Abstract
Objectives. We sought to quantify the impact of the 1998 US Food and Drug Administration (FDA) folic acid fortification policy by estimating folate intake at the population level.
Methods. We analyzed total folate intake levels (from food and supplements) according to gender, age, and race/ethnicity, using data from 2 National Health and Nutrition Examination Surveys. We measured pre- and postfortification folate intake distributions, adjusted for measurement error, and examined proportions of the population who reached certain thresholds of daily total folate intake.
Results. Mean daily food and total folate intake increased by approximately 100 μg/day after fortification. The proportion of women aged 15–44 years who consume more than 400 μg/day of folate has increased since fortification, but has not yet reached the FDA’s 50% target and varies by race/ethnicity from 23% to 33%. Among persons aged 65 years and older who may be at risk for masking a vita-μg/day (the min B12 deficiency, the percentage who consume more than 1000 “tolerable upper intake level”) has at least doubled among Whites and Black men, but has remained less than 5% for all groups.
Conclusions. Since fortification, folic acid intake among the US population has increased, and there are substantial variations by age, gender, and race/ethnicity.
Research in the early 1990s established that increases in prenatal intakes of folate or folic acid early in pregnancy can significantly reduce the risk of neural tube defects (NTDs) in newborns.1,2 NTDs are one of the most common birth defects in this country and affect approximately 2500 live births3 and 1500 terminated pregnancies4 each year. In an effort to reduce the number of NTDs, the US Food and Drug Administration (FDA) in 1998 implemented the most significant fortification policy since the 1940s, and mandated that all enriched grain products be fortified with folic acid.5 This policy was expected to add approximately 100 μg of folic acid per day to the diet of the average American. In addition, the percentage of women of childbearing age who consumed at least 400 μg/day of the vitamin was expected to increase from 29% to 50%.5–10
In addition to reducing the risk of NTDs, folic acid fortification has the potential to decrease risks of heart disease and colon cancer.11–22 However, excessive intake may mask anemia that results from a vitamin B12 deficiency, which could delay diagnosis and treatment in elderly persons. Masking can facilitate the progression of irreversible neurological manifestations of the deficiency, such as numbness, balance problems, weakness, and paralysis.23 The FDA currently recommends that adults do not consume more than 1000 μg of folate per day, an amount defined as a “tolerable upper intake level.”24
Studies have estimated the effects of the FDA fortification policy at a population level, and showed that the average age-specific total folate intake has increased by approximately 100 μg/day for both adult men and women from the early 1990s to the end of the decade.25–37 However, these analyses did not correct for the fact that the dietary intake data represents what the respondents ate in a single day but is not necessarily representative of an average day of the respondents’ dietary intake. Without such correction, it is not possible to accurately estimate the number of women who reach the FDA’s 400 μg/ day threshold. In addition, no study has quantified national and population-based intakes by age, gender, and racial/ethnic subgroups.38 Our analysis provides national, population-based estimates of folate consumption levels by age, gender, and racial/ethnic subgroups and accounts for food and supplement intake, corrected for measurement error because of within-person variation.
METHODS
Data
We analyzed food and dietary supplement data from 2 periods of the National Health and Nutrition Examination Surveys (NHANES III, 1988–1994; NHANES 1999–2000). NHANES are surveys conducted by the National Center for Health Statistics of the Centers for Disease Control and Prevention and are designed to monitor trends in risk behaviors, environmental exposures, diet, nutrition, and health. Data are collected from personal interviews and physical health exams. Nutrient intake data are based primarily on one 24-hour dietary recall measure from the interview component. Data on supplement use are collected during the physical examination component and entail detailed information on specific types and amounts of supplement use over the previous month. Nutrient intake values are calculated by coding the survey data with the US Department of Agriculture’s Survey Nutrient Database, which incorporates folate values and other nutrients that have been added by fortification.
We used data from NHANES III (1988–1994) to represent prefortification folate intake levels and the 1999–2000 data release of the current NHANES for postfortification estimates. We assessed folate intake levels for men and women aged 15 years and older, to incorporate women of childbearing age as defined by the Centers for Disease Control and Prevention (aged 15–44 years)4,39–41 who may be at risk for a NTD-affected pregnancy, as well as adults who may be at risk for colon cancer, coronary heart disease, and B12 deficiency. Age was categorized into 3 groups: 15–44 years, 45–64 years, and 65 years and older. Only subjects who had complete data for both food folate and supplemental folic acid intake levels were included in our analysis, which excluded 19% of subjects for NHANES III and 16% of subjects for NHANES 1999–2000. We included non-Hispanic Whites—hereafter “Whites”—(N = 11332, NHANES III; 2062, NHANES 1999–2000) and non-Hispanic Blacks—hereafter “Blacks”—(N = 4908, NHANES III; 1114, NHANES 1999–2000) as well as Mexican Americans (N = 554, NHANES III; 1655, NHANES 1999–2000); other racial/ ethnic groups were of insufficient sample size. All analyses used sampling weights to adjust for survey design.
