Abstract
To investigate the role of intracellular Ca stores in generating spontaneous excitation of the urethra, the effects of cyclopiazonic acid (CPA) on spontaneous contractions, transient increases in intracellular calcium concentration ([Ca2+]i; Ca transients) and depolarizations were examined in smooth muscles of the rabbit urethra.
In about 90% of circular smooth muscle (CSM) preparations, CPA (10 μM) increased the amplitude of spontaneous contractions by about 180% and reduced their frequency to some 25% of control values (CPA-resistant), while it readily abolished the contractions in the remaining preparations.
In about 70% of CSM preparations, CPA prevented the generation of spontaneous depolarizations termed slow waves, but increased their amplitude and duration in the remainder. CPA also prevented the generation of spontaneous Ca transients in about 40% of CSM preparations, while increasing their amplitude and duration in the remaining preparations. In CPA-resistant preparations that had been exposed to nicardipine (1 μM), subsequent CPA invariably abolished residual spontaneous depolarizations or Ca transients. CPA abolished caffeine-induced Ca transients in Ca-free solutions, suggesting that it effectively depleted intracellular Ca stores.
Longitudinal smooth muscles generated spontaneous action potentials, which had a shape distinct from that of slow waves in CSM. Spontaneous action potentials were abolished by nicardipine but not CPA.
Transmural nerve stimulation increased the frequency of Ca transients to give a sustained rise in [Ca2+]i, but inhibited their generation after blocking α-adrenoceptors with phentolamine (1 μM). These nerve-evoked responses were preserved in preparations that had been exposed to CPA. Similarly, both in control and CPA-treated CSM preparations, spontaneous Ca transients were accelerated by noradrenaline (NAd, 1 μM) and were suppressed by 3-morpholino-sydnonimine (SIN-1, 10 μM), a nitric oxide (NO) donor.
In conclusion, CSM of the urethra generates spontaneous activity, which depends on Ca release from intracellular Ca stores. However, after blocking this primary pacemaking mechanism, L-type Ca channel-dependent action potentials may drive CSM. Irrespective of the origin of pacemaking, neurally-released NAd and NO are capable of modulating spontaneous excitation.
Keywords: Urethra, spontaneous excitation, intracellular Ca store, L-type Ca channel, cyclopiazonic acid
Introduction
Smooth muscle of the urethra plays an important role in maintaining urinary continence by generating a sufficiently high tone to exceed intravesical pressure (Bridgewater et al., 1993). Urethral smooth muscles themselves are capable of developing spontaneous contractions which are augmented by neurally-released noradrenaline (NAd) which tonically acts on α1-adrenoceptors (Andersson & Wein, 2004). During micturition, nitric oxide (NO) released from non-adrenergic, non-cholinergic nerves relaxes urethral smooth muscle, so that the intravesical pressure overcomes the outlet closure pressure (Andersson et al., 1992).
The cellular mechanisms underlying spontaneous contractions in the urethral smooth muscles are considered to be spontaneous depolarizations. Smooth muscles of the urethra exhibit two types of spontaneous depolarization, that is, small, irregular events termed spontaneous transient depolarizations (STDs) and larger, synchronous events termed slow waves (Hashitani et al., 1996; Hashitani & Edwards, 1999). STDs may result from Ca release from intracellular stores which opens Ca-activated chloride channels as they are readily blocked by either cyclopiazonic acid (CPA), an inhibitor of the sarcoplasmic-endoplasmic reticulum Ca (SERCA) pump, or niflumic acid, a blocker of Ca-activated chloride channels. Summed STDs result in larger depolarizations which reach the threshold for the opening of L-type Ca channels (Hashitani & Edwards, 1999). The activation of L-type Ca channels contributes to the plateau phase of slow waves that lead to spontaneous contractions (Hashitani et al, 1996; Hashitani & Edwards, 1999).
Extensive studies using isolated cells taken from the urethra revealed that spontaneous ‘myogenic' excitation in fact originates from interstitial cells rather than from smooth muscle cells. Freshly isolated interstitial cells exhibit spontaneous transient inward currents (STICs) and corresponding Ca oscillations which depend on Ca release from intracellular Ca stores and subsequent activation of Ca-activated chloride channels (Sergeant et al., 2000; 2001; Johnston et al., 2005). On the other hand, urethral smooth muscle cells are only rarely spontaneously active, although they generate action potentials in response to stimulation (Sergeant et al., 2000). Therefore, interstitial cells are now considered to be a primary pacemaker for spontaneous excitation in the urethra.
As interstitial cells are non-contractile, spontaneous excitation originating from these cells may be transmitted to smooth muscles, presumably via gap junction, to initiate spontaneous contractions. STDs recorded both from intact urethral smooth muscles and STICs in isolated interstitial cells are virtually insensitive to blockers of L-type Ca channels (Hashitani et al., 1996; Hashitani & Edwards, 1999; Sergeant et al., 2001), however L-type Ca channels in urethral smooth muscles play a principal role in generating spontaneous contractions. Hence, inhibition of pacemaking function of interstitial cells should lead to a loss of activation of smooth muscle L-type Ca channels, and thus suppress spontaneous contractions.
Intramuscular interstitial cells of Cajal (ICC-IM) receive both excitatory and inhibitory neuronal inputs, and transmit them to gastrointestinal smooth muscles (Hirst & Ward, 2003). Consistently, STICs recorded from urethral interstitial cells are facilitated by NAd, suggesting that these cells may also play an important role in the neurally-mediated regulation of spontaneous excitation (Sergeant et al., 2002). Furthermore, the frequency of slow waves recorded from intact circular smooth muscles (CSMs) of the rabbit urethra was increased by NAd, and was reduced by sodium nitroprusside, a NO donor (Hashitani et al., 1996). However, it still remains to be established whether or not neuronal regulation of spontaneous excitation in the urethra only results from modulation of pacemaking activity in interstitial cells.
The aim of the present study was to investigate the cellular mechanisms underlying spontaneous contractions in CSM of the rabbit urethra, particularly focusing on the role of intracellular Ca stores in their generation. The effects of CPA on spontaneous contractions, Ca transients and slow waves were examined. The possible role of Ca store-dependent pacemaking mechanisms in nerve-mediated modulation of spontaneous excitation in the urethra was also examined.
