Abstract
This study examined whether Paeoniflorin (PF), the major active components of Chinese herb Paeoniae alba Radix, has neuroprotective effect in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model of Parkinson's disease (PD).
Subcutaneous administration of PF (2.5 and 5 mg kg−1) for 11 days could protect tyrosine hydroxylase (TH)-positive substantia nigra neurons and striatal nerve fibers from death and bradykinesia induced by four-dose injection of MPTP (20 mg kg−1) on day 8.
When given at 1 h after the last dose of MPTP, and then administered once a day for the following 3 days, PF (2.5 and 5 mg kg−1) also significantly attenuated the dopaminergic neurodegeneration in a dose-dependent manner. Post-treatment with PF (5 mg kg−1) significantly attenuated MPTP-induced proinflammatory gene upregulation and microglial and astrocytic activation.
Pretreatment with 0.3 mg kg−1 8-cyclopentyl-1,3-dipropylxanthine, an adenosine A1 receptor (A1AR) antagonist, 15 min before each dose of PF, reversed the neuroprotective and antineuroinflammatory effects of PF.
In conclusion, this study demonstrated that PF could reduce the MPTP-induced toxicity by inhibition of neuroinflammation by activation of the A1AR, and suggested that PF might be a valuable neuroprotective agent for the treatment of PD.
Keywords: Paeoniflorin, Parkinson's diease, mice, neuroprotection, neuroinflammation, adenosine A1 receptor, MPTP
Introduction
Parkinson's disease (PD) is characterized by the selective loss of dopaminergic neurons in the substantia nigra pars compacta (SNc). Even after numerous studies, the cause of dopaminergic cell degeneration in SNc of PD patients still has not been identified with certainty. Recently, increasing evidence from human and animal studies has suggested that neuroinflammation is an important contributor to the neuronal loss in PD (McGeer et al., 1988; Hunot & Hirsch, 2003). The hallmark of brain inflammation is the activation of glia, particularly microglia. Microglia, the resident immune cells in the brain, are sensitive to even minor disturbances in central nervous system (CNS) homeostasis and become readily activated during most neuropathological conditions, such as Alzheimer's disease (AD), multiple sclerosis, AIDS dementia, trauma, stroke and PD (Kreutzberg, 1996; Liu & Hong, 2003). Activated microglia are thought to contribute to neuronal damage, particularly in neurodegenerative diseases, via the release of proinflammatory and neurotoxic factors. These factors include proinflammatory cytokines such as tumor necrosis factor-alpha (TNF-α) and interleukin 1 beta (IL-1β), reactive nitrogen species, reactive oxygen species (ROS), eicosanoids and excitatory amino acids (Merrill & Benveniste, 1996; Liu & Hong, 2003). Moreover, microglia-derived neurotoxic factors might act in concert to trigger or exacerbate the neurodegeneration. For example, TNF-α induces the expression of inducible nitric oxide synthase (iNOS) in glia, thus amplifying NO-mediated neuronal damage (Merrill & Benveniste, 1996). Additionally, in the mature brain, the higher density of resting microglia in the SNc compared with other brain regions (Lawson et al., 1996; Kim et al., 2000) might be one of the reasons why dopamine-containing neurons are extremely vulnerable to oxidative stress in PD. Recently, growing experimental evidence demonstrated that inhibition of the inflammatory response could, in part, prevent degeneration of nigrostriatal dopamine-containing neurons in several animal models of PD, suggesting that inhibition of inflammation might become a promising therapeutic intervention for PD (Mogi et al., 1998; Liberatore et al., 1999; Gao et al., 2003; Teismann et al., 2003; Ferger et al., 2004; Furuya et al., 2004; Zhou et al., 2005).
Paeoniae alba Radix (red peony root; Chishao), the dried root of Paeonia lactiflora Pallas or Paeonia veitchii Lynch, is one of the Chinese traditional crude drugs. It has been widely used as a component of traditional Chinese prescriptions to regulate the amenorrhea, to treat traumatic injuries, epistaxis, inflammation, boils and sores, and to relieve pain in the chest and costal regions. Paeoniflorin (PF), a characteristic main principal bioactive component of P. alba Radix (PF, Liu et al., 2005), has been reported to exhibit many pharmacological effects such as anti-inflammatory and antiallergic effects (Yamahara et al., 1982), antihyperglycemic effects (Hsu et al., 1997), analgesic effects (Sugishita et al., 1984), neuromuscular blocking effects (Dezaki et al., 1996), cognition-enhancing effects (Takeda et al., 1995) and inhibitory effects on steroid protein binding (Tamaya et al., 1986). Moreover, recent studies indicated that PF might exert their activities by activation of adenosine A1 receptors (A1AR) (Lai et al., 1998; Cheng et al., 1999; Yang et al., 2001; Tang et al., 2003; Liu et al., 2005).
A1AR has been found to be involved in nigrostriatal dopaminergic neurodegeneration. The administration of adenosine A1AR agonists improved the impairment of dopaminergic system caused by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) (Lau & Mouradian, 1993) or methamaphetamine (DelleDonne & Sonsalla, 1994; Gol'embiowska & Źylewska, 1998), while the antagonists for this receptor subtype enhanced the damage of dopaminergic system caused by mitochondrial inhibitor malonate (Alfinito et al., 2003). Adenosine was also found to regulate the suppression of inflammation (Bouma et al., 1994; Cronstein, 1994; Hasko et al., 1996; Le Moine et al., 1996; Sajjadi et al., 1996; Schwaninger et al., 1997; Tsutsui et al., 2004). In the CNS, A1AR is highly expressed on microglia/macrophages and neurons (Johnston et al., 2001). It has been found that activation and upregulation of A1AR could attenuate neuroinflammation and demyelination in the mice model of multiple sclerosis (Tsutsui et al., 2004). These data indicated that the activation of A1AR might have anti-inflammatory and neuroprotective effects on doparminergic neurons. Recently, we demonstrated that PF has neuroprotective effects in cerebral ischemic rat, and the neuroprotective effects were mediated by A1AR (Liu et al., 2005). In the present studies, we investigated whether PF could prevent the neuroinflammation and dopaminergic neurodegeneration in a MPTP mouse model of PD. Moreover, A1AR antagonist 8-cyclopentyl-1,3-dipropylxanthine (DPCPX) was used to investigate whether A1AR was involved in the protective effects of PF.
Methods
Chemicals and animals
PF was extracted from the dried and powdered roots of P. alba, one species in Paeony. The purity of PF is above 98% (Liu et al., 2005). DPCPX and MPTP were purchased from Sigma Chemical Co. (St Louis, MO, U.S.A.). MPTP was dissolved in saline, and DPCPX was dissolved in saline with 5% dimethyl-sulfoxide (DMSO).
Male C57BL/6 mice, weighing 18–22 g, were used in the present studies. The animals had free access to solid food and water ad libitum under standard conditions of temperature, humidity and light. The study was performed in compliance with National Institutes of Health (NIH) guidelines and was approved by Animal Care and Use Committee, Shanghai Institute of Materia Medica, Chinese Academy of Science.
Experimental protocols
Two experimental paradigms of MPTP delivery have been used as described by Iwashita et al. (2004) with minor modifications. One is the four-dose paradigm of MPTP intoxication to induce severe cell injury, and the other is the two-dose paradigm to induce milder cell injury. To determine the effect of pre-MPTP treatment with PF, the four-dose paradigm (severe model) was used in the present studies. PF in saline was administered at 2.5 or 5 mg kg−1 s.c. for 11 days, on day 8, the animals received 4 × 20 mg kg−1 MPTP at 2 h intervals for the severe model. To determine the effect of post-MPTP treatment with PF, two-dose paradigm of MPTP (mild model) was used. The animals were injected with 2 × 20 mg kg−1 MPTP at 2 h intervals. Daily treatment with 2.5 or 5 mg kg−1 of PF was started at 1 h after the second injection of MPTP, and continued to day 3 after MPTP injection. Furthermore, to determine whether A1AR was involved in the protective effects of PF, DPCPX, the selective A1AR antagonist (0.3 mg kg−1, i.p.) was given to MPTP-treated animals at 15 min before each PF administration in the post-MPTP treatment. In all the experiments, each group contained eight mice.