Analysis Overview
We estimated population distributions of total folate consumption (from food and supplements) by grouping intake levels into categories of 100 μg/day. For each age, gender, and race/ethnicity, we estimated intake frequencies and medians, the percentage of the population taking folic acid–containing supplements, and the proportions of the population taking folate at levels above 200 μg/day, 400 μg/day, and 1000 μg/day. All analyses were performed using SAS version 9.1.3 (SAS Institute Inc, Cary, NC) unless otherwise noted.
Dietary Data
Because a 24-hour recall measure for nutrient intake does not represent an individual’s average long-term daily intake, population distribution estimates of dietary folate intake (folate consumption from all food sources, including fortified foods) will be distorted by measurement error.42 The result is an exaggerated standard deviation caused by overestimating the number of observations in the distribution tails. Such error causes particular problems when estimating the proportions of a population above or below certain thresholds, as was done in our analysis.
We adjusted for measurement error using a subsample of NHANES III subjects who had provided 2 separate 24-hour recalls. There were 1271 observations (7.5%) in the sub-sample who had complete food folate data for both 1-day measures. Second 24-hour recalls were provided at least 1 week after initial measures for over 96% of the sample, and at least 5 days after initial measure for over 99% of the sample.
To correct for measurement error, we log-transformed all food folate data and conducted visual tests of normality (histograms, straight normal probability plots, and comparable means and medians) to verify the normality assumption. We used results from 1-way analyses of variance to estimate the within-subject and between-subject variances for the log-transformed 2-measure sample. We calculated the “true” between-subject variance by assuming that the ratio of within-subject to between-subject variances is the same for the subjects with only 1 day of data as it is for those with 2 days of data, and to thereby remove the effects of the day-to-day variation in intake. This “true” between-person variance was then used to obtain the corrected food folate intake frequency distributions.
We used gender-, age-, and race-specific within-subject variances for the 2-measure, between-subject variance estimates among Whites and Blacks, and applied gender- and age-specific variances from combined racial/ ethnic groups (Whites, Blacks, and Mexican Americans) to the Mexican American subgroup because of small sample sizes of Mexi-can Americans with two 24 recalls. Because of restricted availability of the 2-measure data for NHANES 1999–2000, we used the same within-person variability estimates from NHANES III to correct for measurement error in the postfortification sample.
Total Folate Data
Participants were asked about their supplement use over 1 month, and therefore, the supplemental folate intake is not subject to measurement error caused by day-to-day variability. To obtain the corrected distributions for total folate intake (food and supplement sources), we created a data set of food folate observations from the distributions corrected for measurement error. We used a random process to draw from these food intake distributions based on the estimates of subgroup means and standard deviations, and summed each food folate observation with one chosen from the supplemental intake distribution. We assumed no correlation between supplemental and food folate intake on the basis of estimating Spearman rank correlation coefficients of 0.05 or less from the data.
Statistical Inference
To determine the significance of differences in folate intake between the pre- and postfortification time periods, we performed multiple linear regression analyses of the natural log of total daily folate (μg/day) on each time period. Estimates of the statistical significance of pre- to postfortification differences in supplement use were based on logistic regression. Both regression analyses were conducted using SUDAAN statistical software version 9.0 (Research Triangle Institute, Research Triangle Park, NC) and incorporated NHANES sampling weights, strata, and primary sampling units. Age (continuous), gender, and race/ethnicity were included in both models, and all possible 2-way interactions were considered. Manual forward selection was performed to find the best fitting models, and we also assessed the significance and validity of models by race/ethnicity, gender, and age-category subgroups. We used a 2-sample test for binomial proportions to determine the statistical significance of the pre- to postfortification differences in the proportions of the population for which total folate intake levels were more than 200 μg/day, 400 μg/day, and 1000 μg/day.
RESULTS
Measurement Error Correction
After correcting for measurement error, food folate intake distributions had smaller standard deviations both before and after implementation of the fortification policy. Larger percentages of both gender groups consumed higher levels of food folate after fortification than before, and the distribution modes shifted from approximately 200 μg/day to 300 μg/day.
Total Folate Intake
Total folate intake distributions before and after the fortification policy, corrected for measurement error, are shown in Figure 1 ▶ according to gender and race/ethnicity. The distributions shifted to the right (higher intake levels) after fortification for all population subgroups. The mean increases were larger for Whites than for Blacks and Mexican Americans.
