Abstract
Nitrate tolerance is associated with an enhanced superoxide anion (O2−) production and may be attenuated by statins as they interact with the two main endothelial NO synthase (eNOS) and NAD(P)H oxidase pathways involved in this oxidative stress.
Groups of wild-type (wt, C57Bl/6J) and eNOS knock-out mice (eNOS−/−) received rosuvastatin (20 mg kg−1 day−1 p.o.) for 5 weeks and a cotreatment with the statin plus nitroglycerin (NTG; 30 mg kg−1 day−1, subcutaneous injections b.i.d.) for the last 4 days. Another group received only NTG (30 mg kg−1 d−1, b.i.d. for 4 days) and finally control mice from both strains received no treatment.
Rings of thoracic aortas from these groups were studied in organ baths. Relaxations to NTG (0.1 nM–0.1 mM) were determined on thromboxane analogue (U44619)-precontracted rings and O2− production (RLU 5 s−1 mg−1 of total protein content) was assessed in aorta homogenates with the lucigenin-enhanced chemiluminescence technique. Reverse transcriptase–polymerase chain reaction analysis was performed on aortas from both mice strains.
In vivo NTG treatment induced a significant rightward shift of the concentration–effect curve to NTG compared to control group. There was, however, no cross-tolerance with non-nitrate sources of NO (unaltered response to acetylcholine in wt group). The rosuvastatin+NTG cotreatment was able to protect against the development of nitrate tolerance in both mice strains and L-mevalonate abolished this protective effect of rosuvastatin.
In vivo treatment with apocynin, a purported NAD(P)H oxidase inhibitor, also produced a similar protection to that observed with rosuvastatin in both strains.
Superoxide anion formation was increased after NTG treatment in both mice strains and the rosuvastatin+NTG cotreatment was able to reduce that production.
Moreover, rosuvastatin treatment abolished the increase in gp91phox mRNA (an endothelial membrane NAD(P)H oxidase subunit) expression induced by in vivo exposure to NTG.
These findings suggest that long-term rosuvastatin treatment protects against nitrate tolerance by counteracting NTG-induced increase in O2− production, probably via a direct interaction with the NAD(P)H oxidase pathway.
Keywords: Nitrate tolerance, rosuvastatin, eNOS knockout mice, NAD(P)H oxidase, oxidative stress
Introduction
Long-term treatment with HMG-CoA reductase inhibitors (statins) appears to upregulate the expression and the activity of the vascular endothelial NO synthase (eNOS) pathway and increases nitric oxide availability, resulting in not only a downregulation of oxidative enzymes but also a direct scavenging of superoxide anion. As oxygen radical production is increased in various clinical settings such as hypercholesterolaemia, diabetes and hypertension (Kojda & Harrison, 1999), this statin-induced eNOS upregulation may play a foremost role in the vascular protective effects of these drugs. Moreover, sustained nitroglycerin (NTG) treatment is associated with an increased bioavailability of superoxide anion (Munzel et al., 1996), likely playing a major role in the development of nitrate tolerance. The triggering events leading to this redox imbalance remain controversial as several cellular enzyme systems have been shown to be impaired by sustained in vivo exposure to NTG, including membrane bound oxidases (Munzel et al., 1996), endothelial NOS (Munzel et al., 2000; Gori et al., 2001) and arginine transporters (Ogonowski et al., 2000).
With regard to the statin-induced protection against oxidative stress, we recently found that rats treated with rosuvastatin are protected against the vascular tolerance to NTG (Otto et al., 2005). However, this protection does not seem to be directly related to the eNOS pathway. Indeed, the rosuvastatin treatment decreased the expression of the NAD(P)H oxidase essential subunit, p22phox, without alteration in eNOS abundance. However, we have been unable to formally exclude an eNOS-dependent effect as the statin-induced protection was abolished by administration of the eNOS inhibitor, N-nitro-L-arginine methyl ester (L-NAME). As pointed out by Landmesser et al. (2003), important interactions exist between eNOS and NAD(P)H oxidase pathways whereby increased production of reactive oxygen species by the latter impedes the NO production and stimulates superoxide anion formation by the former enzyme.
Therefore, the role of the eNOS pathway in rosuvastatin-induced protection against nitrate tolerance was examined by assessing whether or not this vascular protection persists in eNOS knockout (eNOS−/−) mice. By comparing the mechanisms in both mouse strains, namely wild-type (wt) (eNOS+/+) and eNOS−/−, this study demonstrates a direct interaction between rosuvastatin and the vascular NAD(P)H oxidase pathway, and the contribution of NAD(P)H oxidase to nitrate tolerance.
Methods
Experimental model
Male and female eNOS-deficient mice (eNOS−/−) and wt mice (eNOS+/+, C57Bl/6J) were purchased from Jackson Laboratories (Bar Harbor, ME, U.S.A.) and were bred in the animal house of the Institute of Pharmacy (Université Libre de Bruxelles). Mice were maintained in a temperature (21°C)- and relative humidity (60%)-controlled room with a 12-h light and dark cycle and were allowed food and tap water ad libitum. All procedures were performed according to protocols approved by the ethical committee of the Faculty of Medicine and Pharmacy from the ‘Université Libre de Bruxelles'.
Drug treatments
Male mice from both strains, aged between 4 and 6 months and weighing 20–30 g, were divided into four groups: group 1 received rosuvastatin (20 mg kg−1 day−1) added to drinking water for 5 weeks and for the last 4 days they received a statin+nitroglycerin (NTG; 30 mg kg−1 day−1) cotreatment by subcutaneous injections (b.i.d.). Group 2 received rosuvastatin alone for 5 weeks; group 3 received NTG alone for 4 days and group 4 served as control (no treatment). The rosuvastatin and NTG doses were chosen according to previous studies in rats (Wang et al., 2002; Fontaine et al., 2003; Nangle et al., 2003; Rader et al., 2003; Otto et al., 2005) and preliminary experiments in mice. The NTG doses were slightly smaller than those used in our previous experiments in the rats (Otto et al., 2005) since higher doses were not tolerated in mice.
In some experiments, mice from both strains were treated for 6 days with apocynin (5 mg kg−1 day−1) (Ben-Shaul et al., 2001; Van den Worm et al., 2001; Sonta et al., 2004) added in the drinking water and for the last 4 days they received a cotreatment with apocynin+NTG (30 mg kg−1 day−1 by subcutaneous injection, b.i.d.).
