Abstract
Sensory neurons are a major site of opioid analgesic action, but the effect of chronic morphine treatment (CMT) on μ-opioid receptor function in these cells is unknown. We examined μ-opioid receptor modulation of calcium channel currents (ICa) in small trigeminal ganglion (TG) neurons from mice chronically treated with morphine and measured changes in μ-opioid receptor mRNA levels in whole TG.
Mice were injected subcutaneously with 300 mg kg−1 of morphine base in a slow release emulsion three times over 5 days, or with emulsion alone (vehicles). CMT mice had a significantly reduced response to the acute antinociceptive effects of 30 mg kg−1 morphine compared with controls (P=0.035).
Morphine inhibited ICa in neurons from CMT (EC50 300 nM) and vehicle (EC50 320 nM) mice with similar potency, but morphine's maximum effect was reduced from 36% inhibition in vehicle to 17% in CMT (P<0.05). Similar results were observed for the selective μ-opioid agonist Tyr-D-Ala-Gly-N-Me-Phe-Gly-ol enkephalin (DAMGO). Inhibition of ICa by the GABAB agonist baclofen was unaffected by CMT.
In neurons from CMT mice, there were significant reductions in P/Q-type (P=0.007) and L-type (P=0.002) ICa density.
μ-Opioid receptor mRNA levels were not altered by CMT.
These data demonstrate that CMT produces a significant reduction of the effectiveness of μ-opioid agonists to inhibit ICa in TG sensory neurons, accompanied by a reduction in ICa density. Thus, adaptations in sensory neurons may mediate some of the tolerance to the antinociceptive effects of morphine that develop during systemic administration.
Keywords: Morphine tolerance, trigeminal ganglion, neuroadaptation, chronic opioids, analgesia
Introduction
Tolerance to the analgesic effects of opioids such as morphine is an important problem in the management of chronic pain (McQuay, 1999; Buntin-Mushock et al., 2005). Studies in animals have suggested that prolonged administration of morphine leads to a modest reduction in the effectiveness of μ-opioid receptor coupling to cellular effectors (reviewed by Johnson et al., 2005), and a significant number of adaptations in cellular signalling cascades and opioid-sensitive neural circuits (reviewed by Williams et al., 2001; Ossipov et al., 2004; Bailey & Connor, 2005).
μ-Opioid receptors located on the peripheral and central terminals of primary afferent neurons can mediate some of the antinociceptive effects of morphine as well as being part of endogenous analgesic systems. Tolerance to the peripheral antinociceptive effects of morphine has been reported in animals treated with morphine systemically (Honore et al., 1997), and chronic intrathecal morphine treatment results in an abolition of the ability of morphine to inhibit stimulus-induced substance P release from sensory neurons (Gu et al., 2005). Repeated injections of morphine have also been shown to lead to a decrease in μ-opioid receptor mRNA in dorsal root ganglia (Meuser et al., 2003; Starowicz et al., 2005). Although these findings suggest that there is likely to be a reduction in the signalling capacity of μ-opioid receptors on sensory neurons in chronically morphine-treated animals, this has never been directly tested.
In this study, we have examined the changes in signalling of μ-opioid receptors expressed on sensory neurons isolated from the trigeminal ganglion (TG) of mice. We have focused on a population of small TG neurons that have previously been shown to express ion channels and receptors associated with nociceptors, in particular the vanilloid receptor TRPV1 and the Gs-coupled prostaglandin EP3 receptor (Borgland et al., 2001; 2002). We have used μ-opioid receptor inhibition of voltage-gated calcium channel currents (ICa), which occurs via a direct interaction between the receptor-activated G-protein βγ-subunits and the channels (De Waard et al., 2005), as a sensitive assay of receptor function with which to address the hypothesis that chronic morphine treatment (CMT) of mice leads to a reduction in signalling by μ-opioid receptors expressed in sensory neurons.
Methods
Experiments were carried out on male C57Bl6/J mice. All experiments were carried out under protocols approved by the local Animal Ethics Committee.
