Abstract
Adhesion of polymorphonuclear cells (PMNs) to vascular endothelial cells (EC) is a critical step in recruitment and infiltration of leukocytes into tissues during inflammation. High doses of butyric acid have been shown to ameliorate inflammation in inflammatory bowel diseases (IBD). Cholesteryl-butyrate solid lipid nanoparticles (chol-but SLN) as prodrug are a possible delivery system for butyric acid.
Sodium butyrate or chol-but SLN were coincubated with human PMNs and human umbilical vein EC (HUVEC); adhesion was quantified by computerized microimaging fluorescence analysis. Both chol-but SLN and sodium butyrate displayed antiadhesive effects on FMLP- and IL-1β-stimulated cells in a concentration–response curve (10−8–10−5 M), but chol-but SLN were in all cases more active. Moreover, chol-but SLN inhibited FMLP-induced adhesion of PMNs to FCS-coated plastic wells, thus showing a direct effect on PMNs, while sodium butyrate had little effect. Confocal microscopy showed that fluorescent SLN entered PMNs and HUVEC after 10 min incubation. Chol-but SLN acted either on activated PMN or HUVEC.
Chol-but SLN inhibited O2−· production and myeloperoxidase release by PMNs evoked by FMLP, in a dose-dependent, but not time-dependent, manner and were more active than sodium butyrate.
In conclusion, in all tests chol-but SLN were more active than sodium butyrate. Thus, chol-but SLN might be a valid alternative to sodium butyrate in the anti-inflammatory therapy of ulcerative colitis, avoiding complications related to the administration of sodium butyrate.
Keywords: Chol-but SLN, sodium butyrate, HUVEC, PMNs, neutrophil, adhesion, GPR41 and GPR43 receptors
Introduction
Migration of leukocytes from the blood stream to sites of infection is a key event in cellular immune and inflammatory responses, mediated by numerous types of molecules including several adhesion receptors (Bendas, 2005). Dysregulation, that is responding to autogenous or nonthreatening agents, can lead to the uncontrolled infiltration of leukocytes into healthy tissue, and such a mechanism contributes, in part, to the pathology of certain inflammatory and autoimmune reactions, such as rheumatoid arthritis, asthma, multiple sclerosis, Crohn's disease, Ulceratice Cholitis (UC), psoriasis and many others (Bendas, 2005).
Enteric bacteria do not generally induce a destructive inflammatory response in the gut, and products of bacterial fermentation are known to play an active and beneficial role in epithelial colonic homeostasis. Short-chain fatty acids (SCFAs: acetate, propionate and n-butyrate) derived from the enteric bacterial fermentation of nondigested dietary carbohydrates provide an essential energy substrate for the colonic epithelium: a deficiency of SCFA can trigger the development of diversion colitis (Dyer et al., 2005), resulting from diversion of the fecal stream and inducing crypt abscesses, epithelial cell degeneration, acute and chronic inflammation in the lamina propria, and regenerative changes in the crypts (Glotzer et al., 1981).
Among SCFAs, butyrate is particularly interesting owing to its anti-inflammatory effects, studied in various in vitro systems (Segain et al., 2000; Klampfer et al., 2003; Menzel et al., 2004; Scheppach & Weiler, 2004; Zapolska-Downar et al., 2004). Butyrate enemas were found to be cytoprotective in experimental rat colitis (D'Argenio et al., 1996; Venkatraman et al., 2003). Dietary fiber supplementation ameliorated colonic damage in HLA-B7 transgenic rats and this effect was associated with an increased production of SCAFs, which can synergistically inhibit the production of proinflammatory mediators (Rodriguez-Cabezas et al., 2003). A double-blind multicenter clinical trial demonstrated that combined treatment with topical butyrate and 5-ASA is significantly more effective than 5-ASA alone in the management of refractory distal colitis (Vernia et al., 2003). Another pilot study showed that patients with quiescent UC can safely take a diet rich in oat bran, specifically to increase the fecal butyrate levels (Hallert et al., 2003).
Unfortunately, the clinical use of sodium butyrate is limited by its short half-life, owing to its rapid metabolism and excretion (Pellizzaro et al., 1999). The requirement for large intravenous doses of the drug means that continuous administration is needed to produce therapeutic blood concentrations. In addition, anemia, headache, nausea, diarrhea, abdominal cramps and a strong odor limit patient compliance (Conley et al., 1998; Patnaik et al., 2002). Prodrugs, such as tributyrin or Pivanex, have been produced to partially overcome some of these problems, and are now in phase I or II clinical trials for their antitumoral effects (Edelman et al., 2003; Reid et al., 2004), but additional studies are needed to further reduce these side effects.
Solid lipid nanoparticles (SLN) are being extensively studied as promising alternative carriers for drugs and diagnostics (Westesen et al., 1997; Gasco, 2001; Manjunath et al., 2005). SLN, prepared from a warm microemulsion and carrying a drug (either hydrophilic or lipophilic) have been tested in vivo on laboratory animals via duodenal, intravenous and ocular administration. SLN can increase bioavailability and modify pharmacokinetic parameters and tissue distribution of the incorporated drug. Normally, the lipophilic matrix consists of fatty acids and/or triglycerides (Gasco, 2001). Cholesteryl butyrate (chol-but SLN) was employed as SLN lipid matrix (Gasco, 2004) in tests to evaluate its function as a prodrug of butyric acid. In several tumor cell-lines, chol-but SLN displayed a higher antineoplastic effect than butyrate (Pellizzaro et al., 1999; Salomone et al., 2001; Serpe et al., 2004) and fluorescent SLN showed chol-but SLN to be internalized within a few minutes (Pellizzaro et al., 1999; Salomone et al., 2001).
Part of sodium butyrate's activity may be ascribed to a direct effect on leukocytes and microvascular endothelial cells (EC) that are crucial for leukocyte extravasation during the inflammatory response. Indeed, sodium butyrate and other SCFAs exert multiple effects on leukocytes, particularly polymorphonuclear cells (PMNs), altering cytoplasmic pH, calcium concentration, oxygen metabolism, phagocytosis, cell proliferation, cytoskeletal actin distribution and chemotaxis (Eftimiadi et al., 1987; Brunkhorst et al., 1992). These effects may partly be due to sodium butyrate's capacity to interact with the GPR43 receptor (Brown et al., 2003; Le Poul et al., 2003; Nilsson et al., 2003; Xiong et al., 2004), a G-protein-coupled receptor that is highly expressed in PMNs and monocytes (Le Poul et al., 2003; Senga et al., 2003). By contrast, GPR41, another G-protein-coupled receptor that can interact with sodium butyrate, is more widely expressed (Brown et al., 2003), but not present on PMNs or monocytes (Le Poul et al., 2003). Moreover, sodium butyrate may also modulate inflammation by a direct effect on human intestinal microvascular EC, in which it can modulate gene transcription by inhibiting histone deacetylase (Ogawa et al., 2003).