Systolic blood pressure measurements
Blood pressure was measured noninvasively using a tail-cuff method (Apollo 179, IITC Life Science Instruments, Woodland Hills, CA, U.S.A.) on conscious, restrained mice when the animals were between 4 and 6 months of age. The mice have been accommodated to the restrainers during 1 h for 4 consecutive days without taking any blood pressure measurements. On day ‘five', the first blood pressure measurements were performed and all blood pressures were calculated as the average of 5–10 measurements per day for 2 consecutive days (so day 5 and day 6).
Plasma lipid analysis
Total cholesterol and triglycerides were measured colorimetrically in plasma collected at the time of euthanasia of mice from both strains. Samples were stored at −20°C prior to analysis and commercially available kits (Roche) were used.
Vascular relaxation experiments
Mice (between 4 and 6 months of age) were euthanased with CO2 and the thoracic aortas were dissected, cleaned of adherent tissue, and cut into segments. The aortic rings were mounted in microvessel organ chambers (EMKA, Paris), filled with Krebs–Henseleit solution (in mM: NaCl, 118.4; KCl, 4.7; MgSO4, 1.2; KH2PO4, 1.2; CaCl2, 2.5; NaHCO3, 25; glucose, 5), bubbled with 95% O2 and 5% CO2 and maintained at 37°C. The rings were connected to a force transducer to measure isometric tensions, and recorded on a transducer data acquisition system (EMKA, Paris). Resting tension was increased stepwise to reach a final tension of 0.80 g and rings were allowed to equilibrate for 60 min. Krebs–Henseleit solution was changed, before and twice after each concentration–response curve. Cumulative concentration–response curves for acetylcholine (ACh) (10−9–10−4 M) and NTG (10−10–10−4 M) were generated after precontraction of vessels with 9,11-dideoxy-11α,9α-epoxymethanoprostaglandin F2α (U-46619). The used concentration of 0.1 μmol l−1 gave similar plateaus in both mice strains (in wt mice aortas (n=8): 0.76±0.02 versus 0.77±0.09 g in eNOS−/− mice aortas (n=8)), thereby allowing an accurate comparison of the vasodilator effect of a drug. In some experiments, L-mevalonate (100 μM) was incubated for 1 h in the bath after the concentration–response curve to ACh. After having washed out this HMG-CoA reductase metabolite, the concentration–response curves to NTG were constructed.
All experiments were performed in the absence of a cyclooxygenase inhibitor, according to previous studies (Levy, 1980) and in our preliminary experiments, indomethacin did not affect endothelium-dependent relaxations in the mouse aorta.
NAD(P)H oxidase activity measured by the lucigenin-enhanced chemiluminescence technique
The thoracic aortas from the different mice groups of both strains were isolated and cleaned of fat and loose connective tissue. The aortas were then homogenized on ice in a lysis buffer containing Tris–HCl (50 mM), EDTA (1 mM), dithiothreitol (DTT) (0.5 mM) and protease inhibitor cocktail (pepstatinA, E-64, bestatin, leupeptin, aprotinin, 4-(2aminoethyl)benzenesulfonyl fluoride). The samples were centrifuged; 20 μl of the supernatant were used for protein assay and 40 μl were added to the vials containing Dulbecco's modified Eagle's medium (DMEM, Cambrex, Belgium) with 5 μM of lucigenin at 37°C. The background signal with lucigenin alone was not modified upon addition of the homogenates. The reaction was initiated by addition of NADPH (100 μM) to the vials containing aortic homogenates. NADPH did not modify the basal signal in blank vials (without homogenates). The peak count was assessed in a Lumat LB 9507 Luminometer (EG&G Berthold) every 5 s for 45 min. At the end of the experiment, diphenylene iodonium (DPI), an NAD(P)H oxidase inhibitor (100 μM), or L-NAME, an eNOS inhibitor (10 μM), was added into the vials. A buffer blank was subtracted from each reading and the NAD(P)H oxidase activity was expressed as relative light units (RLU)/5 s mg−1 of total protein content.
RNA extractions and reverse transcriptase polymerase chain reaction (RT–PCR)
Aortas from the different mice groups of both strains were snap-frozen in liquid nitrogen and total RNA was extracted with the ‘SV total RNA isolation system' (Promega, Belgium). The extracted RNA was used for reverse transcription (Fermentas, Germany) in a total volume of 20 μl. The reverse transcription products were amplified by PCR for the four genes of interest (p22phox, gp91phox, rac-1 and eNOS) and the internal standard gene, GAPDH. The PCR conditions were as follows: initial denaturation step: 94°C, 5 min; then the PCR was followed during 27, 30, 33 and 36 cycles, respectively, for each gene, with the following steps: denaturation 94°C, 1 min; annealing 62°C, 1 min; elongation 72°C, 1 min.
The primers employed for p22phox amplification were: TGG CCT GAT TCT CAT CAC TGG sense and GGG ACA ACT CCA CAG AAA CTC antisense (product size 580 bp); for gp91phox amplification: GCT GGG ATC ACA GGA ATT GTC sense and GCT TAT CAC AGC CAC AAG CAT T antisense (product size: (597 bp); the primers for rac-1 amplification were: TGAAGT ATT CTG TTC CTA GTT GTG sense and TGC ATT CTT GCC AGT GAG TTA G antisense (product size: 740 bp) and for eNOS amplification the primers were: TGT GTG CAT GGA TCT GGA CAC CAG GAC AA sense and GAA TGG TTG CCT TCA CAC GCT CG CCA T antisense (product size: 427 bp). The same cDNA samples were used for GAPDH cDNA amplification to confirm that equal amounts of RNA were reverse transcribed (sense: AGG TCG GTG TGA ACG GAT TTG GCC GTA T and antisense: CCT TCT CCA TGG TGG TGA AGA CAC CAG TA; product size: 308 bp).
Equal amounts of RT–PCR products were loaded on 1.5% agarose gels and absorbance of ethidium bromide-stained DNA bands were quantified by AIDA Image Analyser. The intensity of expression of the genes of interest was normalized to the intensity of expression of their respective internal standard gene GAPDH. The mRNA expression of these normalized genes from the control mice of both strains was set as 100%.
