Abstract
Calcitonin gene-related peptide (CGRP) released from trigeminal afferents is known to play an important role in the control of intracranial blood flow. In a rat preparation with exposed cranial dura mater, periods of electrical stimulation induce increases in meningeal blood flow. These responses are due to arterial vasodilatation mediated in part by the release of CGRP. In this preparation, the effect of a CGRP-binding mirror-image oligonucleotide (Spiegelmer NOX-C89) was examined.
Spiegelmer NOX-C89 applied topically at concentrations between 10−7and 10−5 M to the exposed dura mater led to a dose-dependent inhibition of the electrically evoked blood flow increases. The highest dose reduced the mean increases in flow to 56% of the respective control levels. A nonfunctional control Spiegelmer (not binding to CGRP) was ineffective in changing blood flow increases. Intravenous injection of NOX-C89 (5 mg kg−1) reduced the evoked blood flow increases to an average of 65.5% of the control. The basal blood flow was not changed by any of the applied substances.
In addition, an ex vivo preparation of the hemisected rat skull was used to determine CGRP release from the cranial dura mater caused by antidromic activation of meningeal afferents. In this model, 10−6 M of NOX-C89 reduced the evoked CGRP release by about 50%.
We conclude that increases in meningeal blood flow due to afferent activation can be reduced by sequestering the released CGRP and thus preventing it from activating vascular CGRP receptors. Moreover, the Spiegelmer NOX-C89 may inhibit CGRP release from meningeal afferents. Therefore, the approach to interfere with the CGRP/CGRP receptor system by binding the CGRP may open a new opportunity for the therapy of diseases that are linked to excessive CGRP release such as some forms of primary headaches.
Keywords: Neuropeptide release, vasodilatation, dura mater encephali, laser Doppler, trigeminal afferents, Spiegelmer, aptamer, mirror-image oligonucleotide, migraine, headache
Introduction
Calcitonin gene-related peptide (CGRP) is a neuropeptide that plays an important role in the regulation of meningeal blood flow under pathological conditions, which are associated with headache (Hargreaves & Shepheard, 1999). CGRP is expressed in a major proportion of trigeminal afferents innervating intracranial tissues (Edvinsson & Uddman, 1981; Tsai et al., 1988; Edvinsson et al., 1989; Uddman et al., 1989; Keller & Marfurt, 1991) and is released upon afferent activation from peripheral and central terminals contributing to neurogenic inflammation and nociceptive transmission (Sugimoto et al., 1997; Holzer, 1998). The functional system of trigeminal afferents and intracranial blood vessels, which has been called the trigemino-vascular system (Buzzi & Moskowitz, 1992), is particularly activated during vascular headaches (May & Goadsby, 1999). Migraine and other primary headaches have been shown to be accompanied by a significant elevation in CGRP concentration in the venous outflow from the head (Goadsby et al., 1990; Edvinsson & Goadsby, 1995). Also, CGRP has been found to be the most potent vasodilatory peptide in intracranial arteries both in vitro and in situ (McCulloch et al., 1986; Edvinsson et al., 1987).
In several animal experiments, the exposed cranial dura mater has been used as an in vivo preparation to study the role of CGRP in vasomotor activity (measured as arterial diameter) and meningeal perfusion (measured as arterial blood flow) (Williamson et al., 1997; Petersen et al., 2004). In a previous study, our group has shown that activation of meningeal afferents by local electrical stimulation of the exposed cranial dura mater evokes increases in meningeal blood flow that can be dose dependently inhibited through local application of the CGRP receptor antagonist CGRP8–37 (Kurosawa et al., 1995). Activation of meningeal afferents is thus linked to the release of CGRP, which binds to CGRP receptors on meningeal arteries causing vasodilatation and increased blood flow. Additionally, an ex vivo preparation of the hemisected rat skull with intact dura mater has been developed as a simple model for measuring CGRP release from meningeal afferents activated by electrical or chemical stimuli (Ebersberger et al., 1999; Strecker et al., 2002).
In the present study, we used the meningeal blood flow preparation in order to test the hypothesis that the direct inactivation of CGRP released from meningeal afferents will result in reduced blood flow increases after local electrical stimulation of the cranial dura mater. The CGRP inhibitor NOX-C89 used in these experiments is based on a biostable mirror-image RNA oligonucleotide, a so-called Spiegelmer. The Spiegelmer, built from non-natural L-RNA nucleotides, forms a three-dimensional structure that binds to the CGRP peptide with high affinity. Owing to its mirror-image nature, Spiegelmer NOX-C89 cannot be degraded by natural endonucleases. NOX-C89 has been shown to block CGRP-induced receptor activation linked to cyclic adenosine 3′,5′ monophosphate (cAMP) formation with an inhibitory concentration (IC50) of ≈3 nM in a cell culture assay (Vater et al., 2003). In the present experiments, the Spiegelmer reduced electrically evoked increases in meningeal blood flow in a dose-dependent manner. Unexpectedly, the Spiegelmer also reduced the CGRP release caused by electrical stimulation in the hemisected skull preparation.
Methods
Anaesthesia and general preparation
Adult male Wistar rats with a body weight of 330–450 g bred and housed at our institute were used. The experiments were performed in accordance with the ethical guidelines of the International Association for the Study of Pain and the German animal protection laws. The experimental protocol was reviewed by an ethics committee and approved by the local district government. The animals were anaesthetized with an initial intraperitoneal dose of 120–150 mg kg−1 thiopentone (Trapanal®, Altana Pharma AG, Konstanz, Germany), followed by additional doses of 25 mg kg−1 thiopentone when required. Depth of anaesthesia was routinely assessed and held at a level in which noxious stimuli (pinching of earlobes) failed to elicit motor reflexes or changes of the systemic arterial pressure, which ranged from 80 to 120 mmHg. The right femoral artery was cannulated to monitor systemic arterial blood pressure. Another catheter was inserted into the right femoral vein for infusion of solutions. The animals were tracheotomized and artificially ventilated with oxygen-enriched room air. Expiratory CO2 was monitored and maintained at 4.5–5%, which suppressed spontaneous breathing. Body temperature was maintained at 37–37.5°C with a feedback-controlled homoeothermic system (FMI, Germany). Vital parameters (blood pressure, heart rate, expiratory CO2 level and body temperature) were continuously recorded throughout the experiment.