The aim of this study was to compare the effect of sodium butyrate and that of chol-but SLN on PMN function, by assessing their capacity to modulate adhesion to EC, superoxide anion (O2−·) production, and myeloperoxidase release in vitro. The results showed chol-but SLN to inhibit all PMN activities and to be much more active than sodium butyrate.
Methods
Cell cultures and reagents
Dextran T500 was from Pharmacia Biotech (Uppsala, Sweden). Bovine calf serum (BCS, endotoxin tested) was from Hyclone Laboratories Inc. (Logan, UT, U.S.A.). Trypsin was from Difco Laboratories Inc., Detroit, MI, U.S.A. Histopaque®1077, fluorescein diacetate, M199 (endotoxin tested), n-FMLP, IL-1β, PMA, cytochrome c, superoxide dismutase, cytochalasin B, MTT and HSA were from Sigma-Aldrich (St Louis, MO, U.S.A.).
Epikuron 200 (soya phosphatidylcholine 95%) and sodium taurocholate were kind gifts from Lucas Meyer (Hamburg, Germany) and from PCA (Basaluzzo, Italy), respectively. Cholesteryl butyrate, sodium butyrate and butanol were from Fluka (Buchs, CH). 6-coumarin was from Acros (New Jersey, U.S.A.).
All other reagents and solvents were from Merck (Darmstadt, Germany).
Stock solutions were prepared daily and diluted in M199 to the appropriate concentrations before each experiment.
PMNs were prepared from citrated venous blood obtained from healthy volunteers at a local hospital blood bank, using the standard techniques of dextran sedimentation followed by Histopaque®1077 gradient centrifugation. Residual erythrocytes were removed by hypotonic lysis and PMNs were resuspended in buffered salt solution (BSS) (138 mmol l−1 NaCl, 2.7 mmol l−1 KCl, 8.1 mmol l−1 Na2HPO4, 1.5 mmol l−1 KH2PO4, 1 mmol l−1 MgCl2, 1 mmol l−1 CaCl2, pH 7.4) supplemented with 1 mg ml−1 glucose and 1 mg ml−1 human serum albumin (HSA). Purity of the final cell suspension and cell viability, assessed by the Trypan blue exclusion test, were >95% in all cases. Cell viability was not affected by drug treatment.
Human umbilical vein endothelial cells (HUVEC) were isolated from human umbilical veins by collagenase treatment (0.1%) and cultured in M199 with the addition of 20% BCS, 100 U ml−1 penicillin, 100 μg ml−1 streptomycin, 5 U.I. m−1 heparin, 12 μg ml−1 bovine brain extract and 200 mM glutamine. Purity of the HUVEC preparation, evaluated by morphology and immunostaining for factor VIII, was >95%. Contaminant leukocytes were detected by immunostaining for CD45.
HUVEC were grown to confluence in flasks and used at the III-V passage. Informed consent was obtained from all volunteers.
Preparation of chol-but SLN and sodium butyrate solutions
Chol-but SLN were prepared by the microemulsion method, reported elsewhere (Pellizzaro et al., 1999). Briefly, a warm oil-in-water microemulsion was prepared from cholesterylbutyrate, Epikuron 200, butanol and water. The warm microemulsion was dispersed in cold water and the resulting chol-but SLN aqueous dispersion was washed twice by diaultrafiltration using a TCF2 system Amicon (Danvers, U.S.A.) with a Diaflo YM 100 membrane (cutoff 100,000 Da). The aqueous dispersions of chol-but SLN were sterilized by autoclaving (15 min, 121°C, 2 bar) before use. Fluorescent chol-but SLN were prepared as above but adding 6-coumarin (0.04% w w−1) to the formulation.
Sodium butyrate solutions were freshly prepared in sterile water or in a mixture of sterile water and ethanol (50 : 50 v v−1) before each experiment, at a concentration of 5 M.
Characterization and stability of chol-but SLN
The average diameters and polydispersity indices of fluorescent and nonfluorescent chol-but SLN were determined by photon correlation spectroscopy (PCS) using an N4 MD instrument (Coulter) at a fixed angle of 90° and at a temperature of 25°C. Each value reported with its s.e.m. is the average of 10 measurements and is calculated by the Coulter software using cumulant analysis and an exponential sampling method. The polydispersity index is a measure of the size distribution of a nanoparticle population (Koppel, 1972) and is calculated using a software package (Coulter Scientific Instruments, U.S.A.).
The electrophoretic mobility and zeta potential were measured using a 90 PLUS instrument (Brookhaven Instrument Corporation, NY, U.S.A.). To determine the zeta potential, SLN samples were diluted with KCl 0.1 mM and placed in the electrophoretic cell, where an electric field of 15.2 V cm−1 was applied. Each sample was analyzed at least in triplicate. The measured electrophoretic mobility was converted into zeta potential using the Smoluchowsky equation (Müller, 1991). The processing was performed by the software included in the system. The zeta potential is a measure of the surface charge of nanoparticles when they are dispersed in aqueous solutions; the parameter is employed to assess colloidal dispersion stability.
The stability of chol-but SLN was evaluated in physiological phosphate buffer (pH=7.4) and in BSS. The SLN were dispersed in one or other medium and average diameter and polydispersity index values were determined by PCS after 1, 2, 3 and 4 h.
Quantitative determination of chol-but was by a reverse-phase HPLC method (Duncan, 1979) using a UV detector.
The concentration of chol-but in the SLN was determined on weighed amounts of chol-but SLN dissolved in the mobile phase and then injected.
Visualization of chol-but SLN internalization
Internalization of chol-but-loaded SLN was determined by confocal fluorescence microscopy. 30,000 HUVEC were seeded in 3 ml of culture medium and allowed to attach for 24 h on glass coverslips in a Petri dish. 30,000 PMNs were seeded in 3 ml of culture medium and placed on glass coverslips in a Petri dish. Cells were incubated with 10−5 M chol-but-loaded SLN for 10 min. HUVEC and PMNs were washed with PBS, then coverslips were inverted and mounted on glass slides. The sections were observed with a fluorescence microscope (Eclipse 800, Nikon, Japan) equipped with a CCD camera (Axiocam HRc; Zeiss) and with a confocal laser scanning microscope (CLSM; Zeiss LSM 510 Meta). The intensity of emission lines (568 nm) was adjusted individually to use the full dynamic range of the photomultiplier. Typically, stacks of six to ten confocal sections (512 × 512 pixels) spaced at 600 nm were acquired at a depth of eight bits, using a × 100 lens (numerical aperture 1.4) and a pinhole set at 1 Airy unit. Cells were analyzed with fluorescence microscopy using the 568 nm laser line as excitation, and fluorescence was collected through a red long-pass filter.