Materials
Acetylcholine chloride (ACh), apocynin, 9,11-dideoxy-11α,9α-epoxymethano-prostaglandin F2α (U-46619), NTG, NADPH, mevalonic acid lactone, bovine superoxide dismutase (SOD), DTT, DPI and L-NAME were purchased from Sigma Chemical Company (Bornem, Belgium). NTG was purchased from Therabel-Pharmal, Brussels, Belgium and rosuvastatin was generously provided by AstraZeneca (Cheshire, England).
Statistical analysis
Results were expressed as means±s.e.m., n representing the number of different animals studied. Relaxations are expressed as the percentage inhibition of tension developed by U-46619. For each concentration–effect curve, the area under the curve (AUC) was calculated and expressed in arbitrary units; the concentration causing half-maximal relaxation expressed as negative log molar concentration (pD2) was also assessed. An unpaired Student's t-test was used for assessing differences between the two strains. Intergroup multiple comparisons within each strain were performed by a one-way ANOVA followed by Tukey's post hoc test in order to accurately assess the rosuvastatin effect (in each strain). For comparison of rings from the same animals (in vitro mevalonate incubation), a paired Student's t-test was used. Significance was accepted at P<0.05.
Results
Plasma lipids, weights and arterial tension
Total cholesterol and triglyceride levels and body weights were not significantly modified by the different treatments in both mice strains. There was no significant difference when comparing the wt mice groups with the eNOS−/− mice groups (Table 1).
Table 1.
Plasma lipids, weights and arterial tension in wt and eNOS−/− mice
| Control wt (n=8) | Rosuvastatin wt (n=8) | Control eNOS−/− (n=8) | Rosuvastatin eNOS−/− (n=8) | |
|---|---|---|---|---|
| Body weight (g) |
28.0±0.9 |
27.5±0.7 |
27.3±0.7 |
26.5±0.6 |
| Triglycerides (mg dl−1) |
130.0±10.1 |
131.7±11.3 |
126.7±15.2 |
109.7±13.3 |
| Cholesterol (mg dl−1) |
71.6±4.2 |
71.7±3.3 |
67.2±2.7 |
68.1±3.2 |
| Systolic blood pressure | 90±5 | 89±2 | 115±3* | 119±4* |
P<0.05 versus the corresponding wt group.
Systolic blood pressure was significantly elevated in eNOS−/− mice and this difference persisted after rosuvastatin treatment (Table 1). Similar findings of blood pressure measurements were obtained by Anning et al. (2005) and Arruda et al. (2005).
Organ bath study: ACh-induced relaxation
In contrast to aortas from control wt mice, no relaxations to ACh were observed in control eNOS−/− mice aortas.
In wt mice, the in vivo exposure to NTG slightly but not significantly altered the pD2 and AUC values (Table 2).
Table 2.
Concentration–effect curves to ACh after the different in vivo treatments in wt mice
| Pharmacological parameters | Control (n=7) | Rosuvastatin (n=8) | Rosuvastatin-NTG (n=8) | NTG (n=7) |
|---|---|---|---|---|
| Maximal response (%) |
68±3 |
70±3 |
75±2 |
63±4 |
| pD2 value |
6.83±0.12 |
6.85±0.14 |
6.70±0.06 |
6.44±0.15 |
| AUC | 707±31 | 679±31 | 654±20 | 774±30 |
Rosuvastatin treatment alone did not modify the concentration–response curve to ACh (nonsignificant (NS) versus control group) (Table 2).
Organ bath study: NTG-induced vasorelaxation
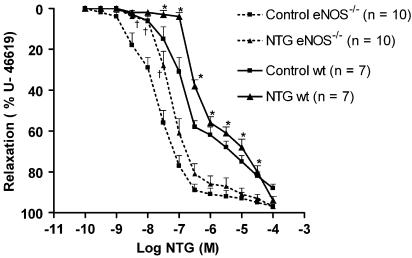
The concentration–effect curves to NTG were significantly shifted to the right after 4 days in vivo NTG treatment in wt and also in eNOS−/− mice aortas. In wt mice, the pD2 and the AUC values of the NTG group were significantly different from those of the control group: pD2: 6.25±0.06 and AUC: 891±17 (n=7) versus 6.71±0.05 and 773±17.7 (n=7) in the control group (P<0.05 for both parameters). In preparations from eNOS−/− mice, the pD2 and AUC values of the NTG group were: 7.17±0.09 and 665±32 (n=10) versus 7.72±0.12 and 558±30 (n=10) for the control group (P<0.05 for both parameters). The concentration–response curves to NTG in preparations from control and NTG groups in eNOS−/− mice were significantly shifted to the left compared to the corresponding groups of the wt mice (Figure 1). However, the development of nitrate tolerance occurred at a similar degree in both strains as the EC50 ratio of control and NTG groups of wt mice was not significantly different to the EC50 ratio of the corresponding eNOS−/− mice groups (EC50 NTG wt/EC50 control wt=4.05±1.89 (n=7) versus EC50 NTG eNOS−/−/EC50 control eNOS−/−: 2.86±1.90 (n=10), NS).
Figure 1.
Concentration–response curves to NTG in wt mice aortas. Preparations were precontracted with U-46619 (10−7 M) and NTG was given cumulatively. *P<0.05 versus control wt and †P<0.05 versus control eNOS−/−. Results are expressed as mean±s.e.m.
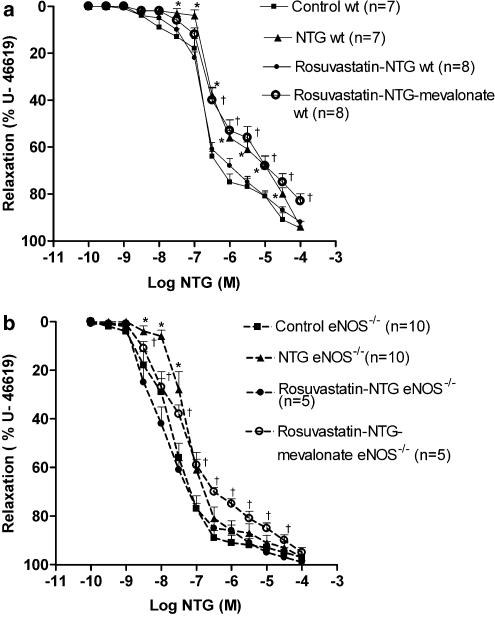
Rosuvastatin protected against the development of nitrate tolerance in wt and also in eNOS−/− mice aortas; the pD2 values were 6.67±0.05 (n=8) and 7.94±0.18 (n=5), respectively, (NS versus respective control groups).
