Abstract
Long chain fatty acids have recently been identified as agonists for the G protein-coupled receptors GPR40 and GPR120. Here, we present the first description of GW9508, a small-molecule agonist of the fatty acid receptors GPR40 and GPR120. In addition, we also describe the pharmacology of GW1100, a selective GPR40 antagonist. These molecules were used to further investigate the role of GPR40 in glucose-stimulated insulin secretion in the MIN6 mouse pancreatic β-cell line.
GW9508 and linoleic acid both stimulated intracellular Ca2+ mobilization in human embryonic kidney (HEK)293 cells expressing GPR40 (pEC50 values of 7.32±0.03 and 5.65±0.06, respectively) or GPR120 (pEC50 values of 5.46±0.09 and 5.89±0.04, respectively), but not in the parent HEK-293 cell line.
GW1100 dose dependently inhibited GPR40-mediated Ca2+ elevations stimulated by GW9508 and linoleic acid (pIC50 values of 5.99±0.03 and 5.99±0.06, respectively). GW1100 had no effect on the GPR120-mediated stimulation of intracellular Ca2+ release produced by either GW9508 or linoleic acid.
GW9508 dose dependently potentiated glucose-stimulated insulin secretion in MIN6 cells, but not in primary rat or mouse islets. Furthermore, GW9508 was able to potentiate the KCl-mediated increase in insulin secretion in MIN6 cells. The effects of GW9508 on insulin secretion were reversed by GW1100, while linoleic acid-stimulated insulin secretion was partially attenuated by GW1100.
These results add further evidence to a link between GPR40 and the ability of fatty acids to acutely potentiate insulin secretion and demonstrate that small-molecule GPR40 agonists are glucose-sensitive insulin secretagogues.
Keywords: GPR40, agonist, antagonist, small molecule, GPR120, GPCR, Type 2 diabetes, insulin, MIN6
Introduction
GPR40 and GPR120 exemplify a growing number of G protein-coupled receptors (GPCRs) that have been shown to be activated by free fatty acids (Brown et al., 2005). To date, the specificity of plasma membrane receptors for fatty acids appears to be differentiated by the subset of fatty acids that they are activated by, with GPR41 and 43 being activated by short chain fatty acids (carbon chain <6) (Brown et al., 2003; Le Poul et al., 2003; Nilsson et al., 2003), GPR120 being activated by long chain unsaturated fatty acids (Hirasawa et al., 2005) and GPR40 being activated by saturated and unsaturated long chain fatty acids (Briscoe et al., 2003; Itoh et al., 2003; Kotarsky et al., 2003;). The lack of specificity of these receptors for a single fatty acid implies that selectivity may be determined by the local tissue-specific environment.
GPR40 is located downstream of CD22 on chromosome 19q13.1 (Sawzdargo et al., 1997), close to a region that has shown linkage to elevated serum triglycerides in families with type 2 diabetes (Elbein & Hasstedt, 2002). Two polymorphisms, an Arg211His substitution and a rare Asp175Asn mutation have been identified in the GPR40 gene (Black & Leff, 1983; Haga et al., 2002). A recent study was unable to link any variations in the coding region of GPR40 to type 2 diabetes or insulin release alterations in Danish patient populations (Hamid et al., 2005), although the Arg211His polymorphism was significantly correlated to HOMA-β in healthy Japanese men (Ogawa et al., 2005). It is well established that fatty acids function acutely to maintain basal insulin secretion and to ‘prime' the islet β-cells to respond to glucose following a prolonged fast (Stein et al., 1997; Dobbins et al., 1998a, 1998b; Gravena et al., 2002). The mechanisms underlying these effects remain unclear, but have been hypothesized to involve intracellular metabolism of the fatty acid molecule itself (Prentki et al., 1992; 1997). GPR40 mRNA was reported by several groups to be expressed in human and rodent pancreatic islets. Furthermore the finding that activation of the receptor resulted in elevation of intracellular Ca2+ via coupling to Gαq/11, leading to activation of PKC suggested a possible role for GPR40 in insulin secretion (Briscoe et al., 2003; Itoh et al., 2003; Kotarsky et al., 2003). A key role for GPR40 in the acute effects of fatty acids on pancreatic β-cells was suggested based on the finding that downregulation of GPR40 expression in the mouse insulinoma cell line MIN6 (Miyazaki et al., 1990) or in Ins-1E cells resulted in a decrease in the ability of fatty acids to potentiate insulin secretion (Itoh et al., 2003; Shapiro et al., 2005). As fatty acids potentiate insulin secretion in a glucose-sensitive manner it is conceivable that if the effects of fatty acids on insulin secretion are mediated at least in part through GPR40, a small-molecule GPR40 agonist may act as a glucose-sensitive secretagogue.
The use of fatty acids themselves to elucidate the role of fatty acid-binding receptors is complicated by the ability of the fatty acids to be metabolized within the cells, rather than to act as extracellular-signaling molecules. Moreover, the ability of certain fatty acids to activate more than one receptor, including nuclear receptors such as peroxisome proliferator-activated receptor (PPAR)α (Gottlicher et al., 1992), necessitates the use of molecular tools to dissect out the mechanism. Furthermore, although certain thiazolidinediones (TZD's) have been shown to activate GPR40 (Kotarsky et al., 2003), interpretation of the results from these experiments are hampered by the accompanying PPAR activity of the tool compounds.
In this paper, we present data detailing the pharmacology of a novel small-molecule agonist of GPR40 and GPR120, together with a selective antagonist of GPR40. Using these compounds, we verify that the potentiation of insulin secretion by fatty acids appears to be mediated at least partially through GPR40, and that GPR40 agonists can function as glucose-sensitive secretagogues in vitro.
Methods
Materials and compound nomenclature
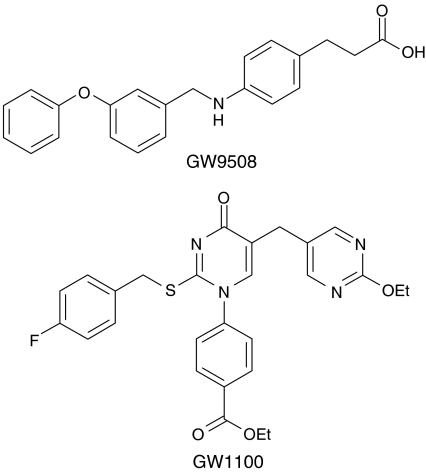
GW9508 (3-[4-({[3-(phenyloxy)phenyl]methyl}amino)phenyl] propanoic acid) and GW1100 (ethyl 4-[5-{[2-(ethyloxy)-5-pyrimidinyl]methyl}-2-{[(4-fluorophenyl) methyl]thio}-4-oxo-1(4H)-pyrimidinyl] benzoate), were originally identified from a high-throughput screen of the GlaxoSmithKline chemical collection. Structures are shown in Figure 1. Fatty acids were obtained from Sigma (St Louis, Missouri, MO, U.S.A.) and were dissolved in 100% DMSO. The final concentration of DMSO was 1% in all in vitro studies.
Figure 1.
Chemical structures of the GPR40/GPR120 agonist GW9805 and selective GPR40 antagonist GW1100.
