. 2003;51:4247–4253. doi: 10.1021/jf0300745. [DOI] [PubMed] [Google Scholar]
- GARCIA-ZEPEDA E., ROTHENBERG M.E., OWNBEY R.T., CELESTIN J., LEDER P., LUSTER A.D. Human eotaxin is a specific chemoattractant for eosinophil cells and provides a new mechanism to explain tissue eosinophilia. Nat. Med. 1996;2:449–456. doi: 10.1038/nm0496-449. [DOI] [PubMed] [Google Scholar]
- GERBER B.O., ZANNI M.P., UGUCCIONI M. Functional expression of the eotaxin receptor CCR3 in T lymphocytes co-localizing with eosinophils. Curr. Biol. 1997;7:836–843. doi: 10.1016/s0960-9822(06)00371-x. [DOI] [PubMed] [Google Scholar]
- GHOSH S., MAY M.J., KOPP E.B. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- GOLDMAN F.D., GILMAN A.L., HOLLENBACK C. Hydroxychloroquine inhibits calcium signals in T cells: a new mechanism to explain its immunomodulatory properties. Blood. 2000;95:3460–3466. [PubMed] [Google Scholar]
- GUTIERREZ-RAMOS J.C., LLOYD C., GONZALO J.A. Eotaxin: from an eosinophilic chemokine to a major regulator of allergic reactions. Immunol. Today. 1999;20:500–504. doi: 10.1016/s0167-5699(99)01522-4. [DOI] [PubMed] [Google Scholar]
- HE L., GRAMMER A.C., WU X., LIPSKY P.E. TRAF3 forms heterotrimers with TRAF2 and modulates its ability to mediate NF-{kappa}B activation. J. Biol. Chem. 2004;279:55855–55865. doi: 10.1074/jbc.M407284200. [DOI] [PubMed] [Google Scholar]
- HUBER M.A., DENK A., PETER R.U., WEBER L., KRAUT N., WIRTH T. The IKK-2/IκBα/NF-κB Pathway Plays a key role in the regulation of CCR3 and eotaxin-1 in Fibroblasts. J. Biol. Chem. 2002;272:1268–1275. doi: 10.1074/jbc.M109358200. [DOI] [PubMed] [Google Scholar]
- HUBER M.A., KRAUT N., ADDICKS T., PETER R.U. Cell-type-dependent induction of eotaxin and CCR3 by ionizing radiation. Biochem. Biophys. Res. Commun. 2000;269:546–552. doi: 10.1006/bbrc.2000.2287. [DOI] [PubMed] [Google Scholar]
- JOEL L.P., DAVID B. Two pathways to NF-κB. Mol. Cell. 2002;10:693–701. [Google Scholar]
- KABOURIDIS P.S., MAGEE A.I., LEY S.C. S-acylation of LCK protein tyrosine kinase is essential for its signalling function in T lymphocytes. EMBO J. 1997;16:4983–4998. doi: 10.1093/emboj/16.16.4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KANG M.A., YUN S.Y., WON J. Rosmarinic acid inhibits Ca2+-dependent pathway of T-cell antigen receptor-mediated signaling by inhibiting the PLC-γ1 and Itk activity. Blood. 2003;101:3534–3542. doi: 10.1182/blood-2002-07-1992. [DOI] [PubMed] [Google Scholar]
- KARIN M., LIN A. NF-kappaB at the crossroads of life and death. Nat. Immunol. 2002;3:221–227. doi: 10.1038/ni0302-221. [DOI] [PubMed] [Google Scholar]
- KARIN M., YAMAMOTO Y., WANG Q.M. The IKK NF-kappa B system: a treasure trove for drug development. Nat. Rev. Drug Discov. 2004;3:17–26. doi: 10.1038/nrd1279. [DOI] [PubMed] [Google Scholar]
- KELM M.A., NAIR M.G., STRASBURG G.M. Antioxidant and cyclooxygenase inhibitory phenolic compounds from Ocimum sanctum Linn. Antioxidant Phytomed. 2000;7:7–13. doi: 10.1016/S0944-7113(00)80015-X. [DOI] [PubMed] [Google Scholar]