Vitality and proliferation of cells treated with sodium butyrate and chol-but SLN
Sodium butyrate and chol-but SLN (10−8–10−5 M) were incubated with PMNs for times between 10 min and 4 h: PMN viability was assessed by Trypan blue exclusion. The effects on cell proliferation were measured by MTT assay, a colorimetric method based on the ability of the HUVEC to convert tetrazolium salt into formazan product (Mosmann, 1983). The cells were treated with chol-but SLN (10−8–10−5 M) for 10 min or 4 h. All measurements were performed in triplicate. Cholesterol, equimolarly present in chol-but, does not affect cell growth (Pellizzaro et al., 1999; Serpe et al., 2004).
Adhesion assay
HUVEC were grown to confluence in 24-well plates, washed, and rested for 1 day in M199 plus 10% BCS without bovine brain extract. PMNs (107 cells ml−1) were labeled with fluorescein diacetate (5 μg ml−1) for 30 min at 37°C, washed with BSS, and plated at 106 cells well−1 in a final volume of 0.25 ml BSS on HUVEC. Sodium butyrate or chol-but SLN (both at 10−8–10−5 M) and 10−7 M FMLP were added to HUVEC simultaneously with PMNs, and left in place for 10 min. In the experiments with IL-1β, EC were preincubated for 10 min with sodium butyrate or chol-but SLN (both at 10−8–10−5 M) and then stimulated with IL-1β (0.05 ng ml−1) for 4 h, PMNs being added in the last 10 min.
In the time-course test, HUVEC were pretreated with sodium butyrate or chol-but SLN (both at 10−8–10−5 M, for 10, 30, 50, 110 or 230 min), and then challenged with 10−7 M FMLP and PMNs for a further 10 min. After incubation, nonadherent PMNs were removed by washing three times with 1 ml BSS. The center of each well was analyzed by fluorescence image analysis (Dianzani et al., 2003). Adherent cells were counted using Image Pro Plus Software for microimaging (Media Cybernetics, version 5.0). Single experimental points were assayed in quadruplicate, and the standard error of the four replicates was below 10% in all cases. Data are presented as percentage adhesion versus the control value, control adhesion being measured on untreated HUVEC. Control adhesion was 51±10 cells microscope fields−1 (n=26).
The direct effect on HUVEC was assessed by pretreating cells with chol-but SLN (10−5 M) and IL-1β (0.05 ng ml−1) for 4 h, washing three times and challenging with PMNs for a further 10 min.
The direct effect on PMNs was assessed by seeding cells on 24-well ECs-free plates for 10 min at 37°C, in the presence of sodium butyrate or chol-but SLN and 10−7 M FMLP. The plates had previously been coated with heat-inactivated calf serum for 3 h to reduce spontaneous adhesion to the plastic wells.
Percentage inhibition of adhesion was calculated as follows: (100−(a)/(b)) × 100, where a is adhesion measured in the presence of butyrate or chol-but SLN plus stimulus minus basal adhesion, and b is adhesion elicited by stimulus minus basal adhesion.
Superoxide anion (O2−·) production
PMNs (1 × 10−6 cells ml−1) suspended in a buffered salt solution were pretreated with cytochalasin B (5 μg ml−1) for 5 min at 37°C, to maximize the measured response, and then challenged with sodium butyrate or chol-but SLN for 5–20 min at 37°C before exposure to 10−7 M FMLP for a further 5 min, or to 10−8 M PMA for 15 min. O2−· production was determined spectrophotometrically by measuring the superoxide dismutase-inhibitable reduction of cytochrome c reduced per 106 PMNs min−1. None of the compounds tested interfered with the spectophotometric assay; they were checked for interference in the assay by measuring their effects on cytochrome c reduction with a xanthine oxidase superoxide generating system. Assays were carried out in the same buffer, with 100 μM cytochrome c, 150 μM hypoxanthine, 0.01 units of xanthine oxidase per milliliter and appropriate concentrations of each drug.
Myeloperoxidase assay
PMNs (2 × 106 cells) were incubated in BSS to a final volume of 1 ml with cytochalasin B and sodium butyrate or chol-but SLN for 5 min, then 10−8 M FMLP was added for a further 5 min. Myeloperoxidase release was assayed as described by Henson et al. (1978). Release of enzymes was expressed as percentage of total cell enzyme activity.
Statistical analysis
Results are expressed as means±s.e.m.; n indicates the number of experiments. Data in Figures 3, 4, 5, 6 and 7 were analyzed by two-way analysis of variance; the Bonferroni multiple comparison post-test was then applied to determine significant differences between specific pairs of means. The molar concentration of a substance that reduces response to the stimulus by 50% (IC50) was calculated with a nonlinear regression model using Origin version 6.0 software (Microcal Software, Northampton, U.S.A.). The IC50 data in Figure 6 were analyzed using the Student's t-test. Differences were considered to be statistically significant at P<0.05. All statistical analyses were performed using GradPadPrism 3.0 software (GraphPad Software, San Diego, CA, U.S.A.).
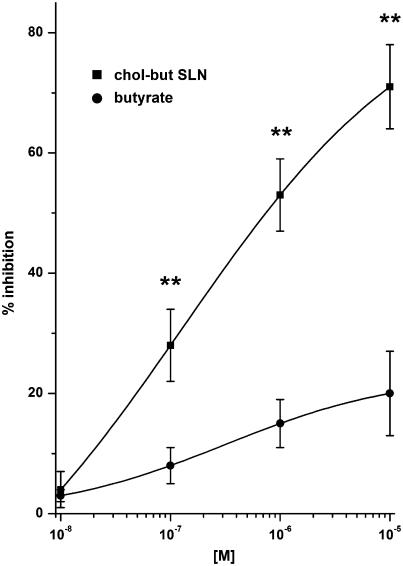
Figure 3.
Effects of butyrate and chol-but SLN on PMNs adhesion to HUVEC evoked by FMLP. PMNs and HUVEC were incubated with increasing concentrations (10−8–10−5 M) of the tested compound and with 10−7 M FMLP for 10 min at 37°C. Data are expressed as percentage inhibition of control adhesion, as means±s.e.m.; n=5. Asterisks mark statistically significant inhibition of chol-but SLN versus butyrate (**P<0.001). Control adhesion (i.e. adhesion in the absence of FMLP) was 51±10 cells microscope field−1 (mean±s.e.m., n=26), adhesion induced by 10−7 M FMLP was 351±25% of the control.
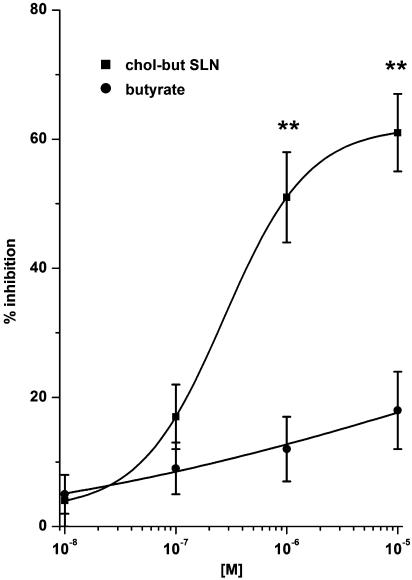
Figure 4.
