Abstract
Dopamine is an appetite suppressant, while neuropeptide Y (NPY), an appetite stimulant in the brain, is reported to be involved in anorectic action induced by a combined administration of D1/D2 agonists in normal rats. In diabetic rats, however, these factors have not been studied.
Rats (including normal, diabetic and insulin-treated diabetic rats) were given daily injections of saline or D1/D2 agonists for 6 days. Changes in food intake and hypothalamic NPY content of these rats were assessed and compared.
The D1/D2 agonist-induced anorectic responses were altered in diabetic rats compared to normal rats treated similarly. Both the anorectic response on the first day of dosing and the tolerant response on the subsequent days were attenuated.
This alteration was independent of the neuroendocrine disturbance on feeding behavior since the basic pattern of food intake during the time course of a 24-h day/night cycle was similar in normal and diabetic rats; the decrease of food intake following drug treatment was only shown at the initial interval of 0–6 h in both groups of rats.
However, this alteration coincided with changes in NPY content following D1/D2 coadministration. The replacement of insulin in diabetic rats could normalize both NPY content and D1/D2 agonist-induced anorexia.
It is demonstrated that the response of D1/D2 agonist-induced appetite suppression is attenuated in diabetic rats compared to normal rats and that elevated hypothalamic NPY content may contribute to this alteration.
Keywords: Anorectic agent, orexigenic peptide, food intake, insulin, brain
Introduction
Neuropeptide Y (NPY) is the most potent appetite transducer known and widely distributed in the central nervous system with high concentrations found in hypothalamus (Leger et al., 1987). NPY plays a functional role in the regulation of feeding behavior (Billington et al., 1994; Woods et al., 1998) and is postulated to control the energy balance by inhibiting thermogenesis, especially under conditions of energy deficiency such as food restriction, intense exercise, obesity, and diabetes (Richard, 1995; Kotz et al., 1998; Kalra & Kalra, 2004). The NPY system is upregulated, as shown by mRNA overexpression and increased peptide release, through a mechanism of genetic defect in either the NPY receptor (Zucker fa/fa rat, cp/cp rat, and db/db mouse) or the peptide itself (ob/ob mouse) (Beck, 2000), or a mechanism of drug induction by administering the streptozotocin (STZ), a pancreatic β-cell toxin, into normal animals (Frankish et al., 1995).
It has been postulated that dopaminergic activities comprise a major functional component of a central regulatory system for metabolism (Cincotta & Meier, 1995). Evidence indicates that a timed daily administration of DA agonists in obese mice (type-II diabetes) can decrease hyperinsulinemia, hyperglycemia, hyperlipidemia and obesity (i.e. metabolic syndrome) partly due to the effect of drug on hypothalamic NPY neurons (Scislowski et al., 1999; Bina & Cincotta, 2000). Moreover, administration of DA agonists in normal rats can decrease the feeding behavior by an antagonistic action on hypothalamic NPY-containing neurons (Gillard et al., 1993; Kuo, 2003; Hsieh et al., 2005). However, it is unclear if this effect is still applicable in STZ-induced diabetic conditions (type-I diabetes) in which neuroendocrine function is disturbed leading to hyperphagia, hyperglycemia, and an increased hypothalamic NPY content (Williams et al., 1989; Sahu et al., 1992). A recent report from our laboratory indicated that diabetic conditions could modulate the anorectic response induced by amphetamine, an indirect DA agonist, due to the elevated NPY level in hypothalamus (Kuo, 2005). Therefore, one might hypothesize that D1/D2 agonist-induced anorectic responses might be modified in diabetic rats and that this modulation might be relevant to hypothalamic NPY gene expression.
Although DA receptors have been classified as several subtypes (Sibley & Monsma, 1992), the D1 and D2 subtypes are widely accepted as the major forms (Missale et al., 1998). In this study, a combined administration of D1/D2 agonists was selected since it can additively decrease food intake (Kuo, 2003) and in certain instances, maximal central D2 agonist responses (including inhibition of feeding) require endogenous DA to activate the D1 receptor site (Jackson et al., 1988; Cooper & Al-Naser, 1993). DA agonists used in this study, including the selective D1 agonist SKF 38393 (SKF) and the selective D2 agonist quinpirole (QNP), are agents that have been proved to have central inhibitory action on food intake following systemic administration (Samanin & Garattini, 1993).
Methods
Materials
SKF 38393 was from Research Biochemical Inc. (Natick, MA, U.S.A.), insulin solution and STZ was obtained from Sigma Chemical Co. (St Louis, MO, U.S.A.). NPY-RIA kits were purchased from Peninsula Labs Inc. (Belmont, CA, U.S.A.). cDNA synthesis kits were purchased from Boehringer Mannheim GmbH, Germany. Glucose kits were purchased from BioSystem Inc. (Barcelona, Spain). STZ and SKF 38393 were dissolved in 0.9% saline. Quinpirole (Eli Lilly Co., U.S.A.) was dissolved in distilled water. All drugs were freshly dissolved to a final volume of 1 mg ml−1.
Animal treatments
Male rats of the Wistar strain, weighing 200–300 g, were obtained from the animal center of National Cheng Kung University Medical College. They were housed individually in cages, maintained at 22±2°C in a room with a 12-h light–dark cycle (light on at 06:00), and habituated to frequent handling. The administration of drugs and checking of food intake were performed daily at the beginning of the dark period (18:00). This study was carried out in accordance with the Guide for the Care and Use of Laboratory Animals as adopted by the National Institutes of Health.
