Abstract
Cancer registries are a valuable resource for recruiting participants for public health–oriented research, although such recruitment raises potentially competing concerns about patient privacy and participant accrual.
We surveyed US central cancer registries about their policies for research contact with patients, and results showed substantial variation. The strategy used most frequently (37.5% of those that allowed patient contact), which was among the least restrictive, was for investigators to notify patients’ physicians and then contact patients with an opt-out approach. The most restrictive strategy was for registry staff to obtain physician permission and contact patients with an opt-in approach.
Population-based studies enhance cancer control efforts, and registry policies can affect researchers’ ability to conduct such studies. Further discussion about balanced recruitment approaches that protect patient privacy and encourage beneficial research is needed.
POPULATION-BASED CANCER registries provide information that is essential for identifying public health priorities and for planning and monitoring cancer-related programs.1–5 In the United States, cancer reporting occurs without patients’ consent because of the collective benefit derived from complete case reporting. All 50 states mandate cancer reporting, and state authorities specify regulations that govern central cancer registries. Additionally, registries receive funding from 1 or both of 2 federal programs: the Surveillance, Epidemiology, and End Results (SEER) program6 and the National Program of Cancer Registries (NPCR).7 Together, NPCR and SEER registries collect cancer data for virtually the entire US population.
Cancer registries also play a vital role in cancer research. They provide not only statistical data for analyzing patterns and trends but also an unbiased source of participants for population-based studies. However, recruiting research participants through cancer registries raises 2 related concerns. First, investigators could obtain personal information about individuals in the registry before the individuals have agreed to participate in the research, perhaps before they are even aware that a cancer registry exists. Second, recruitment strategies that maximize privacy protections (e.g., ensuring investigators have no personal information about patients in the registry without their permission) may compromise participant accrual and, thus, the validity and generalizability of research findings.
A key policy challenge is to balance the needs of patients, investigators, health care providers, and registries to protect privacy while promoting the scientific usefulness of registry.3 Cancer registries use a variety of policies for addressing this challenge:
Educating patients about the registry: Some registries use passive methods (e.g., Web sites and brochures displayed in waiting rooms) to inform the public about the registry. Others actively notify individuals that information about their cancer diagnosis has been reported to the registry and that researchers may contact them.
Involving treating physicians: Some registries require physician permission before investigators can contact patients, and nonresponse is considered to be passive refusal. Others require only physician notification of planned patient contact, and nonresponse is considered to be passive permission.
Initiating patient contact: After potential participants receive an introductory letter about a study, some registries require an opt-in approach, where investigators follow-up only with those patients who take an action (e.g., return a postcard) to indicate their interest. Others allow investigators to follow-up with all patients except those who opt-out of further contact.
Thus, procedures for identifying and contacting potential research participants through US central cancer registries are not uniform, and systematically collected data about these procedures are not available. We surveyed cancer registries to define the range of practice with regard to recruitment for observational research, including policies about educating patients, involving physicians, and initiating patient contact. We also asked about rapid case ascertainment, a critical process for many population-based studies.8,9 A secondary objective was to elucidate changes contemplated or implemented in response to the Health Insurance Portability and Accountability Act (HIPAA) or to privacy concerns in general. Although HIPAA does not obstruct state laws that mandate disease reporting for public health purposes (45 CFR 164.203), it has brought attention to the issue of health information privacy.
METHODS
We drafted a survey instrument on the basis of state cancer registry laws and regulations, literature about cancer registries, and literature about research recruitment. We revised the survey after a review by experts in cancer registration; cancer epidemiology; and health law, policy, and ethics. We then submitted an application to survey members of the North American Association of Central Cancer Registries (NAACCR),10 an umbrella organization that includes all US and Canadian central cancer registries. The NAACCR board of directors approved our application in January 2004, and board members from US registries—in their capacity as registry directors—pretested the survey and offered their feedback. The final survey (available from the corresponding author) comprised 40 questions and included a section for comments and clarification. Additionally, respondents were asked to review and correct information we had obtained in a summary of the main survey results (available from the corresponding author).
In May 2004, we used a NAACCR e-mail distribution list to send the survey electronically to the directors of 59 registries: each of the 50 state registries, the District of Columbia registry, and SEER area registries in Alaska, California (three registries), Georgia, Michigan, Oklahoma, and Washington. A reminder was e-mailed 3 weeks later, and a second reminder was sent by US mail with signature confirmation 6 weeks later. Data were analyzed with Stata software, version 8.0 (Stata Corp, College Station, Tex).
