Abstract
Clozapine, a commonly used antipsychotic drug, can induce QT prolongation, which may lead to torsades de pointes and sudden death. To investigate the arrhythmogenic side effects of clozapine, we studied the impact of clozapine on human ether-a-go-go-related gene (HERG) channels expressed in Xenopus oocytes and HEK293 cells, and on the delayed rectifier K+ currents of guinea-pig cardiomyocytes.
Clozapine dose-dependently decreased the amplitudes of the currents at the end of voltage steps, and the tail currents of HERG. The IC50 for the clozapine blockade of HERG currents in Xenopus oocytes progressively decreased relative to depolarization (39.9 μM at −40 mV, 28.3 μM at 0 mV and 22.9 μM at +40 mV), whereas the IC50 for the clozapine-induced blockade of HERG currents in HEK293 cells at 36°C was 2.5 μM at +20 mV.
The clozapine-induced blockade of HERG currents was time dependent: the fractional current was 0.903 of the control at the beginning of the pulse, but declined to 0.412 after 4 s at a test potential of 0 mV.
The clozapine-induced blockade of HERG currents was use-dependent, exhibiting more rapid onset and greater steady state blockade at higher frequencies of activation, with a partial relief of blockade observed when the frequency of activation was decreased.
In guinea-pig ventricular myocytes held at 36°C, treatment with 1 and 5 μM clozapine blocked the rapidly activating delayed rectifier K+ current (IKr) by 24.7 and 79.6%, respectively, but did not significantly block the slowly activating delayed rectifier K+ current (IKs).
Our findings collectively suggest that blockade of HERG currents and IKr, but not IKs, may contribute to the arrhythmogenic side effects of clozapine.
Keywords: Antipsychotic drug, clozapine, HERG channel, LQT, rapidly activating delayed rectifier K+ current, Torsades de pointes
Introduction
The antipsychotic drugs are a heterogeneous group of chemical compounds whose therapeutic efficacies are derived from interactions with dopamine and/or serotonin receptors. Typical antipsychotics, which have been used for treatment of schizophrenia, can cause extrapyramidal syndrome, tardive dyskinesia and hyperprolactinemia, whereas the so-called atypical antipsychotics have been associated with a lower incidence of extrapyramidal effects and hyperprolactinemia (Calderone et al., 2005). Clozapine, the prototype atypical antipsychotic, is useful for the management of patients who prove unresponsive to typical antipsychotics. However, the rate of sudden death has been suggested to be 3.8 times higher in clozapine-treated patients versus those treated with other antipsychotics (Modai et al., 2000). Clozapine has cardiovascular side effects, including myocarditis and cardiomyopathy (Killian et al., 1999), and appears to change cardiac rhythm by inducing ventricular tachycardia (Varma & Achan, 1999) and lengthening of the rate-corrected QT (QTc) at therapeutic dosages (Tanner & Culling, 2003). These alterations in normal cardiac rhythm could induce the polymorphic ventricular arrhythmias known as torsades de pointes, and sudden death.
QTc prolongation generally results from delayed or prolonged repolarization. This may be caused by reduced functioning of the delayed rectifier cardiac K+ current (IK), which has both rapidly and slowly activating components, called IKr and IKs, respectively (Sanguinetti & Jurkiewicz, 1990). Both the components are responsible for initiating repolarization of the cardiac action potential thereby terminating the plateau phase of the action potential (Sanguinetti & Jurkiewicz, 1990). The major protein underlying IKr is encoded by the human ether-a-go-go-related gene (HERG) (Sanguinetti et al., 1995); mutations in HERG have been shown to cause chromosome 7-linked inherited long QT syndrome (LQT2) (Curran et al., 1995). Several drugs that block IKr and HERG have been shown to cause acquired LQT and torsades de pointes (Suessbrich et al., 1996; Suessbrich et al., 1997). Indeed, the cardiotoxicity of numerous drugs is mainly due to their interaction with HERG K+ channels (Taglialatela et al., 1998).
Clozapine was reported to delay repolarization by prolonging phase 3 of the action potential, induce early after depolarizations (EADs), and/or trigger the spontaneous depolarization of non-pacemaker myocytes (Adamantidis et al., 1994). Furthermore, QTc was found to be prolonged in a patient taking clozapine (Tanner & Culling, 2003). Although no previous study has conclusively proven that clozapine causes sudden death through cardiac arrhythmia, the noted associations prompted us to examine the effects of clozapine on cardiac electrophysiological properties. We hypothesized that this atypical antipsychotic drug may prolong action potential duration (APD) in vivo and cause LQT by inhibiting IKr, HERG channels, and/or IKs. To address these possibilities, we examined the effect of clozapine on HERG channels expressed in Xenopus oocytes and stably expressed in HEK293 cells. To confirm that clozapine blocks HERG channels, we further tested the effect of this agent on native IKr in guinea-pig ventricular myocytes.
Methods
Expression of HERG in oocytes
HERG (accession no. U04270) cRNA was synthesized by in vitro transcription from 1 μg of linearized cDNA using a T7 message machine kit (Ambion, Austin, TX, U.S.A.), and was stored in 10 mM Tris-HCl (pH 7.4) at −80°C. Stage V–VI oocytes were surgically removed from female Xenopus laevis (Nasco, Modesto, CA, U.S.A.) anesthetized with 0.17% tricane methanesulphonate (Sigma, St Louis, MO, U.S.A.). The theca and follicle layers were manually removed from the oocytes using fine forceps, and each oocyte was injected with 40 nl of cRNA (0.1–0.5 μg μl−1). The injected oocytes were maintained in modified Barth's solution containing 88 mM NaCl, 1 mM KCl, 0.4 mM CaCl2, 0.33 mM Ca(NO3)2, 1 mM MgSO4, 2.4 mM NaHCO3, 10 mM HEPES (pH 7.4), and 50 μg ml−1 gentamicin sulphonate. Currents were studied 2–7 days after injection.
Solutions and voltage clamp recording from oocytes
The utilized normal Ringer's solution contained 96 mM NaCl, 2 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, and 10 mM HEPES (pH adjusted to 7.4 with NaOH). The antipsychotic drug clozapine and all salts were purchased from Sigma. A stock solution of clozapine was prepared in distilled water and added to the external solutions at the indicated concentrations. Solutions were applied to oocytes by continuous chamber perfusion during recordings; solution exchanges were completed within 3 min, and HERG currents were recorded 5 min after the solution exchange. Each oocyte was used for multiple recordings at different drug concentrations. In between recordings, drugs were washed with normal Ringer's solution for 10 or 20 min to wash out ⩽20 μM and ⩾50 μM of the drug, respectively. If the oocyte did not recover current after 30 min of washing with normal Ringer's solution, it was excluded from further experiments. In general, each oocyte could be tested with 4–6 different concentrations of clozapine. Currents were measured at room temperature (21–23°C) with a two-microelectrode voltage clamp amplifier (Warner Instruments, Hamden, CT, U.S.A.). The electrodes were filled with 3 M KCl and had a resistance of 2–4 MΩ for voltage-recording electrodes and 0.6–1 MΩ for current-passing electrodes. Stimulation and data acquisition were controlled with the Digidata and pCLAMP software packages (Axon Instruments, Sunnyvale, CA, U.S.A.). Data were expressed as mean±s.e.m.