Methods
Tissue preparation
Male rabbits, weighing 2–3 kg, were killed by exsanguination under pentobarbitone anaesthesia. This procedure has been approved by the animal experimentation ethics committee of the Japanese Society for Physiology. The urethra and bladder were removed, and the urethra was dissected free of the bladder approximately 3 cm distal to the bilateral ureter entry. The dorsal wall of the urethra was then opened longitudinally; the mucosa and periurethral connective tissues were then dissected away. The outer striated muscle and longitudinal smooth muscle were carefully removed leaving circular muscle layers. However, the division into circular and longitudinal smooth muscle layers is not as clear as in the gastrointestinal tract wall.
For tension recordings, CSM strips of approximately 1 × 2 × 10 mm were used. For Ca measurements or intracellular recordings, smaller preparations, approximately 3–6 mm long and 1–2 mm wide, were dissected and then a few smooth muscle layers were removed leaving CSM preparations with a few muscle bundles. To standardize experimental conditions, tension recordings were also carried out using smaller preparations.
Isometric tension recordings
For isometric tension recordings, preparations were transferred to 2 ml organ baths and were superfused with warmed (36°C) Krebs' solution at a constant flow rate (2 ml min−1). Silk threads were tied around both ends of a strip, one of them was fixed at the bottom of the organ bath and the other was connected to an isometric force transducer that was connected to a bridge amplifier. Isometric tension changes were digitized using Digidata 1200 interface and stored on a personal computer for later analysis. After setting up, the preparations were allowed to equilibrate for 60–90 min; during this period, a basal tension of approximately 2 mN was applied.
To detect tension changes of smaller preparations, one end was pinned out on a Sylgard plate, and the other end was tied by a nylon thread that connected to a force transducer.
Intramural nerves were selectively stimulated by passing brief pulses of constant current (duration 100 μs) between two parallel sliver plate electrodes placed in the organ baths. The neural selectivity was confirmed by sensitivity to tetrodotoxin (1 μM).
Intracellular calcium measurements
For the measurement of changes in [Ca2+]i, small preparations were pinned out on a Sylgard plate (silicone elastomer, Dow Corning Corporation, Midland, MI, U.S.A.) at the bottom of a recording chamber (volume, approximately 1 ml) which was mounted on the stage of an inverted microscope. After 1 h incubation with warmed (36°C) Krebs' solution, spontaneous movements of the tissues were observed. Subsequently, the preparations were loaded with a fluorescent dye, by incubation in nominally Ca2+-free solution containing fura-2 AM (10 μM; Molecular Probes) and cremphor EL (0.01%, Sigma) for 1 h at room temperature. After loading, preparations were superfused with dye-free warmed (36°C) Krebs' solution at a constant flow (about 2 ml min−1) for 30 min. Preparations, loaded with fura-2, were illuminated with ultraviolet light, wave lengths 340 and 380 nm, alternating at a frequency higher than 40 Hz. The ratio of the emission fluorescence (R340/380) in a desired size of rectangular window was measured through a barrier filter (peak transmission 510 nm; sampling time 150–210 ms), using a micro-photoluminescence measurement system (ARGUS/HiSCA, Hamamatsu Photonics, Hamamatsu, Japan), and was taken as an index of [Ca2+]i.
For transmural nerve stimulation, the preparations were placed between a pair of platinum electrodes in the recording chamber, and were stimulated by passing brief pulses of constant current (duration 50 μs). The neural selectivity was confirmed by sensitivity to tetrodotoxin (1 μM).
Intracellular recordings
For the recording of the membrane potential, preparations were pinned out in the same type of recording chamber used for the calcium experiments and were superfused with warmed (36°C) Krebs' solution at a constant flow rate (2 ml min−1). After 1 h equilibration, individual urethral smooth muscle cells were impaled with glass capillary microelectrodes, filled with 0.5 M KCl (tip resistance, 150–250 MΩ). Membrane potential changes were recorded using a high-input impedance amplifier (Axoclamp-2B, Axon Instruments, Inc., Foster City, CA, U.S.A.), and displayed on a cathode-ray oscilloscope (SS-9622, Iwatsu, Tokyo, Japan). After low-pass filtering (cutoff frequency, 1 kHz), membrane potential changes were digitized with a Digidata 1200 interface (Axon Instruments, Inc., Union City, CA, U.S.A.), and stored on a personal computer for later analysis.
Solutions and drugs
The ionic composition of Krebs' solution was as follows (mM); NaCl, 119; KCl, 5.0; CaCl2, 2.5; MgCl2, 2.0; NaHCO3, 25.0; NaH2PO4, 1.0 and glucose, 11.0. The solution was bubbled with 95% O2 and 5% CO2 to maintain pH in the recording bath at approximately 7.4. Nominally Ca-free solution was prepared by omitting CaCl2 from the composition of Krebs' solution.
Drugs used were 3-morpholino-sydnonimine (SIN-1) hydrochloride, Nω-nitro-L-arginine (L-nitro-arginine), caffeine, CPA, nicardipine, noradrenaline hydrochloride, phentolamine, tetrodotoxin and thapsigargin (all from Sigma, St Louis, MI, U.S.A.). These drugs were dissolved in distilled water except CPA, nicardipine and thapsigargin which were dissolved in dimethyl sulphoxide (DMSO). Caffeine was directly dissolved into nominally Ca-free solution to obtain its final concentration. The final concentration of these solvents in physiological saline did not exceed 1 : 1000.
Calculations and statistics
Measured values are expressed as mean±standard deviation. Statistical significance was tested using Student's t-test, and probabilities of less than 5% were considered significant.
Results
Effects of CPA on spontaneous contractions
Circular smooth muscle preparations of the rabbit urethra developed phasic contractions (Figure 1Aa and b). Spontaneous contractions had a frequency ranging between 12.2 and 23.4 min−1 (mean 15.9±2.9 min−1) and had a peak amplitude ranging between 1.9–3.4 mN (mean 2.7±0.7 mN, n=14). To investigate the role of intracellular Ca stores, the generation of these spontaneous contractions, and the effect of blocking the SERCA pumps using CPA on phasic contractions was examined.
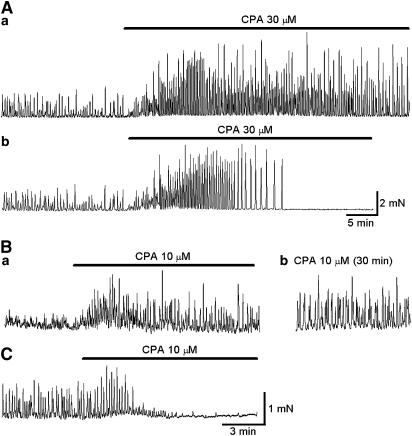
Figure 1.