To compare the differences in D1/D2 agonist-induced anorectic response between normal and diabetic rats, repeated applications of the D1 agonist SKF (1 or 2 mg kg−1) plus D2 agonist QNP (0.1 or 0.2 mg kg−1), at dose ratio of 1/0.1 or 2/0.2 mg kg−1, was administered IP to normal or diabetic rats (n=6–8 each group) at the beginning of the dark phase (18:00) daily for 6 days. The first injection of D1/D2 agonists was at the end of Day 0 (18:00), a time that is also regarded as the beginning of Day 1. Amounts of food intake were measured at 24 h after drug treatment daily for 6 days.
To examine the effect of insulin replacement on the D1/D2 agonist-induced anorectic response in diabetic rats, SKF/QNO (0/0, 1/0.1, or 2/0.2 mg kg−1, i.p.; n=6–8 per group) was administered to insulin-treated diabetic rats at the beginning of Day 1 (i.e., at 18:00 of Day 0) daily for 6 days. The preliminary study revealed that repeated treatments of insulin (1 U kg−1, i.p.) twice a day for at least 7 days were necessary for the restoration of feeding behavior and NPY content in diabetic rats. Therefore, insulin was administered to diabetic rats twice daily for 7 days (Day −6–Day 0) before the treatment of D1/D2 agonists and was continued for 6 days during repeated treatments of D1/D2 agonists. The daily amount of food intake of each rat during repeated treatments of D1/D2 agonists was recorded.
To examine the impact of D1/D2 agonists on NPY content in rats (including normal, diabetic and, insulin-treated diabetic rats), SKF/QNO coadministration (0/0 or 2/0.2 mg kg−1, i.p.; n=6–8 per group) was administered to each rats daily for 6 days. Rats were injected with the drug once a day for 1, 2, 3, 4, 5 or 6 days depending on the group of rats (n=5–6 for each group). To be more specific, rats in Day-1 group were treated with D1/D2 agonists for 1 day and then killed at the end of Day 1 (at 18:00), and rats in Day-2 group were treated with D1/D2 agonists for 2 days and then killed at the end of Day 2 (at 18:00), and so on. On the day of killing, rats received a supplemental treatment of 2/0.2 mg kg−1 D1/D2 agonists 60 min before killing to enhance the effect of the drugs. NPY contents of these rats were determined by methods described in appropriate sections.
To assess the effect of D1/D2 agonists (0/0 or 2/0.2 mg kg−1; i.p.) on hypothalamic NPY mRNA levels, rats were injected with the drug at 18:00 of Day 0 (n=5–6 for each group). On the day of killing (Day 1), rats received a supplemental treatment of 2/0.2 mg kg−1 D1/D2 agonists 60 min before killing to enhance the effect of drug. Rats were then anesthetized and decapitated, and eventually the hypothalamus was removed from their brain and subjected to quantification of NPY mRNA.
To compare the differences in feeding behavior induced by a single treatment of D1/D2 agonists (0/0 or 2/0.2 mg kg−1; i.p.; n=6–8 per group) among normal rats, diabetic rats and tolerant diabetic rats, the amounts of food intake was measured every 6 h, including at 18:00, at 24:00, at 06:00, at 12:00 and at 18:00 (the next day), over a 24-h period after drug treatment.
Induction of diabetic rats
For the induction of the diabetic condition, rats were fasted for 24 h and then given a single injection of STZ (65 mg kg−1, i.v.) into the femoral vein under pentobarbital anesthesia (Nembutal, 40 mg kg−1, i.p.). A fasting blood glucose level >350 mg dl−1 at 72 h after STZ injection confirmed the presence of the diabetic condition. Hyperglycemia was confirmed once for every 3 days during this stage and sustained for at least 3 weeks. Clinical features of the disease (polyuria, polydipsia, polyphagia, weight loss, and malaise) were also observed during this stage. Blood glucose levels were measured using the glucose oxidase method as described in the manufacturers instructions contained in Glucose Kits (BioSystem Inc., Barcelona, Spain). At 1 week after establishing basic hyperglycemia, the animals were divided into several groups for further studies.
Hypothalamic NPY determinations
Rats were anesthetized and decapitated 24 h after drug or vehicle administration. The hypothalamus was removed immediately and placed in 2 N acetic acid followed by sonication for 20 s at 4°C. After sonication, an aliquot (50 μl) of the disrupted tissue was dissolved in 1 N NaOH and assayed for protein content (Lowry et al., 1951). The remainder of tissue sample was immediately boiled for 5 min, and then cooled on ice followed by centrifugation for 20 min at 3000 × g. The supernatants were stored at −20°C until further assay. Each sample was then measured by a radioimmunoassay (RIA) specific for NPY as described previously (Allen et al., 1984). Authentic antibody against NPY and radioactive tracer 125I-labeled NPY were obtained from Peninsula Labs Inc. (San Carios, CA, U.S.A.).
Hypothalamic NPY mRNA determinations
RNA extraction
Rats were anesthetized and decapitated 24 h after drug administration. The hypothalamus was removed immediately. Hypothalamic NPY mRNA levels in a block of mediobasal hypothalamic tissue were measured as described previously (Morris, 1989). In brief, total RNA was isolated from this tissue block using the modified guanidinium thiocyanate–phenol–chloroform method (Chomczynski & Sacchi, 1987). Each hypothalamic block was homogenized in 1 ml of TRIZOL reagent (Life Technologies Inc., Grand Island, U.S.A.) using an Ultrasonic Processor (Vibra Cell, Model CV17; Sonics & Materials Inc., Danbury, CT, U.S.A.). After an incubation at 22°C for 5 min, 0.2 ml of chloroform was added to each sample, shaken vigorously for 15 s, incubated at 22°C for 3 min, then centrifuged at 12,000 × g for 15 min at 4°C. After removal of aqueous phase and precipitation with 0.5 ml isopropanol, samples were incubated at 22°C for 10 min and centrifuged at 12,000 × g for 15 min at 4°C. The gel-like RNA pellets were washed with 75% ethanol by vortexing and centrifugation at 7500 × g for 5 min at 4°C. Thereafter, RNA pellets were dried briefly, dissolved in RNase-free water, and stored at −80°C. The content of RNA was determined spectrophotometrically at 260 nm.
