Abstract
The present study evaluated the participation of tumour necrosis factor-α (TNF-α) in the inflammatory and nociceptive responses evoked by carrageenan in the mouse paw.
The intraplantar injection of carrageenan (300 μg paw−1) induced a marked and biphasic paw oedema formation (peaks at 6 and 72 h), which was accompanied by a long-lasting mechanical allodynia (that remained elevated for up to 72 h) and a significant increase of myeloperoxidase (MPO) activity (peak at 6 h) in both Swiss and C57/BL6 mice.
The paw oedema, the elevation of MPO activity and to a lesser extent the mechanical allodynia elicited by carrageenan were found to be significantly reduced in TNF-α p55 receptor knockout mice.
Of interest, the systemic administration of an anti-TNF-α antibody produced a significant inhibition of paw oedema, mechanical allodynia and MPO activity. A noteworthy decrease in inflammatory and nociceptive responses caused by carrageenan was also observed when mice were previously treated with the preferential inhibitor of TNF-α synthesis, thalidomide.
The present results clearly indicate that the proinflammatory cytokine TNF-α plays a critical role in the oedema formation, as well as in the mechanical allodynia and the neutrophil migration, following carrageenan administration into the mouse paw. Intraplantar injection of carrageenan in mice could constitute a useful model for assessment of the in vivo effects of potential inhibitors of TNF-α-related pathways.
Keywords: Carrageenan, mouse paw, TNF-α, oedema, nociception, cell migration
Introduction
A great number of studies have attempted to evaluate nociceptive and inflammatory alterations in rodents. Winter et al. (1962) introduced the paw oedema model arising from the intraplantar injection of carrageenan in rats. It has been demonstrated that carrageenan produces a monophasic oedematogenic response in the rat paw, which is accompanied by inflammatory cell migration and marked nociceptive alterations. Further studies have shown that carrageenan-induced rat paw oedema is largely associated with the production of several inflammatory mediators such as histamine, prostaglandins, kinins, nitric oxide (NO) and cytokines, among others (Di Rosa et al., 1971; Salvemini et al., 1986; Tabo et al., 1998; Pinheiro and Calixto, 2002; Morris, 2003; Esposito et al., 2005). In mice, the intraplantar injection of carrageenan produces a peculiar oedematogenic response, which possesses a biphasic profile, with peaks at 6 and 72 h (Levy, 1969; Sugishita et al., 1981; Henriques et al., 1987; Ianaro et al., 1994; Posadas et al., 2004). Carrageenan-induced mouse paw oedema is also associated with paw nociceptive changes and the migration of inflammatory cells to the site of injection (Posadas et al., 2004; Cunha et al., 2005). It has been demonstrated recently that inflammatory responses following carrageenan injection into mice paw are associated with the production of prostaglandins (formed by the action of both COX-1 and COX-2) and NO (derived from both eNOS and iNOS) (Posadas et al., 2004; Bucci et al., 2005).
Tumour necrosis factor-α (TNF-α) is a potent proinflammatory cytokine that possesses multiple effects, including the activation of inflammatory cells, the induction of several inflammatory proteins, cytotoxicity, etc. (Feldmann & Saklatvala, 2001; Haddad, 2002; Hopkins, 2003). The present study was designed to investigate the contribution of TNF-α production to the inflammatory responses (oedema and neutrophil migration) and also the nociceptive response (mechanical allodynia) caused by the intraplantar injection of carrageenan into the mouse paw.
Methods
Animals
Male Swiss, C57/BL6 and TNF-α p55−/− receptor knockout mice (30–35 g) were used throughout this study. Animals were housed under conditions of optimum light, temperature and humidity (12 h light–dark cycle, 22±1°C, 60–80% humidity), with food and water provided ad libitum. Swiss mice were obtained from the Department of Pharmacology, Universidade Federal de Santa Catarina (UFSC, Florianópolis, Brazil). C57/BL6 wild-type or TNF-α p55−/− receptor knockout mice were obtained from the Universidade Federal de Minas Gerais (UFMG, Belo Horizonte, Brazil). The TNF-α p55−/− receptor-deficient mice used in the present study were C57/BL6 inbred. Experiments were conducted in accordance with the current guidelines for the care of laboratory animals and ethical guidelines for the investigation of experimental pain in conscious animals laid down by Zimmermann (1983). The Ethics Committee of the Federal University of Santa Catarina approved all the experimental procedures (protocol numbers 262/CEUA and 23080.035334/2003-16/UFSC). The numbers of animals and intensities of noxious stimuli used were the minimum necessary to demonstrate the consistent effects.