Effects of CPA on spontaneous contractions in circular smooth muscles of the rabbit urethra. (Aa) A large circular smooth muscle strip generating spontaneous phasic contractions. CPA (30 μM) initially increased both the frequency and amplitude of phasic contractions, but subsequently reduced their frequency. (Ab) In another large preparation that developed spontaneous contractions, CPA (10 μM) initially increased both the frequency and amplitude of contractions and then abolished their generation after about 15 min. (Ba) In a smaller circular smooth muscle strip, spontaneous phasic contractions were developed. CPA increased the amplitude and frequency of the contractions. (Bb) In the same preparations that had been exposed to CPA for 30 min, spontaneous contractions with increased amplitude and reduced frequency were still generated. (C) In another smaller circular smooth muscle strip that exhibited spontaneous contractions, CPA initially increased the amplitude and frequency of the contractions, but then abolished them.
In 12 out of 14 preparations, CPA (10 μM) initially increased the frequency of spontaneous contractions and often induced a sustained rise in the basal tension. After prolonged exposure to CPA (about 30 min), spontaneous phasic contractions had an increased amplitude (7.3±1.4 mN, P<0.05) and a reduced frequency (3.9±1.2 min−1, P<0.05). CPA-resistant spontaneous contractions were abolished by nicardipine (1 μM, n=4). As one might expect that higher concentrations of CPA are required to disrupt Ca store function in large muscle strips, we examined the effects of a higher concentration of CPA (30 μM) on phasic contractions for up to 1 h. However, increasing the concentration of CPA did not cause further suppression of the contractile responses (n=5, Figure 1Aa). In the remaining two preparations, CPA (10 μM) initially increased the frequency and amplitude of phasic contractions, but then prevented their generation within some 15 min (Figure 1Ab).
To assess possible effects of the disruption of internal calcium stores on neurotransmitter release, the effects of CPA (10 μM) on spontaneous contractions were also examined in the presence of phentolamine (1 μM), tetrodotoxin (1 μM) and L-nitro arginine (0.1 mM). In the presence of these agents, CPA had similar effects to those in control solution. Briefly, CPA initially increased both the amplitude and frequency of spontaneous contractions. After a prolonged application of CPA (20–30 min), it increased the amplitude and duration of phasic contractions but reduced their frequency (n=3).
To further examine the role of Ca stores in generating spontaneous contractions, the effects of thapsigargin, another blocker of SERCA pumps, were examined in five preparations. Thapsigargin (5 μM) initially increased both the amplitude and frequency of spontaneous contractions, and also increased basal tension. After prolonged application of thapsigargin (60 min), tension gradually returned to the original level, but the amplitude and duration of the phasic contractions remained increased although their frequency was reduced (n=5).
As CPA unexpectedly reinforced rather than suppressed spontaneous contractions in a majority of urethral smooth muscle preparations, its effects on spontaneous contractions were also investigated using smaller muscle preparations.
In 12 out of 18 small preparations studied, CPA (10 μM) again increased the amplitude and duration of spontaneous contractions while reducing their frequency (Figure 1Ba and b). In preparations that had been exposed to CPA for 30 min, phasic contractions had an increased peak amplitude (mean 1.1±0.2 mN in control; 3.9±1.1 mN in CPA, P<0.05) and a reduced frequency (mean 15.5±2.3 min−1 in control; 3.9±0.9 min−1 in CPA, P<0.05). In the remaining six preparations, CPA (10 μM) initially increased the amplitude and frequency of spontaneous contractions but prevented their generation within 5 min (Figure 1C).
Effects of CPA on spontaneous slow waves
The mechanisms underlying spontaneous contractions are considered to involve spontaneous electrical activity, namely STDs and slow waves, and therefore the effects of CPA on the depolarizations were examined. As STDs do not appear to be associated with spontaneous contractions, only slow waves were considered in the following experiments.
In 11 out of 17 preparations, CPA (10 μM) initially increased the frequency of slow waves but prevented their generation within 5 min (Figure 2A). CPA also depolarized the membrane by about 6 mV (6.2±1.9 mV, n=11). In control conditions, urethral smooth muscle cells had a resting membrane potential, determined at the most stable negative potential between each slow waves, ranging between −52.4 and −45.4 mV (mean −49.8±2.7 mV, n=11). Slow waves had an amplitude (30±1.7 mV, n=11), a duration (505.6±105.6 ms) and a frequency (10.8±2.9 min−1).
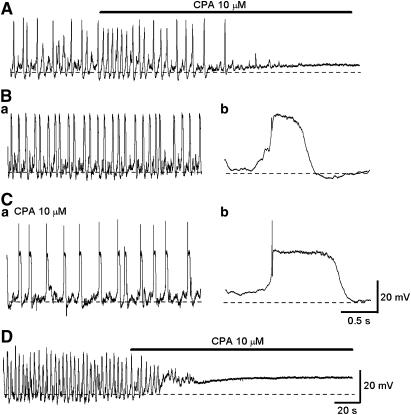
Figure 2.
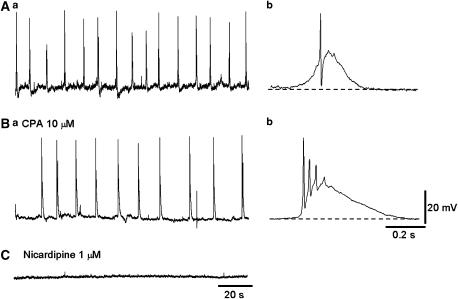
Effects of CPA on spontaneous slow waves in circular smooth muscle of the rabbit urethra. (A) Slow waves and spontaneous transient depolarizations in a circular smooth muscle strip. CPA (10 μM) initially increased the frequency of slow waves but then abolished them. (Ba) In the other circular smooth muscle strip, spontaneous depolarizations were generated. (Ca) In the same preparation after exposure to CPA for 30 min, spontaneous slow waves with increased amplitude and duration were generated at a lower frequency. (Bb, Cb) With a fast time scale, slow waves in the presence of CPA had a more rapid upstroke and longer duration than in control solution. (D) In a preparation that had been exposed to nicardipine (1 μM) for 30 min, spontaneous depolarizations were generated, and CPA abolished them.
