Abstract
The rapidly activating delayed-rectifying K+ current (IKr) in heart cells is an important determinant of repolarisation, and decreases in its density are implicated in acquired and inherited long QT syndromes. The objective of the present study on IKr in guinea-pig ventricular myocytes was to evaluate whether the current is acutely regulated by tyrosine phosphorylation.
Myocytes configured for ruptured-patch or perforated-patch voltage-clamp were depolarised with 200-ms steps to 0 mV for measurement of IKr tail amplitude on repolarisations to −40 mV.
IKr in both ruptured-patch and perforated-patch myocytes was only moderately (14–20%) decreased by 100 μM concentrations of protein tyrosine kinase (PTK) inhibitors tyrphostin A23, tyrphostin A25, and genistein. However, similar-sized decreases were induced by PTK-inactive analogues tyrphostin A1 and daidzein, suggesting that they were unrelated to inhibition of PTK.
Ruptured-patch and perforated-patch myocytes were also treated with promoters of tyrosine phosphorylation, including phosphotyrosyl phosphatase (PTP) inhibitor orthovanadate, exogenous c-Src PTK, and four receptor PTK activators (insulin, insulin-like growth factor-1, epidermal growth factor, and basic fibroblast growth factor). None of these treatments had a significant effect on the amplitude of IKr.
We conclude that Kr channels in guinea-pig ventricular myocytes are unlikely to be regulated by PTK and PTP.
Keywords: Delayed-rectifier IKr, guinea-pig ventricular myocytes, tyrphostin A1, tyrphostin A23, tyrphostin A25, genistein, daidzein, orthovanadate, c-Src, growth factors
Introduction
The rapidly activating delayed-rectifier K+ current (IKr) has a critical role in the electrical activity of cardiac ventricular myocytes. Outward IKr is a major factor in the termination of the plateau phase of the action potential and in driving repolarisation of the cell membrane (Sanguinetti & Jurkiewicz, 1990; Zeng et al., 1995; Jones et al., 1998). Downregulation of the channels that carry IKr contributes to the prolongation of action potential duration observed in failing heart cells (Priebe & Beuckelmann, 1998; Tomaselli & Marban, 1999), and dysfunction of the channels is implicated in certain forms of inherited and acquired long QT syndrome (Sanguinetti, 1999).
Cardiac IKr is regulated by intracellular serine/threonine phosphorylation systems. The current is negatively regulated by protein kinase A (Karle et al., 2002) and positively regulated by protein kinase C (Heath & Terrar, 2000). Although there is evidence that other cardiac currents regulated by these kinases are also acutely regulated by protein tyrosine kinase (PTK) (e.g., Wang & Lipsius, 1998; Ogura et al., 1999; Wang et al., 2003), there is no information (to our knowledge) on whether cardiac IKr is regulated by PTK. The objective of this study was to investigate that possibility by measuring IKr in guinea-pig ventricular myocytes that were exposed to inhibitors and stimulators of tyrosine phosphorylation. The compounds used to inhibit tyrosine phosphorylation were the broadspectrum PTK inhibitors tyrphostin A23, tyrphostin A25, and genistein (Gazit et al., 1989; Akiyama & Ogawara, 1991), whereas the compounds used to stimulate tyrosine phosphorylation were the phosphotyrosyl phosphatase (PTP) inhibitor orthovanadate (Swarup et al., 1982), exogenous c-Src PTK, and four activators of receptor PTK (insulin, insulin-like growth factor-1 (IGF-1), epidermal growth factor (EGF), and basic fibroblast growth factor (bFGF)).
Methods
Preparation of myocytes
Adult guinea-pigs (225–275 g) were killed by cervical dislocation and exsanguination in accordance with the national and university regulations on animal experimentation. Hearts were quickly removed, mounted on a Langendorff column, and perfused through the coronary artery for 10–15 min. The Ca2+-free perfusate (37°C) contained (in mM) NaCl 125, KCl 5, MgCl2 1.2, taurine 20, glucose 20, and N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES) 5 (pH 7.4), as well as 0.08–0.12 mg ml−1 collagenase (Yakult Pharmaceutical Co., Tokyo, Japan). On completion of collagenase digestion, the heart tissue was minced, and myocytes dispersed in a high-K+, nutrient-supplemented storage solution (22°C) that contained KCl 30, KOH 80, KH2PO4 30, MgSO4 3, glutamic acid 50, taurine 20, glucose 20, ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA) 0.5, and HEPES 10 (pH 7.4 with KOH).
Electrophysiology
Myocytes were voltage-clamped using either the standard ruptured-patch method or the nystatin perforated-patch method. Recording pipettes were fabricated from thick-walled borosilicate glass capillaries (H15/10/137, Jencons Scientific Ltd, Leighton Buzzard, U.K.), and had resistances of 2–3 MΩ when filled with dialysate. Pipette offsets were nulled before patch formation, and liquid-junction potentials (≈ −10 mV) were offset during data analysis. In the ruptured-patch experiments, series resistance ranged between 4 and 8 MΩ and was compensated by 60–80%. In the perforated-patch experiments, series resistance declined to 8–25 MΩ within 10–20 min of seal formation, and experiments were initiated when it was stable over a 10-min period. Series resistance compensation was used in most of the experiments such that uncompensated resistance was reduced below 10 MΩ (typically 2–6 MΩ). Membrane currents were recorded with an EPC-9 amplifier (HEKA Electronics, Mahone Bay, NS, Canada). The electrical signals were low-pass filtered at 3 kHz, and digitized with an A/D converter (Digidata 1200A, Axon Instruments, Foster City, CA, U.S.A.) and pCLAMP software (Axon Instruments) at a sampling rate of 8–10 kHz before analysis. All experiments were conducted at 36°C.
Superfusates and pipette solutions
The standard superfusate was a Tyrode's solution that contained (in mM) NaCl 140, KCl 5.4, CaCl2 1.8, MgCl2 1.2, glucose 10, and HEPES 5 (pH 7.4 with NaOH), as well as 3 μM glibenclamide (Sigma-Aldrich, Oakville, ON, Canada) to suppress any ATP-sensitive K+ current that might arise following application of TK inhibitors (Harvey & Ashford, 1998; Stadnicka et al., 2002). In some experiments, the superfusate was a K+-, Ca2+-free Cd2+ solution that contained (in mM) NaCl 140, Cd2+ 0.2, MgCl2 1.2, glucose 10, and HEPES 5 (pH 7.4 with NaOH). The standard pipette solution used for ruptured-patch experiments contained (in mM) KCl 30, potassium aspartate 110, MgATP 5, EGTA 5, and HEPES 5 (pH 7.2 with KOH). The pipette solution used in the perforated-patch experiments contained (in mM) KCl 30, potassium aspartate 110, MgCl2 5, and HEPES 5 (pH 7.2 with KOH). Nystatin (Sigma-Aldrich) was added to this pipette-filling solution from a stock solution (100 mg ml−1 in dimethyl sulphoxide (DMSO)) to give a final concentration of 50–100 μg ml−1. The nystatin stock solution was freshly prepared and used for up to 2 h.
