Abstract
This study investigates the role of nitric oxide (NO) and reactive oxygen species (ROS) on endothelial function of pulmonary arteries in a mice model of hypoxia-induced pulmonary hypertension.
In pulmonary arteries from control mice, the NO-synthase inhibitor Nω-nitro-L-arginine methyl ester (L-NAME) potentiated contraction to prostaglandin F2α (PGF2α) and completely abolished relaxation to acetylcholine. In extrapulmonary but not intrapulmonary arteries, acetylcholine-induced relaxation was slightly inhibited by polyethyleneglycol-superoxide dismutase (PEG-SOD) or catalase.
In pulmonary arteries from hypoxic mice, ROS levels (evaluated using dihydroethidium staining) were higher than in controls. In these arteries, relaxation to acetylcholine (but not to sodium nitroprusside) was markedly diminished. L-NAME abolished relaxation to acetylcholine, but failed to potentiate PGF2α-induced contraction. PEG-SOD or catalase blunted residual relaxation to acetylcholine in extrapulmonary arteries, but did not modify it in intrapulmonary arteries. Hydrogen peroxide elicited comparable (L-NAME-insensitive) relaxations in extra- and intrapulmonary arteries from hypoxic mice.
Exposure of gp91phox–/– mice to chronic hypoxia also decreased the relaxant effect of acetylcholine in extrapulmonary arteries. However, in intrapulmonary arteries from hypoxic gp91phox–/– mice, the effect of acetylcholine was similar to that obtained in mice not exposed to hypoxia.
Chronic hypoxia increases ROS levels and impairs endothelial NO-dependent relaxation in mice pulmonary arteries. Mechanisms underlying hypoxia-induced endothelial dysfunction differ along pulmonary arterial bed. In extrapulmonary arteries from hypoxic mice, endothelium-dependent relaxation appears to be mediated by ROS, in a gp91phox-independent manner. In intrapulmonary arteries, endothelial dysfunction depends on gp91phox, the latter being rather the trigger than the mediator of impaired endothelial NO-dependent relaxation.
Keywords: Chronic hypoxia, endothelial dysfunction, gp91phox, hydrogen peroxide, NADPH oxidase, nitric oxide, pulmonary artery, reactive oxygen species
Introduction
In pulmonary circulation as in other vascular beds, endothelial cells play a critical role in maintaining homeostasis, via the release of vasculoprotective factors such as nitric oxide (NO). Indeed, overexpression of endothelial NO synthase (eNOS) prevents remodeling of the pulmonary vasculature induced by chronic hypoxia (Ozaki et al., 2001), and conversely, eNOS-deficient mice display enhanced pulmonary vascular remodeling and hypertension in response to hypoxia (Steudel et al., 1998). Endothelial dysfunction, which is mainly characterized by impaired synthesis and/or bioactivity of endothelium-derived NO, is an early event in pathogenesis of many cardiovascular disorders (Li & Forstermann, 2000) and appears as a key event in pulmonary hypertension (Chen & Oparil, 2000; Hampl & Herget, 2000; Budhiraja et al., 2004). Although upregulation of eNOS and/or enhanced relaxation to endothelium-derived NO have been reported (Isaacson et al., 1994; Resta et al., 1997; Shirai et al., 2003; Jernigan et al., 2004a), numerous studies rather show a decrease of endothelium-dependent relaxation in the pulmonary arterial bed during pulmonary hypertension (Adnot et al., 1991; Murata et al., 2002; Sauzeau et al., 2003; Abe et al., 2004; Elmedal et al., 2004). An increase in arginase activity (Xu et al., 2004) and in pulmonary level of the endogenous NOS inhibitor assymmetric dimethylarginine (Millatt et al., 2003), as well as alterations in eNOS/caveolin-1 interaction (Murata et al., 2002) are potential mechanisms contributing to endothelial dysfunction in pulmonary hypertension.
In many vascular beds, reactive oxygen species (ROS) play a key role in endothelial dysfunction. Elevated superoxide anions may decrease both synthesis and bioactivity of endothelium-derived NO (Cai & Harrison, 2000; Li & Forstermann, 2000). Hydrogen peroxide, the dismutation product of superoxide, is an endothelium-derived relaxing factor in some arteries (Matoba et al., 2000), and under pathological situations such as systemic hypertension, eNOS-derived hydrogen peroxide mediates endothelium-dependent vasodilatation (Landmesser et al., 2003). Because hydrogen peroxide possesses a longer half-life than superoxide, and not only modulates tone but also promotes growth and hypertrophy of vascular smooth muscle cells (Zafari et al., 1998; Taniyama & Griendling, 2003), there is growing interest for this mediator in pathophysiology of many cardiovascular diseases. A potential role of ROS in the development of pulmonary hypertension is supported by experimental data showing that several antioxidants prevent some cardiopulmonary alterations triggered by chronic hypoxia (Lai et al., 1998; Hoshikawa et al., 2001; Elmedal et al., 2004). Moreover, it has been shown in different models of pulmonary hypertension that diminished pulmonary vasorelaxation to exogenous NO is related to increased level of ROS (Wanstall et al., 1997; Brennan et al., 2003; Jernigan et al., 2004b). Recently, evidence has been provided using genetic mice models that NADPH oxidase-dependent superoxide production enhances responsiveness of pulmonary arteries to contractile agonists in hypoxic pulmonary hypertension (Liu et al., 2006). In addition, depletion in tetrahydrobiopterin (an important NOS cofactor regulating eNOS coupling) results in increased superoxide synthesis, hypertension and remodeling within pulmonary vasculature even under normoxic conditions, and to a greater susceptibility to hypoxia-induced pulmonary hypertension (Khoo et al., 2005). However, there is very few direct evidence indicating that ROS participate to and/or modulate endothelial function in pulmonary arteries, particularly in the course of pulmonary hypertension.
Therefore, the present study investigates endothelial function, and more specially the role of NO and ROS, in pulmonary arteries from mice exposed or not to chronic hypoxia. In this species, little information exists concerning endothelium-dependent reactivity of isolated pulmonary arteries, and studies on the pulmonary effects of chronic hypoxia mainly focus on changes in the hemodynamics of perfused lungs or muscularization of pulmonary arterioles. As there exist differences along pulmonary arterial bed in NO-related alterations in animals subjected to chronic hypoxia (Shirai et al., 2003; Elmedal et al., 2004), both extra- and intrapulmonary arteries were examined for endothelium-dependent reactivity and ROS levels. The role of superoxide anions and hydrogen peroxide was investigated using specific decomposing enzymes, superoxide dismutase (SOD) and catalase, respectively. The potential role of NADPH oxidase was studied in mice with genetic deletion of the gp91phox subunit, which mediates endothelial dysfunction in some models of systemic hypertension (Jung et al., 2004). A preliminary report of this study has been presented in abstract form (Fresquet et al., 2005).
