Abstract
We used okadaic acid (OA), a potent preferential inhibitor of PP2A and PP5 but not PP1 (PP subfamilies), to examine the involvement of serine/threonine protein phosphatase (PP) in behavioral sensitization stimulated by treatment with cocaine in mice.
Repeated administration of cocaine (10 mg kg−1) once a day for five consecutive days produced a progressive increase in locomotor activity that was maintained after the cessation of cocaine treatment, as revealed by the fact that a challenge dose of cocaine given on day 7 of withdrawal reproduced an enhanced stimulant effect.
On the seventh day of withdrawal, OA-sensitive PP activity and expression of PP2A and PP5, but not PP1γ, were increased in whole-cell extract of the nucleus accumbens and the ventral tegmental area in cocaine-sensitized mice, compared to saline-treated mice.
Restraint stress increased locomotor activity in cocaine-sensitized mice on day 7 after drug administration was ceased. The locomotor activity was more susceptible to restraint-elicited enhancement in cocaine-sensitized mice than in saline-treated mice. The restraint-induced hyperlocomotion was suppressed by a single intracerebroventricular injection of OA immediately before restraint in cocaine-sensitized mice, but this suppression did not occur in saline-treated mice.
The membrane fraction of the whole brain in cocaine-sensitized mice showed that OA-sensitive activity levels rise after mice are subjected to restraint, and this is concomitant with an increase in expression levels of PP2A and PP5, but not PP1γ.
These results suggest that the upregulated OA-sensitive PPs are involved in stress-induced hyperlocomotion in cocaine-sensitized mice. There may be intracellular mechanisms mediating psychostimulant cross-sensitization to stress underlying the spontaneous recurrence of its psychosis.
Keywords: Addiction, behavioral sensitization, cocaine, cross-sensitization, locomotor activity, mesolimbic system, okadaic acid, restraint stress, serine/threonine protein phosphatase, translocation
Introduction
Psychostimulants, such as cocaine, produce psychomotor excitation via the mesolimbic dopamine system, which consists of the ventral tegmental area (VTA), the nucleus accumbens (NAc) and the frontal cortex. Repeated administration of psychostimulants elicits enduring neuroadaptation. This is associated with enhanced behavioral responsiveness to drug administration and may be correlated with drug addiction and the maintenance of paranoid psychosis in human stimulant abusers. Once abstinent from psychostimulants, addicted individuals can experience intense craving induced by a single drug-taking experience or an environmental stimulus, which may lead to relapse to abuse (Jaffe et al., 1989; Ehrman et al., 1992). Animal models of addiction have provided substantial information about drug-induced neuroadaptations, including behavioral sensitization to the stimulant effect of repeated drug administration and the reinstatement of drug-seeking behavior in animals extinguished from drug self-administration (Ujike, 2002).
Chronic administration of large doses of psychomotor stimulants can result in the development of psychosis in humans (Sato et al., 1992; Jaffe, 2002). Although abstinent from stimulants, stressful experiences may elicit a spontaneous recurrence of the psychoses (Yui et al., 1999). The roles of stress and the subsequent activation of the hypothalamo–pituitary–adrenal (HPA) axis in relapse have been under investigation. It has been shown that corticotropin-releasing factor and glucocorticoid hormone, stress-coping molecules released from the activated HPA axis, both play an important role in relapse, and can induce reinstatement of drug-seeking behavior (Sarnyai et al., 2001; Marinelli & Piazza, 2002). However, the intracellular signal transduction systems underlying stress-induced relapse remain unclear.
The phosphorylation state of functional protein molecules determines their physiological activity in many cellular events. The protein kinases involved in drug addiction are well recognized, but only one subfamily of serine (Ser)/threonine (Thr) protein phosphatase (PP), called PP1 (Svenningsson et al., 2004; Valjent et al., 2005), is known to contribute to drug addiction. A better understanding of the role PPs play in addiction could provide useful information in the development of strategies for preventing relapse.
In the present study, we first examined the stimulant effect of repeated administration of cocaine, which induced behavioral sensitization to its psychostimulant effects, on the expression level of PPs in the NAc and the VTA, primary brain regions related to the development of drug addiction. Next, we used okadaic acid (OA), a potent preferential inhibitor for PP2A and PP5 but not PP1, to test the involvement of OA-sensitive PPs in cocaine-induced behavioral sensitization to restraint, during cocaine withdrawal. Furthermore, because subcellular translocation of PPs is believed to be the primary mode of activation in mammalian cells, we asked whether stress induced by a period of restraint altered the subcellular distribution and activity of PPs. Thus, we examined the influence of restraint on the expression level and activity of OA-sensitive PPs in membrane and cytosol fractions extracted from whole brain.
Methods
A total of 187 male ICR mice (SLC, Hamamatsu, Japan) weighing 25–35 g were used. They were housed five to a cage in an air-conditioned (23–24°C, 60% humidity) and light-controlled (light from 08:00 to 20:00 hours) room. All cocaine injections (10 mg kg−1) and behavioral tests were performed during the light cycle. Mice were given saline or cocaine subcutaneously once a day for five consecutive days, and then given no further injections for 6 days. On day 7 of this withdrawal period, animals received a single injection of saline or cocaine, referred to as challenge, or were subjected to restraint. This involved placing an animal in a 50-ml centrifuge tube (Corning, New York, NY, U.S.A.) with a breathing hole for 5, 30 or 60 min. Control animals were not subjected to restraint but were handled for a few seconds. Intracerebroventricular (i.c.v) injections of OA or saline were given immediately before exposure to restraint. Locomotor activity was measured in a 31 × 36-cm cage in a chamber with an array of fresnel lenses above the cage, which monitored motion in multiple zones of the cage (CompACT AMS, Muromachi Kikai, Tokyo, Japan). One day before cocaine treatment commenced, the animals received a single subcutaneous injection of saline and were left in the measurement cages for 3 h in order to adapt them to the measurement environment and injection procedure. The animals were further habituated to the measuring cages for 3 h on all cocaine injection days and on day 7 of the withdrawal period. After habituation, animals received drug injections and/or restraint, and then locomotor activity was monitored in the measuring cages for 60 or 180 min. All procedures were approved by Animal Research Committee of Wakayama Medical University in accordance with Japanese Government Animal Protection and Management Law, Japanese Government Notification on Feeding and Safekeeping of Animals and The Guidelines for Animal Experiments in Wakayama Medical University (approval number 64).