FIGURE 1—
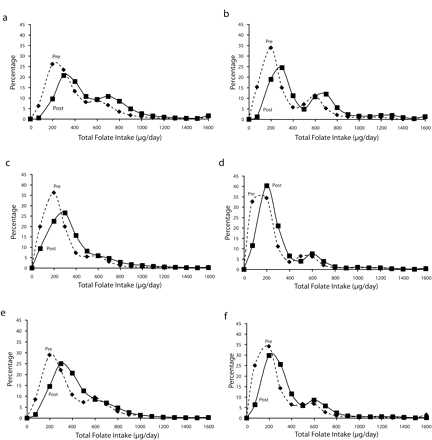
Daily total folate intake distributions, pre- and postfortification, for non-Hispanic White men (a), non-Hispanic White women (b), non-Hispanic Black men (c), non-Hispanic Black women (d), Mexican American men (e), and Mexican American women (f), corrected for measurement error. Includes folate consumption from all food and supplement sources, with food data adjusted for measurement error. Points represent midpoints of categories, and plots are smoothed between points. Upper endpoint of 1600 μg/day represents all intake more than 1500 μg/day.
Median total folate intakes for before and after fortification are shown in Figure 2 ▶ according to age, gender, and race/ethnicity. Median folate intake for all Whites increased by more than 100 μg/day after fortification. All women of reproductive age increased their median intake by at least 100 μg/day. Among Blacks and Mexican Americans, the median intake among persons aged 65 years or older either increased by less than 100 μg/ day (Black men and women), or decreased (Mexican American men and women) following fortification.
FIGURE 2—
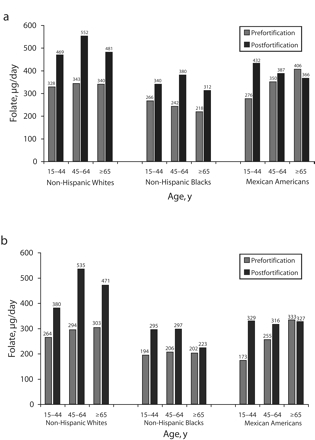
Total folate intake medians, pre- and postfortification, for men (a) and women (b) aged ‡15 years. Medians are presented by cohort and race/ethnicity, and includes folate consumption from all food and supplement sources. The effect of fortification was found to be highly significant (P < .001), after control for age, age-squared, gender, and race/ ethnicity. The effect of age varied significantly by race/ethnicity and gender, and the effect of gender varied significantly by race/ethnicity.
The effect of survey period (i.e., pre- vs postfortification) on the log of total daily folate consumption was significant (P < .0001) in a multiple linear regression model with control for age, age-squared, gender, race/ ethnicity, and significant interactions.
Threshold Estimates
Table 1 ▶ shows the percentage of women of childbearing age who consumed more than 200 μg/day and 400 μg/day of total folate, and the percentage of older people who consumed more than 1000 μg/day. The within-person variation in food folate intake is shown, as well as the threshold estimates with and without the correction for this variation. Overall, Whites were reaching all thresholds in greater proportions than were Blacks and Mexican Americans; Blacks were least likely to have had intakes above these levels.
TABLE 1—
Population Total Folate Consumption Pre- and Postfortification, With and Without Measurement Error Correction
| % Women Aged 15–44 Years Consuming > 200 μg/day | % Women Aged 15–44 Years Consuming > 400 μg/day | % Men Aged ≥65 Years Consuming > 1000 μg/day | % Women Aged ≥ 65 Years Consuming>1000 μg/day | ||||||||||||||||
| Uncorrected | Corrected | Uncorrected | Corrected | Uncorrected | Corrected | Uncorrected | Corrected | ||||||||||||
| Within-Person Variance | Pre | Post | Pre | Post | Pre | Post | Pre | Post | Within-Person Variance | Pre | Post | Pre | Post | Within-Person Variance | Pre | Post | Pre | Post | |
| Non-Hispanic Whites | 0.4827 | 62 | 82 | 60 | 91*** | 36 | 52 | 30 | 39*** | 0.2036 | 5% | 8% | 2% | 4%** | 0.2616 | 4% | 8% | 2% | 4%*** |
| Non-Hispanic Blacks | 0.3684 | 49 | 70 | 43 | 69*** | 27 | 31 | 20 | 26*** | 0.4904 | 3% | 3% | 1% | 3%** | 0.4138 | 2% | 1% | 1% | 1% |
| Mexican Americans | 0.4331 | 45 | 77 | 42 | 88*** | 25 | 37 | 17 | 28*** | 0.2692 | 15% | 5% | 6% | 2%* | 0.2901 | 12% | 2% | 4% | 1%* |
*P < .1; **P < .05; ***P < .01 (2-sample test for binomial proportions).