Methods
Animals
The investigation conforms with the Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1996). Agreement (number A 33409) was obtained by French authorities. Male C57BL6 (24–30 g, 11–14 weeks old; Elevage Janvier, Le Genest St Isle, France) or gp91phox–/– mice (25–27 g, 11–12 weeks old, C57BL6 background (Jung et al., 2004)) were exposed to chronic hypoxia in a hypobaric chamber (380 mmHg) for 21 days. Control mice (normoxic group) were housed in room air at ambient atmospheric pressure. For comparison, one group of mice was placed for 21 days in a chamber under atmospheric pressure. All groups were kept at the same light/dark cycle. The chamber was open third a week for 30 min for animal care and cleaning.
Hematocrit and right ventricular (RV) hypertrophy
Mice were killed by cervical dislocation. In some experiments, blood sample was rapidly removed by heart puncture and hematocrit was determined using standard capillary tube technique. The heart and lungs were removed and placed in physiological salt solution (PSS) containing (in mM): NaCl 119; KCl 4.7; CaCl2 1.5; MgSO4 1.17; KH2PO4 1.18; NaHCO3 25; and glucose 5.5. The RV was dissected from the left ventricle and septum (LV+S). Tissues were weighed and the weight ratio RV/LV+S was calculated.
Vascular reactivity
Extrapulmonary arteries (left and right main branches) and intrapulmonary arteries (first-order branch) were dissected free of connective tissue. Segments of pulmonary artery (1.6–2.0 mm length) were mounted in a Mulvany myograph (Multi Myograph System, model 610M, J.P. Trading, Aarhus, Denmark) as described previously (Leblais et al., 2004). Passive length–tension relationship demonstrated that the optimal resting tension for both extrapulmonary and intrapulmonary arteries corresponded to an equivalent transmural pressure of 15 and 30 mmHg, in arteries from control and hypoxic mice, respectively. This is in accordance with previous data obtained in rat, showing that optimal resting tension was higher in pulmonary arteries from hypoxic animals, compared to controls (Bonnet et al., 2001). Thus, unless otherwise indicated, arteries from control and hypoxic mice were studied under a resting tension corresponding to an equivalent transmural pressure of 15 and 30 mmHg, respectively. Internal diameters of extra- and intrapulmonary arteries mounted under these conditions were in the range of 0.8–1.2 and 0.5–0.7 mm, respectively (see Table 1). After a 60 min equilibration period under resting tone, viability of arteries was evaluated using PSS containing 80 mM KCl (equimolar substitution with NaCl). Arteries developing a wall tension below 1 mN mm−1 (extrapulmonary arteries) or 0.5 mN mm−1 (intrapulmonary arteries) were discarded.
Table 1.
Contractile effect of PGF2α (in the absence or presence of 300 μM L-NAME) in extrapulmonary and intrapulmonary arteries from normoxic and hypoxic mice
| |
Contraction to PGF2α |
|
|---|---|---|
| pD2 | Emax (mN mm−1) | |
|
Extrapulmonary arteries |
|
|
| Normoxia (int ∅: 0.79–0.85 mm) |
|
|
| Alone |
5.20±0.05 |
1.19±0.06 |
| +L-NAME |
5.44±0.04* |
1.23±0.04 |
| |
|
|
| Hypoxia (int ∅: 1.04–1.10 mm) |
|
|
| Alone |
5.73±0.11+++ |
1.82±0.10+++ |
| +L-NAME |
5.82±0.09 |
1.74±0.13 |
| |
|
|
|
Intrapulmonary arteries |
|
|
| Normoxia (int ∅: 0.53–0.57 mm) |
|
|
| Alone |
5.10±0.09 |
0.90±0.05 |
| +L-NAME |
5.36±0.09** |
0.98±0.06 |
| |
|
|
| Hypoxia (int ∅: 0.69–0.73 mm) |
|
|
| Alone |
5.54±0.05++ |
1.69±0.09++ |
| +L-NAME | 5.61±0.13 | 1.63±0.05 |
Arteries from normoxic and hypoxic mice were studied under a resting tension corresponding to an equivalent transmural pressure of 15 or 30 mmHg, respectively (see Methods). Corresponding internal diameters (int ∅) of arterial segments are indicated.
P<0.05,
P<0.01: significant difference compared to respective controls (without L-NAME; paired Student's t-test).
P<0.01,
P<0.001: significant difference compared to normoxia (unpaired Student's t-test). n=6–9.
Concentration–response curves to prostaglandin F2α (PGF2α, added in a cumulated manner from 10 nM to 100 μM) were constructed, in the absence or presence of the NOS inhibitor Nω-nitro-L-arginine methyl ester (L-NAME, 300 μM, added 30 min before PGF2α). The effect of endothelium-dependent (acetylcholine, added in a cumulated manner from 1 nM to 100 μM) or endothelium-independent (sodium nitroprusside, SNP, added in a cumulated manner from 0.1 nM to 10 μM) relaxing agents was investigated in arteries submaximally precontracted with PGF2α (using a concentration producing about 90% of the response to 80 mM KCl). Concentration–response curves to acetylcholine were performed in the absence or presence of L-NAME (300 μM), the soluble guanylyl cyclase inhibitor 1H-(1,2,4)oxadiazolo[4,3a]quinoxalin-1-one (ODQ, 1 μM), SOD (200 U ml−1), permeant polyethyleneglycol-SOD (PEG-SOD, 120 U ml−1) or catalase (250 U ml−1). All agents were added 30 min before PGF2α. In some other experiments, hydrogen peroxide (0.1 μM to 1 mM, added in a cumulated manner) was applied to arteries submaximally precontracted with PGF2α. Concentration–response curves to hydrogen peroxide were performed in the absence or presence of catalase (250 U ml−1), L-NAME (300 μM) or ODQ (1 μM).
Detection of ROS
Fixed frozen ring segments of extra- and intrapulmonary arteries were cut into 10 μm-thick sections and placed on a glass slide. Slides were first preincubated on ice in phosphate-buffered saline, in the absence or presence of PEG-SOD (135 U ml−1) or PEG-catalase (250 U ml−1) for 30 min, and then exposed to the fluorescent dye dihydroethidium (DHE, 2.5 μM) in the dark for a further 30 min period. Slides were rinsed and examined with a Nikon microphot microscope equipped with a camera (excitation wave length 530 nm, emission wave length 610 nm). Fluorescence intensity was quantified with MetaMorph Offline® software.
Drugs
Acetylcholine chloride, L-NAME, SOD, PEG-SOD, catalase and PEG-catalase were supplied by Sigma Chemical Co. (St Quentin-Fallavier, France). ODQ was supplied by Tocris (Bristol, U.K.), Dinoprost tromethamine (PGF2α) by Pharmacia (Puurs, Belgique), DHE by Molecular Probes (Cergy Pontoise, France) and hydrogen peroxide by Cooper (Melun, France). All drugs were prepared in distilled water, except ODQ and DHE (stock solution in DMSO).