Chemicals and drugs
All chemicals used in making solutions were purchased from Sigma-Aldrich and were of the highest purity grade available. Daidzein, genistein, glibenclamide, and tyrphostins A1, A23, A51, and A63 were purchased from Calbiochem (La Jolla, CA, U.S.A.), and nisoldipine was kindly provided by Bayer (Etobicoke, ON, Canada). These compounds were dissolved in DMSO (Sigma-Aldrich), stored in the dark at −20°C, and added to experimental solutions as required. The highest eventual concentration of DMSO in bathing solutions was 0.1%, a concentration that had no effect on IKr; nevertheless, when experiments were conducted with drug-containing bathing solution that had ⩾0.05% DMSO, the control bathing solution generally contained the same concentration of DMSO. Sodium orthovanadate (Fisher Scientific, Nepeon, ON, Canada) was freshly prepared in water before experiments, and added to superfusates whose pH was then adjusted with HCl. E4031 (Eisai, Tokyo, Japan) was added to the bathing solution, and active c-Src PTK (human, recombinant) (Upstate Biotechnology, Lake Placid, NY, U.S.A.) was added to the pipette solution. Insulin (human, recombinant), IGF-1 (human, recombinant), EGF (human, recombinant), and bFGF (recombinant) were obtained from Calbiochem and added to the bathing solution.
Statistics
Experimental data are expressed as means±s.e.m.; n represents the number of experiments. Statistical comparisons were made using Student's paired or unpaired t-test. Differences were considered significant when P<0.05.
Results
The effects of modulators of tyrosine phosphorylation on IKr were investigated in myocytes that were bathed with standard Tyrode's solution, and pretreated with 1 μM nisoldipine to suppress L-type Ca2+ current. The myocytes were pulsed with short (200 ms) low-amplitude (–40 to 0 mV) steps that selectively activate IKr over IKs (Sanguinetti & Jurkiewicz, 1990; Jones et al., 2000), and changes in IKr were monitored by measuring the amplitude of tail currents on repolarisations to −40 mV. Under control conditions, the IKr tails in eight ruptured-patch myocytes deactivated in a biexponential fashion (τ1=299±21 ms, amplitude 76±9 pA; τ2=3155±262 ms, amplitude 66±5 pA) as described in earlier studies on guinea-pig (Heath & Terrar, 1996) and canine (Liu & Antzelevitch, 1995; Varró et al., 2000) ventricular myocytes.
Effects of PTK inhibitors and their inactive analogues on IKr
Experiments on ruptured-patch myocytes
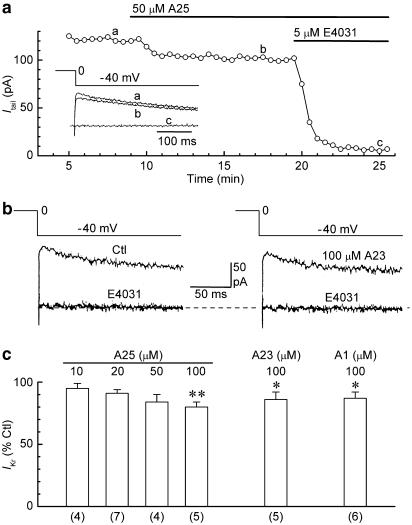
Figure 1a shows the data obtained from a ruptured-patch myocyte that was exposed to PTK inhibitor tyrphostin A25 (50 μM). The amplitude of IKr was only slightly reduced by the inhibitor, whereas it was reduced to almost zero by subsequent addition of 5 μM E4031, a specific inhibitor of cardiac IKr (Sanguinetti & Jurkiewicz, 1990). On average, application of 50 μM tyrphostin A25 for ≈ 10 min reduced the amplitude of the current by 16±6% (n=4), and application of 100 μM reduced it by 20±4% (n=5) (P<0.01) (Figure 1c). Similar-sized reductions in the amplitude of IKr were measured in myocytes that were exposed for 10 min to PTK inhibitor tyrphostin A23 (100 μM) (14±6%, n=5) (P<0.05). For comparison with these results, myocytes were exposed to tyrphostin A1 (100 μM), a PTK-inactive analogue of tyrphostins A23 and A25 (Gazit et al., 1989). The analogue reduced the amplitude of IKr by 13±5% (n=6) (P<0.05) (Figure 1c).
Figure 1.
Effects of tyrphostins A25, A23, and A1 on IKr in ruptured-patch myocytes. The myocytes were pulsed with 200-ms steps from −40 to 0 mV for measurement of the amplitude of the IKr tail on repolarisation to −40 mV. (a) Effects of 50 μM tyrphostin A25 and subsequent 5 μM E4031 on IKr tail amplitude in a representative myocyte. Inset: superimposed current records obtained at the times indicated on the plot. (b) Tail current records obtained before (Ctl) (left) and 10 min after application of 100 μM tyrphostin A23 (A23) (right) to a representative myocyte. Two traces obtained after addition of E4031 (as in panel a) are superimposed on the control and A23 records. (c) Summary of the results obtained with the three tyrphostin compounds. *P<0.05, **P<0.01, versus predrug control amplitude. Numbers of myocytes in parentheses.
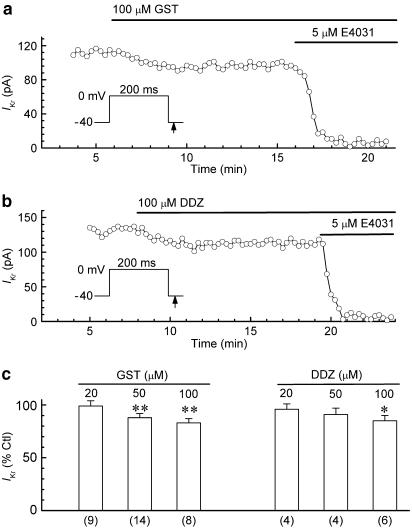
Similar experiments were conducted with genistein, a PTK inhibitor that is structurally and mechanistically different than the tyrphostins used here (Gazit et al., 1989; Davis et al., 2001). As illustrated by the results obtained from a myocyte exposed to 100 μM genistein for 10 min (Figure 2a), the inhibitor had relatively small effects on IKr. Overall, genistein (20, 50, and 100 μM) decreased the amplitude of the IKr tail by 1±5% (n=9), 12±4% (n=14) (P<0.01), and 17±4% (n=8) (P<0.01), respectively (Figure 2c). As a negative control for the involvement of PTK in the inhibitory action of genistein, we evaluated the effects of daidzein (20, 50, and 100 μM). Like genistein, the inactive analogue had a modest concentration-dependent inhibitory effect on IKr; at 100 μM, the current amplitude was reduced by 15±5% (n=6) (P<0.05) (Figure 2b and c).