The fractional electrical distance (δ), that is, the fraction of the transmembraneous electrical field sensed by a single positive charge at the binding site, was determined with half-blocking concentrations (KD) obtained from the fractional current (fo) as the current with 20 μM clozapine and under control conditions at the end of the voltage step with the equation KD=(fo/(1–fo)) × 20 (in μM). The value of δ was obtained by fitting the KD values with the equation KD=KD 0 mV × exp(−zδ FV/RT), where KD 0 mV represents the half-blocking concentration at the reference potential of 0 mV. V represents the membrane potential and z, R, F and T have their usual meaning (Snyders et al., 1992).
Solutions and voltage clamp recording from guinea-pig ventricular myocytes
Single ventricular myocytes were isolated from guinea-pig hearts by a standard enzymatic technique (Choi et al., 2005). Isolated cells were superfused at 36°C with normal Tyrode's solution containing 140 mM NaCl, 4.4 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, 5 mM HEPES and 10 mM glucose (adjusted to pH 7.4 with 4 M NaOH). Inward rectifier K+ currents were inhibited by the addition of 5 mM CsCl. The patch pipettes (outer diameter 1.5 mm, World Precision Instruments, U.S.A.) had resistances of around 1–2 MΩ. The pipette solution for the potassium current measurement contained 140 mM KCl, 1 mM MgCl2, 5 mM EGTA, 5 mM MgATP, 2.5 mM diTris-phosphocreatine, and 2.5 mM disodium phosphocreatine (adjusted to pH 7.4 with KOH). The ‘pipette-to-bath' liquid junction potential was small (−3.5 mV), and was left uncorrected. Membrane capacitance (the time integral of the capacitive response to a 10 mV hyperpolarizing pulse from a holding potential of 0 mV, divided by the voltage drop) averaged 121.5±24.5 pF (n=10). Measurements were taken using an Axopatch 200A amplifier (Axon Instruments) and a CV-201 headstage. Voltage-clamp commands were generated using the WinWCP (John Dempster, Strathclyde University, U.K.) or pClamp (ver. 5.1, Axon Instruments) software programs. The current signals were filtered via a 1–10 kHz, 8-pole Bessel-type low-pass filter and digitized with an AD-DA converter (Digidata 1200, Axon Instruments) for subsequent analysis using the pCLAMP software ver. 6.0.3. All chemicals were obtained from Sigma except for E-4031, which was kindly provided by Eisai Co. (Tokyo, Japan).
HEK293 cell culture and whole-cell patch clamp recording
HEK293 cells stably expressing HERG channels, a kind gift from Dr C. January (Zhou et al., 1998), were cultured in minimum essential medium (MEM) supplemented with 10% fetal bovine serum, 1 mM sodium pyruvate, 0.1 mM non-essential amino-acid solution, 100 units ml−1 penicillin G sodium, 100 μg ml−1 streptomycin sulfate, and 400 μg ml−1 geneticin. Whole-cell currents were recorded using standard patch clamp techniques. The external solution contained 136 mM NaCl, 5.4 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, 10 mM glucose and 10 mM HEPES (adjusted to pH 7.4 with NaOH). The intracellular solution contained 130 mM KCl, 1 mM MgCl2, 10 mM EGTA, 5 mM MgATP and 10 mM HEPES (adjusted to pH 7.2 with KOH). The pipettes had resistances of 2–4 MΩ. All experiments were performed at 36±1°C.
Statistical evaluations
All data are expressed as mean±s.e.m. Unpaired or paired Student's t-tests, or ANOVA were used for statistical comparisons when appropriate, and differences were considered significant at P<0.05.
Results
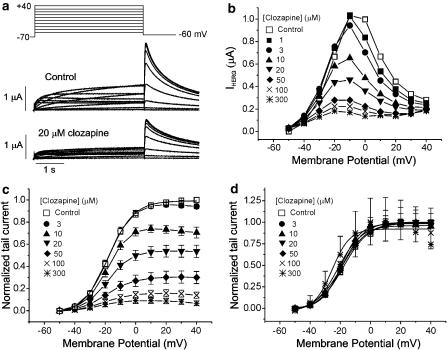
We first investigated the effect of clozapine on HERG currents using a Xenopus oocyte expression system. Throughout the experiments, the holding potential was maintained at −70 mV, and the repolarization potential was maintained at a constant of −60 mV for the analysis of tail currents (Itail). Figure 1a shows an example of a voltage-clamp recording from a Xenopus oocyte, with representative current traces given under control conditions and after exposure to 20 μM clozapine. Under control conditions, the depolarizing steps activated time-dependent outward currents. The amplitude of the outward currents measured at the end of the pulse (IHERG) increased with increasingly positive voltage steps, peaked at −10 mV and decreased thereafter, resulting in a negative slope of the I–V curve (Figure 1b). The current–voltage relationships for IHERG obtained at various concentrations of clozapine are shown in Figure 1b. As the concentration of clozapine progressively increased, the amplitude of IHERG decreased dose-dependently.
Figure 1.
Effect of clozapine on human ether-a-go-go-related gene (HERG) currents (IHERG) elicited by depolarizing voltage pulses. (a) Superimposed current traces elicited by 4-s depolarizing voltage pulses in 10 mV steps (upper panel) from a holding potential of −70 mV in the absence of clozapine (control, middle panel) and in the presence of 20 μM clozapine (lower panel). (b) Plot of the HERG current (IHERG) measured at the end of depolarizing pulses against the pulse potential seen with different concentrations of clozapine. (c) Plot of the normalized tail current measured at its peak just after repolarization. The amplitude of the tail current in the absence of the drug was taken as 1. Control data were fitted to the Boltzmann equation, y=1/{1+exp[(−V+V1/2) /dx]} with V1/2 of −18.0 mV. (d) Activation curves with values normalized to the respective maximum value at each concentration of clozapine. Symbols with error bars represent means±s.e.m (n=8).
After the depolarizing steps, repolarization to −60 mV induced an outward Itail, which had an amplitude even larger than that of IHERG during depolarization. This characteristic property of HERG currents is due to the rapid recovery from inactivation and a slow deactivation mechanism (Sanguinetti et al., 1995). The amplitude of Itail increased with depolarizing steps from −60 to +10 mV and was then superimposed on further depolarizing steps to +40 mV. When 20 μM clozapine was added to the perfusate, both IHERG and Itail were suppressed (Figure 1a, bottom panel). To examine the effects of clozapine on activation curve, the amplitude of Itail was normalized to the peak amplitude obtained under control conditions at maximum depolarization, and was plotted against the potential of the step depolarization (Figure 1c). The normalized Itail reflects the voltage-dependent activation of the HERG channels. Data obtained under control conditions were well fitted by the Boltzmann equation, with half-maximal activation (V1/2) at −18.0 mV. As the concentration of clozapine increased, the peak Itail amplitude decreased, indicating that clozapine treatment decreased the maximum conductance of HERG channels in this experimental system.
To examine whether clozapine shifted the activation curve, we normalized the values in Figure 1c to the respective maximum values at each concentration. As shown in Figure 1d, the activation curves in the control oocytes, as well as those treated with 1, 3, 10, 20, 50, and 100 μM clozapine basically overlapped, whereas the curve representing the highest concentration of clozapine (300 μM) was shifted leftward. The V1/2 calculations were consistent with this finding, yielding values of −18.0±0.37, −18.8±0.08, −18.6±0.23, −19.4±0.56, −18.7±0.10, −19.1±0.26, −21.65±1.42, and −25.4±2.64 mV in the control and 1, 3, 10, 20, 50, 100 and 300 μM clozapine-treated groups, respectively (n=8). Thus, the V1/2 values for experiments run in the presence of 1–100 μM clozapine were not significantly different from each other, whereas that for experiments run in the presence of 300 μM were significantly lower than controls. These findings indicate that clozapine does not change activation gating at 1–100 μM.