GPR40 and GPR120 BacMam baculovirus generation
The complete coding sequences of human GPR40 (GenBank Accession no. NM-005303) and GPR120 (GenBank Accession no. NM-181745) were inserted into the baculovirus shuttle vector, pFNcmv, under the transcriptional control of the CMV promoter (Ames et al., 2004). Plasmid preparations (Qiagen, Valencia, CA, U.S.A.) were performed after the plasmids were amplified in Escherichia coli strain TOP10 (Invitrogen, Carlsbad, CA, U.S.A.). The fidelity of the inserted sequences was confirmed by nucleotide sequencing using an ABI Prism Model 377 automated sequencer (Foster City, CA, U.S.A.). Recombinant BacMam baculoviruses were constructed from the BacMam shuttle plasmid by using the bacterial cell-based Bac-to-Bac system (Invitrogen, Carlsbad, CA, U.S.A.) (Luckow et al., 1993). The viruses were propagated in Sf9 (Spodoptera frugiperda) cells cultured in Excel420 (JRH Biosciences, Lenexa, KS, U.S.A.) and supplemented with 5% (v v−1) fetal bovine serum (JRH Biosciences) according to established protocols (O'Reilly et al., 1992).
Measurement of intracellular Ca2+ release
Human embryonic kidney (HEK-293) cells, stably transfected to express the human macrophage scavenging receptor (Class A, type 1; GenBank Accession no. D90187) were maintained in Dulbecco's modified Eagles medium (DMEM; high glucose) (Invitrogen, Carlsbad, CA, U.S.A.) containing 10% (v v−1) heat-inactivated fetal calf serum. The expression of this receptor by the HEK-293 cells enhances their ability to stick to tissue culture treated plasticware.
Cells were harvested using an enzyme-free cell dissociation buffer (Invitrogen, Carlsbad, CA, U.S.A.). Of this solution, 5 ml, was added and allowed to wash over the cells for 30 s. The solution was then removed and the cells returned to the incubator for 5 min. Following a gently tapping of the flask the cells were resuspended in media.
For GPR40 studies cells were transduced with GPR40 BacMam baculovirus at a multiplicity of infection (MOI) of 25. Control cells were not transduced with virus. The cells were plated at a concentration of 10,000 cells per well in black 384-well clear bottom tissue culture treated plates (Corning Costar, Cambridge, MA, U.S.A.). The cells were cultured for 24 h at 37°C in a humidified 5% CO2/95% air environment to allow for protein expression. Subsequently, the medium was removed from the wells and replaced with 40 μl of Calcium-3 dye (Molecular Devices, Sunnyvale, CA, U.S.A.). The cells were allowed to load with dye for 1 h at 37°C in a cell culture incubator. The plates were then allowed to cool to room temperature for 20 min. All compounds were diluted to appropriate concentrations in Calcium-3 dye. Compounds were added during the assay by the FLIPR3 instrument (Molecular Devices, Sunnyvale, CA, U.S.A.) in 10 μl per well volumes and the wells were read by the instrument every 2 s for 90 s. Antagonist studies were performed in a similar format. Antagonist was added to the well in a 10 μl volume and the plates were incubated at room temperature for 15 min before the second addition of 10 μl per well of an ED80 concentration of agonist.
For GPR120 studies cells were transduced with GPR120 BacMam baculovirus at a MOI of 25. Control cells were not transduced with virus. The cells were plated at a concentration of 40,000 cells per well in black 96-well clear bottom poly-D-lysine coated tissue culture plates (Corning Costar, Cambridge, MA, U.S.A.). The cells were cultured for 24 h at 37°C in a humidified 5% CO2/95% air environment to allow for protein expression. Subsequently, the medium was removed from the wells. The wells were washed with 50 μl of serum-free media before 50 μl of Calcium Plus dye (Molecular Devices, Sunnyvale, CA, U.S.A.) was added. The cells were allowed to load with dye for 1 h at 37°C in a cell culture incubator. The plates were then allowed to cool to room temperature for 20 min. All compounds were diluted to appropriate concentrations in Calcium Plus dye. Compounds were added during the assay by the FLIPR3 instrument (Molecular Devices, Sunnyvale, CA, U.S.A.) in 50 μl per well volumes and the wells were read by the instrument every 1 s for 60 s. Antagonist studies were performed in a similar format. Antagonist was added to the well in a 50 μl volume and the plates were incubated at room temperature for 15 min before the second addition of 50 μl per well of an ED80 concentration of agonist.
GPR40 reporter assay
The hGPR40-5 × Gal/Gal4-Elk1-luc+ CHO cell line was generated as described previously (Briscoe et al., 2003). The cells were maintained in DMEM/F12 (Invitrogen, Carlsbad, CA, U.S.A.) containing 5% FBS (v v−1) and 2 mM glutamine. 48 h before the assay, hGPR40-Gal4-Elk1/5 × Gal4-luc+ CHO cells were plated at a concentration of 10,000 cells per well in a 96-well black-view plate (Costar, Cambridge, MA, U.S.A.) in complete medium. At 18 h before the assay, the medium was replaced with 90 μl per well of serum-free complete medium. At the time of the assay, the medium was replaced with 80 μl of serum-free medium and 10 μl of antagonist was added. Following a 30 min incubation, 10 μl of agonist was added to each well and the plates were incubated for 5 h at 37°C. Subsequently, media was removed and replaced with 50 μl of a 1 : 1 mixture of LucPlus™ (Packard, Boston, MA, U.S.A.) and Dulbecco's phosphate buffered saline containing 1 mM CaCl2 and 1 mM MgCl2. Plates were incubated in the dark at room temperature for 10 min before luciferase activity was determined using a TopCount™ microplate luminescence counter (Perkin-Elmer, Boston, MA, U.S.A.) using 3 s per well count time.
MIN6 insulin secretion assay
MIN6 cells were maintained in DMEM (high glucose) (Invitrogen, Carlsbad, CA, U.S.A.) containing 15% heat-inactivated fetal calf serum and 72 μM β-mercaptoethanol.
Cells were harvested using a nonenzymatic cell dissociation liquid (Invitrogen, Carlsbad, CA, U.S.A.). Cells were resuspended in the above media and were dissociated before being seeded into 96-well plates (40,000 cells per well). The cells were subsequently cultured for 48 h at 37°C in a humidified 5% CO2/95% air environment. Media was removed from the cells and they were washed twice with glucose-free Krebs (NaCl 119 mM, KCl 4.74 mM, CaCl2 2.54 mM, MgCl2 1.19 mM, KH2PO4 1.19 mM, NaHCO3 25 mM and HEPES (pH 7.4) 10 mM) containing 0.05% (w v−1) insulin-free bovine serum albumin (BSA). A measure of 100 μl of the above Krebs solution containing 2.5 mM glucose was added to each well and the plates were returned to the incubator for 30 min. Subsequently, the plates were washed a further two times with glucose-free Krebs and 200 μl of Krebs containing glucose at the desired concentration, plus or minus compound, was added. Following 2 h incubation the Krebs solution in each well was removed and the insulin content of each sample was determined.
Insulin determination
Insulin concentrations were determined using the ORIGEN™ system (Bioveris, Gaithersburg, MD, U.S.A.). In brief, samples were added to a 96-well plate. To each sample was added 25 μl of biotinylated anti-rat insulin monoclonal antibody (3 μg ml−1; Biogensis, Kingston, NH, U.S.A.) dissolved in assay buffer (phosphate buffered saline (PBS), containing 1% (w v−1) insulin-free BSA, 1.5% Tween 20 (v v−1), 0.5% Sodium Azide (w v−1)). Subsequently, 25 μl of a ruthenium tagged anti-rat insulin monoclonal antibody (3 μg ml−1; Biogenesis, Kingston, NH, U.S.A.), was added. Following 2 h incubation at room temperature, 25 μl of streptavidin coated beads (40 μg ml−1; Dynabeads M280 Streptavidin, Bioveris, Gaithersburg, MD, U.S.A.) were added. After a final 30 min incubation, 100 μl assay buffer was added to each well and the insulin content was determined using a M-series M8 Analyzer (IGEN, Gaithersburg, MD, U.S.A.).
