Abstract
Although previous reports have suggested that the sigma 1 (σ1) receptor may be involved in pain sensation, its specific site of action has not been elucidated. The aim of present study was to determine the role of the spinal σ1 receptor in formalin-induced pain behavior, spinal cord Fos expression and phosphorylation of N-methyl-D-aspartate receptor subunit 1 (pNR1).
Intrathecal (i.t.) pretreatment with the selective σ1 receptor antagonist, BD-1047 (N-[2-(3,4-dichlorophenyl)ethyl]-N-methyl-2-(dimethylamino)ethylamine dihydrobromide) (10–100 nmol) dose dependently reduced formalin-induced pain behaviors in second phase, but not first phase, of the formalin test. I.t. injection of BD-1047 also reduced formalin-evoked Fos expression and pNR1 at the protein kinase C-dependent site, serine-896 (Ser896) and the protein kinase A-dependent site, serine-897 (Ser897) in spinal dorsal horn.
i.t. BMY-14802 ((α-(4-fluorophenyl)-4-(5-fluoro-2-pyrimidinyl)-1-piperazinebutanol hydrochloride) (10–100 nmol, σ1 receptor antagonist and 5-HT1A receptor agonist) dose dependently reduced formalin-induced pain behaviors in both phases. However, the 5-HT1A receptor might not be involved in the antinociceptive effect of BMY-14802 on the second phase, since i.t. pretreatment with the 5-HT1A receptor antagonist propranolol ((S)-1-isopropylamino-3-(1-naphthyloxy)-2-propanol hydrochloride) (injected 10 min prior to i.t. BMY-14802) partially blocked the effect of BMY-14802 on the first phase of the formalin test but did not affect the inhibitory effect of BMY-14802 on the second phase. In addition, i.t. BMY-14802 significantly reduced formalin-evoked Fos expression and pNR1 (Ser896 and Ser897) expression in spinal dorsal horn.
The results of this study suggest that selective blockage of spinal σ1 receptors can reduce pain behaviors, spinal cord Fos expression and pNR1 (Ser896 and Ser897) expression associated with the second phase of the formalin test.
Keywords: σ1 receptor, BD-1047, BMY-14802, formalin test, Fos, NMDA phosphorylation
Introduction
The sigma (σ) receptor has been classified into two distinct subtypes called sigma 1 (σ1) and σ2, which differ in their affinities for σ ligands (Guitart et al., 2004). Sigma binding sites are distinct from opiate, phencyclidine and dopamine (D2) receptors (Su, 1982; Tam & Cook, 1984; Walker et al., 1990). Recently, it has been shown in σ1 receptor knockout mice that both phases of formalin-induced paw licking/biting behavior are reduced by approximately 55% in comparison to wild-type animals, suggesting that the σ1 receptor is involved in formalin-induced pain (Cendan et al., 2005b). Systemic injection of haloperidol and its metabolites has been shown to consistently produce antinociceptive effects in both phases of the formalin test via the suppression of σ1 receptor activity (Cendan et al., 2005a). These findings suggest that σ1 receptor antagonists can produce antinociception in the formalin test. However, neither the precise site of action nor the mechanism of action of σ drugs has been clearly defined with respect to nociception. Since σ1 receptors have the potential to modulate biological systems related to learning and memory, psychostimulant-induced sensitization, cocaine-induced conditioned place preference and pain perception (Ueda et al., 2001; Guitart et al., 2004), it seems likely that systemic injection of σ1 ligands may affect normal physiological functions. However, little has been reported regarding the linkage of sigma receptors to specific signaling pathways. Thus, it is very important to determine both the site of action and mechanism of action of σ1 receptor ligands as they relate to nociception.
Because σ1 receptors have such a wide distribution (Alonso et al., 2000), it is very difficult to discriminate the actual sites of action of systemically administered sigma drugs on pain sensation or which location is important for sigma effects on nociception in σ1 receptor knockout mice. For these reasons, the present study was aimed at testing the hypothesis that spinal cord σ1 receptors are involved with central nociceptive processing and sensitization in a formalin-induced pain model.
In the present study, we determined the role of spinal σ1 receptor antagonism on formalin-induced paw licking/biting behavior and formalin-induced spinal Fos expression by intrathecal (i.t.) pretreatment of mice with selective σ1 receptor antagonists BD-1047 (N-[2-(3,4-dichlorophenyl)ethyl]-N-methyl-2-(dimethylamino)ethylamine dihydrobromide) or BMY-14802 (α-(4-fluorophenyl)-4-(5-fluoro-2-pyrimidinyl)-1-piperazinebutanol hydrochloride). The BD-1047 antagonist shows a high affinity for σ1 receptor (Ki=0.93 nM) and thus is commonly used to evaluate the role of the σ1 receptor (Matsumoto et al., 1995; McCracken et al., 1999; Wang et al., 2005). BMY-14802 is also regarded as a potent σ1 receptor antagonist (IC50=75 nM), but in addition it acts as a partial agonist at the 5-HT1A receptor (IC50=199 nM; Matos et al., 1996). Since i.t. injection of a 5-HT1A receptor agonist has previously been shown to significantly reduce formalin-induced pain behavior and spinal cord Fos expression (Buritova et al., 2005), we pretreated animals with the 5-HT1A receptor antagonist, propranolol ((S)-1-isopropylamino-3-(1-naphthyloxy)-2-propanol hydrochloride), 10 min prior to the i.t. injection of BMY-14802. This allowed us to remove any 5-HT1A receptor effects and to specifically examine the spinal σ1 receptor antagonism of BMY-14802 on formalin-induced nociception (Ernberg et al., 2000). Behavioral experiments were carried out by measuring formalin-induced nociceptive paw licking time following i.t. pretreatment with the σ1 receptor antagonists.
N-methyl-D-aspartate (NMDA) receptor activation plays an important role in formalin-induced nociception (Coderre & Melzack, 1992; Chaplan et al., 1997) and in particular NR1 is known to be an essential component of functional NMDA receptors (Masu et al., 1993; Mori & Mishina, 1995). The σ1 receptors appear to be functionally coupled to NMDA receptors (Bermack et al., 2002; Nuwayhid & Werling, 2003). This is further supported by the work of Wang & Takigawa (2002) showing that the selective σ1 ligands, MS-377 and 3-PPP, attenuate the PCP blockade of NMDA-induced increases in intracellular calcium in cultured neocortical neurons. Collectively, these results support the current interpretation that sigma ligands may directly or indirectly modulate NMDA receptor complex functions. The function and localization of NMDA receptors is also modulated by post-translational modifications including phosphorylation, glycosylation and nytrosylation. NMDA receptors are phosphorylated on serines of both the NR1 and NR2 subunits and on tyrosines of the NR2 subunits (Llansola et al., 2005). Recent work has shown that lumbar spinal cord NR1 subunits are phosphorylated on serine residues within 2 h of the induction of hind paw inflammation with carrageenan (Caudle et al., 2005). Because of the known interaction between the σ1 and NMDA receptors, we chose to investigate whether the i.t. administration of σ1 receptor antagonists reduced formalin-induced phosphorylation of the NMDA receptor subunit 1 (pNR1) in the spinal cord dorsal horn.