Mice were killed by instantaneous dislocation of the neck, an appropriate method of humane killing, on day 7 of withdrawal, followed by removal of the whole brains for preparation of whole-cell extract. Thick coronal sections of the forebrain and the midbrain were made, and then the ventral striatum, including the NAc, and the ventral part of the midbrain, including the VTA, were isolated from the coronal sections. These dissected tissues were subject to whole-cell extraction, as reported previously (Maeda et al., 2005a). In brief, the tissues were lysed by brief sonication in ice-cold lysis buffer: 50 mM Tris (pH 7.0), 0.1 mM EDTA, 0.1 mM EGTA, 1 mM dithiothreitol, 0.1% Triton X-100, 0.1 mM p-amino-benzamide, 10 μg ml−1 leupeptin, 1 μg ml−1 pepstatin and 1 mM phenylmethylsulfonyl fluoride. The lysed tissues were centrifuged at 15,000 × g at 4°C for 30 min. The supernatants were used as a whole-cell extract of the NAc and the VTA.
In another experiment, the whole brain was used for the preparation of subcellular fractions. The whole brains of mice were quickly removed immediately after the cessation of restraint, the peak time for producing restraint-induced hyperlocomotion, as had been revealed by measurements of locomotor activity (Figure 4a). The whole brain was homogenized using a motor-driven glass-Teflon homogenizer in ice-cold buffer A containing 20 mM Tris-HCl (pH 7.5), 2 mM EDTA, 0.5 mM EGTA, 1 mM phenylmethylsulfonyl fluoride, 25 μg ml−1 leupeptin, 0.1 mg ml−1 aprotinin and 0.32 M sucrose. The homogenate was centrifuged at 1000 × g for 10 min and the resultant supernatant was recentrifuged at 100,000 × g for 30 min at 4°C. The pellets were washed with buffer A without sucrose and dissolved in 0.5 ml of the lysis buffer, and then retained as the crude membrane fraction, referred to as the membrane fraction. The resulting supernatant was subjected to acetone precipitation in order to extract and concentrate proteins in the cytosol fraction. Then, nine volumes of cold acetone were added to one volume of the supernatant. This was shaken vigorously and kept at −70°C for 10 min and then centrifuged at 1000 × g at 4°C for 10 min. The resulting supernatant was discarded and the resulting pellet left under dry air to eliminate any acetone residue. The dried pellet was dissolved in 0.25 ml of the lysis buffer and retained as the cytosol fraction.
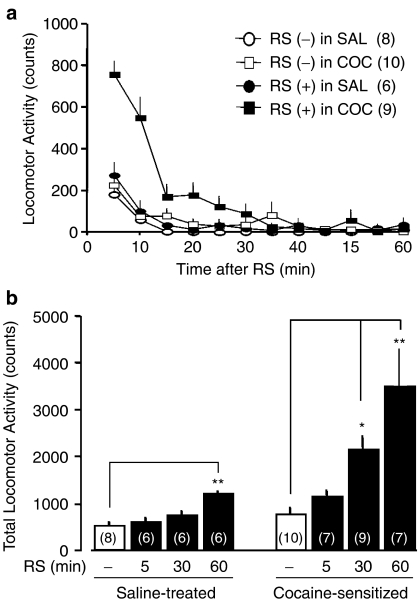
Figure 4.
Exposure to restraint stress (RS) elicits greater hyperlocomotion in cocaine (COC)-sensitized mice. (a) Time course of locomotor activity in mice following 30 min of restraint. (b) Hyperlocomotion depends on the duration of RS. Fifty-nine animals went through locomotor testing as described in Figure 2. Saline (SAL) or COC (10 mg kg−1) was administered to 26 or 39 animals, respectively, but six animals did not develop behavioral sensitization and were thus omitted from the data. The omitted six animals were exposed to RS for 30 min and subsequent locomotor activity was measured on withdrawal day 7 as described earlier. Twenty-six mice exposed to the SAL regimen and the remaining 33 mice with behavioral sensitization were assigned randomly with respect to length of time subjected to restraint (parenthesis in b). A single period of restraint (RS (+)) or handling (control treatment; RS (−)) was performed on withdrawal day 7. Locomotor activity was subsequently monitored in 5 min bin for 60 min. A one-way ANOVA followed by Dunnett's test revealed a significant increase in locomotor activity in SAL-treated mice (F(3,22)=8.84, P=4.91 × 10−4; q=4.87, P<0.01 in 60 min-RS) compared to COC-sensitized mice (F(3,29)=9.19, P=1.97 × 10−4; q=2.69, P<0.05 in 30 min-RS; q=4.93, P<0.01 in 60 min-RS). *P<0.05 and **P<0.01, determined by Dunnett's test.
The PP activity was measured according to our methods reported previously (Maeda et al., 2005a). To remove endogenous phosphate, the prepared extracts were passed once through Micro Bio-Spin Chromatography Columns (Bio-Rad, Tokyo, Japan), and the flow-through extract was retained as the sample. The protein concentration of sample was evaluated by the Bradford method (Coomassie Protein Assay Kit). Phosphatase assays were performed using the molybdate, malachite green, phosphate complex assay kit with synthetic phosphopeptide as substrate (Serine/Threonine Phosphatase Assay System), as described below. Five micrograms of sample was incubated at 37°C for 30 min in 50 μl reaction buffer including 5 nmol synthetic phosphopeptide: 50 mM imidazole (pH 7.2), 0.2 mM EGTA, 0.02% β-mercaptoethanol and 0.1 mg ml−1 BSA. Reactions were terminated by adding the molybdate dye buffer and incubated at room temperature for 30 min. Absorbance was determined at 600 nm. The liberated free phosphate was detected neither in the reaction mixture lacking the synthetic phosphopeptide nor in the reaction mixture without the tissue sample (data not shown). We regarded the free phosphate detected after incubation as being liberated from the synthetic phosphopeptide that resulted from dephosphorylation by PP. OA-sensitive PP activity was determined as the difference in PP activity in the presence or absence of 100 nM OA.
