Abstract
Since the 1960s, scientists and pharmaceutical representatives have called for the advancement and development of new antimicrobial drugs to combat infectious diseases. In January 2005, Senate Majority Leader Bill Frist (R-TN), MD, introduced a biopreparedness bill that included provisions for patent extensions and tax incentives to stimulate industry research on new antimicrobials.
Although government stimulus for private development of new antimicrobials is important, it does not resolve long-standing conflicts of interest between private entities and society. Rising rates of antimicrobial resistance have only exacerbated these conflicts. We used methicillin-resistant Staphylococcus aureus as a case study for reviewing these problems, and we have suggested alternative approaches that may halt the vicious cycle of resistance and obsolescence generated by the current model of antimicrobial production.
THE DEVELOPMENT AND USE of antimicrobials exemplify a classic problem in public health ethics: the conflict between the private interests of individual entities (patients, physicians, and corporations) and the public interest of society at large. In the United States, the commodification of antimicrobials exacerbates this conflict. Antimicrobials are produced and distributed by private interests (pharmaceutical and biotechnology companies) for the purpose of generating profit. They are consumed by individuals who have the financial means to purchase them and they are developed and allocated on the basis of market criteria (generation of profit and ability to pay) rather than on the basis of benefit to the public at large.
An increase in antimicrobial resistance intensifies the problems generated by these conflicts of interest. We used methicillin-resistant Staphylococcus aureus (MRSA) as a case study for examining these problems. We focus on the vicious cycle of resistance and obsolescence generated by the current use of antimicrobial drugs and propose some alternative approaches to future antimicrobial development.
EMERGENCE OF RESISTANCE
Staphylococcus aureus (S aureus) is a gram-positive bacteria that can either transiently or persistently colonize the anterior nares within the nasal cavity. S aureus colonization is present in 30% to 50% of adults.1 Life-threatening infections, such as bacteremia and pneumonia, may occur when mucosal or cutaneous barriers are breached and thereby provide an opportunity for S aureus to spread and infect various sites within the body.1
Shortly after the widespread use of penicillin that followed World War II, penicillin-resistant strains of S aureus began to emerge in hospitals. By 1969, penicillin-resistant S aureus had been identified among individuals who had not been hospitalized.2 Today, more than 90% of S aureus strains are resistant to penicillin and other penicillin-related antibiotics.3 Methicillin-resistant S aureus (MRSA) emerged only 1 year after methicillin was introduced, and it now causes about 50% of all nosocomial infections.4 The recent emergence of vancomycin intermediate–resistant S aureus and vancomycin/methicillin resistant S aureus is especially worrisome because there are few alternative antibiotic therapies for treating these multidrug-resistant infections.5–7
The emergence of community-acquired MRSA (CMRSA) has been identified among otherwise healthy individuals who do not have traditional risk factors for carriage of antibiotic-resistant organisms.8–10 CMRSA strains differ in antimicrobial susceptibility compared with hospital-acquired MRSA, because CMRSA strains often lack resistance to several various non-ß-lactam antibiotics, a feature most likely attributable to a lower frequency of antibiotic-selective pressures in the community compared with the hospital environment.2,11 However, with the migration of CMRSA strains into hospital settings and movement of these strains back out to the community, an agile CMRSA strain with multidrug resistance traits may emerge.12 Many of the CMRSA strains carry the Panton-Valentine leukocydin toxin gene, which has been correlated with more aggressive and potentially fatal CMRSA infections.13–15
Despite the continuing emergence of resistant pathogens, development of new classes of antimicrobials has been at a virtual standstill since the late 1970s (Figure 1 ▶).16,17 Only 2 new classes of antibiotics have been introduced during the past 24 years. There are already concerns about resistance to 2 recently introduced antibiotics in the lipopeptide and oxazolidinone classes. Several reports have described cases of clinically significant resistance to daptomycin (a lipopeptide) for the treatment of MRSA infections,18–20 and there are currently 7 case reports of resistance to linezolid (an oxazolidinone) among individuals with S aureus infections.21 In 2002, there were 506 new molecular entities in the research and development pipeline at the 15 major pharmaceutical companies; however, only 6 were antibacterial agents.16
FIGURE 1—
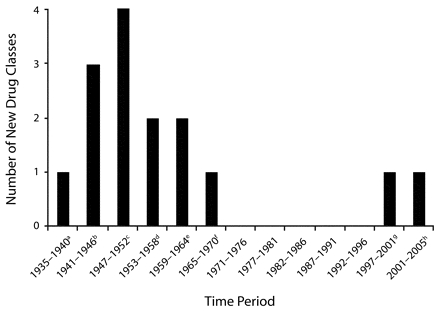
Number of new drug classes introduced between 1935 and 2005
a Sulfanomides.
b Penicillins, aminoglycosides, cephalosporins.
c Chloramphenicol, tetracyclines, macrolides, lincosamides, streptograms.
d Glycopeptides, rifamycins.
e Nitroimidiazoles, quinolones.
f Trimethoprim.
gOxazolidinones
hLipopeptides.