Morphine treatment
CMT consisted of a series of three subcutaneous injections of morphine base (300 mg kg−1) in a sustained release emulsion on alternate days over a 5-day period. The sustained release preparation consisted of 50 mg of morphine base suspended in 1 ml of emulsion (0.1 ml of Arlacel A (mannide monooleate), 0.4 ml of light liquid paraffin and 0.5 ml of 0.9% (w v−1) NaCl). Injections of warmed suspension were made under light halothane anaesthesia. Vehicle mice were injected with suspension lacking morphine. Vehicle and morphine treatments were performed in parallel, on littermates. Animals were used on day 6 or 7 for electrophysiology and day 7 for mRNA quantification.
Nociceptive testing
Two days after the final treatment with morphine base or vehicle, each mouse was placed on the floor inside a cylindrical metal container (dimensions 23 cm height × 16 cm diameter), with the floor heated to 52°C. The time until the mouse either licked its hind paw or jumped in an attempt to escape the hot plate was measured (latency to respond), and then the mouse was removed and returned to its home cage. If mice did not respond on the hot plate within 90 s, they were removed and their latency recorded as 90 s. For each mouse, three hot plate readings, 20 min apart, were collected to establish a stable baseline latency. The mice were then injected with 30 mg kg−1 morphine intraperitoneally (i.p.) and were tested on the hot plate after 20 min. The differences between treatments were assessed by a factorial ANOVA followed by a Scheffe F-test (Statview, SAS Institute, Cary, NC, U.S.A.).
Cell isolation
Cells were isolated from trigeminal ganglia essentially as described in Roberts et al. (2002). Briefly, mice were anaesthetized with halothane (4%), and killed by decapitation. The trigeminal ganglia were removed and placed in physiological saline containing (mM) NaCl 126, KCl 2.5, CaCl2 2.5, MgCl2 10, NaH2PO4 1.2, NaHCO3 24 and glucose 10, gassed with 95% O2–5% CO2. Ganglia were cut up with iridectomy scissors and incubated at 32–34°C for 30 min in physiological saline. The ganglia were then transferred to oxygenated modified HEPES-buffered saline (HBS) containing 20 U ml−1 papain and incubated at 32–34°C for 20–25 min. The modified HBS contained (mM): NaCl 140, KCl 2.5, CaCl2 2.5, MgCl2 10, HEPES 10, glucose 10, pH 7.3 (NaOH), 330±5 mosmol. The digestion was terminated by the addition of HBS containing 1 mg ml−1 BSA and 1 mg ml−1 trypsin inhibitor. Minced ganglia were washed free of enzyme and enzyme inhibitors with room temperature modified HBS. Cells were released by gentle trituration through decreasing bore, silanized Pasteur pipettes with fire-polished tips. The cells were plated onto plastic culture dishes and kept at room temperature in modified HBS. Unless otherwise noted, none of the incubation buffers contained morphine, which means that the cells from CMT animals were withdrawn on killing, and the cells from the vehicles did not see morphine until superfused with it in the experiments described below. Cells were used for up to 8 h after dissociation.
Electrophysiological recording
Ionic currents from mouse trigeminal neurons were recorded in the whole-cell configuration of the patch-clamp method (Hamill et al., 1981) at room temperature (22–24°C), as described by Borgland et al. (2002). Dishes were continually perfused with HBS (mM): NaCl 140, KCl 2.5, CaCl2 2.5, MgCl2 1, HEPES 10, glucose 10, pH 7.3 (NaOH), 330±5 mosmol l−1. For recording ICa, the extracellular solution contained (mM): tetraethylammonium chloride 140, CsCl 2.5, HEPES 10, MgCl2 1, CaCl2 2.5, glucose 10, BSA 0.05%, pH 7.3 (CsOH), 330±5 mosmol l−1. Recordings were made with fire-polished borosilicate pipettes filled with (in mM): CsCl 120; MgATP 5, NaCl 5, Na2GTP 0.2, EGTA 10, CaCl2 2 and HEPES 20, pH 7.3, 285±5 mosmol l−1, resistance approximately 2 MΩ.