Paw oedema
The experiments were conducted according to the procedures originally described by Henriques et al. (1987). Briefly, the animals received a 50 μl subcutaneous (s.c.) injection, into one hindpaw (the right), of phosphate-buffered saline (PBS, composition, in mmol l−1: NaCl 137, KCl 2.7 and phosphate buffer 10) containing carrageenan (300 μg paw−1). The contralateral paw (left paw) received 50 μl of PBS and was used as the control. Paw oedema was measured by means of a plethysmometer, modified for mice (Ugo Basile), at several time points (1/2, 1, 2, 4, 6, 24, 48, 72 and 96 h) after the injection of carrageenan. Paw oedema is expressed in microlitres as the difference between the right and left paws.
Hindpaw withdrawal response induced by von Frey hairs
The mechanical allodynia was carried out according to the procedures described before by Quintão et al. (2005) with minor modifications. The animals received a 50 μl s.c. injection, into the right hindpaw, of PBS containing carrageenan (300 μg paw−1). Following several intervals of time (1, 3, 4, 6, 24, 48 and 72 h), the mice were placed individually in clear Plexiglas boxes (9 × 7 × 11 cm) on elevated wire mesh platforms to allow access to the ventral surface of the right hindpaw. The animals were acclimatized for 30 min before behavioural testing. The withdrawal response frequency was measured following 10 applications (duration of 1 s each) of von Frey hairs (VFH, Stoelting, Chicago, IL, U.S.A.). Stimuli were delivered from below to the plantar surface of the right hindpaw. The VFH of 0.6 g produces a mean withdrawal frequency of about 15%, which is considered to be an adequate value for the measurement of mechanical hypernociception (Bortolanza et al., 2002). Therefore, 0.6 g VFH was used throughout this study. In order to determine the basal mechanical thresholds, all the groups were evaluated before carrageenan injection.
Neutrophil myeloperoxidase assay
The neutrophil recruitment to the mouse paw was measured by means of tissue myeloperoxidase (MPO) activity, which was determined according to the method described by da Cunha et al. (2004), with some adjustments. The animals received a 50 μl s.c. injection, into the right paw, of PBS containing carrageenan (300 μg paw−1) and were killed 6 h later. PBS-treated paws were used as control. At the time of killing, the s.c. tissue of the paws was removed, homogenized at 5% (weight volume−1) in ethylenediaminetetraacetic acid (EDTA)/NaCl buffer (pH 4.7) and centrifuged at 10,000 r.p.m. for 15 min at 4°C. The pellet was re-suspended in hexadecyltrimethyl ammonium bromide (HTAB) 0.5% buffer (pH 5.4) and the samples were frozen and thawed three times in liquid nitrogen. Upon thawing, the samples were re-centrifuged (10,000 r.p.m., 15 min, at 4°C) and 25 μl of the supernatant were used for the MPO assay. The enzymatic reaction was assessed with 1.6 mM tetramethylbenzidine (TMB), 80 mM NaPO4 and 0.3 mM hydrogen peroxide. The absorbance was measured at 690 nm and the results are expressed in optical density per milligram of tissue.
Effect of anti-TNF-α strategies
To evaluate the role of TNF-α in the nociceptive and inflammatory responses induced by carrageenan in the mouse paw, different groups of Swiss mice were systemically treated with an anti-TNF-α antibody (100 μg kg−1, i.v.) or with the known inhibitor of TNF-α synthesis, thalidomide (30 mg kg−1, s.c.), both administered 5 min before carrageenan (300 μg paw−1) injection. A separate group of mice received the anti-TNF-α antibody (100 μg kg−1, i.v.) 5 min before and 3 h after the application of carrageenan. The control animals received the vehicle. The doses of inhibitors were chosen on the basis of previous studies (Hattori et al., 1998; Karrow et al., 2003; Arruda et al., 2004; Rocha et al., 2005; Quintão et al., 2006).
In other groups of experiments, the relevance of TNF-α was assessed using TNF-α p55 receptor knockout mice. C57/BL6 mice were used as the control group for this series of experiments. The paw oedema, the mechanical allodynia and the MPO activity were measured as described in the preceding sections.
Drugs and reagents
The following drugs were used: carrageenan, PBS tablets, EDTA, HTAB, TMB (all from Sigma-Aldrich, St Louis, MO, U.S.A.), NaPO4, hydrogen peroxide and Tween 20 (all from Merck, Germany). Anti-murine neutralizing antibody anti-TNF-α (Lot CT101) was obtained from R&D Systems Inc. (Minneapolis, MN, U.S.A.). Thalidomide was from Tocris (Cookson Inc., Ellisville, MO, U.S.A.). The anti-TNF-α antibody was prepared daily in 0.9% (w v−1) NaCl solution. Thalidomide was diluted in a 1% DMSO/saline solution.