Data analysis
Contractile effects are expressed in mN mm−1. Relaxant effects are expressed as the percentage of the initial tone induced by PGF2α. To determine the potency of PGF2α, the EC50 value (concentration that produces 50% of the maximum response) was estimated in each individual concentration–response curve using the Boltzman equation fit and converted into pD2. Data are given as mean±s.e.m. of n experiments (n: number of mice). Concentration–response curves were compared using analysis of variance (ANOVA) for repeated measures. Other parameters were compared using Student's t-test. Differences were considered statistically significant when P<0.05.
Results
Hypoxia-induced pulmonary arterial hypertension
Exposure of C57BL6 mice to hypobaric hypoxia for 3 weeks resulted in a significant increase in both hematocrit (from 34.1±2.6%, n=7 control mice, to 45.8±3.2%, n=14 hypoxic mice, P<0.05) and RV/LV+S ratio (from 0.27±0.01, n=82 control mice, to 0.43±0.01, n=119 hypoxic mice, P<0.001). In mice placed for 3 weeks in the chamber under atmospheric pressure, RV/LV+S ratio (0.35±0.04; n=4) was not significantly different compared to mice housed in room air at ambient atmospheric pressure, but significantly differed (P<0.01) from ratio obtained in hypoxic mice.
Role of NO in modulating contractile responses in pulmonary arteries from normoxic and hypoxic mice
Exposure of C57BL6 mice to chronic hypoxia induced an increase in maximal effect and potency of PGF2α, in both extra- and intrapulmonary arteries (Table 1). Hypoxia also induced a marked increase in the contractile responses to 80 mM KCl (from 1.30±0.02 to 1.90±0.03 mN mm−1 in extrapulmonary arteries, P<0.001; and from 0.74±0.02 to 1.22±0.03 mN mm−1 in intrapulmonary arteries, P<0.001; n=67–120). In arteries removed from mice placed for 3 weeks in the chamber under atmospheric pressure, the effects of PGF2α and 80 mM KCl were not significantly different compared to those obtained in arteries from control mice (data not shown).
The relaxant influence of ‘basal' NO release was assessed by comparing PGF2α-induced contraction, in the presence or absence of the NOS inhibitor, L-NAME (300 μM). In extra- and intrapulmonary arteries from control mice, sensitivity to PGF2α (but not its maximal effect) was significantly increased in the presence of L-NAME (Table 1). In contrast, in arteries from hypoxic mice, L-NAME failed to potentiate PGF2α-induced contraction (Table 1).
Pulmonary arteries from normoxic and hypoxic mice were examined under different resting tension (equivalent transmural pressure of 15 and 30 mmHg, respectively, see Methods). When arteries from normoxic mice were studied under the same resting tension than arteries from hypoxic mice (i.e. 30 mmHg), PGF2α-induced contraction was still potentiated by L-NAME. The pD2 values for PGF2α were 5.42±0.10 and 5.97±0.12, in the absence or presence of 300 μM L-NAME, respectively, in extrapulmonary arteries (n=11; P<0.001), and 5.50±0.06 and 5.86±0.12, in the absence or presence of 300 μM L-NAME, respectively, in intrapulmonary arteries (n=9; P<0.05). This demonstrates that the loss of potentiating effect of L-NAME on PGF2α-induced contraction in arteries from hypoxic mice was related to chronic hypoxia, but not to increased resting tension.
Role of NO and ROS in the relaxant effect of acetylcholine in pulmonary arteries from normoxic and hypoxic mice
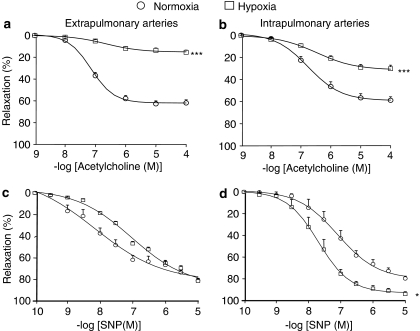
In extra- and intrapulmonary arteries of control mice (submaximally precontracted with 10–40 μM PGF2α), acetylcholine elicited a concentration-dependent relaxant effect (Figure 1a and b). In arteries removed from mice placed for 3 weeks in the chamber under atmospheric pressure, the effect of acetylcholine was not significantly different from that obtained in arteries of control mice (data not shown). In arteries from hypoxic mice, the concentration of PGF2α used for precontraction was decreased to 1–10 μM, in order to obtain a precontraction similar in amplitude to that in arteries of control mice. In these conditions, the effect of acetylcholine was markedly reduced in both extra- and intrapulmonary arteries from hypoxic mice in comparison to controls (Figure 1a and b), whereas the relaxant effect of SNP (endothelium-independent NO-donating agent) was either not significantly modified (extrapulmonary arteries, Figure 1c) or even significantly increased (intrapulmonary arteries, Figure 1d).
Figure 1.
Relaxant effect of acetylcholine (a, b) and sodium nitroprusside (c, d) in extrapulmonary (a, c) and intrapulmonary (b, d) arteries from wild-type mice exposed or not to hypoxia. In each panel, the response is expressed as the percentage of relaxation of the tone induced by PGF2α. *P<0.05, ***P<0.001: significant difference compared to normoxia (ANOVA). n=15–53 (a, b) or 5–12 (c, d).
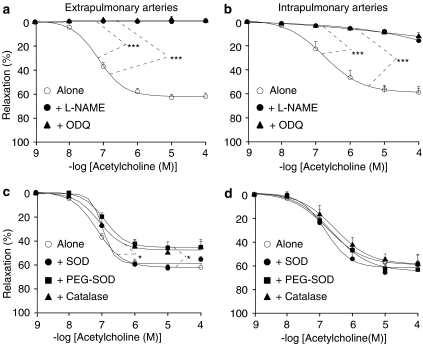
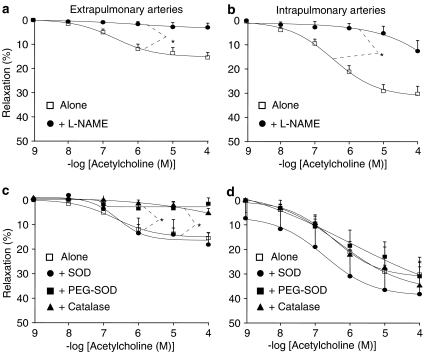
To investigate the participation of NO, the effect of acetylcholine was studied in the absence or presence of L-NAME or ODQ (an inhibitor of the activation of soluble guanylyl cyclase by NO). In control mice, L-NAME (300 μM) or ODQ (1 μM) almost completely abolished the effect of acetylcholine, in both extra- and intrapulmonary arteries (Figure 2a and b). To investigate the role of ROS in participating and/or modulating endothelium-dependent relaxation, the effect of acetylcholine was studied in the absence or presence of SOD (which dismutes superoxide anion into hydrogen peroxide), PEG-SOD (a permeant SOD) or catalase (which decomposes hydrogen peroxide into water and oxygen). SOD (200 U ml−1) did not significantly modify acetylcholine-induced relaxation, neither in extra- nor in intrapulmonary arteries of control mice (Figure 2c and d). PEG-SOD (120 U ml−1) and catalase (250 U ml−1) slightly but significantly diminished (by about 30%) the effect of acetylcholine in extrapulmonary, but not in intrapulmonary arteries from control mice (Figure 2c and d).