Behavioral observations
The pole test was used to measure bradykinesia, a typical symptom of parkinsonism (Matsuura et al., 1997; Araki et al., 2001; Kato et al., 2004). In the present studies, pole test was conducted 4 days after MPTP treatments with each experimental paradigm. The mice were placed head upward near the top of a vertical rough-surfaced pole (diameter 8 mm, height 55 cm). The time taken to turn completely downward (time to turn; T-turn) and the time until all four feet of the mouse reached the floor (locomotion activity time; T-LA) were recorded with the cutoff limit of 30 s. The test was performed five times for each mouse.
Immunohistochemistry
After anesthetization with pentobarbital, the mice were perfused by the intracardiac route with PBS, followed by 4% paraformaldehyde in PBS. The mice were then decapitated, and the brains were removed and immersed for 48 h in 4% paraformaldehyde for fixation. Midbrain and striatum coronal sections (25 μm thick) were then prepared with a cryostat. For all immunostaining, the sections were first rinsed with PBS containing 0.1% Triton-X (PBS-T), and then were immersed in a solution of 0.5% H2O2 for 30 min. After incubated overnight with primary antibody in PBS-T containing 10% normal serum at 4°C, the sections were washed three times in PBS-T and incubated with a biotinylated secondary antibody (Vector Laboratories, Burlingame, CA, U.S.A.) in PBS-T for 2 h at room temperature. Then, sections were incubated in avidin–biotin peroxidase complex (Vector Laboratories) for 1 h. A final incubation in DAB was performed for visualization. All the sections were then washed in PBS, mounted on amino propyltriethoxy silan-coated slides, dried, dehydrated in a graded series of ethanol, cleared in xylene, and coverslipped. Following primary antibodies were used in this experiment: mouse monoclonal anti-tyrosine hydroxylase (TH, dilution 1 : 1000, Sigma Chemical Co., St Louis, MO, U.S.A.) for dopaminergic neurons, mouse monoclonal anti-glial fibrillary acidic protein (GFAP, dilution 1 : 1000; Chemicon International, Temecula, CA, U.S.A.) for astrocytes, mouse monoclonal anti-cd11b (1 : 200; Serotec, Raleigh, NC, U.K.) for activated microglia. For the microglial activation assay, the mice were killed 24 h after the last dose of MPTP. Whereas for astrocytic activation examination, mice were killed 96 h after the last MPTP injection.
Assessment of neuronal loss
Loss of neurons in the SNc of mice was determined by serial section analysis of the total number of TH-positive neurons at day 4 after MPTP treatment as described previously (Conti et al., 2005). Briefly, total numbers of TH-immunoreactive and Nissl-stained SNc neurons were counted by using stereology as previously described (Coggeshall, 1992; Gundersen, 1992; Volpe et al., 1998; Sugama et al., 2003). Non-neuronal cells were excluded by counting clearly defined nucleus, cytoplasm and prominent nucleolus by Nissl-staining. This procedure was carried out on four sections at a periodicity of 100 μm in the SNc. Average neuron density was obtained by summing the number of neuron profiles divided by the calculated volume. The total number of neurons was calculated as the product of the neuron density and the volume of SNc as described previously (Coggeshall, 1992; Gundersen, 1992; DeGiorgio et al., 1998; Volpe et al., 1998; Sugama et al., 2003). With this procedure, the number of cells counted is not affected by the volume of the SNc or the size of the neurons.
To determine the gray density of the TH-immunoreactive staining in the striatum, a square frame of 700 × 700 μm was placed in the dorsal part of the striatum. A second square frame of 200 × 200 μm was placed in the region of the corpus callosum to measure background values. To control for variations in background illumination, the average of the background density readings from the corpus callosum was subtracted from the average of density readings of the striatum for each section. TH optical density was given in arbitrary units (a.u.).
Real-time reverse transcription PCR
Total RNA was extracted from the ventral midbrain (VM) of mice with TRIzol reagent (Invitrogen, Gaithersburg, MD, U.S.A.) 24 h after the last dose of MPTP. The concentration and purity of the RNA preparations were determined by measuring the absorbance at 260 and 280 nm in a spectrophotometer. First-strand cDNA was synthesized from total RNA using M-MLV reverse transcriptase (Promega, Madison, WI, U.S.A.). The cDNA template was then amplified by PCR using Ex-Taq (TaKaRa, Kyoto, Japan). The nucleotide sequences of the primers were based on published cDNA sequences (Table 1). Semiquantitative analysis was performed by monitoring in real-time the increase of fluorescence of the SYBR-green dye (Molecular Probes, Carlsbad, CA, U.S.A.) on DNA Engine Opticon 2 thermal cycler (MJ Research, Waltham, MS, U.S.A.). Real-time fluorescence measurements were performed, and a threshold cycle value for each gene of interest was determined, as reported previously (Power et al., 2003). All data were normalized to the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA level and expressed as mRNA relative fold change (RFC).
Table 1.
The specific primers for interested genes
| Oligonucleotides designed for RT–PCR | Sequence |
|---|---|
| IL-1β forward |
5′-CTGTGTCTTTCCCGTGGACC-3′ |
| IL-1β reverse |
5′-CAGCTCATATGGGTCCGACA-3′ |
| iNOS forward |
5′-TCACTGGGACAGCACAGAAT-3′ |
| iNOS reverse |
5′-TGTGTCTGCAGATGTGCTGA-3′ |
| TNF-α forward |
5′-GCGGTGCCTATGTCTCAGCC-3′ |
| TNF-α reverse |
5′-TGAGGAGCACGTAGTCGGGG-3′ |
| GAPDH forward |
5′-GGTTGTCTCCTGCGACTTCA-3′ |
| GAPDH reverse | 5′-TGGTCCAGGGTTTTTACTCC-3′ |
N-methyl-4-phenylpyridinium ion measurements
1-methyl-4-phenyl-pyridiniumion (MPP+) measurements were performed as described previously (Iwashita et al., 2004). Briefly, PF 5 mg kg−1 was injected subcutaneously (s.c.) 7 days before or 1 h after a single dose of MPTP (20 mg kg−1 i.p.). At 2 h after MPTP dosing, striatal concentrations of MPP+ was measured by HPLC as described previously (Crocker et al., 2003).
Statistical analysis
Data were presented as the mean±s.e.m. Statistical differences were determined by Paired student's t-test or one-way analysis of variance (ANOVA) followed by Dunnett's post hoc comparison. For all cases, significance of differences were accepted at P<0.05.
Results
Effects of pre- and post-treatment with PF on MPTP-induced dopaminergic neurodegeneration and motor deficit
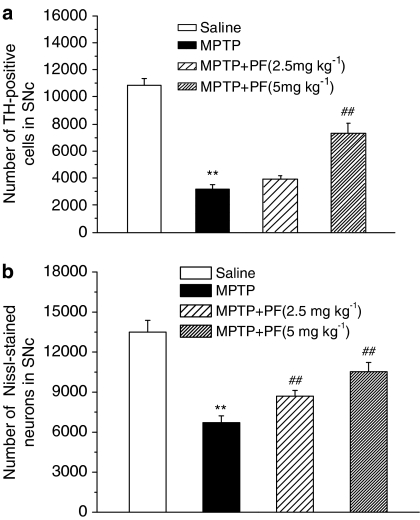
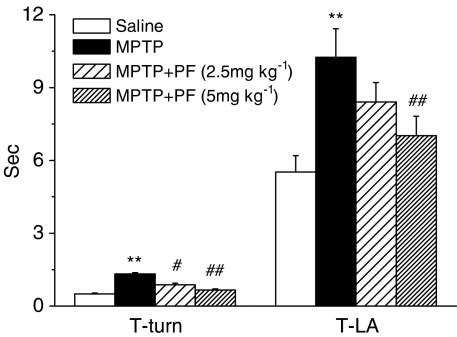
To ascertain whether PF exerts the neuroprotective effects in the MPTP mouse model, PF was first evaluated on the four-dose paradigm of MPTP delivery (severe model). PF was administered 11 days to C57BL/6 mice (2.5 or 5 mg kg−1 s.c.), and MPTP was injected to mice on day 8. In this model, pretreatment of PF significantly and dose dependently ameliorated the loss of the nigrostriatal dopaminergic neurons in the SNc (Figure 1) and terminals in the striatum (Figure 2). The MPTP injection resulted in significant motor deficits in the pole test. The time of T-turn of the pole test was increased by 4.6-fold, the time T-LA was increased by 1.9-fold (all with significance at P<0.01 compared with normal control). Treatment with PF significantly reduced the motor abnormalities in the pole tests in a dose-dependent manner (Figure 3).