Head surgery and blood flow recording
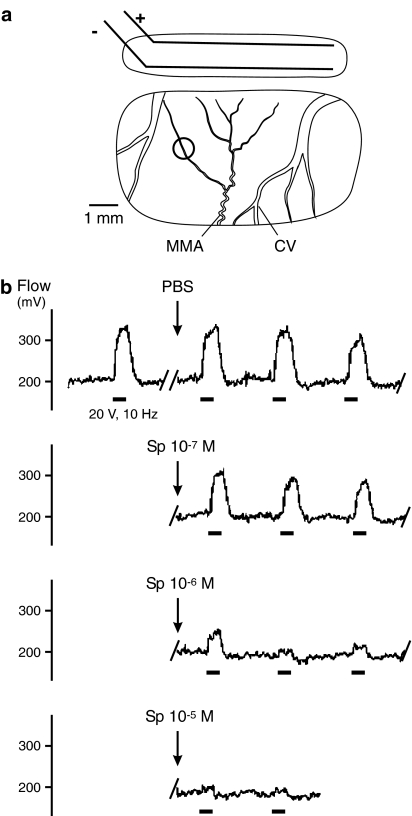
The preparation for dural blood flow recording has been reported previously in detail (Kurosawa et al., 1995). The animals were placed in a stereotaxic frame with the head held in a fixed position by ear bars. The eyes were covered with a protecting ointment (Bepanthen®, Roche, Mannheim, Germany). An incision was made along the midline of the scalp, and the left parietal region of the skull was exposed. Using a dental drill and liquid cooling with drops of saline, a cranial window of about 4 × 7 mm2 was drilled into the parietal bone to expose the dura mater about the medial meningeal artery (MMA; Figure 1a). Arterial and venous vessels in the dura mater could clearly be differentiated from cortical arteries. Blood flow was recorded by a laser Doppler system (DRT4, Moor Instruments, Axminster, U.K.) at a sampling frequency of 10 Hz via needle type probes (tip diameter 0.8 mm) that were positioned over branches of the MMA. The dura in the recording window was protected from drying with pieces of cotton soaked with isotonic saline. A second slit-like window (about 1.5 × 6 mm2) was drilled apically along the superior sagittal sinus for electrical stimulation (Figure 1a). In this stimulation window a pair of parallel wire electrodes (diameter 0.2 mm, length 5 mm, distance 1 mm) was attached to the dura and covered with mineral oil.
Figure 1.
(a) Stimulation and recording window in the parietal bone showing bipolar electrodes (±) and the recording site (ring) over a branch of the MMA; cortical veins (CV). (b) Sections from a continuous recording of meningeal blood flow showing increases in flow upon electrical stimulation (bars) and their changes after topical application of PBS and Spiegelmer NOX-C89 (10−7–10−5 M).
Stimulation and drug administration
The experiment was started not earlier than 60 min after trepanning the skull to ensure that the basal (unstimulated) blood flow was stable. The dura was stimulated at intervals of 3–5 (mostly 4) min for periods of 30 s with rectangular pulses of 0.5 ms duration, 8–24 V at 10 Hz. The stimulation strength was optimized at the beginning of each experiment to elicit clear increases in blood flow without changing the systemic blood pressure or causing motor responses of the head muscles. After three control stimulation periods, the saline in the cranial window was replaced with 25–30 μl of Dulbecco's phosphate-buffered saline with Ca2+/Mg2+ (PBS) or solutions of Spiegelmer NOX-C89 (10−7–10−5 M) in PBS. Small pieces of cotton arranged around the recording probe prevented the solution from running off. After six further stimulation periods, the first solution was replaced by a second solution with a higher concentration of NOX-C89. In four experiments one single dose, in three experiments two doses and in six experiments three doses of Spiegelmer were applied at increasing concentrations and left on the dura for 6–8 stimulation periods each. In additional experiments, a nonfunctional Spiegelmer consisting of the same nucleotides but with an inverse sequence was used as a control.
In a separate set of experiments and after three control stimulation periods, PBS (vehicle control) or a solution of 5 mg kg−1 of NOX-C89 in PBS (0.5 ml) was slowly intravenously (i.v.) injected, followed by six other stimulation intervals.
Spiegelmer synthesis and sequence
The Spiegelmer NOX-C89 used in this study is a variant of the previously published CGRP-binding Spiegelmer STAR-F12 (Vater et al., 2003). Briefly, from a large D-RNA library short D-RNA molecules with high affinity to synthetic D-CGRP were selected and amplified by polymerase chain reaction. After several cycles of in vitro selection, the enriched pool was sequenced and the corresponding mirror-image L-RNAs (Spiegelmers) were synthesized. The Spiegelmer with the highest affinity for CGRP (STAR-F12) was identified by receptor inhibition assays. Additional truncation of six 3′-terminal nucleotides (STAR-F12Δ43–48) and substitution of adenosines 30 and 35 to uridines, synthesis yields could be increased without loss of affinity to CGRP (unpublished data). The resulting candidate sequence of NOX-C89, an all-L-RNA oligonucleotide, is: 5′-GGACUGAUGG CGCGGUCCUA UUACGCCGAU AGGGUGAGGGGA-3′. The nonfunctional control Spiegelmer has the inverse sequence of NOX-C89. The Spiegelmers have been synthesized at NOXXON Pharma AG using standard phosphoramidite chemistry.
Data collection and analysis
Blood flow and vital parameter data were stored and processed with the DRTsoft program (Moore Instruments). As blood flow has no dimension, the mV readings of the flow monitor were taken as arbitrary units. Increases in blood flow caused by electrical stimulation were calculated as mean flow over a period of 60 s from the onset of stimulation (spanning over the stimulation period and a further 30 s) diminished by the basal flow during the 60 s period before each individual stimulation. Flow values were normalised in each experiment to the mean of the three control stimulation values before application of the first substance. Means of normalised stimulated flow values within and between groups of experiments (grouped by concentration of substance) were statistically compared using one- and two-way analysis of variance (ANOVA) with repeated measurements followed by Fisher's least significance difference (LSD) test with multiple comparisons for post-hoc analysis (Statistica® software package, StatSoft, U.S.A.). Significance was accepted at the 5% level. Basal flow values were also normalised and their variation analysed statistically using the same tests. All flow values are given as mean±standard error of the mean (s.e.m.).