Determination of antagonist KB value for GW1100
Agonist concentration–response curves generated in the presence of increasing concentrations of antagonist in the hGPR40-5 × Gal/Gal4-Elk1-luc+ CHO cell line, were fitted to a model of insurmountable antagonism (Ehlert, 1988). Responses were modeled by the standard operational model (Black & Leff, 1983) as described by the following equation:
The following values were assumed τ=6; Emax=105; KA=500 nM; n=2; α=1.5; KB=1 μM. τ represents a fitting parameter to model efficacy of the agonist, receptor number and efficiency of receptor coupling. The magnitude of this parameter has no bearing on the estimate of antagonist affinity and serves only to model the control response with respect to agonist receptor occupancy. α represents the cooperativity factor for binding of agonist imposed by occupancy of the antagonist. n represents the Hill coefficient for control and subsequent concentration-response curves and has no bearing on the antagonist potency estimate. This reflects the efficiency of stimulus-response coupling in the system. Emax represents the maximal response capability of the system. In addition, this model assumes that the insurmountable antagonism observed results from an inability of the antagonist-bound receptor to respond to the agonist.
Statistical analysis
Results are presented as mean±s.e.m., n represents the number of individual experiments performed. Differences between results were analyzed by Student's t-test (unpaired) (*P<0.05; **P<0.01; ***P<0.001), unless otherwise stated. Studies to examine the K+ATP channel dependency of GW9508 in vitro were analyzed by ANOVA, followed by the Tukey–Kramer multiple comparison test.
Results
Pharmacological characterization of GW9508 and GW1100
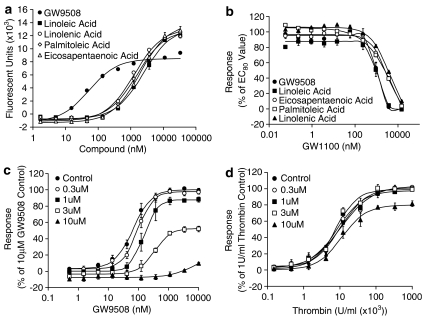
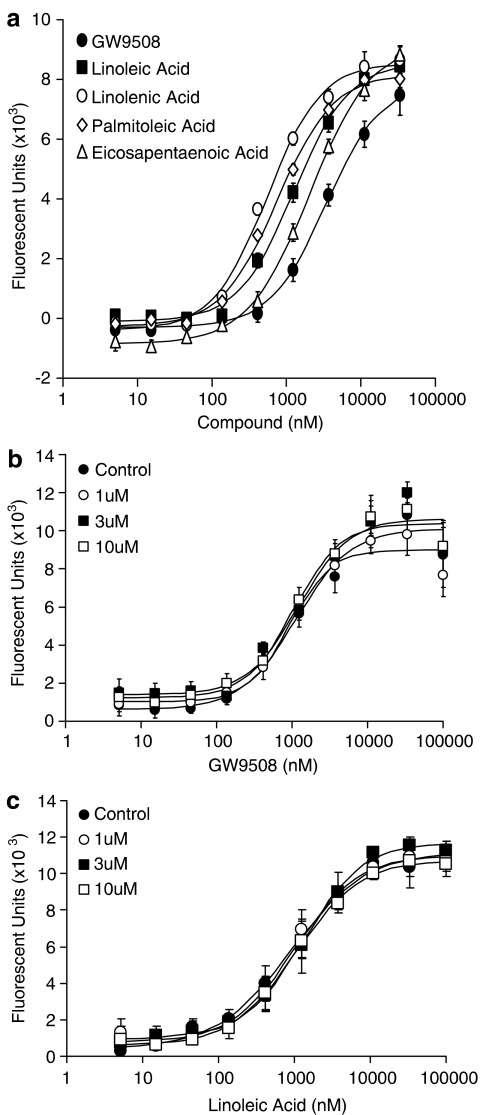
Activation of GPR40 by fatty acids has been shown to result in an increase in intracellular Ca2+ levels, an action mediated via Gαq/11 (Briscoe et al., 2003; Itoh et al., 2003; Kotarsky et al., 2003). Similarly GPR120, also a receptor for fatty acids, has also been shown to couple to intracellular Ca2+ signaling (Hirasawa et al., 2005). Using a HEK-293 cell line transiently transduced to express GPR40 or GPR120, we have characterized the pharmacology of GW9508, a novel GPR40 and GPR120 agonist. GW9508 has been shown in either functional or binding assays to be at least 100-fold selective against 220 other GPCRs, 60 kinases, 63 proteases, seven integrins and 20 nuclear receptors including PPARα, δ and γ (pEC50 4.0, 4 and 4.9, respectively, in transient transfection assays) (Data generated internally at GlaxoSmithKline). In the same selectivity panel, the novel GPR40 antagonist GW1100 was selective with the exception of the oxytocin receptor, for which it acted as an antagonist with a pIC50 6.9. GW9508 produced a concentration-dependent increase in intracellular Ca2+ concentrations via GPR40 receptor activation (pEC50=7.32±0.03; n=4) and the GPR120 receptor (pEC50=5.46±0.09; n=4) (Figures 2a and 3a). GW9508 was inactive against other receptors of the GPR40 family, GPR41 and GPR43 (pEC50<4.3), which are activated by short chain fatty acids. The potency of GW9508 was significantly greater than that observed for long chain fatty acids at the GPR40 receptor, including linoleic acid (pEC50=5.65±0.06; n=4), α-linolenic acid (pEC50=5.94±0.03; n=4), palmitoleic acid (pEC50=5.86±0.05; n=4) and cis-5,8,11,14,17-eicosapentaenoic acid (pEC50=5.73±0.04; n=4) (Figure 2a). However, at the GPR120 receptor the potency of the long chain fatty acids examined was similar to that observed for GW9508. The potencies of the fatty acids were, linoleic acid pEC50=5.89±0.04; n=4, α-linolenic acid pEC50=6.26±0.04; n=4, palmitoleic acid pEC50=6.12±0.08; n=4, cis-5,8,11,14,17-eicosapentaenoic acid pEC50=5.68±0.06; n=4) (Figure 3a). At the GPR120 receptor the magnitude of the responses for GW9508 and all the fatty acids tested were identical, whereas at the GPR40 receptor GW9508 appeared to be a partial agonist with respect to the fatty acids tested. No response was observed for either GW9508 or any of the fatty acids in untransduced HEK cells, confirming that the responses observed were indeed GPR40 or GPR120 mediated (data not shown).
Figure 2.
Pharmacological profile of the GW9508 and GW1100 at the GPR40 receptor. (a) The effect of GW9508, linoleic acid, α-linolenic acid, palmitoleic acid and cis-5,8,11,14,17-eicosapentaenoic acid on intracellular Ca2+ levels was examined in HEK-293 cells expressing the human GPR40 receptor. pEC50 values obtained were GW9508 (pEC50=7.32±0.03; n=4), linoleic acid (pEC50=5.65±0.06; n=4), α-linolenic acid (pEC50=5.94±0.03; n=4), palmitoleic acid (pEC50=5.86±0.05; n=4) and cis-5,8,11,14,17-eicosapentaenoic acid (pEC50=5.73±0.04; n=4). (b) Inhibition of GW9508 and linoleic acid induced intracellular Ca2+ increase by GW1100, a selective GPR40 receptor antagonist. GW1100 produced a concentration-dependent decrease in intracellular Ca2+ response to an EC80 concentration of GW9508 (pIC50=5.99±0.03; n=4) and linoleic acid (pIC50=5.99±0.06; n=4). (c) The effect of increasing concentrations of GW1100 on GW9508-induced luciferase activity in a human GPR40-5 × Gal/Gal4-Elk1-luc+ CHO cell line reporter assay. Cells were preincubated with GW1100 for 15 min before addition of GW9508. GW1100 produced a concentration-dependent rightward shift in the GW9508 curve followed by a depression of the signal. This is consistent with a non-competitive mode of antagonism in the presence of a significant GPR40 receptor reserve. (d) The selectivity of GW1100 in the human GPR40-5 × Gal/Gal4-Elk1-luc+ CHO cell line reporter assay was confirmed using Thrombin as a control stimulus. Data represent the mean±s.e.m.