Figure 2.
Effects of genistein (GST) and daidzein (DDZ) on IKr in ruptured-patch myocytes. The myocytes were pulsed with 200-ms steps from −40 to 0 mV for measurement of the amplitude of the IKr tail on repolarisation to −40 mV. (a, b) Effects of 100 μM GST and 100 μM DDZ on IKr tail amplitude in representative myocytes. (c) Summary of the results obtained with 8–10 min applications of the drugs. *P<0.05, **P<0.01 versus predrug control amplitude. Numbers of myocytes in parentheses.
Experiments on perforated-patch myocytes
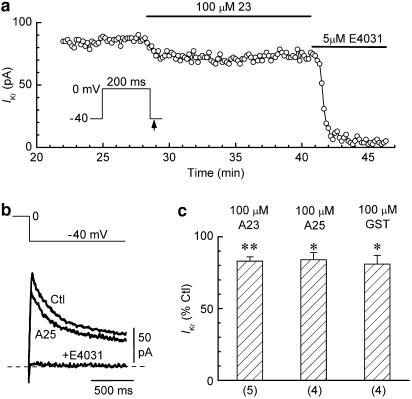
The ‘true' responses of whole-cell IKr to kinase modulators may be better preserved in myocytes investigated using the perforated-patch technique than in those investigated using the ruptured-patch technique (Heath & Terrar, 2000). To determine whether this was the case in the present study, we patched myocytes with pipettes that were filled with a nystatin solution (Korn & Horn, 1989; Missan et al., 2004). The representative data and summary presented in Figure 3 indicate that the effects of 100 μM concentrations of tyrphostin A23, tyrphostin A25, and genistein on IKr in perforated-patch myocytes were similar to those observed in ruptured-patch myocytes.
Figure 3.
Effects of TK inhibitors on IKr in perforated-patch myocytes. (a) Moderate reduction of IKr amplitude during application of 100 μM tyrphostin A23 to a representative myocyte. (b) Superimposed records of IKr tails obtained from a myocyte before (Ctl), 12 min after the addition of 100 μM tyrphostin A25, and 4 min after the subsequent addition of 5 μM E4031. (c) Summary of the results obtained with the tyrphostins and genistein. *P<0.05, **P<0.01 versus predrug control amplitude. Numbers of myocytes in parentheses.
A test of the ‘activity' of PTK inhibitors
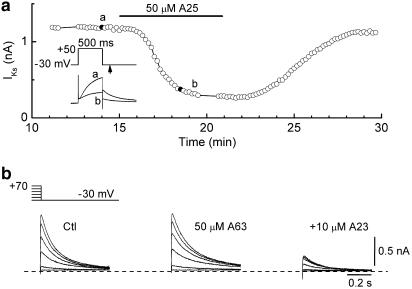
The relatively small effects of PTK inhibitors on IKr raised the question of whether these agents were indeed ‘active', that is, did they modulate other membrane currents in a manner expected from the results of earlier studies? To address that question, we investigated the effects of PTK inhibitors on slowly-activating IKs because it has been suggested that at least part of the rapid inhibitory effect of 50 μM genistein on canine (Zhou et al., 1997) and guinea-pig (Hool et al., 1998) ventricular IKs is related to an inhibition of PTK. The experiments on IKs were performed on ruptured-patch myocytes under conditions (K+-free Cd2+ external solution; long depolarisations) that enhance IKs and minimise IKr (Sanguinetti & Jurkiewicz, 1990; Missan et al., 2003). The myocytes were depolarised from −30 to +50 mV for 500 ms at 0.1 Hz except for sequences of 2-s depolarisations at appropriate intervals. Figure 4a shows the time course of the amplitude of the IKs tail (−30 mV) recorded from a myocyte that was exposed to 50 μM tyrphostin A25. The inhibitor lowered the amplitude by ≈75%, and this action was fully reversed during the 10-min washout period. In five experiments of this type, the IKs tail after 2-s depolarisations to +70 mV was reduced to 23±5% of its control amplitude (P<0.001). This action of tyrphostin A25 may well be related to an inhibition of PTK because IKs was insensitive to PTK-inactive tyrphostin A63 (50 μM) but strongly suppressed by 10 μM tyrphostin A23 (n=3; e.g., Figure 4b).
Figure 4.
Effects of tyrphostin compounds on slowly-activating IKs in two ruptured-patch myocytes. The myocytes were bathed in a K+-free Cd2+ solution and depolarised from −30 mV at 0.1 Hz. (a) Time plot of the amplitude of IKs tails following 500-ms depolarisations to +50 mV in a myocyte exposed to 50 μM tyrphostin A25. Inset: records obtained at the times indicated in the plot. (b) Families of IKs tails following 2-s depolarisations from −30 mV. The tails were recorded before drug treatment (Ctl), after 10-min exposure to PTK-inactive tyrphostin A63 (50 μM) (middle), and after a subsequent 10-min exposure to combined tyrphostin A63 and tyrphostin A23 (10 μM).
Lack of effect of PTP inhibitor orthovanadate on IKr
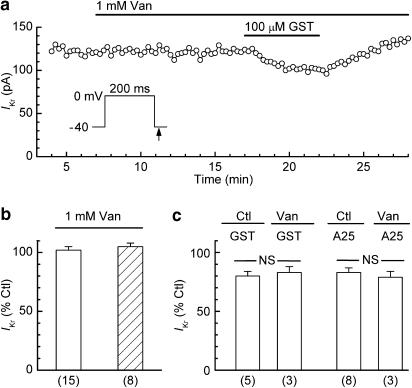
The foregoing results suggested that IKr was little affected by treatments aimed at lowering the basal activity of PTK. To evaluate whether the current was affected by an inhibition of basal PTP activity, myocytes were treated with 1 mM orthovanadate for 10 min. These treatments had no significant effect on the amplitude of IKr in either ruptured-patch myocytes (increase of 2±3%; n=15) or perforated-patch myocytes (increase of 5±3%; n=8) (Figure 5a and b). In some of the experiments on ruptured-patch myocytes, the orthovanadate pretreatments were followed by cotreatments with 100 μM tyrphostin A25 or 100 μM genistein to determine whether the inhibition of PTP blocked the inhibition of IKr by the PTK inhibitors. As indicated by the data in Figure 5a and c, pretreatment with orthovanadate had no effect on the inhibitory actions of tyrphostin A25 and genistein.