The FDA’s goal to have 50% of women of childbearing age consume at least 400 μg/ day of folate has not been met for any racial/ ethnic group, but the percentages have significantly increased since fortification. At 30% prefortification and 39% postfortification, more White women have reached the 400 μg/day threshold than have Black women (20% pre, 26% post) and Mexican-American women (17% pre, 28% post), both before and after fortification. There were also large increases in the percentage of women of all races/ethnicities who are consuming more than 200 μg/day of total folate: approximately 90% for both Whites and Mexican Americans, and 70% for Blacks. This represents an almost 100% increase for Mexican Americans.
Among people aged 65 years or older who may be at higher risk for B12 deficiency, the proportions of the population who consumed more than 1000 μg/day after fortification increased for White men and women and Black men, decreased for Mexican-American men and women, and remained constant for Black women. Corrected for measurement error, only 1%–4% of persons aged 65 and older were at high risk for masking after fortification.
Supplemental Folate Intake
Figure 3 ▶ shows the percentage of men and women who take supplements that contain folic acid before and after fortification. More Whites took supplements containing folic acid than did Blacks and Mexican Americans, more people older than 44 years took supplements than did younger people, and more than half of the subgroups showed postfortification decreases in the proportion of people taking supplements. For all women of childbearing age, fewer people were taking supplements after fortification than before; the same result was found for Mexican American men of all ages. The largest absolute change was among Mexican American men older than 64 years, for whom the percentage taking supplements decreased by more than 50% after fortification. In a logistic regression model, the effect of survey period (i.e., pre- vs postfortification) on consumption of folic acid supplements was found to be significant, and the effect was modified by age (P < .0001) and race/ ethnicity (P < .05). The model controls for age, age-squared, gender, race/ethnicity, and significant interactions.
FIGURE 3—
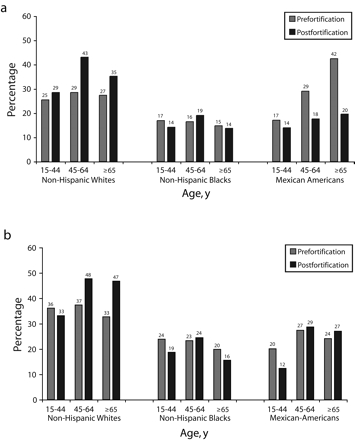
Percentage of population taking supplements that contain folic acid, pre- and postfortification, among men (a) and women (b). The change after fortification was significant and dependent on age (P< .001) and race (P< .05), after control for age, age-squared, gender, race/ethnicity, and the interaction between gender and race/ethnicity.
DISCUSSION
We used population-based survey data adjusted for measurement error to examine the effect of the FDA folic acid fortification policy. Although our analysis showed a substantial increase of total folate intake levels after fortification among US adults, we found considerable variations in intake by age, gender, and race/ethnicity. Although all women of childbearing age—the target population for the policy—increased their median total folate intake by at least 100 μg/day following fortification, Black and Mexican American women still lagged behind White women in total consumption and may be target populations for additional fortification or supplementation policies. Among older people who may be at risk for B12 deficiencies, there are higher percentages of Whites and Blacks consuming more than 1000 μg/day of folate (the “tolerable upper intake level”) since fortification. At the same time, higher folate intake levels among these populations may help to reduce risks of heart disease and colon cancer.
The total intake distributions found in our analysis describe the typical American diet: most people consume approximately 200–300 μg/day (pre- to postfortification) of folate from food sources (legumes, leafy greens, fortified cereals, etc.), which is depicted in Figure 1 ▶ by the first and larger mode in the distributions. The second, smaller peaks at approximately 600 μg/day represent those people who consume 400 μg/ day from supplemental sources in addition to an average diet. Peaks at 1200–1300 μg/day in women aged 15–44 years may represent women who take a supplement (400 μg) in addition to a prenatal vitamin (800 μg); that this peak exists primarily among Whites but not Blacks and Mexican Americans of this age group may indicate that the latter groups are not receiving comparable prenatal care.
Several studies have estimated the changes in folate intake as a result of fortification.27–37 Our results corroborate the findings from those studies and show that folate intake levels have increased for the average person by approximately 100 μg/day. However, our analysis also corrects for dietary intake data measurement error when estimating folate intake levels. Because NHANES uses a single 24-hour dietary recall per individual when collecting food and nutrient intake data, estimated distributions of population intakes will have artificially high standard deviations. Although these incorrect values may not affect the estimated means, the error can have important effects on estimates of the proportion of a population above or below certain thresholds.42
Our results show that failing to take measurement error into account would have overestimated the percentage of women of child-bearing age who reached the 400 μg/day goal set by the US Public Health Service in folic acid fortification.43 For example, we would have incorrectly concluded that the FDA target had already been met (50% of White women; 52% consuming more than 400 μg/day). At the same time, we would have overestimated the percentage of older people consuming more than 1000 μg/day, and incorrectly concluded that more people may be at risk for masking B12 deficiency than truly is the case.