Methods
Animals
Male ICR mice (24–30 g B.W.) were purchased from the Laboratory Animal Center of Seoul National University (SNU). A total number of 109 mice were used for these studies and were maintained under the following conditions: 12 h light/dark cycle, room temperature (20–25°C) and 40–60% humidity. Food and water were available ad libitum. The experimental protocols for animal usage were reviewed and approved by the SNU Animal Care and Use Committee and conform to NIH guidelines (NIH publication no. 86-23, revised 1985). This study was carried out in accordance with the ethical guidelines for investigations of experimental pain in conscious animals (Zimmermann, 1983).
i.t. injection
Drugs were dissolved in 5 μl of sterile saline and an i.t. injection was performed according to the procedure of Hylden & Wilcox (1980), using a 50 μl Hamilton syringe with a 30-gauge needle. The flick of the tail was considered indicative of a successful i.t. administration. The control group received an i.t. injection of sterile saline solution.
The σ1 receptor antagonists, BD-1047 and BMY-14802, were purchased from Tocris (Avonmouth, U.K.). These drugs were dissolved in sterile saline solution and intrathecally injected 10 min before formalin injection, respectively. The 5-HT1A receptor antagonist, propranolol, was purchased from the Sigma (St Louis, MO, U.S.A.) and was dissolved in sterile saline. The i.t. injection of propranolol (in an injection volume of 5 μl) was performed 10 min before the i.t. treatment of BMY-14802.
Formalin test
The formalin test was carried out as previously described (Kim et al., 2005). Formalin injected subcutaneously into the hind paw produces a biphasic pain response: an acute phase of short duration followed by a longer-lasting tonic phase. After a 30 min acclimation period in an observation chamber (with a mirror placed under the floor at a 45° angle to allow an unobstructed view of the paw), mice were injected with 20 μl of 1% formalin solution into the plantar surface of the right hind paw using a 30-gauge needle. After the formalin injection, animals were immediately placed on a temperature-regulated acrylic observation chamber (height: 40 cm, diameter: 20 cm), and their behavior was recorded for a 30 min period using a video camera. Following the video-taping, paw licking time (in seconds per each 5 min increment) was calculated by two experienced investigators, blinded to the experimental conditions, during both the first phase (0–10 min post-formalin injection) and the second phase (10–30 min post-formalin injection) of the formalin test. Subsequently, the mean licking time was calculated from the data obtained by these two experimenters. To determine the possible antinociceptive effect of σ1 receptor antagonists on formalin-induced pain behavior, data were expressed as the percent antinociception, which was calculated using the formula: 100-((post-drug licking time/mean licking time of control group) × 100). Mice in the control group received an i.t. injection of saline without any further treatment. A total of 56 mice were used for formalin test experiments (n=7 in each group).
Immunohistochemistry for Fos and pNR1 (Ser896 and Ser897)
All immunohistochemical procedures used in the present study are based on the methods previously described by Kwon et al. (2001). Animals were deeply anesthetized with 5% isoflurane and perfused transcardially with calcium-free Tyrode's solution followed by a fixative containing 4% paraformaldehyde and 0.2% picric acid in 0.1 M phosphate buffer (pH 6.9). Animals that were used for Fos immunohistochemistry were killed 2 h after formalin injection, while those used for pNR1 immunohistochemistry were killed 30 min after formalin injection as previously described (Brenner et al., 2004; Fukuda et al., 2006). The spinal cord (L4–6) was removed immediately after perfusion, postfixed in the same fixative for 4 h and then cryoprotected in 30% sucrose in PBS (pH 7.4).
A series of frozen sections (40 μm thickness) were cut through the L4–6 segments of the lumbar spinal cord using a cryostat (Microm, Germany). After elimination of endogenous peroxidase activity with 3% hydrogen peroxide in PBS and preblocking with 1% normal goat serum and 0.3% Triton X-100 in PBS, the sections were incubated in rabbit anti-Fos antiserum (1 : 10,000, cat# PC38, Calbiochem, CA, U.S.A.) or in rabbit anti-pNR1 antisera (Ser896 cat# 06–640 or Ser897 cat# 06–641, 1 : 1000, Upstate Biotechnology, NY, U.S.A.) overnight. The sections were rinsed in PBS and processed with the avidin–biotin–peroxidase technique as previously described (Kwon et al., 2001). Finally, visualization was performed using 3,3′-diaminobenzidine (DAB, Sigma, St Louis, MO, U.S.A.). The DAB reaction was intensified with 0.2% nickel chloride. The sections were mounted on gelatin-subbed slides and the slides were dried, dehydrated in ethanol (70–100% gradually), cleared in xylene, and cover-slipped. A total of 25 mice were used for Fos and pNR1 (Ser896 and Ser897) immunohistochemistry (n=5 in each group).
Image analysis
Tissue sections were first examined using dark field microscopy (Zeiss Axioscope, Germany) to determine the segmental level according to Abbadie & Besson (1994) and to identify specific gray matter landmarks in order to define individual spinal cord laminae. For quantitative analysis, sections were scanned and the five with the greatest number of labeled cells at the L4–6 level were selected from each animal. Individual sections were digitized with 4096 gray levels using a cooled CCD camera (Micromax Kodak 1317; Princeton Instruments, AZ, U.S.A.) connected to a computer-assisted image analysis system (Metamorph; Universal Imaging, PA, U.S.A.). In order to maintain a constant threshold for each image and to compensate for subtle variability of the immunostaining, we only counted neurons that were at least 50% darker than the average gray level of each image after background subtraction and shading correction were performed in two areas of dorsal horn, the superficial layer (laminae I–II) and the deep dorsal horn (laminae III–VI). The microscope illumination and data acquisition settings were fixed throughout the entire analysis procedure. The average number of Fos and pNR1-immunoreactive neurons was calculated per section from each animal. These values obtained from at least six animals in each group were averaged and presented as group data. All analysis procedures described above were performed blindly with regard to the experimental condition.