Figure 1.
Effects of PF on MPTP-induced dopaminergic neuron loss in the SNc with pretreatment. Quantitative analysis of the TH-positive cells (a) and Nissl-stained neurons (b) in the SNc of mice. Each column and vertical bar represents the mean±s.e.m. of results from eight mice. **P<0.01 compared with saline-treated mice, ##P<0.01 compared with MPTP-treated mice (one-way ANOVA followed by Dunnett's post hoc comparison).
Figure 2.
Effects of PF on MPTP-induced dopaminergic fiber loss in the striatum with pretreatment. Each column and vertical bar represents the mean±s.e.m. of results from eight mice. **P<0.01 compared with saline-treated mice, ##P<0.01 compared with MPTP-treated mice (one-way ANOVA followed by Dunnett's post hoc comparison).
Figure 3.
Effects of PF on MPTP-induced motor deficit in mice with pretreatment. Each column and vertical bar represents the mean±s.e.m. of results from eight mice. **P<0.01 compared with saline-treated mice, #P<0.05, ##P<0.01 compared with MPTP-treated mice (one-way ANOVA followed by Dunnett's post hoc comparison).
To determine whether the neuroprotective effect of PF would also be obtained with delayed treatment, post-treatment of PF after MPTP injections was conducted in C57BL/6 mice. On the two-dose paradigm of MPTP delivery (mild model), MPTP was injected twice at a 2 h interval. Administration of MPTP with this regimen resulted in mild-to-moderate reduction of SNc dopaminergic neuron survive (approximately 39% of normal level; Figure 4) and striatal dopaminergic terminals level (approximately 23% of normal level; Figure 5). When PF (2.5 or 5 mg kg−1) was subcutaneously administered 1 h after second MPTP injections and administered once a day for the following 3 days, post-treatment of PF also significantly attenuated the motor abnormalities in the pole tests (Figure 6), neuronal loss assessed by the quantification of TH-positive cells and Nissl-stained neurons in the SNc (Figure 4) and TH-positive terminals in the striatum (Figure 5). The loss of TH-positive neurons in the SNc was significant but relatively mild compared with TH positive in the striatum. Thus, the depletion of TH-positive terminals in the striatum was coincident with cell death in the SNc.
Figure 4.
Effects of PF on MPTP-induced dopaminergic neuron loss in the SNc with post-treatment. Quantitative analysis of the TH-positive cells (a) and Nissl-stained neurons (b) in the SNc of mice. Each column and vertical bar represents the mean±s.e.m. of results from eight mice. **P<0.01 compared with saline-treated mice, ##P<0.01 compared with MPTP-treated mice (one-way ANOVA followed by Dunnett's post hoc comparison).
Figure 5.
Effects of PF on MPTP-induced dopaminergic fiber loss in the striatum with post-treatment. Each column and vertical bar represents the mean±s.e.m. of results from eight mice. **P<0.01 compared with saline-treated mice, ##P<0.05 compared with MPTP-treated mice (one-way ANOVA followed by Dunnett's post hoc comparison).
Figure 6.
Effects of PF on MPTP-induced motor deficit in mice with post-treatment. Each column and vertical bar represents the mean±s.e.m. of results from eight mice. **P<0.01 compared with saline-treated mice, #P<0.05, ##P<0.01 compared with MPTP-treated mice (one-way ANOVA followed by Dunnett's post hoc comparison).
Effect of PF on glial activation and upregulation of proinflammatory molecules
As PF has been reported to have an anti-inflammatory effect, we determined whether PF treatment could attenuated the glial-mediated inflammatory response in the MPTP mouse model of PD. Potent microglial activation in SNc and striatum was observed at 24 h after the last dose of MPTP (Figure 7b and e), while the astrocytic activation was at observed 96 h (Figure 8b and e). In terms of morphological characteristics, both the cd11b and GFAP-positive cells appeared more compact, rounded, and with obvious cellular thickening, indicative of an activated state (Soltys et al., 2001). Post-MPTP treatment of PF (5 mg kg−1) significantly attenuated the microglial and astrocytic activation (Figures 7 and 8).
Figure 7.
Effects of PF on MPTP-induced microglial activation in the SNc and striatum. Cd11b immunohistochemistry in the SNc (a–c) and striatum (d–f) of mice. (a, d) saline-treated mice, (b, e) MPTP-treated mice, (c, f) MPTP plus PF-treated mice. Microglial activation after MPTP were attenuated by PF treatment in both the SNc and striatum, n=8, scale bar, 100 μm.
Figure 8.
Effects of PF on MPTP-induced astrocytic activation in the SNc and striatum. GFAP immunohistochemistry in the SNc (a–c) and striatum (d–f) of mice, (a, d) saline-treated mice, (b, e) MPTP-treated mice, (c, f) MPTP plus PF-treated mice. Astrocytic activation after MPTP were attenuated by PF treatment in both the SNc and striatum, n=8, scale bar, 100 μm.
It has been reported that the selective expression and release of inflammatory mediators, such as cytokines and NO, from activated glial cells contribute to the pathogenesis of PD (Mogi et al., 1994a, 1994b; Przedborski & Jackson-Lewis, 1998). Given the effect of PF on MPTP-induced glial activation, we examined whether the production of known proinflammatory molecules was also inhibited by PF. The RFC in mRNA expression of IL-1β of MPTP-treated mice showed a significant 5.3-fold and 1.4-fold increase in the SNc and striatum, respectively, compared with that of saline-treated mice. Similarly, treatment of MPTP significantly augmented the expression of TNF-α and iNOS compared with saline group. The upregulation of these proinflammatory molecules was reduced by post-MPTP treatment of PF (5 mg kg−1) (Table 2). These data suggested that the anti-inflammatory property of PF might be contributed to its neuroprotective effect.
Table 2.
Effects of PF on the mRNA upregulation of proinflammatory molecules induced by MPTP
| Saline | MPTP | PF (5 mg kg−1)+MPTP | DPCPX (0.3 mg kg−1)+PF (5 mg kg−1)+MPTP | |
|---|---|---|---|---|
|
VM | ||||
| iNOS |
1.00±0.17 |
2.98±0.50** |
1.02±0.17## |
2.23±0.37$ |
| TNF-α |
1.00±0.17 |
2.69±0.45** |
1.12±0.19## |
1.74±0.29 |
| IL-1β |
1.00±0.09 |
5.26±0.84** |
2.12±0.03## |
3.51±0.14$$ |
| |
|
|
|
|
|
Striatum | ||||
| iNOS |
1.00±0.03 |
1.95±0.32* |
1.17±0.27 |
1.68±0.07 |
| TNF-α |
1.00±0.08 |
3.17±0.26** |
1.30±0.02## |
2.63±0.03$$ |
| IL-1β | 1.00±0.18 | 1.42±0.06* | 1.06±0.17 | 1.24±0.04 |
All data were normalized to the GADPH mRNA level and expressed as RFC±s.e.m. (n=8).
P<0.05,
P<0.01 compared with saline-treated mice;
P<0.01 compared with MPTP-treated mice;
P<0.05,
P<0.01 compared with PF (5 mg kg−1)+MPTP-treated mice (one-way ANOVA followed by Dunnett's post hoc comparison).
