Abstract
The aim of this study was to assess the effects of treatment with isoproterenol (ISO, 0.3 mg kg−1 day−1, s.c.) for 7 days on the vascular reactivity of rat-isolated aortic rings. Additionally, potential mechanisms underlying the changes that involved the endothelial modulation of contractility were investigated.
Treatment with ISO induced cardiac hypertrophy without changes in haemodynamic parameters. Aortic rings from ISO-treated rats showed an increase in the contraction response to phenylephrine (PHE) and serotonin, but did not change relaxations produced by acetylcholine or isoproterenol. Removal of the endothelium increased the responses to PHE in both groups. However, this procedure was less effective in ISO-treated as compared with control rats. Endothelial cell removal abolished the increase in the response to PHE in ISO-treated rats. The presence of Nω-nitro-L-arginine methyl ester shifted the concentration–response curve to PHE to the left in both groups of rats. However, this effect was more pronounced in the ISO group. In addition, aminoguanidine (50 μM) potentiated the actions of PHE only in the ISO group. ISO treatment increased nitric oxide synthase (NOS) activity and neuronal NOS and endothelial NOS protein expression in the aorta.
Neither losartan (10 μM) nor indomethacin (10 μM) abolished the effects of ISO on the actions of PHE. Superoxide dismutase (SOD, 150 U ml−1) and L-arginine (5 mM), but neither catalase (300 U ml−1) nor apocynin (100 μM), blocked the effect of ISO treatment. In addition, we observed an increase in superoxide anion levels as measured by ethidium bromide fluorescence and of copper and zinc superoxide dismutase protein expression in ISO-treated rats.
In conclusion, our data suggest that ISO treatment alters the endothelial cell-mediated modulation of the contraction to PHE in rat aorta. The increased maximal response of PHE seems to be due to an increase in superoxide anion generation, which inactivates some of the basal NO produced and counteracts NO-mediated negative modulation even in the presence of high NO production and antioxidant defence.
Keywords: Isoproterenol, β-adrenoceptor, vascular reactivity, endothelium, nitric oxide, superoxide anions
Introduction
An increased sympathetic tone resulting in sustained stimulation of adrenoceptors has been associated with cardiovascular diseases, such as essential hypertension, cardiac hypertrophy and heart failure (Bristow, 2000; Esler & Kaye, 2000). In these situations, it is believed that elevated levels of circulating and tissue catecholamines produce chronic stimulation of cardiac β-adrenoceptors that might result in desensitisation, downregulation and signalling abnormalities in cardiac myocytes (Castellano & Bohm, 1997; Badenhorst et al., 2003). However, little is known about the effects of chronic stimulation of β-adrenoceptors on vascular function and its endothelial modulation.
In smooth muscle cells, β-adrenoceptor agonists, such as isoproterenol (ISO), promote vasodilatation by activation of an adenylyl cyclase-cAMP-PKA pathway that causes hyperpolarisation and decreases the sensitivity of myofilaments to Ca2+ (Rembold & Chen, 1998). In addition, activation of β-adrenoceptors on the endothelium produces vasodilatation (Gray & Marshall, 1992; Brawley et al., 2000) that is mediated by the synthesis of nitric oxide (NO) (Ferro et al., 1999). It is possible that chronic β-adrenergic stimulation could induce adaptive alterations of vascular β-adrenoceptor signalling pathways resulting in endothelial dysfunction, impaired peripheral vasodilatation and/or inappropriate vasoconstriction.
Treatment with ISO for several days is used as a model for prolonged β-adrenoceptor stimulation. This treatment results in ventricular hypertrophy (Rona et al., 1959; Taylor & Tang, 1984), increased ventricular collagen content (Benjamin et al., 1989) and a reduced inotropic response to ISO (Chang et al., 1982; Kenakin & Ferris, 1983; Vassallo et al., 1988).
However, the effects of chronic β-adrenergic stimulation on vascular function have not been studied as extensively as those on the heart. Hayes et al. (1986) demonstrated that ISO infusion (400 μg kg−1 h−1) for 4 days attenuates β-adrenoceptor-mediated vasodilatation without altering the vasoconstriction produced by norepinephrine in the rat aorta and portal vein. In a subsequent study from that laboratory, Cohen & Schenck (1987), using the same treatment with ISO, reported that vascular β1-adrenoceptor subtype seems to be more susceptible to downregulation than are β2-adrenoceptors. However, the effects of the treatment with ISO upon endothelial function were not evaluated.
The purpose of the present study was to assess the effect of treatment for 7 days with ISO on the vasodilator responses to acetylcholine and ISO, and on the contractile response to PHE and serotonin. Additionally, we evaluated the potential role of the endothelium and of the vasoactive factors NO, cyclooxygenase (COX)-derived products, angiotensin II and superoxide anions on the response to phenylephrine (PHE).
Methods
Animals
Male Wistar rats (270–300 g) were obtained from colonies maintained at the Animal Quarters of the Programa de Pós-Graduação em Ciências Fisiológicas of the Universidade Federal do Espírito Santo. Rats were housed at a constant room temperature and light cycle (12 : 12 h light–dark), with free access to standard rat chow and tap water. All experimental procedures complied with the guidelines for biomedical research as stated by the Brazilian Societies of Experimental Biology. The rats were randomly assigned to one of the following groups: (1) ISO-treated rats (n=23) that received (−)-ISO (0.3 mg kg−1 day−1, s.c.), once daily, suspended in 0.1 ml of soy bean oil, for 7 days and (2) Control rats (n=24) that received only the vehicle (0.1 ml, s.c.) for 7 days.
Haemodynamic and morphologic index
On the 6th day of treatment, rats were anaesthetised with sodium pentobarbital (60 mg kg−1, i.p.) and the right carotid artery was cannulated to determine arterial pressure and heart rate. These measurements were obtained 24 h later in conscious animals. Animals were then anaesthetised with ether and killed by exsanguination. The heart and thoracic aorta were removed. The right and left ventricles of the heart were weighed, and normalised to body weight (mg g−1). This ratio was used as an index of ventricular hypertrophy.
Thoracic aortas were carefully dissected out and cleaned of connective tissue. For reactivity experiments, the aortas were divided into cylindrical segments 4 mm in length. For the analysis of nitric oxide synthase (NOS) activity and NOS and copper and zinc superoxide dismutase (Cu/Zn-SOD) protein expression, arteries were rapidly frozen in liquid nitrogen and kept at −70°C until the day of analysis. For the analysis of superoxide anion production thoracic aortic segments were first immersed in an embedding medium (TBS Tissue Freezing Medium) and then frozen and kept at −70°C until superoxide anion was measured.
Vascular reactivity experiments
Segments of thoracic aorta (4 mm in length), free of fat and connective tissue, were mounted in an isolated tissue chamber containing Krebs–Henseleit solution (in mM: NaCl 118; KCl 4.7; NaHCO3 25; CaCl2-2H2O 2.5; KH2PO4 1.2; MgSO4–7H2O 1.2; glucose 11 and ethylenediamine-tetraacetic acid (EDTA) 0.01), gassed with 95% O2 and 5% CO2, and maintained at a resting tension of 1 g at 37°C. Isometric tension was recorded using an isometric force transducer (GRASS FT03, RI, U.S.A.) connected to an acquisition system (MP100 Biopac Systems, Inc., Santa Barbara, CA, U.S.A.).