In the remaining six preparations, CPA (10 μM) initially increased and then reduced the frequency of slow waves, but did not prevent their generation (Figure 2B and C). CPA also depolarized the membrane by about 3 mV (3.4±0.6 mV, n=6). In preparations that had been exposed to CPA for some 30 min, slow waves had an increased amplitude (29.3±2.1 mV in control; 48.9±2.6 mV CPA, n=6, P<0.05), an increased duration (558±118 ms in control; 1511±435 ms in CPA, P<0.05) and a reduced frequency (19.3±3.3 min−1 in control; 2.8±0.7 min−1 in CPA, P<0.05). The resting membrane potential in the CPA-resistant urethral smooth muscle ranged between −52 and −46.7 mV (mean −49.8±2.2 mV, n=6), and these values were not different from those of CPA-sensitive preparations. In four preparations that were resistant to CPA, spontaneous depolarizations persisted in the presence of nicardipine (1 μM). However, subsequent CPA (10 μM) depolarized the membrane by about 8 mV and prevented the generation of residual electrical activity (Figure 2D).
Effects of CPA on spontaneous Ca transients in the urethra
Circular smooth muscle of the urethra generated spontaneous transient increases in [Ca2+]i (Ca transients). In the majority of preparations, two types of spontaneous Ca transient were generated, namely large Ca transients that presumably result from slow waves and smaller events, which may correspond to STDs. On some occasions, bursting Ca transients were also generated.
In 6 out of 21 preparations, spontaneous Ca transients were generated at 8.9±2.7 min−1 and had a mean amplitude of 0.29±0.07 R340/380 and a half-width of 872±88 ms. CPA (10 μM) initially increased the frequency of Ca transients and caused a sustained rise in the basal Ca level (mean 0.19±0.03 R340/380), and prevented the generation of Ca transients within 5 min (Figure 3Aa). After washing out CPA, Ca returned to the original level and the generation of spontaneous Ca transients was restored (Figure 3Ab).
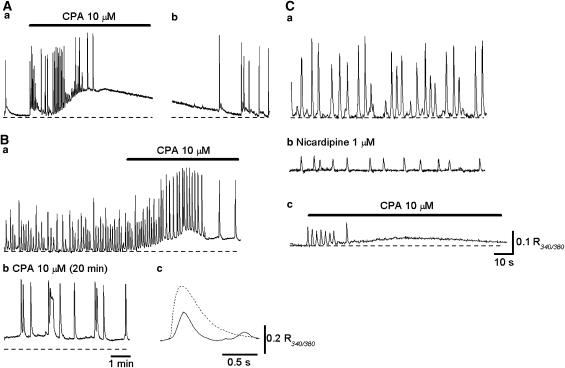
Figure 3.
Effects CPA on spontaneous Ca transients in circular smooth muscle of the rabbit urethra. (Aa) Spontaneous Ca transients in a circular smooth muscle strip. CPA (10 μM) initially increased the frequency of Ca transients but then abolished them. (Ab) After washing out CPA, spontaneous Ca transients were restored. (Ba) In another preparation, spontaneous Ca transients were generated, and CPA increased their frequency and amplitude. (Bb) In the same preparation that had been exposed to CPA for 30 min, Ca transients with increased amplitude and duration were generated. (Bc) With a fast time scale, Ca transients in the presence of CPA (dotted line) had a larger amplitude and longer duration than in control solution (full line). (Ca) In a preparation that generated CPA-resistant spontaneous Ca transients, (Cb) nicardipine (1 μM) dramatically diminished Ca transients. (Cc) Subsequent CPA abolished the residual Ca transients.
In the remaining 15 preparations, Ca transients were generated at 7.3±1.4 min−1 and had a mean amplitude of 0.24±0.08 R340/380 and a half-width of 912±132 ms. CPA (10 μM) initially increased and then reduced the frequency of Ca transients, but did not prevent their generation for up to 30 min (Figure 3Ba). CPA also increased the amplitude and duration of Ca transients. In preparations that had been exposed to CPA for some 30 min, spontaneous Ca transients had an increased amplitude (0.26±0.08 R340/380, n=15, P<0.05), an increased duration (3928±1966 ms, n=15, P<0.05) and a reduced frequency (3.1±1.1 min−1, n=15, P<0.05; Figure 3Bb). In five preparations that generated CPA-resistant Ca transients (Figure 3Ca), nicardipine (1 μM)) attenuated spontaneous Ca transients (Figure 3Cb), and the subsequent application of CPA (10 μM) invariably abolished spontaneous Ca transients (Figure 3Cc).
Effects of CPA on caffeine-induced Ca transients
To confirm that CPA effectively depleted intracellular Ca stores, the effects of CPA on caffeine-induced Ca transients were examined in nominally Ca-free solution.
Switching from Krebs' solution to nominally Ca-free solution reduced basal Ca levels and abolished spontaneous Ca transients (Figure 4a). After reaching a stable Ca level, caffeine (30 mM) produced a Ca transient and then further reduced the basal Ca level (Figure 4a). In preparations that had been exposed to CPA (10 μM) for 30 min, switching from Krebs' solution to nominally Ca-free solution reduced the basal Ca level (Figure 4b), but subsequent addition of caffeine failed to produce a Ca transient and further reduced the basal Ca level (Figure 4b). After washing out CPA for over 30 min, switching from Krebs' solution to nominally Ca-free solution reduced the basal Ca level, and caffeine-induced Ca transients were restored (Figure 4c).
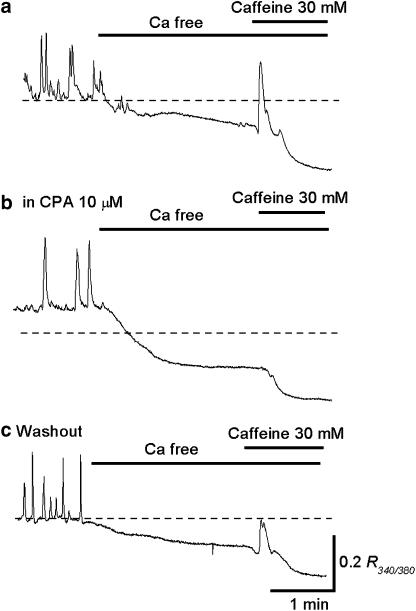
Figure 4.