Recordings were made using an Axopatch 1D amplifier using pCLAMP acquisition software (Axon Instruments, Union City, CA, U.S.A.). Currents were filtered at 2 kHz, sampled at 5–20 Hz, and recorded on hard disk for later analysis. Series resistance ranged from 2 to 7 MΩ and was compensated by 80% in all experiments. An approximate value of whole-cell capacitance was determined by nulling the amplifier capacitance compensation circuit. Leak current was subtracted on-line using a P/8 protocol. Cells were exposed to drugs via a series of flow pipes positioned about 200 μM from the cells.
RNA isolation
Whole trigeminal ganglia were dissected on day 7 and total RNA isolated with Tri Reagent (Sigma, Australia) as per the manufacturer's instructions. The final RNA was re-suspended in 0.1% v v−1 diethyl pyrocarbonate (DEPC)-treated H2O. RNA quantity and quality were assessed using an ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, Delaware, U.S.A.) and 1.2% formaldehyde gel electrophoresis.
Reverse transcription
Total RNA (0.5 μg) was subject to reverse transcription using oligo(dT)15 (Promega, Madison, WI, U.S.A.). RNA secondary structures were removed by preheating to 70°C for 10 min before the addition of 40 U rRNasin (Promega, U.S.A.), 0.40 μl 100 mM dNTPs, 4 μl 5 × Reaction buffer and 0.25 μl BioScript (all Bioline Australia Pty Ltd, Alexandria, NSW, Australia) in DEPC-H2O. The reactions were incubated for 60 min at 50°C before inactivating the BioScript for 10 min at 70°C. cDNA was stored at 4°C until further use.
Real-time reverse transcription–polymerase chain reaction (RT–PCR)
Changes in μ-opioid receptor mRNA expression were assessed using RT–PCR (Bustin et al., 2005) on a Rotorgene 3000 (Corbett Research Pty Ltd, Australia). Thermal cycling conditions were as follows: initial denaturation at 95°C for 10 min, followed by 40 cycles of 95°C for 20 s, 55°C for 20 s and 72°C for 20 s. Melt analysis consisted of an initial 45 s at 72°C followed by increasing steps (1°C increments) for 5 s to 95°C. Fluorescence measurements were recorded throughout the run and the data were analysed by the proprietary software accompanying the system. A standard curve was generated with 10-fold dilutions of total RNA and a negative template control was included for each set of primers used.
The cDNA was coamplified with exon spanning primers for μ-opioid receptor (forward, 5′-TTC-TGC-ATC-CCA-ACT-TCC-TC-3′; reverse, 5′-CTG-ACA-GCA-ACC-TGA-TTC-CA-3′, Ensembl gene ENSMUSG00000000766) and hypoxanthine phosphoribosyltransferase (HPRT, forward, 5′-TAA-GTT-CTT-TGC-TGA-CCT-GCT-G-3′; reverse, 5′-TAT-GTC-CCC-CGT-TGA-CTG-AT-3′, Ensembl gene ENSMUST00000026723), the latter being a constitutively expressed gene chosen for consistency. The primers for both the μ-opioid receptor and HPRT were generated from sequences in exons 3 and 4. Each RT–PCR amplification, with a total reaction volume of 25 μl, contained 1 μl cDNA, 12.5 μl 2 × ImmoMix (Bioline Australia Pty Ltd), 0.75 μl forward/reverse primers for gene of interest (Sigma Genosys Australia Pty Ltd) and 2.50 μl SYBR Green I (Invitrogen Australia Pty Ltd) supplemented with MilliQ H2O. RT–PCR products were separated on 1.5% agarose gel and stained with ethidium bromide to confirm amplicon size and number.
Data analysis
Significant differences between means were tested using unpaired, Student's t-test as noted. All data are expressed as mean±s.e., unless otherwise indicated.
Drugs and chemicals
Morphine base and morphine hydrochloride were from Glaxo, U.K. DAMGO and ω-conotoxin GVIA were from Auspep (Melbourne, Australia). Nimodipine and baclofen were from Research Biochemicals International (Natick, MA, U.S.A.). ω-Agatoxin IVA was from the Peptide Institute (Osaka, Japan). Buffer salts were from either BDH or Sigma. BSA and trypsin inhibitor (chicken egg white ovomucoid, type II-O) were from Sigma, Australia. Papain was from Worthington Biochemical Corporation (Freehold, NJ, U.S.A.).