Experimental protocols
After a 45 min equilibration period, all aortic rings were initially exposed twice to 75 mM KCl, the first time to check their functional integrity and the second time to assess the maximal tension developed. Afterwards, endothelial integrity was tested by the action of acetylcholine (10 μM) in segments previously contracted with PHE (∼10 μM). A relaxation equal to or greater than 90% was considered as demonstrative of the functional integrity of the endothelium. After a washout period, increasing concentrations of PHE (0.1 nM–30 μM) or serotonin (10 nM–30 μM) were applied, and concentration–response curves to these contractile agonists were obtained. In other aortic rings previously contracted with PHE (∼10 μM), concentration–response curves to ISO (0.1 nM–3 μM) or acetylcholine (0.01 nM–100 μM) were obtained.
The influence of the endothelium on the response to PHE was investigated after its mechanical removal performed by rubbing the lumen with a needle. The absence of endothelium was confirmed by the inability of 10 μM acetylcholine to produce relaxation. The role of some local vasoactive factors on the PHE-elicited contractile response was investigated. In order to do this, the effects of the following drugs were evaluated: (1) the nonselective NOS inhibitor Nω-nitro-L-arginine methyl ester (L-NAME, 100 μM); (2) the inducible NO synthase (iNOS) inhibitor aminoguanidine (50 μM); (3) the COX inhibitor indomethacin (10 μM); (4) the angiotensin type 1 receptor (AT1)-receptor blocker losartan (10 μM); (5) the superoxide anion scavenger superoxide dismutase (SOD, 150 U ml−1); (6) the hydrogen peroxide scavenger catalase (300 U ml−1); (7) the NAD(P)H oxidase inhibitor apocynin (100 μM) and (8) the NO precursor L-arginine (5 mM). These drugs were added 30 min before the construction of the concentration–response curve to PHE.
NOS activity
NOS activity was measured by the [3H]L-citrulline assay method as previously described (McKee et al., 1994) with slight modifications for the aortic tissue. This assay is based on the biochemical conversion of L-arginine to L-citrulline by NOS. Isolated thoracic aortas with and without endothelium were suspended in hydroxyethylpiperazine ethanesulphonic acid (HEPES) buffer (in mM: HEPES 20, sucrose 320, DTT 1, EDTA 1, PMSF 1) and lysed with the aid of a sonifier (Thorton). The homogenates were centrifuged at 500 × g for 10 min at 4°C. Aliquots of the supernatant were incubated with hydroxyethylpiperazine ethanesulphonic acid buffer (30 mM HEPES, pH=7.4) containing 1 mM EDTA, 1.25 mM CaCl2, 4 μM FAD, 4 μM FMN, 25 μM 5,6,7,8-tetrahydrobiopterin (BH4), 1 mM NADPH, 10 μg ml−1 calmodulin, 120 nM L-arginine and 0.5 μCi [3H]L-arginine for 60 min at 37°C in a total volume of 200 μl. The reactions were stopped by the addition of 1 ml of a solution containing 20 mM HEPES, pH=5.5. These samples were applied to a 0.3 ml DOWEX 50WX8-400 (Na+ form) column and [3H]L-citrulline was eluted with 1 ml HEPES buffer (20 mM, pH 5.5). Citrulline, ionically neutral at pH 5.5, passes through the column completely. The radioactivity corresponding to [3H]L-citrulline in the eluate was measured by liquid scintillation counting. Protein was determined by the Bio-Rad protein assay.
Western blot analysis for eNOS, nNOS and iNOS and Cu/Zn-SOD
Proteins from homogenised aorta (100 μg) and prestained molecular sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) standards were separated by 7.5% (endothelial nitric oxide synthase (eNOS), neuronal nitric oxide synthase (nNOS) and inducible nitric oxide synthase (iNOS)) or 12% (Cu/Zn-SOD) SDS–PAGE. The proteins were then transferred to polyvinyl difluoride membranes overnight at 4°C by using a Mini Trans-Blot Cell system (Bio-Rad) containing 25 mM Tris, 190 mM glycine, 20% methanol and 0.05% SDS. Human endothelial cells, stimulated mouse macrophages and rat brain were used as the positive controls for eNOS, iNOS, and for Cu/Zn-SOD and nNOS, respectively.
The membrane was blocked for 60 min at room temperature in Tris-buffered solution (10 mM Tris, 100 mM NaCl and 0.1% Tween 20) with 5% powdered nonfat milk. Then, the membrane was incubated for 1 h at room temperature with a rabbit polyclonal antibody for eNOS (1 : 1000 dilution), or mouse monoclonal antibody for nNOS (1 : 1000 dilution), or iNOS (1 : 2000 dilution), or Cu/Zn-SOD (1 : 1000 dilution). After washing was completed, the membrane was incubated with a 1 : 1500 dilution of anti-mouse or anti-rabbit IgG antibody conjugated to horseradish peroxidase.
The membrane was thoroughly washed, and the immunocomplexes were detected by using an enhanced horseradish peroxidase-luminol chemiluminescence system (ECL Plus, Amersham International; Little Chalfont, U.K.) and subjected to autoradiography (Hyperfilm ECL, Amersham International). Signals on the immunoblot were quantified with the Scion Image software. The same membrane was used to determine α-actin protein expression using a monoclonal antibody anti α-actin (1 : 1500 dilution), and its content was used to normalize NOS and SOD protein expression in each sample.
Superoxide anion measurement in aorta
The oxidative fluorescent dye hydroethidine was used to evaluate the in situ production of superoxide anions as described previously (Hernanz et al., 2004). Hydroethidine permeates cells freely and, in the presence of superoxide anions, is oxidized to ethidium bromide, which is trapped by intercalation into DNA.
Transverse aortic sections (14 μm) were obtained on a cryostat from previously frozen aorta and collected on glass slides and equilibrated for 30 min at 37°C in Krebs–Henseleit buffer. Fresh buffer containing hydroethidine (2 μM) was topically applied to each tissue section and coverslipped. Slides were incubated in a light-protected, humidified chamber at 37°C for 30 min. Control sections received the same volume of Krebs–Henseleit buffer. Images were obtained with a Nikon E1000 microscope equipped for epifluorescence (excitation at 488 nm; emission 610 nm). Fluorescence was detected with a 585-nm long-pass filter.
Drugs and reagents
All chemicals used to prepare the Krebs–Henseleit solution (KHS) and losartan were purchased from Merck & Co., Inc. (Whitehouse Station, NJ, U.S.A.). (−)-Isoproterenol, (±)-isoproterenol hydrochloride, acetylcholine hydrochloride, phenylephrine hydrochloride, 5-hydroxytryptamine hydrochloride, L-NAME dihydrochloride, aminoguanidine hemisulphate, L-arginine, indomethacin, superoxide dismutase (bovine erythrocyte), catalase (bovine liver), apocynin, HEPES, EDTA, nicotinamide adenine dinucleotide phosphate (NADPH), riboflavin monophosphate (FMN), flavin adenine dinucleotide (FAD), tetrahydrobiopterin (BH4), dithiothreitol (DTT), phenylmethanesulphonyl fluoride (PMSF), were purchased from Sigma Chemical (St Louis, MO, U.S.A.). TBS Tissue Freezing Medium was from Triangle Biomedical Sciences (Durhan, NC, U.S.A.). [3H]L-Arginine was from NEN Products (Boston, MA, U.S.A.). Liquid scintillation Ultima Gold was from Packard (Meriden, CT, U.S.A.) and Tween 20, Tris, SDS and acrylamide from Bio-Rad (Hercules, CA, U.S.A.). Anti-NOS was purchased from Transduction Laboratories (Lexington, U.K.); anti-Cu/Zn-SOD and monoclonal antibody anti-α-actin from Sigma (Steinheim, Germany); anti-mouse or anti-rabbit IgG antibody conjugated to horseradish peroxidase from Bio-Rad (Hercules, CA, U.S.A.). Hydroethidine was from Polysciences Inc. (Warrington, PA, U.S.A.).