RESULTS
Response Rates and Registry Characteristics
Surveys were completed by 42 of the 59 (71.2%) invited registries, including 38 of 51 (74.5%) statewide and District of Columbia registries, and 4 of 8 (50%) SEER area registries. According to publicly available information,6,7,10 nonresponding registries were older than responding registries (mean year established 1972 vs 1982; P = 0.01), but there were no significant differences in federal funding source (any SEER funding vs NPCR funding only) or NAACCR certification (a dichotomous variable reflecting data quality).
Among responding registries, NPCR was the most common source of federal funding for basic registry operations. Most were housed in their state’s health department; other locations included universities, a cancer center not affiliated with a university, a hospital association, and a tribal government (Table 1 ▶). However, even when not housed in the health department, state laws and regulations generally govern registries’ activities, including whether and how patients may be contacted for research purposes.
TABLE 1—
Cancer Registry Information (n = 42)
| No. (%) | |
| Year registry established (mean = 1982; range = 1949–1999) | |
| NAACCR certifieda | |
| In 3 of last 3 years | 26 (61.9) |
| In 2 of last 3 years | 7 (16.7) |
| In 1 of last 3 years | 2 (4.8) |
| In 0 of last 3 years | 7 (16.7) |
| Federal funding (direct) | |
| NPCR | 31 (73.8) |
| SEER | 7 (16.7) |
| SEER/NPCR | 4 (9.5) |
| Location | |
| State health department | 29 (69.1) |
| University | 10 (23.8) |
| Cancer center | 1 (2.4) |
| Other | 2 (4.8) |
| Patient education | |
| Passive | 25 (59.5) |
| Active and passive | 5 (11.9) |
| Neither | 12 (28.6) |
| Patient contact allowed | |
| Yes | 33 (78.6) |
| No | 9 (21.4) |
| Rapid case ascertainment capability | |
| Yes | 14 (33.3) |
| No | 28 (66.7) |
Note. NAACCR = North American Association of Central Cancer Registries; NPCR = National Program of Cancer Registries; SEER = Surveillance, Epidemiology, and End Results program.
aGold or silver certification by the North American Association of Central Cancer Registries in 2001 for 1999 incidence data, in 2002 for 2000 incidence data, or in 2003 for 2001 incidence data.
Patient Education
Active and passive education.
Thirty (71.4%) registries used passive approaches to patient education; 5 of these registries also used active approaches with at least 1 subset of patients (Table 1 ▶). Among the 5 registries that used active education, 3 said the registry most often informed patients about cancer reporting. Two said the diagnosing provider or facility most often informed patients, although 1 noted it had no resources for enforcement and was aware that the requirement was regularly not applied.
Between 2000 and 2004, 2 registries made or considered a change in their approach to patient education. The North Carolina registry was the only respondent that said HIPAA concerns (and privacy concerns in general) were a primary motivation; in 2003, it implemented a policy of active education for the subset of patients who were identified as potentially eligible for a particular study.
Before July 2001, patients in Virginia were not actively notified; from July 2001 to July 2003, state law required the registry to notify patients that it had received information. However, effective July 2003, the law (§32.1–71.02) was changed to make the diagnosing physician responsible for notifying patients. The reported motivations for this change were multiple negative consequences that resulted from direct notification and concern about upholding the physician–patient relationship.
Educational materials.
Twenty-seven (64.3%) registries had educational materials, including brochures, pamphlets, or information sheets (88.9%); Web pages specifically designed for patients and the public (70.4%); and other items, such as annual reports and community outreach activities (18.5%). Although 15 (35.7%) registries did not have educational materials, passive education may still occur through laws that require lists of reportable conditions be made available to the public. For example, Florida law requires hospitals to provide notification of the reportability of cancer. The Florida Cancer Data System also requires researchers to include disclosures about the registry in their initial mailing to patients and to ensure that study personnel provide a clear description of cancer registration in the state.11
Disclosures about the possibility of researcher contact.