The levels of PPs were measured with methods similar to those reported previously (Maeda et al., 2005a). The protein concentration of the prepared extract was evaluated by Bradford method and adjusted to 5 μg μl−1. The following sample buffer was added in equal proportion to the extract, followed by boiling for 7 min: 0.5 M Tris, 10% sodium dodecyl sulfate (SDS), 10% glycerol, 2-mercaptoethanol and 1% bromophenol blue. Proteins in the sample were separated by size on 10% SDS–polyacrylamide gel and transferred to nitrocellulose membranes in blotting buffer: 25 mM Tris, 192 mM glycine, 0.05% SDS and 20% methanol. The transferred membranes were blocked in phosphate-buffered saline (PBS) containing 5% non-fat dried milk at room temperature for 2 h. The membrane was incubated in primary antibody diluted in PBS containing 5% BSA at 4°C overnight: anti-PP1γ1 catalytic subunit antibody, anti-PP2A catalytic α subunit antibody or anti-PP5/PPT antibody, followed by 2 h incubation at room temperature with secondary antibody, horseradish peroxidase-conjugated goat anti-mouse IgG diluted in PBS containing 5% non-fat dried milk and 0.05% Tween 20. Chemiluminescence of the antigen–antibody peroxidase complex was performed by LumiGLO Reagent and Peroxide, and detected by Chemiluiminator (ATTO, Tokyo, Japan) and analyzed by NIH image (NIH, Bethesda, MD, U.S.A.).
Drugs
Cocaine hydrochloride (Takeda Pharmaceutical Company, Osaka, Japan) and OA sodium salt (Sigma-Aldrich, St Louis, MO, U.S.A.) were dissolved in saline. The following chemicals and reagents were used in the present study: anti-PP1γ1 catalytic subunit antibody (Exalpha Biologicals, Watertown, MA, U.S.A.), anti-PP2A catalytic α subunit antibody and anti-PP5/PPT antibody (BD Bioscience, Tokyo, Japan), horseradish peroxidase-conjugated goat anti-mouse IgG (Zymed Laboratories, San Francisco, CA, U.S.A.), LumiGLO Reagent and Peroxide (Cell Signaling Technology, Beverly, MA, U.S.A.), Coomassie Protein Assay Kit (Pierce, Rockford, IL, U.S.A.) and Serine/Threonine Phosphatase Assay System (Promega, Madison, WI, U.S.A.). All other chemicals were purchased from Nacalai Tesque (Kyoto, Japan) or Wako Pure Chemical Industries (Osaka, Japan).
Data analysis
Data are represented as mean±s.e.m. Statistical significance between unpaired groups was assessed using an unpaired Student's t-test for comparing two columns (Figures 2 and 3) or a one-way ANOVA test followed by Dunnett's post hoc test (Figures 1 and 4) or Bonferroni post hoc test (Figures 5 and 6) for multiple comparisons. For the analysis of locomotor activity during the test day (Figure 1), a one-way ANOVA for repeated measures was used to ascertain differences, followed by Dunnett's post hoc test for multiple comparisons. Significance was set at the P<0.05 level.
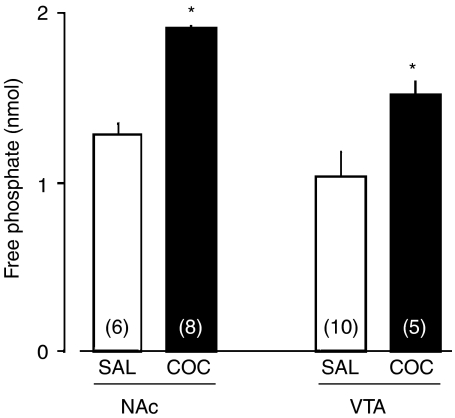
Figure 2.
OA-sensitive PP activity was increased in the brains of mice receiving repeated administration of saline (SAL) or cocaine (COC; 10 mg kg−1). Thirty-one animals went through locomotor testing once a day on day 1 and day 5 of repeated administration; SAL or COC was administered to 16 or 15 animals once a day for 5 days, respectively. Of those receiving COC regimen, only two animals did not develop behavioral sensitization and were removed from the data shown in this figure and were used as nonsensitization animals in experiments shown in Figure 5. Of the remaining 13 animals receiving COC, the NAc was isolated on withdrawal day 7 and another five animals were used to isolate VTA tissue. In SAL-treated animals, the NAc and the VTA was isolated from six and 10 out of 16 animals receiving SAL, respectively. Mice did not go through drug challenge and locomotor testing on withdrawal day 7 before tissue collection. The whole-cell extract was prepared from the NAc and the VTA of mice on day 7 of withdrawal. The free phosphate liberated by dephosphorylation was measured for evaluation of PP activity. OA-sensitive PP activity was determined as the difference in PP activity in the absence of and in the presence of 100 nM OA. The values in the parenthesis are the experimental number of mice. Student's t-test revealed the significant difference between SAL and COC treatment in the NAc (df=12, t=2.79, P=0.0164) and the VTA (df=13, t=2.31, P=0.0376). *P<0.05 vs SAL, determined by Student's t-test.
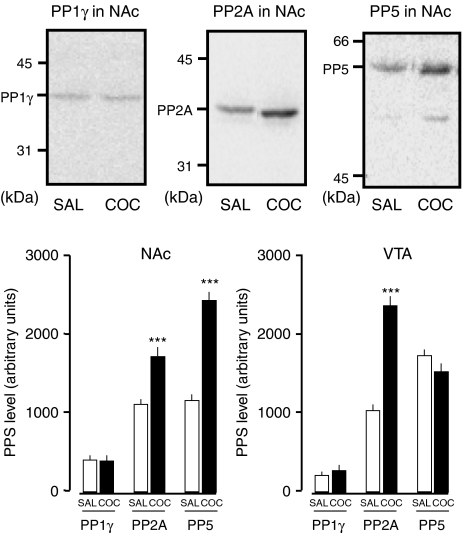
Figure 3.
Upregulation of OA-sensitive PPs in the brains of mice receiving repeated administration of cocaine (COC; 10 mg kg−1). Photographs are representative immunoblots of PP1γ, PP2A and PP5 protein in the NAc. Twelve animals went through locomotor testing using the method described for Figure 2. Saline (SAL) or COC was administered to six animals as described in Figure 2. All the animals subjected to the cocaine regimen developed behavioral sensitization. Mice did not go through drug challenge and locomotor testing on withdrawal day 7 before tissue collection. The NAc and the VTA were both isolated from all animals on day 7 of withdrawal. The individual prepared extracts were used in immunoblots using antibody against all examined subtypes of PPs. Student,s t-test revealed that PP2A content in the COC-treated group was significantly different in the NAc (df=10, t=4.69, P=8.61 × 10−4) and the VTA (df=10, t=8.65, P=5.92 × 10−6) compared to those given SAL, whereas PP5 content in the COC-treated group was significantly different only in the NAc (df=10, t=9.58, P=2.36 × 10−6) compared to mice given SAL. n=6. ***P<0.001 vs SAL, determined by Student's t-test.