Figure 2.
Relaxant effect of acetylcholine in extrapulmonary (a, c) and intrapulmonary (b, d) arteries from control mice (wild-type). The effect of acetylcholine was studied in the absence or presence of 300 μM L-NAME, 1 μM ODQ (a, b), 200 U ml−1 SOD, 120 U ml−1 PEG-SOD or 250 U ml−1 catalase (c, d). In each panel, the response is expressed as the percentage of relaxation of the tone induced by PGF2α. *P<0.05, ***P<0.001: significant difference compared to controls (ANOVA). n=3–15 (a, b) or 5–15 (c, d).
In pulmonary arteries from hypoxic mice, L-NAME inhibited the remaining effect of acetylcholine (Figure 3a and b). In extrapulmonary arteries from hypoxic mice, addition of SOD did not modify acetylcholine-induced relaxation, whereas both PEG-SOD and catalase blunted it (Figure 3c). In contrast, in intrapulmonary arteries from hypoxic mice, relaxant effect of acetylcholine remained unaffected in the presence of SOD, PEG-SOD or catalase (Figure 3d).
Figure 3.
Relaxant effect of acetylcholine in extrapulmonary (a, c) and intrapulmonary (b, d) arteries from hypoxic mice (wild-type). The effect of acetylcholine was studied in the absence or presence of 300 μM L-NAME (a, b), 200 U ml−1 SOD, 120 U ml−1 PEG-SOD or 250 U ml−1 catalase (c, d). In each panel, the response is expressed as the percentage of relaxation of the tone induced by PGF2α. *P<0.05: significant difference compared to controls (ANOVA). n=7–53 (a, b) or 4–53 (c, d).
Relaxant effect of hydrogen peroxide in pulmonary arteries from normoxic and hypoxic mice
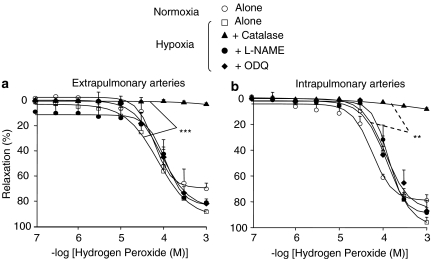
To determine whether differences in the influence of catalase between extra- and intrapulmonary arteries could be owing to differential sensitivity to hydrogen peroxide, the relaxant effect of this agent was compared in both types of arteries. After precontraction with PGF2α, exogenously applied hydrogen peroxide elicited a similar concentration-dependent relaxant effect in both extra- and intrapulmonary arteries, from either control or hypoxic mice (Figure 4a and b). Hydrogen peroxide-induced relaxation was insensitive to L-NAME or ODQ, but abolished by catalase (Figure 4a and b).
Figure 4.
Relaxant effect of hydrogen peroxide in extrapulmonary (a) and intrapulmonary (b) arteries from wild-type mice exposed or not to hypoxia. The effect of hydrogen peroxide was studied in the absence or presence of 250 U ml−1 catalase, 300 μM L-NAME or 1 μM ODQ. In each panel, the response is expressed as the percentage of relaxation of the tone induced by PGF2α. **P<0.01, ***P<0.001: significant difference compared to controls (ANOVA). n=4–23.
ROS content in pulmonary arteries from normoxic and hypoxic mice
To determine the influence of chronic hypoxia on ROS levels of pulmonary arteries, in situ staining with the fluorescent probe DHE was applied on tissue slides. Extra- and intrapulmonary arteries from hypoxic mice exhibited a significant increase in DHE staining (of about 2.5 to four-fold) throughout the vascular wall compared to arteries from controls (Figure 5a and b). Staining was blunted by PEG-SOD and PEG-catalase, indicating that it effectively originated from superoxide and hydrogen peroxide. Comparison of absolute values of fluorescence obtained under the same experimental conditions revealed that following chronic hypoxia, extrapulmonary artery displayed greater ROS levels than intrapulmonary arteries (7.0±0.4 and 4.0±1.3 × 104 arbitrary units, respectively; n=3; P<0.01).
Figure 5.
Representative photomicrographs and histograms of DHE staining in extrapulmonary (a) and intrapulmonary (b) arteries from wild-type mice exposed or not to hypoxia. Slides were preincubated in the absence or presence of 135 U ml−1 PEG-SOD or 250 U ml−1 PEG-catalase before the addition of DHE. In histograms, fluorescent intensity is expressed as relative values (percentage of fluorescence obtained in arteries from normoxic mice). *P<0.05, **P<0.01: significant difference (Student's t-test). n=4.
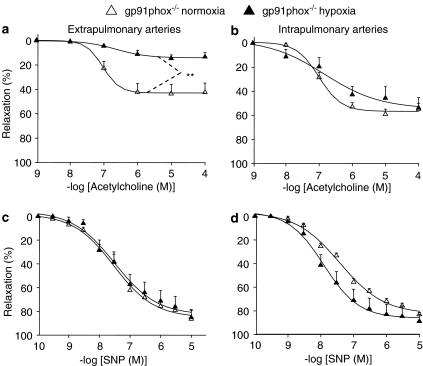
Relaxant effect of acetylcholine in pulmonary arteries from hypoxic gp91phox–/– mice
To further document a role of ROS in hypoxia-induced endothelial dysfunction, and also to determine whether differences between extra- and intrapulmonary arteries could be related to differential role of NADPH oxidase, some experiments were performed in arteries from mice with genetic deletion of the gp91phox subunit. Like those from wild-type, extrapulmonary arteries from hypoxic gp91phox–/– mice exhibited a decrease in the relaxant effect of acetylcholine, in comparison to those from gp91phox–/– mice not subjected to chronic hypoxia (Figure 6a). In contrast, unlike those from wild-type, intrapulmonary arteries from hypoxic gp91phox–/– mice displayed relaxation to acetylcholine that was not significantly different from that obtained in intrapulmonary arteries from mice not exposed to hypoxia (Figure 6b). Exposure of gp91phox–/– mice to chronic hypoxia did not modify the relaxant effect induced by SNP in extra- and intrapulmonary arteries (Figure 6c and d).
Figure 6.
Relaxant effect of acetylcholine (a, b) and sodium nitroprusside (c, d) in extrapulmonary (a, c) and intrapulmonary (b, d) arteries from gp91phox−/− mice exposed or not to hypoxia. In each panel, the response is expressed as the percentage of relaxation of the tone induced by PGF2α. **P<0.01: significant difference (ANOVA). n=7–8.