After correcting for measurement error, we found that the percentage of women of childbearing age now consuming more than 400 μg/day of total folate has increased to between 26% and 38%, but has not yet reached 50% for any racial/ethnic group. Such results are consistent with data that indicate NTD rates have decreased 20%–32% after the policy,7,44–49 compared with the potential to prevent 50%–70% of NTDs by additional folate intake.1,2,50 By correcting for measurement error among age, racial, and gender subgroups, our analysis is able to more accurately determine the percentages of target and at-risk populations who have reached the thresholds of interest for drawing policy-level conclusions.
One unexpected finding from our analysis is that within all racial/ethnic groups, fewer women of childbearing age are now taking supplements that contain folic acid than were taking them before fortification. This finding indicates a potential need for increased awareness among these populations and their physicians regarding multivitamins. Ray and colleagues found evidence of suboptimal use of periconceptional folic acid supplements globally among women of childbearing age.51
The substantial racial/ethnic variations in folate consumption found in our analysis are also seen in serum and red blood cell folate levels. Population-level analyses of NHANES surveys indicate that serum and red blood cell folate concentrations for women of childbearing age (both before and after fortification) are lowest among Blacks and highest among Whites; Mexican Americans fall in between those groups.39,52 Our results suggest that differences in supplement use are driving the disparities, because food folate intake means and medians are very similar among all three racial/ethnic groups both before and after fortification. Further understanding of the basis for these disparities will provide policymakers more insight into ways to achieve their target: 50% of all women of childbearing age consuming at least 400 μg/day of folate.
The results of our analysis must be considered in light of its limitations. Because of restrictions in data availability, we used within-person variance estimates from NHANES III data to correct for measurement error in NHANES 1999–2000. This may have caused the analysis to underestimate the degree of measurement error correction needed postfortification, because the consumption of fortified grains will increase the within-person variability. Our results may have thereby overestimated the percentages of our target populations that reached the 400 μg/day and 1000 μg/day thresholds.
There is also some evidence that when estimating the true between-person variance from repeat measurements, more accurate results may be derived by including all subjects (those with and without repeat measurements) in the repeated-measures analysis of variance.53 We tested the affect of this assumption on the percentages of women of childbearing age who reached the 400 μg/ day threshold. We found that the new distributions did have different standard deviations, but there was no pattern among subgroups as to whether the change in methods increased or decreased the distribution spread. The changes in percentage of women of childbearing age who reached the 400 μg/ day threshold before and after fortification decreased for Whites and Mexican Americans but increased for Blacks. However, neither the significance of the changes nor the conclusions drawn regarding racial/ethnic variations were affected.
Other factors that may cause imprecise estimates include the use of interview data and uncertainty about the actual amounts of folate added to foods. Possible underreporting biases and underestimation of food folate content27,37 could cause our results to underestimate true intake levels, which could be accentuated after fortification as a result of the added effect of fortified foods. Estimating dietary folate intake alone to evaluate the fortification program is also limited in that total folate consumption is not the only factor that influences folate status and its affect on disease, both of which can also be influenced by smoking, body mass index, history of NTD, and variations in individual responses to folate intake.38,54,55 Future analyses of the folate biochemical indicator data from NHANES III and NHANES 1999–2000, in conjunction with folate intake data, may ultimately provide better estimates of the effects of folic acid fortification.52
Given the uncertainty of the minimum effective dose of folate for preventing NTDs, the safety concerns for the elderly, and the magnitude of a national fortification mandate, there is great interest in evaluating folate intake and health outcomes after folic acid fortification.24,30,31,56–58 Although most of the FDA policy’s focus has been on NTDs and masking anemia caused by vitamin B12 deficiency, increased folate consumption is also believed to reduce the risk of coronary heart disease and colon cancer. It would help to better guide policy evaluation if future research could incorporate the existing epidemiological evidence on the relation of folate intake with myocardial infarctions and colon cancer, into an analysis that weighs the risks versus benefits of folic acid fortification on these multiple health outcomes. Such knowledge could contribute to the continuing debate about fortification levels,23,38,46,57–59 as would a better understanding of the dose–response group relationships between folate intake and the progression of these health outcomes.
Since implementation of folic acid fortification in the United States, folate intake among the general population has increased, but there are substantial variations by age, gender, and race/ethnicity. Although the goal of the fortification policy (50% of women of childbearing age consuming at least 400 μg/ day of folate) has not been met, the policy has successfully increased the percentage of women aged 14–44 years who consume at least 400 μg/day of folate. Targeted interventions to promote use of supplements among women of childbearing age may be needed to further increase these proportions and to further reduce the risk of NTDs in the United States.