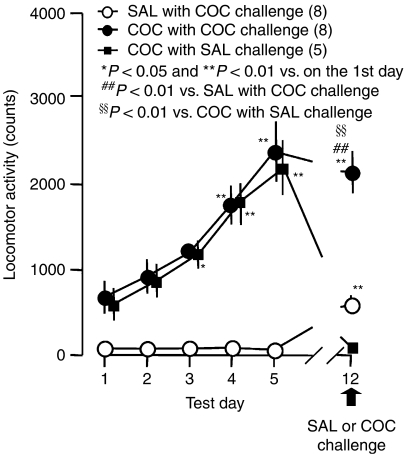
Figure 1.
Repeated administration of cocaine (COC; 10 mg kg−1) induced behavioral sensitization in mice. Twenty-two animals were administered the drug followed by locomotor testing once a day for 5 days. Saline (SAL) with COC challenge was administered to eight mice and COC was administered to14 animals once a day from day 1 to day 5. In mice receiving COC regimen, only one animal did not develop behavioral sensitization and was thus removed from these data but was used in experiments shown in Figure 5 as a nonsensitized animal. Of the remaining 13 mice, those with behavioral sensitization were challenged with cocaine or saline on day 7 of withdrawal: five mice were in the COC with SAL challenge group and eight were in the COC with COC challenge group (10 mg kg−1). The ordinate indicates the total amount of locomotor activity for 3 h after administration. A one-way ANOVA for repeated measures revealed that the significant difference on the test day in SAL with COC challenge (F(5,35)=109.23, P<0.0001), COC with SAL challenge (F(5,20)=42.88, P<0.0001) and COC with COC challenge (F(5,35)=89.83, P<0.0001). *P<0.05 and **P<0.01 vs on test day 1 in each group (Dunnett's test). ##P<0.01 vs SAL with COC challenge and §§P<0.01 vs COC with SAL challenge (F(2,18)=78.75, P<0.0001, a one-way ANOVA followed by Dunnett's test).
Figure 5.
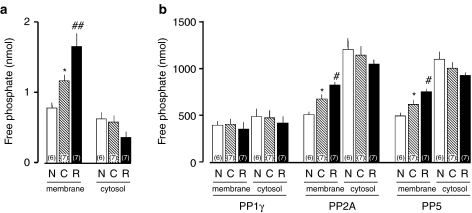
Restraint stress exposure for 30 min increased the activated form of OA-sensitive PPs in the brain membrane fraction of cocaine-sensitized mice. Fourteen animals were given the cocaine regimen followed by locomotor testing, resulting in the behavioral sensitization of all the animals. On withdrawal day 7, seven animals received control treatment (C), and the other seven animals were subjected to restraint for 30 min (R). All the sensitized animals were killed immediately at the end of the restraint period. In addition, six nonsensitized mice (N) removed in experiments described in Figures 1, 2 and 6 were killed. The membrane fraction and the cytosol fraction were prepared from the collected whole brain. (a) The OA-sensitive PP activity. The free phosphate liberated by dephosphorylation was measured for PP activity. OA-sensitive PP activity was determined as the difference in PP activity in the absence of and in the presence of 100 nM OA. The one-way ANOVA followed by Bonferroni test revealed a significant difference in PP activity in the membrane fraction (F(2,17)=25.47, P<0.0001; t=3.14, P<0.05 in C vs N; t=4.11, P<0.01 in R vs C), but not in the cytosol fraction (F(2,17)=2.95, P=0.080). (b) The contents of PPs in membrane and cytosol were assayed by Western blot. The one-way ANOVA followed by Bonferroni test revealed the following: in the membrane PP1γ (F(2,17)=0.217, P=0.807); in the cytosol PP1γ (F(2,17)=0.169, P=0.846); in the membrane PP2A (F(2,17)=17.38, P<0.0001; t=3.13, P<0.05 in C vs N; t=2.88, P<0.05 in R vs C); in the cytosol PP2A (F(2,17)=1.05, P=0.372); in the membrane PP5 (F(2,17)=15.26, P=0.0002; t=2.67, P<0.05 in C vs N; t=2.96, P<0.05 in R vs C); in the cytosol PP5 (F(2,17)=0.827, P=0.454). *P<0.05 vs N, #P<0.05 and ##P<0.01 vs C, determined by Bonferroni test.
Figure 6.
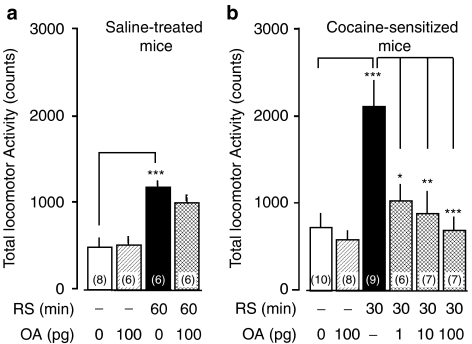
Restraint stress (RS) elicits OA-sensitive hyperlocomotion in cocaine-sensitized mice. Forty animals were given the cocaine regimen followed by locomotor testing as described earlier, but three animals did not develop behavioral sensitization and thus were omitted from the data. Three omitted animals were used in experiments described in Figure 5 as nonsensitized animals. The remaining 37 mice with behavioral sensitization were assigned randomly as shown by the number in the parenthesis in the treatment groups. Intracerebroventricular administration of OA or saline was given to saline-treated (a) and cocaine-sensitized (b) mice immediately before restraint on day 7 of withdrawal, and subsequent locomotor activity was monitored for 60 min. A one-way ANOVA followed by Bonferroni test revealed a significant increase in locomotor activity induced by 60 min-RS (F(3,22)=11.40, P=1.03 × 10−4; t=5.06, P<0.001) which was not altered in saline-treated mice by 100 pg OA immediately before RS (t=1.25, P>0.05). On the other hand, OA (1–100 pg) suppressed the significant increase in locomotor activity induced by 30 min-RS in cocaine-sensitized mice (F(5,41)=8.23, P=1.88 × 10−5; t=5.17, P<0.001) in a dose-dependent manner (t=3.52, P<0.05 at 1 pg; t=4.17, P<0.01 at 10 pg; t=4.84, P<0.001 at 100 pg, vs 30-min RS without OA in cocaine-sensitized mice). *P<0.05, **P<0.01 and ***P<0.001 determined by Bonferroni test.