Results
CMT produces antinociceptive tolerance
The CMT schedule our laboratory has used over several years has been shown to produce significant physical dependence on morphine, and we have also previously shown antinociceptive tolerance to a single dose of morphine (10 mg kg−1) in the CMT animals challenged with a noxious thermal stimulus (52°C hot plate; Bagley et al., 2005a). We confirmed this finding by administering a near maximally effective concentration of morphine (30 mg kg–1 i.p.) on day 7 of treatment with saline or morphine and examining the time it took for the mice to respond to the same noxious thermal stimulus. Baseline hot plate latencies did not differ between vehicle (21±2 s, n=9) and CMT (24±5 s, n=11) animals, but 20 min after treatment with 30 mg kg−1 of morphine CMT animals showed a significantly decreased latency to respond to the noxious thermal stimulus (55±8 vs 82±6 s for vehicles, P=0.035).
CMT inhibits μ-opioid receptor signalling
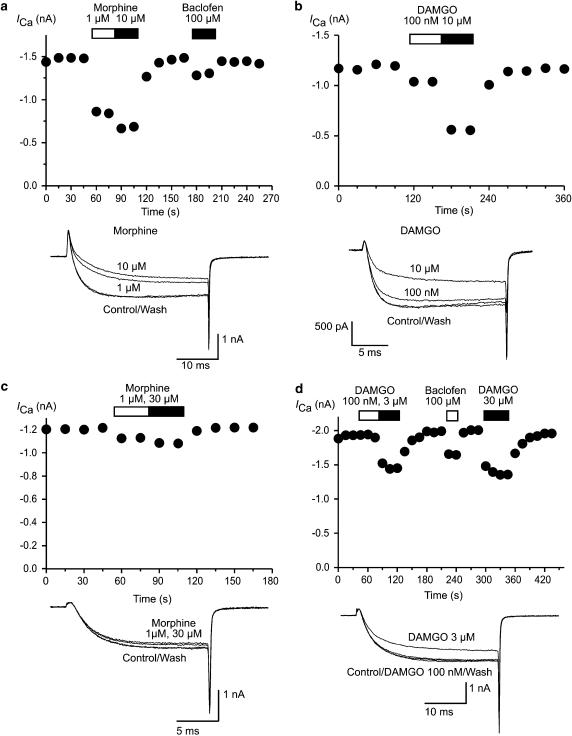
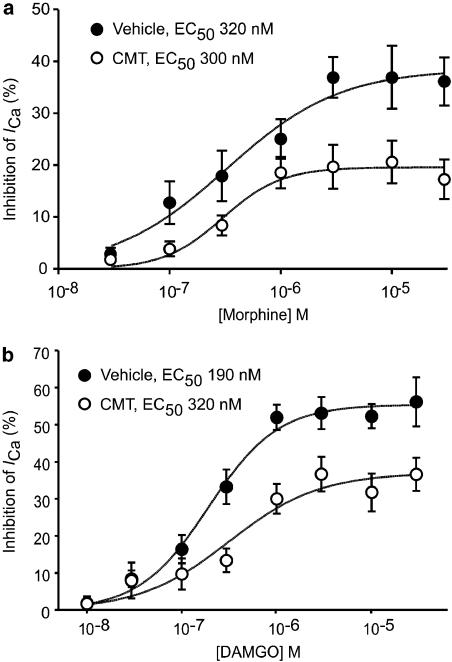
We examined the changes in opioid receptor signalling in small Type 1 neurons (capacitance <12 pF diameter−1 <20 μM). To measure drug inhibition of ICa, cells were voltage clamped at −90 mV and repetitively stepped to 0 mV. Superfusion of the μ-opioid agonists DAMGO or morphine continued to inhibit ICa in a similar proportion of neurons from CMT mice compared to cells isolated from vehicle-treated mice (171/208 cells, 82% in CMT cells, versus 160/196 cells, 82% in vehicles, χ2, P=0.87; Figure 1). The capacitance of the cells examined in the two groups did not differ (8.3±0.2 pF for vehicles, 8.2±0.2 pF for CMT cells, P=0.52). The potency of DAMGO and morphine to inhibit ICa was determined by exposing the cells to two or more concentrations of agonist and then fitting the pooled data to a logistic function in the program Graphpad Prism 4. DAMGO potency was similar in neurons from CMT mice (pD2 of 6.50, 95% confidence interval 6.96–6.04, EC50=320 nM) and vehicle-treated animals (pD2 of 6.72, 95% confidence interval 6.86 to 6.57, EC50=190 nM; Figure 2). A two-way ANOVA comparing the DAMGO concentration response curves in vehicle and CMT animals showed a significant main effect of concentration (P<0.0001) and treatment (P<0.0001), but no significant interaction between concentration and treatment (P=0.09). The inhibition of ICa by maximally effective concentrations of DAMGO (30 μM) was significantly reduced in TG neurons taken from chronically morphine-treated animals (37±4 versus 56±7% in vehicle, P<0.05, Bonferroni post hoc test corrected for multiple comparisons; Figure 2).