Measurement of motor performance
In order to rule out possible drug effects on motor coordination, mice that received i.t. BD-1047 (100 nmol) or BMY-14802 (100 nmol) were evaluated with a rotarod apparatus as previously described (Lee et al., 2000). The apparatus consisted of a horizontal bar (diameter=6 cm), subdivided into four compartments (model# DJ-4009, Dae-Jong Engineering & Clean Technology, Korea). All mice were placed on the horizontal bar rotating at a speed of 4 r.p.m. 24 h before the actual rotarod test and those that were able to remain on the rod for at least 120 s were included in the study. The mice were divided into four groups (n=7 per group) and were treated with the i.t. saline, i.t. BD-1047 (100 nmol), i.t. BMY-14802 (100 nmol) or the reference sodium pentobarbital (40 mg kg−1, i.p.). Each animal was subsequently tested on the rotarod and performance time on the bar (in sec) during a 2-min test period was recorded before, just after (0 h), and at 1 and 2 h after drug administration.
Statistical analysis
All experimental results are shown as the mean±s.e.m. The levels of statistical significance were determined by an unpaired Student's t-test for comparisons between two means, and by analysis of variance (ANOVA) followed by Newman–Keuls test for multiple comparisons. P-values of less than 0.05 were considered to be statistically significant.
Results
The antinociceptive effect of σ1 receptor antagonists on the formalin-induced pain behavior
Mice that received i.t. saline and intraplantar formalin injection exhibited typical biphasic pain behaviors during the 30 min observation period (first phase: 0–10 after formalin injection and second phase: 10–30 min after formalin injection). Formalin-induced paw licking/biting time was 75.6±5.6 s during the first phase and 140±18.2 s during second phase. Specific pain-related behaviors were not evoked in control mice, receiving only i.t. saline.
Intrathecal pretreatment with the σ1 receptor antagonist BD-1047 significantly inhibited formalin-induced pain behavior during the second phase of the formalin test, except at the lowest dose (10 nmol) of BD-1047 tested (Figure 1). It is noteworthy that BD-1047 had no effect on formalin-induced pain behavior during the first phase of the formalin test.
Figure 1.
The antinociceptive effect of i.t. BD-1047 on formalin-induced pain behavior. BD-1047 administration significantly reduced the paw licking/biting behavior in the second but not in the first phase of the formalin test as compared with control mice receiving i.t. vehicle injection, *P<0.05 and ***P<0.001. The number of mice was seven in each group.
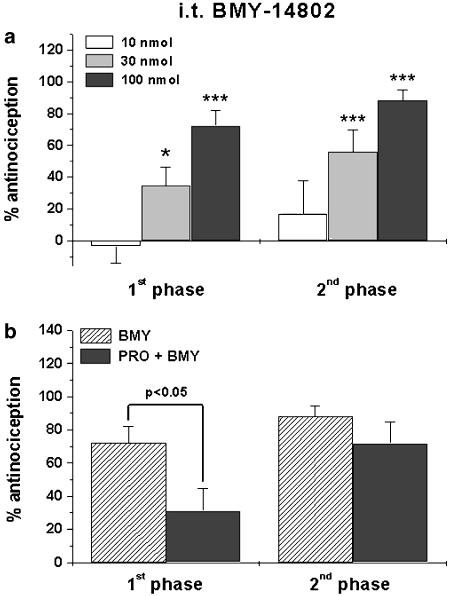
I.t. BMY-14802 dramatically reduced formalin-induced pain behaviors in both phases of the formalin test. Similar to BD-1047, the lowest dose of BMY-14802 (10 nmol) used in this study had no antinociception effect (panel a in Figure 2). I.t. pretreatment with the 5-HT1A receptor antagonist propranolol significantly reduced the antinociceptive effect of BMY-14802 in the first, but not in the second, phase of the formalin test, while i.t. propranolol itself did not affect formalin-induced paw licking/biting behavior (81.7±7 s in the first phase and 132±19.1 s in the second phase).
Figure 2.
The antinociceptive effect of i.t. BMY-14802 (BMY) on formalin-induced pain behavior. BMY-14802 significantly suppressed formalin-induced pain behavior in both the first and second phases of the formalin test (a). This strong antinociceptive effect of BMY-14802 (100 nmol) in the first phase, but not in the second phase of the formalin test was significantly reversed by i.t. pretreatment with propranolol (PRO, 100 nmol) (b). *P<0.05 and ***P<0.001 as compared to control mice receiving i.t. saline injection and formalin. The number of mice was seven in each group.
The suppressive effect of σ1 receptor antagonists on formalin-induced spinal Fos expression
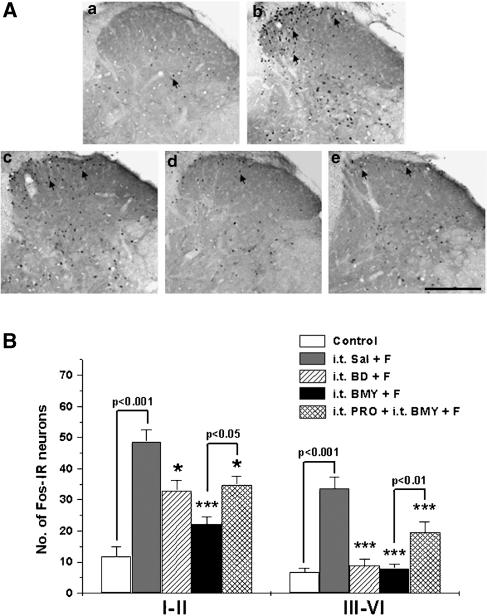
As shown in Figure 3, 2 h after the formalin injection, the number of Fos-immunoreactive (IR) neurons in the spinal cord dorsal horn was significantly increased in laminae I–II (48.7±3.9) and laminae III–VI (33.7±3.6) as compared with that of control mice (11.8±3.2 in laminae I–II and 6.7±1.5 in laminae III–VI). I.t. injection of BD-1047 (100 nmol) significantly reduced this formalin-induced increase in Fos expression in the dorsal horn. The i.t. administration of BMY-14802 (100 nmol) also caused a significant reduction in formalin-induced Fos expression in laminae I–II and in laminae III–VI. As illustrated in Figure 3B, i.t. injection of propranolol partially reduced this suppressive effect of BMY-14802 on formalin-induced Fos expression.
Figure 3.