Data analysis
Results are presented as means±s.e.m. ‘n' denotes the number of animals used in each experiment. Vasoconstrictor responses to PHE and serotonin are expressed as a percentage of the contraction produced by 75 mM KCl for each ring. Relaxation responses to acetylcholine and ISO are expressed as the percentage of relaxation relative to the maximum contractile response. The maximum response (Emax values) and the negative log of the agonist concentrations producing 50% of maximum response (pD2 values) were estimated by an iterative nonlinear regression analysis of each individual concentration–response curve using GraphPad Prism Software (San Diego, CA, U.S.A.). To compare the effect of different drugs on the response to PHE in segments from control or ISO-treated rats, some results were expressed as ‘differences of area under the concentration–response curves' (dAUC) in control and experimental situations. AUCs were calculated from the individual concentration–response curves and the differences were expressed as a percentage of the AUC of the corresponding control. NOS activity results are presented as picomoles of citrulline per milligram protein per minute. Protein expression results are expressed as the ratio between signals on the immunoblot corresponding to each NOS isoform or Cu/Zn-SOD bands and the corresponding α-actin band.
The data were analysed statistically by Student's t-test and by one- or two-way analyses of variance (ANOVA) followed by Tukey's post hoc test. P<0.05 was considered significant.
Results
Blood pressure, heart rate and ventricular weight in control and ISO-treated rats
Treatment with ISO for 7 days resulted in hypertrophy of both right (control: 0.49±0.02 vs ISO-treated rats: 0.68±0.05 mg g−1, P<0.05) and left ventricles (control: 1.88±0.08 vs ISO-treated rats: 2.45±0.08 mg g−1, P<0.05). There were no statistically significant differences of body weight gain in either group. Control animals gained 3±1 g while ISO-treated rats gained 2±2 g (P>0.05). At the end of the treatment, no significant changes were observed for mean arterial pressure (control: 108±3 vs ISO-treated rats: 109±2 mmHg, P>0.05) or heart rate (control: 332±9 vs ISO-treated rats: 362±11 b.p.m., P>0.05).
Vascular reactivity in isolated aorta from control and ISO-treated rats
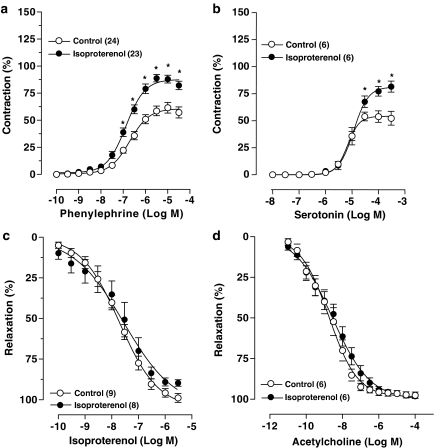
The contraction induced by 75 mM KCl was smaller in aortas from ISO-treated rats when compared to control rats (control: 2.19±0.07 vs ISO-treated rats: 1.85±0.07 g; P<0.05). In contrast, the Emax for PHE increased in rings obtained from rats treated with ISO. This occurred without significant changes in the pD2 for PHE (Figure 1a and Table 1). The increased Emax was also observed when the contraction was expressed in absolute values (control: 1.39±0.10 vs ISO-treated rats: 1.74±0.11 g, P<0.05). The contractile response to serotonin in the aortas from ISO-treated rats was also enhanced as compared to its action in aortas from control rats (Figure 1b and Table 1).
Figure 1.
Concentration–response curves to PHE (a), serotonin (b), isoproterenol (c) and acetylcholine (d) in endothelium-intact segments of thoracic aortas from control and isoproterenol-treated rats. Contraction responses are expressed as a percentage of the response to 75 mM KCl and relaxation responses are expressed as percentage of precontraction induced by PHE (∼1 μM). Number of rats is indicated in parentheses. The values are means±s.e.m. 2-way ANOVA; *P<0.05 vs Control.
Table 1.
Effects of the treatment with isoproterenol for 7 days on sensitivity (pD2) and maximal response (Emax) values to phenylephrine, serotonin, isoproterenol and acetylcholine in intact rat thoracic aortic rings
| |
Control |
Isoproterenol-treated rats |
||
|---|---|---|---|---|
| pD2 | Emax (%) | pD2 | Emax (%) | |
| Phenylephrine |
6.68±0.07 |
63.1±4.9 (24) |
6.68±0.08 |
90.9±3.4* (23) |
| Serotonin |
5.16±0.08 |
54.9±5.3 (6) |
4.94±0.08 |
82.8±4.3* (6) |
| Isoproterenol |
7.70±0.12 |
98.9±2.7 (9) |
7.49±0.21 |
88.2±2.3 (8) |
| Acetylcholine | 9.16±0.47 | 97.7±1.9 (6) | 9.01±0.38 | 98.6±1.5 (6) |
Values are mean±s.e.m.; number of animals is indicated in parentheses. Emax is expressed as a percentage of the response to 75 mM KCl. Student's t-test,
P<0.05 vs Control.
In aortic rings contracted with PHE, ISO produced a concentration-dependent relaxation in both control and ISO-treated rats. ISO treatment did not induce significant changes in Emax or sensitivity to ISO in isolated aortas (Figure 1c and Table 1). The endothelium-dependent relaxation to acetylcholine was similar between the two groups (Figure 1d and Table 1).
Effect of endothelium removal on the vasoconstrictor response to phenylephrine
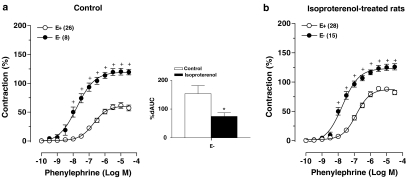
Removal of the endothelium enhanced Emax and pD2 values for PHE in aortas from control and ISO-treated rats (Figure 2a and b and Table 2). After endothelial denudation, the contraction to PHE was similar in both groups. As indicated by the comparison of dAUC values in rings without endothelium, the negative endothelial modulation in the PHE-induced contraction is impaired in arteries from the ISO-treated rats (Figure 2).
Figure 2.
Effects of endothelium removal (E−) on concentration–response curves to PHE in thoracic aortic rings from control (a) and isoproterenol-treated rats (b). Contraction responses are expressed as a percentage of the response to 75 mM KCl. Number of rats is indicated in parentheses. The values are means±s.e.m. 2-way ANOVA, +P<0.05 vs E+. Inset, dAUC to PHE in E− and E+ aortic rings from control and isoproterenol-treated rats; dAUC are expressed as a percentage of the corresponding AUC for E+ aortic rings. Student's t-test, *P<0.05 vs Control.
Table 2.