Figure 1.
μ-Opioid agonists continue to inhibit ICa in trigeminal ganglion sensory neurons from CMT mice. ICa was evoked by repetitively stepping TG neurons from −90 to 0 mV. Time plots of the ICa amplitude of TG neurons taken from vehicle-treated mice illustrate the effect of application of several morphine (a) and DAMGO (b) concentrations on ICa. These can be compared with responses of TG neurons from CMT mice to morphine (c) and DAMGO (d). In all experiments, drugs were applied for the duration of the bars. Example traces from each experiment are reproduced below the respective time plots.
Figure 2.
Maximum effect of morphine and DAMGO on ICa are reduced in TG neurons from CMT mice. This figure illustrates concentration–response relationships for morphine (a) and DAMGO (b) inhibition of ICa in TG neurons from vehicle- and morphine-treated mice. Each point represents the mean±s.e.m. of at least six cells, and curves were fit to the pooled data. The maximum effect of both morphine and DAMGO was reduced in neurons from CMT animals (P<0.05, two-way ANOVA followed by Bonferroni post hoc test).
Morphine potency was also similar in neurons from CMT mice (pD2 of 6.52, 95% confidence interval 6.79–6.26, EC50=300 nM) and vehicle-treated animals (pD2 of 6.49, 95% confidence interval 6.87–6.11, EC50=320 nM; Figure 2). A two-way ANOVA comparing the morphine concentration response curves in vehicle and CMT animals showed a significant main effect of concentration (P<0.0001) and treatment (P<0.0001), but no significant interaction between concentration and treatment (P=0.28). The inhibition of ICa by maximally effective concentrations of morphine (30 μM) was significantly reduced in TG neurons taken from chronically morphine-treated animals (17±4 versus 36±5% in vehicle, P<0.05, Bonferroni post hoc test corrected for multiple comparisons; Figure 2).
A limited number of recordings were made from TG neurons with a capacitance >12 pF diameter−1 >20 μM to assess the effect of CMT on μ-opioid receptor coupling in larger Type 1 neurons, which are also mostly μ-opioid sensitive (Borgland et al., 2001). Superfusion of maximally effective concentrations of morphine (10–30 μM) inhibited ICa by 38±4% in neurons from vehicle-treated mice (n=13) and 22±4% in neurons from CMT mice (n=16, P=0.012). The capacitance of the cells examined in the two groups did not differ (16±1 pF for vehicle cells, 17±2 pF for CMT cells).
It has been reported that morphine treatment of rats can increase the surface expression of δ-opioid receptor in several tissues, including sensory neurons (Gendron et al., 2006). Acute application of the δ-opioid receptor agonist deltorphin II (1 μM) did not inhibit ICa in any TG neurons examined; the ICa amplitude in μ-opioid receptor-sensitive TG neurons from vehicle mice was 101±0.5% of control (n=14), and in cells from CMT mice the ICa amplitude was 102±0.5% of control (n=13).