Figure 5.
Effects of orthovanadate and PTK inhibitors on IKr. The myocytes were pulsed with 200-ms steps from −40 to 0 mV. (a) Time plot of the amplitude of the IKr tail in a ruptured-patch myocyte that was treated with 1 mM orthovanadate (Van) and then co-treated with 100 μM genistein (GST). The pretreatment did not prevent inhibition by GST. (b) Lack of effect of 1 mM orthovanadate on the amplitude of IKr in control experiments on ruptured-patch (open bar) and perforated-patch (hatched bar) myocytes. (c) Lack of effect of orthovanadate pretreatment on the responses of IKr to 100 μM tyrphostin A25 and 100 μM GST in ruptured-patch myocytes. NS: no significant difference between the two groups (unpaired t-test). The Van data obtained from myocytes in the Van/A25 and Van/GST experiments are included in the Van (1 mM) data of panel b. The control A25 and GST data are from Figures 1c and 2c, respectively. Numbers of myocytes in parentheses.
Effects of stimulators of tyrosine phosphorylation on IKr
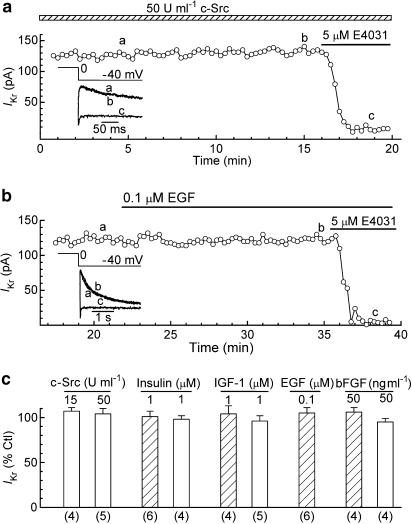
The first approach used to stimulate tyrosine phosphorylation was to dialyse ruptured-patch myocytes with pipette solution that contained 15 or 50 U ml−1 c-Src PTK. From earlier whole-cell studies on the role of c-Src in the regulation of other ion channel types (e.g., Caraiscos et al., 2002; Strauss et al., 2002), it seemed likely that maximal effects of the kinase would be observed in 5–10 min. In the present study, myocytes were dialysed with the kinase solution for 10–15 min before the addition of 5 μM E4031. As indicated by the data shown in Figure 6a and c, the kinase dialysate had no detectable effect on the amplitude of IKr.
Figure 6.
Effects of stimulators of tyrosine phosphorylation on IKr in ruptured-patch and perforated-patch myocytes. The myocytes were pulsed from −40 to 0 mV for 200 ms for measurement of the amplitude of the IKr tail on repolarisation to −40 mV before (control) and 10–15 min after the initiation of treatment. (a) IKr in a ruptured-patch myocyte dialysed with pipette solution that contained 50 U ml−1 c-Src. Inset: superimposed records obtained at the times indicated in the plot. (b) IKr in a perforated-patch myocyte treated with 0.1 μM EGF. Inset: superimposed records obtained at the post-pipette-attachment times indicated in the plot. (c) Summary of the data obtained on ruptured-patch (open bars) and perforated-patch (hatched bars) myocytes. In the experiments with c-Src dialysate, the amplitude of IKr measured during the first minute of dialysis was taken as the control amplitude. Numbers of myocytes in parentheses.
The second approach used to stimulate tyrosine phosphorylation was to treat myocytes with ligands that on binding their receptors cause activation of receptor PTK. In the heart, these receptors include those that bind insulin (Eckel et al., 1985; Gupta et al., 1989), IGF-1 (Engelmann et al., 1989), EGF (Rabkin et al., 1987; Yu et al., 1992), and bFGF (Schneider & Parker, 1990; Akiyama et al., 1999). The 10–15 min duration of the treatments with these four ligands was as long or longer than that required for full development of acute responses in earlier studies on cardiac preparations (e.g., Cittadini et al., 1998; Quintaje et al., 1998; Aulbach et al., 1999; Wu et al., 2000). The experiments were conducted on both ruptured-patch and perforated-patch (e.g., Figure 6b) myocytes, and the results are summarised in Figure 6c. Neither insulin (1 μM), IGF-1 (1 μM) nor bFGF (50 ng ml−1) had any significant effect on the amplitude of IKr in ruptured-patch myocytes. Similarly, neither these ligands nor EGF (0.1 μM) had any significant effect on the current in perforated-patch myocytes.
Discussion
It is now well established that a wide variety of ion channels are acutely regulated by PTK (for reviews, see Davis et al., 2001; Cohen, 2005). We have investigated the possible involvement of PTK in the acute regulation of Kr channels in guinea-pig ventricular myocytes by measuring the responses of IKr to applications of broadspectrum PTK inhibitors, PTK-inactive analogues, PTP inhibitor orthovanadate, pipette c-Src PTK, insulin, and growth factors IGF-1, EGF, and bFGF. As discussed below, the results suggest that PTK does not have a significant role in the regulation of these channels.
We used 10–100 μM concentrations of tyrphostin A23, tyrphostin A25, and genistein to evaluate whether inhibition of basal PTK activity had an effect on IKr. Similar concentrations of these inhibitors have previously been employed to evaluate PTK regulation of L-type Ca2+ channels (Wijetunge & Hughes, 1995; Ogura et al., 1999; Wijetunge et al., 2000), Na+ channels (Wang et al., 2003), hyperpolarisation-activated cation channels (Wu & Cohen, 1997; Wu et al., 2000), and swelling-activated Cl− channels (Du et al., 2004). We found that the PTK inhibitors reduced the amplitude of IKr in a concentration-dependent manner, but that the degree of inhibition was small, that is, 100 μM concentrations only reduced the current by 14–20%. However, even this small reduction in IKr is more likely to have been due to low-affinity channel block than to an inhibition of PTK because (i) similar-sized reductions were observed with 100 μM concentrations of PTK-inactive analogues tyrphostin A1 and daidzein and (ii) pretreatment of myocytes with PTP inhibitor orthovanadate had no significant effect on the reduction of IKr by PTK inhibitors.