Statistical analysis
The results are presented as the mean±s.e.m. of 6–10 animals. The percentages of inhibition are reported as the mean±s.e.m. of inhibitions obtained in each individual experiment. Statistical comparison of the data was performed by analysis of variance followed by Dunnett's test or Student's unpaired t-test. P-values less than 0.05 (P<0.05) were considered significant.
Results
Carrageenan-induced paw oedema
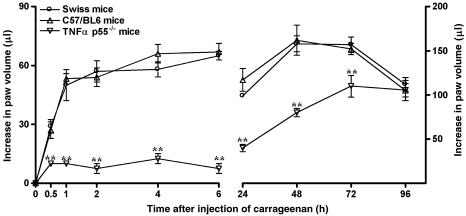
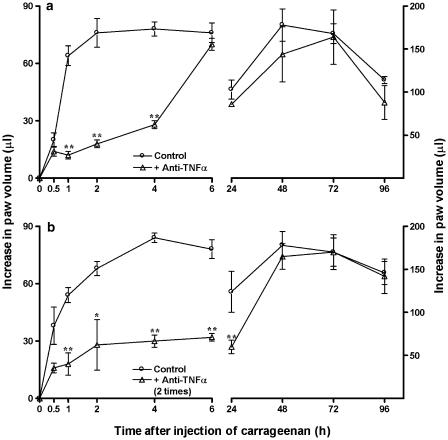
As described before (Henriques et al., 1987), the intraplantar injection of carrageenan (300 μg paw−1) into the mouse paw produced a biphasic and marked paw oedema formation, which peaked at 6 and 72 h, decreasing after 96 h. The oedematogenic response caused by carrageenan in Swiss mice was similar to that observed in C57/BL6 mice. Interestingly, both the early and the late phases of carrageenan-induced oedema were found to be strikingly reduced in TNF-α p55 receptor knockout mice. The percentages of inhibition were 89±4 and 50±3% at 6 and 48 h, respectively (Figure 1). The treatment of animals with a single dose of the anti-TNF-α antibody (100 μg kg−1, i.v., 5 min) significantly reduced the early, but not the late, phase of carageenan-evoked oedema, with inhibitions of 76±3 and 64±3% at 2 and 4 h, respectively (Figure 2a). In addition, the administration of anti-TNF-α antibody (100 μg kg−1, i.v.) 5 min before and 3 h after the injection of carrageenan was able to markedly reduce the oedematogenic response caused by administration of carrageenan, an effect that was observed for up to 24 h. The inhibitions observed were 59±3 and 51±6%, at 6 and 24 h, respectively (Figure 2b). Finally, the systemic treatment of mice with the preferential blocker of TNF-α synthesis thalidomide (30 mg kg−1, s.c., 5 min) significantly prevented both the early and the late phases of carrageenan-induced paw oedema. The percentages of reduction were, respectively, 70±7 and 41±10%, at 6 and 48 h (Figure 3).
Figure 1.
Mouse paw oedema formation induced by carrageenan (300 μg paw−1) in Swiss, C57/BL6 wild-type or TNF-α p55−/− receptor knockout mice. Each point represents the mean of 10 animals and vertical lines show the s.e.m. Asterisks denote the significance levels when the values obtained for C57/BL6 wild type were compared to those for TNF-α p55−/− receptor knockout mice: **P<0.01.
Figure 2.
Effect of systemic treatment with anti-TNF-α antibody (100 μg kg−1, i.v.), given 5 min before carrageenan (a) or 5 min before and 3 h after carrageenan (b), on the mouse paw oedema formation induced by carrageenan (300 μg paw−1) in Swiss mice. Each point on the curve represents the mean of six animals and vertical lines show the s.e.m. Asterisks denote the significance levels in comparison to control values: *P<0.05; **P<0.01.
Figure 3.
Effect of systemic treatment with thalidomide (30 mg kg−1, s.c., 5 min before carrageenan) on the mouse paw oedema formation induced by carrageenan (300 μg paw−1) in Swiss mice. Each point on the curve represents the mean of six animals and vertical lines show the s.e.m. Asterisks denote the significance levels in comparison to control values: *P<0.05; **P<0.01.