Figure 3.
Pharmacological profile of the GW9508 and GW1100 at the GPR120 receptor. (a) linoleic acid, α-linolenic acid, palmitoleic acid and cis-5,8,11,14,17-eicosapentaenoic acid on intracellular Ca2+ levels was examined in HEK-293 cells expressing the human GPR120 receptor. pEC50 values obtained were GW9508 (pEC50=5.46±0.09; n=4), linoleic acid (pEC50=5.89±0.04; n=4), α-linolenic acid (pEC50=6.26±0.04; n=4), palmitoleic acid (pEC50=6.12±0.08; n=4) and cis-5,8,11,14,17-eicosapentaenoic acid (pEC50=5.68±0.06; n=4). (b) The effect of increasing concentrations of GW1100 on GW9508 induced increases in intracellular Ca2+ levels. Cells were preincubated with GW1100 for 15 min before addition of GW9508. GW1100 had no effect on the GW9508 response. (c) The effect of increasing concentrations of GW1100 on linoleic acid induced increases in intracellular Ca2+ levels. Cells were preincubated with GW1100 for 15 min before addition of linoleic acid. GW1100 had no effect on the linoleic acid response. Data represent the mean±s.e.m.
GW1100 produced a concentration-dependent inhibition of the GW9508-stimulated increase in intracellular Ca2+ levels mediated via GPR40 (Figure 2b and c). A similar effect of GW1100 was observed for linoleic acid, α-linolenic acid, palmitic acid and cis-5,8,11,14,17-eicosapentaenoic acid-stimulated increase in intracellular Ca2+ levels. In the presence of approximate EC80 concentrations of GW9508 (1 μM), linoleic acid (10 μM), α-linolenic acid (5 μM), palmitoleic acid and cis-5,8,11,14,17-eicosapentaenoic acid (5 μM), GW1100 completely reversed the agonist response for all the ligands with similar pIC50 values (GW9508 pIC50=5.99±0.03; n=4, linoleic acid pIC50=5.99±0.06; n=4, α-linolenic acid pIC50=5.57±0.02; n=4, palmitoleic acid pIC50=5.74±0.03; n=4, cis-5,8,11,14,17-eicosapentaenoic acid pIC50=5.56±0.02; n=4) (Figure 2b.) GW1100 alone had no effect in this system. In contrast, GW1100 had no effect on the ability of GW9508, linoleic acid, α-linolenic acid, palmitoleic acid or cis-5,8,11,14,17-eicosapentaenoic acid to stimulate intracellular Ca2+ release via GPR120 in this system at concentrations up to and including 10 μM. The effect of GW1100 addition on GW9508 and linoleic acid-mediated intracellular Ca2+ levels via GPR120 is shown in Figure 3b and c.
To further examine the pharmacology and selectivity of the antagonist GW1100, a stable human GPR40-5 × Gal/Gal4-Elk1-luc+ CHO reporter cell line was employed. Consecutive concentration–response curves were generated to the GPR40 agonist GW9508 in the presence of increasing concentrations of GW1100 (Figure 2c). GW1100 at a concentration of 1 μM produced a significant rightward shift in the concentration–response curve to GW9508 (pEC50=7.17±0.08 in the absence and pEC50=6.79±0.09 in the presence of 1 μM GW1100; P<0.05; n=3). At this concentration of GW1100 no significant decrease in maximal response was observed. However, at concentrations of GW1100 of 3 μM and higher a significant decrease in the maximal response was observed with a continuing rightward shift in the pEC50 response. This insurmountable antagonism is consistent with noncompetitive antagonism of the receptor in the presence of an excess of spare receptors. The calculated KB value for GW1100 was approximately 1 μM.
To confirm that the inhibition of the GW9508 response by GW1100 was specific to GPR40, a similar experiment was carried out using thrombin as the agonist (Figure 2d). A reduction of the thrombin response would suggest that the antagonist may not be directly inhibiting the GPR40 receptor, but instead may be functionally antagonizing the GPR40-mediated response. In contrast to the effect of GW1100 on the GW9508 concentration–response curve, GW1100 at concentrations up to 10 μM had no significant effect on the pEC50 value of the thrombin concentration–response curve. A small 19.3±3.6% (P<0.01; n=3) suppression of the maximal response was also observed with 10 μM GW1100. In addition, no response was observed upon addition of GW9508 to the host 5 × Gal/Gal4-Elk1-luc+ CHO cell line that did not express GPR40 (data not shown). These results demonstrate that GW1100 is a selective GPR40 receptor antagonist up to 10 μM in this system.
Effect of GW9508 and GW1100 on glucose-stimulated insulin secretion from MIN6 cells
Previous studies have shown that free fatty acids such as linoleic acid can produce a glucose-dependent increase in insulin secretion from perfused pancreas, rat or human islets and insulin secreting cells (Stein et al., 1997; Gravena et al., 2002; Itoh et al., 2003). GPR40 siRNA has been shown to attenuate the response to linoleic acid in MIN6 cells, suggesting that the action of free fatty acids is partially mediated via this receptor (Itoh et al., 2003). Here, we used the agonist GW9508 and antagonist GW1100 to examine further the role GPR40 might play in glucose-dependent insulin secretion using the MIN6 mouse insulinoma cell line.
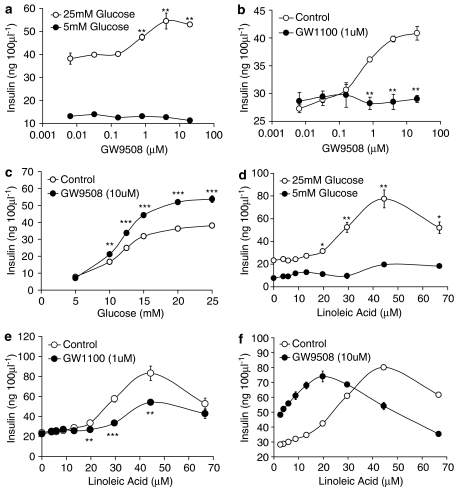
GW9508 produced a concentration-dependent increase (pEC50=6.14±0.03; n=3) in glucose-stimulated insulin secretion at high glucose levels (25 mM). This resulted in a 1.52±0.04-fold (n=3) increase in insulin secretion with 20 μM GW9508 in the presence of 25 mM glucose, compared with 25 mM glucose alone (Figure 4a). In contrast, no effect of GW9508 on insulin release was observed at low glucose levels (5 mM). The magnitude of the potentiation of insulin secretion observed at high glucose following GPR40 activation was similar to that reported in MIN6 cells and in islets following the activation of GLP-1 receptor (Parkes et al., 2001; Tsuboi et al., 2003). This enhancement of glucose-stimulated insulin secretion by GW9508 was completely inhibited by the selective GPR40 antagonist GW1100 (1 μM), demonstrating that the action of GW9508 was indeed mediated through the GPR40 receptor and not from activation of GPR120 (Figure 4b). A more comprehensive study examining the effect of GW9508 at multiple glucose concentrations clearly demonstrated the glucose-dependency of the GW9508 response. The ability of GW9508 (10 μM) to enhance insulin secretion from MIN6 cells was significantly enhanced as glucose concentrations were increased (Figure 4c). Thus, in this system the GPR40 agonist GW9508 acts as a glucose-dependent insulin secretagogue.