The GABAB receptor agonist baclofen also inhibits ICa in Type 1 trigeminal neurons (Borgland et al., 2002). In small opioid-sensitive neurons from CMT mice, a high concentration of baclofen (100 μM) inhibited ICa by 18±2%, the inhibition in cells from vehicle mice was 19±4% (n=12 for each).
CMT reduces ICa density
CMT resulted in modest reduction in the peak ICa density in μ-opioid sensitive, Type 1 trigeminal neurons. The peak ICa density of small Type 1 neurons was 175±6 pA pF−1 in opioid-sensitive neurons from CMT mice, which is significantly less than the peak ICa density in neurons from vehicles (200±6 pA pF−1, P=0.005). The peak current density of small, opioid-insensitive, Type 1 neurons was similar in neurons from CMT and vehicle-treated mice (139±10 pA pF−1, n=37 and 153±14 pA pF−1, n=36 respectively, P=0.49). The peak current density of the Type 2, opioid-insensitive trigeminal neurons was not affected by CMT (CMT, 105±4 pA pF−1, n=175; vehicle, 101±4 pA pF−1, n=171; P=0.45). In Type 2 cells, the current at −40 mV is almost entirely carried by T-type ICa (Borgland et al., 2001). There was no difference in the amount of T-type ICa in neurons from CMT mice and vehicle-treated mice (41±1 versus 42±1 pA pF−1, P=0.35). Current at −40 mV represents about 1% of the peak current in small, opioid-sensitive Type 1 neurons (i.e. about 10–20 pA; Borgland et al., 2001), and we did not attempt to isolate any T-type component of these currents.
To investigate whether the decreased amount of ICa in Type 1 trigeminal neurons from CMT mice occurred during CMT or resulted from processes subsequent to withdrawal, the density of ICa in small trigeminal neurons was examined after in vitro withdrawal. In these experiments, cells were isolated and maintained in buffers containing 5 μM morphine and then rapidly (<2 s) switched to morphine-free recording solution, while the cell was repetitively stepped from −90 to 0 mV. Removal of morphine resulted in a rapid increase in the ICa amplitude that stabilized within 60 s. The ICa amplitude increased by 42±6%, in neurons from vehicle-treated animals, and 14±1% in cells from CMT animals. The peak ICa density of neurons from CMT mice immediately following morphine withdrawal was 163±12 pA pF–1 (n=25), which was significantly less than the ICa density of neurons from vehicle animals immediately following morphine withdrawal, which was 203±13 pA pF–1 (n=30, P=0.03).
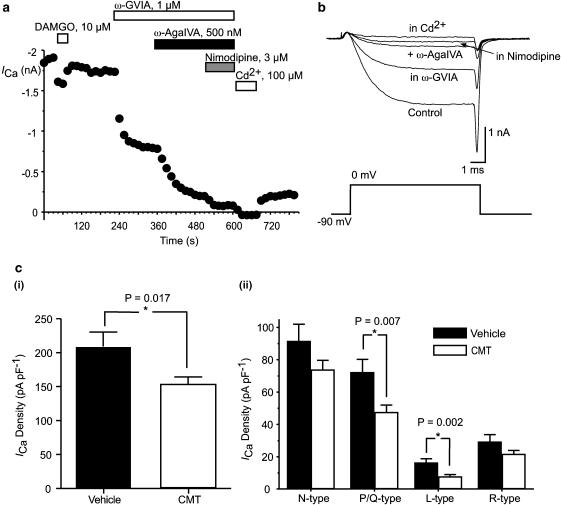
The contributions of pharmacologically defined calcium channel subtypes to the ICa in small trigeminal neurons from vehicle and CMT mice was examined by repetitively stepping cells from −90 to 0 mV in the presence of selective inhibitors of N-type channels (ω-conotoxin GVIA, 1 μM), P/Q-type channels (ω-agatoxin IVA, 500 nM), L-type channels (nimodipine, 3 μM) and resistant channels (Cd2+, 100 μM), as described previously (Borgland et al., 2001). In this subset of experiments, the ICa density at 0 mV was also significantly less in neurons from CMT mice than in those from vehicle-treated animals (P=0.017; Figure 3). In neurons from CMT mice, there was a significant decrease in the amount of P/Q- and L-type ICa when compared with neurons from vehicle-treated animals (P=0.007 and 0.002, respectively; Figure 3). However, there was no difference in the proportion of the whole-cell ICa contributed by N- and P/Q-type channels; they comprised 78% of the total ICa in neurons from vehicle-treated animals and 81% in neurons from CMT mice.