Effect of DPCPX on the neuroprotective and anti-inflammatory effects of PF
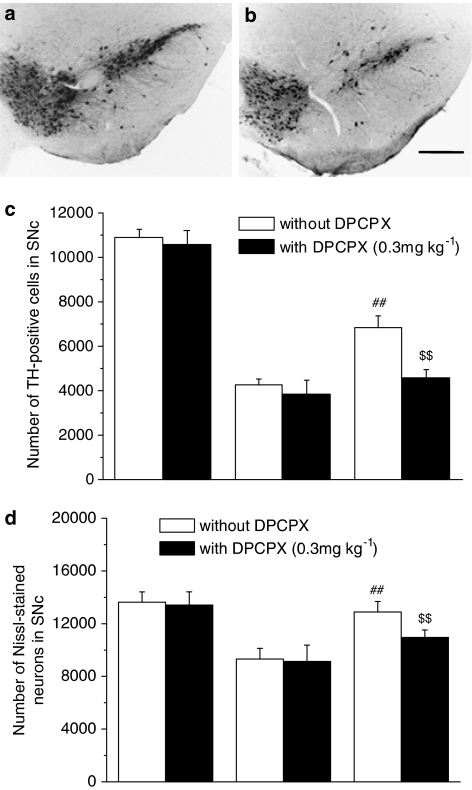
To determine whether A1AR is involved in the neuroprotective effects of PF, DPCPX (0.3 mg kg−1), a selective A1AR antagonist, was injected i.p. at 15 min before PF administration in the MPTP mild model. DPCPX alone did not cause or enhance the dopaminergic neurodegeneration and neuroinflammation in saline or MPTP-treated mice (Figures 9 and 10). However, the protective effect of PF (5 mg kg−1) was abolished by pretreatment with the selective A1AR antagonist DPCPX (0.3 mg kg−1) (Figures 9 and 10). Notably, the anti-gliosis effect of PF was reversed (Figures 11 and 12). The mRNA expression of proinflammatory molecules was also elevated by DPCPX administration compared with PF plus MPTP treatment (Table 2).
Figure 9.
Effects of DPCPX on the dopaminergic neuron-protective effects of PF in the SNc. TH immunohistochemistry in the SNc (a, b), quantitative analysis of the TH-positive cells (c) and Nissl-stained neurons (d) in the SNc of mice. (a) MPTP plus PF-treated mice, (b) MPTP plus PF-treated mice that pretreated with DPCPX before PF administration. The protective effect of PF (5 mg kg−1) on dopaminergic neurons in the SNc was abolished by pretreatment with the selective A1AR antagonist DPCPX (0.3 mg kg−1). Each column and vertical bar represents the mean±s.e.m. of results from eight mice. ##P<0.01 compared with MPTP-treated mice, $$P<0.01 compared with MPTP plus PF-treated mice (one-way ANOVA followed by Dunnett's post hoc comparison), scale bar, 200 μm.
Figure 10.
Effects of DPCPX on the dopaminergic fiber-protective effects of PF in the striatum. TH immunohistochemistry in the striatum (a, b), quantitative analysis of the TH optical density in the striatum of mice (c). (a) MPTP plus PF-treated mice, (b) MPTP plus PF-treated mice that pretreated with DPCPX before PF administration. The protective effect of PF (5 mg kg−1) on dopaminergic fibers in the striatum was abolished by pretreatment with the selective A1AR antagonist DPCPX (0.3 mg kg−1). Each column and vertical bar represents the mean±s.e.m. of results from eight mice. ##P<0.01 compared with MPTP-treated mice, $$P<0.05 compared with MPTP plus PF-treated mice (one-way ANOVA followed by Dunnett's post hoc comparison), scale bar, 500 μm.
Figure 11.
Effect of DPCPX on the microglia-modulating effects of PF. Cd11b immunohistochemistry in the SNc (a, b) and striatum (c, d), (a, c) MPTP plus PF-treated mice, (b, d) MPTP plus PF-treated mice that pretreated with DPCPX before PF administration. The microglia-modulating effects of PF (5 mg kg−1) were reversed by pretreatment with DPCPX (0.3 mg kg−1), n=8, scale bar, 100 μm.
Figure 12.
Effect of DPCPX on the astrocyte-modulating effects of PF. GFAP immunohistochemistry in the SNc (a, b) and striatum (c, d) of mice. (a, c) MPTP plus PF-treated mice, (b, d) MPTP plus PF-treated mice that pretreated with DPCPX before PF administration. The astrocyte-modulating effects of PF (5 mg kg−1) were reversed by pretreatment with DPCPX (0.3 mg kg−1), n=8, scale bar, 100 μm.
Effect of PF on MPTP metabolism
To confirm that the neuroprotective effect of PF is not caused by reducing metabolism of MPTP to MPP+, MPP+ level in the brain was measured after PF treatment. At 2 h after the MPTP treatment, MPP+ levels in the striatal were 2.21±0.25 μg g−1 (pretreated with 5 mg kg−1 PF for 7 days) and 2.38±0.11 μg g−1 (PF was injected 1 h after MPTP) in PF-treated mice, or 2.23±0.17 μg g−1 in vehicle treated mice, respectively. Thus, PF treatment had no effect on the concentration of MPP+ in the brain of MPTP-treated C57BL/6 mice.
Discussion
In the present studies, two experimental paradigms of MPTP delivery (Iwashita et al., 2004) were used to determine the neuroprotective properties of PF. Both the severe and mild MPTP treatment caused a selective dopaminergic neurodegeneration in SNc and striatum (Figures 1, 2, 4 and 5), which result in the motor deficit in the pole test in mice (Figures 3 and 6). Our results were similar to those reported by Liberatore et al. (1999), Benner et al. (2004), Kurosaki et al. (2004) and Conti et al. (2005). In the present studies, we demonstrated that 11 days' treatment with PF attenuated the dopaminergic neurotoxicity and bradykinesia induced by four-dose injection of MPTP on day 8 in mice (Figures 1, 2 and 3). Since two-dose paradigm of MPTP has been proved to be an excellent PD model for drug evaluation with delayed treatment (Iwashita et al., 2004), this paradigm was also used to determine the effects of post-MPTP treatment with PF in the present studies. We demonstrated that the neuroprotective effects were also obtained with post-MPTP treatment of PF (Figures 4, 5 and 6). In addition, the HPLC-UV assay indicated that PF administration 7 days before or 1 h after MPTP had no effect on the striatal MPP+ content in mice, suggesting that the neuroprotection afforded by PF was not attributable to impairment of MPTP metabolism. Thus, the key finding of the present studies was that PF, a monoterpene glucoside isolated from the Chinese herbal Paeony radix, could potently protected dopaminergic neurons against MPTP-induced degeneration and motor deficit in mice (Figures 1, 2, 3, 4, 5 and 6).
It is known that inflammation has an important role in the pathogenesis of PD (McGeer et al., 1988; Hunot & Hirsch, 2003). The hallmark of brain inflammation is the activation of glia, particularly microglia. Activation of microglia is thought to contribute to neuronal damage by the release of proinflammatory and neurotoxic factors. These factors include proinflammatory cytokines such as TNF-α and IL-1β, reactive nitrogen species, ROS, eicosanoids and excitatory amino acids (Mogi et al., 1994a, 1994b; Merrill & Benveniste, 1996; Przedborski & Jackson-Lewis, 1998; Liu & Hong, 2003). In the MPTP mouse model of PD, reactive gliosis has been characterized with both reactive astrogliosis and microgliosis observed in the SNc and striatum (Francis et al., 1995; Kohutnicka et al., 1998; Kurkowska-Jastrzebska et al, 1999; Kay & Blum, 2000). It has been reported that the expression of cd11b, the marker of activated microglia, reached maximum at 24–48 h after acute MPTP injection (Kohutnicka et al., 1998; Ferger et al., 2004; Furuya et al., 2004). While GFAP, the marker of astrocytes, has been reported peaked at 4–7 days after MPTP injection (Schneider & Denaro, 1988; Kohutnicka et al., 1998; Kato et al., 2004). Therefore, in the present studies, mice were killed at either 24 h or 96 h after the last dose of MPTP, respectively, in order to examine the activation of either microglia or astrocytes. We demonstrated that MPTP treatment induced a marked increase in microglial and astrocytic activation within the SNc and striatum, and that the reactive gliosis was reduced in the PF (5 mg kg−1)-treated animals (Figures 7 and 8). Upregulation of the proinflammatory molecules such as IL-1β, TNF-α and iNOS has been reported in MPTP-treated mice (Grunblatt et al., 2000; Wu et al., 2002; Ferger et al., 2004; Furuya et al., 2004; Hebert et al., 2005; Marchetti et al., 2005; Shen et al., 2005). The inhibition of these upregulation has been clarified to be neuroprotective (Mogi et al., 1998; Liberatore et al., 1999; Ferger et al., 2004). Consistent with these reports, we demonstrated that the mRNA levels of IL-1β, TNF-α, and iNOS were significantly upregulated in VM and striatum by MPTP treatment in the present studies. Moreover, the upregulation of these proinflammatory molecules was significantly reversed by PF (5 mg kg−1) (Table 2). These results strongly suggested that the neuroprotective effect of PF observed in the present investigation is most probably derived from its anti-inflammatory property.