Pathways involved in the contraction induced by phenylephrine in thoracic aortic rings from control and rats treated with isoproterenol for 7 days
| |
Control |
Isoproterenol-treated rats |
||
|---|---|---|---|---|
| pD2 | Emax (%) | pD2 | Emax (%) | |
| E+ |
6.68±0.07 |
63.1±4.9 (26) |
6.86±0.08 |
90.9±3.4* (28) |
| E− |
7.76±0.13+ |
121.1±5.6+ (8) |
7.70±0.11+ |
126.8±5.2+ (15) |
| E+/L-NAME |
7.48±0.08+ |
134.7±12.9+ (8) |
8.69±0.21+* |
176.4±10.3+* (8) |
| E+/Aminoguanidine |
6.66±0.08 |
65.8±7.4 (12) |
7.22±0.08+ |
117.2±7.2+* (6) |
| E+/Indomethacin |
6.63±0.12 |
65.4±4.1 (10) |
6.81±0.12 |
95.8±5.7* (8) |
| E+/Losartan |
6.40±0.26 |
40.2±5.3+ (12) |
6.67±0.17 |
69.9±8.6+* (7) |
| E+/SOD |
6.26±0.07+ |
40.3±9.4+ (9) |
6.19±0.06+ |
46.6±7.6+ (8) |
| E+/Catalase |
6.16±0.08+ |
52.0±6.1 (7) |
6.31±0.07+ |
77.3±4.2* (7) |
| E+/Apocynin |
6.67±0.10 |
53.6±3.7 (7) |
6.74±0.11 |
82.9±3.0* (7) |
| E+/L-arginine | 6.47±0.11 | 72.0±8.6 (10) | 6.67±0.09 | 72.1±6.4+ (7) |
Values are mean±s.e.m.; number of animals is indicated in parentheses. E+, intact endothelium; E−, endothelial denudation; L-NAME, Nω-nitro-L-arginine methyl ester; SOD, superoxide dismutase; pD2, negative log of the agonist concentration producing 50% of maximum response, Emax, maximal response expressed as percentage of the response to 75 mM KCl. One-way ANOVA,
P<0.05 vs Control;
P<0.01 vs E+.
Evaluation of endothelial pathways involved in the vasoconstrictor response to PHE in aortic rings from ISO-treated rats
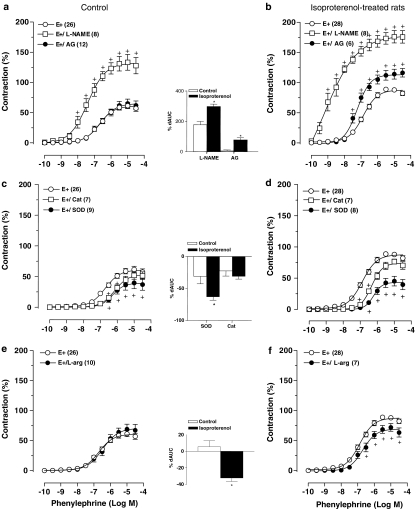
The nonselective NOS inhibitor L-NAME increased pD2 and Emax values for PHE in intact thoracic aortic rings from both groups (Figure 3a and b and Table 2). dAUCs (Figure 3a and b) showed higher values in arteries from ISO-treated rats than in those from the control group. At the concentration of L-NAME used in these experiments, the vasorelaxation produced by acetylcholine was abolished (data not shown).
Figure 3.
Effects of L-NAME (100 μM) or aminoguanidine (AG, 50 μM) (a and b) or SOD (150 U ml−1) or catalase (CAT, 300 U ml−1) (c and d) or L-arginine (L-arg, 5 mM) (e and f) on concentration–response curves to PHE in intact (E+) thoracic aortic rings from control and isoproterenol-treated rats. Contraction responses are expressed as a percentage of the response to 75 mM KCl. Number of rats is indicated in parentheses. The values are means±s.e.m. Two-way ANOVA, +P<0.05 vs E+. Inset, dAUC to PHE in E+/treatments and E+ aortic rings from control and isoproterenol-treated rats; dAUC are expressed as a percentage of the corresponding AUC for E+ aortic rings. Student's t-test, *P<0.05 vs Control.
In the presence of the selective iNOS inhibitor aminoguanidine, there was no significant alteration of the concentration–response curve for PHE in aortic rings from control animals (Figure 3a and Table 2). In contrast, aminoguanidine was able to shift to the left the concentration–response curves for PHE in aortic segments from ISO-treated animals, increasing the maximal response to this vasoconstrictor (Figure 3b and Table 2). At the concentration of aminoguanidine used, there was no significant inhibition of the vasorelaxation produced by acetylcholine (data not shown).
Indomethacin did not significantly modify the concentration–response curves to PHE in vascular rings obtained from both groups of rats (Table 2). Pretreatment with losartan reduced Emax for PHE in both groups of rats, but the Emax value for PHE was still higher in aorta from rats treated with ISO (Table 2).
SOD incubation was able to reduce the Emax and pD2 values for PHE in aortic rings from both control and ISO-treated rats (Figure 3c and d, and Table 2). However, in the presence of SOD, there was no significant difference between the concentration–response curves for PHE between both groups of rats (Table 2). In addition, there was a greater reduction in the dAUC for PHE in rings obtained from ISO-treated rats in the presence of SOD (Figure 3c and d).
On the other hand, in the presence of catalase, there was a reduction in pD2 values for PHE in both groups, with no changes in Emax (Figure 3c and d and Table 2). A comparison of dAUCs indicated that the effect of catalase was similar in aortic rings from control and ISO-treated rats (Figure 3c and d).
To investigate if NAD(P)H oxidase was a source of elevated superoxide anion production in ISO-treated rats, aortic rings with intact endothelium were incubated with apocynin. This inhibitor did not change significantly either Emax or pD2 for PHE in both groups of rats. Thus, the contractile response of the ISO group was still significantly higher than the response of the control group (Table 2).
L-Arginine did not induce significant changes in the concentration–response curve for PHE in aortic rings from control animals (Figure 3e and Table 2). In contrast, it was able to shift to the right the concentration–response curves for PHE in aortic segments from ISO-treated animals by reducing the Emax (Figure 3f and Table 2). In addition, there was a greater reduction in the dAUC for PHE in rings obtained from ISO-treated rats in the presence of L-arginine (Figure 3e and f).
It is important to emphasize that in all experiments in which enzyme inhibitors or receptor blockers were used, the basal tone of aortic rings obtained in both groups of rats did not change (results not shown).
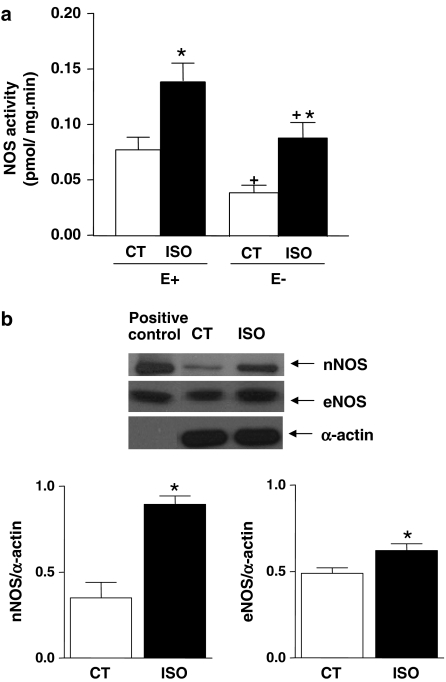
Activity and protein expression of NOS
As shown in the Figure 4a, NOS activity was increased in aortic segments with and without endothelium from ISO-treated rats. In agreement with this finding, we also observed an increase in the protein expression for the endothelial and neuronal isoforms of NOS (Figure 4b). We did not detect protein expression for the inducible isoform of NOS in aortas from either group of rats (data not shown).
Figure 4.