The suppressive effect of i.t. injection of the spinal σ1 receptor antagonists, BD-1047 (BD) and BMY-14802 (BMY), on formalin (F)-induced spinal Fos expression. Representative spinal cord sections illustrating the typical pattern of Fos expression observed in each of the five groups are shown in the upper panel (A). At 2 h after the formalin injection, the number of Fos immunoreactive (IR) neurons is remarkably increased both in the superficial dorsal horn (laminae I–II) and the intermediate dorsal horn (laminae III–VI) in i.t. saline (Sal)-pretreated mice (spinal cord section ‘b') as compared to control mice (spinal cord section ‘a'). Both i.t. BD-1047 and BMY-14802 significantly suppressed the formalin-induced Fos expression, while i.t. propranolol (PRO) reversed but did not completely abolish the inhibitory effect of BMY-14802 (B). In panel (A), a: control, b: i.t. Sal+F, c: i.t. BD+F, d: i.t. BMY+F, e: i.t. PRO+i.t. BMY+F. Arrows: Fos-IR neurons. Scale bar=200 μm. *P<0.05 and ***P<0.001 as compared with those of i.t. Sal+F group. The number of mice was five in each group.
The inhibitory effect of i.t. BD-1047 and BMY-14802 on formalin-induced pNR1 (Ser896) expression in the dorsal horn
In control animals, only a small number of pNR1 (Ser896) IR neurons was found in the dorsal horn and these were scattered randomly throughout laminae I–VI (Figure 4). At 30 min after the formalin injection, pNR1 Ser896-positive neurons were mainly found in the deep dorsal horn (36.4±1.5 in laminae III–VI) with relatively low level of immunostained neurons in the superficial layer (3.2±0.6 in laminae I–II). Photomicrographs illustrating the typical distribution pattern of pNR1 (Ser896) in the dorsal horn of each treatment group are shown in panel A of Figure 4.
Figure 4.
The suppressive effect of the i.t. injected σ1 receptor antagonists, BD-1047 (BD) and BMY-14802 (BMY), on the formalin (F)-induced spinal pNR1 (Ser896) expression. Typical expression and distribution pattern of pNR1 (Ser896) immunostaining in representative spinal cord sections for each experimental and control group is shown in the upper panel (A). The formalin-induced increase in the number of pNR1-immunoreactive (IR) neurons was mainly observed in the intermediate dorsal horn rather than in the superficial layer (spinal cord section ‘b' compared to control section ‘a'). The i.t. injection of BD-1047 and BMY-14802 significantly suppressed formalin-induced pNR1 expression particularly in laminae III and IV. However, i.t. BMY-14802, but not BD-1047, suppressed the small increases in pNR1 (Ser896) expression in laminae I–II (B). Interestingly, the inhibitory effect of BMY-14802 on formalin-induced pNR1 (Ser896) expression was not changed by i.t. propranolol (PRO) administration. In panel (A), a: control, b: i.t. Sal+F, c: i.t. BD+F, d: i.t. BMY+F, e: i.t. PRO+i.t. BMY+F. Arrows: pNR1 (Ser896)-IR neurons. Scale bar=200 μm. *P<0.05 and ***P<0.001 as compared to the i.t. Sal+F group. The number of mice was 5 in each group.
I.t. administration of BD-1047 significantly reduced formalin-induced pNR1 (Ser896) expression in laminae III–IV. In contrast, formalin-injected mice that received an i.t. injection of the σ1 receptor antagonist, BMY-14802, showed a significant reduction in the number of pNR1 (Ser896)-positive neurons in both laminae I–II (0.9±0.5) and in laminae III–VI (21.3±2.9). This inhibitory effect of BMY-14802 on formalin-induced pNR1 (Ser896) immunostaining was not significantly changed by i.t. propranolol administration (# of pNR1 neurons=1.5±0.6 in laminae I–II and 25.1±2.3 in laminae III–VI), suggesting that the 5-HT1A receptor was not involved in the inhibitory effect of BMY-14802 on formalin-induced pNR1 (Ser896) immunostaining.
The inhibitory effect of i.t. BD-1047 and BMY-14802 on formalin-induced pNR1 (Ser897) expression in the dorsal horn
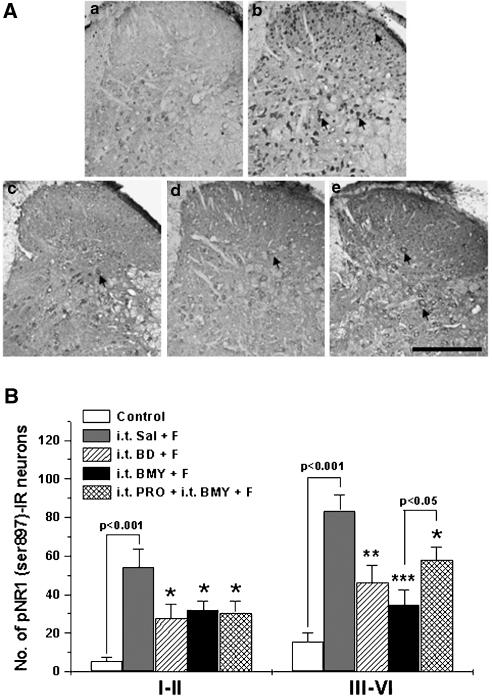
At 30 min after the formalin injection, the number of pNR1 (Ser897) IR neurons was significantly increased in the spinal dorsal horn (54.3±9.3 in laminae I–II and 83.6±7.9 in laminae III–VI) as compared with that of control mice (5.5±1.72 in laminae I–II and 15.5±4.8 in laminae III–VI) (see Figure 5). The typical distribution pattern of pNR1 (Ser897) in each treatment group is illustrated in panel A of Figure 5.
Figure 5.
The suppressive effect of i.t. injected σ1 receptor antagonists, BD-1047 (BD) and BMY-14802 (BMY), on formalin (F)-induced spinal pNR1 (Ser897) expression. Typical expression and distribution pattern of pNR1 (Ser897) immunostaining in representative spinal cord sections for each experimental and control group (panel A). Formalin injection remarkably increased the number of pNR1 (Ser897)-immunoreactive (IR) neurons in both the superficial layer and intermediate dorsal horn. The i.t. administration of BD-1047 and BMY-14802 significantly suppressed formalin-induced pNR1 (Ser897) expression in the spinal cord dorsal horn (laminae I–VI) (B). I.t. propranolol partially reversed but did not abolish the inhibitory effect of BMY-14802 on formalin-induced pNR1 (Ser897) expression in laminae III–VI. In panel (A), a: control, b: i.t. Sal+F, c: i.t. BD+F, d: i.t. BMY+F, e: i.t. PRO+i.t. BMY+F. Arrows: pNR1 (Ser897)-IR neurons. Scale bar=200 μm. *P<0.05, **P<0.01 and ***P<0.001 as compared with those of i.t. Sal+F group. The number of mice was five in each group.