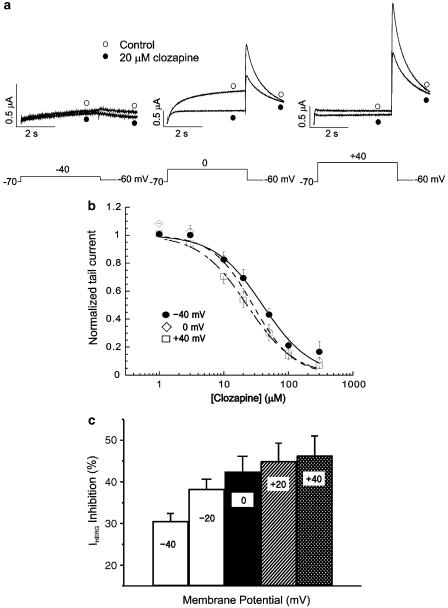
To test whether the effect of clozapine is voltage-dependent, we compared the clozapine-induced decrease of Itail at different potentials (Figure 2). Our results revealed that a higher degree of blockade was present at more positive voltages (Figure 2a). At −40 mV, 20 μM clozapine reduced the amplitude of normalized Itail by 30.6% (from 0.04±0.01 to 0.03±0.01; n=8, P<0.05), while at 0 and +40 mV, the same concentrations of clozapine reduced the amplitudes of normalized Itail by 42.5% (from 0.87±0.01 to 0.50±0.05; n=8, P<0.05) and 46.4% (from 1.00±0.01 to 0.54±0.06; n=8, P<0.05), respectively. Dose–response relationships were obtained at +40, 0 and −40 mV. The data were fitted by Hill equations (Figure 2b), giving IC50 values of 39.9±3.90, 28.3±3.42 and 22.9±1.16 μM, respectively, and Hill coefficients of 1.17±0.13, 1.38±0.21 and 1.18±0.07 at −40, 0 and +40 mV, respectively (n=8). As shown in Figure 2c, the HERG current inhibition by 20 μM clozapine at different voltages (−40, −20, 0, +20, and +40 mV) was 30.6±1.8, 38.3±2.3, 42.5±3.6, 45.0±4.3, and 46.3±4.6%, respectively, suggesting that the clozapine-induced blockade of HERG currents progressively increases with increasing depolarization. Collectively, these findings indicate that the clozapine-induced blockade of HERG current is voltage-dependent.
Figure 2.
Voltage dependence of the clozapine-induced HERG current blockade. (a) Current traces from a cell depolarized to −40 mV (left panel), 0 mV (middle panel) and +40 mV (right panel), before and after exposure to 20 μM clozapine, showing increased blockade of HERG current at the more positive potential. The protocol consisted of 4-s depolarizing steps to −40, 0 or +40 mV from a holding potential of −70 mV, followed by repolarization to −60 mV. (b) Concentration-dependent block of IHERG by clozapine at different membrane potentials. At each depolarizing voltage step (−40, 0 or +40 mV), the tail currents in the presence of various concentrations of clozapine were normalized to the tail current obtained in the absence of drug, and then plotted against clozapine concentrations. Symbols with error bars represent means±s.e.m (n=8). The line represents that the data fits to the Hill equation. (c) Clozapine-induced HERG current inhibition at different voltages. At each depolarizing voltage step (−40, −20, 0, +20 or +40 mV), the tail currents in the presence of 20 μM clozapine were normalized to the tail current obtained in the absence of drug. Bars with error bars represent means±s.e.m (n=8).
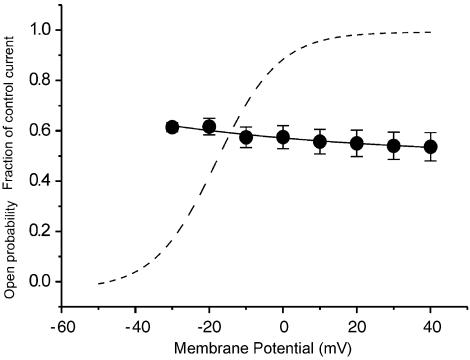
For further analysis, the relative current under clozapine (20 μM) was calculated for each potential (Figure 3; filled circles; n=8). Then, the relative currents with the drug, as fractions of the control current were fitted with a monoexponential function (Figure 3, solid line). As the relative conductance of the HERG control current reached more than 90% of its maximal value at potentials positive to +10 mV (Figure 3, dashed line; mean open probability at +10 mV obtained by Boltzmann fit: 0.95), the range between +10 and +40 mV was taken to estimate the fractional electrical distance (δ), that is, the fraction of the transmembranous electrical field sensed at the receptor site of clozapine. From the fraction of control current achieved with clozapine, half-blocking concentrations (KD) were calculated. Fitting the mean KD values in the potential range from +10 to +40 mV with the mean KD at the reference potential of 0 mV (KD 0 mV=27.0 μM) yielded a fractional electrical distance of δ=0.56.
Figure 3.
Voltage dependence of clozapine-induced blockade of HERG tail currents. The filled circles represent the mean relative currents in the presence of 20 μM clozapine (data from Figure 2), given as a fraction of the control current in the potential range from −30 to +40 mV. Symbols with error bars represent means±s.e.m (n=8). The solid line shows the data points fitted with a monoexponential function. The dashed line gives the open probability curve obtained by fitting a Boltzmann equation to the control tail-current amplitude.
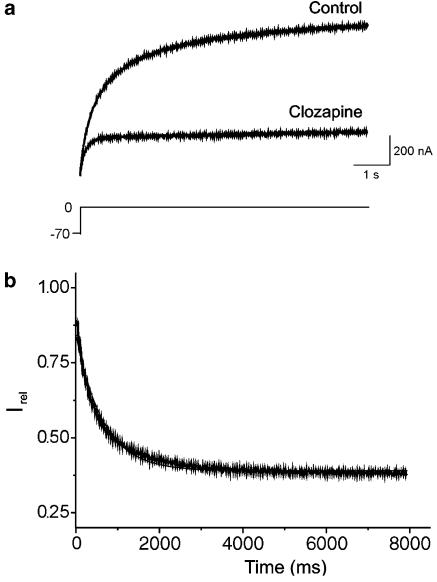
To assess whether the clozapine-dependent blockade of HERG currents was time-dependent, we activated currents in the absence of the drug using a single depolarizing step to 0 mV for 8 s (Figure 4a). After obtaining the control measurement, we perfused the system with 10 μM clozapine for 13 min while holding the channels in the closed state at −70 mV. We then applied the same single-step protocol to assess changes in the HERG currents over time in the presence of clozapine. Our results revealed that clozapine time-dependently blocked the HERG current, up to a maximum of 73% at 8 s in a representative cell (Figure 4a). The fractional sustained current (obtained by normalizing the experimental versus control currents) decreased with ongoing depolarization (Figure 4b). At the beginning of the pulse, the fractional current was 0.903±0.047 of the control, but it declined to 0.412±0.067 after 4 s at a test potential of 0 mV (Figure 4b; n=5). These findings indicate that HERG channels are only partially blocked by clozapine when they are maintained at the holding potential.
Figure 4.