Effects CPA on caffeine-induced Ca transients in circular smooth muscle of the rabbit urethra. (a) Switching from Krebs' solution to nominally Ca-free solution reduced the basal Ca level and prevented the generation of spontaneous Ca transients. Subsequent caffeine (30 mM) produced a Ca transient and then further reduced the basal Ca level. (b) In the same preparation that had been exposed to CPA (10 μM) for 30 min, caffeine failed to produce Ca transient in nominally Ca-free solution. (c) After washing out CPA, caffeine-induced Ca transients were restored.
Properties of spontaneous depolarizations in longitudinal smooth muscles
As the L-type Ca channels may be involved in the generation of CPA-resistant electrical activity, the possible contribution of longitudinal smooth muscles to circular smooth muscle function were examined.
In control solutions, longitudinal smooth muscle cells generated spontaneous action potentials which were very different from slow waves recorded from CSM preparations (Figure 5A). Longitudinal smooth muscle cells had resting membrane potentials ranging between −53 and −45 mV (mean −49.5±3.7 mV, n=6). Spontaneous action potentials had a peak amplitude ranging between 46 and 49 mV (mean 47.6±2.3 mV, n=6), half-widths ranging between 416 and 646 ms (mean 510±97 ms) and a frequency ranging between 4.6 and 6.1 min−1 (5.3±1.2 min−1).
Figure 5.
Properties of spontaneous depolarizations in longitudinal smooth muscles of the rabbit urethra. (Aa) Longitudinal smooth muscle of the urethra generated spontaneous depolarizations consisting of slow depolarizations and a superimposed spike-like depolarization. (Ba) In the same preparation that had been exposed to CPA for 30 min, spontaneous depolarizations were still generated. (Ab, Bb) In the presence of CPA, spontaneous depolarizations had a longer duration and the number of superimposed spike-potentials was increased. (C) After washing out CPA, control depolarizations were restored, and subsequent nicardipine (1 μM) abolished them.
CPA (10 μM) increased the amplitude and duration of spontaneous action potentials but reduced their frequency (Figure 5B). In preparations that had been exposed to CPA for some 30 min, spontaneous action potentials had an increased amplitude (52.5±3.5 mV, n=6, P<0.05), an increased duration (959±323 ms, n=6, P<0.05) and a reduced frequency (2.8±0.7 min−1, n=6, P<0.05). The membrane potential in CPA ranged between −52 and −44.5 mV (−48.6±3.5 mV, n=6). CPA also increased the number of spike-like depolarizations, which were superimposed on the slow depolarizations. Spontaneous action potentials were invariably blocked by nicardipine (1 μM, n=4; Figure 5C).
Nerve-evoked modulation of spontaneous Ca transients
Since CPA-sensitive and CPA-resistant spontaneous excitation was observed in CSM of the urethra, neuronal modulation of both pattern of responses was investigated. The effects of CPA on nerve-mediated changes in spontaneous Ca transients in the urethral smooth muscle were examined.
In control conditions, transmural nerve stimulation increased [Ca2+]i. Brief stimulation at a high frequency (10 Hz, 10 s) initiated transient increases in [Ca2+]i, while prolonged stimulation at a low frequency (2 Hz, 30 s) increased the frequency of spontaneous Ca transients to cause a sustained rise in [Ca2+]i (Figure 6Aa). These excitatory responses were abolished by either phentolamine (1 μM, n=5) or guanethidine (3 μM, n=3), indicating that they resulted from the activation of α-adrenoceptors by neurally-released NAd. In six preparations that had been exposed to CPA (10 μM) for 30 min, transmural nerve stimulation still increased [Ca2+]i (Figure 6Ab). However, the peak amplitude of nerve-evoked increase in [Ca2+]i (10 Hz, 10 s) was reduced from 208.3±19.8% (n=6) to 121.9±12.8% (n=6) of that of corresponding spontaneous Ca transients.
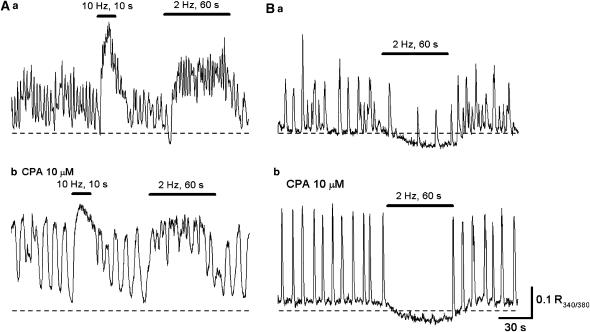
Figure 6.
Nerve-mediated modulation of Ca transients in circular smooth muscle of the rabbit urethra. (Aa) In spontaneously active circular smooth muscle, transmural nerve stimulation (10 Hz, 10 s and 2 Hz, 60 s) increased the frequency of Ca transients to form a sustained rise in Ca. (Ab) In the same preparation that had been exposed to CPA for 30 min, spontaneous Ca transients were generated, and transmural stimulation increased the Ca level. (Ba) In another spontaneously active preparation, which had been exposed to phentolamine (1 μM), transmural stimulation inhibited Ca transients and reduced the Ca level. (Bb) In the same preparation that had been exposed to CPA for 30 min, spontaneous Ca transients were generated, and transmural stimulation suppressed their generation.
After blocking α-adrenergic transmission with phentolamine (1 μM), nerve stimulation at a low frequency (2 Hz, 30 s) either reduced both the frequency and amplitude of Ca transients (Figure 6Ba; n=3) or prevented their generation (n=5). Nerve stimulation also reduced the basal Ca level. All these inhibitory responses were dramatically reduced by L-nitro-arginine (0.1 mM), suggesting that they are mediated by neurally released NO. In five preparations that had been exposed to both CPA (10 μM) and phentolamine, nerve stimulation was still capable of suppressing spontaneous Ca transients, and reduced the basal Ca level (Figure 6Bb).
Effects of exogenous NAd and SIN-1 on spontaneous Ca transients
As CPA may affect transmitter release, the effects neurotransmitters applied to the organ bath were investigated.
NAd (1 μM) applied to the organ bath increased the frequency of Ca transients and caused sustained increases in [Ca2+]i (Figure 7Aa). The peak amplitude of NAd-induced increases in [Ca2+]i was 234.5±43.5% (n=8) of the mean amplitude of corresponding spontaneous Ca transients. In preparations that had been exposed to CPA (10 μM) for 30 min, NAd still caused a sustained increase in [Ca2+]i (Figure 7Ab). However, the peak amplitude of NAd-induced increase in [Ca2+]i was reduced to 123.6±21.3% (n=6) of that of spontaneous Ca transients, suggesting that CPA had effectively disrupted the function of intracellular Ca stores.