Discussion
The present study investigates for the first time endothelial function of isolated pulmonary arteries from hypoxic mice. It shows that chronic hypoxia increases ROS levels and impairs endothelial NO-dependent relaxation in these arteries. Moreover, it shows that the mechanisms underlying hypoxia-induced impairment of acetylcholine-induced relaxation markedly differed between extra- and intrapulmonary arteries. This study provides also the first evidence for a role of gp91phox in hypoxia-induced endothelial dysfunction of intrapulmonary arteries.
In control mice, the potentiating effect of L-NAME on PGF2α-induced contraction (Table 1) indicates that in pulmonary arteries as in many other vascular beds, ‘basal' (i.e. non agonist-stimulated) NOS activity attenuates contractile responses. L-NAME also almost totally abolished endothelium-dependent relaxation to acetylcholine (Figure 2), indicating that eNOS-derived NO is the major endothelium-derived relaxing factor in mice pulmonary arteries. The lack of effect of exogenous SOD on acetylcholine-induced relaxation (Figure 2) shows that extracellular superoxide anions did not diminish the biological activity of endothelium-derived NO in pulmonary arteries from control mice. In contrast to SOD, permeant PEG-SOD or catalase induced a slight inhibition of acetylcholine-induced relaxation in extrapulmonary arteries from control mice (Figure 2). This inhibitory effect of both PEG-SOD and catalase is consistent with the view that, upon stimulation with acetylcholine, intracellular superoxide serves as a substrate for synthesis of hydrogen peroxide, which reaches the extracellular space and induces smooth muscle relaxation. Conversion of intracellular superoxide into hydrogen peroxide during preincubation period with PEG-SOD and subsequent inactivation of hydrogen peroxide by endogenous catalase might explain the inhibitory effect of PEG-SOD on acetylcholine-induced relaxation. The relaxant properties of exogenously applied hydrogen peroxide have been demonstrated in various vascular beds, including murine ones (Matoba et al., 2000; Ellis et al., 2003; Rabelo et al., 2003) and are also demonstrated here in extrapulmonary arteries from control mice (Figure 4). Moreover, in mice mesenteric arteries, hydrogen peroxide appears as an endothelium-derived relaxing factor, for which eNOS is an important source (Matoba et al., 2000). The full inhibition of acetylcholine-induced relaxation by L-NAME in extrapulmonary arteries from control mice (Figure 2) is consistent with this mechanism. The complete inhibition also obtained in the presence of ODQ (Figure 2), despite the contribution of hydrogen peroxide to acetylcholine-induced relaxation, is striking and deserves further investigations. Similar data have been previously reported in mice aorta (Ellis et al., 2003). ODQ interfered rather with the production of hydrogen peroxide than its biological activity, as relaxation elicited by exogenously applied hydrogen peroxide was unaffected by ODQ (Figure 4). It has been shown that ODQ displays other pharmacological properties than guanylyl cyclase inhibition (Muller et al., 1998) and interferes with the mechanism of vasorelaxation mediated by acetylcholine through inhibition of NOS (Feelisch et al., 1999). Whereas acetylcholine-induced relaxation was diminished by PEG-SOD and catalase in extrapulmonary arteries, it remained unaffected in intrapulmonary arteries (Figure 2). This not only argues against nonspecific effects of the exogenously applied enzymes but also demonstrates that in mice the mechanisms underlying endothelium-dependent relaxation differed between extra- and intrapulmonary arteries. Differences between extra- and intrapulmonary arteries are unlikely owing to differential sensitivity to hydrogen peroxide, as this agent exerted similar effects in both types of arteries (Figure 4). Altogether, data obtained in control mice suggest that in intrapulmonary arteries, endothelium-dependent relaxation to acetylcholine is mediated by eNOS-derived NO, but not by ROS. However, in extrapulmonary arteries, eNOS-derived NO and to a limited extent ROS (especially hydrogen peroxide) mediate endothelium-dependent relaxation.
Pulmonary hypertension from hypoxic origin was demonstrated here by RV hypertrophy and increase in hematocrit in mice subjected to hypoxia. Extra- and intrapulmonary arteries removed from hypoxic mice exhibited enhanced contractile response to KCl and PGF2α (Table 1). Differential results have been reported in the literature concerning hypoxia-induced alteration of reactivity to vasoconstrictors in rat pulmonary arteries. They might be explained by differences in applied passive tension, vessel sizes or presence/absence of endothelium. Nevertheless, hyper-reactivity of pulmonary arteries to some vasoconstrictors was previously observed in models of pulmonary hypertension (McCulloch et al., 1998; Keegan et al., 2001; Abe et al., 2004). Hypoxia-induced hyper-reactivity of pulmonary arteries to PGF2α was associated with a loss of the potentiating effect of L-NAME on contraction (Table 1), but not with a decrease in the relaxant effect of SNP (Figure 1). This suggests that ‘basal' NO production, rather than NO relaxant effect, was diminished in pulmonary arteries from hypoxic mice, and that endothelial dysfunction may be partially responsible for hyper-reactivity to contractile agonists. As responses to receptor-dependent (PGF2α) and -independent (KCl) contractile agents were both modified, contractile mechanisms downstream to elevation of cytosolic calcium in smooth muscle cells were likely affected following chronic hypoxia. Various mechanisms may contribute to hyper-reactivity to vasoconstrictors, including activation of the Rho-kinase pathway (Abe et al., 2004), and enhanced generation of superoxide or cyclooxygenase-derived constricting products (Cortes et al., 1996; Miller et al., 2000; Pannirselvam et al., 2005; Liu et al., 2006).
Hypoxia-induced endothelial dysfunction was further demonstrated in both extra- and intrapulmonary arteries by a profound decrease in the relaxant effect of acetylcholine, whereas relaxation to SNP was either not diminished (extrapulmonary artery) or even increased (intrapulmonary arteries) (Figure 1). The latter effect may be owing to the upregulation of soluble guanylyl cyclase or cGMP-dependent protein kinase following chronic hypoxia (Li et al., 1999; Jernigan et al., 2003). Endothelial dysfunction was associated with an increased level of ROS in all vascular layers of pulmonary arteries from hypoxic mice, as demonstrated using DHE (Figure 5), a fluorescent dye that reacts with both superoxide and hydrogen peroxide (Munzel et al., 2002). Despite elevation of ROS (including superoxide, presumably), relaxant responses to acetylcholine were not amplified in the presence of SOD or PEG-SOD (Figure 3) and relaxant responses to SNP were not modified (Figure 1) in pulmonary arteries from hypoxic mice. This might be explained by differential subcellular localization of superoxide and NO production, or to a major contribution of hydrogen peroxide (which does decrease NO bioavailability) in ROS elevation, as suggested by the inhibitory effect of PEG-catalase on DHE staining.