Of the 21 registries that had patient education materials and allowed patient contact (see next section), 14 (66.7%) said their materials described the possibility that researchers may contact patients about participating in a study. Of these, 1 said patients were specifically asked to let the registry know whether they were willing to be contacted, and another said patients were told they could inform the registry if they did not want to be contacted. The majority (78.6%) said the disclosure included only a general statement about how to contact the registry with questions or concerns.
Contacting Patients About Participation in Observational Research
Allowing contact for research purposes.
Thirty-three (78.6%) responding registries said contact with patients was allowed for the purposes of research recruitment (Table 1 ▶). These registries reported a total of 171 observational studies (mean = 5.9; range = 0–15) for which potential participants had been identified through their registry between 2000 and mid-2004.
Research approvals.
Of the 33 registries that allowed patient contact, all reported 1 or more approval processes. These included approval by the institutional review board of the investigator’s home institution (87.9%); the institutional review board or other research committee associated with the registry (78.8%); a specific person associated with the registry, for example, the director (57.6%); and other approvals, for example, from an advisory committee (39.4%). One registry required approval by the institutional review board of each reporting facility in the proposed study area.
Physician involvement.
Of the 33 respondents that allowed patient contact, 29 (87.9%) required or strongly recommended contact with patients’ physicians before contact with patients (Table 2 ▶); however, approximately 20% did not routinely collect information that identified patients’ physicians. Several noted that a physician code was collected only if hospitals chose to report it; furthermore, although registries encouraged hospitals to use a physician license number that would have meaning statewide, most used hospital-specific codes.
TABLE 2—
Research Contact With Patients Among Respondents Who Allowed Contact (n = 33)
| No. (%)a | |
| Is physician involved in recruitment process? | |
| Yes, required | 25 (75.8) |
| Yes, strongly recommended | 4 (12.1) |
| No | 4 (12.1) |
| If yes (n = 29), physician contacted by | |
| Investigator | 17 (58.6) |
| Registry staff | 11 (37.9) |
| If yes (n = 29), type of physician involvement | |
| Notification | 24 (82.8) |
| Permission | 4 (13.8) |
| Patient contacted by | |
| Investigator | 20 (60.6) |
| Registry staff | 12 (36.4) |
| Patient response | |
| Opt out | 21 (63.6) |
| Opt in | 11 (33.3) |
aPercentages may not sum to 100% because of missing information.
Of the 29 registries that involved physicians, most (58.6%) allowed researchers to contact patients’ physicians, and the majority (82.8%) used physician notification (Table 2 ▶). Among those that used notification, the mean length of time allowed for physician response was 2.7 weeks (range = 2–4 weeks).
Between 2000 and 2004, 5 registries made or considered a change in physician involvement, all of which were toward less physician involvement. The Massachusetts and North Carolina registries both changed from physician permission to physician notification (in 2000 and 2003, respectively); the Louisiana and Utah registries were considering such a change. In 2001, the Wisconsin registry changed from physician permission to physician notification and was considering a further change to no involvement. With the exception of North Carolina, neither HIPAA nor general privacy concerns were primary motivations. One registry described a general sense that physicians should not have the right to decide if patients can be contacted. The North Carolina registry noted some physicians were concerned about whether actively providing permission for researcher contact with patients—rather than being notified but not required to respond—could be a violation of HIPAA.
Initiating patient contact.
After a research project was approved, and physician input was obtained, if applicable, most (60.6% of the 33 that allowed patient contact) allowed investigators to initiate contact with patients (Table 2 ▶). Most (63.6%) also allowed an opt-out approach.
Three registries made a change in how patients were contacted between 2000 and 2004. The Kentucky registry, citing both HIPAA and general privacy concerns, implemented procedures in 2003 that allow patients to either participate in research projects or ask that their names not be released. The New Jersey registry said that, although it occasionally allowed investigators to contact patients before 1999, it implemented procedures in 2002 that require registry staff to initiate contact because of general privacy concerns.
Patient preferences about researcher contact.
Respondents were asked to estimate the percentage of patients reported between 2000 and 2004 who were flagged to prevent researcher contact between 2000 and 2004. Patients communicated this preference to the registry in response to either disclosures in educational materials or having been contacted by researchers. Of the 33 respondents that allowed patient contact, 10 (30.3%) said no patients were flagged; 17 (51.5%) said less than 1% of patients were flagged; and 3 (9.1%) said more than 1% of patients were flagged.