Relative changes in sustained HERG currents in response to clozapine. (a) Original recording of currents under control conditions (Control) and in the presence of clozapine (10 μM) during voltage steps to 0 mV. After the control measurement was recorded, the oocyte was clamped at −70 mV and superfused with the drug solution for 13 min. (b) Relative current (Irel) obtained by dividing the clozapine current by the control current of the recording in (a), and fitting the result with a monoexponential function. Time 0 ms corresponds to the beginning of the depolarizing voltage step.
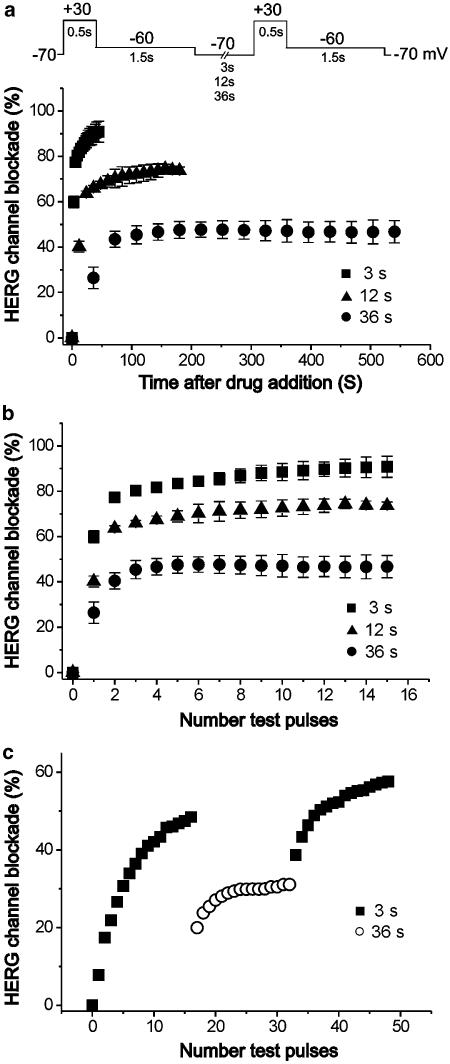
To examine whether the clozapine-induced blockade of HERG is use-dependent, we activated HERG channels with 0.5 s depolarizing steps to +30 mV at intervals of 3, 12 or 36 s in the presence of 30 μM clozapine (n=7). The voltage protocol was shown in the upper panel of Figure 5a. We found that the time course of the channel blockade was dramatically dependent on the activation frequency, with the clozapine-induced blockade of HERG occurring much more quickly at higher activation frequencies (Figure 5a). When the data were plotted as a function of the number of test pulses (Figure 5b), we found that after the same number of test pulses, the block by 30 μM clozapine was stronger at higher versus lower activation frequencies, indicating that binding of clozapine was favored at higher frequencies. We also found that steady-state HERG channel blockade by clozapine was initially obtained with depolarization at 3 s intervals (Figure 5c), and that a subsequent increase of the depolarization intervals to 36 s resulted in a partial relief of clozapine-induced HERG channel blockade (n=6). These results indicate that the clozapine-induced blockade of HERG channels is strongly use-dependent.
Figure 5.
Use-dependent HERG channel blockade by clozapine. (a) Oocytes were exposed to 30 μM clozapine and tail currents were recorded at −60 mV for 1.5 s after a 0.5-s depolarizing pre-pulse to +30 mV from a holding potential of −70 mV every 3, 12, or 36 s. (b) The HERG channel blockade data from (a) were plotted against the number of test pulses. (c) Steady-state HERG channel blockade by 10 μM clozapine after 16 pulses at 3-s intervals. Increasing the depolarization intervals to 36 s in the presence of clozapine resulted in a partial relief, while returning to 3-s intervals again increased HERG channel blockade. Symbols with error bars represent means±s.e.m (n=6–7 each).
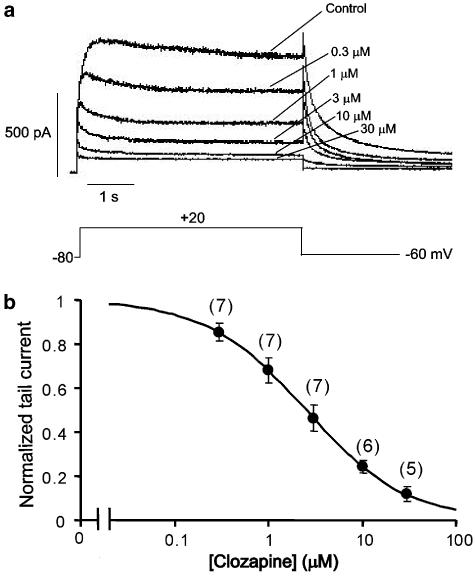
The IC50 values of many HERG channel blockers have been shown to differ depending on whether the HERG channels are expressed in Xenopus oocytes or mammalian cells, an effect probably due to the sequestration of blockers in the large ooplasm of oocytes (Choi et al., 2005). We therefore tested the effects of clozapine in HEK293 cells expressing HERG channels at 36°C (Figure 6). HERG currents were activated by a 4.8-s depolarizing pulse to +20 mV from a holding potential of −80 mV, and the tail current was recorded at −60 mV. The cells were treated with steadily increasing concentrations of clozapine, from 0.3 to 30 μM (Figure 6a). We observed that during each depolarizing pulse, the current amplitude gradually declined as the drug blocked the HERG channels, until a steady state was reached. Once the current amplitude reached steady state, we increased the drug concentration. Our results revealed that the dose–response curve for the clozapine-induced blockade of the normalized tail current showed an IC50 of 2.5 μM and a Hill coefficient of 0.82 (n=5–7) (Figure 6b). These results indicate that the clozapine-induced inhibition of HERG channels stably expressed in HEK293 cells took place at an eightfold lower concentration than that required by HERG channels expressed in Xenopus oocytes.
Figure 6.
Clozapine-induced blockade of HERG channels constitutively expressed in HEK293 cells. (a) Representative current traces recorded from HEK293 cells after application of clozapine. (b) Concentration-dependent blockade of HERG current. HERG tail-current amplitude was normalized to the control and plotted as a function of clozapine concentration. Data were fitted with the Hill equation, giving a half-maximal inhibitory concentration (IC50) of 2.5 μM and a Hill coefficient of 0.82 at +20 mV. Numbers in parentheses represent the number of cells.