The responses of IKr to PTK inhibitors, PTK-inactive analogues, and PTK inhibitors in the presence of orthovanadate, all suggested that a lowering of basal tyrosine phosphorylation has little effect on the activity of Kr channels in guinea-pig ventricular myocytes. However, the primary results were obtained on ruptured-patch myocytes, and this raised the possibility that the effects of the signalling probes had been distorted by the washout of a critical cytoplasmic constituent. This appears not to have been the case, because the effects of the three PTK inhibitors and orthovanadate on IKr in perforated-patch myocytes (minimal washout) were not different than those in ruptured-patch myocytes.
To our knowledge, there have been no previous reports on the effects of PTK inhibitors on cardiac IKr. However, it is of interest to compare the present results with those obtained in two studies on the role of PTK in the acute regulation of ‘ether-a-go-go' related gene (ERG) K+ channels. Cayabyab & Schlichter (2002) recorded native ERG current in a rat microglial cell line (MLS-9), and found that 50 μM genistein reduced current amplitude by ≈60%, whereas 50 μM daidzein only reduced it by an insignificant 19±8% (see also Schlichter et al., 2006). On the other hand, Schledermann et al. (2001) recorded currents carried by rat ERG1 α-subunits expressed in GH3/B6 cells and found that acute application of 100 μM tyrphostin A23 only reduced the maximal amplitude of the current by about 18%. Our results with 100 μM tyrphostin A23 are in agreement with those of the Schledermann et al. (2001) study.
An important objective of the present study was to determine whether pharmacological treatments designed to produce a stimulation of tyrosine phosphorylation caused a change in the amplitude of IKr. The compounds used for this purpose included recombinant c-Src PTK and four activators of receptor PTK (insulin, IGF-1, EGF, and bFGF). c-Src PTK was supplied via the pipette at strengths (15 and 50 U ml−1) similar to those (20–30 U ml−1) used in earlier whole-cell studies on ion channel regulation (e.g., Caraiscos et al., 2002; Strauss et al., 2002; Feranchak et al., 2003). However, dialysis of the kinase for up to 15 min caused little change in IKr. The trials with insulin and IGF-1 were conducted with a concentration (1 μM) that rapidly stimulated cardiac contraction and L-type Ca2+ current (Strömer et al., 1996; Cittadini et al., 1998; Aulbach et al., 1999), whereas those with bFGF used a concentration (50 ng ml−1) equal to or higher than those which rapidly stimulated Ca2+ currents in glial and neuronal cells (Puro & Mano, 1991; Koike et al., 1993) and mitogen-activated protein kinase (MAPK) activity in cardiomyocytes (Eppenberger-Eberhardt et al., 1997). The outcome of these trials on ruptured-patch and perforated-patch myocytes was an unchanged IKr. EGF was applied at a maximally effective concentration of 0.1 μM (Lorita et al., 2002) (see also Wu et al., 2000) and, like the other receptor-PTK activators, had no significant effect on IKr in perforated-patch myocytes.
A common action of insulin and the three growth factors studied here is they stimulate the MAPK pathway (Pawson & Scott, 1997; Quintaje et al., 1998; Siddle et al., 2001). There is accumulating evidence that activation of two terminal MAPKs, ERK (extracellular-regulated kinase) 1 and 2, has a stimulatory effect on an array of ion channel types, including Ca2+ channels (Ma et al., 1996), volume-sensitive Cl− channels (Crepel et al., 1998), ATP-sensitive K+ channels (O'Malley et al., 2003), large conductance Ca2+-activated K+ channels (O'Malley et al., 2003; O'Malley & Harvey, 2004), and Kv4.2 channels (Schrader et al., 2005). The lack of effect of insulin and growth factors on myocyte IKr suggests that in the absence of other perturbations, ERK1 and 2 have limited involvement in the regulation of cardiac Kr channels.
Recent studies on the effects of tyrosine phosphorylation modulators on cardiac myocytes suggest that hyperpolarisation-activated pacemaker current (Yu et al., 2000, 2004), L-type Ca2+ current (Hool et al., 1998; Wang & Lipsius, 1998; Ogura et al., 1999), Na+ current (Wang et al., 2003), transient outward current (Wang et al., 2002), and volume-sensitive Cl− current (Du et al., 2004; Ren & Baumgarten, 2005) are under the acute regulatory influence of PTK. The results of the present study indicate that this is unlikely to be the case for IKr.
Acknowledgments
We are grateful to Ms Gina Dickie for excellent technical assistance. This work was supported by the Heart and Stroke Foundation of New Brunswick, and by the Canadian Institutes of Health Research.
Abbreviations
- bFGF1
basic fibroblast growth factor
- DMSO
dimethyl sulphoxide
- EGF
epidermal growth factor
- EGTA
ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid
- ERG
‘ether-a-go-go'-related gene
- ERK
extracellular-regulated kinase
- HEPES
N-2-hydroxyethylpiperazine-N′-2-ethanesulphonic acid
- IGF-1
insulin-like growth factor-1
- IKr
rapidly activating delayed-rectifier K+ current
- IKs
slowly activating delayed-rectifier K+ current
- I–V
current–voltage
- MAPK
mitogen-activated protein kinase
- PTP
phosphotyrosyl phosphatase
- PTK
protein tyrosine kinase
References
- AKIYAMA T., OGAWARA H. Use and specificity of genistein as inhibitor of protein-tyrosine kinases. Methods Enzymol. 1991;201:362–370. doi: 10.1016/0076-6879(91)01032-w. [DOI] [PubMed] [Google Scholar]
- AKIYAMA Y., ASHIZAWA N., SETO S., OHTSURU A., KURODA H., ITO M., YAMASHITA S., YANO K. Involvement of receptor-type tyrosine kinase gene families in cardiac hypertrophy. J. Hypertens. 1999;17:1329–1337. doi: 10.1097/00004872-199917090-00014. [DOI] [PubMed] [Google Scholar]