Mechanical allodynia caused by carrageenan
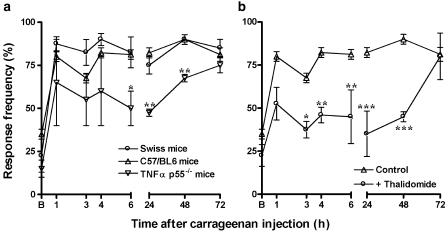
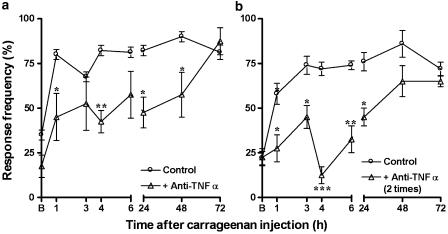
The application of carrageenan (300 μg paw−1) caused a sustained mechanical allodynia, as indicated by a large increase from baseline values in response to 0.6 g VFH stimulation, which was observed as early as 1 h and persisted for up to 72 h (Figure 4). The results show that the mechanical allodynia following carrageenan injection was not significantly different between Swiss and C57/BL6 mice, but that it was found significantly reduced in mice with genic deletion of the TNF-α p55 receptor (Figure 4a). The percentages of reduction observed were 39±12, 37±3 and 25±2% at 6, 24 and 48 h, respectively. The mechanical allodynia caused by carrageenan was also significantly inhibited in mice pretreated with the anti-TNF-α antibody (100 μg kg−1, i.v., 5 min before) by 48±8, 42±10 and 36±14%, at 4, 24 and 48 h, respectively (Figure 5a). The repeated treatment with anti-TNF-α antibody (100 μg kg−1, i.v.), 5 min before and 3 h after the injection of carrageenan, resulted in a marked decrease of the allodynic response, with percentages of inhibition of 39±9, 82±6, 56±10 and 41±6% at 3, 4, 6 and 24 h, respectively (Figure 5b). Likewise, the pretreatment of animals with thalidomide (30 mg kg−1, s.c., 5 min) was capable of significantly reducing carrageenan-induced mechanical allodynia, with percentages of inhibition of 44±5, 45±19, 57±16 and 50±3% at 4, 6, 24 and 48 h, respectively (Figure 4b).
Figure 4.
Mechanical allodynic response induced by carrageenan (300 μg paw−1) in Swiss, C57/BL6 wild-type or TNF-α p55−/− receptor knockout mice (a). Effect of systemic treatment with thalidomide (30 mg kg−1, s.c.), given 5 min before carrageenan, on the mechanical allodynia induced by carrageenan injection (b). Each point represents the mean of 6–10 animals and vertical lines show the s.e.m. Asterisks denote the significance levels when the values obtained for C57/BL6 wild type were compared to those for TNF-α p55−/− receptor knockout mice or in comparison to Swiss mice (thalidomide experimental group): *P<0.05; **P<0.01.
Figure 5.
Effect of systemic treatment with anti-TNF-α antibody (100 μg kg−1, i.v.), given 5 min before carrageenan (a) or 5 min before and 3 h after carrageenan (b), on the mechanical allodynia induced by carrageenan injection. Each point represents the mean of 6–10 animals and vertical lines show the s.e.m. Asterisks denote the significance levels in comparison to control values: *P<0.05; **P<0.01.
Increase in MPO activiy
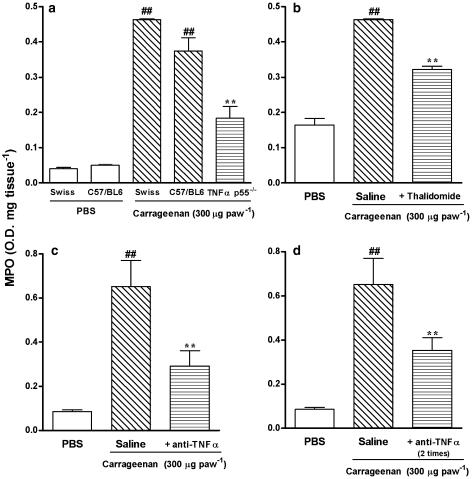
Carrageenan injection is associated with a massive migration of neutrophils, as indicated by an increase of about 10-fold in the MPO activity in the s.c. tissue of the mouse paw at 6 h. The increase of MPO activity induced by carrageenan was not significantly different between Swiss and C57/BL6 mice, but was markedly reduced in TNF-α p55 receptor knockout mice (51±9%) (Figure 6a). A significant inhibition of increased MPO activity was also achieved by treating animals with anti-TNF-α (100 μg kg−1) in either single or repeated schedules of treatment (Figure 6c and d). The percentages of inhibition were 55±11 and 46±9%, respectively. Finally, thalidomide (30 mg kg−1, s.c.), given 5 min before carrageenan injection (Figure 6b), also significantly reduced carrageenan-induced increase in MPO activity (30±2%).
Figure 6.
Increase in MPO activity induced by carrageenan (300 μg paw−1) in Swiss, C57/BL6 wild-type or TNF-α p55−/− receptor knockout mice (a). Effect of systemic treatment with thalidomide (30 mg kg−1, s.c.), given 5 min before carrageenan, on the increase in MPO activity induced by carrageenan injection (b). Effect of systemic treatment with anti-TNF-α antibody (100 μg kg−1, i.v.), given 5 min before carrageenan (c) or 5 min before and 3 h after carrageenan (d), on the MPO activity induced by carrageenan injection. Each point represents the mean of six animals and vertical lines show the s.e.m. Asterisks denote the significance levels when the values obtained for C57/BL6 wild type were compared to those for TNF-α p55−/− receptor knockout mice or in comparison to Swiss mice (anti-TNF-α or thalidomide experimental groups): **P<0.01 and significantly different to PBS-treated group (#) values, ##P<0.01.