The i.t. administration of BD-1047 significantly reduced formalin-induced pNR1 (Ser897) expression in laminae I–II (27.6±7.2) and in laminae III–VI (46.4±8.9) of the spinal cord dorsal horn. Similarly, i.t. injection of BMY-14802 significantly reduced formalin-induced pNR1 (Ser897) expression in the dorsal horn (32±4.5 in laminae I–II and 34.6±7.8 in laminae III–VI). I.t. injection of propranolol did not significantly change the inhibitory effect of BMY-14802 on pNR1 (Ser897) immunostaining in the superficial dorsal horn (laminae I–II), However, propranolol partially reversed, but did not completely abolish the inhibitory effect of i.t. BMY-14802 on the formalin-induced pNR1 (Ser897) expression in dorsal horn laminae III–VI, suggesting that the 5-HT1A receptor might not play a major role in the inhibitory effect of BMY-14802 on formalin-induced phosphorylation of the NMDA NR1 subunit.
The effect of BD-1047 and BMY-14802 on normal motor function in the rotarod test
The mean rotarod performance time of animals given an intrathecal injection of BD-1047 or BMY-14802 was not statistically different from the vehicle-treated control group at any of the time points (0, 30 and 60 min) tested (Table 1). Only mice receiving sodium pentobarbital (40 mg kg−1, i.p.) as a positive control showed a significant decrease in rotarod performance.
Table 1.
The i.t. injection of BD-1047 or BMY-14802 did not affect motor coordination during the 60 min post-treatment period in the mouse rotarod test
|
Group |
Time of permanence (s) |
|||
|---|---|---|---|---|
| |
Before treatment |
After treatment (min) |
||
| 0 | 30 | 60 | ||
| Control |
118±1.8 |
116±2.4 |
114±4.7 |
120±0 |
| BD-1047 (100 nmol, i.t.) |
118±2.6 |
116±2.5 |
114±5.7 |
120±0 |
| BMY-14802 (100 nmol, i.t.) |
114±3.7 |
112±8.1 |
118±2.2 |
120±0.3 |
| Pentobarbital sodium (40 mg kg−1, i.p.) |
120±0 |
47.3±13*** |
0.9±0.5*** |
107±6.0 |
Control mice received i.t. saline only.
P<0.001 as compared with that of the control group. The number of mice was seven in each group. (i.t.: intrathecal, i.p.: intraperitoneal).
Discussion
The results of this study demonstrate that i.t. pretreatment with the σ1 receptor antagonist BD-1047 significantly reduces formalin-induced paw licking/biting behavior in the second phase of the formalin test. In addition, i.t. injection of BD-1047 was shown to reduce formalin-induced spinal Fos expression both in superficial layers (laminae I–II) and in deeper layers (laminae III–VI) of the dorsal horn. In this regard, it is important to point out that spinal cord Fos expression induced by formalin injection represents neuronal activity mainly associated with the second phase of the formalin test (Miyata et al., 2003). On the other hand, administration of the σ1 receptor antagonist BMY-14802 strongly blocked formalin-induced pain behavior in both phases of the formalin test. However, while BMY 14802 has been shown to exhibit its most potent binding at the σ1 binding site, it shows some degree of serotonin subtype 1A binding but negligible dopamine receptor binding (Gewirtz et al., 1994). In this regard, we have found that the antinociceptive effect of BMY-14802 on formalin-induced pain behavior associated with the first phase of the formalin test is due predominantly to activation of 5-HT1A receptors, since i.t. pretreatment with propranolol (a 5-HT1A receptor antagonist) significantly reversed the suppressive effect of BMY-14802 on the paw licking/biting behavior associated with the first phase. In the second phase of the formalin test, propranolol partially reduced the antinociceptive effect of BMY-14802, however there was no statistical significance between these groups. With respect to the effect of BMY-14802 on Fos expression, propranolol was found to partially inhibit the suppressive effect of BMY-14802 on formalin-induced spinal Fos expression. Since formalin-induced Fos expression is associated with both the first and second phases of the formalin test (Abbadie et al., 1997), one possibility is that BMY-14802 acts via 5-HT1A to reduce Fos expression associated with the first phase of the formalin test. Alternatively, the reduction in Fos expression might be partially due to the action of BMY-14802 on 5-HT1A receptors associated with the second phase of the formalin test. While our data do not support such an effect, it is possible that higher doses of propranolol might have shown that effect of BMY-14802 on 5-HT1A receptors during the second phase of the formalin test may be involved. With this caveat in mind, our results suggest that spinal σ1 receptors play a pivotal role in formalin-induced nociception associated with the second, but not the first, phase of the formalin test. These results are interesting in light of the fact that both σ1 receptor knockout mice and mice receiving systemic administration of haloperidol to block σ1 receptors have been reported to exhibit significant antinociception in both phases of the formalin test (Cendan et al., 2005a, 2005b). In contrast, we demonstrate in the present study that inhibition of spinal σ1 receptors only produces antinociception in the second phase. This discrepancy may be due to the possibility that the antinociception associated with the first phase of the formalin test induced by σ1 receptor antagonism is mediated by the inhibition of σ1 receptors located in supra-spinal brain regions or in the periphery. Thus, spinal σ1 receptor activation may be more important for the pain sensation associated with the second phase of the formalin test rather than the first phase.
NMDA receptors are composed of three related families of subunits: NR1, NR2 and NR3 (Ozawa et al., 1998). All functional NMDA receptors include at least one NR1 subunit and NR1 is required for receptor activity (Hollmann & Heinemann, 1994; Mori & Mishina, 1995). NMDA receptors are phosphorylated and dephosphorylated by a variety of kinases (Yamakura & Shimoji, 1999) that regulated NMDAR function (Liao et al., 2001). The NR1 subunit undergoes a protein kinase C (PKC)-mediated phosphorylation at Ser896, as well as a cyclic AMP-dependent protein kinase (PKA)-mediated phosphorylation at Ser897 (Leonard & Hell, 1997). Both of these PKC- and PKA-mediated NMDA phosphorylation pathways are important for nociceptive processing in the spinal cord (Fukuda et al., 2002; Yajima et al., 2003). Recently, it has been shown that pNR1 contributes to central sensitization after intradermal capsaicin injection (Zou et al., 2002) and to spinal nerve ligation-induced neuropathic pain (Gao et al., 2005). Furthermore, Caudle et al. (2005) have demonstrated that lumbar spinal cord NR1 subunits are phosphorylated on serine residues within 2 h of the induction of hind paw inflammation with carrageenan. Thus, a variety of reports have begun to surface suggesting that phosphorylation of the NMDA NR1 subunit is associated with both acute and persistent pain and perhaps it is not surprising that this process is also associated with formalin-induced pain behaviors.