- AULBACH F., SIMM A., MAIER S., LANGENFELD H., WALTER U., KERSTING U., KIRSTEIN M. Insulin stimulates the L-type Ca2+ current in rat cardiac myocytes. Cardiovasc. Res. 1999;42:113–120. doi: 10.1016/s0008-6363(98)00307-1. [DOI] [PubMed] [Google Scholar]
- CARAISCOS V.B., MIHIC S.J., MACDONALD J.F., ORSER B.A. Tyrosine kinases enhance the function of glycine receptors in rat hippocampal neurons and human alpha(1)beta glycine receptors. J. Physiol. 2002;539:495–502. doi: 10.1113/jphysiol.2001.013508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAYABYAB F.S., SCHLICHTER L.C. Regulation of an ERG K+ current by Src tyrosine kinase. J. Biol. Chem. 2002;277:13673–13681. doi: 10.1074/jbc.M108211200. [DOI] [PubMed] [Google Scholar]
- CITTADINI A., ISHIGURO Y., STROMER H., SPINDLER M., MOSES A.C., CLARK R., DOUGLAS P.S., INGWALL J.S., MORGAN J.P. Insulin-like growth factor-1 but not growth hormone augments mammalian myocardial contractility by sensitizing the myofilament to Ca2+ through a wortmannin-sensitive pathway: studies in rat and ferret isolated muscles. Circ. Res. 1998;83:50–59. doi: 10.1161/01.res.83.1.50. [DOI] [PubMed] [Google Scholar]
- COHEN D.M. SRC family kinases in cell volume regulation. Am. J. Physiol. Cell. Physiol. 2005;288:C483–C493. doi: 10.1152/ajpcell.00452.2004. [DOI] [PubMed] [Google Scholar]
- CREPEL V., PANENKA W., KELLY M.E., MACVICAR B.A. Mitogen-activated protein and tyrosine kinases in the activation of astrocyte volume-activated chloride current. J. Neurosci. 1998;18:1196–1206. doi: 10.1523/JNEUROSCI.18-04-01196.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS M.J., WU X., NURKIEWICZ T.R., KAWASAKI J., GUI P., HILL M.A., WILSON E. Regulation of ion channels by protein tyrosine phosphorylation. Am. J. Physiol. Heart Circ. Physiol. 2001;281:H1835–H1862. doi: 10.1152/ajpheart.2001.281.5.H1835. [DOI] [PubMed] [Google Scholar]
- DU X.L., GAO Z., LAU C.P., CHIU S.W., TSE H.F., BAUMGARTEN C.M., LI G.R. Differential effects of tyrosine kinase inhibitors on volume-sensitive chloride current in human atrial myocytes: evidence for dual regulation by Src and EGFR kinases. J. Gen. Physiol. 2004;123:427–439. doi: 10.1085/jgp.200409013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECKEL J., VAN ECHTEN G., REINAUER H. Adult cardiac myocytes in primary culture: cell characteristics and insulin-receptor interaction. Am. J. Physiol. 1985;249:H212–H221. doi: 10.1152/ajpheart.1985.249.2.H212. [DOI] [PubMed] [Google Scholar]
- ENGELMANN G.L., BOEHM K.D., HASKELL J.F., KHAIRALLAH P.A., ILAN J. Insulin-like growth factors and neonatal cardiomyocyte development: ventricular gene expression and membrane receptor variations in normotensive and hypertensive rats. Mol. Cell. Endocrinol. 1989;63:1–14. doi: 10.1016/0303-7207(89)90076-2. [DOI] [PubMed] [Google Scholar]
- EPPENBERGER-EBERHARDT M., AIGNER S., DONATH M.Y., KURER V., WALTHER P., ZUPPINGER C., SCHAUB M.C., EPPENBERGER H.M. IGF-I and bFGF differentially influence atrial natriuretic factor and alpha-smooth muscle actin expression in cultured atrial compared to ventricular adult rat cardiomyocytes. J. Mol. Cell. Cardiol. 1997;29:2027–2039. doi: 10.1006/jmcc.1997.0408. [DOI] [PubMed] [Google Scholar]
- FERANCHAK A.P., KILIC G., WOJTASZEK P.A., QADRI I., FITZ J.G. Volume-sensitive tyrosine kinases regulate liver cell volume through effects on vesicular trafficking and membrane Na+ permeability. J. Biol. Chem. 2003;278:44632–44638. doi: 10.1074/jbc.M301958200. [DOI] [PubMed] [Google Scholar]
- GAZIT A., YAISH P., GILON C., LEVITZKI A. Tyrphostins I: synthesis and biological activity of protein tyrosine kinase inhibitors. J. Med. Chem. 1989;32:2344–2352. doi: 10.1021/jm00130a020. [DOI] [PubMed] [Google Scholar]
- GUPTA M.P., LEE S.L., DHALLA N.S. Activation of heart sarcoplasmic reticulum Ca++-stimulated adenosine triphosphatase by insulin. J. Pharmacol. Exp. Ther. 1989;249:623–630. [PubMed] [Google Scholar]
- HARVEY J., ASHFORD M.L. Role of tyrosine phosphorylation in leptin activation of ATP-sensitive K+ channels in the rat insulinoma cell line CRI-G1. J. Physiol. 1998;510 (Part 1):47–61. doi: 10.1111/j.1469-7793.1998.047bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEATH B.M., TERRAR D.A. The deactivation kinetics of the delayed rectifier components IKr and IKs in guinea-pig isolated ventricular myocytes. Exp. Physiol. 1996;81:605–621. doi: 10.1113/expphysiol.1996.sp003962. [DOI] [PubMed] [Google Scholar]
- HEATH B.M., TERRAR D.A. Protein kinase C enhances the rapidly activating delayed rectifier potassium current, IKr, through a reduction in C-type inactivation in guinea-pig ventricular myocytes. J. Physiol. 2000;522 (Part 3):391–402. doi: 10.1111/j.1469-7793.2000.t01-2-00391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOOL L.C., MIDDLETON L.M., HARVEY R.D. Genistein increases the sensitivity of cardiac ion channels to beta-adrenergic receptor stimulation. Circ. Res. 1998;83:33–42. doi: 10.1161/01.res.83.1.33. [DOI] [PubMed] [Google Scholar]
- JONES S.E., OGURA T., SHUBA L.M., MCDONALD T.F. Inhibition of the rapid component of the delayed-rectifier K+ current by therapeutic concentrations of the antispasmodic agent terodiline. Br. J. Pharmacol. 1998;125:1138–1143. doi: 10.1038/sj.bjp.0702173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JONES S.E., SHUBA L.M., ZHABYEYEV P., MCCULLOUGH J.R., MCDONALD T.F. Differences in the effects of urinary incontinence agents S-oxybutynin and terodiline on cardiac K+ currents and action potentials. Br. J. Pharmacol. 2000;131:245–254. doi: 10.1038/sj.bjp.0703595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KARLE C.A., ZITRON E., ZHANG W., KATHOFER S., SCHOELS W., KIEHN J. Rapid component IKr of the guinea-pig cardiac delayed rectifier K+ current is inhibited by beta1-adrenoreceptor activation, via cAMP/protein kinase A-dependent pathways. Cardiovasc. Res. 2002;53:355–362. doi: 10.1016/s0008-6363(01)00509-0. [DOI] [PubMed] [Google Scholar]