Discussion
TNF-α is a key proinflammatory cytokine that has been widely implicated in both the establishment and the perpetuation of the inflammatory process. Most of the TNF-α effects are mediated by the interaction with two receptors called p55 (type I) and p75 (type II), and they are clearly dependent on the activation of transcriptional factors and de novo synthesis of several proteins involved in the inflammatory response (Feldmann & Saklatvala, 2001; Haddad, 2002; Hopkins, 2003; Sommer & Kress, 2004). In the present study, we have assessed the role played by TNF-α in the inflammatory and nociceptive responses evoked by carrageenan injection into the mouse paw.
The model of mouse oedema induced by the intraplantar injection of carrageenan has been widely employed in recent years in order to assess the effects of new anti-inflammatory drugs. For this purpose, different concentrations of carrageenan and distinct mouse strains have been employed (Levy, 1969; Sugishita et al., 1981; Henriques et al., 1987; Ianaro et al., 1994; Posadas et al., 2004). From these studies, it is possible to observe that in the mouse, carrageenan evokes a very characteristic oedematogenic response with a clear biphasic profile. In spite of the systematic use of this inflammation model, few studies have evaluated the possible mechanisms implicated in the early and late phases of oedema formation induced by carrageenan in the mouse paw. For instance, Siqueira-Junior et al. (2003) demonstrated that treatment with the preferential inhibitor of COX-1 valeryl-salicylate produced a marked reduction of the early phase of carrageenan-induced mouse paw oedema, whereas it significantly increased the late phase. Moreover, it has recently been demonstrated that COX-2 is upregulated only in the second phase of the paw oedema caused by carrageenan (Posadas et al., 2004). Conversely, it has been shown that NO (derived from both the endothelial and inducible NO synthase isoforms) is apparently implicated in the two phases of carrageenan-evoked mouse paw oedema (Posadas et al., 2004; Bucci et al., 2005).
The results of the present study indicate, for the first time, that TNF-α is likely to be involved in both the first and second phases of mouse carrageenan oedema. This conclusion is supported by the results showing that the oedema formation induced by carrageenan was strikingly reduced in TNF-α p55 receptor knockout mice, in comparison to C57/BL6 mice, whereas no significant difference was observed between Swiss and C57/BL6 mouse strains. In addition, the pretreatment with thalidomide (given 5 min before carrageenan) was also able to markedly reduce the early and late stages of the oedematogenic response caused by carrageenan. Of note, thalidomide and its analogues have been shown to largely reduce the increase of TNF-α synthesis, and to a lesser extent, of other proinflammatory cytokines (for review see Teo et al., 2005). Finally, our results showed that treatment of animals with a single dose of anti-TNF-α antibody significantly decreased the early phase of carrageenan oedema, without affecting the late phase. Furthermore, the repeated administration of anti-TNF-α antibody, 5 min before and 3 h after carrageenan, markedly reduced the oedema caused by carrageenan for up to 24 h. It is worth noting that anti-TNF-α strategies adopted in the present study were always more effective against the first, in comparison to the second phase of carrageenan oedema. Therefore, the present data allow us to suggest that TNF-α plays an essential role in the early, and to a lesser extent in the late, stage of the paw oedema induced by carrageenan in mice. Further studies are necessary to establish whether TNF-α interferes with the production of other mediators of inflammation following the intraplantar injection of carrageenan into the mouse paw.
It has been widely demonstrated that oedema formation caused by carrageenan is accompanied by a reduction in the threshold to nociceptive stimuli, resulting from primary and central mechanisms of sensitization in both rats and mice (Tabo et al., 1998; Parada et al., 2003; Cunha et al., 2005; Quintão et al., 2005). In the present study, we have demonstrated that carrageenan injection induces a long-lasting mechanical allodynic response, which is evident after as little as 1 h and remains elevated for up to 72 h. In addition, the results obtained herein clearly indicate the involvement of the proinflammatory cytokine TNF-α in the mechanical allodynia evoked by carrageenan in mice. Our conclusion is derived from two sets of experimental evidence: (1) the mechanical allodynia induced by carrageenan was partially reduced in TNF-α p55 receptor knockout mice, in comparison to C57/BL6 mice, and (2) the pretreatment with both anti-TNF-α antibody (single or repeated administration) and thalidomide prevented, in a significant manner, the allodynic responses evoked by carrageenan in Swiss mice. These data extend and confirm the previous work conducted by Cunha et al. (2005) showing that carrageenan-induced mechanical hypernociception is significantly reduced in mice with genic deletion of TNF-α p55 (type 1) receptor, according to assessment with an electronic version of the von Frey test. The relevance of TNF-α to the nociceptive responses elicited by carrageenan has also been demonstrated in rats. For example, both thalidomide and anti-TNF-α antibody are highly effective in preventing the mechanical hyperalgesia caused by carrageenan in the Randall–Sellito test (Cunha et al., 1992; Ribeiro et al., 2000). It has also been shown that the inhibition of TNF-α by treatment with thalidomide, the anti-TNF-α antibody or the antisense to TNF-α p55 receptor markedly prevented the mechanical hyperalgesia and priming responses induced by carrageenan in rats (Parada et al., 2003). Furthermore, TNF-α has also been deeply implicated in the central and peripheral mechanisms involved in neuropathic pain in both mice and rats (Sommer & Kress, 2004; Quintão et al., 2006). Taken together, the literature and present data clearly indicate the importance of TNF-α for the inflammatory nociception induced by carrageenan.