Acknowledgments
We thank Bernard Rosner of the Harvard Medical School, Channing Laboratory for his statistical advice. T. Bentley is a recipient of a training grant from the National Cancer Institute (grant R25 CA92203) as part of the Dana-Farber/Harvard Cancer Center Program in Cancer Outcomes Research Training. This research was also funded by the National Cancer Institute (grant UOI CA88204).
Human Participant Protection No protocol approval was needed for this study.
Peer Reviewed
Contributors K. M. Kuntz originated the study and supervised all aspects of its implementation. T.G. K. Bentley conducted all analyses and led the writing. W.C. Willett and M. C. Weinstein helped to conceptualize ideas, interpret findings, and review drafts of the article.
References
- 1.Czeizel AE, Dudas I. Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. N Engl J Med. 1992;327: 1832–1835. [DOI] [PubMed] [Google Scholar]
- 2.Medical Research Council Vitamin Study Research Group. Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. Lancet. 1991; 338:131–137. [PubMed] [Google Scholar]
- 3.US Food and Drug Administration. Folic acid fortification fact sheet: Office of Public Affairs. 1996. Available at: http://vm.dfsan.fda.gov/~dms/wh-folic.html. Accessed January 7, 2003.
- 4.Johnston R, Staples D. Knowledge and use of folic acid by women of childbearing age—United States, 1997. MMWR Recommendations and Reports. 1997;46: 721–723. [PubMed] [Google Scholar]
- 5.US Food and Drug Administration. Food standards: amendment of standards of identity for enriched grain products to require addition of folic acid. Federal Register. 1996;61:8781–8797. [Google Scholar]
- 6.US Food and Drug Administration. Food standards: amendment of standards of identity for enriched grain products to require addition of folic acid. Federal Register. 1993;58:53305–53312. [Google Scholar]
- 7.Honein MA, Paulozzi LJ, Mathews TJ, Erickson JD, Wong LY. Impact of folic acid fortification of the US food supply on the occurrence of neural tube defects. JAMA. 2001;285:2981–2986. [DOI] [PubMed] [Google Scholar]
- 8.Romano PS, Waitzman NJ, Scheffler RM, Pi RD. Folic acid fortification of grain: an economic analysis. Am J Public Health. 1995;85:667–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rader JI. Folic acid fortification, folate status and plasma homocysteine. J Nutrition. 2002;132: 2466S–2470. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control. Recommendations for the use of folic acid to reduce the number of cases of spina bifida and other neural tube defects. MMWR Recommendations and Reports. 1992;41(RR-14):001. [PubMed] [Google Scholar]
- 11.Abby SL, Harris IM, Harris KM. Homocysteine and cardiovascular disease. J Am Board Fam Practice. 1998;11:391–398. [DOI] [PubMed] [Google Scholar]
- 12.Giovannucci E, Rimm EB, Ascherio A, Stampfer MJ, Colditz GA, Willett WC. Alcohol, low-methionine–low-folate diets, and risk of colon cancer in men. J Natl Cancer Inst. 1995;87:265–273. [DOI] [PubMed] [Google Scholar]
- 13.Giovannucci E, Stampfer MJ, Colditz GA, Hunter DJ, Fuchs C, Rosner BA, Speizer FE, Willett WC. Multivita-min use, folate, and colon cancer in women in the Nurses’ Health Study. Ann Intern Med. 1998;129: 517–524. [DOI] [PubMed] [Google Scholar]
- 14.Glynn SA, Albanes D, Pietinen P, et al. Colorectal cancer and folate status: a nested case-control study among male smokers. Cancer Epidemiol Biomarkers Prev. 1996;5:487–494. [PubMed] [Google Scholar]
- 15.Freudenheim JL, Graham S, Marshall JR, Haughey BP, Cholewinski S, Wilkinson G. Folate intake and carcinogenesis of the colon and rectum. Intl J Epidemiol. 1991;20:368–374. [DOI] [PubMed] [Google Scholar]
- 16.Kato I, Dnistrian AM, Schwartz M, et al. Serum folate, homocysteine and colorectal cancer risk in women: a nested case-control study. Br J Cancer. 1999; 79:1917–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morris CD, Carson S. Routine vitamin supplementation to prevent cardiovascular disease: a summary of the evidence for the US Preventive Services Task Force [see comment]. Ann Intern Med. 2003;139:56–70. [DOI] [PubMed] [Google Scholar]
- 18.Rimm EB, Willett WC, Hu FB, et al. Folate and vitamin B6 from diet and supplements in relation to risk of coronary heart disease among women. JAMA. 1998;279:359–364. [DOI] [PubMed] [Google Scholar]
- 19.Su LJ, Arab L. Nutritional status of folate and colon cancer risk: evidence from NHANES I epidemiologic follow-up study. Ann Epidemiol. 2001;11:65–72. [DOI] [PubMed] [Google Scholar]
- 20.Thun MJ, Calle EE, Namboodiri MM, et al. Risk factors for fatal colon cancer in a large prospective study. J Natl Cancer Inst. 1992;84:1491–1500. [DOI] [PubMed] [Google Scholar]
- 21.White E, Shannon JS, Patterson RE. Relationship between vitamin and calcium supplement use and colon cancer. Cancer Epidemiol Biomarkers Prev. 1997; 6:769–774. [PubMed] [Google Scholar]
- 22.Willett WC. Diet, nutrition, and avoidable cancer. Environ Health Perspect. 1995;103:165–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bentley JR, Ferrini RL, Hill LL. American College of Preventive Medicine public policy statement. Folic acid fortification of grain products in the US to prevent neural tube defects. Am J Preventive Med. 1999;16: 264–267. [DOI] [PubMed] [Google Scholar]
- 24.National Institutes of Health Clinical Nutrition Service. Facts about dietary supplements: folate. Bethesda, MD: National Institutes of Health; 2001. Available at: http://ods.od.nih.gov/factsheets/cc/folate.html. Accessed April 22, 2004.