Reverse transcription–polymerase chain reaction
Using the 1st Strand cDNA Synthesis Kit (Boehringer Mannheim GmbH, Germany), RNA preparation was reversely transcribed into single-stranded cDNA. For each sample, 8 μl of sterile DEPC water containing 2 μg of RNA were added to oligo-p(dT)15 primer (0.8 μg μl−1) followed by being denatured at 65°C for 15 min, cooled at 25°C for 10 min, then added to a reaction mixture consisting of 10 × reaction buffer (100 mM Tris, 500 mM KCl; pH 8.3), dNTPs (10 mM each), MgCl2 (25 mM), RNase inhibitor (40 U μl−1), and AMV reverse transcriptase (25 U μl−1). Reaction mixtures were incubated at 42°C for 2 h and then brought to 95°C for 5 min to terminate the reaction followed by soaking at 16°C. PCR was subsequently carried out by mixing 3 μl of cDNA product with mastermix solution consisting of DEPC water, 10 × reaction buffer, MgCl2 (25 mM), dNTPs (10 mM each), P1 and P2 primers (1 μg μl−1 each), and Taq polymerase (5 U μl−1). Sequences of primers used in this experiment were 5′-GGGCTGTGTGGACTGACC-3′ and 5′-GGAAGGGTCTTCAAGCCT-3′ for sense and antisense strands, respectively. PCR reactions for NPY were carried out on a PCR thermocycler (Perkin–Elmer GeneAmp 2400) for 28 cycles with the following steps: 91°C for 1 min (denaturing), 60°C for 1 min (annealing), and 72°C for 30 s (extension), followed by a final elongation step at 72°C for 7 min, and finally the PCR products were soaked at 16°C. GAPDH was used as the internal standard calibrator. PCR reaction for the GAPDH was carried out in steps similar to those described above except the changes of two steps annealing (52°C) and cycles (25).
Gel electrophoresis
At the completion of RT-PCR, 8 μl of each PCR product was subsequently separated by flat-bed gel electrophoresis on a 2% agarose gel. Gels stained by ethidium bromide were visualized under UV light, photographed, and then scanned densitometrically. The ratio of NPY/GAPDH mRNA was measured by digital densitometry (Hoefer, San Francisco, CA, U.S.A.) to determine relative NPY mRNA levels. The calculated NPY mRNA level was compared with the control group and then expressed as percentage of control.
Statistical analysis
Statistical data were assessed by t-test, one-way or two-way ANOVA followed by post hoc Dunnett's test. P<0.05 was considered to be statistically significant. Values are represented as mean±standard error (s.e.m.).
Results
The effect of D1/D2 agonist coadministration on feeding behavior
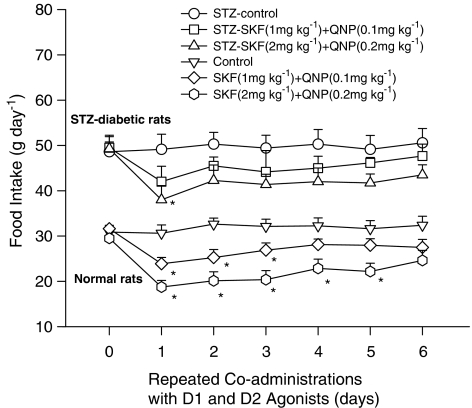
Changes of food intake in normal rats receiving D1/D2 agonists are shown in the lower part of Figure 1. Using two-way ANOVA to repeatedly measure the effect of D1/D2 agonists on feeding behavior from Days 0 to 6, significant dose-dependent (F(2,20)=8.5, P<0.01) and time-dependent (F(6,49)=5.5, P<0.01) effects were revealed, but the interaction-dependent effect was not included. Dunnett's test (P<0.05) revealed that coadministrations with D1/D2 agonist (at dose ratio 1/0.1 mg kg−1) led to a reduction of food intake on Days 1, 2 and 3, while coadministrations with D1/D2 agonist (at dose ratio 2/0.2 mg kg−1) exerted significant effects of food intake from Days 1 to 5. These results indicated that repeated coadministrations of D1/D2 agonist decreased markedly the food intake on Day 1 and restored gradually the food intake to normal level (tolerance effect) on the followings in normal rats.
Figure 1.
The comparison of daily food intake between diabetic and normal rats during a 6-day repeated coadministration of D1/D2 agonists. SKF/QNP at a dose ratio of 1/0.1 or 2/0.2 mg kg−1 was injected at 18:00 of each treatment day. Comparisons were made using two-way ANOVA followed by Dunnett's test. *Indicated P<0.05 compared to the control group of each treatment day.
Changes of food intake in diabetic rats receiving D1/D2 agonists are shown in the upper part of Figure 1. Using two-way ANOVA to repeatedly measure the effect of D1/D2 agonists on feeding behavior from Days 0 to 6, a significant dose-dependent (F(2,20)=4.1, P<0.05) but not time- and interaction-dependent effect was observed. Dunnett's test revealed that D1/D2 agonist (1/0.1 mg kg−1) coadministrations had no significant effect on the food intake, but D1/D2 agonist (2/0.2 mg kg−1) coadministrations exerted a significant effect on Day 1. These results indicated that D1/D2 agonist coadministrations could dose-dependently induce an anorectic effect in diabetic rats.