Preparation for CGRP release
The preparation has previously been described in detail (Ebersberger et al., 1999; Strecker et al., 2002). Briefly, male Wistar rats (160–200 g) were quickly killed in an CO2 atmosphere. The head was separated from the body at the atlanto-occipital joint. The skin was cut along the midline and retracted from the skull, which was divided into two equal halves along the sagittal plane. The two cerebral hemispheres were removed without lesioning the dura mater, which remained attached to the skull. The skull cavities with the dura were washed for 30 min at room temperature with carbogen-gased synthetic interstitial fluid (SIF, pH 7.4) containing (in mM): 108 NaCl, 3.48 KCl, 3.5 MgSO4, 26 NaHCO3, 11.7 NaH2PO4, 1.5 CaCl2, 9.6 sodium gluconate, 5.55 glucose and 7.6 sucrose. The skull halves were mounted in a water bath forming a humid chamber with a temperature of 37°C. During the experiments, the cavities were repetitively filled with SIF to a level that completely covered the supratentorial dura but not the posterior fossa. Five consecutive samples of eluate were collected at intervals of 5 min by evacuating the fluid from the skull without touching the dura.
For stimulation an isolated steel needle electrode (cathode, diameter 250 μm) was introduced with its blank tip to a depth of 1 mm into the trigeminal ganglion near the branching region of V1 and V2/3. The anode was connected to the tissue at the occipital bone. The skull cavity was filled with 300 μl SIF for the first sampling period (basal release), 5 min later (second period) replaced with SIF (control experiments) or with NOX-C89 (10−6 M) in SIF. In the third period, the skull was again filled either with SIF or Spiegelmer and the trigeminal ganglion was stimulated with pulses of 0.5 ms length at 40 V and 10 Hz for 5 min. In the fourth and fifth (poststimulation) periods, the same solutions were applied as in the second and the first period.
CGRP assay and data analysis
The method for detecting CGRP in the eluate with an enzyme-linked immunoassay (EIA) has been previously published in detail (Averbeck & Reeh, 2001). Briefly, incubation fluids were processed immediately after the experiment using commercial CGRP-EIA buffers and kits (Cayman, distributed by SPIbio, France). Peptide degradation was prevented by adding distinct volumes of the EIA buffer that contains peptidase inhibitors. The antibody is directed against human α/β-CGRP but has 100% cross-reactivity to rat and mouse CGRP. The detection level of immunoreactive CGRP (iCGRP) in this assay is about 2 pg ml−1. To control a possible interaction of the Spiegelmer–CGRP complex with the immunoassay, commercial human α-CGRP (Calbiochem®, Merck Biosciences, Germany) was diluted with 20% EIA buffer to final concentrations of 10−10 and 10−11 M. One-half of the CGRP samples were spiked either with EIA buffer or with 10−6 M Spiegelmer in EIA buffer and analysed like the eluates.
Values of iCGRP release from subsequent sampling periods were compared using one-way ANOVA with repeated measurements followed by the post-hoc LSD test. Differences in iCGRP concentrations between Spiegelmer-treated and control samples were analysed using a Mann–Whitney U-test (Statistica® software package, StatSoft, U.S.A.). Differences were considered significant at P<0.05. All values are given as mean±s.e.m.
Results
Basal blood flow
Blood flow was recorded from branches of the MMA that were visible in the recording window (Figure 1a). Before electrical stimulation, the influence of either PBS, Spiegelmer NOX-C89 or control Spiegelmer on the basal blood flow was analysed. When comparing 1–2 min intervals before and after the application of substances, no significant effects on the basal blood flow were detected. Mean changes in basal blood flow after topical administration of PBS were 0.2±1.5%, after NOX-C89 administration 0.6±0.8% (10−7 M), −1.1±1.2% (10−6 M) and −1.9±1.6% (10−5 M), and after control Spiegelmer (10−5 M) 1.3±1.7%. Following i.v. injection of PBS, the basal flow changed by 4.6±1.3%, after NOX-C89 (5 mg kg−1) by 2.3±1.7%. Furthermore, neither topical nor i.v. administration of Spiegelmer caused significant changes in arterial blood pressure or other systemic parameters.
Stimulated blood flow
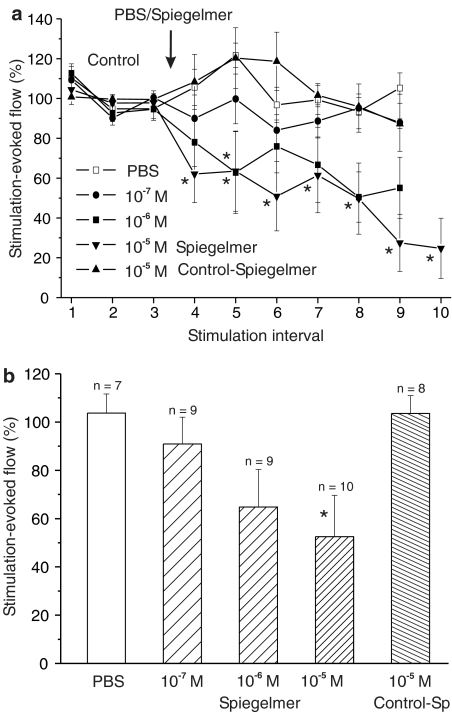
Electrical field stimulation of the dura mater was carried out with bipolar stimulus electrodes (Figure 1a). Stimulation for 30 s caused increases in meningeal blood flow that typically started after a short delay of 2–5 s, then increased steeply to a plateau-like state within 10–15 s and, after switching off the stimulus, returned to the baseline within 20–40 s (Figure 1b). These responses were fairly stable before the application of substances and served as control values. Topical application of PBS as a vehicle (n=7) or the lowest concentration of NOX-C89 (10−7 M; n=8) onto the exposed dura mater did not cause significant changes in stimulated flow values compared to the control (Figures 1b and 2a). Following topical application of higher concentrations of NOX-C89, the evoked increases in flow were reduced, although there was considerable variation between experiments (Figure 2a). After application of 10−6 M, NOX-C89 (n=9) stimulated flow values in the fifth stimulation interval (second after the application) were reduced to 62.8±20.5% of the control and significantly smaller than each of the control values (P<0.05, two-way repeated measures ANOVA and LSD test). Immediately after the application of 10−5 M NOX-C89 (n=10), stimulated flow values were smaller than each control value (P<0.01) and reached a minimum of 27.4±14.3% during the ninth stimulation interval (sixth after NOX-C89 application; Figure 2a). In four experiments, in which the stimulation periods were continued up to 45 min after 10−5 M NOX-C89, there was no recovery of the stimulated flow responses (data not shown). In contrast, after application of the control Spiegelmer at 10−5 M (n=8), the stimulated flow values were not different compared to the vehicle (Figure 2a).