- 25.Bialostosky K, Wright J, Kennedy-Stephenson J, McDowell M, Johnson C. Dietary intake of macronutrients, micronutrients and other dietary constituents: United States 1988–94. Hyattsville, MD: National Center for Health Statistics; 2002. Vital Health Statistics Series No. 11. [PubMed]
- 26.Wright J, Wang C, Kennedy-Stephenson J, Ervin R. Dietary intake of ten key nutrients for public health, United States: 1999–2000. Hyattsville, MD: National Center for Health Statistics; 2003. Advance Data from Vital and Health Statistics No. 334. [PubMed]
- 27.Choumenkovitch SF, Selhub J, Wilson PW, Rader JI, Rosenberg IH, Jacques PF. Folic acid intake from fortification in United States exceeds predictions. J Nutr. 2002; 132:2792–2798. [DOI] [PubMed] [Google Scholar]
- 28.Cuskelly GJ, McNulty H, Scott JM. Fortification with low amounts of folic acid makes a significant difference in folate status in young women: implications for the prevention of neural tube defects. Am J Clin Nutr. 1999;70:234–239. [DOI] [PubMed] [Google Scholar]
- 29.Jacques PF, Selhub J, Bostom AG, Wilson PW, Rosenberg IH. The effect of folic acid fortification on plasma folate and total homocysteine concentrations. N Engl J Med. 1999;340:1449–1454. [DOI] [PubMed] [Google Scholar]
- 30.Rader J, Weaver C, Angyal G. Total folate in enriched cereal-grain products in the United States following fortification. Food Chem. 2000;70: 275–289. [Google Scholar]
- 31.Lewis CJ, Crane NT, Wilson DB, Yetley EA. Estimated folate intakes: data updated to reflect food fortification, increased bioavailability, and dietary supplement use [see comment]. Am J Clinical Nutrition. 1999; 70:198–207. [DOI] [PubMed] [Google Scholar]
- 32.Malinow MR, Duell PB, Hess DL, et al. Reduction of plasma homocyst(e)ine levels by breakfast cereal fortified with folic acid in patients with coronary heart disease. N Engl J Med. 1998;338:1009–1015. [DOI] [PubMed] [Google Scholar]
- 33.Pawlosky RJ, Flanagan VP. A quantitative stable-isotope LC-MS method for the determination of folic acid in fortified foods. J Agric Food Chem. 2001;49: 1282–1286. [DOI] [PubMed] [Google Scholar]
- 34.Quinlivan EP, Gregory JF, III. Effect of food fortification on folic acid intake in the United States. Am J Clinical Nutrition. 2003;77:221–225. [DOI] [PubMed] [Google Scholar]
- 35.Tice JA, Ross E, Coxson PG, et al. Cost-effectiveness of vitamin therapy to lower plasma homocysteine levels for the prevention of coronary heart disease: effect of grain fortification and beyond. JAMA. 2001;286: 936–943. [DOI] [PubMed] [Google Scholar]
- 36.Lawrence JM, Petitti DB, Watkins M, Umekubo MA. Trends in serum folate after food fortification. Lancet. 1999;354:915–916. [DOI] [PubMed] [Google Scholar]
- 37.Afman L, Bagley P, Selhub J. Determination of folic acid in fortified cereal products using the affinity/ HPLC method. FASEB J. 1999;13:A891. [Google Scholar]
- 38.Neuhouser ML, Beresford SA. Folic acid: are current fortification levels adequate? Nutrition. 2001;17: 868–872. [DOI] [PubMed] [Google Scholar]
- 39.Erickson JD, Mulinare J, Yang Q, et al. Folate status in women of childbearing age, by race/ethnicity—United States, 1999–2000. MMWR Weekly. 2002;51: 808–810. [PubMed] [Google Scholar]
- 40.Centers for Disease Control and Prevention. Folate status in women of childbearing age—United States, 1999. MMWR. Weekly. 2000;.49:962–965. [PubMed] [Google Scholar]
- 41.Petrini J, Damus K, Johnston R, Mattison D. Knowledge and use of folic acid by women of child-bearing age—United States, 1995 and 1998. MMWR Recommendations and Reports. 1999;48:325–327. [Google Scholar]
- 42.Willet W. Nutritional epidemiology. New York, NY: Oxford University Press; 1998.