(a) NOS activity (pmol mg min−1) in isolated aortas from control (CT, n=7) and isoproterenol-treated rats (ISO, n=6) with (E+) and without (E−) endothelium. (b) Top: representative Western blot of nNOS and eNOS protein expression in thoracic aorta of control (CT) and ISO. Left lane, corresponding positive control for each protein (rat brain and human endothelial cells, respectively). Expression of α-actin is also shown as a loading control. Bottom: quantitative analysis of Western blot for eNOS and nNOS protein expression in thoracic aortas of control (CT, n=7) and isoproterenol-treated rats (ISO, n=7). Results (means±s.e.m.) are expressed as the ratio between signal for the NOS protein and signal for α-actin in the corresponding aorta. Student's t-test, *P<0.05 vs Control.
Superoxide anion generation and antioxidant defence mechanisms in isolated aorta from ISO-treated rats
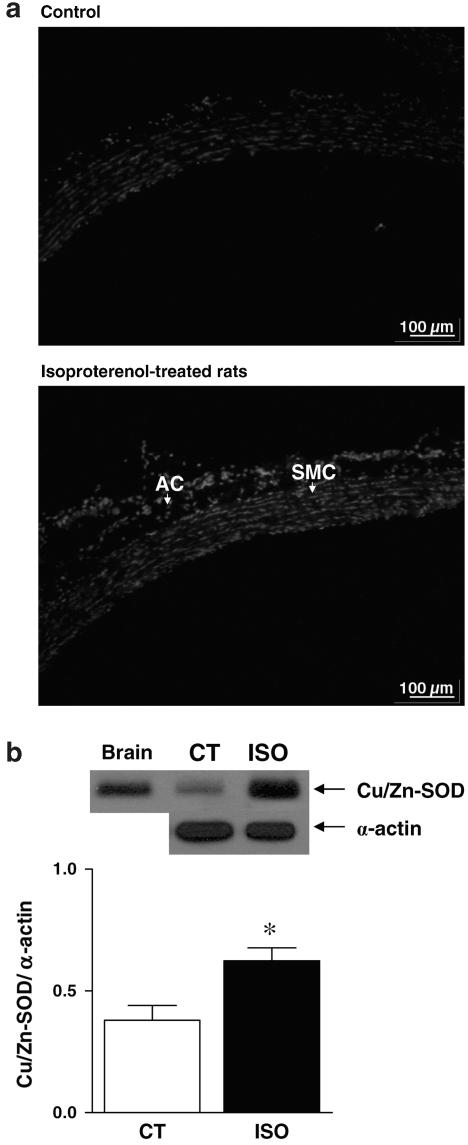
An increase of ethidium bromide fluorescence was observed in aortas from ISO-treated rats as compared with control rats, reflecting an increased superoxide anion generation induced by ISO treatment (Figure 5a). To assess antioxidant defense mechanisms in these vessels, we analysed the Cu/Zn-SOD protein expression. Cu/Zn-SOD protein expression was detected in arteries from both groups and its expression was higher in aortas from ISO-treated rats (Figure 5b).
Figure 5.
(a) Representative fluorescence photomicrographs of microscopic sections of thoracic aorta from control (a, n=3) and isoproterenol-treated rats (b, n=3). Vessels were labelled with the oxidative dye hydroethidine, which produces a red fluorescence when oxidized to ethidium bromide by superoxide anion. AC, adventitial cells; SMC, smooth muscle cells. (b) Representative Western blot (Top) and quantitative analysis (Bottom) for Cu/Zn-SOD protein expression in thoracic aortas of control (CT, n=4) and isoproterenol-treated rats (ISO, n=4). Results (means±s.e.m.) are expressed as the ratio between signal for the Cu/Zn-SOD protein and signal for α-actin in the corresponding aorta. Student's t-test, *P<0.05 vs Control.
Discussion
In the present study, we evaluated the effects of chronic β-adrenergic stimulation produced by seven daily injections of ISO on the vascular reactivity of isolated aortas. Isolated aortas obtained from treated animals had an increased reactivity to the vasoconstrictor actions of PHE and serotonin, but not to the relaxant actions of acetylcholine and ISO. Our results suggest that the changes in the reactivity to the vasoconstrictors are due to an alteration of endothelial modulation of the contractile responses associated with NO and superoxide anion imbalance.
ISO treatment produced right and left ventricular hypertrophy but no significant changes in heart rate, mean arterial pressure and body weight. Previous studies from our group also reported that this treatment produced ventricular hypertrophy with no changes in left ventricular systolic and end diastolic pressures, mean arterial pressure, heart rate or body weight (Vassallo et al., 1988; Busatto et al., 1999). These results suggest that the treatment used in the present study did not produce cardiac failure.
The lack of change in the vasorelaxation produced by ISO administration to the aortas from rats treated with isoproterenol for 7 days was unexpected because previous reports have shown that a continuous stimulation of β-adrenoceptors induces desensitisation followed by downregulation of these adrenoceptors (Harden, 1983; Kimura et al., 1993). On the heart, this effect results in reduced inotropic and chronotropic effects evoked by β1-adrenoceptor stimulation (Chang et al., 1982; Kenakin & Ferris, 1983; Hayes et al., 1986). Hayes et al. (1986) and Cohen & Schenck (1987) demonstrated that prolonged infusion of ISO impaired the vasodilator response to ISO in isolated aortas. In the present study we did not observe this effect, despite the fact that the dose of ISO that we used was 32-fold smaller than that used by those authors. It has been shown that β2-adrenoceptors, which are the main β-adrenoceptor subtype responsible for the vasodilator response induced by nonspecific β-agonists (O'Donnell & Wanstall, 1984), are less susceptible than β1-adrenoceptors to downregulation (Cohen & Schenck, 1987). In addition, β3-adrenoceptors can be upregulated when β1- and β2-adrenoceptor subtypes are downregulated (Rohrer et al., 1999; Moniotte et al., 2001). Moreover, it is known that high doses of ISO and other catecholamines can induce toxic effects (Balta et al., 1995; Banerjee et al., 2003). These toxic effects could mask the vascular changes present in aortic rings after chronic β-adrenergic stimulation. Together, the relative resistance of β2-adrenoceptors to downregulation, a possible upregulation of β3-adrenoceptors, and the smaller dose of ISO might explain the differences between our results and those obtained by Hayes et al. (1986) and Cohen & Schenck (1987).
The increased vasoconstrictor response to PHE and serotonin described in the present work is in agreement with previous reports from our group. This effect was observed when the contractile responses to PHE were normalized to the KCl response and expressed in grams of tension. These reports, using the same ISO treatment protocol, including the dose range as used in the current protocol, showed an increase in the pressor responses to α-adrenergic agonists in vivo, in rats (Trindade et al., 1992) and mice (Gava et al., 2004). In contrast, previous reports have demonstrated no changes in vasoconstrictor responses to norepinephrine and serotonin in aorta and portal vein isolated from ISO-treated rats (Hayes et al., 1986). Again, these discrepant results might be related to the higher dose of ISO used by those authors (9.6 mg kg−1 day−1) as compared with the dose used in the present study and by Trindade et al. (1992) and Gava et al. (2004). However, we could not exclude a possible limitation of the present results using contractile responses to PHE normalised by KCl response, since this parameter varies between aortas from control and ISO-treated groups in the presence of the endothelium.