When comparing the anorectic effect of D1/D2 agonist coadministrations between normal and diabetic rats, it was revealed that (1) there was a significant decrease of anorectic response on Day 1 in diabetic rats (36% in normal rats vs 23% in diabetic rats), and (2) D1/D2 agonist coadministrations produced a faster tolerant effect on the anorectic response in diabetic rats. These results indicated that the diabetic condition could modify the anorectic responses induced by D1/D2 agonists.
The effect of insulin replacement on the feeding response in diabetic rats
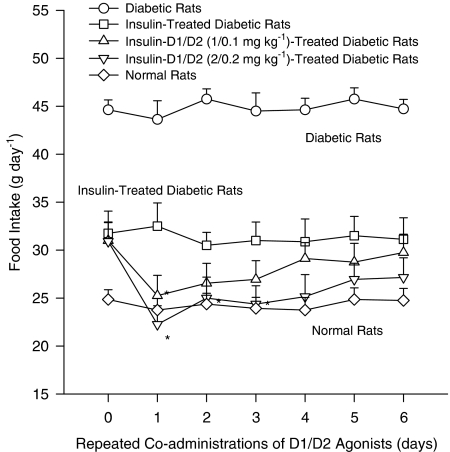
The effect of insulin replacement on D1/D2 agonist-induced anorectic response in diabetic rats is shown in Figure 2. Using two-way ANOVA to repeatedly measure the effect of D1/D2 agonist coadministrations on feeding behavior from Days 0 to 6, it revealed significant dose-dependent (F(2,18)=6.2, P<0.01) and time-dependent effects (F(6,47)=4.2, P<0.01), but an interaction effect was not included. Dunnett's test revealed that coadministrations with D1/D2 agonist (1/0.1 mg kg−1) led to a significant effect on Day 1, while coadministrations with D1/D2 (2/0.2 mg kg−1) resulted in a significant effect from Days 1 to 3. This result revealed the efficiency of insulin replacement to normalize both the feeding behavior and the anorectic response to D1/D2 agonist coadministrations.
Figure 2.
The effect of insulin replacement on D1/D2 agonists-induced daily food intake in diabetic rats over a 6-day period. To normalize the daily food intake before SKF/QNP coadministrations, insulin (1 U kg−1; i.p.) was administered to diabetic rats twice a day for 7 days and was continuously administered to these rats for 6 days during SKF/QNP coadministrations. SKF/QNP at a dose ratio of 1/0.1 or 2/0.2 mg kg−1 was injected at 18:00 of each treatment day. Comparisons were made using two-way ANOVA followed by Dunnett's test. *Indicated P<0.05 compared to the insulin-treated control group of each treatment day.
The effect of D1/D2 agonists on hypothalamic NPY contents
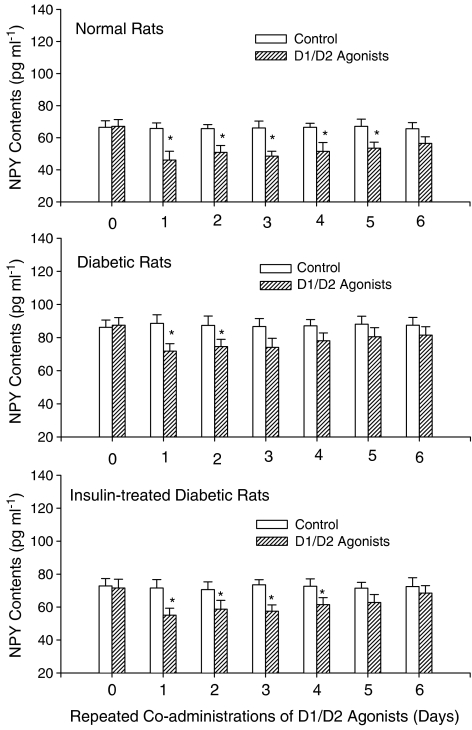
Results shown in upper part of Figure 3 reveal that D1/D2 agonist coadministrations in normal rats can lead to a significant decrease of NPY content. Using one-way ANOVA (F(6,42)=3.8, P<0.01) followed by a Dunnett's test, it was revealed that D1/D2 agonists could decrease NPY content for 5 days (from Days 1 to 5). Both the anorectic response on Day 1 and the tolerant response on the subsequent days were related to changes of NPY content in normal rats treated with D1/D2 agonists.
Figure 3.
Effects of repeated coadministrations of D1/D2 agonists on changes of hypothalamic NPY content in normal, diabetic and insulin-treated diabetic rats. Levels of NPY were measured by a radioimmunoassay technique. SKF/QNP (2/0.2 mg kg−1; i.p.) was injected at 18:00 of each treatment day. Values are represented as mean±standard error (s.e.m.), n=6–8 per group. *P<0.05 vs the control group of each treatment day in normal or diabetic rats.
Results shown in the middle part of Figure 3 reveal that D1/D2 agonist coadministrations in diabetic rats can result in a significant decrease of NPY content. Statistical analysis showed that D1/D2 agonists could decrease NPY content for 2 days (Day 1 and Day 2) (F(6,35)=2.6, P<0.05). Moreover, the treatment of STZ could increase NPY content about 30% in diabetic rats (88 pg ml−1) comparing to normal ones (68 pg ml−1) (t-test, P<0.05). These results showed that the hypothalamic NPY content was elevated in diabetic rats and that the attenuation of anorectic response in diabetic rats treated with D1/D2 agonists was related to the change of NPY content.