- KIMURA Y., OKUDA H., OKUDA T. Studies on the activities of tannins and related compounds, X: effects of caffeetannins and related compounds on arachidonate metabolism in human polymorphonuclear leukocytes. J. Nat. Prod. 1987;50:392–399. doi: 10.1021/np50051a009. [DOI] [PubMed] [Google Scholar]
- KINTZIOS S., MAKRI O., PANAGIOTOPOULOS E., SCAPETI M. In vivo rosmarinic acid accumulation in sweet basil (Ocimum basilicum L.) Biotechnol. Lett. 2003;25:405–408. doi: 10.1023/a:1022402515263. [DOI] [PubMed] [Google Scholar]
- LEE J., JUNG E., PARK J., JUNG K., LEE S., HONG S., PARK J., PARK E., KIM J., PARK S., PARK D. Anti-inflammatory effects of magnolol and honokiol are mediated through inhibition of the downstream pathway of MEKK-1 in NF-kappaB activation signaling. Planta Med. 2005;71:338–343. doi: 10.1055/s-2005-864100. [DOI] [PubMed] [Google Scholar]
- LUKACS N.W., OLIVERIRA S.H.P., HOGABOAM C.M. Chemokines and asthma: redundancy of function or a coordinated effort. J. Clin. Invest. 1999;104:995–999. doi: 10.1172/JCI8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATSUKURA S., STELLATO C., PLITT J.R., BICKEL C., MIURA K., GEORAS S.N., CASOLARO V., SCHLEIMER R.P. Activation of eotaxin gene transcription by NF-kappa B and STAT6 in human airway epithelial cells. J. Immunol. 1999;163:6876–6883. [PubMed] [Google Scholar]
- MATTOLI S., STACEY M.A., SUN G., BELLINI A., MARTINI M. Eotaxin expression and eosinophilic inflammation in asthma. Biochem. Biophys. Res. Commun. 1997;236:299–301. doi: 10.1006/bbrc.1997.6958. [DOI] [PubMed] [Google Scholar]
- MERCURIO F., ZHU H., MURRAY B.W., SHEVCHENKO A., BENNETT B.L., LI J., YOUNG D.B., BARBOSA M., MANN M., MANNING A., RAO A. IKK-1 and IKK-2: cytokine-activated IkappaB kinases essential for NF-kappaB activation. Science. 1997;278:860–866. doi: 10.1126/science.278.5339.860. [DOI] [PubMed] [Google Scholar]
- NEURATH M.F., FUSS I., SCHURMANN G., PETTERSSON S., ARNOLD K., MULLER-LOBECK H., STROBER W., HERFARTH C., BUSCHENFELDE K.H. Cytokine gene transcription by NF-kappa B family members in patients with inflammatory bowel disease. Ann. NY Acad. Sci. 1998;859:149–159. doi: 10.1111/j.1749-6632.1998.tb11119.x. [DOI] [PubMed] [Google Scholar]
- OSAKABE N., YASUDA A., NATSUME M. Rosmarinic acid, a major polyphenolic component of Perilla frutescens, reduces lipopolysaccharide (LPS)-induced liver injury in D-galactosamine (D-GalN)-sensitized mice. Free Radic. Biol. Med. 2002;33:798–806. doi: 10.1016/s0891-5849(02)00970-x. [DOI] [PubMed] [Google Scholar]
- PACCANI S.R., BONCRISTIANO M., ULIVIERI C. Nonsteroidal anti-inflammatory drugs suppress T cell activation by inhibiting p38MAPK induction. J. Biol. Chem. 2002;277:1509–1513. doi: 10.1074/jbc.M110676200. [DOI] [PubMed] [Google Scholar]