In contrast, in vitro L-mevalonate incubation abolished this protective effect in both mice strains (Figure 2a and b). This L-mevalonate-induced reversal of rosuvastatin effect was similar in both strains: the EC50 ratio was 2.41±1.51 (n=8) versus 3.19±0.34 (n=5) in eNOS−/− mice (NS).
Figure 2.
(a) Concentration–response curves to NTG in wt mice aortas. Preparations were precontracted with U-46619 (10−7 M) and NTG was given cumulatively. *P<0.05 versus control and †P<0.05 versus rosuvastatin–NTG. Results are expressed as mean±s.e.m. (b) Concentration–effect curve to NTG in eNOS−/− mice aortas. Preparations were precontracted with U-46619 (10−7 M) and NTG was given cumulatively. *P<0.05 versus control and †P<0.05 versus rosuvastatin–NTG. Results are expressed as mean±s.e.m.
In both strains, rosuvastatin alone did not modify the concentration–response curve to NTG compared to the control group and L-mevalonate incubation did not alter the response to NTG in aortas from control and rosuvastatin groups (data not shown).
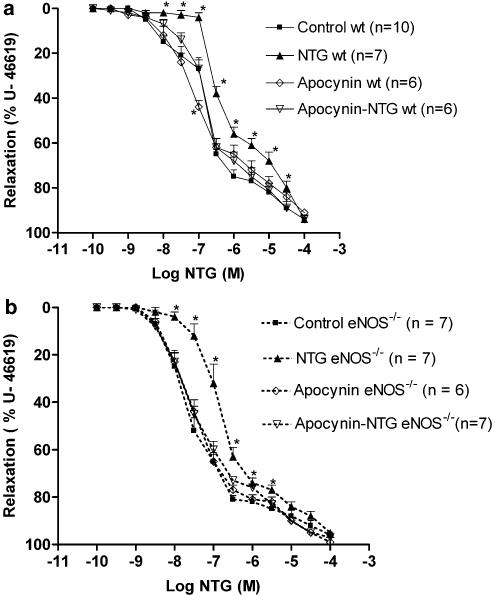
Apocynin, an NAD(P)H oxidase inhibitor, was also able to protect against the development of nitrate tolerance in wt and eNOS−/− mice (Figure 3a and b). The pD2 and AUC values of the apocynin–NTG-treated wt mice were: 6.72±0.06 and 778±28 (n=6) versus 6.80±0.10 and 767±20 (n=10) in the wt mice control group, respectively (NS). In eNOS−/− mice, the pD2 and AUC values of the apocynin–NTG group were: 7.43±0.11 and 647±13 (n=7) versus 7.59±0.15 and 627±22 (n=7) in the control group, respectively (NS). Apocynin treatment alone did not modify the concentration–effect curves to NTG in both strains (Figure 3a and b).
Figure 3.
(a) Concentration–effect curve to NTG in wt mice aortas. Preparations were precontracted with U-46619 (10−7 M) and NTG was given cumulatively. *P<0.05 versus control. Results are expressed as mean±s.e.m. (b) Concentration–effect curve to NTG in eNOS−/− mice aortas. Preparations were precontracted with U-46619 (10−7 M) and NTG was given cumulatively. *P<0.05 versus control. Results are expressed as mean±s.e.m.
NAD(P)H oxidase activity
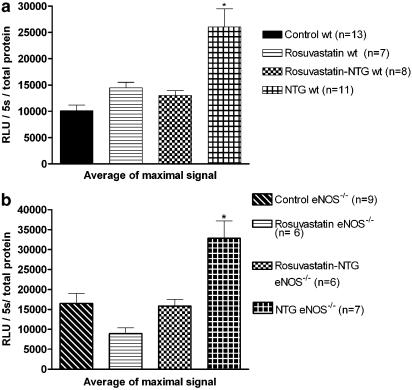
The NAD(P)H oxidase activity was significantly (P<0.05) increased in preparations from the eNOS−/− mice control group compared to those from the wt mice control group: 16,488±2487 (n=9) versus 10,114±1057 (n=13).
In the NTG group of both strains, a significant increase in the NAD(P)H oxidase activity was observed, whereas preparations from the group cotreated with rosuvastatin+NTG behaved similarly to those from the control group (Figure 4a and b). In the rosuvastatin groups of both strains, the NAD(P)H oxidase activity was also similar to that observed in preparations from the control group (Figure 4a and b) . The addition of DPI, a nonspecific NAD(P)H oxidase inhibitor, completely abolished the NAD(P)H oxidase activity, whereas L-NAME had no effect.
Figure 4.
(a) NAD(P)H oxidase activity assay in aortas from wt mice. Data are expressed as RLU and presented as mean±s.e.m. NTG treatment for 4 days significantly increased the NAD(P)H oxidase activity, which was abolished by rosuvastatin treatment (rosuvastatin–NTG group). *P<0.05 versus all other groups. (b) NAD(P)H oxidase activity in aortas from eNOS−/− mice. Data are expressed as RLU and presented as mean±s.e.m. NTG treatment for 4 days significantly increased the NAD(P)H oxidase activity, which was abolished by rosuvastatin treatment (rosuvastatin–NTG group). *P<0.05 versus all other groups.
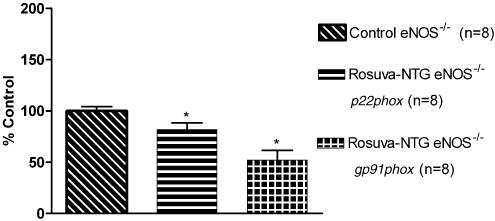
RT–PCR
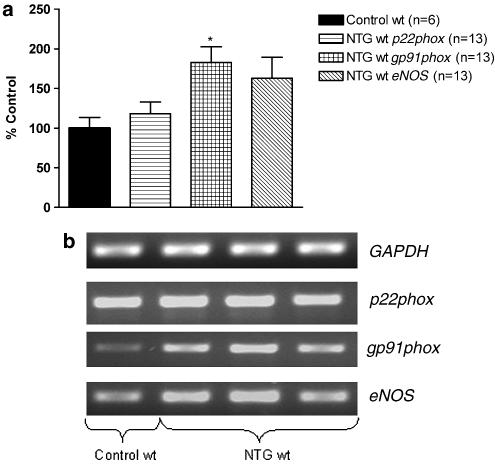
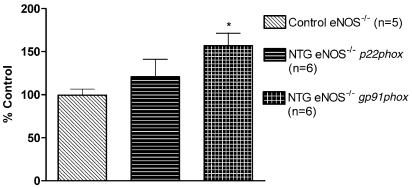
In wt and eNOS−/− mice aortas, in vivo NTG treatment induced a significant increase of gp91phox mRNA expression compared to the respective mRNA expression in aortas from the control mice, whereas p22phox and rac-1 mRNA expressions were not altered (Figure 5, 6 and 10). In wt mice aortas, eNOS mRNA expression was also not modified after in vivo NTG exposure (Figure 5).