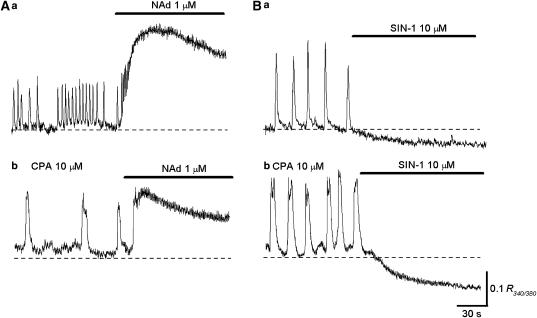
Figure 7.
Effects of NAd and SIN-1 on Ca transients in circular smooth muscles of the rabbit urethra. (Aa) Circular smooth muscle developed spontaneous Ca transients, and NAd (1 μM) applied to the organ bath increased their frequency to form a sustained rise in Ca. (Ab) In the same preparation that had been exposed to CPA for 30 min, spontaneous Ca transients were generated, and CPA evoked a sustained rise in Ca. (Ba) In another preparation, spontaneous Ca transients were generated, and SIN-1 (10 μM) prevented their generation and reduced the basal Ca level. (Bb) In the same preparation that had been exposed to CPA for 30 min, Ca transients were generated. Subsequent SIN-1 prevented their generation and reduced the basal Ca level.
SIN-1 (10 μM) either reduced the frequency and amplitude of Ca transients (n=3) or prevented their generation (Figure 7Ba; n=5). SIN-1 also reduced the basal Ca level. In preparations that had been exposed to CPA (10 μM) for 30 min (n=5), SIN-1 was still capable of suppressing spontaneous Ca transients, and reduced the basal Ca level (Figure 7b).
Discussion
In CSM of the rabbit urethra, spontaneous excitation originates from interstitial cells by means of the spontaneous release of Ca2+ from intracellular stores, which activates Ca-activated Cl channels (Hashitani et al., 1996; Hashitani & Edwards, 1999; Sergeant et al., 2000; 2001; Johnston et al., 2005). Resultant depolarizations may be transmitted to smooth muscle cells, presumably via gap junctions, to activate L-type Ca channels and so contract urethral smooth muscle. Thus, spontaneous contractions may be inhibited as a consequence of the inhibition of the primary step of spontaneous excitation, that is, Ca release from stores in interstitial cells or by blocking Ca entry.
CPA is known to inhibit Ca uptake into intracellular stores via SERCA pumps, and is known to suppress both STICs in isolated interstitial cells and STDs in intact CSM of the urethra (Hashitani et al., 1996; Sergeant et al, 2001). Therefore, blockade of SERCA pumps with CPA would be expected to suppress spontaneous contractions of the urethral smooth muscles. In lymphatic smooth muscle that generate Ca store-dependent spontaneous excitation, CPA indeed blocked the generation of STDs (Ferrusi et al., 2004) and also strongly suppressed lymphatic pumping (Atchison et al., 1998). However, CPA increased the amplitude and duration of phasic contractions in 70–90% of urethral smooth muscle preparations, although it prevented their generation in the remaining preparations. As CPA abolished caffeine-induced Ca transients in nominally Ca-free solution, it clearly had depleted intracellular Ca stores. Furthermore, thapsigargin also failed to block spontaneous contractions. Thus, it is unlikely that CPA-treated preparations generated spontaneous contractions through a Ca store-dependent pacemaker, that is, through interstitial cells.
Consistent with the results of contractile studies, CPA increased the amplitude and duration of spontaneous depolarizations and corresponding Ca transients in some 40–60% of preparations, while suppressing their generation in the remaining preparations. In detrusor smooth muscles of the bladder in which spontaneous action potentials result from the opening of L-type Ca channels, CPA increased the amplitude and duration of action potentials (Hashitani & Brading, 2003). This may result from a diminished activation of large conductance Ca-activated potassium (BK) channels via calcium-induced calcium release as a consequence of the disruption of Ca store function with CPA. In the presence of CPA, slow waves in CSM of the urethra had a faster upstroke than under control conditions, as well as having an increased amplitude and duration, suggesting that Ca-dependent negative feedback on L-type Ca channels had been diminished by CPA. Indeed, the blockade of BK channels increased the amplitude and duration of action potentials in isolated smooth muscle cells of the sheep urethra (Hollywood et al., 2000).
In longitudinal smooth muscle of the urethra, spontaneous depolarizations are generated: these are inhibited by nicardipine but not by CPA, suggesting that L-type Ca channels play a principal role in their generation. CPA increased the duration and number of superimposed spike-like depolarizations. Thus, after disrupting primary pacemaking by interstitial cells that depend on the Ca release, L-type Ca channel-dependent pacemaking depolarizations, arising from longitudinal smooth muscles may drive the CSMs. Alternatively, small populations of circular smooth muscles that are capable of generating spontaneous depolarization might dominate the pacemaking system. Indeed, isolated smooth muscle cells from the rabbit and sheep urethra are capable of generating spontaneous depolarizations, although only a small population of cells (3–10%) has this capability (Cotton et al., 1997; Sergeant et al., 2000). In the gastrointestinal tract, several studies indicate that longitudinal smooth muscles have their own pacemaking mechanisms (Liu & Huizinga, 1993; Malysz et al., 1996; Boddy et al., 2004; Daniel et al., 2004). Longitudinal smooth muscles generate spike-like action potentials which depend on the opening of L-type Ca channels, and L-type Ca channel-dependent action potentials dominate spontaneous activity when the primary pacemaking mechanisms, which rely on the Ca handling in myenteric ICC (ICC-MY), are suppressed (Malysz et al., 1996; Daniel et al., 2004).
CPA effectively diminished spontaneous depolarizations in both CSM of the rabbit urethra and longitudinal smooth muscle of the guinea-pig urethra (Hashitani et al., 1996; Hashitani & Edwards, 1999). We do not have a clear explanation for this discrepancy between previous and present experiments; however, we may need to consider the heterogeneity of the preparations. CPA blocked spontaneous contractions in only 10% of large preparations, but abolished the contractions in about 30% of smaller preparations. Therefore, increases in the size of preparations may increase the heterogeneity of cell types that contribute to the overall spontaneous excitation, and thus increase the possible contribution of L-type Ca channel-dependent pacemaking. For example, spike-like action potentials had been recorded from rabbit urethral smooth muscles by some investigators (Callahan & Creed, 1985; Bradley et al., 2004), although others have recorded slow waves and STDs from the same muscles (Hashitani et al., 1996; Waldeck et al., 1998).