Figure 2.
Mean increases in flow evoked by electrical stimulation before (control) and after topical application of PBS, Spiegelmer NOX-C89 and control Spiegelmer onto the exposed dura. (a) Flow increases (normalised to the mean of the control responses) and their variation after application of PBS, NOX-C89 (10−7–10−5 M) and control Spiegelmer (10−5 M); *significant difference to all three control values (P<0.05, ANOVA). (b) Normalised and averaged flow responses of six stimulation intervals after application of PBS, NOX-C89 and control Spiegelmer, respectively; *significant difference to PBS.
For comparison of the effects between different NOX-C89 concentrations, the six consecutive stimulated flow values after each application were averaged. These mean values of stimulated flow decreased with increasing NOX-C89 concentration and reached the level of significance at 10−5 M (P<0.05; one-way ANOVA and LSD test). The relative means for PBS were 103.7±5.6%, for NOX-C89 92.2±9.9% (10−7 M), 71.0±16.0% (10−6 M) and 55.9±16.4% (10−5 M), and for the control Spiegelmer 103.6±7.4% of the preapplication control (Figure 2b).
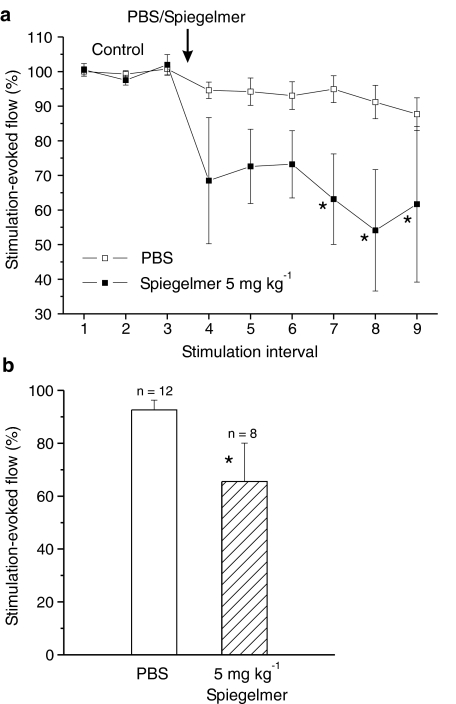
After i.v. injection of NOX-C89 (5 mg kg−1, n=8), the electrically evoked increases in flow were reduced and reached a minimum of 54.1±17.5% during the eighth stimulation interval (fifth after NOX-C89 application; Figure 3a). Although there was considerable variation, the values of the stimulation periods 7–9 were significantly smaller than each of the control values (P<0.05, two-way repeated measures ANOVA and LSD test). In contrast, injection of PBS (n=12) did not change the stimulation evoked flow (Figure 3a). The mean of all consecutive flow values after NOX-C89 injection was 65.5±14.5%, which was significantly different from the mean of the respective values after application of PBS (P<0.05; one-way ANOVA; Figure 3b).
Figure 3.
Mean increases in flow evoked by electrical stimulation before (control) and after i.v. injection of PBS or CGRP-binding Spiegelmer NOX-C89 onto the exposed dura. (a) Flow increases (normalised to the mean of the control responses) and their variation after administration of PBS or NOX-C89 (5 mg kg−1); *significant difference to all three control values (P<0.05, ANOVA). (b) Normalised and averaged flow responses of six stimulation intervals after administration of PBS or NOX-C89; *significant difference to PBS.
Stimulation evoked iCGRP release ex vivo
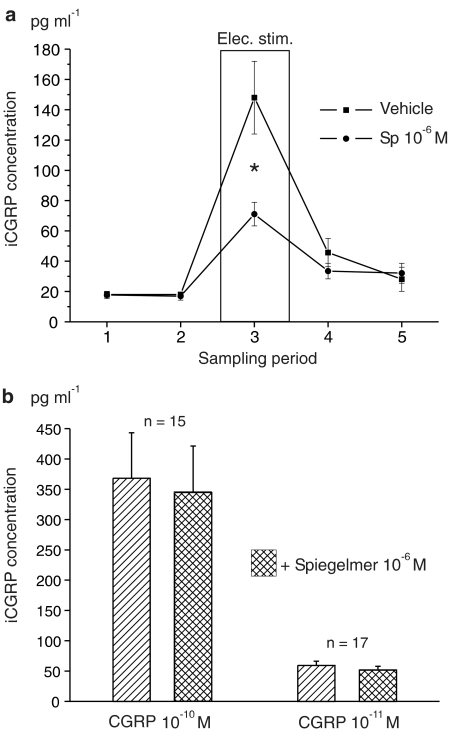
CGRP release was measured in eight skull halves incubated with Spiegelmer and compared with eight control halves. The basal level of iCGRP concentration in the eluates (first sample) was 17.7±2.4 pg ml−1. After addition of NOX-C89 (10−6 M) in the second period, the iCGRP concentration was not significantly different (16.9±2.6 pg ml−1). In the control experiments, the respective values in the first and the second period were both 18.0 pg ml−1. Electrical stimulation of the trigeminal ganglion in the third sampling period caused significant increases in CGRP release both in the NOX-C89-treated and the control preparations (one-way ANOVA and LSD test; Figure 4a). In the NOX-C89 incubated skull halves, the iCGRP concentration increased to 71.1±7.8 pg ml−1 (402% of the basal level), while in the control experiments it was 148.0±24.0 pg ml−1 (822% of the basal level). The difference between these stimulated release values was highly significant (P<0.01, U-test) and indicates that the Spiegelmer NOX-C89 has partly inhibited CGRP release. In the fourth (poststimulation) period, the iCGRP concentration was 33.5±5.2 pg ml−1 in the NOX-C89-treated samples and 45.7±9.3 pg ml−1 in the control samples, which was no longer significantly different. In the fifth sampling period, there was no difference (32.1±6.6 vs 28.1±8.0 pg ml−1; Figure 4a).