Figure 5.
RT–PCR results in aortas from wild-type (wt) mice. (a) The bar graph corresponds to the densitometric analysis and (b) the stained agarose gel contains the RT–PCR products. The intensity of expression of the three genes of interest (p22phox, gp91phox and eNOS) normalized to the intensity of expression of the internal standard gene GAPDH from the same aorta sample. The normalized mRNA of the three genes of interest from the control mice was set as 100%. *P<0.05 versus control wt.
Figure 6.
RT–PCR analysis in aortas from eNOS−/− mice. The intensity of expression of the two genes of interest (p22phox, gp91phox) was normalized to the intensity of expression of the internal standard gene GAPDH from the same aorta sample. The normalized mRNA expression of the two genes of interest from the control mice was set as 100%. *P<0.05 versus control eNOS−/−.
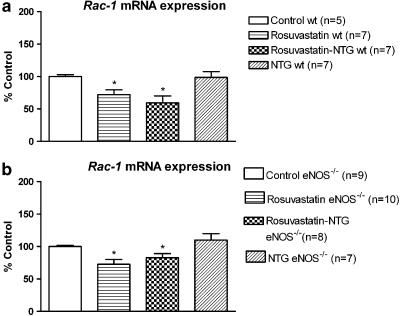
Figure 10.
(a) RT–PCR analysis in wt mice. The intensity of expression of rac-1 mRNA expression was normalized to the intensity of expression of the internal standard gene GAPDH from the same aorta sample. The normalized mRNA expression of rac-1 from the control mice was set as 100%. *P<0.05 versus control wt. (b) RT–PCR analysis in eNOS−/− mice. The intensity of expression of rac-1 mRNA expression was normalized to the intensity of expression of the internal standard gene GAPDH from the same aorta sample. The normalized mRNA expression of rac-1 from the control mice was set as 100%. *P<0.05 versus control eNOS−/−.
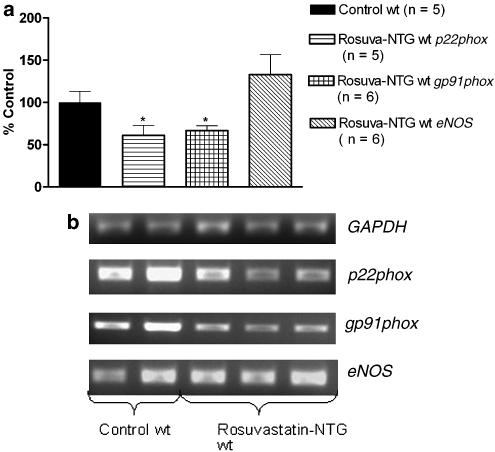
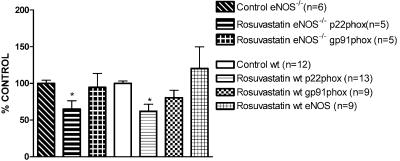
Of note, in both strains, rosuvastatin+NTG cotreatment reduced the mRNA expression of the NAD(P)H oxidase subunits, p22phox, gp91phox (Figures 7 and 8) and rac-1 (Figure 10) compared to respective control groups. Rosuvastatin treatment alone was also able to reduce p22phox and rac-1 mRNA expressions, but not gp91phox mRNA expression in both strains (Figure 9 and 10). In wt mice, eNOS mRNA expression was not modified after rosuvastatin treatment alone (Figure 9) or after the rosuvastatin+NTG cotreatment (Figure 7a).
Figure 7.
RT–PCR analysis in aortas from wt mice. (a) The bar graph corresponds to the densitometric analysis and (b) the stained agarose gel contains the RT–PCR products. The intensity of expression of the three genes of interest (p22phox, gp91phox and eNOS) was normalized to the intensity of expression of the internal standard gene GAPDH from the same aorta sample. The normalized mRNA expressions of the three genes of interest from the control mice were set as 100%. *P<0.05 versus control wt.
Figure 8.
RT–PCR analysis in aortas from eNOS−/− mice. The intensity of expression of the two genes of interest (p22phox, gp91phox) was normalized to the intensity of expression of the internal standard gene GAPDH from the same aorta sample. The normalized mRNA expressions of the two genes of interest from the control mice were set as 100%. *P<0.05 versus control eNOS−/−.
Figure 9.
RT–PCR analysis in aortas from eNOS−/− and wt mice. The intensity of expression of p22phox, gp91phox and eNOS mRNA expression was normalized to the intensity of expression of the internal standard gene GAPDH from the same aorta sample. The normalized mRNA expressions of p22phox, gp91phox and eNOS from the control mice were set as 100%. *P<0.05 versus the respective control.
Discussion
Our results show that rosuvastatin protects against the development of nitrate tolerance via a direct interaction with the NAD(P)H oxidase pathway. Indeed, the NAD(P)H oxidase activity was significantly increased after in vivo NTG exposure in both wt and eNOS−/− strains, and rosuvastatin counteracted this oxidative stress by downregulating membrane subunits, namely p22phox and g91phox and the small G protein, rac-1, which plays a pivotal role in assembly of the active oxidase. Interestingly, we show that NTG altered only the g91phox but not the p22phox, which is one of the main targets of angiotensin II. This suggests that angiotensin II is probably not the first step towards nitrate tolerance and superoxide anion production. The action of rosuvastatin via these NAD(P)H oxidase subunits probably entails a downregulation of this oxidase pathway and therefore, counteracts the oxidative stress induced by in vivo exposure to NTG. In line with this hypothesis, apocynin, which impairs the assembly of the cytosolic subunits p47phox and p67phox with the membrane-bound subunit p22phox (Brandes, 2003), was also able to inhibit the development of nitrate tolerance. The effect of rosuvastatin is likely related to an inhibition of mevalonate synthesis, leading to changes in isoprenoid metabolism. Indeed, in the present study, addition of mevalonate into the organ bath completely abolished the protective effect of the statin. As previously shown (Bokoch & Prossnitz, 1992), the oxidase activation is highly sensitive to inhibition of protein isoprenylation. Accordingly, in cultured endothelial cells and in rat aortas, statins seem to inhibit endothelial superoxide anion formation by blocking rac-mediated assembly of NAD(P)H oxidase (Wagner et al., 2000).