Rapid Case Ascertainment
Almost all respondents (95.2%) said laws or regulations in their state required that cancer cases be reported within 6 months of diagnosis, treatment, or discharge. Fourteen (33.3%) had rapid case ascertainment capability (Table 2 ▶). The average definition of rapid was within 44 days of diagnosis (range = 30–90 days). Of the registries that had rapid-reporting capability, 4 (28.6%) said more than 10% of cases were reported by entities either not willing or not able to participate in rapid case ascertainment. Five (35.7%) said 1% to 10% of cases were reported by such entities, and 1 (7.1%) said less than 1% of cases had been reported by such entities.
DISCUSSION
Population-based studies improve the public’s health through the development of new knowledge about cancer etiology, outcomes, and quality of care, and investigators have begun to use cancer registries to identify and contact potential participants for such research.12–16 However, research recruitment through cancer registries raises scientific and ethical concerns.
Investigators’ ability to identify, contact, and achieve high participation rates among potential participants increases the validity and the generalizability of findings.17 These recruitment activities must take place within the context of well-established requirements for ethically responsible research,18–20 which include an obligation to minimize risks to participants. When assessing risks, however, it is important to distinguish between risks associated with recruiting participants—identifying and contacting individuals about their interest in research participation—and risks associated with participating in the research.21 When contacted by researchers, individuals have a number of options, including not responding, expressing disinterest at the outset, or learning more about the research and making an informed decision about whether to take part.
The primary risk associated with research recruitment through cancer registries is invasion of privacy, which can sometimes rise to the level of serious harm (e.g., insurance or employment discrimination) if sensitive medical information is misused. Harm also might ensue if information of personal significance is introduced as part of the offer to participate in research. For example, some patients listed in a cancer registry may be unaware of their diagnosis before researcher contact.
Invasion of privacy also can be a wrong rather than a harm.22 Wrongs are generally seen as less grievous than harms, because wrongs seldom touch on matters of physical or even emotional well-being.22 When researchers recruit through cancer registries, an individual may perceive that his or her privacy has been invaded when an investigator gains access to personal information before that person has agreed to participate in the research. Although such an invasion may be contrary to some patients’ preferences about whether and how they are contacted, it does not necessarily result in harm. Committing a wrong against a person violates the ethical principle of respect for persons, that is, allowing individuals to make autonomous choices rather than imposing choices upon them.22 At the same time, every person who seeks medical or preventive care is a direct beneficiary of the knowledge and insights gained from research.23
Our survey sought to describe strategies cancer registries use to address these potentially competing concerns. The results showed substantial variation across registries. Approximately 20% did not allow research contact with patients. Of the registries that did, 9 of the 12 theoretically possible models for research recruitment were actually used (Figure 1 ▶). The most common model, which is among the least restrictive, allows investigators to notify patients’ physicians and then contact patients with an opt-out approach. Registries that used this model at the time of the survey accounted for 76 (44.4%) of the 171 studies that were conducted between 2000 and mid-2004. In comparison, registries that used the most restrictive model (registry staff obtain physician permission and then contact patients with an opt-in approach) accounted for only 9 studies (5.3%).
FIGURE 1—
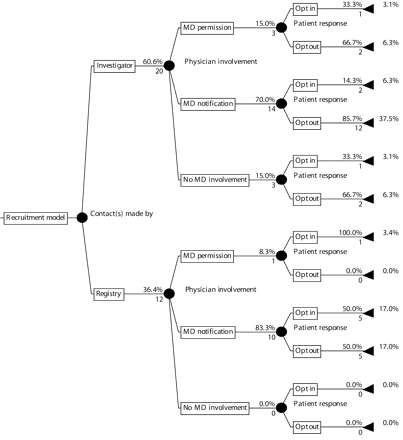
Models for research recruitment through cancer registries
Note. This figure depicts recruitment models from the 33 (78.6%) registries through which research contact with patients was allowed; 9 (21.4%) registries did not allow such contact.
The number below each “branch” is the number of registries in that category.
The percentage above each branch is the proportion of registries from the immediately preceding node that were in that category. The percentages for the set of branches emanating from each node may not total 100% because of missing information. The percentages at the far right are the proportion of registries that used the model defined by following that branch back to the far left of the diagram.