Results shown in the lower part of Figure 3 indicate the impact of D1/D2 agonist coadministrations on the decrease of NPY content in insulin-treated diabetic rats. Statistical analysis (F(6,38)=3.1, P<0.01) showed that D1/D2 agonists could decrease NPY content for 4 days (from Days 1 to 4). Moreover, the treatment of insulin alone could normalize NPY content from Days 1 to 6 in insulin-treated diabetic rats (73 pg ml−1) comparing to normal ones (68 pg ml−1) (t-test, P<0.05). These results revealed that the treatment of insulin replacement in diabetic rats could normalize either the NPY content in hypothalamus or the anorectic effect of D1/D2 agonists.
The effect of D1/D2 agonists on hypothalamic NPY mRNA levels
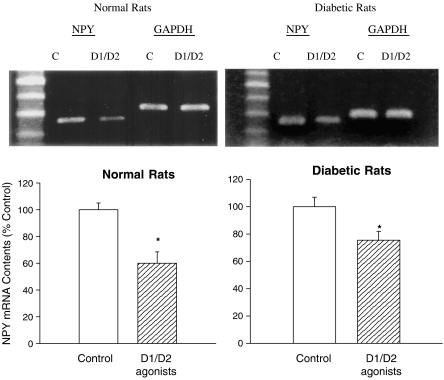
Results shown in Figure 4 reveal the effect of D1/D2 agonist coadministrations on the change of NPY mRNA level in both diabetic and normal rats. Using GAPDH as the internal standard, the ratio of NPY mRNA over GAPDH mRNA in D1/D2 agonist-treated rats was markedly decreased as compared with the saline-treated group (P<0.05, t-test). In normal rats, the ratio of NPY/GAPDH mRNA measured by digital densitometry was 85±5% in saline-treated group and 45±4% in D1/D2 agonist-treated group. The relative ratio between D1/D2 agonist- and saline-treated groups was 53±5%. In diabetic rats, the ratio of NPY/GAPDH mRNA measured was 88±6% in saline-treated group and 60±3% in D1/D2 agonists-treated group. The relative ratio between D1/D2 agonist- and saline-treated groups was 68±5%. These results revealed that levels of hypothalamic NPY mRNA were decreased following drug treatments in both diabetic and normal rats, revealing the involvement of NPY gene in D1/D2 agonist-induced anorexia.
Figure 4.
Upper part: the effect of D1/D2 agonists (2/02 mg kg−1; i.p.) on hypothalamic NPY mRNA level in diabetic and normal rats. NPY mRNA levels were measured using RT–PCR. Lower part: relative values between D1/D2 agonist- and saline-treated groups. Contents of NPY mRNA in D1/D2 agonist-treated group were indicated as the percentage of control. Bars are mean±s.e.m. N=6–8 each group. *Indicates P<0.05 compared to control group.
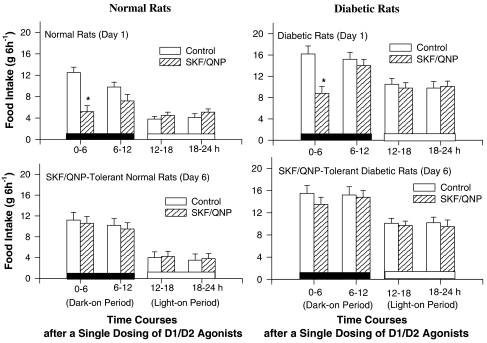
Time courses for the change of feeding after a single injection of D1/D2 agonists
Results shown in Figure 4 reveal that: (1) basic patterns of feeding response are similar in normal, tolerant, and diabetic rats. They eat more in the dark period (0–12 h after a single treatment of D1/D2 agonist) but less in the light period; (2) a single treatment of D1/D2 agonist (2/0.2 mg kg−1) leads to a decrease of food intake only at the first 0–6 h time interval in normal and diabetic rats (t-test, P<0.05) (Figure 5). There are no changes during the other time intervals after drug treatment in both rats; and (3) the feeding behavior on Day 6 is restored to normal level in both diabetic and normal rats, revealing that the development of tolerance to D1/D2 agonist-induced anorexia is occurred at the initial 0–6 h time interval. These results revealed that the basic pattern of D1/D2 agonist-induced anorexia in diabetic rats was similar to that of normal ones and that the tolerant effect induced by repeated treatments of D1/D2 agonists was occurred at the initial 6-h period in both rats.
Figure 5.
Comparisons for the time course of a 24-h feeding behavior between normal (left panel) and diabetic rats (right panel) after a single dosing of SKF/QNP coadministration. A 24-h food intake was divided into four intervals (0–6, 6–12, 12–18 and 18–24 h). Rats were given with SKF/QNP (2/0.2 mg kg−1; i.p.) or saline at 18:00 (i.e. at the beginning of dark-on period) on each testing day. Comparisons were made using t-test. *Indicated P<0.05 compared to the control group of each time intervals.
Discussion
The STZ diabetic rats provide an interesting model, in which overactivity of hypothalamic NPY neuron has been demonstrated and can explain the marked hyperphagia in this condition (Williams, 1989; Sindelar et al., 2002). The present results revealed that the anorectic response induced by D1/D2 agonists was attenuated in STZ diabetic rats as compared with normal rats treated similarly. Both the anorectic effect on Day 1 and the tolerant effect on the subsequent days were attenuated. This attenuation was regulated by NPY since the alteration of NPY content following drug treatment was consistent with the change of feeding behavior. Moreover, this attenuation was relevant to the enhanced activity of hypothalamic NPY neuron in diabetic rats since their NPY contents were increased (approximately by 30%) and the lasting duration of anorectic response was shortened (5 days in normal rats vs 2 days in diabetic rats) during a 6-day drug treatment. However, this attenuation was normalized in insulin-treated diabetic rats due to the restoration of NPY content in hypothalamus. These results support the hypothesis that hypothalamic NPY is involved in the regulation of D1/D2 agonist-induced anorexia in STZ diabetic rats.