Several lines of evidence indicate that adenosine may be an endogenous neuroprotective agent in the CNS (Rudolphi et al., 1992; Ongini & Schubert, 1998; Von Lubitz, 1999; De Mendonça et al., 2000). A1AR agonists were shown to attenuate dopaminergic neurodegeneration in different models of PD (Lau & Mouradian, 1993; DelleDonne & Sonsalla, 1994; Gol'embiowska & Źylewska, 1998), while blockade of A1AR has been found to enhance the dopaminergic system damage (Alfinito et al., 2003). To investigate whether A1AR was involved in the neuroprotection of PF, DPCPX, a selective A1AR antagonist, was used in the present studies. Since it has been reported that doses higher than 0.5 mg kg−1 DPCPX tended exacerbate MPTP toxicity (Chen et al., 2001), the lower but effective dose of 0.3 mg kg−1 DPCPX (Zarrindast et al., 1999) was chosen here. We demonstrated that DPCPX alone did not cause dopaminergic neurodegeneration in saline-treated mice or enhance the neurotoxicities in MPTP-treated mice. However, pretreatment with DPCPX reversed the neuroprotective effects of PF (Figures 9 and 10), indicating that A1AR might play an important role in the PF-induced neuroprotection. In addition, our previous studies reported that PF was able to displace the binding of [3H] N-ethylcarboxamidoadenosine (NECA), the A1AR agonist, in competitive binding assays (Liu et al., 2005).
Recent studies have demonstrated that activation of adenosine receptors on immune cells suppressed the production of proinflammatory mediators, including TNF-α (Bouma et al., 1994; Hasko et al., 1996; Sajjadi et al., 1996) and matrix metalloproteinases (Boyle et al., 1996). Adenosine receptor agonists appear to influence other macrophage properties, such as phagocytosis and chemotaxis, thereby reducing leukocyte accumulation at sites of inflammation (Cronstein, 1994; Olah & Stiles, 1995). In the CNS, the A1AR is highly expressed on microglia/macrophages and neurons (Johnston et al., 2001). However, A1AR has not been detected in dopaminergic neurons in SNc (Alexander & Reddington, 1989), indicated that the activation of A1AR might not have direct effects on dopaminergic neurons. It has been reported that activation or upregulation of A1AR which located in glial cells could attenuate neuroinflammation and demyelination in the mice model of multiple sclerosis, another disease of the CNS characterized by neuroinflammation (Tsutsui et al., 2004). In the present studies, we demonstrated that treatment with DPCPX (0.3 mg kg−1) alone did not cause reactive gliosis or upregulation of proinflammatory molecules in saline-treated mice. Treatment with DPCPX (0.3 mg kg−1) alone did not potentiate the neuroinflammation in MPTP-treated mice either (data not shown). However, pretreatment with DPCPX reversed the anti-inflammatory effects of PF (Figures 11 and 12; Table 2). The results suggested that A1AR might be involved in the anti-inflammatory effects of PF.
None of the classical A1AR agonists was used as the positive control in the present studies. One reason is that the classical A1AR agonists caused severe cardiovascular side effects (Daval et al., 1991; Collis & Hourani, 1993; Van Schaick et al., 1997). Another and more important reason is that the mechanism of PF was different from that of the classical A1AR receptor agonists (Liu et al., 2005).
In conclusion, our results demonstrated that PF, a characteristic monoterpene glucoside isolated from the root of P. alba, had a potent neuroprotective effect on dopaminergic neurons in the MPTP mouse model of PD. The neuroprotective effects of PF might be mediated though its modulation of neuroinflammation by activation of the A1AR. Our results suggested that PF might represent a promising candidate for the prevention and treatment of PD.
Acknowledgments
We thank Dr Chang-Qiang Ke for his measurement of MPP+ by HPLC-UV assay. This work was supported by research grants from the Ministry of Science and Technology of China (2004CB720305) and the Shanghai Metropolitan Fund for Research and Development (04DZ14005).
Abbreviations
- A1AR
adenosine A1 receptor
- AD
Alzheimer's disease
- CNS
central nervous system
- DMSO
dimethyl-sulfoxide
- DPCPX
8-cyclopentyl-1,3-dipropylxanthine
- GFAP
glial fibrillary acidic protein
- IL-1β
interleukin 1 beta
- iNOS
inducible nitric oxide synthase
- MPP+
1-methyl-4-phenyl-pyridiniumion
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- NECA
N-ethylcarboxamidoadenosine
- PD
Parkinson's disease
- PF
paeoniflorin
- RFC
relative fold change
- ROS
reactive oxygen species
- SNc
substantia nigra pars compacta
- TH
tyrosine hydroxylase
- TNF-α
tumor necrosis factor-alpha
- VM
ventral midbrain
References
- ALEXANDER S.P., REDDINGTON M. The cellular localization of adenosine receptors in rat neostriatum. Neuroscience. 1989;3:645–651. doi: 10.1016/0306-4522(89)90011-0. [DOI] [PubMed] [Google Scholar]
- ALFINITO P.D., WANG S.P., MANZINO L., RIJHSINGHANI S., ZEEVALK G.D., SONSALLA P.K. Adenosinergic protection of dopaminergic and GABAergic neurons against mitochondrial inhibition through receptors located in the substantia nigra and striatum, respectively. J. Neurosci. 2003;23:10982–10987. doi: 10.1523/JNEUROSCI.23-34-10982.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARAKI T., MIZUTANI H., MATSUBARA M., IMAI Y., MIZUGAKI M., ITOYAMA Y. Nitric oxide synthase inhibitors cause motor deficits in mice. Eur. Neuropsycho. Pharmacol. 2001;11:125–133. doi: 10.1016/s0924-977x(01)00077-3. [DOI] [PubMed] [Google Scholar]
- BENNER E.J., MOSLEY R.L., DESTACHE C.J., LEWIS T.B., JACKSON-LEWIS V., GORANTLA S., NEMACHEK C., GREEN S.R., PRZEDBORSKI S., GENDELMAN H.E. Therapeutic immunization protects dopaminergic neurons in a mouse model of Parkinson's disease. Proc. Natl. Acad. Sci. U.S.A. 2004;101:9435–9440. doi: 10.1073/pnas.0400569101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOUMA M.G., STAD R.K., VAN DEN WILDENBERG F.A., BUURMAN W.A. Differential regulatory effects of adenosine on cytokine release by activated human monocytes. J. Immunol. 1994;153:4159–4168. [PubMed] [Google Scholar]
- BOYLE D.L., SAJJADI F.G., FIRESTEIN G.S. Inhibition of synoviocyte collagenase gene expression by adenosine receptor stimulation. Arthritis. Rheum. 1996;39:923–930. doi: 10.1002/art.1780390608. [DOI] [PubMed] [Google Scholar]