Figure 4.
Effect of GW9508 and GW1100 on insulin secretion from the MIN6 mouse insulinoma cell line. (a) GW9508 produced a concentration-dependent increase in glucose-stimulated insulin secretion in the presence of 25 mM glucose (pEC50=6.14±0.03; **P<0.01 vs 25 mM glucose Control; n=3). No effect of GW9508 was observed in the presence of 5 mM glucose. (b) The effect of GW9508 on insulin secretion in the presence of 25 mM glucose was significantly reduced in the presence of 1 μM GW1100 (**P<0.01 vs GW9508 alone; n=3). (c) Glucose dependency of GW9508 effect on insulin secretion. Insulin secretion was significantly enhanced in the presence of GW9508 (10 μM) at higher glucose concentrations compared with lower glucose concentrations (**P<0.01; ***P<0.001; n=3). (d) Linoleic acid produced a concentration-dependent increase in glucose-stimulated insulin secretion in the presence of 25 mM glucose (*P<0.05; **P<0.01 vs 25 mM glucose Control; n=4). A significantly smaller increase in insulin secretion was observed for linoleic acid in the presence of 5 mM glucose. (e) The effect of linoleic acid on insulin secretion in the presence of 25 mM glucose was significantly reduced in the presence of 1 μM GW1100 (**P<0.01; ***P<0.001 vs linoleic acid alone; n=4). (f) GW9508 and linoleic acid effects on glucose-stimulated (25 mM) insulin secretion are additive (**P<0.01; ***P<0.001 vs linoleic acid alone; n=4). Data represent the mean±s.e.m.
Consistent with previous studies, linoleic acid produced a concentration-dependent increase in glucose-stimulated (25 mM) insulin secretion in the MIN6 cells (Figure 4d). The response was bell-shaped in nature with a maximum response occurring at around 44 μM. Accurate pEC50 values could not be determined due to the form of the response, however, a pEC50 value of approximately 4.50 (n=4) was determined. Addition of linoleic acid (44 μM) resulted in a 3.34±0.32 (n=4) fold increase in insulin secretion in the presence of 25 mM glucose compared with 25 mM glucose alone. This response was significantly larger than that described above for GW9508. Linoleic acid also produced a small increase in glucose-stimulated insulin secretion in the presence of 5 mM glucose compared with the 5 mM glucose controls. The increases observed were only significant at the higher linoleic acid concentrations of 44 μM (P<0.001) and 67 μM (P<0.001). The enhancement of glucose-stimulated insulin secretion by linoleic acid was significantly but only partially inhibited by the selective GPR40 antagonist GW1100 (1 μM) (Figure 4e). These data demonstrate that this action of linoleic acid was indeed mediated, at least in part, by the GPR40 receptor.
Combination studies were performed to examine whether the effects of GW9508 and linoleic acid on glucose-stimulated insulin secretion would be additive (Figure 4f). The addition of GW9508 (10 μM) to a concentration–response curve for linoleic acid produced a leftward shift and slight increase in the slope of the curve suggesting that linoleic acid and GW9508 are additive, if not mildly synergistic, in their action. No change in the maximum response was observed upon addition of GW9508, however, the asymptote of the linoleic acid curve was also shifted to the left in the presence of GW9508 (44 μM in the absence vs 20 μM in the presence of GW9508).
Effect of GPR40 activation on KCl-stimulated insulin secretion from MIN6 cells
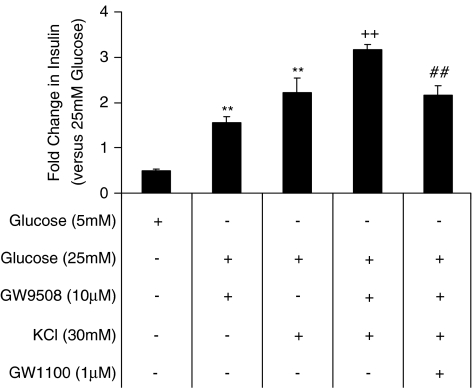
A recent report suggested that free palmitate was able to augment insulin secretion at the level of exocytosis through augmentation of L-type Ca2+ channel activity and an increase in the size of the readily releasable pool (RRP) of granules (Olofsson et al., 2004). Furthermore, Itoh et al. (2003) demonstrated that blockage of the L-type Ca2+ channel with nifedipine prevented the ability of linoleic acid to stimulate insulin secretion from MIN6 cells. The mechanism underlying the effect of GW9508 on insulin secretion in MIN6 cells was investigated in the presence or absence of diazoxide, to prevent closure of the K+ATP channel, or in the presence or absence of KCl, a depolarizing stimulus. In the presence of diazoxide (80 μM) and 25 mM glucose GW9508 was unable to elevate insulin release above that observed at 5 mM glucose (data not shown). Attempts to study the KATP+-independent nature of the GW9508 response were hampered by the observed potentiation of insulin secretion in the presence of 80 μM diazoxide and 30 mM KCl at 25 mM glucose. In the presence of 30 mM KCl and 25 mM glucose, conditions under which the cell would be depolarized and the L-type Ca2+ channels would be open, GW9508 significantly enhanced the insulin release compared to that produced by KCl alone (Figure 5) (from 37.7±7.6 ng 100 μl−1 to 51.9±12.2 ng 100 μl−1 insulin in the presence of 25 mM glucose; P<0.01; n=3). In the presence of the GPR40 antagonist GW1100, the enhancement of KCl-stimulated insulin release by GW9508 was prevented (Figure 5).
Figure 5.
Effect of KCl on GW9508-potentiatiation of insulin secretion. Potentiation of KCl-stimulated insulin secretion in the presence of GW9508 at 25 mM glucose. In the presence of 25 mM glucose, insulin release was significantly potentiated by either 10 μM GW9508 or 30 mM KCl **P<0.01 vs 25 mM glucose-treated MIN6 cells;n=3). The presence of GW9508 (10 μM) significantly enhanced KCl or GW9508-stimulated insulin secretion in the presence of 25 mM glucose (++P<0.01 vs KCl or GW9508-treated MIN6 cells; n=3). Addition of the antagonist GW1100 (1 μM) prevented the effect of GW9508 on KCl or GW9508-mediated insulin release (##P<0.01 vs GW9508 and KCl-treated MIN6 cells; n=3). Data represent mean±s.e.m. Analysis of results was by ANOVA, followed by Tukey–Kramer multiple comparison test.