Results
The total number of mice used in the present study was 187, with 68 treated with repeated administration of saline and 119 treated with cocaine. Repeated administration of saline did not affect locomotor activity measured after daily saline injection: the locomotor activity on day 1 was not significantly different from that on days 2–5 (F(5,35)=109.23, P<0.0001, one-way ANOVA for repeated measurements; q=0.14, 0.08, 0.23 and 0.62 on days 2, 3, 4 and 5, respectively; P>0.05 on the second day to the fifth day vs on the first day; Dunnett's test; Figure 1). One hundred and seven out of 119 mice receiving repeated administration of cocaine showed more counts of locomotor activity on day 5 than on day 1, and were regarded as animals with behavioral sensitization to the stimulant effect of cocaine. The remaining 12 mice that showed no behavioral sensitization were removed from the experimental group unless otherwise noted and were used only in restraint studies as described below. Repeated administration of cocaine induced a progressive increase in locomotor activity in mice receiving repeated administration of cocaine followed by saline challenge (F(5,20)=42.88, P<0.0001) and cocaine challenge (F(5,35)=89.83, P<0.0001), revealed by a one-way ANOVA for repeated measurements (Figure 1). The locomotor activity on days 3–5 was significantly greater than on day 1 in mice receiving repeated administration of cocaine followed by saline challenge (q=3.05, P<0.05 on day 3; q=6.76, P<0.01 on day 4; q=9.35, P<0.01 on day 5; Dunnett's test) and by cocaine challenge (q=5.163, P<0.01 on day 3; q=10.61, P<0.01 on day 4; q=16.67, P<0.01 on day 5; Dunnett's test). To test whether our regimen of cocaine administration produced long-lasting locomotor sensitization, the mice pretreated with saline or cocaine were challenged with 10 mg kg−1 cocaine on day 7 of withdrawal, the 12th test day (Figure 1). Mice receiving repeated administration of cocaine showed enhanced augmentation of locomotor activity on day 12, revealed by cocaine challenge (q=8.02, P<0.01 vs on day 1, Dunnett's test). The locomotor activity was also significantly greater on day 12 in cocaine-treated mice with cocaine challenge (F(2,18)=78.75, P<0.0001, one-way ANOVA), compared to saline-treated animals given cocaine challenge (q=9.48, P<0.01; Dunnett's test) and in cocaine-treated animals with saline challenge (q=11.55, P<0.01, Dunnett's test).
On day 7 of withdrawal, we assessed OA-sensitive PP activity in the whole-cell extract of the NAc and the VTA (Figure 2). The PP activity was observed in the NAc and the VTA of mice receiving repeated administration of saline. Student's t-test revealed a significant difference between saline and cocaine treatment in the NAc (df=12, t=2.79, P=0.0164) and the VTA (df=13, t=2.31, P=0.0376). We examined the effect of repeated administration of cocaine on the protein levels of PPs in the whole-cell extract of the NAc and the VTA in mice on day 7 of withdrawal (Figure 3). Repeated administration of cocaine produced a significant increase in the level of PP2A in the NAc (df=10, t=4.69, P=8.61 × 10−4) and in the VTA (df=10, t=8.65, P=5.92 × 10−6). The level of PP5 was significantly greater in the NAc (df=10, t=9.58, P=2.36 × 10−6), but not in the VTA (df=10, t=0.919, P=0.380), of the cocaine-treated group compared with the saline-treated group. The level of PP1γ was not changed in the NAc (df=10, t=0.201, P=0.845) or the VTA (df=10, t=2.01, P=0.0726) in cocaine-treated mice, as compared to saline-treated mice.
To test whether PPs are involved in the cross-sensitization between cocaine and stress, we exposed cocaine-treated mice to restraint on day 7 of withdrawal (Figure 4). Control mice, which were handled for a few seconds without restraint, showed negligible locomotor activity, whether or not they had received the cocaine regimen. Exposure to 30 min of restraint elicited a marked increase in locomotor activity in cocaine-treated mice, but not in saline-treated mice. The locomotor activity was maximal immediately after the end of restraint (Figure 4a). The restraint-induced hyperlocomotion was dependent on the duration of restraint in both saline- (F(3,22)=8.84, P=4.91 × 10−4, one-way ANOVA) and cocaine-treated mice (F(3,29)=9.19, P=1.97 × 10−4, one-way ANOVA). The locomotor activity was, however, more responsive to 30 min restraint in cocaine-treated animals (q=2.69, P<0.05 vs control, Dunnett's test) than in saline-treated mice (q=1.60, P>0.05 vs control, Dunnett's test) (Figure 4b). We also examined the effect of restraint on locomotor activity using mice in which behavioral sensitization had not developed even though they have been given the cocaine regimen, as described above. The locomotor activity was unchanged by restraint for 30 min in the mice (total locomotor activity of 658±260 counts, n=6; df=14, P=0.735 vs control mice showing behavioral sensitization (n=10), Student's t-test).
To examine the effect the development of behavioral sensitization has on the PPs in the subcellular fraction, we first compared basal functioning of PPs of the whole brain. The activity and the protein levels of PPs in the membrane fraction and the cytosol fraction was compared between non-cocaine-sensitized mice and cocaine-sensitized mice with control treatment. The PP activity was significantly greater in the membrane fraction (F(2,17)=25.47, P<0.0001, one-way ANOVA; t=3.14, P<0.05, Bonferroni test), but not in the cytosol fraction (F(2,17)=2.95, P=0.080, one-way ANOVA), in cocaine-sensitized mice than in non-cocaine-sensitized animals (Figure 5a). Significant increases were observed in PP2A levels (F(2,17)=17.38, P<0.0001, one-way ANOVA; t=3.13, P<0.05, Bonferroni test) and PP5 (F(2,17)=15.26, P=0.0002, one-way ANOVA; t=2.67, P<0.05, Bonferroni test) in the membrane fraction, but not in the cytosol fraction (F(2,17)=1.05, P=0.372 in PP2A; F(2,17)=0.827, P=0.454 in PP5), in cocaine-sensitized mice, compared to non-cocaine-sensitized mice (Figure 5b). On the other hand, the level of PP1γ was not changed in the membrane fraction (F(2,17)=0.217, P=0.807, one-way ANOVA) or the cytosol fraction (F(2,17)=0.169, P=0.846, one-way ANOVA). Whether restraint affected the subcellular localization and activity of OA-sensitive PPs was examined in the subcellular fraction of whole brain in cocaine-sensitized mice. The PP activity was significantly greater in the membrane fraction in restraint-exposed mice than in control animals (t=4.11, P<0.01, Bonferroni test), whereas restraint did not significantly change the PP activity in the cytosol fraction (Figure 5a). Immunoblots for OA-sensitive PPs revealed that restraint induced significant increases in the amount of PP2A measured (t=2.88, P<0.05, Bonferroni test) and PP5 (t=2.96, P<0.05, Bonferroni test) in the membrane fraction, but not in the cytosol fraction, in cocaine-sensitized mice (Figure 5b). On the other hand, the PP1γ level was not changed by restraint in the membrane fraction or the cytosol fraction.