- KOIKE H., SAITO H., MATSUKI N. Effect of fibroblast growth factors on calcium currents in acutely isolated neuronal cells from rat ventromedial hypothalamus. Neurosci. Lett. 1993;150:57–60. doi: 10.1016/0304-3940(93)90107-v. [DOI] [PubMed] [Google Scholar]
- KORN S.J., HORN R. Influence of sodium-calcium exchange on calcium current rundown and the duration of calcium-dependent chloride currents in pituitary cells, studied with whole cell and perforated patch recording. J. Gen. Physiol. 1989;94:789–812. doi: 10.1085/jgp.94.5.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU D.W., ANTZELEVITCH C. Characteristics of the delayed rectifier current (IKr and IKs) in canine ventricular epicardial, midmyocardial, and endocardial myocytes. A weaker IKs contributes to the longer action potential of the M cell. Circ. Res. 1995;76:351–365. doi: 10.1161/01.res.76.3.351. [DOI] [PubMed] [Google Scholar]
- LORITA J., ESCALONA N., FARAUDO S., SOLEY M., RAMIREZ I. Effects of epidermal growth factor on epinephrine-stimulated heart function in rodents. Am. J. Physiol. Heart Circ. Physiol. 2002;283:H1887–H1895. doi: 10.1152/ajpheart.00217.2002. [DOI] [PubMed] [Google Scholar]
- MA H., MATSUNAGA H., LI B., SCHIEFFER B., MARRERO M.B., LING B.N. Ca2+ channel activation by platelet-derived growth factor-induced tyrosine phosphorylation and Ras guanine triphosphate-binding proteins in rat glomerular mesangial cells. J. Clin. Invest. 1996;97:2332–2341. doi: 10.1172/JCI118676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MISSAN S., ZHABYEYEV P., DYACHOK O., JONES S.E., MCDONALD T.F. Block of cardiac delayed-rectifier and inward-rectifier K+ currents by nisoldipine. Br. J. Pharmacol. 2003;140:863–870. doi: 10.1038/sj.bjp.0705518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MISSAN S., ZHABYEYEV P., DYACHOK O., OGURA T., MCDONALD T.F. Inward-rectifier K+ current in guinea-pig ventricular myocytes exposed to hyperosmotic solutions. J. Membr. Biol. 2004;202:151–160. doi: 10.1007/s00232-004-0726-3. [DOI] [PubMed] [Google Scholar]
- OGURA T., SHUBA L.M., MCDONALD T.F. L-type Ca2+ current in guinea pig ventricular myocytes treated with modulators of tyrosine phosphorylation. Am. J. Physiol. Heart Circ. Physiol. 1999;276:H1724–H1733. doi: 10.1152/ajpheart.1999.276.5.H1724. [DOI] [PubMed] [Google Scholar]
- O'MALLEY D., HARVEY J. Insulin activates native and recombinant large conductance Ca2+-activated potassium channels via a mitogen-activated protein kinase-dependent process. Mol. Pharmacol. 2004;65:1352–1363. doi: 10.1124/mol.65.6.1352. [DOI] [PubMed] [Google Scholar]
- O'MALLEY D., SHANLEY L.J., HARVEY J. Insulin inhibits rat hippocampal neurones via activation of ATP-sensitive K+ and large conductance Ca2+-activated K+ channels. Neuropharmacology. 2003;44:855–863. doi: 10.1016/s0028-3908(03)00081-9. [DOI] [PubMed] [Google Scholar]
- PAWSON T., SCOTT J.D. Signaling through scaffold, anchoring, and adaptor proteins. Science. 1997;278:2075–2080. doi: 10.1126/science.278.5346.2075. [DOI] [PubMed] [Google Scholar]
- PRIEBE L., BEUCKELMANN D.J. Simulation study of cellular electric properties in heart failure. Circ. Res. 1998;82:1206–1223. doi: 10.1161/01.res.82.11.1206. [DOI] [PubMed] [Google Scholar]
- PURO D.G., MANO T. Modulation of calcium channels in human retinal glial cells by basic fibroblast growth factor: a possible role in retinal pathobiology. J. Neurosci. 1991;11:1873–1880. doi: 10.1523/JNEUROSCI.11-06-01873.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- QUINTAJE S.B., REBSAMEN M., CHURCH D.J., VALLOTTON M.B., LANG U. MAP kinase mediates epidermal growth factor- and phorbol ester-induced prostacyclin formation in cardiomyocytes. J. Mol. Cell. Cardiol. 1998;30:933–945. doi: 10.1006/jmcc.1998.0657. [DOI] [PubMed] [Google Scholar]
- RABKIN S.W., SUNGA P., MYRDAL S. The effect of epidermal growth factor on chronotropic response in cardiac cells in culture. Biochem. Biophys. Res. Commun. 1987;146:889–897. doi: 10.1016/0006-291x(87)90614-0. [DOI] [PubMed] [Google Scholar]
- REN Z., BAUMGARTEN C.M. Antagonistic regulation of swelling-activated Cl− current in rabbit ventricle by Src and EGFR protein tyrosine kinases. Am. J. Physiol. Heart Circ. Physiol. 2005;288:H2628–H2636. doi: 10.1152/ajpheart.00992.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANGUINETTI M.C. Dysfunction of delayed rectifier potassium channels in an inherited cardiac arrhythmia. Ann. N.Y. Acad. Sci. 1999;868:406–413. doi: 10.1111/j.1749-6632.1999.tb11302.x. [DOI] [PubMed] [Google Scholar]
- SANGUINETTI M.C., JURKIEWICZ N.K. Two components of cardiac delayed rectifier K+ current. Differential sensitivity to block by class III antiarrhythmic agents. J. Gen. Physiol. 1990;96:195–215. doi: 10.1085/jgp.96.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHLEDERMANN W., WULFSEN I., SCHWARZ J.R., BAUER C.K. Modulation of rat erg1, erg2, erg3 and HERG K+ currents by thyrotropin-releasing hormone in anterior pituitary cells via the native signal cascade. J. Physiol. 2001;532:143–163. doi: 10.1111/j.1469-7793.2001.0143g.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHLICHTER L.C., JIANG J., NEWELL E.W., WANG J., TSUI F., WANG Y.The C-terminus of EAG-family K+ channels binds to and trans-activates SHP-1 tyrosine phosphatase: SHP-1 down-regulates and Src up-regulates the current Biophys. J. 200690101aAbstracts Issue16227505 [Google Scholar]