It has been recently reported that intraplantar injection of carrageenan into the mouse paw produces a marked neutrophil influx to the s.c. tissue, as indicated by a 10-fold increase in the MPO activity, which peaks at 6 h (da Cunha et al., 2004; Posadas et al., 2004). In this connection, another aspect investigated in the present work was the possible relevance of TNF-α for the neutrophil migration induced by carrageenan in the mouse paw. Interestingly, as demonstrated for the oedema and the allodynia, TNF-α also seems to play a relevant role in the neutrophil migration following carrageenan injection. Accordingly, carrageenan-induced increase in MPO activity was reduced to a great extent in mice with genic deletion of the TNF-α p55 receptor, in comparison to C57/BL6 mice. Likewise, the rise in MPO activity in the mouse paw was also markedly reduced by the single and by the repeated treatment with anti-TNF-α. Interestingly, the increased MPO activity was also prevented by thalidomide. These results are in accordance with previous data showing that neutrophil migration observed in the model of pleurisy induced by carrageenan is significantly blocked by anti-TNF-α strategies (Fröde et al., 2001). Collectively, the results allow us to suggest that TNF-α is a key molecule for the process of cell migration induced by carrageenan in mice.
In summary, the present data clearly indicate the proinflammatory cytokine TNF-α as a crucial mediator for oedema formation, neutrophil migration and allodynic responses following carrageenan injection into the mouse paw. These pieces of evidence cast new light on the mechanisms responsible for mediating the inflammatory and nociceptive actions of carrageenan in the mouse paw.
Acknowledgments
This work was supported by grants from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brazil), the Programa de Apoio aos Núcleos de Excelência (PRONEX) (Brazil) and the Fundação de Apoio à Pesquisa de Santa Catarina (FAPESC). A.C.C.R. is an undergraduate medical student receiving a grant from PIBIC/CNPq/BIP/UFSC (Brazil). E.S.F. and N.L.M.Q. are post-graduate students in pharmacology receiving grants from CAPES and CNPq, respectively. M.M.C. holds a post-doctoral fellowship from CAPES. We thank Dr Leda Quercia Vieira very much indeed for her kind donation of C57/BL6 and TNF-α p55−/− receptor knockout mice.
Abbreviations
- MPO
myeloperoxidase
- NO
nitric oxide
- PBS
phosphate-buffered saline
- s.c.