We examined the effect of i.c.v. injection of OA on restraint-induced hyperlocomotive activity. The significant increase in locomotor activity induced by 60 min restraint (F(3,22)=11.40, P=1.03 × 10−4, one-way ANOVA; t=5.06, P<0.001 vs control without OA, Bonferroni test) was not altered in saline-treated mice by 100 pg OA administered immediately before restraint (t=1.25, P>0.05, Bonferroni test) (Figure 6a). On the other hand, OA (1–100 pg) suppressed the significant increase in locomotor activity induced by 30 min restraint in cocaine-sensitized mice (F(5,41)=8.23, P=1.88 × 10−5, one-way ANOVA; t=5.17, P<0.001 vs control without OA, Bonferroni test) in a dose-dependent manner (t=3.52, P<0.05 at 1 pg; t=4.17, P<0.01 at 10 pg; t=4.84, P<0.001 at 100 pg, vs 30 min-restraint without OA in cocaine-sensitized mice; Bonferroni test) (Figure 6b). The locomotor activity was not affected by 100 pg OA in control animals treated with saline (t=1.50, P>0.05 vs control without OA in saline-treated mice, Bonferroni test; Figure 6a) or cocaine (t=0.50, P>0.05 vs control without OA in cocaine-sensitized mice, Bonferroni test; Figure 6b).
Discussion
There have been fewer studies into the role of the PP family in drug addiction, compared to studies of the role of Ser/Thr protein kinases. PP1, although a PP subfamily with low affinity for OA, is possibly involved in drug addiction, as indicated by studies using animals with genetically modified dopamine- and cAMP-regulated phosphorylation of Mr 32,000 (DARPP-32), which is an intrinsic inhibitor for PP1 and has been most extensively studied (Hiroi et al., 1999; Valjent et al., 2005). On the other hand, it is unclear whether OA-sensitive PPs are involved in drug addiction.
To elucidate the involvement of OA-sensitive PPs, PP2A and PP5, in behavioral sensitization to the stimulant effect of cocaine, we first examined the effect of repeated administration of cocaine, which produced behavioral sensitization on the expression levels of PP2A and PP5 in the whole-cell extract of the NAc and the VTA of mice (Figure 3). PP1γ, PP2A and PP5 were all expressed in both the NAc and the VTA in mice receiving repeated administration of saline, as reported previously (Abe et al., 1994; Becker et al., 1994; da Cruz E Silva et al., 1995). Repeated administration of cocaine altered the expression levels of PP2A in both brain regions, whereas the amount of PP5 was increased only in the NAc. In contrast, a change in the level of PP1γ was not observed in either brain region (Figure 3). These results suggest that the changes that occur after cessation of daily cocaine administration are due to previous treatment, the last of which was performed on day 5. The increase in OA-sensitive PP expression was associated with an enhancement of OA-sensitive PP activity in the whole-cell extract of both regions (Figure 2). These results suggest that repeated administration of cocaine upregulates the activated form of PP2A and PP5 in the brain region related to drug addiction, although the reason for the regional difference in the change in expression level of PP5 remains unclear. Backes & Hemby (2003) found that the PP2A catalytic subunit mRNA, but not the PP1 family, were upregulated after 1 day and 20 days of self-administration of cocaine in the VTA dopamine neurons of rats, which may support in part the present results. This led us to consider the role OA-sensitive PPs may play in the withdrawal period in cocaine-sensitized mice.
As yet, there are no studies that have examined the influence of restraint on spontaneous locomotor activity during withdrawal from repeated administration of cocaine in mice. We think it is likely related to stress-induced reinstatement of drug seeking or drug taking and stress-induced recurrence of psychostimulant psychosis (Ujike, 2002). Understanding the mechanisms underlying cocaine-induced behavioral sensitization to stress may therefore throw more light on the biological basis of addiction. The present study showed that restraint induced hyperlocomotion in both saline-treated and cocaine-sensitized mice on day 7 of withdrawal in a manner dependent on the duration of restraint exposure. The locomotor activity, however, was more susceptible to restraint-elicited augmentation in cocaine-sensitized mice than in saline-treated mice. Thirty minutes of restraint did not alter locomotor activity in mice receiving repeated administration of saline or in mice that did not show behavioral sensitization even though they were given repeated administration of cocaine. The results suggest that cocaine-sensitized mice are predisposed to stress-induced hyperlocomotion, which is referred to as cross-sensitization and is maintained over the withdrawal period (Kalivas & Stewart, 1991).
The dosage of OA used in the present study was determined based on our previous study, which demonstrated that i.c.v. injection of OA-blocked morphine-induced antinociception via the inhibition of PP2A and/or PP5 in mice (Maeda et al., 2005a). Another study indicates the possibility that similar doses of OA preferentially block PP2A rather than PP1 when injected i.c.v. to mice (Moncada et al., 2003). Therefore, the doses of OA used in the present study seem sufficient to specifically inhibit OA-sensitive PPs in the mouse brain. Using this dosage of OA, we examined the effect of i.c.v. injection of OA on restraint-induced hyperlocomotion. Restraint-induced hyperlocomotion in cocaine-sensitized mice, but not in saline-treated mice, was blocked by i.c.v. injection of OA before restraint in a dose-dependent manner. This led us to propose that OA-sensitive PPs work specifically on the expression of cross-sensitization in mice with behavioral sensitization to stimulant effect of cocaine.