- SCHNEIDER M.D., PARKER T.G. Cardiac myocytes as targets for the action of peptide growth factors. Circulation. 1990;81:1443–1456. doi: 10.1161/01.cir.81.5.1443. [DOI] [PubMed] [Google Scholar]
- SCHRADER L.A., BIRNBAUM S.G., NADIN B.M., REN Y., BUI D., ANDERSON A.E., SWEATT J.D. ERK/MAPK regulates the Kv4.2 potassium channel by direct phosphorylation of the pore-forming subunit. Am. J. Physiol. Cell Physiol. 2005;290:C852–C861. doi: 10.1152/ajpcell.00358.2005. [DOI] [PubMed] [Google Scholar]
- SIDDLE K., URSO B., NIESLER C.A., COPE D.L., MOLINA L., SURINYA K.H., SOOS M.A. Specificity in ligand binding and intracellular signalling by insulin and insulin-like growth factor receptors. Biochem. Soc. Trans. 2001;29:513–525. doi: 10.1042/bst0290513. [DOI] [PubMed] [Google Scholar]
- STADNICKA A., KWOK W.M., WARLTIER D.C., BOSNJAK Z.J. Protein tyrosine kinase-dependent modulation of isoflurane effects on cardiac sarcolemmal KATP channel. Anesthesiology. 2002;97:1198–1208. doi: 10.1097/00000542-200211000-00025. [DOI] [PubMed] [Google Scholar]
- STRAUSS O., ROSENTHAL R., DEY D., BENINDE J., WOLLMANN G., THIEME H., WIEDERHOLT M. Effects of protein kinase C on delayed rectifier K+ channel regulation by tyrosine kinase in rat retinal pigment epithelial cells. Invest. Ophthalmol. Vis. Sci. 2002;43:1645–1654. [PubMed] [Google Scholar]
- STRÖMER H., CITTADINI A., DOUGLAS P.S., MORGAN J.P. Exogenously administered growth hormone and insulin-like growth factor-I alter intracellular Ca2+ handling and enhance cardiac performance. In vitro evaluation in the isolated isovolumic buffer-perfused rat heart. Circ. Res. 1996;79:227–236. doi: 10.1161/01.res.79.2.227. [DOI] [PubMed] [Google Scholar]
- SWARUP G., COHEN S., GARBERS D.L. Inhibition of membrane phosphotyrosyl-protein phosphatase activity by vanadate. Biochem. Biophys. Res. Commun. 1982;107:1104–1109. doi: 10.1016/0006-291x(82)90635-0. [DOI] [PubMed] [Google Scholar]
- TOMASELLI G.F., MARBAN E. Electrophysiological remodeling in hypertrophy and heart failure. Cardiovasc. Res. 1999;42:270–283. doi: 10.1016/s0008-6363(99)00017-6. [DOI] [PubMed] [Google Scholar]
- VARRÓ A., BALATI B., IOST N., TAKACS J., VIRAG L., LATHROP D.A., CSABA L., TALOSI L., PAPP J.G. The role of the delayed rectifier component IKs in dog ventricular muscle and Purkinje fibre repolarization. J. Physiol. 2000;523 (Part 1):67–81. doi: 10.1111/j.1469-7793.2000.00067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG Y., KUMAR R., WAGNER M.B., CHENG J., MISHRA M., JOYNER R.W. Regulation of transient outward current in human atrial myocytes by protein tyrosine kinase pathway. J. Cardiovasc. Electrophysiol. 2002;13:927–935. doi: 10.1046/j.1540-8167.2002.00927.x. [DOI] [PubMed] [Google Scholar]
- WANG Y., WAGNER M.B., KUMAR R., CHENG J., JOYNER R.W. Inhibition of fast sodium current in rabbit ventricular myocytes by protein tyrosine kinase inhibitors. Pflügers Arch. 2003;446:485–491. doi: 10.1007/s00424-003-1061-8. [DOI] [PubMed] [Google Scholar]
- WANG Y.G., LIPSIUS S.L. Genistein elicits biphasic effects on L-type Ca2+ current in feline atrial myocytes. Am. J. Physiol. Heart Circ. Physiol. 1998;275:H204–H212. doi: 10.1152/ajpheart.1998.275.1.H204. [DOI] [PubMed] [Google Scholar]
- WIJETUNGE S., HUGHES A.D. Effect of platelet-derived growth factor on voltage-operated calcium channels in rabbit isolated ear artery cells. Br. J. Pharmacol. 1995;115:534–538. doi: 10.1111/j.1476-5381.1995.tb16367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIJETUNGE S., LYMN J.S., HUGHES A.D. Effects of protein tyrosine kinase inhibitors on voltage-operated calcium channel currents in vascular smooth muscle cells and pp60(c-src) kinase activity. Br. J. Pharmacol. 2000;129:1347–1354. doi: 10.1038/sj.bjp.0703186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WU J.Y., COHEN I.S. Tyrosine kinase inhibition reduces If in rabbit sinoatrial node myocytes. Pflügers Arch. 1997;434:509–514. doi: 10.1007/s004240050430. [DOI] [PubMed] [Google Scholar]
- WU J.Y., YU H., COHEN I.S. Epidermal growth factor increases If in rabbit SA node cells by activating a tyrosine kinase. Biochim. Biophys. Acta. 2000;1463:15–19. doi: 10.1016/s0005-2736(99)00233-3. [DOI] [PubMed] [Google Scholar]
- YU H., GAO J., WANG H., WYMORE R., STEINBERG S., MCKINNON D., ROSEN M.R., COHEN I.S. Effects of the renin-angiotensin system on the current I(to) in epicardial and endocardial ventricular myocytes from the canine heart. Circ Res. 2000;86:1062–1068. doi: 10.1161/01.res.86.10.1062. [DOI] [PubMed] [Google Scholar]
- YU H.G., LU Z., PAN Z., COHEN I.S. Tyrosine kinase inhibition differentially regulates heterologously expressed HCN channels. Pflügers Arch. 2004;447:392–400. doi: 10.1007/s00424-003-1204-y. [DOI] [PubMed] [Google Scholar]
- YU Y., NAIR B.G., PATEL T.B. Epidermal growth factor stimulates cAMP accumulation in cultured rat cardiac myocytes. J. Cell. Physiol. 1992;150:559–567. doi: 10.1002/jcp.1041500317. [DOI] [PubMed] [Google Scholar]
- ZENG J., LAURITA K.R., ROSENBAUM D.S., RUDY Y. Two components of the delayed rectifier K+ current in ventricular myocytes of the guinea pig type. Theoretical formulation and their role in repolarization. Circ. Res. 1995;77:140–152. doi: 10.1161/01.res.77.1.140. [DOI] [PubMed] [Google Scholar]
- ZHOU Y.Y., YAO J.A., TSENG G.N. Role of tyrosine kinase activity in cardiac slow delayed rectifier channel modulation by cell swelling. Pflügers Arch. 1997;433:750–757. doi: 10.1007/s004240050341. [DOI] [PubMed] [Google Scholar]