Except NPY, it is likely that the reduced anorectic action of D1/D2 agonists in diabetic rats may be due to a decreased inhibitory action of central dopaminergic system. Although DA level in the brain is increased in short-term diabetes (Ramakrishnan et al., 2005), DA synthesis within the brain is reduced and DA level in the hypothalamus is lower in chronic STZ-diabetic rats (Trulson & Himmel, 1983; Chu et al., 1986). The change of cerebral DA content may influence the neuroendocrine system and therefore decrease the anorectic response of D1/D2 agonists. However, this possibility still needs to be investigated further.
As diabetic condition can disturb the function of neuroendocrine system, it is likely that the reduction of D1/D2 agonist-induced anorexia in diabetic rats may be due to a shift of the distribution of feeding episode during a 24-h day/night cycle. Diabetic animals may eat more than controls in day cycle due to the disturbance of endocrine regulation. However, from the time course of a 24-h feeding behavior, it revealed that both the distribution of feeding episode (mainly at night cycle) and the drug-induced anorectic and tolerant responses (at the initial 0–6 h time interval) in diabetic rats were similar to that of normal ones. These results revealed the noninterference of neuroendocrine disturbance on feeding behavior in diabetic rats. A previous report (Luo et al., 1999) indicating that DA agonists could correct abnormalities in the neuroendocrine axis and reduce the autonomic nervous tone, which contributed to obesity and diabetes, might support our finding to some extent.
Evidence indicates that DA agonists can act centrally to reduce appetite (Samanin & Garattini, 1993). Although the specific site of dopaminergic action in the brain is unknown and preliminary investigations suggest that a decrease in ventromedial hypothalamic stimulation of lipolysis and hepatic glucose output may be involved in the effect observed with D1/D2 agonist treatment (Cincotta & Meier, 1995; Kraszewski & Cincotta, 2000), our present results suggest that hypothalamic NPY-containing neurons may be one of the sites on which the DA drug acts and this mechanism may play an essential role in the regulation of appetite suppression in STZ (type-I) diabetic rats. Similar to our findings, it was reported that dopaminergic agonists might improve hyperphagia by normalizing the elevated level of hypothalamic NPY in ob/ob mouse (type II diabetes) (Bina & Cincotta, 2000).
The present finding that the decrease of NPY mRNA content following drug treatment in both normal and diabetic rats indicated that NPY genome was involved in the anorectic action of D1/D2 agonists. This result was different from that of serotonin-treated animals. Serotonin treatment can decrease NPY content in the area of paraventricular nucleus, but the level of NPY mRNA in hypothalamus and the NPY content in the area of arcuate nucleus are not changed, suggesting that NPY gene is not involved in serotonin-induced anorexia (Dryden et al., 1996). Thus, the central appetite-controlled pathway activated by DA agonists may be different from that of serotonin agonists. A previous report indicating that the activation of central melanocortin pathway played a major role in the anorectic action of serotonin might support our view (Heisler et al., 2002).
It has been postulated that dopaminergic agonist treatment may improve obesity and diabetes in a variety of animal models and in humans (Scislowski et al., 1999; Jetton et al., 2001). In the present study, repeated coadministrations of D1/D2 agonist could normalize both the elevated NPY content and hyperphagic effect observed in STZ-diabetic rats, implying the potential efficiency of D1/D2 agonists in the improvement of hyperphagia in type-I diabetes in human. Although the functional role of NPY in human homeostasis remain enigmatic, the animal studies have identified it as a potential target for new drugs to treat obesity and diabetes (Grove & Smith, 2003; Kalra & Kalra, 2004). The metabolic and neuroendocrine responses to D1/D2 agonist are very similar to those of leptin since both treatments can attenuate hyperphagia, obesity and elevated hypothalamic NPY (Krugel et al., 2003; Brunetti et al., 2005). Thus, it is rational to predict that combined administration of D1 and D2 agonists may contribute to the improvement in hyperphagia by lowering the NPY gene expression in human diabetes or obesity.
In conclusion, our results demonstrate that the increased content of hypothalamic NPY plays a major role in attenuating the anorectic response of D1/D2 agonists in STZ-induced diabetic rats as compared to normal ones. Moreover, the present findings provide new information about an important topic in endocrinology of great contemporary interest that is the neuroendocrine regulation of appetite control. An extra dimension has been added in using a type-I diabetic model.
Acknowledgments
The author expresses gratitude to Dr Juei-Tang Cheng, professor of the National Cheng Kung University, for the invaluable suggestions on this article. This research was supported by a grant from National Science Council (NSC 94-2320-B-040-018).