Figure 4.
(a) Mean concentrations of iCGRP in the eluate of skull cavities collected over 5 min intervals before, during and after electrical stimulation of the trigeminal ganglion in the presence of buffer (vehicle) or Spiegelmer NOX-C89 (10−6 M), measured with an ELISA; *significant difference between vehicle and Spiegelmer samples (P<0.01, U-test). (b) Mean iCGRP concentration measured in solutions of α-CGRP (10−10 and 10−11 M) without and in the presence of 10−6 M NOX-C89.
Measurements of CGRP solutions in vitro
To test the possibility that the Spiegelmer NOX-C89 may interfere with the CGRP assay, additional in vitro preparations were made. In 15 independent samples, a 10−10 M solution of α-CGRP was prepared. The mean concentration of iCGRP in these preparations was 368.0±75.1 pg ml−1. In the presence of 10−6 M Spiegelmer, the mean iCGRP content of 15 parallel preparations was 344.7±76.6 pg ml−1 (Figure 4b). Accordingly, 17 preparations of a 10−11 M solution of α-CGRP were compared. The mean iCGRP concentration was 59.2±7.2 pg ml−1 without and 51.6±6.4 pg ml−1 with 10−6 M Spiegelmer. This confirms that although NOX-C89 was given in excess so that nearly all CGRP molecules were bound by Spiegelmer molecules, 93.7% (10−10 M) and 87.1% (10−11 M) of the CGRP could be detected by the immune assay. The values with and without NOX-C89 were not significantly different (t-test of dependent samples) and (after reduction of the blank value) close to the expected CGRP concentrations. The blank value of SIF alone was identical with that of a 10−6 M NOX-C89 solution without CGRP in SIF (15.8 pg ml−1 on average).
Discussion
In the present study, the biological activity of a CGRP-binding mirror-image RNA oligonucleotide, the Spiegelmer NOX-C89, was examined in a rat preparation of stimulated meningeal blood flow, which is known to depend on the release and vasodilatory action of CGRP (Kurosawa et al., 1995; Williamson et al., 1997). Spiegelmers are oligonucleotides that are composed of mirror-image nucleotides and display therefore an extraordinary biostability (Klussmann et al., 1996; Eulberg & Klussmann, 2003). Several Spiegelmer-based peptide antagonists that bind and inhibit their targets with strong potency have been described recently, such as a GnRH Spiegelmer (Leva et al., 2002; Wlotzka et al., 2002), a nociceptin Spiegelmer (Faulhammer et al., 2004), a ghrelin Spiegelmer (Helmling et al., 2004), and a substance P Spiegelmer (Eulberg et al., 2005). The identification and characterisation of the CGRP-binding Spiegelmer is described elsewhere (Vater et al., 2003). In the present study, it was used for the first time in animals to evaluate its CGRP inhibiting effects in vivo by monitoring vasodilatation.
CGRP is a 37 amino-acid neuropeptide that is expressed in a proportion of small to medium diameter spinal and trigeminal primary afferents most of which are classified as nociceptive (Hoheisel et al., 1994; Ichikawa & Sugimoto, 2001). CGRP is released from the peripheral and central terminals of these afferents upon depolarisation and opening of calcium channels (Eltorp et al., 2000; Jenkins et al., 2004). Neuropeptide release from peripheral afferent endings is usually implicated in the phenomenon of neurogenic inflammation, in which CGRP specifically causes vasodilatation, flare and increased perfusion of the tissue (Messlinger et al., 1995; Holzer, 1998). In the CNS neuropeptides released from central terminals of nociceptive afferents seem to contribute to nociceptive transmission in the dorsal horn (Ryu et al., 1988; Sun et al., 2004).
Meningeal and intracerebral blood vessels are densely innervated by CGRP immunoreactive nerve fibres (Edvinsson et al., 1987; Tsai et al., 1988; Messlinger et al., 1993). The high proportion of trigeminal afferents innervating intracranial structures may indicate a particularly important efferent function of this neuropeptide in the trigemino-vascular system. This is also reflected by the measurable increase in CGRP levels in the venous outflow from the head during migraine and other severe attacks of headache, which are believed to be causally linked to the activation of intracranial nociceptive afferents (Goadsby et al., 1990; Edvinsson & Goadsby, 1994). Apart from this clinical link there are several in vitro as well as in vivo studies that clearly show the release and the strong vasodilatory action of CGRP in the meninges, especially the cranial dura mater (Messlinger et al., 1995; Williamson et al., 1997; Eltorp et al., 2000). In a model of meningeal blood flow, our group has previously shown that local electrical stimulation of rat dura mater causes increases in meningeal blood flow. These increases can dose dependently be inhibited by blockade of CGRP receptors with the CGRP receptor antagonist CGRP8–37 (Kurosawa et al., 1995). In the present study, we have used this model to confirm the concept that NOX-C89 which binds directly to the neuropeptide is able to inhibit the vasodilatory action of CGRP in vivo. After local administration of NOX-C89 onto the dura, the evoked increases in flow were reduced. To exclude an unspecific effect of high doses of Spiegelmer NOX-C89, a nonfunctional control-Spiegelmer designed as the inverse sequence of NOX-C89 was applied and turned out to be ineffective in reducing stimulated flow. The inhibition of the stimulated flow increases by the NOX-C89 was concentration-dependent, although somewhat weaker than the inhibition formerly observed with CGRP8–37 (Kurosawa et al., 1995).
Intravenous administration of NOX-C89 at 5 mg kg−1 was less effective than topically applied Spiegelmer. This may indicate limited diffusion of NOX-C89 through vascular and dural tissue structures that could be due to the polyanionic nature of the compound or its high molecular weight of approx. 14 kDa. However, recent experiments in our group indicated that the inhibitory effect of NOX-C89 is comparable to the activity of the nonpeptide CGRP receptor antagonist BIBN4096BS (data to be published).