We might speculate that a major source of oxygen radicals, after in vivo exposure to NTG, is the endothelium, as g91phox (in contrast to p22phox) is selectively expressed in endothelial cells. Nevertheless, with regard to nitrate tolerance and the protective effect of rosuvastatin, the eNOS pathway did not play a major role in the current study: (1) the magnitude of nitrate tolerance was similar in wt and eNOS−/− mice, and similar results have been observed by Wang et al. (2002); (2) rosuvastatin protects in a similar way against nitrate tolerance in both strains; (3) in wt mice, rosuvastatin does not modify the eNOS mRNA expression. Although this absence of eNOS pathway upregulation does not support data from recent experiments performed in myocardial tissue (Di Napoli et al., 2005) or in cultured endothelial cells (Laufs et al., 2002), similar findings have been described in other studies, even with much higher doses of rosuvastatin (Pelat et al., 2003).
One may argue that if rac-1 and p22phox are the potential rosuvastatin targets, the NAD(P)H oxidase activity should be decreased in the rosuvastatin groups. The most likely reason for the absence of rosuvastatin-induced alteration in superoxide anion basal release is some disruption of the enzyme complex during homogenization so that only partial NAD(P)H oxidase activity can be detected.
Considering the assessment of superoxide anion production, it is unlikely that the observed signal might be artificially overexpressed because of a phenomenon known as redox cycling of lucigenin with superoxide anion. It is quite clear from reports in the literature (Li et al., 1998; Munzel et al., 2002) that this redox cycling does not occur with low concentrations of lucigenin such as that used in the present study, 5 μM (Munzel et al., 2002). Furthermore, in the blank vial, NADPH did not modify the chemiluminescent signal, and DPI, a potent inhibitor of flavoprotein containing oxidoreductases (but not of the NOS inhibitor, L-NAME), was able to inhibit the O2− production in all the vials.
In the current study, in vivo NTG exposure did not significantly attenuate the relaxation to acetylcholine. This lack of crosstolerance with non-nitrate sources of NO has also been observed in human vessels (Du et al., 1992). Sage et al. (2000) demonstrated that internal mammary arteries isolated from tolerant subjects exhibited unaltered responses to sodium nitroprusside and calcium ionophore despite an enhanced O2− production. This may be related not only to the extent of tolerance but also very likely to species differences namely nitroglycerin metabolisation and/or the presence of antioxidant enzymes such as extracellular superoxide dismutase (Karlsson & Marklund, 1988).
Finally, the only difference between the reactivity of aortas from the two strains, with regard to the response to NTG, is the leftward shift of the concentration–response curves to NTG in the eNOS−/− mice. According to Brandes et al. (2000), this increased sensitivity to nitrovasodilator agents observed in eNOS−/− mice aortas is related to an upregulation of the soluble guanylate cyclase activity.
As mentioned above, the initial event leading to enhanced superoxide anion production remains unknown. A recent hypothesis proposed by Fung (2004) is that NTG is metabolized into NO by several enzymes, but simultaneously inactivates these enzymes via a thionitrate oxidation. The superoxide production induced by nitrate tolerance would then be a propagating mechanism rather than the initiating oxidation agent. Whatever the nitrate tolerance mechanisms are, the current study demonstrates that rosuvastatin downregulates the NAD(P)H oxidase pathway, which is considered to be the major source of ROS production in endothelial cells and in vascular smooth muscle cells (Bayraktutan et al., 1998; 2000; Griendling et al., 2000).
Like statins, ACE inhibitors can also counteract the oxidative stress induced by in vivo exposure to NTG in a rat model. However, using a similar model, namely a 6-week treatment with ramipril and a cotreatment with NTG 3 days before euthanasia, the protective effect of ramipril seems to be mainly related to the eNOS pathway (Berkenboom et al., 1999). Indeed, the ACE inhibition produces a marked potentiation of the endothelial responses and an increase in eNOS abundance via a bradykinin-dependent mechanism (Berkenboom et al., 1997). Therefore, statins and ACE inhibitors may have complementary actions to counteract the oxidative stress induced by in vivo exposure to NTG. This may hold true in other clinical settings such as diabetes mellitus, hypercholesterolemia and hypertension. Accordingly, some clinical investigations have demonstrated additive effects of ACE inhibitor and statin on the endothelial function (Koh et al., 2006).
In conclusion, long-term treatment with rosuvastatin effectively suppresses vascular oxidative activity via a downregulation of the NAD(P)H oxidase pathway and protects against the oxidative stress induced by exposure to NTG in mice. In contrast, the eNOS pathway is neither critically involved in this beneficial effect nor in the development of nitrate tolerance.
Acknowledgments
We gratefully acknowledge AstraZeneca for providing rosuvastatin. We also thank the Molecular Biology Laboratory of Professor Nathalie Verbruggen (ULB) and the Immunology Laboratory of Professor Michel Goldman (ULB) for their help in RT–PCR adjustments. Anne Otto is a recipient of a doctoral fellowship from the Ministère de la Culture, de l'Enseignement Supérieur et de la Recherche, BRF 02/022 (Luxembourg) and this work was supported by the Fondation pour la Chirurgie Cardiaque.