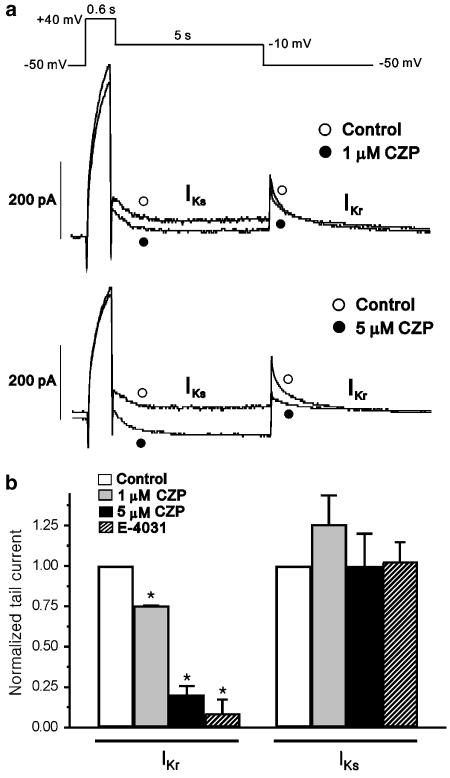
Finally, we tested the effects of clozapine on the rapid and slow components of the delayed rectifier currents in guinea-pig ventricular myocytes at 36°C, using a voltage clamp protocol designed to electrophysiologically separate the currents (Heath & Terrar, 1996) (Figure 7a, inset; stimulation frequency of 0.03 Hz). Our results revealed that depolarization to +40 mV activated both IKr and IKs. Repolarization to −10 mV revealed IKs as a deactivating Itail, and subsequent repolarization to −50 mV resulted in deactivation of IKr. Application of 2 μM E-4031, a selective blocker of IKr (Sanguinetti & Jurkiewicz, 1990), blocked the rapid component of the delayed rectifier K+ current but had no effect on IKs (n=11, Figure 7b). Experiments in the presence and absence of the drug revealed that 1 μM and 5 μM clozapine dose-dependently inhibited IKr by 24.7±1.01 and 79.6±5.2%, respectively (n=6 and 4, respectively, P<0.05; Figure 7a and b), suggesting that native IKr is more sensitive to clozapine than HERG channels expressed in Xenopus oocytes. Neither 1 μM nor 5 μM clozapine had a significant effect on IKs under our experimental conditions (n=6 and 4, respectively). These findings collectively indicate that clozapine preferentially blocked the rapid component of the delayed rectifier K+ current, but not the slow component. Thus, clozapine may prolong APD primarily by blocking IKr but not IKs.
Figure 7.
Effect of clozapine on the slow and rapid components of the delayed rectifier K+ current in guinea-pig ventricular myocytes. (a) Representative traces of the rapid (IKr) and slow (IKs) components of the delayed rectifier K+ channel tail currents before and after treatment with 1 or 5 μM clozapine (CZP). (b) Summary of the effects of 1 and 5 μM CZP and 2 μM E-4031 on IKr and IKs tail currents, normalized relative to the control current (n=6, 4, and 11 for 1 μM CZP, 5 μM CZP, and E-4031, respectively, *P<0.05). The tail-current amplitudes were measured as the difference between peak outward current and the steady state current at the end of the repolarizing voltage pulses.
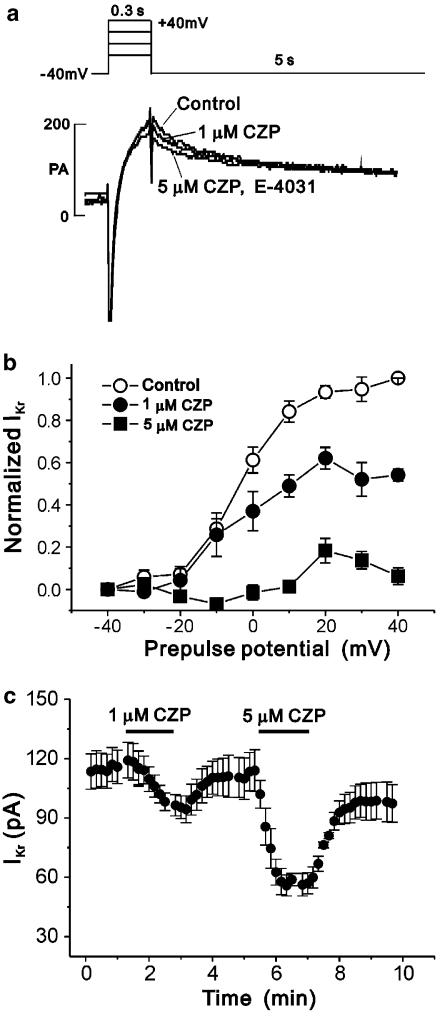
We then assayed the effects of clozapine on the activation curve of IKr, using a voltage protocol that required only short depolarization steps and allowed us to record the current–voltage relationship for IKr deactivating at −40 mV (Figure 8a, inset) (Sanguinetti & Jurkiewicz, 1990; Heath & Terrar, 1996). To prevent possible contamination by IKs, we treated each myocyte with 2 μM E-4031 after clozapine treatment, then used the E-4031-sensitive component for data analysis by subtracting the amplitude of the E-4031-insensitive tail current from that obtained in the absence or presence of clozapine (Figure 8a). Our results revealed that treatment with 1 μM clozapine significantly reduced IKr only at prepulses positive to 0 mV (n=5, Figure 8b), whereas treatment with 5 μM clozapine significantly inhibited the current at all prepulses (n=6). In addition, the degree of blockade increased with more positive voltages; treatment with 5 μM clozapine blocked IKr at −30 and +30 mV by 59.7±4.66 and 85.4±2.48%, respectively (n=6, P<0.05, Figure 8b). Consistent with our findings in the HERG channels, these results indicate that a higher degree of clozapine-induced blockade was present at more positive voltages. Also consistent with the HERG channel-based results, we found that 1 and 5 μM clozapine reduced the amplitude of IKr in a concentration-dependent manner (n=4; Figure 8c).
Figure 8.
Effect of clozapine (CZP) on the IKr of guinea-pig ventricular myocytes. (a) Superimposed recordings showing decay of IKr tail currents in the absence and presence of 1 or 5 μM CZP and 2 μM E-4031 at pre-pulses of +40 mV. (b) Activation curves for IKr measured as E-4031-sensitive tail currents at −40 mV before and after exposure to 1 or 5 μM CZP. IKr normalized to that following a depolarizing pre-pulse to +40 mV in the absence of the drug. Symbols with error bars represent means±s.e.m (n=5 and 6 for 1 μM and 5 μM CZP, respectively). (c) IKr tail currents measured every 10 s are plotted as a function of time (min) showing the dose-dependent effect of CZP. Symbols with error bars represent means±s.e.m (n=4).
Discussion
We herein show that clozapine inhibits HERG channels. The IC50 values of clozapine were 22.9 μM (at +40 mV) for HERG channels heterologously expressed in Xenopus oocytes and 2.5 μM for HERG channels stably expressed in HEK293 cells. Furthermore, 5 μM clozapine inhibited the IKr of guinea-pig ventricular myocytes by 80%. The higher extracellular concentrations of drug required to block HERG channels in the Xenopus oocyte expression system versus HEK293 cells are consistent with previous findings that higher drug concentrations are required for efficacy in bath applications to Xenopus oocytes. For example, blockade of HERG by dofetilide (Kiehn et al., 1996) or the antiarrhythmic drug, BRL-32872 (Thomas et al., 2001), showed 10- to 20-fold higher IC50 values when the drug was applied to the bath compared with its application to the internal membrane surface in inside-out patches of Xenopus oocytes. This may be caused by a reduction of the effective drug concentration at the cell membrane, due to the presence of the vitelline membrane and egg yolk.
The therapeutic plasma concentrations of clozapine typically range from 200 to 400 ng ml−1 (0.61–1.22 μM) (Perry et al., 1991); this corresponds to cardiac concentrations ranging from 1.35 to 2.69 μM, because the average clozapine tissue-to-plasma ratio for myocardium is about 2.2 (Titier et al., 2004). Therapeutic doses of clozapine have been associated with QTc prolongation (Tanner & Culling, 2003) and sudden death (Killian et al., 1999; Modai et al., 2000). Plasma levels of up to 10 μM have been reported in cases of clozapine toxicity (Broich et al., 1998). However, it is not clear whether some or all of the cases of sudden death reported to date were arrhythmia-related, because fatal myocarditis and cardiomyopathy have been associated with clozapine (Killian et al., 1999) and one of the clinical cases displayed pulmonary embolism upon autopsy (Modai et al., 2000). Clozapine is primarily metabolized by CYP3A4 into pharmacologically inactive demethylated, hydroxylated, and N-oxide derivatives. The normal elimination half-life of clozapine is ∼12 h (Ereshefsky, 1996), but this interval may be prolonged in patients with hepatic disease and renal insufficiency (Buckley & Sanders, 2000). Our present results revealed that the IC50 for clozapine-induced blockade of HERG channels was 2.5 μM in HEK293 cells, and that the IC50 for IKr was ∼1 μM in mammalian cardiomyocytes. These values are similar to the serum concentration of clozapine under normal conditions (Broich et al., 1998; Titier et al., 2004). Considering that therapeutic doses of clozapine have been associated with QTc prolongation (Tanner & Culling, 2003), our findings suggest that serological levels of clozapine may be capable of inhibiting HERG currents, perhaps causing QTc prolongation.