The presented results are consistent with our hypothesis that binding of the Spiegelmer NOX-C89 to CGRP reduces the vasodilatory effects of CGRP at meningeal blood vessels. It is likely that NOX-C89 sequesters the CGRP that is released from perivascular trigeminal afferents and thus prevents the neuropeptide from binding to the CGRP receptors on smooth muscle cells of the dural arteries. The inhibitory effect of the NOX-C89 on stimulation-evoked flow increases was more pronounced than the inhibitory effect achieved with topical (40.6 mM) or i.v. administration (214 μg kg−1) of the the 5-HT1B/D agonist sumatriptan (Messlinger et al., 1997). The 5-HT1B/D agonists are believed to reduce the stimulated flow through their inhibitory effect on the CGRP release from meningeal afferents (Martin, 1996; Eltorp et al., 2000).
To evaluate the possibility that NOX-C89 may enhance its inhibitory effect through an additional inhibition of CGRP release from perivascular dural afferents, we used an ex vivo preparation of the hemisected rat skull (Ebersberger et al., 1999). In this model, antidromic electrical stimulation of the trigeminal ganglion causes CGRP release from the cranial dura mater and this was significantly reduced in the presence of the Spiegelmer by about 50%. This effect cannot be explained by an interference of NOX-C89 with the CGRP-EIA. We compared the iCGRP concentration of α-CGRP solutions (10−10 and 10−11 M, which are in the range of the concentrations found after meningeal stimulation in the skull) in the presence and the absence of NOX-C89 (10−6 M) and observed a divergence of about 10% only. The EIA is based on a double-antibody sandwich technique, in which the capture antibody recognises 6–7 amino acids on the C-terminal end and the tracer antibody 6–7 amino acids on the N-terminal end of the CGRP molecule (personal communication of Dr P. Vitaux, SPI Bio). NOX-C89 recognises approx. 13 amino acids at the N-terminal part of the CGRP peptide (unpublished data), but under the conditions of the EIA the Spiegelmer does not seem to interfere with the recognition by the tracer antibody.
We therefore cannot exclude the possibility that part of the inhibitory effect of the anti-CGRP-Spiegelmer NOX-C89 on the evoked blood flow is due to an inhibition of CGRP release from the dura, although the mechanism of such an effect is as yet unknown. There is some evidence for functional CGRP autoreceptors located on primary afferents (Segond et al., 2002; Ma et al., 2003). RCP, the intracellular receptor component protein of the CGRP receptor complex, has been shown by immunohistochemistry to be colocalised with CGRP in dorsal root ganglion cells (Ma et al., 2003). Binding sites for CGRP have been demonstrated to be partly colocalised with CGRP immunoreactivity in cultured dorsal root ganglion cells using a nanogold marking technique (Segond et al., 2002). Using calcium imaging and whole-cell patch-clamp techniques, a proportion of these cells has been shown to respond to administration of CGRP with intracellular calcium increases and inward currents (Segond et al., 2002). If activation of afferent CGRP autoreceptors is one of the mechanisms to facilitate CGRP release, inactivation of CGRP by NOX-C89 could reduce this effect.
During attacks of migraine and cluster headache, the CGRP-concentration of the plasma in the jugular vein is increased, and it is likely that this CGRP is released from intracranial trigeminal afferents (Edvinsson & Goadsby, 1994). It seems that activation of CGRP receptors crucially contributes to the generation or maintenance of headaches, because blockade of CGRP receptors with the novel nonpeptide CGRP receptor antagonist BIBN4096BS has turned out to reduce migraine pain (Olesen et al., 2004). This finding has not only opened a new therapeutic approach for headache but also suggests that the CGRP/CGRP receptor system plays an important role in trigeminal nociception. Therefore, the interference with the CGRP/CGRP receptor system by currently using receptor antagonists such as CGRP8–37 and BIBN4096BS may be well supplemented by approaches that block the ligand CGRP as well. The Spiegelmer NOX-C89 is a suited candidate to address such questions as NOX-C89 displays a high binding affinity to CGRP. Moreover, the present data underline NOX-C89's inhibitory function in vitro as well as in vivo. Studies are underway to further profile Spiegelmer NOX-C89.
Acknowledgments
We thank I. Izydorczyk, A. Kuhn, J. Schramm, M. Schulte and B. Vogler for their excellent technical assistance and Dr R. Carr for reading the manuscript. We also thank the synthesis team at NOXXON Pharma AG for providing the Spiegelmers, and S. Stark and B. Wlotzka for helpful discussions. This work was supported by the DFG (SFB 353) and the BMBF (German Headache Consortium).