subcutaneous
- TMB
tetramethylbenzidine
- TNF-α
tumor necrosis factor-α
- VFH
von Frey hair
References
- ARRUDA M.S., RICHINI V.B., OLIVEIRA S.M., VILANI-MORENO F.R. Experimental murine mycobacteriosis: evaluation of the functional activity of alveolar macrophages in thalidomide-treated mice. Braz. J. Med. Biol. Res. 2004;37:485–492. doi: 10.1590/s0100-879x2004000400005. [DOI] [PubMed] [Google Scholar]
- BORTOLANZA L.B., FERREIRA J., HESS S.C., DELLE MONACHE F., YUNES R.A., CALIXTO J.B. Anti-allodynic action of the tormentic acid, a triterpene isolated from plant, against neuropathic and inflammatory persistent pain in mice. Eur. J. Pharmacol. 2002;453:203–208. doi: 10.1016/s0014-2999(02)02428-7. [DOI] [PubMed] [Google Scholar]
- BUCCI M., ROVIEZZO F., POSADAS I., YU J., PARENTE L., SESSA W.C., IGNARRO L.J., CIRINO G. Endothelial nitric oxide synthase activation is critical for vascular leakage during acute inflammation in vivo. Proc. Natl. Acad. Sci. U.S.A. 2005;102:904–908. doi: 10.1073/pnas.0408906102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CUNHA F.Q., POOLE S., LORENZETTI B.B., FERREIRA S.H. The pivotal role of tumour necrosis factor alpha in the development of inflammatory hyperalgesia. Br. J. Pharmacol. 1992;107:660–664. doi: 10.1111/j.1476-5381.1992.tb14503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CUNHA T.M., VERRI W.A., JR, SILVA J.S., POOLE S., CUNHA F.Q., FERREIRA S.H. A cascade of cytokines mediates mechanical inflammatory hypernociception in mice. Proc. Natl. Acad. Sci. U.S.A. 2005;102:1755–1760. doi: 10.1073/pnas.0409225102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DA CUNHA F.M., DUMA D., ASSREUY J., BUZZI F.C., NIERO R., CAMPOS M.M., CALIXTO J.B. Caffeic acid derivatives: in vitro and in vivo anti-inflammatory properties. Free Radic. Res. 2004;38:1241–1253. doi: 10.1080/10715760400016139. [DOI] [PubMed] [Google Scholar]
- DI ROSA M., GIROUD J.P., WILLOUGHBY D.A. Studies on the mediators of the acute inflammatory response induced in rats in different sites by carrageenan and turpentine. J. Pathol. 1971;104:15–29. doi: 10.1002/path.1711040103. [DOI] [PubMed] [Google Scholar]
- ESPOSITO E., IACONO A., RASO G.M., PACILIO M., COPPOLA A., DI CARLO R., MELI R. Raloxifene, a selective estrogen receptor modulator, reduces carrageenan-induced acute inflammation in normal and ovariectomized rats. Endocrinology. 2005;146:3301–3308. doi: 10.1210/en.2005-0375. [DOI] [PubMed] [Google Scholar]
- FELDMANN M., SAKLATVALA J. Proinflammatory cytokines. Cytokine Ref. 2001;1:291–305. [Google Scholar]
- FRÖDE T.S., SOUZA G.E., CALIXTO J.B. The modulatory role played by TNF-alpha and IL-1 beta in the inflammatory responses induced by carrageenan in the mouse model of pleurisy. Cytokine. 2001;13:162–168. doi: 10.1006/cyto.2000.0816. [DOI] [PubMed] [Google Scholar]
- HADDAD J.J. Cytokines and related-receptor mediated signalling pathways. Biochem. Biophys. Res. Commun. 2002;297:700–713. doi: 10.1016/s0006-291x(02)02287-8. [DOI] [PubMed] [Google Scholar]
- HATTORI K., HIRANO T., MIYAJIMA H., YAMAKAWA N., TATENO M., OSHIMI K., KAYAGAKI N., YAGITA H., OKUMURA K. Differential effects of anti-Fas ligand and anti-tumor necrosis factor alpha antibodies on acute graft-versus-host disease pathologies. Blood. 1998;91:4051–4055. [PubMed] [Google Scholar]
- HENRIQUES M.G., SILVA P.M., MARTINS M.A., FLORES C.A., CUNHA F.Q., ASSREUY-FILHO J., CORDEIRO R.S. Mouse paw edema. A new model for inflammation. Braz. J. Med. Biol. Res. 1987;20:243–249. [PubMed] [Google Scholar]
- HOPKINS S.J. The pathological role of cytokines. Legal Med. 2003;5:S45–S47. doi: 10.1016/s1344-6223(02)00088-3. [DOI] [PubMed] [Google Scholar]
- IANARO A., O'DONNELL C.A., DI ROSA M., LIEW F.Y. A nitric oxide synthase inhibitor reduces inflammation, down-regulates inflammatory cytokines and enhances interleukin-10 production in carrageenin-induced oedema in mice. Immunology. 1994;82:370–375. [PMC free article] [PubMed] [Google Scholar]
- KARROW N.A., GUO T.L., ZHANG L.X., MCCAY J.A., MUSGROVE D.L., PEACHEE V.L., GERMOLEC D.R., WHITE K.L., JR Thalidomide modulation of the immune response in female B6C3F1 mice: a host resistance study. Int. Immunopharmacol. 2003;3:1447–1456. doi: 10.1016/S1567-5769(03)00143-7. [DOI] [PubMed] [Google Scholar]