Effects of butyrate and chol-but SLN on PMN adhesion to FCS-coated plastic wells. PMNs were incubated with increasing concentrations (10−8–10−5 M) of the tested compound and with 10−7 M FMLP and seeded on 24-well EC-free plates for 10 min at 37°C. Data are expressed as percentage inhibition of control adhesion, as means±s.e.m.; n=5. Asterisks mark statistically significant inhibition of chol-but SLN versus butyrate (**P<0.001). Control adhesion (i.e. adhesion in the absence of FMLP) was 108±32 cells microscope−1 field (mean±s.e.m., n=5), adhesion induced by 10−7 M FMLP was 378±25% of the control.
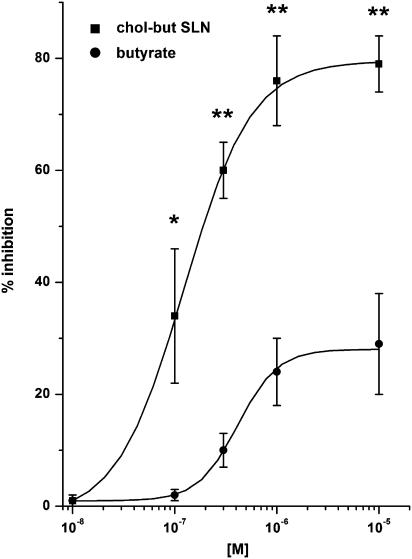
Figure 5.
Effects of butyrate and chol-but SLN on PMNs adhesion to HUVEC evoked by IL-1β. HUVEC were incubated with increasing concentrations (10−9–10−5 M) of the tested compound for 10 min and then stimulated with 0.05 ng ml−1 IL-1β for 4 h at 37°C. Data are expressed as percentage inhibition of control adhesion, as means±s.e.m.; n=5. Asterisks mark statistically significant inhibition of chol-but SLN versus butyrate (**P<0.001; *P<0.01). Adhesion induced by 0.05 ng ml−1 IL-1β was 282±38% of the control.
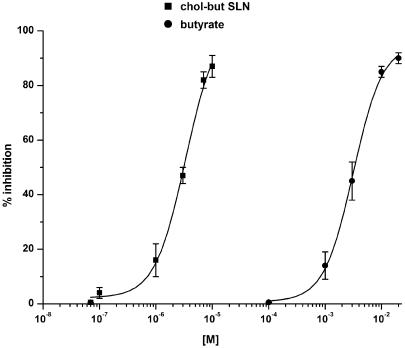
Figure 6.
Effects of butyrate or chol-but SLN effects on FMLP-evoked O2−· production by PMNs. Results are expressed as percentage inhibition of O2−· production evoked by 10−7 M FMLP in the absence of butyrate or chol-but SLN: this production amounted to 2.1±0.15 nmol cytochrome c reduced per 106 PMNs min−1 and was taken as 100%. Data are expressed as means±s.e.m.; n=5.
Figure 7.
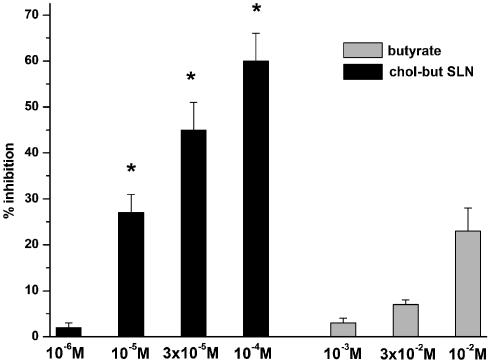
Effects of butyrate or chol-but SLN effects on FMLP-evoked myeloperoxidase release by PMNs. The results are expressed as percentage inhibition of myeloperoxidase release evoked by 10−8 M FMLP in the absence of butyrate or chol-but SLN: this release was 31±5%. Data are expressed as means±s.e.m.; n=5 Asterisks mark statistically significant inhibition of chol-but SLN versus butyrate (*P<0.01).
Results
Characterization of SLN
The chol-but SLN had a diameter of 160±11 nm and a polydispersity index of 0.2±0.02 with a zeta potential of −28 mV. The zeta potential value is high enough to obtain a stable nanoparticle dispersion; the negative charge on SLN surface favors repulsion among nanoparticles, avoiding their aggregation. Chol-but SLN dispersions had an initial chol-but concentration of 12 mM; their average diameter and polydispersity indices were maintained in BSS for 4 h.
The fluorescent SLN prepared to evaluate internalization of chol-but SLN had a diameter of 130±21 nm, a polydispersity index of 0.28 and a zeta potential of −29 mV (Table 1).
Table 1.
Characteristics of chol-but SLN
| Diameter±s.e.m. (nm) | Polydispersity index±s.e.m. | Zeta potential (mV) | Nanoparticles in 1 ml dispersion (approx. number)* | |
|---|---|---|---|---|
| Chol-but SLN |
160±11 |
0.20±0.02 |
−28 |
4.6 × 1014 |
| Chol-but SLN (fluorescent) | 130±21 | 0.28±0.02 | −29 | 8.7 × 1014 |
Computed from average nanoparticle diameter (spherical shape and density=1).
SLN aqueous dispersion concentration: 12 mM.
The average diameter and polydispersity index of chol-but SLN dispersions were maintained in BSS solution for 4 h.
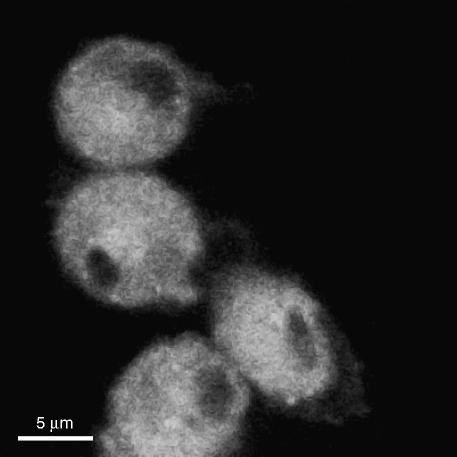
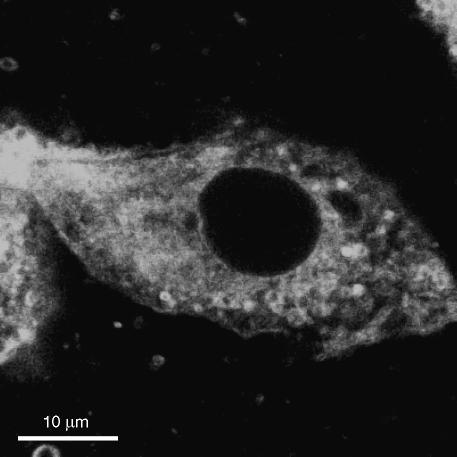
Internalization of fluorescent chol-but SLN was evaluated by confocal microscopy in PMNs; after 10 min incubation, high levels of fluorescence were detectable only in the cytoplasm (Figure 1) and remained there throughout the observation period (10–30 min; data not shown). Similar results were obtained on HUVEC (Figure 2).