The removal of the endothelium enhanced the pD2 and the Emax to PHE in aortas from both groups. However, after endothelium denudation, there were no differences in the contractile response to PHE between control and ISO-treated groups. This result suggests an impairment of the negative endothelial modulation of α-adrenoceptor-mediated contraction in aortas from ISO-treated rats. The possible factors involved in this altered endothelial modulation of PHE-induced contraction were investigated including products derived from the COX-pathway, the local renin–angiotensin system, NO and reactive oxygen species (ROS). The experiments with inhibitors of COX and with AT1-receptor antagonist suggested that neither of these pathways or mechanisms were involved in the enhanced response to PHE. However, our results showed that ISO treatment altered NO availability and ROS generation in rat aorta.
An increased NOS activity and protein expression was observed. The higher nonspecific NOS activity appeared to occur in both endothelial and nonendothelial portions of the aorta. Previous reports have demonstrated an activation of eNOS in isolated endothelial cells (Ferro et al., 1999, Isenovic et al., 2002) and iNOS in cultured vascular smooth cells (Koide et al., 1993) following β-adrenoceptor stimulation. In addition, we found a significant increase in protein concentrations for both eNOS and nNOS in aortas from ISO-treated rats. This change induced by ISO treatment was greater for nNOS compared with eNOS (percentage increase in protein concentrations: eNOS: 27% vs nNOS: 157%). In addition, there was no measurable protein expression for iNOS in arteries from either control or ISO-treated rats.
More pronounced activity and protein concentrations of NOS might be translated into greater NO production. This should be substantiated by the greater capacity of L-NAME to enhance the contraction to PHE when compared with controls. We also showed that pretreatment with aminoguanidine increased the contraction to PHE only in aortas from ISO-treated rats. However, this effect was less than that observed in the presence of L-NAME and does not seem to be explained by greater basal NO modulation induced by iNOS; however, it can be explained by an aminoguanidine action on nNOS. Boer et al. (2000) support this idea as they have shown that aminoguanidine is about nine times more potent at iNOS than at eNOS and almost equipotent at iNOS and nNOS. There are reports showing that the effect of aminoguanidine in endotoxic shock and in aortic rings from ouabain-induced hypertensive rats is most likely due to the inhibition of nNOS (Gocan et al., 2000; Rossoni et al., 2002). In addition, it was postulated that cultured aortic smooth muscle cells might have a marked expression of nNOS but not of iNOS (Papadaki et al., 1998). Together, these results suggest that the increase in the levels of eNOS and nNOS isoforms could be involved in the higher total NOS activity observed in aortic rings with intact endothelium from ISO-treated rats. On the other hand, the nNOS, but not the iNOS isoform seems to be involved in the higher total NOS activity observed in endothelium-free aortic rings from these animals.
Although the evidence suggests that there is an increased basal NO release in arteries from ISO-treated rats, this was not translated into a reduced contractile response to PHE or serotonin. It is interesting to note that the increase in the Emax to both vasoconstrictors was almost identical (i.e. increases of 27 and 28%). This indicates that the contractile response by itself is important to release endothelial factors that counteract a high basal NO production for aortic tissue as proposed by others authors (Kim & Greenburg, 2001). It is well established that NO can be destroyed by the superoxide anion, a ROS. Thus, an increase in superoxide anion concentrations might result in enhanced NO inactivation, which decreases its bioavailability, despite an increased release of NO in arteries (Gryglewski et al., 1986). This mechanism has been associated with endothelial dysfunction and appears to be a common feature of many cardiovascular diseases such as heart failure (Bauersachs et al., 1999).
In the present study, SOD administration reduced the contraction to PHE in aortas from ISO-treated rats to control levels. In addition, an increase in superoxide anion levels was observed in isolated aortas from ISO-treated rats as indicated by the ethidium bromide fluorescence. These results confirm the involvement of superoxide anions in the increased response to PHE in these preparations. An important source of superoxide anion in the vasculature is the enzyme NAD(P)H oxidase. However, pretreatment with apocynin did not modify the PHE-induced contraction in either group of rats, indicating that this pathway of superoxide anion generation is not affected by treatment with ISO. Some studies employing very high doses of ISO suggested that the autooxidation of ISO might be a source of superoxide anions that can induce oxidative stress damage to the myocardium (Balta et al., 1995; Banerjee et al., 2003). Whether or not this is the case with the relatively low dose of ISO used in the present study remains to be determined.
However, if superoxide is able to inactivate NO, it is expected that L-NAME should have a smaller effect on PHE contraction in arteries from ISO-treated rats. However, L-NAME has a greater effect on PHE-induced contractions in arteries from ISO-treated rats. This contradictory effect can be explained by the dual role of NOS producing NO and superoxide anion. Superoxide generation by a purified preparation of nNOS and eNOS has been demonstrated. Superoxide is generated from the dissociation of the heme ferrous–dioxygen complex in the oxygenase domain of eNOS (Vásquez-Vivar et al., 1998) and nNOS (Pou et al., 1992; 1999). For both isoforms, the efficacy of inactive L-arginine analogues, such as L-NAME, to inhibit superoxide production is contradictory. L-NMMA did not inhibit superoxide anion production by eNOS and a similar lack of effect was reported for L-NMMA on nNOS. L-NAME was able to inhibit superoxide production by nNOS only in higher concentrations (>1 mM). In our study, incubation with L-NAME inhibited NO production by NOS. However, it was probably not able to inhibit superoxide anion production by this enzyme.
Superoxide generation by NOS can be regulated by the substrate for NOS, L-arginine, and by cofactors, mainly BH4. In the absence of L-arginine, O2 accepts an electron from NOS, generating superoxide (Pou et al., 1992). In the present study, we incubated L-arginine for 30 min before the concentration–response curves to PHE. L-arginine was able to normalize the hyper-reactivity to PHE in ISO group to control levels. These results suggest that NOS can be a source for increased superoxide anions generation in aortas from ISO-treated animals. Thus, despite the inhibition of NO production, the maintained increase of superoxide anion production might be responsible for the increased contractile response to PHE after L-NAME incubation in ISO arteries compared with controls. This effect was probably mediated by the nNOS and eNOS isoforms, since there were no detectable levels of iNOS in these vessels. However, other sources of superoxide might mediate oxidative ISO effects and cannot be excluded.
When superoxide anion is dismutated by SOD, H2O2 is generated, and this could also participate in the vascular effects induced by ISO. Nevertheless, the H2O2 scavenger catalase did not show a selective effect on the actions of PHE in aortic rings from ISO-treated animals, suggesting that it does not play a role in reducing NO bioavailability.
Although we observed an increased effect of superoxide anion on the contractile response to PHE in aortic rings from ISO-treated rats, there was a lack of effect of ISO treatment on the endothelium-dependent relaxation to acetylcholine and the partially endothelium-dependent relaxation response to ISO. These apparently contradictory findings might be explained by the greater sensitivity of basal vs agonist-stimulated activity of NO to destruction by superoxide anions (Mian & Martin, 1995; Laight et al., 1998). It is hypothesized that basal NO is more sensitive to inactivation because SOD is more effective to preserve agonist-mediated NO release (Mian & Martin, 1995). Here, we observed an increase of Cu/Zn-SOD protein expression in segments of aorta from ISO-treated rats. Thus, enhanced SOD in the ISO group might selectively preserve agonist-mediated NO responses. However, despite increased SOD levels, superoxide generation still appears enhanced and the PHE-induced contraction remained elevated. Thus, the SOD protein expression change may be an ineffective response to enhanced oxidant generation.