- CHEN J.F., XU K., PETZER J.P., STAAL R., XU Y.H., BEILSTEIN M., SONSALLA P.K., CASTAGNOLI K., CASTAGNOLI N., Jr, SCHWARZSCHILD M.A. Neuroprotection by caffeine and A(2A) adenosine receptor inactivation in a model of Parkinson's disease. J. Neurosci. 2001;21:RC143. doi: 10.1523/JNEUROSCI.21-10-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHENG J.T., WANG C.J., HSU F.L. Paeoniflorin reverses guanethidine-induced hypotension viaactivation of central adenosine A1 receptors in Wistar rats. Clin. Exp. Pharmacol. Physiol. 1999;26:815–816. doi: 10.1046/j.1440-1681.1999.03132.x. [DOI] [PubMed] [Google Scholar]
- COGGESHALL R.E. A consideration of neural counting methods. Trends Neurosci. 1992;15:9–13. doi: 10.1016/0166-2236(92)90339-a. [DOI] [PubMed] [Google Scholar]
- COLLIS M.G., HOURANI S.M. Adenosine receptor subtypes. Trends Pharmacol. Sci. 1993;14:360–366. doi: 10.1016/0165-6147(93)90094-z. [DOI] [PubMed] [Google Scholar]
- CONTI B., SUGAMA S., LUCERO J., WINSKY-SOMMERER R., WIRZ S.A., MAHER P., ANDREWS Z., BARR A.M., MORALE M.C., PANEDA C., PEMBERTON J., GAIDAROVA S., BEAL F., SANNA P.P., HORVATH T., BARTFAI T. Uncoupling protein 2 protects dopaminergic neurons from acute 1,2,3,6-methyl-phenyl-tetrahydropyridine toxicity. J. Neurochem. 2005;93:493–501. doi: 10.1111/j.1471-4159.2005.03052.x. [DOI] [PubMed] [Google Scholar]
- CROCKER S.J., SMITH P.D., JACKSON-LEWIS V., LAMBA W.R., HAYLEY S.P., GRIMM E., CALLAGHAN S.M., SLACK R.S., MELLONI E., PRZEDBORSKI S., ROBERTSON G.S., ANISMAN H., MERALI Z., PARK D.S. Inhibition of calpains prevents neuronal and behavioral deficits in an MPTP mouse model of Parkinson's disease. J. Neurosci. 2003;23:4081–4091. doi: 10.1523/JNEUROSCI.23-10-04081.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRONSTEIN B.N. Adenosine, an endogenous anti-inflammatory agent. J. Appl. Physiol. 1994;76:5–13. doi: 10.1152/jappl.1994.76.1.5. [DOI] [PubMed] [Google Scholar]
- DAVAL J.L., NEHLIG A., NICOLAS F. Physiological and pharmacological properties of adenosine: therapeutic implications. Life Sci. 1991;49:1435–1453. doi: 10.1016/0024-3205(91)90043-b. [DOI] [PubMed] [Google Scholar]
- DEGIORGIO L.A., DIBINIS C., MILNER T.A., SAJI M., VOLPE B.T. Histological and temporal characteristics of nigral transneuronal degeneration after striatal injury. Brain Res. 1998;795:1–9. doi: 10.1016/s0006-8993(98)00247-9. [DOI] [PubMed] [Google Scholar]
- DELLEDONNE K.T., SONSALLA P.K. Protection against methamphetamine-induced neurotoxicity to neostriatal dopaminergic neurons by adenosine receptor activation. J. Pharmacol. Exp. Ther. 1994;271:1320–1326. [PubMed] [Google Scholar]
- DE MENDONÇA A., SEBASTIÃO A.M., RIBEIRO J.A. Adenosine: does it have a neuroprotective role after all. Brain Res. Rev. 2000;33:258–274. doi: 10.1016/s0165-0173(00)00033-3. [DOI] [PubMed] [Google Scholar]
- DEZAKI K., KIMURA I., MIYAHARA K., KIMURA M. Complementary effects of paeoniflorin and glycyrrhizin on intracellular Ca2+ mobilization in the nerve-stimulated skeletal muscle of mice. Jap. J. Pharmacol. 1996;69:281–284. doi: 10.1254/jjp.69.281. [DOI] [PubMed] [Google Scholar]
- FERGER B., LENG A., MURA A., HENGERER B., FELDON J. Genetic ablation of tumor necrosis factor-alpha (TNF-alpha) and pharmacological inhibition of TNF-synthesis attenuates MPTP toxicity in mouse striatum. J. Neurochem. 2004;89:822–833. doi: 10.1111/j.1471-4159.2004.02399.x. [DOI] [PubMed] [Google Scholar]
- FRANCIS J.W., VON VISGER J., MARKELONIS G.J., OH T.H. Neuroglial responses to the dopaminergic neurotoxicant 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in mouse striatum. Neurotoxicol. Teratol. 1995;17:7–12. doi: 10.1016/0892-0362(94)00048-i. [DOI] [PubMed] [Google Scholar]
- FURUYA T., HAYAKAWA H., YAMADA M., YOSHIMI K., HISAHARA S., MIURA M., MIZUNO Y., MOCHIZUKI H. Caspase-11 mediates inflammatory dopaminergic cell death in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson's disease. J. Neurosci. 2004;24:1865–1872. doi: 10.1523/JNEUROSCI.3309-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAO H.M., LIU B., ZHANG W., HONG J.S. Novel anti-inflammatory therapy for Parkinson's disease. Trends. Pharmacol. Sci. 2003;24:395–401. doi: 10.1016/S0165-6147(03)00176-7. [DOI] [PubMed] [Google Scholar]
- GOL'EMBIOWSKA K., ŹYLEWSKA A. Agonists of A2A and A2A adenosine receptors attenuate methamphetamine-induced overflow of dopamine in rat striatum. Brain Res. 1998;806:202–209. doi: 10.1016/s0006-8993(98)00743-4. [DOI] [PubMed] [Google Scholar]
- GRUNBLATT E., MANDEL S., YOUDIM M.B. MPTP and 6-hydroxydopamine-induced neurodegeneration as models for Parkinson's disease: neuroprotective strategies. J. Neurol. 2000;247 (Suppl 2):II95–II102. doi: 10.1007/pl00022909. [DOI] [PubMed] [Google Scholar]
- GUNDERSEN H.J. Stereology: the fast lane between neuroanatomy and brain function – or still only a tightrope. Acta. Neurol. Scand. 1992;137 (Suppl):8–13. doi: 10.1111/j.1600-0404.1992.tb05032.x. [DOI] [PubMed] [Google Scholar]
- HASKO G., SZABO C., NEMETH Z.H., KVETAN V., PASTORES S.M., VIZI E.S. Adenosine receptor agonists differentially regulate IL-10, TNF-alpha, and nitric oxide production in RAW 264.7 macrophages and in endotoxemic mice. J. Immunol. 1996;157:4634–4640. [PubMed] [Google Scholar]
- HEBERT G., MINGAM R., ARSAUT J., DANTZER R., DEMOTES-MAINARD J. A role of IL-1 in MPTP-induced changes in striatal dopaminergic and serotoninergic transporter binding: clues from interleukin-1 type I receptor-deficient mice. Brain Res. Mol. Brain. Res. 2005;136:267–270. doi: 10.1016/j.molbrainres.2005.01.009. [DOI] [PubMed] [Google Scholar]
- HSU F.L., LAI C.W., CHENG J.T. Antihyperglycemic effects of paeoniflorin and 8-debenzoylpaeoniflorin, glucosides from the root of Paeonia lactiflora. Planta Medica. 1997;63:323–325. doi: 10.1055/s-2006-957692. [DOI] [PubMed] [Google Scholar]
- HUNOT S., HIRSCH E.C. Neuroinflammatory processes in Parkinson's disease. Ann. Neurol. 2003;53:S49–S60. doi: 10.1002/ana.10481. [DOI] [PubMed] [Google Scholar]
- IWASHITA A., YAMAZAKI S., MIHARA K., HATTORI K., YAMAMOTO H., ISHIDA J., MATSUOKA N., MUTOH S. Neuroprotective effects of a novel poly(ADP-ribose) polymerase-1 inhibitor, 2-[3-[4-(4-chlorophenyl)-1-piperazinyl] propyl]-4(3H)-quinazolinone ( FR255595), in an in vitro model of cell death and in mouse 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson's disease. J. Pharmacol. Exp. Ther. 2004;309:1067–1078. doi: 10.1124/jpet.103.064642. [DOI] [PubMed] [Google Scholar]