Development of Antimicrobials
Why is there so little private development of new antibiotics despite the pressing public need for them? One reason is that the private and public sectors view the balance between risks and incentives very differently. From the private sector’s point of view, the potential risks involved in the development of antimicrobials clearly outweigh the incentives. Conversely, the public sector views the incentives as clearly outweighing the risks.
The primary incentive for private development of new anti-microbials is the existence of a substantial market. The temporal increase in the incidence of infections such as MRSA within hospitals and the emergence of CMRSA suggest that a market for new treatments exists, and that market may increase over time. An additional incentive for pharmaceutical companies is the good public relations that accompany the development of necessary treatments for infections such as MRSA, especially during a time when the companies have been criticized for high prices and heavy investments in so-called lifestyle drugs.17,22
The development of new antimicrobials also entails significant risks. The expense is considerable—from $100 million to $800 million17—and private producers of antimicrobials are exposed to liability claims for adverse events. When successful in treating infections, antimicrobials are used for very short periods and are used relatively infrequently compared with medications for chronic conditions. For example, the clinical course of a MRSA infection is characterized by a limited period of infection, therapy, and recovery or death.
An additional disincentive results from the increasing pressure to shelve available antibiotics to salvage their applicability for treatment of resistant infections, which was the case for vancomycin before the emergence of CMRSA.17 Finally, new antibiotics run the risk of rapid obsolescence, because use leads to greater resistance and the need for new drugs. MRSA in particular has exhibited a “stealth” ability to quickly adapt and acquire new antibiotic resistance traits.23 The more rapidly pathogens develop resistance and thus render new drugs ineffective, the smaller the potential market for those drugs. By contrast, drugs for chronic conditions (e.g., hypercholesterolemia, hypertension, and arthritis) can be marketed to a large segment of the population and will not be shelved; consumers can use them for years or decades, and they rarely become ineffective as a consequence of repeated use.
From the perspective of the public interest, there are 2 major incentives for developing new antimicrobials. First, they may aid in the mitigation or prevention of future epidemics of emerging infectious diseases. Second, novel antimicrobials may reduce mortality and morbidity associated with extant pathogens such as MRSA. This in turn would lead to a reduction in future hospitalizations, health care expenditures associated with resistance, and costs associated with lost productivity due to disability or death. The primary risks are the costs associated with developing and distributing new drugs and potential adverse events.
Consumption of Antimicrobials
Important conflicts of interest also exist between the individual producers and consumers of antimicrobials and the public interest. Use of antimicrobials by individual patients creates externalities—costs or benefits that accrue to someone other than the individual consumer. The costs of excessive or incorrect use of antimicrobials for the treatment of S aureus infections (e.g., the development of multidrug resistance) accrue not only to the individual patient but also to those who may be infected by resultant resistant strains. Similarly, limiting the use of antimicrobials in a particular patient may not benefit that patient, but it does benefit society at large by preventing the development of resistant strains.
Patients seldom take into account the negative externalities that accompany the use of antimicrobials, and most either do not know or do not consider the ecological impacts of resistance for themselves and others. Physicians may be more aware of the social costs of antimicrobial use, but they must weigh the social benefit of discouraging use against their own interest in serving the wishes of their patients.
Private producers of antimicrobials have an incentive to encourage consumption of antimicrobials—particularly as the expiration of their patents approach—to maximize profits.24 By contrast, it is in the public’s interest to limit consumption of available antimicrobials and to preserve new drugs and classes of drugs as long as possible in order to fight multidrug-resistant infections such as MRSA.25
Looking Ahead
When he commented on the development of new drugs 40 years ago, Harry F. Dowling, observed that “Americans are interested in preserving the wholesome principle of competition that is inherent in their system, and at the same time they want to be certain that the [pharmaceutical] companies will compete with the kinds of drugs and with methods that are beneficial to the public.”26(p75) We face similar ethical dilemmas today that are exacerbated by the growing problem of antimicrobial resistance. Do we value the ability of private entities to develop (or fail to develop) and use antimicrobials in accordance with their own interests more highly than we value the benefit that the public would derive from their development and judicious use? Should market forces, public interest, or some combination of the 2 govern the production and consumption of antimicrobials? Who should pay?