Other drugs that cause acquired LQT and torsades de pointes have been shown to voltage-dependently block HERG channels, suggesting that these drugs bind to the open or inactivated states of HERG channels. For example, the antipsychotic drug, haloperidol (Suessbrich et al., 1997), and two histamine receptor antagonists, terfenadine and astemizole (Suessbrich et al., 1996), were found to preferentially bind to the inactivated state of HERG, whereas the gastrointestinal prokinetic agent, cisapride, was shown to block the open state of HERG (Rampe et al., 1997). Our present results revealed that clozapine decreased the amplitudes of the maximum outward current and the maximum peak tail current. Furthermore, the magnitude of the blockade increased at more positive voltages, which are known to increase the open probability and enhance inactivation. Considering that the V1/2 values of the activation curves did not differ significantly in the absence and presence of 1–100 μM clozapine, our findings suggest that clozapine blocks activated state HERG channels without changing their activation gating. The fraction of block began at a very low level and increased with the duration of the voltage step, suggesting that the channel was not blocked in the resting state at hyperpolarized potentials, but rather became blocked during the depolarization-induced opening step. The voltage- and time-dependent nature of the clozapine-induced blockade indicates that the drug preferentially blocks HERG channels in either the open or inactivated states, not the closed state. However, the voltage-dependent block of clozapine could be interpreted in another way. As the clozapine-induced HERG blockade increased continuously even at highly positive potentials, where the open probability was high and showed minimal change with increasing potential, the data could represent an additional voltage-dependent blockade independent from the saturating block caused by the increase of open probability (Figure 3). Clozapine is positively charged at physiological pH values (pK=7.6), so the further increase of blockade at highly positive potentials might be explained by the induction of heightened outward driving forces at the binding site, due to increasing positive intracellular potentials.
While the present work provides evidence that clozapine blocks HERG channels, the molecular mechanism of this blockade remains to be elucidated. A recent mutational study identified several amino acids of the HERG channel that are critical for binding most of the HERG channel blockers (Mitcheson et al., 2000a) except a rare case of fluvoxamine (Milnes et al., 2003). Two of these residues, Tyr-652 and Phe-656, are predicted to orient toward to the lumen of the pore, based on a homology model of HERG channel structure (Mitcheson et al., 2000a). Mutation of Tyr-652 to non-aromatic amino acids and mutation of Phe-656 to non-hydrophobic amino acids dramatically reduced the effects of the inhibitors (Fernandez et al., 2004), suggesting that a van der Waals hydrophobic interaction of Phe-656 and a cation-π interaction of Tyr-652 may be important for the function of the most HERG channel blockers. Future work will be required to determine whether mutants at Tyr-652 and/or Phe-656 show reduced affinity for clozapine, which might suggest that clozapine blocks the HERG current by sitting directly in the HERG channel pores.
Electrophysiologically, clozapine induces a number of changes in various action potential parameters. The drug decreases both the maximal upstroke velocity of the action potential and the depolarized resting membrane potential, potentially leading to slower conduction, prolonged QRS interval durations and eventual incessant wide QRS ventricular tachycardia (Adamantidis et al., 1994). In addition, the prolonged QT interval and subsequent torsades de pointes may arise from a mechanism involving clozapine-induced prolongation of final repolarization and EADs (Adamantidis et al., 1994). Indeed, a number of lines of evidence suggest that clozapine can modulate several different types of cardiac ion channels. Our present results revealed that clozapine inhibited E-4031-sensitive IKr currents and the expressed major component of IKr, HERG, but had no effect on IKs even at concentrations of 5 μM, suggesting that clozapine preferentially blocked the rapid, but not the slow, component of the delayed rectifier K+ current. Previous studies have suggested that clozapine-induced cardiac arrhythmias may also be associated with effects on inward rectifier K+ channels. For example, clozapine was found to block the overexpressed G-protein-activated inward rectifier K+ (GIRK1) channel (Kobayashi et al., 1998), which is present in heart and brain and shares high amino-acid homology with other members of the inward rectifier K+ channel family, including the GIRK subunits (Lesage et al., 1994). In addition, clozapine was shown to block voltage-sensitive Ca2+ channels in bovine adrenal chromaffin cells (Park et al., 2001) and induced decreases in the APD at 50% repolarization (APD50) of Purkinje fibers (Adamantidis et al., 1994), possibly via inhibition of Ca2+ channels. Interestingly, this prompts us to speculate that clozapine-induced inhibition of voltage-sensitive Ca2+ channels might counteract the potential arrhythmogenic effects of HERG channel blockade, perhaps explaining the relatively low incidence of arrhythmia during clozapine treatment. Future work will be required to determine the effect of clozapine on other cardiac ion channels, with an eye toward understanding the mechanisms underlying clozapine-induced electrophysiological changes in action potentials and ECGs.
Our results revealed that the clozapine-induced blockade of HERG and possibly IKr was more prominent at higher stimulation frequencies, which appears inconsistent with the reverse use-dependent repolarization lengthening induced in cardiac cells by other IKr blockers. Reverse use dependence has been implicated in the bradycardia-dependent proarrhythmic effects of various class III antiarrhythmic agents, as well as with various IKr blocking agents, including E-4031, dofetilide, sotalol, and WAY-123,398 (Hondeghem & Snyders, 1990). Therefore, it will be useful to determine whether clozapine prolongs the APD of cardiomyocytes in a frequency-dependent manner. The molecular mechanism underlying use dependence is not yet fully understood. Mitcheson et al. (2000b) demonstrated that drugs may be trapped in the HERG channel pore upon closure of the activation gate, whereas Kiehn et al. (1999) suggested that positive frequency dependence may indicate an accumulation of blockade resulting from slow disassociation properties in the open channel state.
Recently, Abbott et al. (1999) suggested that the native IKr could be reconstituted by co-expression of the regulatory β-subunit MiRP with HERG, but Weerapura et al. (2002) showed that co-expression of HERG with wild-type MiRP1 (in contrast to the long QT syndrome-linked mutant MiRP1 used by Abbott et al., 1999) does not alter its sensitivity to HERG-blocking drugs. These findings are consistent with a report showing that the effects of terfenadine and fexofenadine were not altered by co-expression with MiRP1 (Scherer et al., 2002). In addition, Kamiya et al. (2001) did not detect significantly different IC50 values when HERG channel inhibition by vesnarinone was compared in the presence and absence of MiRP1. Finally, Weerapura et al. (2002) questioned the contention that co-expression HERG and MiRP1 is required to reassemble native IKr, as they did not find that co-expression of HERG and MiRP1 yielded any native IKr properties not seen with expression of HERG alone.