- LEVY L. Carrageenan paw edema in the mouse. Life Sci. 1969;8:601–606. doi: 10.1016/0024-3205(69)90021-6. [DOI] [PubMed] [Google Scholar]
- MORRIS C.J. Carrageenan-induced paw edema in the rat and mouse. Methods Mol. Biol. 2003;225:115–121. doi: 10.1385/1-59259-374-7:115. [DOI] [PubMed] [Google Scholar]
- PARADA C.A., YEH J.J., JOSEPH E.K., LEVINE J.D. Tumor necrosis factor receptor type-1 in sensory neurons contributes to induction of chronic enhancement of inflammatory hyperalgesia in rat. Eur. J. Neurosci. 2003;17:1847–1852. doi: 10.1046/j.1460-9568.2003.02626.x. [DOI] [PubMed] [Google Scholar]
- PINHEIRO R.M., CALIXTO J.B. Effect of the selective COX-2 inhibitors, celecoxib and rofecoxib in rat acute models of inflammation. Inflamm. Res. 2002;51:603–610. doi: 10.1007/pl00012435. [DOI] [PubMed] [Google Scholar]
- POSADAS I., BUCCI M., ROVIEZZO F., ROSSI A., PARENTE L., SAUTEBIN L., CIRINO G. Carrageenan-induced mouse paw oedema is biphasic, age-weight dependent and displays differential nitric oxide cyclooxygenase-2 expression. Br. J. Pharmacol. 2004;142:331–338. doi: 10.1038/sj.bjp.0705650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- QUINTÃO N.L.M., BALZ D., SANTOS A.R.S., CAMPOS M.M., CALIXTO J.B. Long-lasting neuropathic pain induced by brachial plexus injury in mice: role triggered by the pro-inflammatory cytokine TNFα. Neuropharmacology. 2006;50:614–620. doi: 10.1016/j.neuropharm.2005.11.007. [DOI] [PubMed] [Google Scholar]
- QUINTÃO N.L.M., MEDEIROS R., SANTOS A.R.S., CAMPOS M.M., CALIXTO J.B. Effects of diacerhein on mechanical allodynia in inflammatory and neuropathic models of nociception in mice. Anest. Analg. 2005;101:1763–1769. doi: 10.1213/01.ane.0000184182.03203.61. [DOI] [PubMed] [Google Scholar]
- RIBEIRO R.A., VALE M.L., FERREIRA S.H., CUNHA F.Q. Analgesic effect of thalidomide on inflammatory pain. Eur. J. Pharmacol. 2000;391:97–103. doi: 10.1016/s0014-2999(99)00918-8. [DOI] [PubMed] [Google Scholar]
- ROCHA A.C.C., FERNANDES E.S., PASSOS G.F., CALIXTO J.B., CAMPOS M.M. Assessment of TNFα contribution to the functional up-regulation of kinin B1 receptors in the mouse paw after treatment with LPS. Int. Imunopharmacol. 2005;5:1593–1600. doi: 10.1016/j.intimp.2005.04.007. [DOI] [PubMed] [Google Scholar]
- SALVEMINI D., WANG Z.Q., WYATT P.S., BOURDON D.M., MARINO M.H., MANNING P.T., CURRIE M.G. Nitric oxide: a key mediator in the early and late phase of carrageenan-induced rat paw inflammation. Br. J. Pharmacol. 1986;118:829–838. doi: 10.1111/j.1476-5381.1996.tb15475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIQUEIRA-JUNIOR J.M., PETERS R.R., BRUM-FERNANDES A.J., RIBEIRO-DO-VALLE R.M. Effects of valeryl salicylate, a COX-1 inhibitor, on models of acute inflammation in mice. Pharmacol. Res. 2003;48:437–443. doi: 10.1016/s1043-6618(03)00188-9. [DOI] [PubMed] [Google Scholar]
- SOMMER C., KRESS M. Recent findings on how proinflammatory cytokines cause pain: peripheral mechanisms in inflammatory and neuropathic hyperalgesia. Neurosci. Lett. 2004;361:184–187. doi: 10.1016/j.neulet.2003.12.007. [DOI] [PubMed] [Google Scholar]
- SUGISHITA E., AMAGAYA S., OGIHARA Y. Anti-inflammatory testing methods: comparative evaluation of mice and rats. J. Pharmacobiodyn. 1981;4:565–575. doi: 10.1248/bpb1978.4.565. [DOI] [PubMed] [Google Scholar]
- TABO E., EISELE J.H., JR, CARSTENS E. Force of limb withdrawals elicited by graded noxious heat compared with other behavioral measures of carrageenan-induced hyperalgesia and allodynia. J. Neurosci. Methods. 1998;81:139–149. doi: 10.1016/s0165-0270(98)00018-1. [DOI] [PubMed] [Google Scholar]
- TEO S.K., STIRLING D.I., ZELDIS J.B. Thalidomide as a novel therapeutic agent: new uses for an old product. Drug Discov. Today. 2005;10:107–114. doi: 10.1016/S1359-6446(04)03307-0. [DOI] [PubMed] [Google Scholar]
- WINTER C.A., RISLEY E.A., NUSS G.W. Carrageenan-induced edema in hind paw of the rat as an assay for anti-inflammatory drugs. Proc. Soc. Exp. Biol. 1962;111:544–547. doi: 10.3181/00379727-111-27849. [DOI] [PubMed] [Google Scholar]
- ZIMMERMANN M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]