- JOHNSTON J.B., SILVA C., GONZALEZ G., HOLDEN J., WARREN K.G., METZ L.M., POWER C. Diminished adenosine A1 receptor expression on macrophages in brain and blood of patients with multiple sclerosis. Ann. Neurol. 2001;49:650–658. [PubMed] [Google Scholar]
- KATO H., KUROSAKI R., OKI C., ARAKI T. Arundic acid, an astrocyte-modulating agent, protects dopaminergic neurons against MPTP neurotoxicity in mice. Brain Res. 2004;1030:66–73. doi: 10.1016/j.brainres.2004.09.046. [DOI] [PubMed] [Google Scholar]
- KAY J.N., BLUM M. Differential response of ventral midbrain and striatal progenitor cells to lesions of the nigrostriatal dopaminergic projection. Dev. Neurosci. 2000;22:56–67. doi: 10.1159/000017427. [DOI] [PubMed] [Google Scholar]
- KIM W.G., MOHNEY R.P., WILSON B., JEOHN G.H., LIU B., HONG J.S. Regional difference in susceptibility to lipopolysaccharide-induced neurotoxicity in the rat brain: role of microglia. J. Neurosci. 2000;20:6309–6316. doi: 10.1523/JNEUROSCI.20-16-06309.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOHUTNICKA M., LEWANDOWSKA E., KURKOWSKA-JASTRZEBSKA I., CZLONKOWSKI A., CZLONKOWSKA A. Microglial and astrocytic involvement in a murine model of Parkinson's disease induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) Immunopharmacology. 1998;39:167–180. doi: 10.1016/s0162-3109(98)00022-8. [DOI] [PubMed] [Google Scholar]
- KREUTZBERG G.W. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- KURKOWSKA-JASTRZEBSKA I., WRONSKA A., KOHUTNICKA M., CZLONKOWSKI A., CZLONKOWSKA A. The inflammatory reaction following 1-methyl-4-phenyl-1,2,3, 6-tetrahydropyridine intoxication in mouse. Exp. Neurol. 1999;156:50–61. doi: 10.1006/exnr.1998.6993. [DOI] [PubMed] [Google Scholar]
- KUROSAKI R., MURAMATSU Y., KATO H., ARAKI T. Biochemical, behavioral and immunohistochemical alterations in MPTP-treated mouse model of Parkinson's disease. Pharmacol. Biochem. Behav. 2004;78:143–153. doi: 10.1016/j.pbb.2004.03.006. [DOI] [PubMed] [Google Scholar]
- LAI C.W., HSU F.L., CHENG J.T. Stimulatory effect of paeoniflorin on adenosine A1 receptors to increase the translocation of protein kinase C (PKC) and glucose transporter (GLUT 4) in isolated rat white adipocytes. Life Sci. 1998;62:1591–1595. doi: 10.1016/s0024-3205(98)00112-x. [DOI] [PubMed] [Google Scholar]
- LAU Y.S., MOURADIAN M.M. Protection against acute MPTP-induced dopamine depletion in mice by adenosine A1 agonist. J. Neurochem. 1993;60:768–771. doi: 10.1111/j.1471-4159.1993.tb03215.x. [DOI] [PubMed] [Google Scholar]
- LAWSON L.J., PERRY V.H., DRI P., GORDON S. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience. 1996;39:151–170. doi: 10.1016/0306-4522(90)90229-w. [DOI] [PubMed] [Google Scholar]
- LE MOINE O., STORDEUR P., SCHANDENE L., MARCHANT A., DE GROOTE D., GOLDMAN M., DEVIERE J. Adenosine enhances IL-10 secretion by human monocytes. J. Immunol. 1996;156:4408–4414. [PubMed] [Google Scholar]
- LIBERATORE G.T., JACKSON-LEWIS V., VUKOSAVIC S., MANDIR A.S., VILA M., MCAULIFFE W.G., DAWSON V.L., DAWSON T.M., PRZEDBORSKI S. Inducible nitric oxide synthase stimulates dopaminergic neurodegeneration in the MPTP model of Parkinson disease. Nat. Med. 1999;5:1403–1409. doi: 10.1038/70978. [DOI] [PubMed] [Google Scholar]
- LIU B., HONG J.S. Role of microglia in inflammation-mediated neurodegenerative diseases: mechanisms and strategies for therapeutic intervention. J. Pharmacol. Exp. Ther. 2003;304:1–7. doi: 10.1124/jpet.102.035048. [DOI] [PubMed] [Google Scholar]
- LIU D.Z., XIE K.Q., JI X.Q., YE Y., JIANG C.L., ZHU X.Z. Neuroprotective effect of paeoniflorin on cerebral ischemic rat by activating adenosine A1 receptor in a different manner from its classical agonists. Br. J. Pharmacol. 2005;146:604–611. doi: 10.1038/sj.bjp.0706335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARCHETTI B., SERRA P.A., TIROLO C., L'EPISCOPO F., CANIGLIA S., GENNUSO F., TESTA N., MIELE E., DESOLE S., BARDEN N., MORALE M.C. Glucocorticoid receptor-nitric oxide crosstalk and vulnerability to experimental parkinsonism: pivotal role for glia-neuron interactions. Brain Res. Brain Res Rev. 2005;48:302–321. doi: 10.1016/j.brainresrev.2004.12.030. [DOI] [PubMed] [Google Scholar]
- MATSUURA K., KABUTO H., MAKINO H., OGAWA N. Pole test is a useful method for evaluating the mouse movement disorder caused by striatal dopamine depletion. J. Neurosci. Methods. 1997;73:45–48. doi: 10.1016/s0165-0270(96)02211-x. [DOI] [PubMed] [Google Scholar]
- MCGEER P.L., ITAGAKI S., BOYES B.E., MCGEER E.G. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson's and Alzheimer's disease brains. Neurology. 1988;38:1285–1291. doi: 10.1212/wnl.38.8.1285. [DOI] [PubMed] [Google Scholar]
- MERRILL J.E., BENVENISTE E.N. Cytokines in inflammatory brain lesions: helpful and harmful. Trends Neurosci. 1996;19:331–338. doi: 10.1016/0166-2236(96)10047-3. [DOI] [PubMed] [Google Scholar]
- MOGI M., HARADA M., KONDO T., RIEDERER P., INAGAKI H., MINAMI M., NAGATSU T. Interleukin-1 beta, interleukin-6, epidermal growth factor and transforming growth factor-alpha are elevated in the brain from parkinsonian patients. Neurosci. Lett. 1994a;180:147–150. doi: 10.1016/0304-3940(94)90508-8. [DOI] [PubMed] [Google Scholar]
- MOGI M., HARADA M., RIEDERER P., NARABAYASHI H., FUJITA K., NAGATSU T. Tumor necrosis factor-alpha (TNF-alpha) increases both in the brain and in the cerebrospinal fluid from parkinsonian patients. Neurosci. Lett. 1994b;165:208–210. doi: 10.1016/0304-3940(94)90746-3. [DOI] [PubMed] [Google Scholar]
- MOGI M., TOGARI A., OGAWA M., IKEGUCHI K., SHIZUMA N., FAN D., NAKANO I., NAGATSU T. Effects of repeated systemic administration of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) to mice on interleukin- 1beta and nerve growth factor in the striatum. Neurosci. Lett. 1998;250:25–28. doi: 10.1016/s0304-3940(98)00427-3. [DOI] [PubMed] [Google Scholar]
- OLAH M.E., STILES G.L. Adenosine receptor subtypes: characterization and therapeutic regulation. Annu. Rev. Pharmacol. Toxicol. 1995;35:581–586. doi: 10.1146/annurev.pa.35.040195.003053. [DOI] [PubMed] [Google Scholar]
- ONGINI E., SCHUBERT P. Neuroprotection induced by stimulating A1 or blocking A2A adenosine receptors: an apparent paradox. Drug Dev. Res. 1998;45:387–393. [Google Scholar]