OA-sensitive PPs can dephosphorylate some membrane molecules, such as ion channels, neurotransmitter receptors and neurotransmitter transporters, some of which are a possible molecular basis for addiction. That enables us to focus on the effect of restraint on the OA-sensitive PP activity in the subcellular fraction of whole brain in cocaine-sensitized mice. Here, we demonstrate that restraint can activate OA-sensitive PPs in the membrane fraction of whole brain in cocaine-sensitized mice at a peak time to produce the restraint-induced hyperlocomotion. Restraint-induced enhancement of OA-sensitive PP activity was accompanied by an incremental increase in the expression levels of PP2A and PP5, but not PP1γ, in the membrane fraction in cocaine-sensitized mice. These results suggest that exposure to restraint stimulates translocation of PP2A and PP5 to the cell membrane to induce hyperlocomotion in cocaine-sensitized mice, but not in saline-treated mice. The present study leads us to the proposal that increased protein dephosphorylation in the membrane fraction mediated by OA-sensitive PPs is required for restraint-induced hyperlocomotion in cocaine-sensitized mice.
The present results raise two questions about the involvement of OA-sensitive PPs in stress-induced hyperlocomotion in cocaine-sensitized mice. Firstly, which membrane protein molecules are dephospholyrated by OA-sensitive PPs in order to elicit hyperlocomotion in cocaine-sensitized mice subjected to restraint? One candidate molecule is the large conductance calcium-activated potassium channel, referred to as the BK channel. Synthetic glucocorticoid reportedly activates BK channels through PP2A (Tian et al., 1998; 2001). This led us to consider that stress stimuli activate the HPA axis to release glucocorticoid, which in turn stimulates the BK channel through the stimulation and association of PP2A. The activation of the BK channel could result in modification of cell excitability related to the expression of hyperlocomotion. It is, however, an open question whether the candidate is involved in restraint-induced hyperlocomotion only in cocaine-sensitized mice.
The second question is how restraint causes translocation of OA-sensitive PPs to the cell membrane in cocaine-sensitized mice. There are two reports suggesting that PP2A can be translocated to the membrane fraction to dephosphorylate certain membrane molecules. One example is antigen-stimulated transient, reversible translocation and activation of PP2A in the membrane during mast cell secretion (Ludowyke et al., 2000). The other is that PP2A complexes are translocated to the membrane fraction to dephosphorylate retinal membrane protein during dark exposure in mouse retina (Brown et al., 2002). These reports suggest that the physiological stimuli are sufficient to induce PP2A translocation to the cell membrane, which triggers a physiological response in vivo. On the other hand, although it is known that stress has a propensity to augment the psychomotor effects of psychostimulants (Kalivas & Stewart, 1991), what is it that makes the connection between the restraint stimulus and PP2A translocation to induce consequent hyperlocomotion? Synaptic plasticity in the glutamatergic afferents to the NAc has been implicated in behavioral sensitization to the stimulant effect of cocaine by some studies. Injection of an AMPA receptor agonist to the NAc elicited hyperlocomotion only in cocaine-sensitized mice (Pierce et al., 1996). Long-term depression (LTD) at the glutamatergic afferents, an example of synaptic plasticity, was accompanied by behavioral sensitization to the stimulant effect of cocaine (Thomas et al., 2001; Maeda et al., 2005b). Another experiment showed that upregulation in non-NMDA receptors in the NAc inhibited stress-induced reinstatement of cocaine-seeking behavior in animals trained to self-administer cocaine, another model for addiction (Sutton et al., 2003). A further important fact is that the synaptic activation of OA-sensitive PPs is required for the maintenance of LTD (Mulkey et al., 1993). These reports propose that stress alters plasticity of glutamatergic systems through the activation of OA-sensitive PPs to elicit hyperlocomotion in cocaine-sensitized mice, although further studies are required to test those hypotheses.
In conclusion, the present results are the first demonstration of restraint-induced translocation of OA-sensitive PPs to the membrane associated with induction of hyperlocomotion in cocaine-sensitized mice. These results, therefore, have implications for understanding the intracellular mechanisms of psychostimulant-induced cross-sensitization to stress, which might underlie spontaneous recurrence of psychostimulant psychosis in stressful situations in addiction.
Acknowledgments
We are grateful to Dr James H. Woods and Dr Gail Winger for advice concerning this manuscript.
Abbreviations
- HPA
hypothalamo–pituitary–adrenal
- LTD
long-term depression
- NAc
nucleus accumbens
- OA
okadaic acid
- PBS
phosphate-buffered saline
- PP
serine/threonine protein phosphatase
- Ser
serine
- SDS
sodium dodecyl sulfate
- Thr
threonine
- VTA
ventral tegmental area
References
- ABE H., SHIMA H., SEKIGUCHI M., GUO H., NAGAO M., TAMURA S., KONDO H. Localization of mRNA for protein phosphatase 2A in the brain of adult rats. Brain Res. Mol. Brain Res. 1994;22:139–143. doi: 10.1016/0169-328x(94)90041-8. [DOI] [PubMed] [Google Scholar]
- BACKES E., HEMBY S.E. Discrete cell gene profiling of ventral tegmental dopamine neurons after acute and chronic cocaine self-administration. J. Pharmacol. Exp. Ther. 2003;307:450–459. doi: 10.1124/jpet.103.054965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BECKER W., KENTRUP H., KLUMPP S., SCHULTZ J.E., JOOST H.G. Molecular cloning of a protein serine/threonine phosphatase containing a putative regulatory tetratricopeptide repeat domain. J. Biol. Chem. 1994;269:22586–22592. [PubMed] [Google Scholar]