Figure 1.
Uptake of 6-coumarin-chol-but SLN on PMNs.
Figure 2.
Uptake of 6-coumarin-chol-but SLN on HUVEC.
Effect of chol-but SLN on cell viability
Chol-but SLN (10−8–10−4 M) incubated with PMNs for times between 10 and 240 min did not have any effect on the viability of PMNs, which was >98% in all cases. HUVEC treated with chol-but SLN for the same incubation time proliferated with no significant difference versus controls.
Effect of sodium butyrate and chol-but SLN on PMN adhesion to HUVEC induced by FMLP
The effects of sodium butyrate and chol-but SLN on PMN adhesion to HUVEC were evaluated by coincubating both cell types for 10 min with each compound (10−8–10−5 M) in the presence of 10−7 M FMLP, which mimics bacterial chemotaxin and activates the PMN adhesive machinery. The 10−7 M dose of FMLP had previously been found to induce maximal adhesion of PMNs (Avanzi et al., 1998) (351±25% of control adhesion). Chol-but SLN strikingly inhibited adhesion in a dose-dependent manner with a maximal inhibition of 71±7% (P<0.001 versus sodium butyrate) at about 10−5 M and an IC50 at 2.5±0.5 × 10−7 M. The chol-but SLN dose–response curve was not modified by incubation times in the 10–240 min range (data not shown), and thus 10 min incubation was selected for subsequent experiments. By contrast, the inhibitory effect of sodium butyrate was strikingly lower, with a shallow dose–response curve and maximum inhibition of 20±7% at 10−5 M (n=5, Figure 3). Control adhesion (i.e. adhesion in the absence of FMLP) was 51±10 cells microscope field−1 (mean±s.e.m., n=26) and was not inhibited by either sodium butyrate or chol-but SLN (data not shown).
Effect of sodium butyrate and chol-but SLN on PMN adhesion to FCS-coated plastic wells
To understand the selective effect of chol-but SLN on the two different cell populations, we evaluated the effect of both formulations on PMN adhesion to FCS-coated plastic wells, using FMLP as stimulus. Comparison of the results obtained in this experimental system with those obtained previously using EC showed that the concentration–response curves were similar, without any significant difference, for both compounds (Figures 3 and 4). Indeed, the ability of chol-but SLN to inhibit PMN adhesion can be regarded as a direct effect on PMNs, HUVEC being absent in this experimental model (Figure 4).
Effect of sodium butyrate or chol-but SLN on PMN adhesion to HUVEC induced by IL-1β
The analysis was then extended to PMN adhesion induced by IL-1β, which is crucial in the inflammatory response. Experiments were performed using 0.05 ng ml−1 IL-1β, which induced adhesion levels close to those obtained by 10−7 FMLP (282±38% of control). Also in this system, chol-but SLN strikingly inhibited adhesion, achieving a plateau at 10−6–10−5 M; maximum inhibition was of 80±5% and IC50 was 1.4±0.5 × 10−7 M (Figure 5). The inhibitory effect of sodium butyrate was significantly weaker, with maximal inhibition at 10−5 M of 29±8% and IC50 was 4.4±0.6 × 10−7 M. To assess whether the inhibitory effect could be overcome by increasing the amount of proadhesive stimulus, we used higher doses of IL-1β (1 ng ml−1) to induce maximal PMN adhesion (500% of control adhesion), but chol-but SLN displayed a similar dose–response inhibitory curve with shape and peak close to those recorded in the presence of 0.05 ng ml−1 IL-1β (data not shown).
Furthermore, we coincubated IL-1β-stimulated HUVEC with chol-but SLN, washed them three times and added PMNs. In this different situation, chol-but SLN also inhibited PMN adhesion to stimulated HUVEC (% inhibition 76±4%; n=5), demonstrating that chol-but SLN can also act on stimulated EC.
Effect of sodium butyrate or chol-but SLN on O2−· production and myeloperoxidase secretion induced by FMLP on PMNs
To compare the effect of chol-but SLN on other activities of PMNs, we evaluated their effect on O2−· production and myeloperoxidase release evoked by FMLP. Both chol-but SLN and sodium butyrate inhibited O2−· production evoked by 10−7 M FMLP (2.1±0.15 nmol cytochrome c reduced per 106 PMNs min−1) in a dose-dependent manner, but the former was more active by three orders of magnitude, since IC50 values were 2.8±0.3 × 10−6 M for chol-but SLN and 2.8±0.2 × 10−3 M for sodium butyrate (Figure 6). No difference was found when the experiment was prolonged to 25 min (data not shown) or when the amount of FMLP was changed to 10−8 or 10−6 M (inducing 0.5 and 4.0 nmol cit−1 c rid/106 cells min−1 respectively; data not shown). Neither sodium butyrate (10−4–10−2 M), as also demonstrated by Liu et al. (2001), nor chol-but SLN (10−7–10−5 M) inhibited O2−· production evoked by 10−8 M PMA (3.1±0.25 nmol cytochrome c reduced per 106 PMNs min−1), showing a stimulus selectivity in their inhibitory activity (data not shown).
Moreover, chol-but SLN strongly inhibited myeloperoxidase release induced by 10−8 M FMLP; inhibition was recorded in the concentration range 10−6–10−4 M in a dose-dependent manner and was maximal (60±6% inhibition) at 10−4 M (Figure 7). By contrast, sodium butyrate slightly inhibited myeloperoxidase secretion (23±5% of inhibition) and was active only at concentrations of 10−3–10−2 M. Neither compound induced myeloperoxidase release from nonstimulated PMNs (data not shown).
Discussion
The acute phase of UC is characterized histologically by extensive infiltration of PMNs, lymphocytes, and plasma cells into the crypt epithelium. PMNs protect against bacterial and fungal infections by releasing agents that are able to kill the invader microorganisms, but can also be toxic to the host by destroying tissue cells and dissolving the intercellular connective matrix. A key role is played by release of reactive oxygen species, such as O2−·, and drugs decreasing their production or increasing their clearance can attenuate tissue injury at inflammatory sites (Liu et al., 2001).
Butyrate is known for its anti-inflammatory properties, but its infrequent clinical use is related to the short half-life of butyrate salt derivatives, which impairs long-lasting effects in vivo and makes a continuous administration regimen necessary to maintain acceptable plasma levels. To overcome these problems and improve the pharmacological profile, we formulated the drug as SLN, using a butyrate prodrug as lipid matrix. We have shown that incorporation of drugs into SLN changes their pharmacokinetic profile, with increased half-life, area under the concentration versus time curve (AUC), and peak plasma concentration, and concomitant decrease of clearance (Cavalli et al., 2002).