Abbreviations
- DA
dopamine
- NPY
neuropeptide Y
- QNP
quinpirole
- RIA
radioimmunoassay
- RT–PCR
reverse transcription–polymerase chain reaction
- SKF
SKF 38393
- STZ
streptozotocin
References
- ALLEN J.M., YEATS J.C., ADRIAN T.E., BLOOM S.R. Radioimmunoassay of Neuropeptide Y. Regul. Peptides. 1984;8:61–70. doi: 10.1016/0167-0115(84)90029-6. [DOI] [PubMed] [Google Scholar]
- BECK B. Neuropeptides and obesity. Nutrition. 2000;16:916–923. doi: 10.1016/s0899-9007(00)00410-x. [DOI] [PubMed] [Google Scholar]
- BILLINGTON C.J., BRIGGS J.E., HARKER S., GRACE M., LEVINE A.S. Neuropeptide Y in hypothalamic paraventricular nucleus: a center coordinating energy metabolism. Am. J. Physiol. 1994;266:R1765–R1770. doi: 10.1152/ajpregu.1994.266.6.R1765. [DOI] [PubMed] [Google Scholar]
- BINA K.G., CINCOTTA A.H. Dopaminergic agonists normalize elevated hypothalamic neuropeptide Y and corticotropin-releasing hormone, body weight gain, and hyperglycemia in ob/ob mice. Neuroendocrinology. 2000;71:68–78. doi: 10.1159/000054522. [DOI] [PubMed] [Google Scholar]
- BRUNETTI L., DI NISIO C., ORLANDO G., FERRANTE C., VACCA M. The regulation of feeding: a cross talk between peripheral and central signalling. Int. J. Immunopathol. Pharmacol. 2005;18:201–212. doi: 10.1177/039463200501800203. [DOI] [PubMed] [Google Scholar]
- CHOMCZYNSKI P., SACCHI N. Single-step method of RNA isolation by acid quanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- CHU P.C., LIN M.T., SHIAN L.R., LEU S.Y. Alterations in physiologic functions and brain monoamine content in streptozotocin-diabetic rats. Diabetes. 1986;35:481–485. doi: 10.2337/diab.35.4.481. [DOI] [PubMed] [Google Scholar]
- CINCOTTA A.H., MEIER A.H. Bromocriptine inhibits in vivo free fatty acid oxidation and hepatic glucose output in seasonally obese hamsters (Mesocricetus auratus) Metabolism. 1995;44:1349–1355. doi: 10.1016/0026-0495(95)90041-1. [DOI] [PubMed] [Google Scholar]
- COOPER S.J., AL-NASER H.A.D1:D2 dopamine receptor interactions in relation to feeding responses and food intake D1:D2 Dopamine Receptor Interactions 1993San Diego: Academic; 203–234.ed. Waddington, J. pp [Google Scholar]
- DRYDEN S., FRANKISH H.M., WANG Q., WILLIAMS G. Increased feeding and neuropeptide Y (NPY) but not NPY mRNA levels in the hypothalamus of the rat following central administration of the serotonin synthesis inhibitor p-chlorophenylalanine. Brain Res. 1996;724:232–237. doi: 10.1016/0006-8993(96)00329-0. [DOI] [PubMed] [Google Scholar]
- FRANKISH H.M., DRYDEN S., HOPKINS D., WANG Q., WILLIAMS G. Neuropeptide Y, the hypothalamus and diabetes: Insights into the control of metabolism. Peptides. 1995;16:757–771. doi: 10.1016/0196-9781(94)00200-p. [DOI] [PubMed] [Google Scholar]
- GILLARD E.R., DANG D.Q., STANLEY B.G. Evidence that neuropeptide Y and dopamine in the perifornical hypothalamus interact antagonistically in the control of food intake. Brain Res. 1993;628:128–136. doi: 10.1016/0006-8993(93)90947-l. [DOI] [PubMed] [Google Scholar]
- GROVE K.L., SMITH M.S. Ontogeny of the hypothalamic neuropeptide Y system. Physiol. Behav. 2003;79:47–63. doi: 10.1016/s0031-9384(03)00104-5. [DOI] [PubMed] [Google Scholar]
- HEISLER L.K., COWLEY M.A., TECOTT L.H., WEI F., LOW M.J., SMART J.L., RUBINSTEIN M., TATRO J.B., MARCUS J.N., HOLSTEGE H.L., CHARLOTTE E., CONE R.D., ELMQUIST J.K. Activation of central melanocortin pathways by fenfluramine. Science. 2002;297:609–611. doi: 10.1126/science.1072327. [DOI] [PubMed] [Google Scholar]
- HSIEH Y.S., YANG S.F., KUO D.Y. Amphetamine, an appetite suppressant, decreases neuropeptide Y immunoreactivity in rat hypothalamic paraventriculum. Regul. Peptides. 2005;127:169–176. doi: 10.1016/j.regpep.2004.11.007. [DOI] [PubMed] [Google Scholar]
- JACKSON D.M., ROSS S.B., HASHIZUME M. Further studies on the interaction between bromocriptine and SKF38393 in reserpine and alpha methyl-para-tyrosine-treated mice. Psychopharmacology. 1988;94:321–327. doi: 10.1007/BF00174683. [DOI] [PubMed] [Google Scholar]
- JETTON T.L., LIANG Y., CINCOTTA A.H. Systemic treatment with sympatholytic dopamine agonists improves aberrant beta-cell hyperplasia and GLUT2, glucokinase, and insulin immunoreactive levels in ob/ob mice. Metabolism. 2001;50:1377–1384. doi: 10.1053/meta.2001.26741. [DOI] [PubMed] [Google Scholar]
- KALRA S.P., KALRA P.S. NPY and cohorts in regulating appetite, obesity and metabolic syndrome: beneficial effects of gene therapy. Neuropeptides. 2004;38:201–211. doi: 10.1016/j.npep.2004.06.003. [DOI] [PubMed] [Google Scholar]