- PIERCE J.W., READ M.A., DING H., LUSCINSKAS F.W., COLLINS T. Salicylates inhibit I kappa B-alpha phosphorylation, endothelial–leukocyte adhesion molecule expression, and neutrophil transmigration. J. Immunol. 1996;156:3961–3969. [PubMed] [Google Scholar]
- PONATH P.D., QIN S., RINGLER D.J. Cloning of the human eosinophil chemoattractant, eotaxin. Expression, receptor binding, and functional properties suggests a mechanism for the selective recruitment of eosinophils. J. Clin. Invest. 1996;97:604–612. doi: 10.1172/JCI118456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAHU A., RAWAL N., PANGBURN M.K. Inhibition of complement by covalent attachment of rosmarinic acid to activated C3b. Biochem. Pharmacol. 1999;57:1439–1446. doi: 10.1016/s0006-2952(99)00044-1. [DOI] [PubMed] [Google Scholar]
- SALLUSTO F., MACKAY C.R., LANZAVECCHIA A. Selective expression of the eotaxin receptor CCR3 by human T helper 2 cells. Science. 1997;277:2005–2007. doi: 10.1126/science.277.5334.2005. [DOI] [PubMed] [Google Scholar]
- SCHRODER J.M., NOSO N., STICHERLING M., CHRISTOPHERS E. Role of eosinophil-chemotactic C–C chemokines in cutaneous inflammation. J. Leuk. Biol. 1996;59:1–5. [PubMed] [Google Scholar]
- UGUCCIONI M., MACKAY C.R., OCHSENBERGER B. High expression of the chemokine receptor CCR3 in human blood basophils. Role in activation by eotaxin, MCP-4, and other chemokines. J. Clin. Invest. 1997;100:1137–1143. doi: 10.1172/JCI119624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN KESSEL K.P., KALTER E.S. Rosmarinic acid inhibits external oxidative effects of human polymorphonuclear granulocytes. J. Verhoef. Agents Actions. 1986;17:375–376. doi: 10.1007/BF01982652. [DOI] [PubMed] [Google Scholar]
- YAMADA H., HIRAI K., MIYAMASU M. Eotaxin is a potent chemotaxin for human basophils. Biochem. Biophys. Res. Commun. 1997;231:365–368. doi: 10.1006/bbrc.1997.6100. [DOI] [PubMed] [Google Scholar]
- YANG J., LIN Y., GUO Z., CHENG J., HUANG J., DENG L., LIAO W., CHEN Z., LIU Z., SU B. The essential role of MEKK3 in TNF-induced NF-kappaB activation. Nat. Immunol. 2001;2:620–624. doi: 10.1038/89769. [DOI] [PubMed] [Google Scholar]
- YAWALKAR N., UGUCCIONI M., SCHARER J., BRAUNWALDER J., KARLEN S., DEWALD B., BRAATHEN L.R., BAGGIOLINI M. Enhanced expression of eotaxin and CCR3 in atopic dermatitis. J. Invest. Dermatol. 1999;113:43–48. doi: 10.1046/j.1523-1747.1999.00619.x. [DOI] [PubMed] [Google Scholar]
- YING S., ROBINSON D.S., MENG Q., ROTTMAN J., KENNEDY R., RINGLER D.J., MACKAY C.R., DAUGHERTY B.L., SPRINGER M.S., DURHAM S.R., WILLIAMS T.J., KAY A.B. Enhanced expression of eotaxin and CCR3 mRNA and protein in atopic asthma. Association with airway hyperresponsiveness and predominant co-localization of eotaxin mRNA to bronchial epithelial and endothelial cells. Eur. J. Immunol. 1997;27:3507–3516. doi: 10.1002/eji.1830271252. [DOI] [PubMed] [Google Scholar]
- ZHENG W., WANG S.Y. Antioxidant activity and phenolic compounds in selected herbs. J. Agric. Food Chem. 2001;49:5165–5170. doi: 10.1021/jf010697n. [DOI] [PubMed] [Google Scholar]