Spinal cord NMDA receptors are intimately involved in the development and maintenance of central sensitization and the generation of pain hypersensitivity at the level of the spinal cord. With respect to the formalin test used in the present study, NMDA receptors are known to be very important for maintaining the spinal sensitization associated with the second phase of the formalin test (South et al., 2003). In this regard, it has been reported that i.t. administration of an antisense oligodeoxynucleotide against NR1 produces an antinociception in the second phase of the formalin test, suggesting that the NR1 subunit plays an important role in formalin-induced nociception associated with the second phase rather than the first phase of the formalin test (Rydh-Rinder et al., 2001). Since NR1 is known to play an important role in nociceptive behaviors associated with the second phase of the formalin test and because we have demonstrated that i.t. administration of σ1 receptor antagonists reduce this behavior, we examined whether i.t. administration of σ1 receptor antagonists also reduce formalin-induced pNR1 (Ser896 and Ser897) expression in the spinal dorsal horn. We found that i.t. injection of either BD-1047 or BMY-14802 significantly suppressed formalin-induced pNR1 (Ser896 and Ser897) expression. The suppressive effect of BMY-14802 on formalin-induced pNR1 (Ser897) expression in laminae III–VI was partially reversed by i.t. pretreatment with propranolol. This suggests that a portion of the inhibitory effect of BMY-14802 is due to activation of spinal 5-HT1A receptors. This is supported by a previous report showing that neurons expressing 5-HT1A receptor mRNA are located predominantly in the deep dorsal horn (laminae III–VI; Zhang et al., 2002) and with data showing that administration of 5-HT1A receptor agonists reduce both phases of the formalin test (Jeong et al., 2004). Therefore, it seems likely that the 5-HT1A receptor agonistic effect of BMY-14802 partially contributed to the reduction of formalin-induced pNR1 (Ser897) expression. However, even in the presence of propranolol to block 5-HT1A receptors, BMY-14802 still produced a strong reductive effect on the formalin-induced pNR1 (Ser897) expression in laminae III–VI. BD-1047 is a very selective σ1 antagonist and i.t. administration produced very similar results to those obtained with BMY-14802. Therefore, we believe that the reduction of formalin-induced pNR1 (Ser896 and Ser897) expression by the two σ1 receptor antagonists used in this study occurs primarily via the inhibition of spinal σ1 receptor activity. The results of the present study strongly suggest that activation of spinal σ1 receptors contributes to spinal sensitization associated with the second phase of the formalin test via phosphorylation of NMDA receptor activity. Thus, we have shown that antagonism of spinal σ1 receptors suppresses phosphorylation of the NR1 subunit of spinal NMDA receptors and suppresses formalin-induced nociceptive behaviors.
In conclusion, the present study shows that i.t. administration of σ1 receptor antagonists reduces formalin-induced nociception associated with the second phase of the formalin test. In addition, these antagonists reduced both formalin-induced spinal Fos and pNR1 (Ser896 and Ser897) expression without affecting normal motor function. The results of this study suggest that the spinal σ1 receptor plays an important role in spinal-mediated nociception associated with the second phase of the formalin test.
Acknowledgments
This work was supported by grant no. (R01-2005-000-10580-0) from the Basic Research Program of the Korea Science & Engineering Foundation. In addition, this research was also supported by a grant (M103KV010009 04K2201 00940) from Brain Research Center of the 21st Century Frontier Research Program funded by the Ministry of Science and Technology, the Republic of Korea.
Abbreviations
- BD-1047
N-[2-(3,4-dichlorophenyl)ethyl]-N-methyl-2-(dimethylamino)ethylamine dihydrobromide
- BMY-14802
(α-(4-fluorophenyl)-4-(5-fluoro-2-pyrimidinyl)-1-piperazinebutanol hydrochloride
- NMDA
N-methyl-D-aspartate
- pNR1
phosphorylation of NMDA receptor subunit 1
- propranolol
(S)-1-isopropylamino-3-(1-naphthyloxy)-2-propanol hydrochloride
- Ser896
serine residue 896
- Ser897
serine residue 897
- σ1
sigma 1
References
- ABBADIE C., BESSON J.M. Chronic treatments with aspirin or acetaminophen reduce both the development of polyarthritis and Fos-like immunoreactivity in rat lumbar spinal cord. Pain. 1994;57:45–54. doi: 10.1016/0304-3959(94)90106-6. [DOI] [PubMed] [Google Scholar]
- ABBADIE C., TAYLOR B.K., PETERSON M.A., BASBAUM A.I. Differential contribution of the two phases of the formalin test to the pattern of c-fos expression in the rat spinal cord: studies with remifentanil and lidocaine. Pain. 1997;69:101–110. doi: 10.1016/s0304-3959(96)03285-x. [DOI] [PubMed] [Google Scholar]
- ALONSO G., PHAN V., GUILLEMAIN I., SAUNIER M., LEGRAND A., ANOAL M., MAURICE T. Immunocytochemical localization of the sigma1 (σ1) receptor in the adult rat central nervous system. Neuroscience. 2000;97:155–170. doi: 10.1016/s0306-4522(00)00014-2. [DOI] [PubMed] [Google Scholar]
- BERMACK J., LAVOIE N., DRYVER E., DEBONNEL G. Effects of sigma ligands on NMDA receptor function in the bulbectomy model of depression: a behavioural study in the rat. Int. J. Neuropsychopharmacol. 2002;5:53–62. doi: 10.1017/S1461145701002760. [DOI] [PubMed] [Google Scholar]