Our results show that chol-but SLN exerts a striking inhibitory effect on PMN activation, evaluated in terms of increased adhesiveness, O2−· production and myeloperoxidase release, and is much more efficient than sodium butyrate. This improvement may be ascribed to the rapid uptake of chol-but SLN, whose cytoplasmic levels are already maximal after 10 min in both PMNs and EC. SLN consist of nontoxic bioacceptable and biodegradable substances, that is solid lipids and phosphatidylcholine, and their rapid internalization is probably due to their size and composition (Pellizzaro et al., 1999; Serpe et al., 2004).
The higher inhibitory activity of chol-but SLN than sodium butyrate might be ascribed to its accumulation within cells and to the release of higher concentrations of butyric acid from the internalized drug by esterase enzymatic activities, as suggested by Serpe et al. (2004) in cancer cells in vitro.
The widespread effect of chol-but SLN on different aspects of PMN activation was not due to a general toxic effect on cell function, as it did not affect cell viability nor inhibit PMA-induced superoxide production.
Chol-but SLN appear to act on early events of FMLP-driven activation of PMNs, as their inhibitory activity is already maximal after 10 min incubation. FMLP mimics bacterial chemotaxin and activates PMN by interacting with a surface G-protein receptor, whose triggering initiates a sequence of protein phosphorylation events, including activation of Ras and MAPK p38 and p42/44. Activation of these MAPKs is maximal within 1–2 min, but is maintained for about 10 min and results in a broad range of rapid functional responses, including calcium influx, actin assembly, superoxide production, granule enzyme release, chemokinesis and increased adhesiveness, most of which are mediated by inside-out signals activating β2-integrin function (Nick et al., 1997). Specific inhibition of p38 MAPK results in selective loss of functional responses to FMLP, with inhibition of PMN adhesion, chemotaxis and superoxide release (McLeish et al., 1998). Therefore, together with the recent observation that butyrate inhibits MAPK (Park et al., 2005), these reports strongly suggests that the chol-but SLN inhibitory effect on FMLP-induced activation of PMN is mediated by inhibition of this signaling pathway. This possibility is also supported by our observation that chol-but SLN has no effect on activating PMN induced by PMA, which is a direct activator of protein kinase C (PKC) and activates MAPK only weakly in PMNs (Nick et al., 1997).
Furthermore, chol-but SLN may act not only on PMN, but also on EC. This possibility is suggested by our finding that chol-but SLN is rapidly taken up by HUVEC and inhibits PMN adhesion to HUVEC more efficiently than their adhesion to FCS-coated plastic wells, moreover chol-but SLN can act even if HUVEC were washed before adding PMNs. EC, indeed, play an active role in PMN recruitment, as they express the ligands for multiple PMN surface molecules involved in adhesion. Moreover, they can actively respond to PMN adhesion due to intracellular signaling events triggered by PMN binding. In cultured EC, ligation of ICAM-1 by β2-integrins stimulates activation of several tyrosine kinases and MAPKs, resulting in the recruitment of several actin-binding proteins and cytoskeleton rearrangement (Wang et al., 2005). As a key role has been ascribed to p38 MAPK, it can be suggested that chol-but SLN may also act at the p38 MAPK level in EC.
In summary, our data show that sodium butyrate and chol-but SLN exert rapid anti-inflammatory effects on PMNs, and that the latter is much more potent than the former, as it acts at three logs lower concentrations and displays a broader inhibitory effect on PMN activation. The stronger effect of chol-but SLN might be ascribed to their rapid internalization in the cytoplasmic compartment, which is maximal within few minutes in both PMN and HUVEC. These results suggest that the use of SLN might be a novel clinical possibility to deliver butyric acid at lower dosage that is still able to produce significant anti-inflammatory effects in patients with UC. Further studies are in progress in our laboratory to elucidate the molecular mechanism of chol-but SLN.
Acknowledgments
This research was supported by the Turin University funding (ex-60%). We thank the Pathology Unit and the Obstetrics and Gynecology Unit, Martini Hospital, Turin, for providing human umbilical cords, and the Blood Bank at the Molinette Hospital, Turin, for providing human blood.
Abbreviations
- HUVEC
human umbilical vein endothelial cells
- PMNs
polymorphonuclear cells
- SLN
solid lipid nanoparticles
References
- AVANZI G.C., GALLICCHIO M., BOTTAREL F., GAMMAITONI L., CAVALLONI G., BUOFIGLIO D., BRAGARDO M., BELLOMO G., ALBANO E., FANTOZZI R., GARBARINO G., VARNUM B., AGLIETTA M., SAGLIO G., DIANZANI U., DIANZANI C. GAS6 inhibits granulocyte adhesion to endothelial cells. Blood. 1998;91:2334–2340. [PubMed] [Google Scholar]
- BENDAS G. Inhibitors of membrane receptors involved with leukocyte extravasation. Mini Rev. Med. Chem. 2005;5:575–584. doi: 10.2174/1389557054023279. [DOI] [PubMed] [Google Scholar]
- BROWN A.J., GOLDSWORTHY S.M., BARNES A.A., EILERT M.M., TCHEANG L., DANIELS D., MUIR A.I., WIGGLESWORTH M.J., KINGHORN I., FRASER N.J., PIKE N.B., STRUM J.C., STEPLEWSKI K.M., MURDOCK P.R., HOLDER J.C., MARSHALL F.H., SZEKERES P.G., WILSON S., IGNAR D.M., FOORD S.M., WISE A., DOWEL S.J. The orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short-chain carboxylic acids. J. Biol. Chem. 2003;278:11312–11319. doi: 10.1074/jbc.M211609200. [DOI] [PubMed] [Google Scholar]