In extrapulmonary arteries from hypoxic mice, residual relaxation to acetylcholine was abolished by PEG-SOD or catalase (Figure 3), suggesting a role of ROS, and most likely of hydrogen peroxide, as mediator of endothelium-dependent relaxation. Comparison of acetylcholine-induced relaxation between normoxic and hypoxic mice suggests that in extrapulmonary arteries, the NO-dependent component was abolished following chronic hypoxia, whereas the ROS-dependent one was preserved. Hypoxia-induced alterations of relaxation to acetylcholine were still observed in extrapulmonary arteries from mice deleted for gp91phox gene (Figure 6). This indicates that hypoxia-induced endothelial dysfunction in extrapulmonary artery is independent of gp91phox. This does not exclude a role of other catalytic subunits of NADPH oxidase. Uncoupled eNOS is another potential source of ROS in systemic and pulmonary hypertension (Landmesser et al., 2003; Khoo et al., 2005). It has been shown that hydrogen peroxide activates eNOS (Cai et al., 2003) and this mechanism might account for the inhibitory effect of L-NAME on acetylcholine-induced relaxation in extrapulmonary arteries from hypoxic mice. However, this appears unlikely as L-NAME inhibited acetylcholine- but not hydrogen peroxide-induced relaxation in these arteries. The hypothesis of eNOS-dependent hydrogen peroxide production requires further investigations. Because hydrogen peroxide also promotes growth and hypertrophy of vascular smooth muscle cells (Zafari et al., 1998; Taniyama & Griendling, 2003), it may represent a key mediator of hypoxia-induced remodeling of the pulmonary vasculature.
One major finding of the present study is that in intrapulmonary arteries (in contrast to extrapulmonary arteries), hypoxia-induced endothelial dysfunction is not observed in mice deleted for gp91phox gene (Figure 6). The gp91phox subunit has been implicated in hypoxia-induced hyper-responsiveness of pulmonary arteries to contractile agonists (Liu et al., 2006). The present study brings the first evidence for a role of this NADPH oxidase subunit in hypoxia-induced endothelial dysfunction in pulmonary arterial bed. Interestingly, potentiation by hypoxia of SNP-relaxant responses was not observed in intrapulmonary artery from gp91phox−/− mice (Figure 6d), suggesting that it is related to NADPH oxidase activity and that it might be an adaptive consequence of endothelial dysfunction. The lack of potentiating effect of SOD and PEG-SOD on acetylcholine-induced relaxation, despite the involvement of gp91phox, is consistent with the idea that NADPH oxidase activity is the trigger, but not the direct mediator of impaired endothelial NO-dependent relaxation. In blood vessels, evidence exists that oxidative stress may initiate the production of cyclooxygenase-derived contractile factors (Pannirselvam et al., 2005), and that the latter may counteract endothelium-dependent relaxation in systemic hypertension (Vanhoutte et al., 2005). The potential contribution of these mechanisms in hypoxia-induced endothelial dysfunction deserves further investigations.
In conclusion, this study gives new insights into chronic hypoxia-induced endothelial dysfunction and contributes to a better understanding of the role of ROS in mice pulmonary vasculature. It provides evidence that in extrapulmonary arteries from hypoxic mice, effect of acetylcholine is rather mediated by ROS, in a gp91phox-independent manner. In intrapulmonary arteries, hypoxia-induced endothelial dysfunction is gp91phox-dependent, but NADPH oxidase activity rather appears as the trigger, but not the mediator of impaired endothelial NO-dependent relaxation. These findings will help to identify new pharmacological targets for pulmonary hypertension, targeted on NO or ROS pathways.
Acknowledgments
We thank Mrs Martine Lacayrerie for excellent animal care, Mr Pierre Techoueyres for maintenance of hypobaric chamber, Pr Christian Doutremepuich (Faculty of Pharmacy, Bordeaux) for facilities in hematocrit determination and Dr Jean-Marie Daniel-Lamazière (INSERM U441, Pessac) for facilities in fluorescence determination. This work was partially supported by grants from Fondation de France (2004002928) and Conseil Régional d'Aquitaine (20030301301A).
Abbreviations
- DHE
dihydroethidium
- L-NAME
Nω-nitro-L-arginine methyl ester
- LV
left ventricular
- NO
nitric oxide
- NOS
nitric oxide synthase
- ODQ
1H-(1,2,4)oxadiazolo[4,3a]quinoxalin-1-one
- PEG
polyethyleneglycol
- PGF2α
prostaglandin F2α
- PSS
physiological salt solution
- ROS
reactive oxygen species
- RV
right ventricular
- S
septum
- SOD
superoxide dismutase
References
- ABE K., SHIMOKAWA H., MORIKAWA K., UWATOKU T., OI K., MATSUMOTO Y., HATTORI T., NAKASHIMA Y., KAIBUCHI K., SUEISHI K., TAKESHIT A. Long-term treatment with a Rho-kinase inhibitor improves monocrotaline-induced fatal pulmonary hypertension in rats. Circ. Res. 2004;94:385–393. doi: 10.1161/01.RES.0000111804.34509.94. [DOI] [PubMed] [Google Scholar]
- ADNOT S., RAFFESTIN B., EDDAHIBI S., BRAQUET P., CHABRIER P.E. Loss of endothelium-dependent relaxant activity in the pulmonary circulation of rats exposed to chronic hypoxia. J. Clin. Invest. 1991;87:155–162. doi: 10.1172/JCI114965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BONNET S., BELUS A., HYVELIN J.M., ROUX E., MARTHAN R., SAVINEAU J.P. Effect of chronic hypoxia on agonist-induced tone and calcium signaling in rat pulmonary artery. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001;281:L193–L201. doi: 10.1152/ajplung.2001.281.1.L193. [DOI] [PubMed] [Google Scholar]
- BRENNAN L.A., STEINHORN R.H., WEDGWOOD S., MATA-GREENWOOD E., ROARK E.A., RUSSELL J.A., BLACK S.M. Increased superoxide generation is associated with pulmonary hypertension in fetal lambs: a role for NADPH oxidase. Circ. Res. 2003;92:683–691. doi: 10.1161/01.RES.0000063424.28903.BB. [DOI] [PubMed] [Google Scholar]