Within this framework, 3 approaches to the problem of antimicrobial resistance are evident.
Commodification.
Antimicrobials are fully commodified, and their development, production, and consumption are entrusted to the private sector and are governed primarily by market forces. Under this system, development of new antibiotics clearly entails greater risks than benefits for the private sector, including limiting their consumption once developed. We do not envision this balance of risks and benefits changing in the near future; thus, we believe that this approach will continue to inadequately address antimicrobial resistance.
Public good.
An alternative approach would treat antimicrobials as public goods, and their development, production, and consumption would be governed solely in accordance with the needs of the public. The state—which already pays for a great deal of basic drug development research—could take over the development and production of antimicrobials directly. Alternatively, the state could adopt a defense contractor model and underwrite the development of particular drugs by private industry through short-term agreements. If sufficient funds are made available, and decisions about the development of new antimicrobials are made on the basis of sound scientific judgments about the risks and benefits to the public, this approach could effectively address antimicrobial resistance.
Partnership.
A third approach is a compromise between the first 2 that would allow the private and public sectors to share the risks and benefits associated with antimicrobial drug development. For example, the public sector could provide additional incentives (market exclusivity, patent extension, tax incentives, and expedient Food and Drug Administration approval times) and mitigation of risks (indemnification against liability and guaranteed markets) to the private sector in return for the successful development of new drugs.27 Precedents for this form of partnership include the 1983 Orphan Drug Act, which extended tax credits and guaranteed 7 years of market exclusivity to developers of drugs for rare conditions. During the 109th Congress, several bills that include some form of public–private partnership for antibiotic development were introduced under the guise of homeland security.28 These bills were still pending.
The first approach has so far failed to address antimicrobial resistance, and the second appears to be unfeasible in the current political climate. As such, several recent reports have recommended adopting the third approach by building on similar public–private partnerships that have been implemented for vaccines and orphan drugs.17 However, while this approach might be appropriate in the short term, it does not resolve the basic conflict of interest between private producers and consumers of pharmaceuticals and society at large. In the long term, we believe that only the second option will suffice, i.e., antimicrobials should be treated as a public good, and their production and consumption should be governed solely by the public interest.
We also believe that new drugs are not the only answer. Although an emerging consensus from the research and industry side has called for technological solutions—including use of genomics and new bacterial molecular targets—and more rapid techniques for diagnosing resistant infections, these techniques have thus far shown minimal yield in identifying novel treatments and drug targets. Even if they are effective, these new technologies will not interrupt the vicious cycle of resistance and obsolescence.
Instead, we recommend a renewed emphasis on basic public health prevention regarding infection control and management. This approach includes (1) the consistent application of hand hygiene and environmental infection control guidelines in clinical settings and within other high-risk environments in the community, (2) more rigorous implementation of strategies for the rational use of antimicrobials in clinical and community settings, and (3) development and use of alternative therapies (e.g., vaccinations, probiotics, and phytomedicines) for prevention of bacterial infections. The first 2 points have been advocated by the Centers for Disease Control and Prevention as part of the Get Smart: Know When Antibiotics Work campaign29 and by the Alliance for the Prudent Use of Antibiotics.30 Yet, fostering rational use of antibiotics and promoting adherence to proper hand hygiene regimens in clinical and community settings continues to be a challenging public health dilemma.31,32 The last of our 3 proposed potential prevention measures—alternative therapies—is rarely discussed within the context of antimicrobial development,16,33–36 but it may provide an important avenue for interrupting the cycle of resistance and obsolescence associated with new antimicrobial use.
CONCLUSIONS
In an article published 40 years ago, scientists and pharmaceutical industry representatives concluded that technological advancement and development of new drugs were required for overcoming the issue of resistance.37 Since then, only 3 new classes of antibiotics have been introduced, and research on new antimicrobials has dwindled. Nevertheless, currently proposed solutions continue to advocate further research and market-based solutions for the development of antimicrobials.16,33–36
These downstream approaches do not address the fundamental causes of antibiotic resistance within clinical and community settings, and they do not interrupt the vicious cycle of obsolescence and resistance that market-based solutions encourage. To address the growing problem of antimicrobial resistance, we must implement public health prevention approaches immediately, and we must consider long-term solutions that do not rely on market forces to govern the production and consumption of antimicrobials.