Our secondary objective was to elucidate any effect of HIPAA on registry recruitment policies. Approximately 15% of responding registries reported changes in patient education, and nearly 25% reported changes in procedures for patient contact. Most changes were motivated by privacy concerns in general rather than HIPAA requirements per se—appropriately so, because cancer registries are not covered entities under HIPAA.
A strength of our study was the high response rate for a survey covering a broad array of policies that had not been empirically documented. We relied on face validity when constructing the survey; however, validity was strengthened by review and feedback from a multidisciplinary group of experts. It was further enhanced by providing respondents the opportunity to review their answers in the context of a registry-by-registry summary of the main survey findings.
Because of the prevalence and the public health consequences of cancer, and the attendant need for population-based studies,24,25 further research on the ethical and scientific acceptability of various approaches to research recruitment through cancer registries is important. For example, research is needed on the most effective and efficient ways of educating patients about the registry and the possibility of researcher contact. Because cancer cases are reported without patients’ consent, many patients may be surprised not only to be contacted by a researcher but also to learn that a registry exists that collects their information. Ideally, educational efforts would remedy this lack of awareness and, thus, be an important precursor to research recruitment. At the same time, cancer registries have limited resources, and patients who are dealing with a recent cancer diagnosis may not read or even recall receiving educational materials.26,27
Research also is needed on the role of the treating physician during research recruitment. This research should address (1) physicians’ motivations for whether and how they respond to investigator requests and whether they prefer to be asked permission, notified, or not involved; (2) patients’ knowledge and preferences about physician involvement; and (3) investigators’ insights and experiences, including the effect of physician involvement on study participation rates.
One advantage to physician involvement is that physicians can help manage privacy risks. If registry staff contact physicians and physicians in turn contact patients to determine their interest, the patient can elect whether or not to be contacted by the investigator without the investigator knowing any personal details a priori. Often, however, investigators are given such details: we found investigators, rather than registry staff, were usually responsible for contacting physicians and patients. Furthermore, whether contacted by registry staff or investigators, the physician is generally not bound by procedural requirements. As noted by Gurwitz et al., if the physician does not ask what the patient’s wishes are, it may be the physician’s “own perceptions regarding a constellation of medical and psychosocial characteristics, as well as personal biases, that determine whether approval is given to contact a patient.” 28(p1344) When physicians do communicate with the patient about the study before researcher contact, there are concerns about how the study is presented29 and whether the patient may feel pressured to participate.30
Another advantage to physician involvement is that physicians can help researchers exclude patients who are deceased, too ill, or otherwise ineligible. However, the ability to provide such information assumes an established physician–patient relationship that may not always exist. According to our results, many registries do not routinely collect information that identifies patients’ physicians. Therefore, the physician of record that is available to researchers is often the surgeon listed on the pathology report, who may have no ongoing responsibility for the patient’s care.
Physician involvement may have a positive influence on some patients,29,31 although others may view it as unnecessary or even paternalistic.32,33 From an investigator’s perspective, physician involvement may facilitate effective physician–researcher relationships,31 but specifically obtaining physician permission can be expensive and time-consuming.31,34,35 Placing the physician in the role of “gatekeeper” may have an adverse effect on participant accrual for otherwise beneficial research, which is itself an ethical concern.21
Finally, research is needed on the impact of alternative approaches to initiating patient contact. Whether patients are asked to opt in or opt out of learning more about a study has implications for both privacy and accrual. On the basis of studies that used an opt-out approach,36,37 it appears that very few individuals take action to prevent further researcher contact and that a relatively low proportion of those who do not opt out are in fact not interested. The probability and magnitude of harm that may result from an opt-out approach must be weighed against the loss of research benefits that may result if a more restrictive opt-in approach is required.21
Future research should examine the preferences of various stakeholders, including patients, physicians, investigators, and cancer registries, as well as the outcomes of different approaches with respect to privacy and participant accrual. Protecting patient privacy and supporting beneficial research need not be mutually exclusive goals; indeed, appropriate protections promote public confidence in, and support for, the research enterprise.38 Care must be taken to both protect patients and avoid overly restrictive policies that have a chilling effect on research and limit opportunities for patients who would like to participate.
Acknowledgments
This project was supported by the National Cancer Institute (grant R25-CA57726) for the University of North Carolina-Chapel Hill Lineberger Cancer Control Education Program. The authors thank the board of directors and the members of the North American Association of Central Cancer Registries, Dale Herman and Karen Knight of the North Carolina Central Cancer Registry, and Drs Robert C. Millikan, Betsy L. Sleath, Bryan J. Weiner, Sara L. Tobin, and Brenda K. Edwards.