- KOTZ C.M., BRIGGS J.E., GRACE M.K., LEVINE A.S., BILLINGTON C.J. Divergence of the feeding and thermogenic pathways influenced by NPY in the hypothalamic PVN of the rat. Am. J. Physiol. 1998;275:R471–R477. doi: 10.1152/ajpregu.1998.275.2.R471. [DOI] [PubMed] [Google Scholar]
- KRASZEWSKI K.Z., CINCOTTA A.H. Increased responsiveness of ventromedial hypothalamic neurons to norepinephrine in obese versus lean mice: relation to the metabolic syndrome. Int. J. Mol. Med. 2000;5:349–355. doi: 10.3892/ijmm.5.4.349. [DOI] [PubMed] [Google Scholar]
- KRUGEL U., SCHRAFT T., KITTNER H., KIESS W., ILLES P. Basal and feeding-evoked dopamine release in the rat nucleus accumbens is depressed by leptin. Eur. J. Pharmacol. 2003;482:185–187. doi: 10.1016/j.ejphar.2003.09.047. [DOI] [PubMed] [Google Scholar]
- KUO D.Y. Further evidence for the mediation of both subtypes of dopamine D1/D2 receptors and cerebral neuropeptide Y (NPY) in amphetamine-induced appetite suppression. Behav. Brain Res. 2003;147:149–155. doi: 10.1016/j.bbr.2003.04.001. [DOI] [PubMed] [Google Scholar]
- KUO D.Y. Involvement of hypothalamic neuropeptide Y in regulating the amphetamine-induced appetite suppression in streptozotocin diabetic rats. Regul. Peptides. 2005;127:19–26. doi: 10.1016/j.regpep.2004.10.008. [DOI] [PubMed] [Google Scholar]
- LEGER L., CHARNAY Y., DANGER J.M., VAUDRY H., PELLETIER G., DUBOIS P.M., JOUVET M. Mapping of neuropeptide Y-like immunoreactivity in the feline hypothalamus and hypophysis. J. Comp. Neurol. 1987;255:283–292. doi: 10.1002/cne.902550211. [DOI] [PubMed] [Google Scholar]
- LOWRY O.H., ROSEBROUGH N.J., FARR A.L., RANDALL R.J. Protein measurement with folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- LUO S., LIANG Y., CINCOTTA A.H. Intracerebroventricular administration of bromocriptine ameliorates the insulin-resistant/glucose-intolerant state in hamsters. Neuroendocrinology. 1999;69:160–166. doi: 10.1159/000054415. [DOI] [PubMed] [Google Scholar]
- MISSALE C., NASH S.R., ROBINSON S.W., JABER M., CARON M.G. Dopamine receptors: from structure to function. Physiol. Rev. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- MORRIS B.J. Neuronal localization of neuropeptide Y gene expression in rat brain. J. Comp. Neurol. 1989;290:358–368. doi: 10.1002/cne.902900305. [DOI] [PubMed] [Google Scholar]
- RAMAKRISHNAN R., KEMPURAJ D., PRABHAKARAN K., JAYAKUMAR A.R., DEVI R.S., SUTHANTHIRARAJAN N., NAMASIVAYAM A. A short-term diabetes induced changes of catecholamines and p38-MAPK in discrete areas of rat brain. Life Sci. 2005;77:1825–1835. doi: 10.1016/j.lfs.2004.12.038. [DOI] [PubMed] [Google Scholar]
- RICHARD D. Exercise and the neurobiological control of food intake and energy expenditure. Int. J. Obes. 1995;19:S73–S79. [PubMed] [Google Scholar]
- SAHU A., SNINSKY C.A., PHELPS C.P., DUBE M.G., KALRA P.S., KALRA S.P. Neuropeptide Y release from the paraventricular nucleus in association with hyperphagia in streptozocin-induced diabetic rats. Endocrinology. 1992;131:1979–1985. doi: 10.1210/endo.131.6.1446635. [DOI] [PubMed] [Google Scholar]
- SAMANIN R., GARATTINI S. Neurochemical mechanism of action of anorectic drugs. Annu. Rev. Pharmacol. Toxicol. 1993;73:63–68. doi: 10.1111/j.1600-0773.1993.tb01537.x. [DOI] [PubMed] [Google Scholar]
- SCISLOWSKI P.W., TOZZO E., ZHANG Y., PHANEUF S., PREVELIGE R., CINCOTTA A.H. Biochemical mechanisms responsible for the attenuation of diabetic and obese conditions in ob/ob mice treated with dopaminergic agonists. Int. J. Obes. Relat. Metab. Disord. 1999;23:425–431. doi: 10.1038/sj.ijo.0800893. [DOI] [PubMed] [Google Scholar]
- SIBLEY D.R., MONSMA F.J. Molecular biology of dopamine receptors. Trends Pharmacol. Sci. 1992;13:61–69. doi: 10.1016/0165-6147(92)90025-2. [DOI] [PubMed] [Google Scholar]
- SINDELAR D.K., MYSTKOWSKI P., MARSH D.J., PALMITER R.D., SCHWARTZ M.W. Attenuation of diabetic hyperphagia in neuropeptide Y--deficient mice. Diabetes. 2002;51:778–783. doi: 10.2337/diabetes.51.3.778. [DOI] [PubMed] [Google Scholar]
- TRULSON M.E., HIMMEL C.D. Decreased brain dopamine synthesis rate and increased (3H)spiroperidol binding in streptozotocin-diabetic rats. J. Neurochem. 1983;40:1456–1459. doi: 10.1111/j.1471-4159.1983.tb13590.x. [DOI] [PubMed] [Google Scholar]
- WILLIAMS G., GILL J.S., LEE Y.C., CARDOSO H.M., OKPERE B.E., BLOOM S.R. Increased neuropeptide Y concentrations in the specific hypothalamic regions of the streptozocin induced diabetic rats. Diabetes. 1989;38:321–327. doi: 10.2337/diab.38.3.321. [DOI] [PubMed] [Google Scholar]
- WOODS S.C., FIGLEWICZ D.P., MADDEN L., PORTE D, JR, SIPOLS A.J., SEELEY R.J. NPY and food intake: discrepancies in the model. Regul. Peptides. 1998;75:403–408. doi: 10.1016/s0167-0115(98)00095-0. [DOI] [PubMed] [Google Scholar]