- BRUNKHORST B.A., KRAUS E., COPPI M., BUDNICK M., NIEDERMAN R. Propionate induces polymorphonuclear leukocyte activation and inhibits formylmethionyl-leucyl-phenylalanine-stimulated activation. Infect. Immun. 1992;60:2957–2968. doi: 10.1128/iai.60.7.2957-2968.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAVALLI R., BARGONI A., PODIO V., MUNTONI E., ZARA G.P., GASCO M.R. Duodenal administration of solid lipid nanoparticles loaded with different percentages of tobramycin. J. Pharmc. Sci. 2002;92:1085–1094. doi: 10.1002/jps.10368. [DOI] [PubMed] [Google Scholar]
- CONLEY B.A., EGORIN M.J, TAIT N., ROSEN D.M., SAUSVILLE E.A., DOVER G., FRAM J.R., VAN ECHO D.A. Phase I study of the orally administered butyrate prodrug, tributyrin, in patients with solid tumors. Clin. Cancer Res. 1998;4:629–634. [PubMed] [Google Scholar]
- D'ARGENIO G., COSENZA V., DELLE CAVE M., IOVINO P., DELLE VALLE N., LOMBARDI G., MAZZACCA G. Butyrate enemas in experimental colitis and protection against large bowel cancer in a rat model. Gastroenterology. 1996;110:1727–1734. doi: 10.1053/gast.1996.v110.pm8964397. [DOI] [PubMed] [Google Scholar]
- DIANZANI C., COLLINO M., LOMBARDI G., GARBARINO G., FANTOZZI R. Substance P increases neutrophil adhesion to human umbelical vein endothelial cells. Br. J. Pharmacol. 2003;139:1103–1110. doi: 10.1038/sj.bjp.0705344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUNCAN I.W. Determination of free, total, and esterified cholesterol by high performance liquid chromatography. J. Chromatogr. 1979;162:281–292. doi: 10.1016/s0378-4347(00)81515-7. [DOI] [PubMed] [Google Scholar]
- DYER J.P., FEATHERSTONE J.M., SOLOMON L.Z., CROOK T.J., COOPER A.J., MALONE P.S. The effect of short-chain fatty acids butyrate, propionate, and acetate on urothelial cell kinetics in vitro: potential therapy in augmentation cystoplasty. Pediatr. Surg. Int. 2005;21:521–526. doi: 10.1007/s00383-005-1440-7. [DOI] [PubMed] [Google Scholar]
- EDELMAN M.J., BAUER K., KHANWANI S., TAIT N., TREPEL J., KARP J., NEMIEBOKA N., CHUNG E.J., VAN ECHO D. Clinical and pharmacologic study of tributyrin: an oral butyrate prodrug. Cancer Chemother. Pharmacol. 2003;51:439–444. doi: 10.1007/s00280-003-0580-5. [DOI] [PubMed] [Google Scholar]
- EFTIMIADI C., BUZZI E., TONETTI M., BUFFA P., BUFFA D., VAN STEENBERGEN M.T.J., DE GRAAF J., BOTTA G.A. Short-chain fatty acids produced by anaerobic bacteria alter the physiological responses of human neutrophils to chemotactic peptide. J. Infection. 1987;14:43–53. doi: 10.1016/s0163-4453(87)90808-5. [DOI] [PubMed] [Google Scholar]
- GASCO M.R. Solid lipid nanoparticles for drug delivery. Pharm. Technol. Eur. 2001;13:32–41. [Google Scholar]
- GASCO M.R.Solid lipid nanospheres suitable to a fast internalization into cells 2004. EP 1133286, (24/11/2004) – U.S. 6.685.960 (3/02/2004)
- GLOTZER D.J., GLICK M.E., GOLDMAN H. Proctitis and colitis following diversion of the fecal stream. Gastroenterology. 1981;80:438–441. [PubMed] [Google Scholar]
- HALLERT C., BJORCK I., NYMAN M., POUSETTE A., GRANNO C., SVENSSON H. Increasing fecal butyrate in ulcerative colitis patients by diet: controlled pilot study. Inflamm. Bowel Dis. 2003;9:116–121. doi: 10.1097/00054725-200303000-00005. [DOI] [PubMed] [Google Scholar]
- HENSON P.M., ZANOLARI B., SCHWARTZMAN N.A., HONG S.R. Intracellular control of human neutrophil secretion I. C5a-induced stimulus-specific desensitization and the effects of cytochalasin B. J. Immunol. 1978;121:851–855. [PubMed] [Google Scholar]
- KLAMPFER L., HUANG J., SASAZUKI T., SHIRASAWA S., AUGENLICHT L. Inhibition of interferon gamma signaling by the short chain fatty acid butyrate. Mol. Cancer Res. 2003;1:855–862. [PubMed] [Google Scholar]
- KOPPEL D.E. Analysis of macromolecular polydispersity in intensity correlation spectroscopy: the method of cumulant. J. Chem. Phys. 1972;57:4814–4818. [Google Scholar]
- LE POUL E., LOISON C., STRUYF S., SPRINGAEL J.-Y., LANNOY V., DECOBECQ M.-E., BREZILLON S., DUPRIEZ V., VASSART G., VAN DAMME J., PARMENTIER M., DETHEUX M. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J. Biol. Chem. 2003;278:25481–25489. doi: 10.1074/jbc.M301403200. [DOI] [PubMed] [Google Scholar]
- LIU Q., SHIMOYAMA T., SUZUKI K., UMEDA T., NAKAJI S., SUGAWARA K. Effect of sodium butyrate on reactive oxygen species generation by human neutrophils. Scand. J. Gastroenterol. 2001;36:744–750. doi: 10.1080/003655201300192012. [DOI] [PubMed] [Google Scholar]
- MANJUNATH K., REDDY J.S., VENKATESWARLU V. Solid lipid nanoparticles as drug delivery systems. Methods Find. Exp. Clin. Pharmacol. 2005;27:127–144. doi: 10.1358/mf.2005.27.2.876286. [DOI] [PubMed] [Google Scholar]
- MCLEISH K.R., KNALL C., WARD R.A., GERWINS P., COXON P.Y., KLEIN J.B., JOHNSON G.L. Activation of mitogen-activated protein kinase cascades during priming of human neutrophils by TNF-alpha and GM-CSF. J. Leukoc. Biol. 1998;64:537–545. [PubMed] [Google Scholar]
- MENZEL T., LUHRS H., ZIRLIK S., SCHAUBER J., KUDLICH T., GERKE T., GOSTNER A., NEUMANN M., MELCHER R., SCHEPPACH W. Butyrate inhibits leukocyte adhesion to endothelial cells via modulation of VCAM-1. Inflamm. Bowel Dis. 2004;10:122–128. doi: 10.1097/00054725-200403000-00010. [DOI] [PubMed] [Google Scholar]
- MOSMANN T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- MÜLLER R.H. Colloidal Carriers for Controlled Drug Delivery and Targeting. Boca raton, Florida, U.S.A.: CRC Press; 1991. [Google Scholar]