Human Participant Protections This project was reviewed and approved by the public health institutional review board of the University of North Carolina at Chapel Hill. Cancer registry directors provided written informed consent.
Peer Reviewed
Contributors L. M. Beskow originated the study, completed the analyses, and led the writing. R. S. Sandler and M. Weinberger supervised all aspects of the study and the analyses. All the authors originated ideas, interpreted findings, and reviewed drafts.
References
- 1.Howe HL, Edwards BK, Young JL, et al. A vision for cancer incidence surveillance in the United States. Cancer Causes Control. 2003;14:663–672. [DOI] [PubMed] [Google Scholar]
- 2.Wingo PA, Jamison PM, Hiatt RA, et al. Building the infrastructure for nationwide cancer surveillance and control–a comparison between the National Program of Cancer Registries (NPCR) and the Surveillance, Epidemiology, and End Results (SEER) program (United States). Cancer Causes Control. 2003;14:175–193. [DOI] [PubMed] [Google Scholar]
- 3.Izquierdo JN, Schoenbach VJ. The potential and limitations of data from population-based state cancer registries. Am J Public Health. 2000;90:695–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hankey BF, Ries LA, Edwards BK. The Surveillance, Epidemiology, and End Results program: a national resource. Cancer Epidemiol Biomarkers Prev. 1999;8:1117–1121. [PubMed] [Google Scholar]
- 5.Swan J, Wingo P, Clive R, et al. Cancer surveillance in the US: can we have a national system? Cancer. 1998; 83:1282–1291. [DOI] [PubMed] [Google Scholar]
- 6.National Cancer Institute. Surveillance, Epidemiology, and End Results (SEER) Program. Available at http:// seer.cancer.gov/. Accessed May 31, 2005.
- 7.Centers for Disease Control and Prevention. National Program of Cancer Registries. Available at http://www.cdc.gov/cancer/npcr/index.htm. Accessed May 31, 2005.
- 8.Hiatt RA, Rimer BK. A new strategy for cancer control research. Cancer Epidemiol Biomarkers Prev. 1999;8: 957–964. [PubMed] [Google Scholar]
- 9.UNC Lineberger Comprehensive Cancer Center. Rapid case ascertainment. Available at http://cancer.med.unc.edu/research/cores/facility.asp?facilityID=17. Accessed May 31, 2005.
- 10.North American Association of Central Cancer Registries. Available at http://www.naaccr.org/. Accessed May 31, 2005.
- 11.Florida Cancer Data System. Procedure guide for studies that utilize FCDS for patient identification and contact. Available at http://fcds.med.miami.edu/inc/datarequest.shtml. Accessed May 31, 2005.
- 12.Newman B, Moorman PG, Millikan R, et al. The Carolina Breast Cancer Study: integrating population-based epidemiology and molecular biology. Breast Cancer Res Treat. 1995;35:51–60. [DOI] [PubMed] [Google Scholar]
- 13.Shaheen NJ, Silverman LM, Keku T, et al. Association between hemo-chromatosis (HFE) gene mutation carrier status and the risk of colon cancer. J Natl Cancer Inst. 2003;95:154–159. [DOI] [PubMed] [Google Scholar]
- 14.Potosky AL, Harlan LC, Stanford JL, et al. Prostate cancer practice patterns and quality of life: the Prostate Cancer Outcomes Study. J Natl Cancer Inst. 1999;91:1719–1724. [DOI] [PubMed] [Google Scholar]
- 15.American Cancer Society Behavioral Research Center. Study of Cancer Survivors I & II. Available at http:// www.cancer.org/docroot/res/content/res_9_1_brc_survivorship_research.asp?sitearea=res. Accessed May 31, 2005.
- 16.National Cancer Institute Cancer Control and Population Sciences. Cancer Care Outcomes Research and Surveillance Consortium. Available at http:// healthservices.cancer.gov/cancors/. Accessed May 31, 2005.
- 17.Moorman PG, Newman B, Millikan RC, Tse CK, Sandler DP. Participation rates in a case-control study: the impact of age, race, and race of interviewer. Ann Epidemiol. 1999;9:188–195. [DOI] [PubMed] [Google Scholar]
- 18.National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research. The Bel-mont Report: Ethical Principles and Guidelines for the Protection of Human Subjects of Research. Washington DC: US Government Printing Office; 1979.