Abbreviations
- CGRP
calcitonin gene-related peptide
- EIA
enzyme-linked immunoassay
- MMA
medial meningeal artery
- SIF
synthetic interstitial fluid
References
- AVERBECK B., REEH P.W. Interactions of inflammatory mediators stimulating release of calcitonin gene-related peptide, substance P and prostaglandin E2 from isolated rat skin. Neuropharmacology. 2001;40:416–423. doi: 10.1016/s0028-3908(00)00171-4. [DOI] [PubMed] [Google Scholar]
- BUZZI M.G., MOSKOWITZ M.A. The trigemino-vascular system and migraine. Pathol. Biol. (Paris) 1992;40:313–317. [PubMed] [Google Scholar]
- EBERSBERGER A., AVERBECK B., MESSLINGER K., REEH P.W. Release of substance P, calcitonin gene-related peptide and prostaglandin E2 from rat dura mater encephali following electrical and chemical stimulation in vitro. Neuroscience. 1999;89:901–907. doi: 10.1016/s0306-4522(98)00366-2. [DOI] [PubMed] [Google Scholar]
- EDVINSSON L., EKMAN R., JANSEN I., MCCULLOCH J., UDDMAN R. Calcitonin gene-related peptide and cerebral blood vessels: distribution and vasomotor effects. J. Cereb. Blood Flow Metab. 1987;7:720–728. doi: 10.1038/jcbfm.1987.126. [DOI] [PubMed] [Google Scholar]
- EDVINSSON L., GOADSBY P.J. Neuropeptides in migraine and cluster headache. Cephalalgia. 1994;14:320–327. doi: 10.1046/j.1468-2982.1994.1405320.x. [DOI] [PubMed] [Google Scholar]
- EDVINSSON L., GOADSBY P.J. Neuropeptides in the cerebral circulation: relevance to headache. Cephalalgia. 1995;15:272–276. doi: 10.1046/j.1468-2982.1995.1504272.x. [DOI] [PubMed] [Google Scholar]
- EDVINSSON L., HARA H., UDDMAN R. Retrograde tracing of nerve fibers to the rat middle cerebral artery with true blue: colocalization with different peptides. J. Cereb. Blood Flow Metab. 1989;9:212–218. doi: 10.1038/jcbfm.1989.31. [DOI] [PubMed] [Google Scholar]
- EDVINSSON L., UDDMAN R. Adrenergic, cholinergic and peptidergic nerve fibres in dura mater – involvement in headache. Cephalalgia. 1981;1:175–179. doi: 10.1046/j.1468-2982.1981.0104175.x. [DOI] [PubMed] [Google Scholar]
- ELTORP C.T., JANSEN-OLESEN I., HANSEN A.J. Release of calcitonin gene-related peptide (CGRP) from guinea pig dura mater in vitro is inhibited by sumatriptan but unaffected by nitric oxide. Cephalalgia. 2000;20:838–844. doi: 10.1046/j.1468-2982.2000.00131.x. [DOI] [PubMed] [Google Scholar]
- EULBERG D., BUCHNER K., MAASCH C., KLUSSMANN S. Development of an automated in vitro selection protocol to obtain RNA-based aptamers: identification of a biostable substance P antagonist. Nucleic Acids Res. 2005;33:e45. doi: 10.1093/nar/gni044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EULBERG D., KLUSSMANN S. Spiegelmers: biostable aptamers. Chembiochem. 2003;4:979–983. doi: 10.1002/cbic.200300663. [DOI] [PubMed] [Google Scholar]
- FAULHAMMER D., ESCHGFALLER B., STARK S., BURGSTALLER P., ENGLBERGER W., ERFURTH J., KLEINJUNG F., RUPP J., DAN V.S., SCHRODER W., VONHOFF S., NAWRATH H., GILLEN C., KLUSSMANN S. Biostable aptamers with antagonistic properties to the neuropeptide nociceptin/orphanin FQ. RNA. 2004;10:516–527. doi: 10.1261/rna.5186504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOADSBY P.J., EDVINSSON L., EKMAN R. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann. Neurol. 1990;28:183–187. doi: 10.1002/ana.410280213. [DOI] [PubMed] [Google Scholar]
- HARGREAVES R.J., SHEPHEARD S.L. Pathophysiology of migraine – new insights. Can. J. Neurol. Sci. 1999;26 (Suppl 3):S12–S19. doi: 10.1017/s0317167100000147. [DOI] [PubMed] [Google Scholar]
- HELMLING S., MAASCH C., EULBERG D., BUCHNER K., SCHRODER W., LANGE C., VONHOFF S., WLOTZKA B., TSCHOP M.H., ROSEWICZ S., KLUSSMANN S. Inhibition of ghrelin action in vitro and in vivo by an RNA-Spiegelmer. Proc. Natl. Acad. Sci. U.S.A. 2004;101:13174–13179. doi: 10.1073/pnas.0404175101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOHEISEL U., MENSE S., SCHEROTZKE R. Calcitonin gene-related peptide-immunoreactivity in functionally identified primary afferent neurones in the rat. Anat. Embryol. (Berlin) 1994;189:41–49. doi: 10.1007/BF00193128. [DOI] [PubMed] [Google Scholar]
- HOLZER P. Neurogenic vasodilatation and plasma leakage in the skin. Gen. Pharmacol. 1998;30:5–11. doi: 10.1016/s0306-3623(97)00078-5. [DOI] [PubMed] [Google Scholar]
- ICHIKAWA H., SUGIMOTO T. VR1-immunoreactive primary sensory neurons in the rat trigeminal ganglion. Brain Res. 2001;890:184–188. doi: 10.1016/s0006-8993(00)03253-4. [DOI] [PubMed] [Google Scholar]
- JENKINS D.W., LANGMEAD C.J., PARSONS A.A., STRIJBOS P.J. Regulation of calcitonin gene-related peptide release from rat trigeminal nucleus caudalis slices in vitro. Neurosci. Lett. 2004;366:241–244. doi: 10.1016/j.neulet.2004.05.067. [DOI] [PubMed] [Google Scholar]
- KELLER J.T., MARFURT C.F. Peptidergic and serotoninergic innervation of the rat dura mater. J. Comp. Neurol. 1991;309:515–534. doi: 10.1002/cne.903090408. [DOI] [PubMed] [Google Scholar]
- KLUSSMANN S., NOLTE A., BALD R., ERDMANN V.A., FURSTE J.P. Mirror-image RNA that binds D-adenosine. Nat. Biotechnol. 1996;14:1112–1115. doi: 10.1038/nbt0996-1112. [DOI] [PubMed] [Google Scholar]
- KUROSAWA M., MESSLINGER K., PAWLAK M., SCHMIDT R.F. Increase of meningeal blood flow after electrical stimulation of rat dura mater encephali: mediation by calcitonin gene-related peptide. Br. J. Pharmacol. 1995;114:1397–1402. doi: 10.1111/j.1476-5381.1995.tb13361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVA S., LICHTE A., BURMEISTER J., MUHN P., JAHNKE B., FESSER D., ERFURTH J., BURGSTALLER P., KLUSSMANN S. GnRH binding RNA and DNA Spiegelmers: a novel approach toward GnRH antagonism. Chem. Biol. 2002;9:351–359. doi: 10.1016/s1074-5521(02)00111-4. [DOI] [PubMed] [Google Scholar]