In terms of cardiac toxicity, clozapine has been shown to cause autonomic dysfunction with increased sympathetic and decreased parasympathetic tone, as reflected by significantly higher heart rate and lower heart rate variability, but this is not related to cardiac arrhythmia (Cohen et al., 2001). Our findings suggest that clozapine directly inhibits HERG channels and IKr, possibly resulting in the prolongation of APD and cardiac arrhythmias. However, it is also possible that clozapine induces cardiac arrhythmias by indirectly inhibiting HERG channels via drug-induced acidosis. Kang et al. (2000) reported that clozapine-treated patients had false-positive ischemic patterns on ECG, and it has been suggested that acidosis under ischemic conditions may cause cardiac arrhythmias (Orchard & Cingolani, 1994), possibly by H+-induced suppression of HERG current (Jo et al., 1999). Thus, future work will be required to assess whether clozapine-induced acidosis mediates or contributes to the clozapine-induced HERG blockade identified herein.
In summary, our present findings show for the first time that the atypical antipsychotic drug, clozapine, blocks HERG channels and the IKr but not IKs of guinea-pig cardiomyocytes at near physiological concentrations, suggesting that the drug-induced arrhythmias observed in clozapine-treated psychiatric patients may be due, at least in part, to inhibition of IKr.
Acknowledgments
This work was supported by a Korea Research Foundation Grant funded by the Korean Government (MOEHRD) (R04-2003-000-10007-0) and by a grant (2004-307) from the Asan Institute for Life Sciences, Seoul, Korea.
Abbreviations
- HERG
human ether-a-go-go-related gene
- IHERG
current at the end of the voltage step
- IKr
rapidly activating delayed rectifier K+ current
- Itail
tail current
- LQT
long QT syndrome
- V1/2
the potential required for half-maximal activation
References
- ABBOTT G.W., SESTI F., SPLAWSKI I., BUCK M.E., LEHMANN M.H., TIMOTHY K.W., KEATING M.T., GOLDSTEIN S.A. MiRP1 forms IKr potassium channels with HERG and is associated with cardiac arrhythmia. Cell. 1999;97:175–187. doi: 10.1016/s0092-8674(00)80728-x. [DOI] [PubMed] [Google Scholar]
- ADAMANTIDIS M.M., KERRAM P., DUPUIS B.A. In vitro electrophysiological detection of iatrogenic arrhythmogenicity. Fundam. Clin. Pharmacol. 1994;8:391–407. doi: 10.1111/j.1472-8206.1994.tb00818.x. [DOI] [PubMed] [Google Scholar]
- BROICH K., HEINRICH S., MARNEROS A. Acute clozapine overdose: plasma concentration and outcome. Pharmacopsychiatry. 1998;31:149–151. doi: 10.1055/s-2007-979318. [DOI] [PubMed] [Google Scholar]
- BUCKLEY N.A., SANDERS P. Cardiovascular adverse effects of antipsychotic drugs. Drug Saf. 2000;23:215–228. [Google Scholar]
- CALDERONE V., TESTAI L., MARTINOTTI E., DEL TACCA M., BRESCHI M.C. Drug-induced block of cardiac HERG potassium channels and development of torsade de pointes arrhythmias: the case of antipsychotics. J. Pharm. Pharmacol. 2005;57:151–161. doi: 10.1211/0022357055272. [DOI] [PubMed] [Google Scholar]
- CHOI S.Y., KOH Y.S., JO S.H. Inhibition of human ether-a-go-go-related gene K+ channel and IKr of guinea pig cardiomyocytes by antipsychotic drug trifluoperazine. J. Pharmacol. Exp. Ther. 2005;313:888–895. doi: 10.1124/jpet.104.080853. [DOI] [PubMed] [Google Scholar]
- COHEN H., LOEWENTHAL U., MATAR M., KOTLER M. Association of autonomic dysfunction and clozapine. Heart rate variability and risk for sudden death in patients with schizophrenia on long-term psychotropic medication. Br. J. Psychiatry. 2001;179:167–171. doi: 10.1192/bjp.179.2.167. [DOI] [PubMed] [Google Scholar]
- CURRAN M.E., SPLAWSKI I., TIMOTHY K.W., VINCENT G.M., GREEN E.D., KEATING M.T. A molecular basis for cardiac arrhythmia: HERG mutations cause long QT syndrome. Cell. 1995;80:795–803. doi: 10.1016/0092-8674(95)90358-5. [DOI] [PubMed] [Google Scholar]
- ERESHEFSKY L. Pharmacokinetics and drug interactions: update for new antipsychotics. J. Clin. Psychiatry. 1996;57 Suppl 11:12–25. [PubMed] [Google Scholar]
- FERNANDEZ D., GHANTA A., KAUFFMAN G.W., SANGUINETTI M.C. Physicochemical features of the HERG channel drug binding site. J. Biol. Chem. 2004;279:10120–10127. doi: 10.1074/jbc.M310683200. [DOI] [PubMed] [Google Scholar]
- HEATH B.M., TERRAR D.A. Separation of the components of the delayed rectifier potassium current using selective blockers of IKr and IKs in guinea-pig isolated ventricular myocytes. Exp. Physiol. 1996;81:587–603. doi: 10.1113/expphysiol.1996.sp003961. [DOI] [PubMed] [Google Scholar]
- HONDEGHEM L.M., SNYDERS D.J. Class III antiarrhythmic agents have a lot of potential but a long way to go: reduced effectiveness and dangers of reverse use dependence. Circulation. 1990;81:686–690. doi: 10.1161/01.cir.81.2.686. [DOI] [PubMed] [Google Scholar]
- JO S.H., YOUM J.B., KIM I., LEE C.O., EARM Y.E., HO W.K. Blockade of HERG channels expressed in Xenopus oocytes by external H+ Pflugers Arch. 1999;438:23–29. doi: 10.1007/s004240050875. [DOI] [PubMed] [Google Scholar]
- KAMIYA K., MITCHESON J.S., YASUI K., KODAMA I., SANGUINETTI M.C. Open channel block of HERG K(+) channels by vesnarinone. Mol. Pharmacol. 2001;60:244–253. doi: 10.1124/mol.60.2.244. [DOI] [PubMed] [Google Scholar]
- KANG U.G., KWON J.S., AHN Y.M., CHUNG S.J., HA J.H., KOO Y.J., KIM Y.S. Electrocardiographic abnormalities in patients treated with clozapine. J. Clin. Psychiatry. 2000;61:441–446. doi: 10.4088/jcp.v61n0609. [DOI] [PubMed] [Google Scholar]
- KIEHN J., LACERDA A.E., WIBLE B., BROWN A.M. Molecular physiology and pharmacology of HERG. Single-channel currents and block by dofetilide. Circulation. 1996;94:2572–2579. doi: 10.1161/01.cir.94.10.2572. [DOI] [PubMed] [Google Scholar]
- KIEHN J., THOMAS D., KARLE CA SCHOLS W., KUBLER W. Inhibitory effects of the class III antiarrhythmic drug amiodarone on cloned HERG potassium channels. Naunyn Schmiedebergs Arch. Pharmacol. 1999;359:212–219. doi: 10.1007/pl00005344. [DOI] [PubMed] [Google Scholar]
- KILLIAN J.G., KERR K., LAWRENCE C., CELERMAJER D.S. Myocarditis and cardiomyopathy associated with clozapine. Lancet. 1999;354:1841–1845. doi: 10.1016/s0140-6736(99)10385-4. [DOI] [PubMed] [Google Scholar]