- 19.US Dept of Health and Human Services. Federal Policy for the Protection of Human Subjects (45 CFR 46). Available at http://www.hhs.gov/ohrp/humansubjects/guidance/45cfr46.htm. Accessed May 31, 2005.
- 20.World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. J Int Bioethique. 2004;15:124–129. [PubMed] [Google Scholar]
- 21.Beskow LM, Botkin JR, Daly M, et al. Ethical issues in identifying and recruiting participants for familial genetic research. Am J Med Genet. 2004;130A: 424–431. [DOI] [PubMed] [Google Scholar]
- 22.Capron AM. Protection of research subjects: do special rules apply in epidemiology? J Clin Epidemiol. 1991; 44(suppl 1):81S–89S. [DOI] [PubMed] [Google Scholar]
- 23.Kulynych J, Korn D. The effect of the new federal medical-privacy rule on research. N Engl J Med. 2002;346: 201–204. [DOI] [PubMed] [Google Scholar]
- 24.National Cancer Institute Surveillance Implementation Group. Cancer Surveillance Research Implementation Plan. Available at http://cancercontrol.cancer.gov/sig/. Accessed May 31, 2005.
- 25.Hewitt M, Simone JV, eds. Ensuring Quality of Cancer Care. National Cancer Policy Board, Institute of Medicine. Washington, DC: National Academy Press; 1999.
- 26.Harris KA. The informational needs of patients with cancer and their families. Cancer Pract. 1998;6:39–646. [DOI] [PubMed] [Google Scholar]
- 27.Moody R. Overcoming barriers to delivering information to cancer patients. Br J Nurs. 2003;12:1281–1287. [DOI] [PubMed] [Google Scholar]
- 28.Gurwitz JH, Guadagnoli E, Landrum MB, Silliman RA, Wolf R, Weeks JC. The treating physician as active gatekeeper in the recruitment of research subjects. Med Care. 2001;39: 1339–1344. [DOI] [PubMed] [Google Scholar]
- 29.Savitz DA, Hamman RF, Grace C, Stroo K. Respondents’ attitudes regarding participation in an epidemiologic study. Am J Epidemiol. 1986;123: 362–366. [DOI] [PubMed] [Google Scholar]
- 30.Sugarman J, Regan K, Parker B, Bluman LG, Schildkraut J. Ethical ramifications of alternative means of recruiting research participants from cancer registries. Cancer. 1999;86:647–651. [DOI] [PubMed] [Google Scholar]
- 31.Boring CC, Brockman E, Causey N, Gregory HR, Greenberg RS. Patient attitudes toward physician consent in epidemiologic research. Am J Public Health. 1984;74:1406–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meslin EM. The recruitment of research participants and the role of the treating physician. Med Care. 2001;39: 1270–1272. [DOI] [PubMed] [Google Scholar]
- 33.Beskow LM, Sandler RS, Millikan RC, Weinberger M. Patient perspectives on research recruitment through cancer registries. Cancer Causes Control. 2005; 16:1171–1175. [DOI] [PubMed] [Google Scholar]
- 34.Dicker BG, Kent DL. Physician consent and researchers’ access to patients. Epidemiology. 1990;1:160–163. [DOI] [PubMed] [Google Scholar]
- 35.Herrmann N, Amsel J, Lynch E. Obtaining hospital and physician participation in a case-control study of colon cancer. Am J Public Health. 1981;71: 1314–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Helmes AW, Bowen DJ, Bowden R, Bengel J. Predictors of participation in genetic research in a primary care physician network. Cancer Epidemiol Biomarkers Prev. 2000;9:1377–1379. [PubMed] [Google Scholar]
- 37.Rimer BK, Schildkraut JM, Lerman C, Lin TH, Audrain J. Participation in a women’s breast cancer risk counseling trial. Who participates? Who declines? High Risk Breast Cancer Consortium. Cancer. 1996;77:2348–2355. [DOI] [PubMed] [Google Scholar]
- 38.National Bioethics Advisory Commission. Research Involving Human Biological Materials: Ethical Issues and Policy Guidance, Volume 1. Rockville, Md: National Bioethics Advisory Commission; 1999.