The degree of stretch of the preparations may also be important. In isolated detrusor smooth muscle cells, only a small proportion of cells are spontaneously active, and stretching cells either increased the action potential frequency or induced action potentials in quiescent cells (Wellner & Isenberg, 1993). In gastrointestinal smooth muscles, spontaneous slow waves originate from ICC-MY. In W/Wv mutant mice in which ICC-MY are not present, slow waves were absent but action potentials depending on L-type Ca channels are generated (Malysz et al., 1996). Inhibition of potassium channels with TEA facilitated the action potentials, while not affecting slow wave frequency in control preparations. These results suggest that the frequency of action potentials but not slow waves are highly sensitive to changes in the membrane conductance, and thus the degree of stretching may alter the occurrence of the L-type Ca channel-dependent action potentials. Alternatively, in addition to its primary effect on SERCA pumps, CPA may activate capacitative Ca entry not only in interstitial cells (Bradley et al., 2005) but also in urethral smooth muscle cells. This Ca entry may account for sustained increases in the basal Ca level and associated depolarizations. Thus in the presence of CPA, L-type Ca channel-dependent pacemaking mechanism could be more readily activated. However, we did not find significant differences in either resting membrane potentials or CPA-induced depolarizations between CPA-sensitive and CPA-resistant preparations.
In preparations, in which spontaneous depolarizations and Ca transients were not abolished by CPA, nicardipine dramatically diminished Ca transients but not transient depolarizatons. After blocking L-type Ca channels, CPA invariably abolished residual depolarizations and Ca transients, suggesting that these events may result from the Ca release from store and subsequent opening of Ca-activated chloride channels. In heterogeneous preparations, Ca store-dependent Ca transients may reflect responses arising from smooth muscle as there are many more smooth muscle cells than interstitial cells. However, these responses in the smooth muscles may be driven by interstitial cells. These results again confirmed that concurrent activation of two types of ion channels, namely Ca-activated chloride and L-type Ca channels. Both are required to excite urethral smooth muscle.
In gastrointestinal smooth muscles, ICC-IM play an important role in neuromuscular transmission, and in W/Wv mice that lacked ICC-IM transmural stimulation does not evoke either excitatory or inhibitory junction potentials (Ward et al., 2000; Suzuki et al., 2003). In the urethra, transmural nerve stimulation increased the frequency of Ca transients in both control and CPA-treated preparations, and thus excitatory innervation may not necessarily be targeting a single population of cells, that is, interstitial cells. After the blockade of α-adrenergic transmission, nerve stimulation suppressed Ca transients by releasing NO from nerves. This inhibitory response was again preserved in CPA-treated preparations. Therefore, both excitatory and inhibitory innervation may be capable of modulating spontaneous excitations irrespective of their origin, and thus alter spontaneous contractions in both circular and longitudinal muscles (Mattiasson et al., 1990). Although nerve-mediated modulation of spontaneous Ca transients was basically preserved in CPA-treated preparations, the amplitude of Ca increase in response to either NAd or nerve stimulation was reduced to the level of spontaneous Ca transients, suggesting that α-adrenergic stimulation no longer caused Ca mobilization from Ca stores in CPA-treated preparations.
In conclusion, spontaneous contractions of CSMs of the urethra may result from the primary pacemaking by interstitial cells which depends on Ca release from intracellular stores. Besides this primary pacemaking, smooth muscles themselves may be capable of generating spontaneous depolarizations that depend on L-type Ca channels. Neurally released NAd and NO can modulate both Ca-store-dependent and L-type Ca channels-dependent spontaneous excitation.
Acknowledgments
We thank Professor A.F. Brading and Dr K.D. Thornbury for their critical reading of the manuscript. This project was supported by research grants from Japan Society for the Promotion of Science (No. 15591704, No. 17390443) to H.H.
Abbreviations
- BK channel
large conductance Ca-activated K channel
- CPA
cyclopiazonic acid
- [Ca2+]i
intracellular concentration of free calcium ions
- CSM
circular smooth muscle
- DMSO
dimethyl sulphoxide
- ICC-IM
intramuscular interstitial cells of Cajal
- ICC-MY
myenteric interstitial cells of Cajal
- NAd
noradrenaline
- SERCA
sarcoplasmic-endoplasmic reticulum Ca pump
- SIN-1
3-morpholino-sydnonimine
- STD
spontaneous transient depolarization
- STIC
spontaneous transient inward current
References
- ANDERSSON K.-E., PASCUAL A.G., PERSSON K., FORMAN A., TØTTRUP A. Electrically-induced, nerve-mediated relaxation of rabbit urethra involves nitric oxide. J. Urol. 1992;147:253–259. doi: 10.1016/s0022-5347(17)37208-7. [DOI] [PubMed] [Google Scholar]
- ANDERSSON K.-E., WEIN A.J. Pharmacology of the lower urinary tract: basis for current and future treatments of urinary incontinence. Pharmacol. Rev. 2004;56:581–631. doi: 10.1124/pr.56.4.4. [DOI] [PubMed] [Google Scholar]
- ATCHISON D.J., RODELA H., JOHNSTON M.G. Intracellular calcium stores modulation in lymph vessels depends on wall stretch. Can. J. Physiol. Pharmacol. 1998;76:367–372. [PubMed] [Google Scholar]
- BODDY G., BONG A., CHO W., DANIEL E.E. ICC pacing mechanisms in intact mouse intestine differ from those in cultured or dissected intestine. Am. J. Physiol. 2004;286:G653–G662. doi: 10.1152/ajpgi.00382.2003. [DOI] [PubMed] [Google Scholar]