Discussion
In the present study, we describe the pharmacology of the small-molecule GW9508. Although GW9508 is active as an agonist at both GPR40 and GPR120, it is approximately 100-fold selective for GPR40 with respect to GPR120. In addition, the small-molecule GW1100 inhibited the Ca2+ elevations stimulated by GW9508 mediated by GPR40, but not those mediated via GPR120, demonstrating that GW1100 was a selective antagonist of the GPR40 receptor. The finding that GW9508 dose dependently stimulated insulin secretion in a glucose-sensitive manner in MIN6 cells suggested that activation of GPR40 or GPR120 resulted in glucose-sensitive insulin secretion. The fact that GW1100 blocked this action of GW9508 is indicative that the observed effect of GW9508 is mediated via GPR40, further confirming the hypothesis that GPR40 may play a role in the priming effects of fatty acids on insulin secretion. We were unable, however, to observe potentiation of glucose-stimulated insulin secretion in isolated rat or mouse islets following incubation with GW9508. The reason for the discrepancy between results from MIN6 cells and islets is unclear, however, primary islet experiments were performed in 0.1% BSA, a level of protein which in preliminary experiments decreased the pEC50 of GW9508 for human GPR40 by approximately 1 log unit. In addition, we did not attempt to examine whether the compound mobilized calcium in isolated islets. It is therefore unclear why such a discrepancy exists, however, physiological differences between the cell line and primary cells could be contributory factors.
Although linoleic acid was less potent than GW9508 in potentiating glucose-stimulated insulin release, it was more efficacious (Figure 4f), this could, in part be attributable to the finding that GW9508 only appeared to be a partial agonist at GPR40 with respect to elevation of Ca2+ in HEK-293 cells (Figure 2a). It is also possible that this finding may reflect the possibility that the fatty acid potentiates insulin secretion through multiple pathways. The cause of the bell-shaped curve for linoleic acid potentiation of insulin secretion is unclear (Figure 4d). One possibility could be that the high levels of fatty acids or GPR40 activity may trigger a feedback-loop to reduce insulin secretion. In support of this proposal, 1 mM palmitate was reported to inhibit Ca2+ currents in isolated mouse β-cells, whereas 0.5 mM palmitate had stimulatory effects (Olofsson et al., 2004). Although GW1100 completely prevented the potentiating effects of GW9508 on glucose-stimulated insulin release, only a partial reduction on the effect of linoleic acid was observed (Figure 4e), again supporting the possibility that more than one pathway may play a role in this response. We did not, however, investigate the effect of GW100 on fatty acid-mediated potentiation of insulin secretion in primary islets. It is probable that the ability of free fatty acids to directly potentiate insulin secretion are due to a combination of mechanisms, including long chain CoA esters (Prentki et al., 1992), GPR40 and conceivably other fatty acid-binding GPCRs. Notably, a complete ablation of fatty acid-stimulated insulin secretion or elevation in [Ca2+]i has been reported following knockdown of GPR40 mRNA expression in MIN6, Ins-1E cells or islets (Itoh et al., 2003; Salehi et al., 2005; Shapiro et al., 2005). It is unclear therefore to what extent genetic ablation of GPR40 expression or small-molecule modulation of receptor activity is reflective of the extent that GPR40 plays in the fatty acid effect on insulin secretion.
In many individuals with impaired glucose tolerance or type 2 diabetes, a compounding factor is the elevation in circulating fatty acids. The ability of a GPR40 agonist to potentiate insulin secretion in the presence of elevated free fatty acids was addressed in MIN6 cells. Addition of GW9508 (10 μM) produced a leftward shift of the asymptote of the linoleic acid curve from 44 to 20 μM without altering the maximal response, suggesting that GW9508 and linoleic acid may have additive effects on insulin secretion under certain situations where fatty acids may be raised (Figure 4f). Notably the bell-shaped nature of the curve observed with linoleic acid alone was maintained and left shifted in the presence of GW9508.
The mechanism behind the potentiation of insulin release by fatty acids remains controversial. Despite evidence for the involvement of long chain CoA esters or diacylglyerol (Corkey et al., 1989; Prentki et al., 1992), a number of reports demonstrate that free fatty acids are able to potentiate insulin secretion, secondary to an increase in cytoplasmic Ca2+ (Warnotte et al., 1994; Remizov et al., 2003; Olofsson et al., 2004). A recent report suggests that free palmitate increases insulin secretion by a K+ATP-channel-independent mechanism (Olofsson et al., 2004). Olofsson et al. (2004) demonstrated that 500 μM palmitate was able to potentiate whole-cell peak Ca2+ currents stimulated by 30 mM KCl and that this effect appeared to be specific to L-type Ca2+ channels. The authors also demonstrated that 500 μM palmitate elevated exocytosis and increased the size of the RRP pool. Moreover, the effects of palmitate on Ca2+ currents and exocytosis were not mimicked by palmitoyl-CoA. Notably, Feng et al. (2006), recently reported that the increase in [Ca2+]i and insulin secretion stimulated by linoleic acid in rat β-cells was mediated via GPR40-elevation of cAMP leading to reduction in voltage-gated K+ currents, thus delaying membrane repolarization and enhancing Ca2+ influx. A recent study in Ins-1E cells supports the idea that GPR40 activated by palmitic acid results in an increase in [Ca2+]i and insulin secretion, mediated via activation of phospholipase C (PLC) resulting in the release of calcium from the endoplasmic reticulum and upregulation of calcium influx via L-type calcium channels preactivated by glucose ((Fujiwara et al., 2005; Shapiro et al., 2005). Similarly, oleic acid resulted in an elevation of intracellular calcium in rat islet β-cells through a PLC and L-type Ca2+ channel-mediated pathway (Fujiwara et al., 2005).
GW9508 was unable to potentiate insulin release in the presence of diazoxide (data not shown) in line with observations of Shapiro et al. (2005) who showed that palmitic acid required cell depolarization and opening of L-type Ca2+ channels for its effects on insulin secretion. Efforts to demonstrate KATP+-independent effects of GW9508 on insulin secretion by depolarizing the cells with 80 μM diazoxide in combination with 30 mM KCl were hampered by the unexpected potentiation of KCl-induced insulin secretion caused by diazoxide alone such that GW9508 was not able to further potentiate insulin release (mean fold-change±s.e.m. in insulin release vs KCl (30 mM) in the presence of 25 mM glucose; diazoxide (80 μM) and KCl 1.34±0.1, GW9508 (10 μM) and KCl 1.39±0.13, diazoxide and GW9508 and KCl 1.42±0.19 (n=3)). Sulfonylureas have been reported to exert a K+ATP-channel independent augmentation of insulin secretion in MIN6 cells, through a mechanism involving inhibition of CPT-1 and PKC activation (Lehtihet et al., 2003). It is possible that in MIN6 cells that diazoxide and GPR40 may both potentiate insulin secretion through pathways converging on PKC. This may explain the inability of GW9508 to further potentiate insulin secretion in the presence of 25 mM glucose, KCl and diazoxide. Moreover, the ability of the GPR40 agonist to potentiate KCl-stimulated insulin secretion (Figure 5) supports a model whereby free fatty acids, acting through GPR40, may augment exocytosis of insulin granules through augmentation of Ca2+ channel activity and an increase in the size of the RRP.
The relationship between the well-documented detrimental chronic effects of fatty acids on pancreatic islets and GPR40 is under debate. Although currently it is thought that the loss of β-cell function caused by exposure to fatty acids may be mediated through ceramide formation and subsequent increase in iNOS activity (Unger & Orci, 2002), chronic effects of GPR40 activation by a small-molecule remain to be investigated. The recent report from Steneberg et al. (2005) suggests that although GPR40 deficient mice demonstrate a reduced first-phase insulin response in a glucose-tolerance test, they are resistant to the development of hepatic steatosis, hypertriglyceridemia, hyperglycaemia and glucose intolerance on a 58% fat diet. Moreover, mice overexpressing GPR40 in islet β-cells, led to impairment of β-cell function and diabetes. The authors suggest that GPR40 mediates both the acute and chronic effects of free fatty acids on islets and that a GPR40 antagonist may be useful for type 2 diabetes.