In conclusion, our results suggest that a prolonged treatment with a low dose of ISO produces an alteration of the endothelial modulation on the actions of PHE in thoracic aortas. The enhanced vascular reactivity seems to be due to an increase in superoxide anion generation by uncoupling of NOS activity and subsequent inactivation of NO that effectively counteracts the increased activity and protein expression of eNOS and nNOS, and an increment of the protein expression of Cu/Zn-SOD. These results suggest a role for β-adrenoceptors in endothelial dysfunction and increased vasoconstriction.
Acknowledgments
We thank Larissa de Sá Lima, Maria do Carmo Franco and Luiz R.G. Britto for technical support. The assistance of L.A. Barker, Emeritus Professor of Pharmacology, LSUHSC-New Orleans, in the preparation of this manuscript is appreciated. This research was supported by grants from FAPESP, CNPq and CAPES.
Abbreviations
- AT1
angiotensin type 1 receptor
- BH4
5,6,7,8-tetrahydrobiopterin
- COX
cyclooxygenase
- Cu/Zn-SOD
copper and zinc superoxide dismutase
- dAUC
difference of area under the concentration–response curves
- EDTA
ethylenediamine-tetraacetic acid
- Emax
maximum response
- eNOS
endothelial nitric oxide synthase
- iNOS
inducible nitric oxide synthase
- ISO
isoproterenol
- KHS
Krebs–Henseleit solution
- L-NAME
Nω-nitro-L-arginine methyl ester
- nNOS
neuronal nitric oxide synthase
- NO
nitric oxide
- pD2
negative logarithm of concentrations producing 50% of maximum response
- PHE
phenylephrine
- SDS–PAGE
sodium dodecyl sulphate–polyacrylamide gel electrophoresis
References
- BADENHORST D., VELIOTES D., MASEKO M., TSOTETSI O.J., BROOKSBANK R., NAIDOO A., WOODIWISS A.J., NORTON G.R. Beta-adrenergic activation initiates chamber dilatation in concentric hypertrophy. Hypertension. 2003;41:499–504. doi: 10.1161/01.HYP.0000056601.29613.DD. [DOI] [PubMed] [Google Scholar]
- BALTA N., DUMITRU I.F., STOIAN G., PETEC G., DINISCHIOTU A. Influence of ISO-induced cardiac hypertrophy on oxidative myocardial stress. Rom. J. Physiol. 1995;32:149–154. [PubMed] [Google Scholar]
- BANERJEE S.K., SOOD S., DINDA A.K., DAS T.K., MAULIK S.K. Chronic oral administration of raw garlic protects against isoproterenol-induced myocardial necrosis in rat. Comp. Biochem. Physiol. C. Toxicol. Pharmacol. 2003;136:377–386. doi: 10.1016/j.cca.2003.10.011. [DOI] [PubMed] [Google Scholar]
- BAUERSACHS J., BOULOUMIE A., FRACCAROLLO D., HU K., BUSSE R., ERTL G. Endothelial dysfunction in chronic myocardial infarction despite increased vascular endothelial nitric oxide synthase and soluble guanylate cyclase expression: role of enhanced vascular superoxide production. Circulation. 1999;100:292–298. doi: 10.1161/01.cir.100.3.292. [DOI] [PubMed] [Google Scholar]
- BENJAMIN I.J., JALIL J.E., TAN L.B., CHO K., WEBER K.T., CLARK W.A. Isoproterenol-induced myocardial fibrosis in relation to myocyte necrosis. Circ. Res. 1989;65:657–670. doi: 10.1161/01.res.65.3.657. [DOI] [PubMed] [Google Scholar]
- BOER R., ULRICH W.R., KLEIN T., MIRAU B., HAAS S., BAUR I. The inhibitory potency and selectivity of arginine substrate site nitric-oxide synthase inhibitors is solely determined by their affinity toward the different isoenzymes. Mol. Pharmacol. 2000;58:1026–1034. [PubMed] [Google Scholar]
- BRAWLEY L., SHAW A.M., MACDONALD A. Role of endothelium/nitric oxide in atypical beta-adrenoceptor-mediated relaxation in rat isolated aorta. Eur. J. Pharmacol. 2000;398:285–296. doi: 10.1016/s0014-2999(00)00319-8. [DOI] [PubMed] [Google Scholar]
- BRISTOW M.R. Beta-adrenergic receptor blockade in chronic heart failure. Circulation. 2000;101:558–569. doi: 10.1161/01.cir.101.5.558. [DOI] [PubMed] [Google Scholar]
- BUSATTO V.C., CUNHA V., CICILINI M.A., MILL J.G. Differential effects of isoproterenol on the activity of angiotensin-converting enzyme in the rat heart and aorta. Braz. J. Med. Biol. Res. 1999;32:355–360. doi: 10.1590/s0100-879x1999000300017. [DOI] [PubMed] [Google Scholar]
- CASTELLANO M., BOHM M. The cardiac beta-adrenoceptor-mediated signaling pathway and its alterations in hypertensive heart disease. Hypertension. 1997;29:715–722. doi: 10.1161/01.hyp.29.3.715. [DOI] [PubMed] [Google Scholar]
- CHANG H.Y., KLEIN R.M., KUNOS G. Selective desensitization of cardiac β-adrenoceptors by prolonged in vivo infusion of catecholamines in rats. J. Pharmacol. Exp. Ther. 1982;221:784–789. [PubMed] [Google Scholar]
- COHEN M.L., SCHENCK K.W. Selective down regulation of vascular β1-adrenergic receptors after prolonged isoproterenol infusion. J. Cardiovasc. Pharmacol. 1987;10:365–368. doi: 10.1097/00005344-198709000-00017. [DOI] [PubMed] [Google Scholar]
- ESLER M., KAYE D. Sympathetic nervous system activation in essential hypertension, cardiac failure and psychosomatic heart disease. J. Cardiov. Pharmacol. 2000;35:S1–S7. doi: 10.1097/00005344-200000004-00001. [DOI] [PubMed] [Google Scholar]
- FERRO A., QUEEN L.R., PRIEST R.M., XU B., RITTER J.M., POSTON L., WARD J.P. Activation of nitric oxide synthase by β2-adrenoceptors in human umbilical vein endothelium in vitro. Br. J. Pharmacol. 1999;126:1872–1880. doi: 10.1038/sj.bjp.0702512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAVA A.L., PEOTTA V.A., CABRAL A.M., MEYRELLES S.S., VASQUEZ E.C. Decreased baroreflex sensitivity in isoproterenol-treated mice with cardiac hypertrophy. Auton. Neuros. 2004;114:47–54. doi: 10.1016/j.autneu.2004.07.003. [DOI] [PubMed] [Google Scholar]
- GOCAN N.C., SCOTT J.A., TYNML K. Nitric oxide produced via neuronal NOS may impair vasodilatation in septic rat skeletal muscle. Am. J. Physiol. Heart Circ. Physiol. 2000;278:1480–1489. doi: 10.1152/ajpheart.2000.278.5.H1480. [DOI] [PubMed] [Google Scholar]
- GRAY D.W., MARSHALL I. Novel signal transduction pathway mediating endothelium-dependent β-adrenoceptor vasorelaxation in rat thoracic aorta. Br. J. Pharmacol. 1992;107:684–690. doi: 10.1111/j.1476-5381.1992.tb14507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRYGLEWSKI R.J., PALMER R.M., MONCADA S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature. 1986;320:454–456. doi: 10.1038/320454a0. [DOI] [PubMed] [Google Scholar]