- BRADLEY E., HOLLYWOOD M.A., MCHALE N.G., THORNBURY K.D., SERGEANT G.P. Pacemaker activity in urethral interstitial cells is not dependent on capacitative calcium entry. Am. J. Physiol. 2005;289:C625–C632. doi: 10.1152/ajpcell.00090.2005. [DOI] [PubMed] [Google Scholar]
- BRADLEY J.E., ANDERSON U.A., WOOLSEY S.M., THORNBURY K.D., MCHALE N.G., HOLLYWOOD M.A. Characterization of T-type calcium current and its contribution to electrical activity in rabbit urethra. Am. J. Physiol. 2004;286:C1078–C1088. doi: 10.1152/ajpcell.00463.2003. [DOI] [PubMed] [Google Scholar]
- BRIDGEWATER M., MACNEILL H.F., BRADING A.F. Regulation of urethral tone in pig urethral smooth muscle. J. Urol. 1993;150:223–228. doi: 10.1016/s0022-5347(17)35451-4. [DOI] [PubMed] [Google Scholar]
- CALLAHAN S.M., CREED K.E. The effects of oestrogens on spontaneous activity and responses to phenylephrine of the mammalian urethra. J. Urol. 1985;358:35–46. doi: 10.1113/jphysiol.1985.sp015538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COTTON K.D., HOLLEYWOOD M.A., MCHALE N.G., THORNBURY K.D. Ca2+ current and Ca2+-activated chloride current in isolated smooth muscle cells of the sheep urethra. J. Physiol. 1997;505:121–131. doi: 10.1111/j.1469-7793.1997.121bc.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DANIEL E.E., BODDY G., BONG A., CHO W.G. A new model of pacemaking in the mouse intestine. Am. J. Physiol. 2004;286:G253–G262. doi: 10.1152/ajpgi.00221.2003. [DOI] [PubMed] [Google Scholar]
- FERRUSI I., ZHAO J., VAN HELDEN D., VON DER WEID P.-Y. Cyclopiazonic acid decreases spontaneous transient depolarizations in guinea pig mesenteric lymphatic vessels in endothelium-dependent and -independent manners. Am. J. Physiol. 2004;286:H2287–H2295. doi: 10.1152/ajpheart.00739.2003. [DOI] [PubMed] [Google Scholar]
- HASHITANI H., BRADING A.F. Ionic basis for the regulation of spontaneous excitation in detrusor smooth muscle cells of the guinea-pig urinary bladder. Br. J. Pharmacol. 2003;140:159–169. doi: 10.1038/sj.bjp.0705320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HASHITANI H., EDWARDS F.R. Spontaneous and neurally activated depolarizations in smooth muscle cells of the guinea-pig urethra. J. Physiol. 1999;514:459–470. doi: 10.1111/j.1469-7793.1999.459ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HASHITANI H., VAN HELDEN D.F., SUZUKI H. Properties of spontaneous depolarizations in circular smooth muscle cells of rabbit urethra. Br. J. Pharmacol. 1996;118:1627–1632. doi: 10.1111/j.1476-5381.1996.tb15584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRST G.D.S., WARD S.M. Interstitial cells: involvement in rhythmicity and neural control of gut smooth muscle. J. Physiol. 2003;550:337–346. doi: 10.1113/jphysiol.2003.043299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLLYWOOD M.A., MCCLOSKEY K.D., MCHALE N.G., THORNBURY K.D. Characterization of outward K+ currents in isolated smooth muscle cells from sheep urethra. Am. J. Physiol. 2000;279:C420–C428. doi: 10.1152/ajpcell.2000.279.2.C420. [DOI] [PubMed] [Google Scholar]
- JOHNSTON L., SERGEANT G.P., HOLLYWOOD M.A., THORNBURY K.D., MCHALE N.G. Calcium oscillations in interstitial cells of the rabbit urethra. J. Physiol. 2005;565:449–461. doi: 10.1113/jphysiol.2004.078097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU L.W., HUIZINGA J.D. Electrical coupling of circular muscle to longitudinal muscle and interstitial cells of Cajal in canine colon. J. Physiol. 1993;470:445–461. doi: 10.1113/jphysiol.1993.sp019868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MALYSZ J., THUNEBERG L., MIKKELSEN H.B., HUIZINGA J.D. Action potential generation in the small intestine of W mutant mice that lack interstitial cells of Cajal. Am. J. Physiol. 1996;271:G387–G399. doi: 10.1152/ajpgi.1996.271.3.G387. [DOI] [PubMed] [Google Scholar]
- MATTIASSON A., ANDERSSON K.-E., ANDERSSON P.O., LARSSON B., SJOGREN C., UVELIUS B. Nerve-mediated functions in the circular and longitudinal muscle layers of the proximal female rabbit urethra. J. Urol. 1990;143:155–160. doi: 10.1016/s0022-5347(17)39901-9. [DOI] [PubMed] [Google Scholar]
- SERGEANT G.P., HOLLYWOOD M.A., MCCLOSKEY K.D., THORNBURY K.D., MCHALE N.G. Specialised pacemaking cells in the rabbit urethra. J. Physiol. 2000;526:359–366. doi: 10.1111/j.1469-7793.2000.t01-2-00359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SERGEANT G.P., HOLLYWOOD M.A., MCCLOSKEY K.D., MCHALE N.G., THORNBURY K.D. Role of IP3 in modulation of spontaneous activity in pacemaker cells of rabbit urethra. Am. J. Physiol. 2001;280:C1349–C1356. doi: 10.1152/ajpcell.2001.280.5.C1349. [DOI] [PubMed] [Google Scholar]
- SERGEANT G.P., THORNBURY K.D., MCHALE N.G., HOLLYWOOD M.A. Characterization of norepinephrine-evoked inward currents in interstitial cells isolated from the rabbit urethra. Am. J. Physiol. 2002;283:C885–C894. doi: 10.1152/ajpcell.00085.2002. [DOI] [PubMed] [Google Scholar]
- SUZUKI H., WARD S.M., BAYGUINOV Y.R., EDWARDS F.R., HIRST G.D.S. Involvement of intramuscular interstitial cells in nitrergic inhibition in the mouse gastric antrum. J. Physiol. 2003;546:751–763. doi: 10.1113/jphysiol.2002.033365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALDECK K., NY L., PERSSON K., ANDERSSON K.-E. Mediators and mechanisms of relaxation in rabbit urethral smooth muscle. Br. J. Pharmacol. 1998;123:617–624. doi: 10.1038/sj.bjp.0701645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARD S.M., BECKETT E.A., WANG X., BAKER F., KHOYI M., SANDERS K.M. Interstitial cells of Cajal mediate cholinergic neurotransmission from enteric motor neurons. J. Neurosci. 2000;20:1393–1403. doi: 10.1523/JNEUROSCI.20-04-01393.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WELLNER M.C., ISENBERG G. Properties of stretch-activated channels in myocytes from the guinea-pig urinary bladder. J. Physiol. 1993;466:213–227. [PMC free article] [PubMed] [Google Scholar]