- POWER C., HENRY S., DEL BIGIO M.R., LARSEN P.H., CORBETT D., IMAI Y., YONG V.W., PEELING J. Intracerebral hemorrhage induces macrophage activation and matrix metalloproteinases. Ann Neurol. 2003;53:731–741. doi: 10.1002/ana.10553. [DOI] [PubMed] [Google Scholar]
- PRZEDBORSKI S., JACKSON-LEWIS V. Experimental developments in movement disorders: update on proposed free radical mechanisms. Curr. Opin. Neurol. 1998;11:35–339. doi: 10.1097/00019052-199808000-00009. [DOI] [PubMed] [Google Scholar]
- RUDOLPHI K., SCHUBERT P., PARKINSON F.E., FREDHOLM B.B. Neuroprotective role of adenosine in cerebral ischaemia. Trends Pharmacol. Sci. 1992;13:439–445. doi: 10.1016/0165-6147(92)90141-r. [DOI] [PubMed] [Google Scholar]
- SAJJADI F.G., TAKABAYASHI K, FOSTER A.C., DOMINGO R.C., FIRESTEIN G.S. Inhibition of TNF-alpha expression by adenosine: role of A3 adenosine receptors. J. Immunol. 1996;156:3435–3442. [PubMed] [Google Scholar]
- SCHNEIDER J.S., DENARO F.J. Astrocytic responses to the dopaminergic neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) in cat and mouse brain. J. Neuropathol. Exp. Neurol. 1988;47:452–458. doi: 10.1097/00005072-198807000-00006. [DOI] [PubMed] [Google Scholar]
- SCHWANINGER M., NEHER M., VIEGAS E., SCHNEIDER A., SPRANGER M. Stimulation of interleukin-6 secretion and gene transcription in primary astrocytes by adenosine. J. Neurochem. 1997;69:1145–1150. doi: 10.1046/j.1471-4159.1997.69031145.x. [DOI] [PubMed] [Google Scholar]
- SHEN Y.Q., HEBERT G., LIN L.Y., LUO Y.L., MOZE E., LI K.S., NEVEU P.J. Interleukine-1beta and interleukine-6 levels in striatum and other brain structures after MPTP treatment: influence of behavioral lateralization. J. Neuroimmunol. 2005;158:14–25. doi: 10.1016/j.jneuroim.2004.06.011. [DOI] [PubMed] [Google Scholar]
- SOLTYS Z., ZIAJA M., PAWLINSKI R., SETKOWICZ Z., JANECZKO K. Morphology of reactive microglia in the injured cerebral cortex. Fractal analysis and complementary quantitative methods. J. Neurosci. Res. 2001;63:90–97. doi: 10.1002/1097-4547(20010101)63:1<90::AID-JNR11>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- SUGAMA S., YANG L., CHO B.P., DEGIORGIO L.A., LORENZL S., ALBERS D.S., BEAL M.F., VOLPE B.T., JOH T.H. Age-related microglial activation in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced dopaminergic neurodegeneration in C57BL/6 mice. Brain Res. 2003;964:288–294. doi: 10.1016/s0006-8993(02)04085-4. [DOI] [PubMed] [Google Scholar]
- SUGISHITA E., AMAGAYA S., OGIHARA Y. Studies on the combination of Glycyrrhizae Radix in Shakuyakukanzo-To. J. Pharmacobiodyn. 1984;7:427–435. doi: 10.1248/bpb1978.7.427. [DOI] [PubMed] [Google Scholar]
- TAKEDA S., ISONO T., WAKUI Y., MATSUZAKI Y., SASAKI H., AMAGAYA S., MARUNO M. Absorption and excretion of paeoniflorin in rats. J. Pharm. Pharmacol. 1995;47:1036–1040. doi: 10.1111/j.2042-7158.1995.tb03293.x. [DOI] [PubMed] [Google Scholar]
- TAMAYA T., SATO S., OKADA H.H. Possible mechanism of steroid action of the plant herb extracts glycyrrhizin, glycyrrhetinic acid, and paeoniflorin: inhibition by plant herb extracts of steroid protein binding in the rabbit. Ameri. J. Obstetrics. Gynecol. 1986;155:1134–1139. doi: 10.1016/0002-9378(86)90365-0. [DOI] [PubMed] [Google Scholar]
- TANG L.M., LIU I.M., CHENG J.T. Stimulatory effect of paeoniflorin on adenosine release to increase the glucose uptake into white adipocytes of Wistar rat. Planta. Med. 2003;69:332–336. doi: 10.1055/s-2003-38878. [DOI] [PubMed] [Google Scholar]
- TEISMANN P., TIEU K., CHOI D.K., WU D.C., NAINI A., HUNOT S., VILA M., JACKSON-LEWIS V., PRZEDBORSKI S. Cyclooxygenase-2 is instrumental in Parkinson's disease neurodegeneration. Proc. Natl. Acad. Sci. U.S.A. 2003;100:5473–5478. doi: 10.1073/pnas.0837397100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TSUTSUI S., SCHNERMANN J., NOORBAKHSH F., HENRY S., YONG V.W., WINSTON B.W., WARREN K., POWER C. A1 adenosine receptor up-regulation and activation attenuates neuroinflammation and demyelination in a model of multiple sclerosis. J. Neurosci. 2004;24:1521–1529. doi: 10.1523/JNEUROSCI.4271-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN SCHAICK E.A., MATH-OT R.A., GUBBENS-STIBBE J.M., LANGEMEIJER M.W., ROELEN H.C., IJZERMAN A.P., DANHOF M. 8-Alkylamino-substituted analogs of N6-cyclopentyladenosine are partial agonists for the cardiovascular adenosine A1 receptors in vivo. J. Pharmacol. Exp. Ther. 1997;283:800–808. [PubMed] [Google Scholar]
- VOLPE B.T., WILDMANN J., ALTAR C.A. Brain-derived neurotrophic factor prevents the loss of nigral neurons induced by excitotoxic striatal–pallidal lesions. Neuroscience. 1998;83:741–748. doi: 10.1016/s0306-4522(97)00424-7. [DOI] [PubMed] [Google Scholar]
- VON LUBITZ D.K.J.E. Adenosine and cerebral ischemia: therapeutic future or death of a brave concept. Eur. J. Pharmacol. 1999;365:9–25. doi: 10.1016/s0014-2999(98)00788-2. [DOI] [PubMed] [Google Scholar]
- WU D.C., JACKSON-LEWIS V., VILA M., TIEU K., TEISMANN P., VADSETH C., CHOI D.K., ISCHIROPOULOS H., PRZEDBORSKI S. Blockade of microglial activation is neuroprotective in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson disease. J. Neurosci. 2002;22:1763–1771. doi: 10.1523/JNEUROSCI.22-05-01763.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAMAHARA J., YAMADA T., KIMURA H., SAWADA T., FUJIMURA H. Biologically active principles of crude drugs. II. Anti-allergic principles in ‘Shoseiryu-To' anti-inflammatory properties of paeoniflorin and its derivatives. J. Pharmacobiodyn. 1982;5:921–929. doi: 10.1248/bpb1978.5.921. [DOI] [PubMed] [Google Scholar]
- YANG J., HE L.N., HE S.B., LI G.R. Protective effect of paeoniflorin on calcium overloading injury in cultured primary cortex neurons. Chin. J. Pharmacol. Toxicol. 2001;15:164–168. [Google Scholar]
- ZHOU H.F., LIU X.Y., NIU D.B., LI F.Q., HE Q.H., WANG X.M. Triptolide protects dopaminergic neurons from inflammation-mediated damage induced by lipopolysaccharide intranigral injection. Neurobiol. Dis. 2005;18:441–449. doi: 10.1016/j.nbd.2004.12.005. [DOI] [PubMed] [Google Scholar]
- ZARRINDAST M.R., NAGHIPOUR B., ROUSHAN-ZAMIR F., SHAFAGHI B. Effects of adenosine receptor agents on the expression of morphine withdrawal in mice. Eur. J. Pharmacol. 1999;369:17–22. doi: 10.1016/s0014-2999(99)00021-7. [DOI] [PubMed] [Google Scholar]