- HARDEN T.K. Agonist-induced desensitization of the β-adrenergic receptor-linked adenylate cyclase. Pharmacol. Rev. 1983;35:5–32. [PubMed] [Google Scholar]
- HAYES J.S., WYSS V.L., SCHENCK K.S., COHEN M.L. Effects of prolonged isoproterenol infusion on cardiac and vascular responses to adrenoceptor agonists. J. Pharmacol. Exp. Ther. 1986;237:757–763. [PubMed] [Google Scholar]
- HERNANZ R., BRIONES A.M., ALONSO M.J., VILA E., SALAICES M. Hypertension alters role of iNOS, COX-2, and oxidative stress in bradykinin relaxation impairment after LPS in rat cerebral arteries. Am. J. Physiol. Heart Circ. Physiol. 2004;287:225–234. doi: 10.1152/ajpheart.00548.2003. [DOI] [PubMed] [Google Scholar]
- ISENOVIC E., WALSH M.F., MUNIYAPPA R., BARD M., DIGLIO C.A., SOWERS J.R. Phosphatidylinositol 3-kinase may mediate isoproterenol-induced vascular relaxation in part through nitric oxide production. Metabolism. 2002;51:380–386. doi: 10.1053/meta.2002.30525. [DOI] [PubMed] [Google Scholar]
- KENAKIN T.P., FERRIS R.M. Effects of in vivo β-adrenoceptor down-regulation on cardiac responses to prenalterol and pirbuterol. J. Cardiovasc. Pharmacol. 1983;5:90–97. doi: 10.1097/00005344-198301000-00014. [DOI] [PubMed] [Google Scholar]
- KIM H.W., GREENBURG A.G. Contraction coupled endothelial nitric oxide release: a new paradigm for local vascular control. J. Surg. Res. 2001;100:93–98. doi: 10.1006/jsre.2001.6213. [DOI] [PubMed] [Google Scholar]
- KIMURA H., MIYAMOTO A., OHSHIKA H. Down-regulation of β-adrenoceptors and loss of Gs alpha subunit levels in ventricular myocardium of rats treated with isoproterenol. Life Sci. 1993;53:171–176. doi: 10.1016/0024-3205(93)90511-z. [DOI] [PubMed] [Google Scholar]
- KOIDE M., KAWAHARA Y., NAKAYAMA I., TSUDA T., YOKOYAMA M. Cyclic AMP-elevating agents induce an inducible type of nitric oxide synthase in cultured vascular smooth muscle cells. Synergism with the induction elicited by inflammatory cytokines. J. Biol. Chem. 1993;268:24959–24966. [PubMed] [Google Scholar]
- LAIGHT D.W., KAW A.V., MARTIN J.C., ANGGARD E.E. Interaction between superoxide anion and nitric oxide in the regulation of vascular endothelial function. Br. J. Pharmacol. 1998;124:238–244. doi: 10.1038/sj.bjp.0701814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCKEE M., SCAVONE C., NATHANSON J.A. Nitric oxide, cGMP, and hormone regulation of active sodium transport. Proc. Natl. Acad. Sci. U.S.A. 1994;91:12056–12060. doi: 10.1073/pnas.91.25.12056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIAN K.B., MARTIN W. Differential sensitivity of basal and acetylcholine-stimulated activity of nitric oxide to destruction by superoxide anion on rat aorta. Br. J. Pharmacol. 1995;115:993–1000. doi: 10.1111/j.1476-5381.1995.tb15909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONIOTTE S., KOBZIK L., FERON O., TROCHU J.N., GAUTHIER C., BALLIGAND J.L. Upregulation of β3-adrenoceptors and altered contractile response to inotropic amines in human failing myocardium. Circulation. 2001;103:1649–1655. doi: 10.1161/01.cir.103.12.1649. [DOI] [PubMed] [Google Scholar]
- O'DONNELL S.R., WANSTALL J.C. β1 and β2 adrenoceptor-mediated responses in preparations of pulmonary artery and aorta from young and aged rats. J. Pharmacol. Exp. Ther. 1984;228:733–738. [PubMed] [Google Scholar]
- PAPADAKI M., TILTON R.G., ESKIN S.G., MCINTIRE L.V. Nitric oxide production by cultured human aortic smooth muscle cells: stimulation by fluid flow. Am. J. Physiol. Heart Circ. Physiol. 1998;274:616–626. doi: 10.1152/ajpheart.1998.274.2.h616. [DOI] [PubMed] [Google Scholar]
- POU S., KEATON L., SURICHAMORN W., ROSEN G.M. Mechanism of superoxide generation by neuronal nitric-oxide synthase. J. Biol. Chem. 1999;274:9573–9580. doi: 10.1074/jbc.274.14.9573. [DOI] [PubMed] [Google Scholar]
- POU S., POU W.S., BREDT D.S., SNYDER S.H., ROSEN G.M. Generation of superoxide by purified brain nitric oxide synthase. J. Biol. Chem. 1992;267:24173–24176. [PubMed] [Google Scholar]
- REMBOLD C.M., CHEN X.-L. Mechanisms responsible for forskolin-induced relaxation of rat tail artery. Hypertension. 1998;31:872–877. doi: 10.1161/01.hyp.31.3.872. [DOI] [PubMed] [Google Scholar]
- ROHRER D.K., CHRUSCINSKI A., SCHAUBLE E.H., BERNSTEIN D., KOBILKA B.K. Cardiovascular and metabolic alterations in mice lacking both β1- and β2-adrenergic receptors. J. Biol. Chem. 1999;274:16701–16708. doi: 10.1074/jbc.274.24.16701. [DOI] [PubMed] [Google Scholar]
- RONA G., CHAPPEL C.i., BALAZS T., GAUDRY R. An infarct-like myocardial lesion and other toxic manifestations produced by isoproterenol in the rat. AMA Arch. Pathol. 1959;67:443–455. [PubMed] [Google Scholar]
- ROSSONI L.V., SALAICES M., MIGUEL M., BRIONES A.M., BARKER L.A., VASSALLO D.V., ALONSO M.J. Ouabain-induced hypertension is accompanied by increases in endothelial vasodilator factors. Am. J. Physiol. Heart Circ. Physiol. 2002;283:2110–2118. doi: 10.1152/ajpheart.00454.2002. [DOI] [PubMed] [Google Scholar]
- TAYLOR P.B., TANG Q. Development of isoproterenol-induced cardiac hypertrophy. Can. J. Physiol. Pharmacol. 1984;62:384–389. doi: 10.1139/y84-061. [DOI] [PubMed] [Google Scholar]
- TRINDADE J.D., CABRAL A.M., VASQUEZ E.C., VASALLO D.V. Cardiovascular effects on conscious rats of pretreatment with isoproterenol for 3 days. Braz. J. Med. Biol. Res. 1992;25:301–304. [PubMed] [Google Scholar]
- VÁSQUEZ-VIVAR J., KALYANARAMAN B., MARTÁSEK P., HOGG N., MASTERS B.S.S., KAROUI H., TORDO P., PRITCHARD K.A., JR Superoxide generation by endothelial nitric oxide synthase: the influence of cofactors. Proc. Natl. Acad. Sci. U.S.A. 1998;95:9220–9225. doi: 10.1073/pnas.95.16.9220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VASSALLO D.V., VASQUEZ E.C., CABRAL A.M. Contractile performance of papillary muscles of renovascular hypertensive and isoproterenol-pretreated rats. Pharmacol. Res. Commun. 1988;20:61–72. doi: 10.1016/s0031-6989(88)80607-6. [DOI] [PubMed] [Google Scholar]