- BROWN B.M., CARLSON B.L., ZHU X., LOLLEY R.N., CRAFT C.M. Light-driven translocation of the protein phosphatase 2A complex regulates light/dark dephosphorylation of phosducin and rhodopsin. Biochemistry. 2002;41:13526–13538. doi: 10.1021/bi0204490. [DOI] [PubMed] [Google Scholar]
- DA CRUZ E SILVA E.F., FOX C.A., OUIMET C.C., GUSTAFSON E., WATSON S.J., GREENGARD P. Differential expression of protein phosphatase 1 isoforms in mammalian brain. J. Neurosci. 1995;15:3375–3389. doi: 10.1523/JNEUROSCI.15-05-03375.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EHRMAN R.N., ROBBINS S.J., CHILDRESS A.R., O'BRIEN C.P. Conditioned responses to cocaine-related stimuli in cocaine abuse patients. Psychopharmacology (Berlin) 1992;107:523–529. doi: 10.1007/BF02245266. [DOI] [PubMed] [Google Scholar]
- HIROI N., FIENBERG A.A., HAILE C.N., ALBURGES M., HANSON G.R., GREENGARD P., NESTLER E.J. Neuronal and behavioural abnormalities in striatal function in DARPP-32-mutant mice. Eur. J. Neurosci. 1999;11:1114–1118. doi: 10.1046/j.1460-9568.1999.00570.x. [DOI] [PubMed] [Google Scholar]
- JAFFE J.H.Cocaine-related disorders Comprehensive Textbook of Psychiatry 2002Philadelphia: Lippincott Williams & Wilkins; 999–1015.ed. Sadock, B.J. & Sadock, V.A., pp [Google Scholar]
- JAFFE J.H., CASCELLA N.G., KUMOR K.M., SHERER M.A. Cocaine-induced cocaine craving. Psychopharmacology (Berlin) 1989;97:59–64. doi: 10.1007/BF00443414. [DOI] [PubMed] [Google Scholar]
- KALIVAS P.W., STEWART J. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res. Brain Res. Rev. 1991;16:223–244. doi: 10.1016/0165-0173(91)90007-u. [DOI] [PubMed] [Google Scholar]
- LUDOWYKE R.I., HOLST J., MUDGE L.M., SIM A.T. Transient translocation and activation of protein phosphatase 2A during mast cell secretion. J. Biol. Chem. 2000;275:6144–6152. doi: 10.1074/jbc.275.9.6144. [DOI] [PubMed] [Google Scholar]
- MAEDA T., HAMABE W., GAO Y., FUKAZAWA Y., KUMAMOTO K., OZAKI M., KISHIOKA S. Morphine has an antinociceptive effect through activation of the okadaic-acid-sensitive Ser/Thr protein phosphatases PP2A and PP5 estimated by tail-pinch test in mice. Brain Res. 2005a;1056:191–199. doi: 10.1016/j.brainres.2005.07.033. [DOI] [PubMed] [Google Scholar]
- MAEDA T., KISHIOKA S., HAMABE W., FUKAZAWA Y., KUMAMOTO K., GAO Y., YAMAMOTO C., OZAKI M., YAMAMOTO H. Repeated exposure to addictive drug elicits synaptic adaptation in rat mesolimbic slice culture. J. Pharmacol. Sci. 2005b;97:114. [Google Scholar]
- MARINELLI M., PIAZZA P.V. Interaction between glucocorticoid hormones, stress and psychostimulant drugs. Eur. J. Neurosci. 2002;16:387–394. doi: 10.1046/j.1460-9568.2002.02089.x. [DOI] [PubMed] [Google Scholar]
- MONCADA A., CENDAN C.M., BAEYENS J.M., DEL POZO E. Effects of serine/threonine protein phosphatase inhibitors on morphine-induced antinociception in the tail flick test in mice. Eur. J. Pharmacol. 2003;465:53–60. doi: 10.1016/s0014-2999(03)01461-4. [DOI] [PubMed] [Google Scholar]
- MULKEY R.M., HERRON C.E., MALENKA R.C. An essential role for protein phosphatases in hippocampal long-term depression. Science. 1993;261:1051–1055. doi: 10.1126/science.8394601. [DOI] [PubMed] [Google Scholar]
- PIERCE R.C., BELL K., DUFFY P., KALIVAS P.W. Repeated cocaine augments excitatory amino acid transmission in the nucleus accumbens only in rats having developed behavioral sensitization. J. Neurosci. 1996;16:1550–1560. doi: 10.1523/JNEUROSCI.16-04-01550.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SARNYAI Z., SHAHAM Y., HEINRICHS S.C. The role of corticotropin-releasing factor in drug addiction. Pharmacol. Rev. 2001;53:209–243. [PubMed] [Google Scholar]
- SATO M., NUMACHI Y., HAMAMURA T. Relapse of paranoid psychotic state in methamphetamine model of schizophrenia. Schizophr. Bull. 1992;18:115–122. doi: 10.1093/schbul/18.1.115. [DOI] [PubMed] [Google Scholar]
- SUTTON M.A., SCHMIDT E.F., CHOI K.H., SCHAD C.A., WHISLER K., SIMMONS D., KARANIAN D.A., MONTEGGIA L.M., NEVE R.L., SELF D.W. Extinction-induced upregulation in AMPA receptors reduces cocaine-seeking behaviour. Nature. 2003;421:70–75. doi: 10.1038/nature01249. [DOI] [PubMed] [Google Scholar]
- SVENNINGSSON P., NISHI A., FISONE G., GIRAULT J.A., NAIRN A.C., GREENGARD P. DARPP-32: an integrator of neurotransmission. Annu. Rev. Pharmacol. Toxicol. 2004;44:269–296. doi: 10.1146/annurev.pharmtox.44.101802.121415. [DOI] [PubMed] [Google Scholar]
- THOMAS M.J., BEURRIER C., BONCI A., MALENKA R.C. Long-term depression in the nucleus accumbens: a neural correlate of behavioral sensitization to cocaine. Nat. Neurosci. 2001;4:1217–1223. doi: 10.1038/nn757. [DOI] [PubMed] [Google Scholar]
- TIAN L., HAMMOND M.S., FLORANCE H., ANTONI F.A., SHIPSTON M.J. Alternative splicing determines sensitivity of murine calcium-activated potassium channels to glucocorticoids. J. Physiol. 2001;537:57–68. doi: 10.1111/j.1469-7793.2001.0057k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TIAN L., KNAUS H.G., SHIPSTON M.J. Glucocorticoid regulation of calcium-activated potassium channels mediated by serine/threonine protein phosphatase. J. Biol. Chem. 1998;273:13531–13536. doi: 10.1074/jbc.273.22.13531. [DOI] [PubMed] [Google Scholar]
- UJIKE H. Stimulant-induced psychosis and schizophrenia: the role of sensitization. Curr. Psychiatry. Rep. 2002;4:177–184. doi: 10.1007/s11920-002-0024-7. [DOI] [PubMed] [Google Scholar]
- VALJENT E., PASCOLI V., SVENNINGSSON P., PAUL S., ENSLEN H., CORVOL J.C., STIPANOVICH A., CABOCHE J., LOMBROSO P.J., NAIRN A.C., GREENGARD P., HERVE D., GIRAULT J.A. Regulation of a protein phosphatase cascade allows convergent dopamine and glutamate signals to activate ERK in the striatum. Proc. Natl. Acad. Sci. U.S.A. 2005;102:491–496. doi: 10.1073/pnas.0408305102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YUI K., GOTO K., IKEMOTO S., ISHIGURO T., KAMADA Y. Increased sensitivity to stress and episode recurrence in spontaneous recurrence of methamphetamine psychosis. Psychopharmacology (Berlin) 1999;145:267–272. doi: 10.1007/s002130051058. [DOI] [PubMed] [Google Scholar]