- KOBAYASHI T., IKEDA K., KUMANISHI T. Effects of clozapine on the delta- and kappa-opioid receptors and the G-protein-activated K+ (GIRK) channel expressed in Xenopus oocytes. Br. J. Pharmacol. 1998;123:421–426. doi: 10.1038/sj.bjp.0701621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LESAGE F., DUPRAT F., FINK M., GUILLEMARE E., COPPOLA T., LAZDUNSKI M., HUGNOT J.P. Cloning provides evidence for a family of inward rectifier and G-protein coupled K+ channels in the brain. FEBS Lett. 1994;353:37–42. doi: 10.1016/0014-5793(94)01007-2. [DOI] [PubMed] [Google Scholar]
- MILNES J.T., CROCIANI O., ARCANGELI A., HANCOX J.C., WITCHEL H.J. Blockade of HERG potassium currents by fluvoxamine: incomplete attenuation by S6 mutations at F656 or Y652. Br. J. Pharmacol. 2003;139:887–898. doi: 10.1038/sj.bjp.0705335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MITCHESON J.S., CHEN J., LIN M., CULBERSON C., SANGUINETTI M.C. A structural basis for drug-induced long QT syndrome. Proc. Natl. Acad. Sci. U.S.A. 2000a;97:12329–12333. doi: 10.1073/pnas.210244497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MITCHESON J.S., CHEN J., SANGUINETTI M.C. Trapping of a methanesulfonanilide by closure of the HERG potassium channel activation gate. J. Gen. Physiol. 2000b;115:229–240. doi: 10.1085/jgp.115.3.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MODAI I., HIRSCHMANN S., RAVA A., KURS R., BARAK P., LICHTENBERG P., RITSNER M. Sudden death in patients receiving clozapine treatment: a preliminary investigation. J. Clin. Psychopharmacol. 2000;20:325–327. doi: 10.1097/00004714-200006000-00006. [DOI] [PubMed] [Google Scholar]
- ORCHARD C.H., CINGOLANI H.E. Acidosis and arrhythmias in cardiac muscle. Cardiovasc. Res. 1994;28:1312–1319. doi: 10.1093/cvr/28.9.1312. [DOI] [PubMed] [Google Scholar]
- PARK T., BAE S., CHOI S., KANG B., KIM K. Inhibition of nicotinic acetylcholine receptors and calcium channels by clozapine in bovine adrenal chromaffin cells. Biochem. Pharmacol. 2001;61:1011–1019. doi: 10.1016/s0006-2952(01)00577-9. [DOI] [PubMed] [Google Scholar]
- PERRY P.J., MILLER D.D., ARNDT S.V., CADORET R.J. Clozapine and norclozapine plasma concentrations and clinical response of treatment-refractory schizophrenic patients. Am. J. Psychiatry. 1991;148:231–235. doi: 10.1176/ajp.148.2.231. [DOI] [PubMed] [Google Scholar]
- RAMPE D., ROY M.L., DENNIS A., BROWN A.M. A mechanism for the proarrhythmic effects of cisapride (Propulsid): high affinity blockade of the human cardiac potassium channel HERG. FEBS Lett. 1997;417:28–32. doi: 10.1016/s0014-5793(97)01249-0. [DOI] [PubMed] [Google Scholar]
- SANGUINETTI M.C., JIANG C., CURRAN M.E., KEATING M.T. A mechanistic link between an inherited and an acquired cardiac arrhythmia: HERG encodes the IKr potassium channel. Cell. 1995;81:299–307. doi: 10.1016/0092-8674(95)90340-2. [DOI] [PubMed] [Google Scholar]
- SANGUINETTI M.C., JURKIEWICZ N.K. Two components of cardiac delayed rectifier K+ current. Differential sensitivity to block by class III antiarrhythmic agents. J. Gen. Physiol. 1990;96:195–215. doi: 10.1085/jgp.96.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHERER C.R., LERCHE C., DECHER N., DENNIS A.T., MAIER P., FICKER E., BUSH A.E., WOLLNIK B., STEINMEYER K. The antihistamine fexofenadine does not affect I(Kr) currents in a case report of drug-induced cardiac arrhythmia. Br. J. Pharmacol. 2002;137:892–900. doi: 10.1038/sj.bjp.0704873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SNYDERS J., KNOTH K.M., ROBERDS S.L., Tamkun M.M. Time-, voltage-, and state-dependent block by quinidine of a cloned human cardiac potassium channel. Mol. Pharmacol. 1992;41:322–330. [PubMed] [Google Scholar]
- SUESSBRICH H., SCHONHERR R., HEINEMANN S.H., ATTALI B., LANG F., BUSCH A.E. The inhibitory effect of the antipsychotic drug haloperidol on HERG potassium channels expressed in Xenopus oocytes. Br. J. Pharmacol. 1997;120:968–974. doi: 10.1038/sj.bjp.0700989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUESSBRICH H., WALDEGGER S., LANG F., BUSCH A.E. Blockade of HERG channels expressed in Xenopus oocytes by the histamine receptor antagonists terfenadine and astemizole. FEBS Lett. 1996;385:77–80. doi: 10.1016/0014-5793(96)00355-9. [DOI] [PubMed] [Google Scholar]
- TAGLIALATELA M., CASTALDO P., PANNACCIONE A., GIORGIO G., ANNUNZIATO L. Human ether-a-gogo related gene (HERG) K+ channels as pharmacological targets: present and future implications. Biochem. Pharmacol. 1998;55:1741–1746. doi: 10.1016/s0006-2952(98)00002-1. [DOI] [PubMed] [Google Scholar]
- TANNER M.A., CULLING W. Clozapine associated dilated cardiomyopathy. Postgrad. Med. J. 2003;79:412–413. doi: 10.1136/pmj.79.933.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THOMAS D., WENDT-NORDAHL G., ROCKL K., FICKER E., BROWN A.M., KIEHN J. High-affinity blockade of human ether-a-go-go-related gene human cardiac potassium channels by the novel antiarrhythmic drug BRL-32872. J. Pharmacol. Exp. Ther. 2001;297:753–761. [PubMed] [Google Scholar]
- TITIER K., CANAL M., DERIDET E., ABOUELFATH A., GROMB S., MOLIMARD M., MOORE N. Determination of myocardium to plasma concentration ratios of five antipsychotic drugs: comparison with their ability to induce arrhythmia and sudden death in clinical practice. Toxicol. Appl. Pharmacol. 2004;199:52–60. doi: 10.1016/j.taap.2004.03.016. [DOI] [PubMed] [Google Scholar]
- VARMA S., ACHAN K. Dysrhythmia associated with clozapine. Aust. N. Z. J. Psychiatry. 1999;33:118–119. doi: 10.1080/0004867900025. [DOI] [PubMed] [Google Scholar]
- WEERAPURA M., NATTEL S., CHARTIER D., CABALLERO R., HEBERT T.E. A comparison of currents carried by HERG, with and without coexpression of MiRP1, and the native rapid delayed rectifier current. Is MiRP1 the missing link. J. Physiol. 2002;540:15–27. doi: 10.1113/jphysiol.2001.013296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHOU Z., GONG Q., YE B., FAN Z., MAKIELSKI J.C., ROBERTSON G.A., JANUARY C.T. Properties of HERG channels stably expressed in HEK 293 cells studied at physiological temperature. Biophys. J. 1998;74:230–241. doi: 10.1016/S0006-3495(98)77782-3. [DOI] [PMC free article] [PubMed] [Google Scholar]