- NICK J.A., AVDI N.J., YOUNG S.K., KNALL C., GERWINS P., JOHNSON G.L., WORTHEN G.S. Common and distinct intracellular signaling pathways in human neutrophils utilized by platelet activating factor and FMLP. J. Clin. Invest. 1997;99:975–986. doi: 10.1172/JCI119263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NILSSON N.E., KOTARSKY K., OWMAN C., OLDE B. Identification of a free fatty acid receptor, FFA2R, expressed on leukocytes and activated by short-chain fatty acids. Biochem. Biophys. Res. Commun. 2003;303:1047–1052. doi: 10.1016/s0006-291x(03)00488-1. [DOI] [PubMed] [Google Scholar]
- OGAWA H., RAFIEE P., FISHER P.J., JOHNSON N.A., OTTERSON M.F., BINION D.G. Butyrate modulates gene and protein expression in human intestinal endothelial cells. Biochem. Biophy. Res. Commun. 2003;309:512–519. doi: 10.1016/j.bbrc.2003.08.026. [DOI] [PubMed] [Google Scholar]
- PARK J.S., WOO M.S., KIM S.Y., KIM W.K., KIM H.S. Repression of interferon-gamma-induced inducible nitric oxide synthase (iNOS) gene expression in microglia by sodium butyrate is mediated through specific inhibition of ERK signaling pathways. J. Neuroimmunol. 2005;168:56–64. doi: 10.1016/j.jneuroim.2005.07.003. [DOI] [PubMed] [Google Scholar]
- PATNAIK A., ROWINSKY E.K., VILLALONA M.A., HAMMOND L.A., BRITTEN C.D., SIU L.L., GOETZ A., FELTON S.A., BURTON S., VALONE F.H., ECKHARDT S.G. A phase I study of pivaloyloxymethyl butyrate, a prodrug of the differentiating agent butyric acid, in patients with advanced solid malignancies. Clin. Cancer Res. 2002;8:2142–2148. [PubMed] [Google Scholar]
- PELLIZZARO C., CORADINI D., MOREL S., UGAZIO E., GASCO M.R., DAIDONE M.G. Cholesteryl butyrate in solid lipid nanospheres as an alternative approach for butyric acid delivery. Anticancer Res. 1999;19:3921–3926. [PubMed] [Google Scholar]
- REID T., VALONE F., LIPERA W., IRWIN D., PAROLY W., NATALE R., SREEDHARAN S., KEER H., LUM B., SCAPPATICCI F., BHATNAGAR A. Phase II trial of the histone deacetylase inhibitor pivaloyloxymethyl butyrate (Pivanex, AN-9) in advanced non-small cell lung cancer. Lung Cancer. 2004;45:381–386. doi: 10.1016/j.lungcan.2004.03.002. [DOI] [PubMed] [Google Scholar]
- RODRIGUEZ-CABEZAS M.E., GALVEZ J., CAMUESCO D., LORENTE M.D., CONCHA A., MARTINEZ-AUGUSTIN O., REDONDO L., ZARZUELO A. Intestinal anti-inflammatory activity of dietary fiber (Plantago ovata seeds) in HLA-B27 transgenic rats. Clin. Nutr. 2003;22:463–471. doi: 10.1016/s0261-5614(03)00045-1. [DOI] [PubMed] [Google Scholar]
- SALOMONE B., PONTI R., GASCO M.R., UGAZIO E., QUAGLINO P., OSELLA-ABATE S., BERNENGO M.G. In vitro effects of cholesteryl butyrate solid lipd nanospheres as a butyric acid pro-drug on melanoma cells: evaluation of antiproliferative activity and apoptosis induction. Clinic. Experim. Metast. 2001;18:663–673. doi: 10.1023/a:1013186331662. [DOI] [PubMed] [Google Scholar]
- SCHEPPACH W., WEILER F. The butyrate story: old wine in new bottles. Curr. Opin. Clin. Nutr. Metab. Care. 2004;7:563–567. doi: 10.1097/00075197-200409000-00009. [DOI] [PubMed] [Google Scholar]
- SEGAIN J.P., RAINGEARD DE LA BLETIERE D., BOURREILLE A., LERAY V., GERVOIS N., ROSALES C., FERRIER L., BONNET C., BLOTTIERE H.M., GALMICHE J.P. Butyrate inhibits inflammatory responses through NFkB inhibition: implications for Crohn's disease. Gut. 2000;47:397–403. doi: 10.1136/gut.47.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SENGA T., IWAMOTO S., YOSHIDA T., YOKOTA T., ADACHI K., AZUMA E., HAMAGUCHI M., IWAMOTO T. LSSIG is a novel murine leukocyte-specific GPCR that is induced by the activation of STAT3. Blood. 2003;101:1185–1187. doi: 10.1182/blood-2002-06-1881. [DOI] [PubMed] [Google Scholar]
- SERPE L., LAURORA S., PIZZIMENTI S., UGAZIO E., PONTI R., CANAPARO R., BRIATORE F., BARRERA G., GASCO M.R., BERNENGO M.G., EANDI M., ZARA G.P. Cholesteryl butyrate solid lipid nanoparticles as a butyric acid pro-drug: effects on cell proliferation, cell cycle distribution and c-myc expression in human leukemic cells. Anti Cancer Drugs. 2004;15:525–536. doi: 10.1097/01.cad.0000127329.83568.15. [DOI] [PubMed] [Google Scholar]
- VENKATRAMAN A., RAMAKRISHNA B.S., SHAJI R.V., KUMAR N.S., PULIMOOD A., PATRA S. Amelioration of dextran sulfate colitis by butyrate: role of the shock protein 70 and NF-kappaB. Am. J. Physiol. Gastrointest. Liver Physiol. 2003;285:G177–G184. doi: 10.1152/ajpgi.00307.2002. [DOI] [PubMed] [Google Scholar]
- VERNIA P., ANNESE V., BRESCI G., D'ALBASIO G., D'INCA R., GIACCARI S., INGROSSO M., MANSI C., RIEGLER G., VALPIANI D., CAPRILLI R. Topical butyrate improves efficacy of 5-ASA in refractory distal ulcerative colitis: results of a multicentre trial. Eur. J. Clin. Invest. 2003;33:244–248. doi: 10.1046/j.1365-2362.2003.01130.x. [DOI] [PubMed] [Google Scholar]
- WANG Q., YERUKHIMOVICH M., GAARDE W.A., POPOFF I.J., DOERSCHUK C.M. MKK3 and -6-dependent activation of p38α MAP kinase is required for cytoskeletal changes in pulmonary microvascular endothelial cells induced by ICAM-1 ligation. Am. J. Physiol. Lung Cell Mol. Physiol. 2005;288:L359–L369. doi: 10.1152/ajplung.00292.2004. [DOI] [PubMed] [Google Scholar]
- WESTESEN K., BUNJES H., KOCH H.J. Physicochemical characterization of lipid nanoparticles and evaluation of their drug loading capacity and sustained release potential. J. Control Rel. 1997;48:223–236. [Google Scholar]
- XIONG Y., MIYAMOTO N., SHIBATA K., VALASEK M.A., MOTOIKE T., KEDZIERSKI R.M., YANAGISAWA M. Short-chain fatty acids stimulate leptin production in adipocytes through the G protein-coupled receptor GPR41. PNAS. 2004;101:1045–1050. doi: 10.1073/pnas.2637002100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZAPOLSKA-DOWNAR D., SIENNICKA A., KACZMARCZYK M., KOLODZIEJ B., NARUSZEWICZ M. Butyrate inhibits cytokine-induced VCAM-1 and ICAM-1 expression in cultured endothelial cells: the role of NF-kB and PPARα. J. Nutr. Biochem. 2004;15:220–228. doi: 10.1016/j.jnutbio.2003.11.008. [DOI] [PubMed] [Google Scholar]