Abbreviations
- ACh
acetylcholine
- eNOS−/−
eNOS knockout
- NS
nonsignificant
- NTG
nitroglycerin
- RT–PCR
reverse transcriptase–polymerase chain reaction
References
- ANNING P.B., COLES B., BERMUDEZ-FAJARDO A., MARTIN P.E., LEVISON B.S., HAZEN S.L., FUNK C.D., KUHN H., O'DONNELL V.B. Elevated endothelial nitric oxide bioactivity and resistance to angiotensin-dependent hypertension in 12/15-lipoxygenase knockout mice. Am. J. Pathol. 2005;166:653–662. doi: 10.1016/S0002-9440(10)62287-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARRUDA R.M., PEOTTA V.A., MEYRELLES S.S., VASQUEZ E.C. Evaluation of vascular function in apolipoprotein E knockout mice with angiotensin-dependent renovascular hypertension. Hypertension. 2005;46:932–936. doi: 10.1161/01.HYP.0000182154.61862.52. [DOI] [PubMed] [Google Scholar]
- BAYRAKTUTAN U., BLAYNEY L., SHAH A.M. Molecular characterization and localization of the NAD(P)H oxidase components gp91-phox and p22-phox in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2000;20:1903–1911. doi: 10.1161/01.atv.20.8.1903. [DOI] [PubMed] [Google Scholar]
- BAYRAKTUTAN U., DRAPER N., LANG D., SHAH A.M. Expression of functional neutrophil-type NADPH oxidase in cultured rat coronary microvascular endothelial cells. Cardiovasc. Res. 1998;38:256–262. doi: 10.1016/s0008-6363(98)00003-0. [DOI] [PubMed] [Google Scholar]
- BEN-SHAUL V., LOMNITSKI L., NYSKA A., ZUROVSKY Y., BERGMAN M., GROSSMAN S. The effect of natural antioxidants, NAO and apocynin, on oxidative stress in the rat heart following LPS challenge. Toxicol. Lett. 2001;123:1–10. doi: 10.1016/s0378-4274(01)00369-1. [DOI] [PubMed] [Google Scholar]
- BERKENBOOM G., FONTAINE D., UNGER P., BALDASSARRE S., PREUMONT N., FONTAINE J. Absence of nitrate tolerance after long-term treatment with ramipril: an endothelium-dependent mechanism. J. Cardiovasc. Pharmacol. 1999;34:547–553. doi: 10.1097/00005344-199910000-00011. [DOI] [PubMed] [Google Scholar]
- BEREKNBOOM G., LANGER I., CARPENTIER Y., GROSFILS K., FONTAINE J. Ramipril prevents endothelial dysfunction induced by oxidized low-density lipoproteins: a bradykinin-dependent mechanism. Hypertension. 1997;30:371–376. doi: 10.1161/01.hyp.30.3.371. [DOI] [PubMed] [Google Scholar]
- BOKOCH G.M., PROSSNITZ Z. Isoprenoid metabolism is required for stimulation of the respiratory burst oxidase of HL-60 cells. J. Clin. Invest. 1992;89:402–408. doi: 10.1172/JCI115599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRANDES R.P. A radical adventure: the quest for specific functions and inhibitors of vascular NAPDH oxidases. Circ. Res. 2003;92:583–585. doi: 10.1161/01.RES.0000066880.62205.B0. [DOI] [PubMed] [Google Scholar]
- BRANDES R.P., KIM D., SCHMITZ-WINNENTHAL F.H., AMIDI M., GODECKE A., MULSCH A., BUSSE R. Increased nitrovasodilator sensitivity in endothelial nitric oxide synthase knockout mice: role of soluble guanylyl cyclase. Hypertension. 2000;35:231–236. doi: 10.1161/01.hyp.35.1.231. [DOI] [PubMed] [Google Scholar]
- DI NAPOLI P., TACCARDI A.A., GRILLI A., DE LUTIIS M.A., BARSOTTU A., FELACO M., DE CATERINA R. Chronic treatment with rosuvastatin modulates nitric oxide synthase expression and reduces ischemia/reperfusion injury in rat hearts. Cardiovasc. Res. 2005;66:462–471. doi: 10.1016/j.cardiores.2005.02.008. [DOI] [PubMed] [Google Scholar]
- DU Z.Y., BUXTON B.F., WOODMAN O.L. Tolerance to glyceryl trinitrate in isolated human internal mammary arteries. J. Thorac. Cardiovasc. Surg. 1992;104:1280–1284. [PubMed] [Google Scholar]
- FONTAINE D., OTTO A., FONTAINE J., BERKENBOOM G. Prevention of nitrate tolerance by long-term treatment with statins. Cardiovasc. Drugs Ther. 2003;17:123–128. doi: 10.1023/a:1025383601304. [DOI] [PubMed] [Google Scholar]
- FUNG H.L. Biochemical mechanism of nitroglycerin action and tolerance: is this old mystery solved. Annu. Rev. Pharmacol. Toxicol. 2004;44:67–85. doi: 10.1146/annurev.pharmtox.44.101802.121646. [DOI] [PubMed] [Google Scholar]
- GORI T., MAK S.S., KELLY S., PARKER J.D. Evidence supporting abnormalities in nitric oxide synthase function induced by nitroglycerin in humans. J. Am. Coll. Cardiol. 2001;38:1096–1101. doi: 10.1016/s0735-1097(01)01510-8. [DOI] [PubMed] [Google Scholar]
- GRIENDLING K.K., SORESCU D., USHIO-FUKAI M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ. Res. 2000;86:494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- KARLSSON K., MARKLUND S.L. Extracellular superoxide dismutase in the vascular system of mammals. Biochem. J. 1988;255:223–228. [PMC free article] [PubMed] [Google Scholar]
- KOJDA G., HARRISON D. Interactions between NO and reactive oxygen species: pathophysiological importance in atherosclerosis, hypertension, diabetes and heart failure. Cardiovasc. Res. 1999;43:562–571. doi: 10.1016/s0008-6363(99)00169-8. [DOI] [PubMed] [Google Scholar]
- KOH K.K., QUON M.J., HAN S.H., CHUNG W.J., LEE Y., SHIN E.K. Anti-inflammatory and metabolic effects of cadesartan in hypertensive patients. Int. J. Cardiol. 2006;108:96–100. doi: 10.1016/j.ijcard.2005.07.040. [DOI] [PubMed] [Google Scholar]