- BRENNER G.J., JI R.R., SHAFFER S., WOOLF C.J. Peripheral noxious stimulation induces phosphorylation of the NMDA receptor NR1 subunit at the PKC-dependent site, serine-896, in spinal cord dorsal horn neurons. Eur. J. Neurosci. 2004;20:375–384. doi: 10.1111/j.1460-9568.2004.03506.x. [DOI] [PubMed] [Google Scholar]
- BURITOVA J., LARRUE S., ALIAGA M., BESSON J.M., COLPAERT F. Effects of the high-efficacy 5-HT1A receptor agonist, F 13640 in the formalin pain model: a c-Fos study. Eur. J. Pharmacol. 2005;514:121–130. doi: 10.1016/j.ejphar.2005.03.016. [DOI] [PubMed] [Google Scholar]
- CAUDLE R.M., PEREZ F.M., DEL VALLE-PINERO A.Y., IADAROLA M.J. Spinal cord NR1 serine phosphorylation and NR2B subunit suppression following peripheral inflammation. Mol. Pain. 2005;1:25. doi: 10.1186/1744-8069-1-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CENDAN C.M., PUJALTE J.M., PORTILLO-SALIDO E., BAEYENS J.M. Antinociceptive effects of haloperidol and its metabolites in the formalin test in mice. Psychopharmacology (Berlin) 2005a;182:485–493. doi: 10.1007/s00213-005-0127-z. [DOI] [PubMed] [Google Scholar]
- CENDAN C.M., PUJALTE J.M., PORTILLO-SALIDO E., MONTOLIU L., BAEYENS J.M. Formalin-induced pain is reduced in sigma (1) receptor knockout mice. Eur. J. Pharmacol. 2005b;511:73–74. doi: 10.1016/j.ejphar.2005.01.036. [DOI] [PubMed] [Google Scholar]
- CHAPLAN S.R., MALMBERG A.B., YAKSH T.L. Efficacy of spinal NMDA receptor antagonism in formalin hyperalgesia and nerve injury evoked allodynia in the rat. J. Pharmacol. Exp. Ther. 1997;280:829–838. [PubMed] [Google Scholar]
- CODERRE T.J., MELZACK R. The role of NMDA receptor-operated calcium channels in persistent nociception after formalin-induced tissue injury. J. Neurosci. 1992;12:3671–3675. doi: 10.1523/JNEUROSCI.12-09-03671.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ERNBERG M., LUNDEBERG T., KOPP S. Effect of propranolol and granisetron on experimentally induced pain and allodynia/hyperalgesia by intramuscular injection of serotonin into the human masseter muscle. Pain. 2000;84:339–346. doi: 10.1016/s0304-3959(99)00221-3. [DOI] [PubMed] [Google Scholar]
- FUKUDA T., NISHIMOTO C., HISANO S., MIYABE M., TOYOOKA H. The analgesic effect of xenon on the formalin test in rats: a comparison with nitrous oxide. Anesth. Analg. 2002;95:1300–1304. doi: 10.1097/00000539-200211000-00037. [DOI] [PubMed] [Google Scholar]
- FUKUDA T., WATANABE K., HISANO S., TOYOOKA H. Licking and C-fos expression in the dorsal horn of the spinal cord after the formalin test. Anesth. Analg. 2006;102:811–814. doi: 10.1213/01.ane.0000197690.19075.bd. [DOI] [PubMed] [Google Scholar]
- GAO X., KIM H.K., CHUNG J.M., CHUNG K. Enhancement of NMDA receptor phosphorylation of the spinal dorsal horn and nucleus gracilis neurons in neuropathic rats. Pain. 2005;116:62–72. doi: 10.1016/j.pain.2005.03.045. [DOI] [PubMed] [Google Scholar]
- GEWIRTZ G.R., GORMAN J.M., VOLAVKA J., MACALUSO J., GRIBKOFF G., TAYLOR D.P., BORISON R. BMY 14802, a sigma receptor ligand for the treatment of schizophrenia. Neuropsychopharmacology. 1994;10:37–40. doi: 10.1038/npp.1994.5. [DOI] [PubMed] [Google Scholar]
- GUITART X., CODONY X., MONROY X. Sigma receptors: biology and therapeutic potential. Psychopharmacology (Berlin) 2004;74:301–319. doi: 10.1007/s00213-004-1920-9. [DOI] [PubMed] [Google Scholar]
- HOLLMANN M., HEINEMANN S. Cloned glutamate receptors. Annu. Rev. Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- HYLDEN J.L., WILCOX G.L. Intrathecal morphine in mice: a new technique. Eur. J. Pharmacol. 1980;67:313–316. doi: 10.1016/0014-2999(80)90515-4. [DOI] [PubMed] [Google Scholar]
- JEONG C.Y., CHOI J.I., YOON M.H. Roles of serotonin receptor subtypes for the antinociception of 5-HT in the spinal cord of rats. Eur. J. Pharmacol. 2004;502:205–211. doi: 10.1016/j.ejphar.2004.08.048. [DOI] [PubMed] [Google Scholar]
- KIM H.W., KWON Y.B., HAN H.J., YANG I.S., BEITZ A.J., LEE J.H. Antinociceptive mechanisms associated with diluted bee venom acupuncture (apipuncture) in the rat formalin test: involvement of descending adrenergic and serotonergic pathways. Pharmacol. Res. 2005;51:183–188. doi: 10.1016/j.phrs.2004.07.011. [DOI] [PubMed] [Google Scholar]
- KWON Y.B., LEE J.D., LEE H.J., HAN H.J., MAR W.C., KANG S.K., BEITZ A.J., LEE J.H. Bee venom injection into an acupuncture point reduces arthritis associated edema and nociceptive responses. Pain. 2001;90:271–280. doi: 10.1016/S0304-3959(00)00412-7. [DOI] [PubMed] [Google Scholar]
- LEE J.H., KIM H.W., KWON Y.B., KANG M.S., CHOI D.W., NA J.H., KWON O.K., YOUN H.J., HAN H.J., BYUN T.H., PARK S.Y., CHUN B.H., PYUN J.H., AN G.H., LEE Y.J., CHO M.H. General pharmacology studies on beta-domain deleted recombinant factor VIII. Arzneimittelforschung. 2000;50:86–92. doi: 10.1055/s-0031-1300170. [DOI] [PubMed] [Google Scholar]
- LEONARD A.S., HELL J.W. Cyclic AMP-dependent protein kinase and protein kinase C phosphorylate N-methyl-D-aspartate receptors at different sites. J. Biol. Chem. 1997;272:12107–12115. doi: 10.1074/jbc.272.18.12107. [DOI] [PubMed] [Google Scholar]
- LIAO G.Y., WAGNER D.A., HSU M.H., LEONARD J.P. Evidence for direct protein kinase-C mediated modulation of N-methyl-D-aspartate receptor current. Mol. Pharmacol. 2001;59:960–964. doi: 10.1124/mol.59.5.960. [DOI] [PubMed] [Google Scholar]
- LLANSOLA M., SANCHEZ-PEREZ A., CAULI O., FELIPO V. Modulation of NMDA receptors in the cerebellum. 1. Properties of the NMDA receptor that modulate its function. Cerebellum. 2005;4:154–161. doi: 10.1080/14734220510007996. [DOI] [PubMed] [Google Scholar]