Acknowledgments
A. E. Aiello and N. B. King received funding from The Robert Wood Johnson Health and Society Scholars Program (grant 045823).
Human Participant Protection No protocol approval was needed for this study.
Peer Reviewed
Contributors A. E. Aiello and N. B. King led the writing, and all authors originated ideas and reviewed drafts of the article.
References
- 1.Lowy FD. Antimicrobial resistance: the example of Staphylococcus aureus. J Clin Invest. 2003;111:1265–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chambers HF. The changing epidemiology of Staphylococcus aureus? Emerg Infect Dis. 2001;7:178–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Institute of Medicine. Antimicrobial Resistance: Issues and Options. Workshop Report. Washington DC: Forum on Emerging Infections, Division of Health Sciences Policy; 1998. [PubMed]
- 4.National Nosocomial Infections Surveillance (NNIS). National Nosocomial Infections Surveillance (NNIS) system report, data summary from January 1990–May 1999, issued June 1999. Am J Infect Control. 1999;27:520–532. [DOI] [PubMed] [Google Scholar]
- 5.Vancomycin-resistant Staphylococcus aureus–New York, 2004. MMWR Morb Mortal Wkly Rep. 2004;53:322–323. [PubMed] [Google Scholar]
- 6.Staphylococcus aureus resistant to vancomycin–United States, 2002. MMWR Morb Mortal Wkly Rep. 2002; 51:565–567. [PubMed] [Google Scholar]
- 7.Staphylococcus aureus with reduced susceptibility to vancomycin–United States, 1997. MMWR Morb Mortal Wkly Rep. 1997;46:765–766. [PubMed] [Google Scholar]
- 8.Adcock PM, Pastor P, Medley F, Patterson JE, Murphy TV. Methicillin-resistant Staphylococcus aureus in two child care centers. J Infect Dis. 1998; 178:577–580. [DOI] [PubMed] [Google Scholar]
- 9.Herold BC, Immergluck LC, Maranan MC, et al. Community-acquired methicillin-resistant Staphylococcus aureus in children with no identified predisposing risk. JAMA. 1998; 279:593–598. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. Summary of notifiable diseases—United States, 1998. MMWR Morb Mortal Wkly Rep. 1999;47:ii–92. [PubMed] [Google Scholar]
- 11.Suggs AH, Maranan MC, Boyle-Vavra S, Daum RS. Methicillin-resistant and borderline methicillin-resistant asymptomatic Staphylococcus aureus colonization in children without identifiable risk factors. Pediatr Infect Dis J. 1999;18:410–414. [DOI] [PubMed] [Google Scholar]
- 12.Carleton HA, Diep BA, Charlebois ED, Sensabaugh GF, Perdreau-Remington F. Community-adapted methicillin-resistant Staphylococcus aureus (MRSA): population dynamics of an expanding community reservoir of MRSA. J Infect Dis. 2004;190: 1730–1738. [DOI] [PubMed] [Google Scholar]
- 13.Wannet WJ, Spalburg E, Heck ME, et al. Emergence of virulent methicillin-resistant Staphylococcus aureus strains carrying Panton-Valentine leucocidin genes in the Netherlands. J Clin Microbiol. 2005;43:3341–3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diep BA, Sensabaugh GF, Somboona NS, Carleton HA, Perdreau-Remington F. Widespread skin and soft-tissue infections due to two methicillin-resistant Staphylococcus aureus strains harboring the genes for Panton-Valentine leucocidin. J Clin Microbiol. 2004;42: 2080–2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Said-Salim B, Mathema B, Braughton K, et al. Differential distribution and expression of Panton-Valentine leucocidin among community-acquired methicillin-resistant Staphylococcus aureus strains. J Clin Microbiol. 2005;43:3373–3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spellberg B, Powers JH, Brass EP, Miller LG, Edwards JE Jr. Trends in antimicrobial drug development: implications for the future. Clin Infect Dis. 2004;38:1279–1286. [DOI] [PubMed] [Google Scholar]
- 17.Infectious Disease Society of America (IDSA). Bad Bugs, No Drugs. As Antibiotic Discovery Stagnates. A Public Health Crisis Brews. Alexandria, Va: IDSA; 2004.