- LANDMESSER U., DIKALOV S., PRICE S.R., McCANN L., FUKAI T., HOLLAND S.M., MITCH W.E., HARRISON D.G. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J. Clin. Invest. 2003;111:1201–1209. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAUFS U., GERTZ K., DIRNAGL U., BOHM M., NICKENIG G., ENDERS M. Rosuvastatin, a new HMG-CoA reductase inhibitor, upregulates endothelial nitric oxide synthase and protects from ischemic stroke in mice. Brain Res. 2002;942:23–30. doi: 10.1016/s0006-8993(02)02649-5. [DOI] [PubMed] [Google Scholar]
- LEVY J.V. Prostacyclin-induced contraction of isolated aortic strips from normal and spontaneously hypertensive rats (SHR) Prostaglandins. 1980;19:517–525. doi: 10.1016/s0090-6980(80)80002-5. [DOI] [PubMed] [Google Scholar]
- LI Y., ZHU H., KUPPUSAMY P., ROUBAUD V., ZWEIER J.L., TRUSH M.A. Validation of lucigenin (bis-N-methylacridinium) as a chemilumigenic probe for detecting superoxide anion radical production by enzymatic and cellular systems. J. Biol. Chem. 1998;273:2015–2023. doi: 10.1074/jbc.273.4.2015. [DOI] [PubMed] [Google Scholar]
- MUNZEL T., AFANAS'EV I.B., KLEYSCHYOV A.L., HARRISON D.G. Detection of superoxide in vascular tissue. Arterioscler. Thromb. Vasc. Biol. 2002;22:1761–1768. doi: 10.1161/01.atv.0000034022.11764.ec. [DOI] [PubMed] [Google Scholar]
- MUNZEL T., KURZ S., RAJAGOPALAN S., THOENES M., BERRINGTON W.R., THOMPSON J.A., FREEMAN B.A., HARRISON D.G. Hydralazine prevents nitroglycerin tolerance by inhibiting activation of a membrane-bound NADH oxidase. A new action for an old drug. J. Clin. Invest. 1996;98:1465–1470. doi: 10.1172/JCI118935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUNZEL T., LI H., MOLLNAU H., HINK U., MATHEIS E., HARTMANN M., OELZE M., SKATCHKOV M., WARNHOLTZ A., DUNCKER L., MEINERTZ T., FORSTERMAN U. Effects of long-term nitroglycerin treatment on endothelial nitric oxide synthase (NOS III) gene expression, NOS III-mediated superoxide production, and vascular NO bioavailability. Circ. Res. 2000;86:E7–E12. doi: 10.1161/01.res.86.1.e7. [DOI] [PubMed] [Google Scholar]
- NANGLE M.R., COTTER M.A., CAMERON N.E. Effects of rosuvastatin on nitric oxide-dependent function in aorta and corpus cavernosum of diabetic mice: relationship to cholesterol biosynthesis pathway inhibition and lipid lowering. Diabetes. 2003;52:2396–2402. doi: 10.2337/diabetes.52.9.2396. [DOI] [PubMed] [Google Scholar]
- OGONOWSKI A.A., KAESEMEYER W.H., JIN L., GANAPATHY V., LEIBACH F.H., CALDWELL R.W. Effects of NO donors and synthase agonists on endothelial cell uptake of L-Arg and superoxide production. Am. J. Physiol. Cell Physiol. 2000;278:C136–C143. doi: 10.1152/ajpcell.2000.278.1.C136. [DOI] [PubMed] [Google Scholar]
- OTTO A., FONTAINE D., FONTAINE J., BERKENBOOM G. Rosuvastatin treatment protects against nitrate-induced oxidative stress. J. Cardiovasc. Pharmacol. 2005;46:177–184. doi: 10.1097/01.fjc.0000167010.98177.78. [DOI] [PubMed] [Google Scholar]
- PELAT M., DESSY C., MASSION P., DESAGER J.P., FERON O., BALLIGAND J.L. Rosuvastatin decreases caveolin-1 and improves nitric oxide-dependent heart rate and blood pressure variability in apolipoprotein E−/− mice in vivo. Circulation. 2003;107:2480–2486. doi: 10.1161/01.CIR.0000065601.83526.3E. [DOI] [PubMed] [Google Scholar]
- RADER D.J., DAVIDSON M.H., CAPLAN R.J., PEARS J.S. Lipid and apolipoprotein ratios: association with coronary artery disease and effects of rosuvastatin compared with atorvastatin, pravastatin, and simvastatin. Am. J. Cardiol. 2003;91:20C–30C. doi: 10.1016/s0002-9149(03)00005-5. [DOI] [PubMed] [Google Scholar]
- SAGE P.R., DE LA LANDE I.S., STAFFORD I., BENNETT C.L., PHILLIPOV G., STUBBERFIELD J., HOROWITZ J.D. Nitroglycerin tolerance in human vessels: evidence for impaired nitroglycerin bioconversion. Circulation. 2000;102:2810–2815. doi: 10.1161/01.cir.102.23.2810. [DOI] [PubMed] [Google Scholar]
- SONTA T., INOGUCHI T., TSUBOUCHI H., SEKIGUCHI N., KOBAYASHI K., MATSUMOTO S., UTSUMI H., NAWATA H. Evalution of oxidative stress in diabetic animals by in vivo electron spin resonance measurement – role of protein kinase C. Diabetes Res. Clin. Pract. 2004;66 (Suppl 1):S109–S113. doi: 10.1016/j.diabres.2004.05.008. [DOI] [PubMed] [Google Scholar]
- VAN DEN WORM E., BEUKELMAN C.J., VAN DEN BERG A.J., KROES B.H., LABADIE R.P., VAN DIJK H. Effects of methoxylation of apocynin and analogs on the inhibition of reactive oxygen species production by stimulated human neutrophils. Eur. J. Pharmacol. 2001;433:225–230. doi: 10.1016/s0014-2999(01)01516-3. [DOI] [PubMed] [Google Scholar]
- WAGNER A.H., KOHLER T., RUECKSCHLOSS U., JUST I., HECKER M. Improvement of nitric oxide-dependent vasodilatation by HMG-CoA reductase inhibitors through attenuation of endothelial superoxide anion formation. Arterioscler. Thromb. Vasc. Biol. 2000;20:61–69. doi: 10.1161/01.atv.20.1.61. [DOI] [PubMed] [Google Scholar]
- WANG E.Q., LEE W.I., FUNG H.L. Lack of critical involvement of endothelial nitric oxide synthase in vascular nitrate tolerance in mice. Br. J. Pharmacol. 2002;135:299–302. doi: 10.1038/sj.bjp.0704532. [DOI] [PMC free article] [PubMed] [Google Scholar]