Our data support the idea that acute activation of GPR40 results in potentiation of glucose-mediated insulin secretion. The use of the GPR40 agonist in vivo may help to determine whether agonist modulation of GPR40 would be beneficial in the treatment of type 2 diabetes.
Acknowledgments
We gratefully acknowledge Mandy Bergquist for assistance with statistical analysis of data and James Weiel for assistance with the figures presented.
Abbreviations
- DMEM
Dulbecco's modified Eagles medium
- GPCRs
G protein-coupled receptors
- MOI
multiplicity of infection
- PPAR
peroxisome proliferator-activated receptor
- PLC
phospholipase C
- RRP
readily releasable pool
- TZDs
thiazolidinediones
References
- AMES R., NUTHULAGANTI P., FORNWALD J., SHABON U., VAN DER KEYL H., ELSHOURBAGY N. Heterologous expression of G protein-coupled receptors in U-2 OS osteosarcoma cells. Receptors Channels. 2004;10:117–124. doi: 10.1080/10606820490515012. [DOI] [PubMed] [Google Scholar]
- BLACK J.W., LEFF P. Operational models of pharmacological agonism. Proc. Roy. Soc. London – Ser. B: Biol. Sci. 1983;220:141–162. doi: 10.1098/rspb.1983.0093. [DOI] [PubMed] [Google Scholar]
- BRISCOE C.P., TADAYYON M., ANDREWS J.L., BENSON W.G., CHAMBERS J.K., EILERT M.M., ELLIS C., ELSHOURBAGY N.A., GOETZ A.S., MINNICK D.T., MURDOCK P.R., SAULS H.R., SHABON U., SPINAGE L.D., STRUM J.C., SZEKERES P.G., TAN K.B., WAY J.M., IGNAR D.M., WILSON S., MUIR A.I. The orphan G protein-coupled receptor GPR40 is activated by medium and long chain fatty acids. J. Biol. Chem. 2003;278:11303–11311. doi: 10.1074/jbc.M211495200. [DOI] [PubMed] [Google Scholar]
- BROWN A.J., GOLDSWORTHY S.M., BARNES A.A., EILERT M.M., TCHEANG L., DANIELS D., MUIR A.I., WIGGLESWORTH M.J., KINGHORN I., FRASER N.J., PIKE N.B., STRUM J.C., STEPLEWSKI K.M., MURDOCK P.R., HOLDER J.C., MARSHALL F.H., SZEKERES P.G., WILSON S., IGNAR D.M., FOORD S.M., WISE A., DOWELL S.J. The orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J. Biol. Chem. 2003;278:11312–11319. doi: 10.1074/jbc.M211609200. [DOI] [PubMed] [Google Scholar]
- BROWN A.J., JUPE S., BRISCOE C.P. A family of fatty acid binding receptors. DNA Cell. Biol. 2005;24:54–61. doi: 10.1089/dna.2005.24.54. [DOI] [PubMed] [Google Scholar]
- CORKEY B.E., GLENNON M.C., CHEN K.S., DEENEY J.T., MATSCHINSKY F.M., PRENTKI M. A role for malonyl-CoA in glucose-stimulated insulin secretion from clonal pancreatic beta-cells. J. Biol. Chem. 1989;264:21608–21612. [PubMed] [Google Scholar]
- DOBBINS R.L., CHESTER M.W., DANIELS M.B., MCGARRY J.D., STEIN D.T. Circulating fatty acids are essential for efficient glucose-stimulated insulin secretion after prolonged fasting in humans. Diabetes. 1998a;47:1613–1618. doi: 10.2337/diabetes.47.10.1613. [DOI] [PubMed] [Google Scholar]
- DOBBINS R.L., CHESTER M.W., STEVENSON B.E., DANIELS M.B., STEIN D.T., MCGARRY J.D. A fatty acid- dependent step is critically important for both glucose- and non-glucose-stimulated insulin secretion. J. Clin. Invest. 1998b;101:2370–2376. doi: 10.1172/JCI1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EHLERT F.J. Estimation of the affinities of allosteric ligands using radioligand binding and pharmacological null methods. Mol. Pharmacol. 1988;33:187–194. [PubMed] [Google Scholar]
- ELBEIN S.C., HASSTEDT S.J. Quantitative trait linkage analysis of lipid-related traits in familial type 2 diabetes – evidence for linkage of triglyceride levels to chromosome 19q. Diabetes. 2002;51:528–535. doi: 10.2337/diabetes.51.2.528. [DOI] [PubMed] [Google Scholar]
- FENG D.D., LUO Z., ROH S., HERNANDEZ M., TAWADROS N., KEATING D.J., CHEN C. Reduction in voltage-gated K+ currents in primary cultured rat pancreatic β-cells by linoleic acids. Endocrinology. 2006;147:674–682. doi: 10.1210/en.2005-0225. [DOI] [PubMed] [Google Scholar]
- FUJIWARA K., MAEKAWA F., YADA T. Oleic acid interacts with GPR40 to induce Ca2+ signaling in rat islet beta-cells: mediation by PLC and L-type Ca2+ channel and link to insulin release. Am. J. Physiol. – Endocrinol. Metab. 2005;289:E670–E677. doi: 10.1152/ajpendo.00035.2005. [DOI] [PubMed] [Google Scholar]
- GOTTLICHER M., WIDMARK E., LI Q., GUSTAFSSON J.A. Fatty acids activate a chimera of the clofibric acid-activated receptor and the glucocorticoid receptor. Proc. Natl. Acad. Sci. USA. 1992;89:4653–4657. doi: 10.1073/pnas.89.10.4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRAVENA C., MATHIAS P.C., ASHCROFT S.J. Acute effects of fatty acids on insulin secretion from rat and human islets of Langerhans. J. Endocrinol. 2002;173:73–80. doi: 10.1677/joe.0.1730073. [DOI] [PubMed] [Google Scholar]
- HAGA H., YAMADA R., OHNISHI Y., NAKAMURA Y., TANAKA T. Gene-based SNP discovery as part of the Japanese Millennium Genome Project: identification of 190562 genetic variations in the human genome. J. Hum. Genet. 2002;47:605–610. doi: 10.1007/s100380200092. [DOI] [PubMed] [Google Scholar]
- HAMID Y.H., VISSING H., HOLST B., URHAMMER S.A., PYKE C., HANSEN S.K., GLUMER C., BORCH-JOHNSEN K., JORGENSEN T., SCHWARTZ T.W., PEDERSEN O., HANSEN T. Studies of relationships between variation of the human G protein-coupled receptor 40 Gene and Type 2 diabetes and insulin release. Diabet. Med. 2005;22:74–80. doi: 10.1111/j.1464-5491.2005.01505.x. [DOI] [PubMed] [Google Scholar]
- HIRASAWA A., TSUMAYA K., AWAJI T., KATSUMA S., ADACHI T., YAMADA M., SUGIMOTO Y., MIYAZAKI S., TSUJIMOTO G. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat. Med. 2005;11:90–94. doi: 10.1038/nm1168. [DOI] [PubMed] [Google Scholar]
- ITOH Y., KAWAMATA Y., HARADA M., KOBAYASHI M., FUJII R., FUKUSUMI S., OGI K., HOSOYA M., TANAKA Y., UEJIMA H., TANAKA H., MARUYAMA M., SATOH R., OKUBO S., KIZAWA H., KOMATSU H., MATSUMURA F., NOGUCHI Y., SHINOBARA T., HINUMA S., FUJISAWA Y., FUJINO M. Free fatty acids regulate insulin secretion from pancreatic beta cells through GPR40. Nature. 2003;422:173–176. doi: 10.1038/nature01478. [DOI] [PubMed] [Google Scholar]