- BUDHIRAJA R., TUDER R.M., HASSOUN P.M. Endothelial dysfunction in pulmonary hypertension. Circulation. 2004;109:159–165. doi: 10.1161/01.CIR.0000102381.57477.50. [DOI] [PubMed] [Google Scholar]
- CAI H., HARRISON D.G. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ. Res. 2000;87:840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- CAI H., LI Z., DAVIS M.E., KANNER W., HARRISON D.G., DUDLEY S.C., JR Akt-dependent phosphorylation of serine 1179 and mitogen-activated protein kinase kinase/extracellular signal-regulated kinase 1/2 cooperatively mediate activation of the endothelial nitric-oxide synthase by hydrogen peroxide. Mol. Pharmacol. 2003;63:325–331. doi: 10.1124/mol.63.2.325. [DOI] [PubMed] [Google Scholar]
- CHEN Y.F., OPARIL S. Endothelial dysfunction in the pulmonary vascular bed. Am. J. Med. Sci. 2000;320:223–232. doi: 10.1097/00000441-200010000-00001. [DOI] [PubMed] [Google Scholar]
- CORTES S.F., ANDRIANTSITOHAINA R., STOCLET J.C. Alterations of cyclo-oxygenase products and NO in responses to angiotensin II of resistance arteries from the spontaneously hypertensive rat. Br. J. Pharmacol. 1996;119:1635–1641. doi: 10.1111/j.1476-5381.1996.tb16083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLIS A., PANNIRSELVAM M., ANDERSON T.J., TRIGGLE C.R. Catalase has negligible inhibitory effects on endothelium-dependent relaxations in mouse isolated aorta and small mesenteric artery. Br. J. Pharmacol. 2003;140:1193–1200. doi: 10.1038/sj.bjp.0705549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELMEDAL B., DE DAM M.Y., MULVANY M.J., SIMONSEN U. The superoxide dismutase mimetic, tempol, blunts right ventricular hypertrophy in chronic hypoxic rats. Br. J. Pharmacol. 2004;141:105–113. doi: 10.1038/sj.bjp.0705580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FEELISCH M., KOTSONIS P., SIEBE J., CLEMENT B., SCHMIDT H.H. The soluble guanylyl cyclase inhibitor 1H-[1,2,4]oxadiazolo[4,3,-a] quinoxalin-1-one is a nonselective heme protein inhibitor of nitric oxide synthase and other cytochrome P-450 enzymes involved in nitric oxide donor bioactivation. Mol. Pharmacol. 1999;56:243–253. doi: 10.1124/mol.56.2.243. [DOI] [PubMed] [Google Scholar]
- FRESQUET F., POURAGEAUD F., LEBLAIS V., SAVINEAU J., MARTHAN R., MULLER B. Endothelial dysfuntion induced by chronic hypoxia in mice pulmonary arteries: role of reactive oxygen species. Fund. Clin. Pharmacol. 2005;19:195. doi: 10.1038/sj.bjp.0706779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMPL V., HERGET J. Role of nitric oxide in the pathogenesis of chronic pulmonary hypertension. Physiol. Rev. 2000;80:1337–1372. doi: 10.1152/physrev.2000.80.4.1337. [DOI] [PubMed] [Google Scholar]
- HOSHIKAWA Y., ONO S., SUZUKI S., TANITA T., CHIDA M., SONG C., NODA M., TABATA T., VOELKEL N.F., FUJIMURA S. Generation of oxidative stress contributes to the development of pulmonary hypertension induced by hypoxia. J. Appl. Physiol. 2001;90:1299–1306. doi: 10.1152/jappl.2001.90.4.1299. [DOI] [PubMed] [Google Scholar]
- ISAACSON T.C., HAMPL V., WEIR E.K., NELSON D.P., ARCHER S.L. Increased endothelium-derived NO in hypertensive pulmonary circulation of chronically hypoxic rats. J. Appl. Physiol. 1994;76:933–940. doi: 10.1152/jappl.1994.76.2.933. [DOI] [PubMed] [Google Scholar]
- JERNIGAN N.L., RESTA T.C., WALKER B.R. Contribution of oxygen radicals to altered NO-dependent pulmonary vasodilation in acute and chronic hypoxia. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004a;286:L947–L995. doi: 10.1152/ajplung.00215.2003. [DOI] [PubMed] [Google Scholar]
- JERNIGAN N.L., WALKER B.R., RESTA T.C. Pulmonary PKG-1 is upregulated following chronic hypoxia. Am. J. Physiol. Lung Cell. Mol. Physiol. 2003;285:L634–L642. doi: 10.1152/ajplung.00328.2002. [DOI] [PubMed] [Google Scholar]
- JERNIGAN N.L., WALKER B.R., RESTA T.C. Endothelium-derived reactive oxygen species and endothelin-1 attenuate NO-dependent pulmonary vasodilation following chronic hypoxia. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004b;287:L801–L880. doi: 10.1152/ajplung.00443.2003. [DOI] [PubMed] [Google Scholar]
- JUNG O., SCHREIBER J.G., GEIGER H., PEDRAZZINI T., BUSSE R., BRANDES R.P. gp91phox-containing NADPH oxidase mediates endothelial dysfunction in renovascular hypertension. Circulation. 2004;109:1795–1801. doi: 10.1161/01.CIR.0000124223.00113.A4. [DOI] [PubMed] [Google Scholar]
- KEEGAN A., MORECROFT I., SMILLIE D., HICKS M.N., MACLEAN M.R. Contribution of the 5-HT(1B) receptor to hypoxia-induced pulmonary hypertension: converging evidence using 5-HT(1B)-receptor knockout mice and the 5-HT(1B/1D)-receptor antagonist GR127935. Circ. Res. 2001;89:1231–1239. doi: 10.1161/hh2401.100426. [DOI] [PubMed] [Google Scholar]
- KHOO J.P., ZHAO L., ALP N.J., BENDALL J.K., NICOLI T., ROCKETT K., WILKINS M.R., CHANNON K.M. Pivotal role for endothelial tetrahydrobiopterin in pulmonary hypertension. Circulation. 2005;111:2126–2133. doi: 10.1161/01.CIR.0000162470.26840.89. [DOI] [PubMed] [Google Scholar]
- LAI Y.L., WU H.D., CHEN C.F. Antioxidants attenuate chronic hypoxic pulmonary hypertension. J. Cardiovasc. Pharmacol. 1998;32:714–720. doi: 10.1097/00005344-199811000-00006. [DOI] [PubMed] [Google Scholar]
- LANDMESSER U., DIKALOV S., PRICE S.R., MCCANN L., FUKAI T., HOLLAND S.M., MITCH W.E., HARRISON D.G. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J. Clin. Invest. 2003;111:1201–1209. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEBLAIS V., POURAGEAUD F., IVORRA M.D., GUIBERT C., MARTHAN R., MULLER B. Role of alpha-adrenergic receptors in the effect of the beta-adrenergic receptor ligands, CGP 12177, bupranolol, and SR 59230A, on the contraction of rat intrapulmonary artery. J. Pharmacol. Exp. Ther. 2004;309:137–145. doi: 10.1124/jpet.103.061192. [DOI] [PubMed] [Google Scholar]