- 43.Centers for Disease Control and Prevention. Recommendations for the use of folic acid to reduce the number of cases of spina bifida and other neural tube defects. MMWR Recommendations and Reports. 1992; 41(RR-14);001. [PubMed] [Google Scholar]
- 44.Mersereau P, Kilker K, Carter H, et al. Spina bifida and anencephaly before and after folic acid mandate—United States, 1995–1996 and 1999–2000. MMWR Weekly. 2004;53:362–365. [PubMed] [Google Scholar]
- 45.Evans MI, Llurba E, Landsberger EJ, O’Brien JE, Harrison HH. Impact of folic acid fortification in the United States: markedly diminished high maternal serum α-fetoprotein values. Obstet Gynecol. 2004;103: 474–479. [DOI] [PubMed] [Google Scholar]
- 46.Erickson JD. Folic acid and prevention of spina bifida and anencephaphly. MMWR Recommendations and Reports. 2002;51:1–3. [PubMed] [Google Scholar]
- 47.Mathews TJ, Honein MA, Erickson JD. Spina bifida and anencephaly prevalence—United States, 1991–2001. MMWR Recommendations and Reports. 2002;51:9–11. [PubMed] [Google Scholar]
- 48.Meyer R, Siega-Riz A. Sociodemographic patterns in spina bifida birth prevalence trends—North Carolina, 1995–1999. MMWR Recommendations and Reports. 2002;51:11–14. [PubMed] [Google Scholar]
- 49.Williams LJ, Mai CT, Edmonds LD, et al. Prevalence of spina bifida and anencephaly during the transition to mandatory folic acid fortification in the United States. Teratology. 2002;66:33–39. [DOI] [PubMed] [Google Scholar]
- 50.Milunsky A, Jick H, Jick SS, et al. Multivitamin/ folic acid supplementation in early pregnancy reduces the prevalence of neural tube defects. JAMA. 1989; 262:2847–2852. [DOI] [PubMed] [Google Scholar]
- 51.Ray JG, Singh G, Burrows RF. Evidence for suboptimal use of periconceptional folic acid supplements globally. BJOG. 2004;111:399–408. [DOI] [PubMed] [Google Scholar]
- 52.Pfeiffer CM, Caudill SP, Gunter EW, Osterloh J, Sampson EJ. Biochemical indicators of B vitamin status in the US population after folic acid fortification: results from the National Health and Nutrition Examination Survey 1999–2000. Am J Clin Nutr. 2005;82: 442–450. [DOI] [PubMed] [Google Scholar]
- 53.Rosner B, Polk BF. Predictive values of routine blood pressure measurements in screening for hypertension. Am J Epidemiol. 1983;117:429–442. [DOI] [PubMed] [Google Scholar]
- 54.Neuhouser ML, Beresford SA, Hickok DE, Monsen ER. Absorption of dietary and supplemental folate in women with prior pregnancies with neural tube defects and controls. J Am Coll Nutr. 1998;17: 625–630. [DOI] [PubMed] [Google Scholar]
- 55.Boddie AM, Dedlow ER, Nackashi JA, et al. Folate absorption in women with a history of neural tube defect-affected pregnancy. Am J Clin Nutr. 2000;72: 154–158. [DOI] [PubMed] [Google Scholar]
- 56.Centers for Disease Control and Prevention. Folic acid now: learn more about folic acid. Atlanta, GA: Division of Birth Defects, Child-Development, Disability and Health; 2001. Available at: http://www.cdc.gov/ncbddd/folicacid/default.htm. Accessed March 11, 2004.
- 57.Daly S, Mills JL, Molloy AM, et al. Minimum effective dose of folic acid for food fortification to prevent neural-tube defects. Lancet. 1997;350:1666–1669. [DOI] [PubMed] [Google Scholar]
- 58.Oakley GP, Jr., Adams MJ, Dickinson CM. More folic acid for everyone, now. J Nutr. 1996;126: 751S–755S. [DOI] [PubMed] [Google Scholar]
- 59.Wald NJ. Folic acid and the prevention of neural-tube defects [comment]. N Engl J Med. 2004;350: 101–103. [DOI] [PubMed] [Google Scholar]