- MA W., CHABOT J.G., POWELL K.J., JHAMANDAS K., DICKERSON I.M., QUIRION R. Localization and modulation of calcitonin gene-related peptide-receptor component protein-immunoreactive cells in the rat central and peripheral nervous systems. Neuroscience. 2003;120:677–694. doi: 10.1016/s0306-4522(03)00159-3. [DOI] [PubMed] [Google Scholar]
- MARTIN G.R. Inhibition of the trigemino-vascular system with 5-HT1D agonist drugs: selectively targeting additional sites of action. Eur. Neurol. 1996;36 (Suppl 2):13–18. doi: 10.1159/000119098. [DOI] [PubMed] [Google Scholar]
- MAY A., GOADSBY P.J. The trigeminovascular system in humans: pathophysiologic implications for primary headache syndromes of the neural influences on the cerebral circulation. J. Cereb. Blood Flow Metab. 1999;19:115–127. doi: 10.1097/00004647-199902000-00001. [DOI] [PubMed] [Google Scholar]
- MCCULLOCH J., UDDMAN R., KINGMAN T.A., EDVINSSON L. Calcitonin gene-related peptide: functional role in cerebrovascular regulation. Proc. Natl. Acad. Sci. U.S.A. 1986;83:5731–5735. doi: 10.1073/pnas.83.15.5731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MESSLINGER K., HANESCH U., BAUMGARTEL M., TROST B., SCHMIDT R.F. Innervation of the dura mater encephali of cat and rat: ultrastructure and calcitonin gene-related peptide-like and substance P-like immunoreactivity. Anat. Embryol. (Berlin) 1993;188:219–237. doi: 10.1007/BF00188214. [DOI] [PubMed] [Google Scholar]
- MESSLINGER K., HANESCH U., KUROSAWA M., PAWLAK M., SCHMIDT R.F. Calcitonin gene related peptide released from dural nerve fibers mediates increase of meningeal blood flow in the rat. Can. J. Physiol. Pharmacol. 1995;73:1020–1024. doi: 10.1139/y95-143. [DOI] [PubMed] [Google Scholar]
- MESSLINGER K., HOTTA H., PAWLAK M., SCHMIDT R.F. Effects of the 5-HT1 receptor agonists, sumatriptan and CP 93,129, on dural arterial flow in the rat. Eur. J. Pharmacol. 1997;332:173–181. doi: 10.1016/s0014-2999(97)01072-8. [DOI] [PubMed] [Google Scholar]
- OLESEN J., DIENER H.C., HUSSTEDT I.W., GOADSBY P.J., HALL D., MEIER U., POLLENTIER S., LESKO L.M. Calcitonin gene-related peptide receptor antagonist BIBN 4096 BS for the acute treatment of migraine. N. Engl. J. Med. 2004;350:1104–1110. doi: 10.1056/NEJMoa030505. [DOI] [PubMed] [Google Scholar]
- PETERSEN K.A., BIRK S., DOODS H., EDVINSSON L., OLESEN J. Inhibitory effect of BIBN4096BS on cephalic vasodilatation induced by CGRP or transcranial electrical stimulation in the rat. Br. J. Pharmacol. 2004;143:697–704. doi: 10.1038/sj.bjp.0705966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RYU P.D., GERBER G., MURASE K., RANDIC M. Calcitonin gene-related peptide enhances calcium current of rat dorsal root ganglion neurons and spinal excitatory synaptic transmission. Neurosci. Lett. 1988;89:305–312. doi: 10.1016/0304-3940(88)90544-7. [DOI] [PubMed] [Google Scholar]
- SEGOND V.B., PASTOR A., BISKUP C., SCHLEGEL C., BENNDORF K., SCHAIBLE H.G. Localization of functional calcitonin gene-related peptide binding sites in a subpopulation of cultured dorsal root ganglion neurons. Neuroscience. 2002;110:131–145. doi: 10.1016/s0306-4522(01)00547-4. [DOI] [PubMed] [Google Scholar]
- STRECKER T., DUX M., MESSLINGER K. Nitric oxide releases calcitonin-gene-related peptide from rat dura mater encephali promoting increases in meningeal blood flow. J. Vasc. Res. 2002;39:489–496. doi: 10.1159/000067206. [DOI] [PubMed] [Google Scholar]
- SUGIMOTO T., FUJIYOSHI Y., XIAO C., HE Y.F., ICHIKAWA H. Central projection of calcitonin gene-related peptide (CGRP)- and substance P (SP)-immunoreactive trigeminal primary neurons in the rat. J. Comp. Neurol. 1997;378:425–442. doi: 10.1002/(sici)1096-9861(19970217)378:3<425::aid-cne9>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- SUN R.Q., TU Y.J., LAWAND N.B., YAN J.Y., LIN Q., WILLIS W.D. Calcitonin gene-related peptide receptor activation produces PKA- and PKC-dependent mechanical hyperalgesia and central sensitization. J. Neurophysiol. 2004;92:2859–2866. doi: 10.1152/jn.00339.2004. [DOI] [PubMed] [Google Scholar]
- TSAI S.H., TEW J.M., MCLEAN J.H., SHIPLEY M.T. Cerebral arterial innervation by nerve fibers containing calcitonin gene-related peptide (CGRP): I. Distribution and origin of CGRP perivascular innervation in the rat. J. Comp. Neurol. 1988;271:435–444. doi: 10.1002/cne.902710310. [DOI] [PubMed] [Google Scholar]
- UDDMAN R., HARA H., EDVINSSON L. Neuronal pathways to the rat middle meningeal artery revealed by retrograde tracing and immunocytochemistry. J. Auton. Nerv. Syst. 1989;26:69–75. doi: 10.1016/0165-1838(89)90109-4. [DOI] [PubMed] [Google Scholar]
- VATER A., JAROSCH F., BUCHNER K., KLUSSMANN S. Short bioactive Spiegelmers to migraine-associated calcitonin gene-related peptide rapidly identified by a novel approach: tailored-SELEX. Nucleic Acids Res. 2003;31:e130. doi: 10.1093/nar/gng130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMSON D.J., HARGREAVES R.J., HILL R.G., SHEPHEARD S.L. Intravital microscope studies on the effects of neurokinin agonists and calcitonin gene-related peptide on dural vessel diameter in the anaesthetized rat. Cephalalgia. 1997;17:518–524. doi: 10.1046/j.1468-2982.1997.1704518.x. [DOI] [PubMed] [Google Scholar]
- WLOTZKA B., LEVA S., ESCHGFALLER B., BURMEISTER J., KLEINJUNG F., KADUK C., MUHN P., HESS-STUMPP H., KLUSSMANN S. In vivo properties of an anti-GnRH Spiegelmer: an example of an oligonucleotide-based therapeutic substance class. Proc. Natl. Acad. Sci. U.S.A. 2002;99:8898–8902. doi: 10.1073/pnas.132067399. [DOI] [PMC free article] [PubMed] [Google Scholar]