- LI H., FORSTERMANN U. Nitric oxide in the pathogenesis of vascular disease. J. Pathol. 2000;190:244–254. doi: 10.1002/(SICI)1096-9896(200002)190:3<244::AID-PATH575>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- LI D., ZHOU N., JOHNS R.A. Soluble guanylate cyclase gene expression and localization in rat lung after exposure to hypoxia. Am. J. Physiol. 1999;277:L841–L847. doi: 10.1152/ajplung.1999.277.4.L841. [DOI] [PubMed] [Google Scholar]
- LIU J.Q., ZELKO I.N., ERBYNN E.M., SHAM J.S., FOLZ R.J. Hypoxic pulmonary hypertension: role of superoxide and NADPH oxidase (gp91phox) Am. J. Physiol. Lung Cell. Mol. Physiol. 2006;290:L2–L10. doi: 10.1152/ajplung.00135.2005. [DOI] [PubMed] [Google Scholar]
- MATOBA T., SHIMOKAWA H., NAKASHIMA M., HIRAKAWA Y., MUKAI Y., HIRANO K., KANAIDE H., TAKESHITA A. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in mice. J. Clin. Invest. 2000;106:1521–1530. doi: 10.1172/JCI10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCCULLOCH K.M., DOCHERTY C., MACLEAN M.R. Endothelin receptors mediating contraction of rat and human pulmonary resistance arteries: effect of chronic hypoxia in the rat. Br. J. Pharmacol. 1998;123:1621–1630. doi: 10.1038/sj.bjp.0701785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLATT L.J., WHITLEY G.S., LI D., LEIPER J.M., SIRAGY H.M., CAREY R.M., JOHNS R.A. Evidence for dysregulation of dimethylarginine dimethylaminohydrolase I in chronic hypoxia-induced pulmonary hypertension. Circulation. 2003;108:1493–1498. doi: 10.1161/01.CIR.0000089087.25930.FF. [DOI] [PubMed] [Google Scholar]
- MILLER A.A., MEGSON I.L., GRAY G.A. Inducible nitric oxide synthase-derived superoxide contributes to hypereactivity in small mesenteric arteries from a rat model of chronic heart failure. Br. J. Pharmacol. 2000;131:29–36. doi: 10.1038/sj.bjp.0703528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MULLER B., KLESCHYOV A.L., MALBLANC S., STOCLET J.C. Nitric oxide-related cyclic GMP-independent relaxing effect of N-acetylcysteine in lipopolysaccharide-treated rat aorta. Br. J. Pharmacol. 1998;123:1221–1229. doi: 10.1038/sj.bjp.0701737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUNZEL T., AFANAS'EV I.B., KLESCHYOV A.L., HARRISON D.G. Detection of superoxide in vascular tissue. Arterioscler. Thromb. Vasc. Biol. 2002;22:1761–1768. doi: 10.1161/01.atv.0000034022.11764.ec. [DOI] [PubMed] [Google Scholar]
- MURATA T., SATO K., HORI M., OZAKI H., KARAKI H. Decreased endothelial nitric-oxide synthase (eNOS) activity resulting from abnormal interaction between eNOS and its regulatory proteins in hypoxia-induced pulmonary hypertension. J. Biol. Chem. 2002;277:44085–44092. doi: 10.1074/jbc.M205934200. [DOI] [PubMed] [Google Scholar]
- OZAKI M., KAWASHIMA S., YAMASHITA T., OHASHI Y., RIKITAKE Y., INOUE N., HIRATA K.I., HAYASHI Y., ITOH H., YOKOYAMA M. Reduced hypoxic pulmonary vascular remodeling by nitric oxide from the endothelium. Hypertension. 2001;37:322–327. doi: 10.1161/01.hyp.37.2.322. [DOI] [PubMed] [Google Scholar]
- PANNIRSELVAM M., WIEHLER W.B., ANDERSON T., TRIGGLE C.R. Enhanced vascular reactivity of small mesenteric arteries from diabetic mice is associated with enhanced oxidative stress and cyclooxygenase products. Br. J. Pharmacol. 2005;144:953–960. doi: 10.1038/sj.bjp.0706121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RABELO L.A., CORTES S.F., ALVAREZ-LEITE J.I., LEMOS V.S. Endothelium dysfunction in LDL receptor knockout mice: a role for H2O2. Br. J. Pharmacol. 2003;138:1215–1220. doi: 10.1038/sj.bjp.0705164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RESTA T.C., GONZALES R.J., DAIL W.G., SANDERS T.C., WALKER B.R. Selective upregulation of arterial endothelial nitric oxide synthase in pulmonary hypertension. Am. J. Physiol. 1997;272:H806–H813. doi: 10.1152/ajpheart.1997.272.2.H806. [DOI] [PubMed] [Google Scholar]
- SAUZEAU V., ROLLI-DERKINDEREN M., LEHOUX S., LOIRAND G., PACAUD P. Sildenafil prevents change in RhoA expression induced by chronic hypoxia in rat pulmonary artery. Circ. Res. 2003;93:630–637. doi: 10.1161/01.RES.0000093220.90027.D9. [DOI] [PubMed] [Google Scholar]
- SHIRAI M., PEARSON J.T., SHIMOUCHI A., NAGAYA N., TSUCHIMOCHI H., NINOMIYA I., MORI H. Changes in functional and histological distributions of nitric oxide synthase caused by chronic hypoxia in rat small pulmonary arteries. Br. J. Pharmacol. 2003;139:899–910. doi: 10.1038/sj.bjp.0705312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEUDEL W., SCHERRER-CROSBIE M., BLOCH K.D., WEIMANN J., HUANG P.L., JONES R.C., PICARD M.H., ZAPOL W.M. Sustained pulmonary hypertension and right ventricular hypertrophy after chronic hypoxia in mice with congenital deficiency of nitric oxide synthase 3. J. Clin. Invest. 1998;101:2468–2477. doi: 10.1172/JCI2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TANIYAMA Y., GRIENDLING K.K. Reactive oxygen species in the vasculature: molecular and cellular mechanisms. Hypertension. 2003;42:1075–1081. doi: 10.1161/01.HYP.0000100443.09293.4F. [DOI] [PubMed] [Google Scholar]
- VANHOUTTE P.M., FELETOU M., TADDEI S. Endothelium-dependent contractions in hypertension. Br. J. Pharmacol. 2005;144:449–458. doi: 10.1038/sj.bjp.0706042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANSTALL J.C., KAYE J.A., GAMBINO A. The in vitro pulmonary vascular effects of FK409 (nitric oxide donor): a study in normotensive and pulmonary hypertensive rats. Br. J. Pharmacol. 1997;121:280–286. doi: 10.1038/sj.bjp.0701105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- XU W., KANEKO F.T., ZHENG S., COMHAIR S.A., JANOCHA A.J., GOGGANS T., THUNNISSEN F.B., FARVER C., HAZEN S.L., JENNINGS C., DWEIK R.A., ARROLIGA A.C., ERZURUM S.C. Increased arginase II and decreased NO synthesis in endothelial cells of patients with pulmonary arterial hypertension. FASEB J. 2004;18:1746–1748. doi: 10.1096/fj.04-2317fje. [DOI] [PubMed] [Google Scholar]
- ZAFARI A.M., USHIO-FUKAI M., AKERS M., YIN Q., SHAH A., HARRISON D.G., TAYLOR W.R., GRIENDLING K.K. Role of NADH/NADPH oxidase-derived H2O2 in angiotensin II-induced vascular hypertrophy. Hypertension. 1998;32:488–495. doi: 10.1161/01.hyp.32.3.488. [DOI] [PubMed] [Google Scholar]