- 18.Hayden MK, Rezai K, Hayes RA, Lolans K, Quinn JP, Weinstein RA. Development of Daptomycin resistance in vivo in methicillin-resistant Staphylococcus aureus. J Clin Microbiol. 2005;43: 5285–5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vikram HR, Havill NL, Koeth LM, Boyce JM. Clinical progression of methicillin-resistant Staphylococcus aureus vertebral osteomyelitis associated with reduced susceptibility to daptomycin. J Clin Microbiol. 2005;43: 5384–5387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mangili A, Bica I, Snydman DR, Hamer DH. Daptomycin-resistant, methicillin-resistant Staphylococcus aureus bacteremia. Clin Infect Dis. 2005; 40:1058–1060. [DOI] [PubMed] [Google Scholar]
- 21.Peeters MJ, Sarria JC. Clinical characteristics of linezolid-resistant Staphylococcus aureus infections. Am J Med Sci. 2005;330:102–104. [DOI] [PubMed] [Google Scholar]
- 22.Angell M. The Truth About Drug Companies: How They Deceive Us and What to Do About It. New York, NY: Random House; 2004.
- 23.Hiramatsu K, Cui L, Kuroda M, Ito T. The emergence and evolution of methicillin-resistant Staphylococcus aureus. Trends Microbiol. 2001;9: 486–493. [DOI] [PubMed] [Google Scholar]
- 24.Horowitz JB, Moehring HB. How property rights and patents affect antibiotic resistance. Health Econ. 2004;13: 575–583. [DOI] [PubMed] [Google Scholar]
- 25.Levy SB. Antibiotic resistance: consequences of inaction. Clin Infect Dis. 2001;33(suppl 3):S124–S129. [DOI] [PubMed] [Google Scholar]
- 26.Dowling HF. The pharmaceutical industry and the doctor. What of the future? N Engl J Med. 1961;264:75–79. [DOI] [PubMed] [Google Scholar]
- 27.Trouiller P, Olliaro P, Torreele E, Orbinski J, Laing R, Ford N. Drug development for neglected diseases: a deficient market and a public-health policy failure. Lancet. 2002;359:2188–2194. [DOI] [PubMed] [Google Scholar]
- 28.Office of Legislative Policy and Analysis. Legislative updates. Available at: http://olpa.od.nih.gov/legislation/109/pendinglegislation/bioterror.asp. Accessed July 26, 2006.
- 29.Centers for Disease Control and Prevention. Get Smart: Know When Antibiotics Work. Available at: http://www.cdc.gov/drugresistance/community. Accessed November 15, 2005.
- 30.Alliance for the Prudent Use of Antibiotics. Home page. Available at: http://www.tufts.edu/med/apua. Accessed November 15, 2005.
- 31.O’Boyle CA, Henly SJ, Larson E. Understanding adherence to hand hygiene recommendations: the theory of planned behavior. Am J Infect Control. 2001;29:352–360. [DOI] [PubMed] [Google Scholar]
- 32.Nash DR, Harman J, Wald ER, Kelleher KJ. Antibiotic prescribing by primary care physicians for children with upper respiratory tract infections. Arch Pediatr Adolesc Med. 2002;156: 1114–1119. [DOI] [PubMed] [Google Scholar]
- 33.Projan SJ. Why is big Pharma getting out of antibacterial drug discovery? Curr Opin Microbiol. 2003;6:427–430. [DOI] [PubMed] [Google Scholar]
- 34.Shlaes DM. The abandonment of antibacterials: why and wherefore? Curr Opin Pharmacol. 2003;3:470–473. [DOI] [PubMed] [Google Scholar]
- 35.Norrby SR, Nord CE, Finch R. Lack of development of new antimicrobial drugs: a potential serious threat to public health. Lancet Infect Dis. 2005;5: 115–119. [DOI] [PubMed] [Google Scholar]
- 36.Wenzel RP. The antibiotic pipeline–challenges, costs, and values. N Engl J Med. 2004;351:523–526. [DOI] [PubMed] [Google Scholar]
- 37.Finland M, Kirby WM, Chabbert YA, et al. Round table: are new antibiotics needed? Antimicrobial Agents Chemother (Bethesda). 1965;5:1107–1114. [PubMed] [Google Scholar]