- KOTARSKY K., NILSSON N.E., FLODGREN E., OWMAN C., OLDE B. A human cell surface receptor activated by free fatty acids and thiazolidinedione drugs. Biochem. Biophys. Res. Commun. 2003;301:406–410. doi: 10.1016/s0006-291x(02)03064-4. [DOI] [PubMed] [Google Scholar]
- LE POUL E., LOISON C., STRUYF S., SPRINGAEL J.Y., LANNOY V., DECOBECQ M.E., BREZILLON S., DUPRIEZ V., VASSART G., VAN DAMME J., PARMENTIER M., DETHEUX M. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J. Biol. Chem. 2003;278:25481–25489. doi: 10.1074/jbc.M301403200. [DOI] [PubMed] [Google Scholar]
- LEHTIHET M., WELSH N., BERGGREN P.O., COOK G.A., SJOHOLM A. Glibenclamide inhibits islet carnitine palmitoyltransferase 1 activity, leading to PKC-dependent insulin exocytosis. Am. J. Physiol. – Endocrinol. Metab. 2003;285:E438–E446. doi: 10.1152/ajpendo.00057.2003. [DOI] [PubMed] [Google Scholar]
- LUCKOW V.A., LEE S.C., BARRY G.F., OLINS P.O. Efficient generation of infectious recombinant baculoviruses by site-specific transposon-mediated insertion of foreign genes into a baculovirus genome propagated in Escherichia coli. J. Virol. 1993;67:4566–4579. doi: 10.1128/jvi.67.8.4566-4579.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIYAZAKI J., ARAKI K., YAMATO E., IKEGAMI H., ASANO T., SHIBASAKI Y., OKA Y., YAMAMURA K. Establishment of a pancreatic beta cell line that retains glucose-inducible insulin secretion: special reference to expression of glucose transporter isoforms. Endocrinology. 1990;127:126–132. doi: 10.1210/endo-127-1-126. [DOI] [PubMed] [Google Scholar]
- NILSSON N.E., KOTARSKY K., OWMAN C., OLDE B. Identification of a free fatty acid receptor, FFA(2)R, expressed on leukocytes and activated by short-chain fatty acids. Biochem. Biophys. Res. Commun. 2003;303:1047–1052. doi: 10.1016/s0006-291x(03)00488-1. [DOI] [PubMed] [Google Scholar]
- O'REILLY D.R., MILLER L.K., LUCKOW V.A. A Laboratory Manual. New York: Oxford University Press; 1992. [Google Scholar]
- OGAWA T., HIROSE H., MIYASHITA K., SAITO I., SARUTA T. GPR40 gene Arg211His polymorphism may contribute to the variation of insulin secretory capacity in Japanese men. Metab.: Clin. Exp. 2005;54:296–299. doi: 10.1016/j.metabol.2004.09.008. [DOI] [PubMed] [Google Scholar]
- OLOFSSON C.S., SALEHI A., HOLM C., RORSMAN P. Palmitate increases L-type Ca2+ currents and the size of the readily releasable granule pool in mouse pancreatic beta-cells. J. Physiol. (London) 2004;557:935–948. doi: 10.1113/jphysiol.2004.066258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARKES D.G., PITTNER R., JODKA C., SMITH P., YOUNG A. Insulinotropic actions of exendin-4 and glucagon-like peptide-1 in vivo and in vitro. Metab.: Clin. Exp. 2001;50:583–589. doi: 10.1053/meta.2001.22519. [DOI] [PubMed] [Google Scholar]
- PRENTKI M., TORNHEIM K., CORKEY B.E. Signal transduction mechanisms in nutrient-induced insulin secretion. Diabetologia. 1997;40 Suppl 2:S32–S41. doi: 10.1007/s001250051395. [DOI] [PubMed] [Google Scholar]
- PRENTKI M., VISCHER S., GLENNON M.C., REGAZZI R., DEENEY J.T., CORKEY B.E. Malonyl-CoA and long chain acyl-CoA esters as metabolic coupling factors in nutrient-induced insulin secretion. J. Biol. Chem. 1992;267:5802–5810. [PubMed] [Google Scholar]
- REMIZOV O., JAKUBOV R., DUFER M., KRIPPEIT D.P., DREWS G., WARING M., BRABANT G., WIENBERGEN A., RUSTENBECK I., SCHOFL C. Palmitate-induced Ca2+-signaling in pancreatic beta-cells. Mol. Cell. Endocrinol. 2003;212:1–9. doi: 10.1016/j.mce.2003.09.026. [DOI] [PubMed] [Google Scholar]
- SALEHI A., FODGREN E., NILSSON N.E., JIMENEZ-FELTSTROM J., MIYAZAKI J., OWMAN C., OLDE B. Free fatty acid receptor 1 (FFA(1)R/GPR40) and its involvement in fatty-acid-stimulated insulin secretion. Cell Tissue Res. 2005;322:207–215. doi: 10.1007/s00441-005-0017-z. [DOI] [PubMed] [Google Scholar]
- SAWZDARGO M., GEORGE S.R., NGUYEN T., XU S.J., KOLAKOWSKI L.F., ODOWD B.F. A cluster of four novel human g protein-coupled receptor genes occurring in close proximity to cd22 gene on chromosome 19q13.1. Biochem. Biophys. Res. Commun. 1997;239:543–547. doi: 10.1006/bbrc.1997.7513. [DOI] [PubMed] [Google Scholar]
- SHAPIRO H., SHACHAR S., SEKLER I., HERSHFINKEL M., WALKER M.D. Role of GPR40 in fatty acid action on the beta cell line INS-1E. Biochem. Biophys. Res. Commun. 2005;335:97–104. doi: 10.1016/j.bbrc.2005.07.042. [DOI] [PubMed] [Google Scholar]
- STEIN D.T., STEVENSON B.E., CHESTER M.W., BASIT M., DANIELS M.B., TURLEY S.D., MCGARRY J.D. The insulinotropic potency of fatty acids is influenced profoundly by their chain length and degree of saturation. J. Clin. Invest. 1997;100:398–403. doi: 10.1172/JCI119546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STENEBERG P., RUBINS N., BARTOOV-SHIFMAN R., WALKER M.D., EDLUND H. The FFA receptor GPR40 links hyperinsulinemia, hepatic steatosis, and impaired glucose homeostasis in mouse. Cell Metab. 2005;1:245–258. doi: 10.1016/j.cmet.2005.03.007. [DOI] [PubMed] [Google Scholar]
- TSUBOI T., XAVIER G.D., HOLZ G.G., JOUAVILLE L.S., THOMAS A.P., RUTTER G.A. Glucagon- like peptide-1 mobilizes intracellular Ca2+ and stimulates mitochondrial ATP synthesis in pancreatic MIN6 beta-cells. Biochem. J. 2003;369:287–299. doi: 10.1042/BJ20021288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNGER R.H., ORCI L. Lipoapoptosis: its mechanism and its diseases. Biochim. Biophys. Acta. 2002;1585:202–212. doi: 10.1016/s1388-1981(02)00342-6. [DOI] [PubMed] [Google Scholar]
- WARNOTTE C., GILON P., NENQUIN M., HENQUIN J.C. Mechanisms of the stimulation of insulin release by saturated fatty acids. A study of palmitate effects in mouse beta-cells. Diabetes. 1994;43:703–711. doi: 10.2337/diab.43.5.703. [DOI] [PubMed] [Google Scholar]