- MASU M., NAKAJIMA Y., MORIYOSHI K., ISHII T., AKAZAWA C., NAKANASHI S. Molecular characterization of NMDA and metabotropic glutamate receptors. Ann. NY Acad. Sci. 1993;707:153–164. doi: 10.1111/j.1749-6632.1993.tb38050.x. [DOI] [PubMed] [Google Scholar]
- MATOS F.F., KORPINEN C., YOCCA F.D. 5-HT1A receptor agonist effects of BMY-14802 on serotonin release in dorsal raphe and hippocampus. Eur. J. Pharmacol. 1996;317:49–54. doi: 10.1016/s0014-2999(96)00699-1. [DOI] [PubMed] [Google Scholar]
- MATSUMOTO R.R., BOWEN W.D., TOM M.A., VO V.N., TRUONG D.D., DE COSTA B.R. Characterization of two novel sigma receptor ligands: antidystonic effects in rats suggest sigma receptor antagonism. Eur. J. Pharmacol. 1995;280:301–310. doi: 10.1016/0014-2999(95)00208-3. [DOI] [PubMed] [Google Scholar]
- MCCRACKEN K.A., BOWEN W.D., DE COSTA B.R., MATSUMOTO R.R. Two novel sigma receptor ligands, BD1047 and LR172, attenuate cocaine-induced toxicity and locomotor activity. Eur. J. Pharmacol. 1999;370:225–232. doi: 10.1016/s0014-2999(99)00113-2. [DOI] [PubMed] [Google Scholar]
- MIYATA M., KASHIWADANI H., FUKAIYA M., HAYASHI T., WU D., SUZUKI T., WATANABE M., KAWAKAMI Y. Role of thalamic phospholipase C[beta]4 mediated by metabotropic glutamate receptor type 1 in inflammatory pain. J. Neurosci. 2003;23:8098–8108. doi: 10.1523/JNEUROSCI.23-22-08098.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORI H., MISHINA M. Structure and function of the NMDA receptor channel. Neuropharmacology. 1995;34:1219–1237. doi: 10.1016/0028-3908(95)00109-j. [DOI] [PubMed] [Google Scholar]
- NUWAYHID S.J., WERLING L.L. Sigma1 receptor agonist-mediated regulation of N-methyl-D-aspartate-stimulated [3H]dopamine release is dependent upon protein kinase C. J. Pharmacol. Exp. Ther. 2003;304:364–369. doi: 10.1124/jpet.102.043398. [DOI] [PubMed] [Google Scholar]
- OZAWA S., KAMIYA H., TSUZUKI K. Glutamate receptors in the mammalian central nervous system. Prog. Neurobiol. 1998;54:581–618. doi: 10.1016/s0301-0082(97)00085-3. [DOI] [PubMed] [Google Scholar]
- RYDH-RINDER M., BERGE O.G., HOKFELT T. Antinociceptive effects after intrathecal administration of phosphodiester-, 2′-O-allyl-, and C-5-propyne-modified antisense oligodeoxynucleotides targeting the NMDAR1 subunit in mouse. Brain Res. Mol. Brain Res. 2001;86:23–33. doi: 10.1016/s0169-328x(00)00248-5. [DOI] [PubMed] [Google Scholar]
- SOUTH S.M., KOHNO T., KASPAR B.K., HEGARTY D., VISSEL B., DRAKE C.T., OHATA M., JENAB S., SAILER A.W., MALKMUS S., MASUYAMA T., HORNER P., BOGULAVSKY J., GAGE F.H., YAKSH T.L., WOOLF C.J., HEINEMANN S.F., INTURRISI C.E. A conditional deletion of the NR1 subunit of the NMDA receptor in adult spinal cord dorsal horn reduces NMDA currents and injury-induced pain. J. Neurosci. 2003;23:5031–5040. doi: 10.1523/JNEUROSCI.23-12-05031.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SU T.P. Evidence for sigma opioid receptor: binding of H SKF-10047 to etorphine-inaccessible sites in guinea-pig brain. J. Pharmacol. Exp. Ther. 1982;223:284–290. [PubMed] [Google Scholar]
- TAM S.W., COOK L. Sigma opiates and certain antipsychotic drugs mutually inhibit q – H SKF10, 047 and H haloperidol binding in guinea pig brain membranes, Proc. Proc. Natl. Acad. Sci. U.S.A. 1984;81:5618–5621. doi: 10.1073/pnas.81.17.5618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UEDA H., INOUE M., YOSHIDA A., MIZUNO K., YAMAMOTO H., MARUO J., MATSUNO K., MITA S. Metabotropic neurosteroid/sigma-receptor involved in stimulation of nociceptor endings of mice. J. Pharmacol. Exp. Ther. 2001;298:703–710. [PubMed] [Google Scholar]
- WALKER J.M., BOWEN W.D., WALKER F.O., MATSUMOTO R.R., DE COSTA B., RICE K.C. Sigma receptors: biology and function. Pharmacol. Rev. 1990;42:355–402. [PubMed] [Google Scholar]
- WANG H., TAKIGAWA M. The selective sigma ligand MS-377 attenuates the blockade by phencyclidine of NMDA-induced intracellular calcium. Int. J. Neuropsychopharmacol. 2002;5:239–242. doi: 10.1017/S1461145702002985. [DOI] [PubMed] [Google Scholar]
- WANG L., PRESCOTT A.R., SPRUCE B.A., SANDERSON J., DUNCAN G. Sigma receptor antagonists inhibit human lens cell growth and induce pigmentation. Invest. Ophthalmol. Vis. Sci. 2005;46:1403–1408. doi: 10.1167/iovs.04-1209. [DOI] [PubMed] [Google Scholar]
- YAJIMA Y., NARITA M., SHIMAMURA M., NARITA M., KUBOTA C., SUZUKI T. Differential involvement of spinal protein kinase C and protein kinase A in neuropathic and inflammatory pain in mice. Brain Res. 2003;992:288–293. doi: 10.1016/j.brainres.2003.08.042. [DOI] [PubMed] [Google Scholar]
- YAMAKURA T., SHIMOJI K. Subunit- and site-specific pharmacology of the NMDA receptor channel. Prog. Neurobiol. 1999;59:279–298. doi: 10.1016/s0301-0082(99)00007-6. [DOI] [PubMed] [Google Scholar]
- ZHANG Y.Q., GAO X., JI G.C., HUANG Y.L., WU G.C., ZHAO Z.Q. Expression of 5-HT1A receptor mRNA in rat lumbar spinal dorsal horn neurons after peripheral inflammation. Pain. 2002;98:287–295. doi: 10.1016/S0304-3959(02)00026-X. [DOI] [PubMed] [Google Scholar]
- ZIMMERMANN M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- ZOU X., LIN Q., WILLIS W.D. Role of protein kinase A in phosphorylation of NMDA receptor 1 subunits in dorsal horn and spinothalamic tract neurons after intradermal injection of capsaicin in rats. Neuroscience. 2002;115:775–786. doi: 10.1016/s0306-4522(02)00490-6. [DOI] [PubMed] [Google Scholar]