Figure 3.
Density of ICa is reduced in TG neurons from CMT mice. ICa was evoked by repetitively stepping TG neurons from −90 to 0 mV. (a) A time plot of ICa amplitude in a DAMGO-sensitive TG neuron from CMT mouse exposed to the N-type ICa antagonist ω-conotoxin GVIA, the P/Q-type ICa antagonist ω-agatoxin IVA, the L-type ICa antagonist nimodipine and the nonspecific ICa antagonist cadmium. Example traces for this cell are reproduced in (b). (c) Mean ICa density in cells subsequently exposed to toxins is illustrated in (i), whereas the contribution of each pharmacologically defined component of ICa is illustrated in (ii). There is a significant reduction in overall ICa in cells from CMT animals, largely as a result of significantly less P/Q and L-type ICa in these neurons. Each bar represents the mean±s.e.m. of at least 24 cells. * indicates a significant difference between neurons from vehicle and CMT treated mice.
CMT does not change μ-opioid receptor mRNA levels
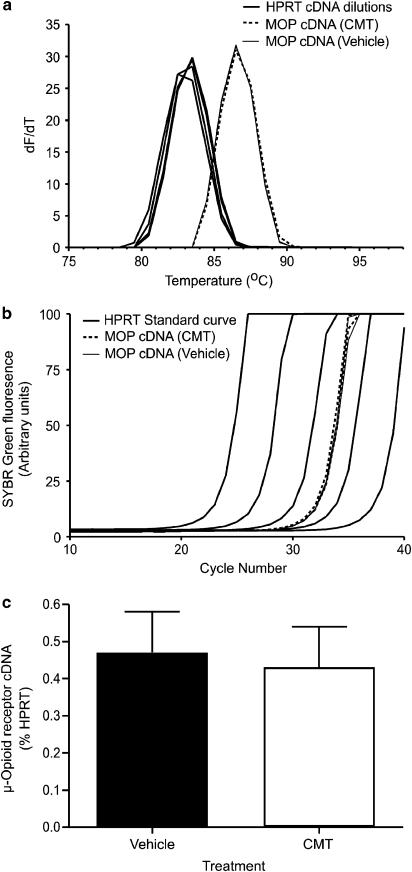
Isolation and separation of the RT–PCR products for the μ-opioid receptor and HPRT confirmed the specificity of the primers chosen as only single bands of predicted size (239 bp for the μ-opioid receptor; 126 bp for HPRT) were visualized on an agarose gel (data not shown). No amplification was observed for either primer in the negative template control. There was no significant difference in μ-opioid receptor mRNA expression relative to HPRT mRNA between trigeminal ganglia from CMT mice (n=10) and vehicles (n=13; P=0.81; Figure 4). As a positive control for our primers, we compared the levels of μ-opioid receptor mRNA in the trigeminal ganglia of male 129SvEv/C57BL6 mice heterozygous for deletion of exon 1 of the receptor with their wild-type controls (Connor et al., 1999b; Schuller et al., 1999). Heterozygous mice had levels of μ-opioid receptor mRNA 0.11±0.02% of HPRT mRNA (n=5), significantly less than the levels of μ-opioid receptor mRNA in wild-type mice (0.18±0.03% of HPRT mRNA, P=0.035, n=4). Both the ‘wild-type' and μ-opioid receptor+/−mice are a mix of the C57BL6 strain used for the rest of this study and the 129SvEv strain used to generate the receptor knockout, and may express lower levels of the μ-opioid receptor than pure C57BL6 mice.
Figure 4.
μ-Opioid receptor mRNA levels are not altered by CMT. mRNA levels were quantified using RT–PCR, following conversion of mRNA to cDNA. (a) Melt curves for the cDNA fragments amplified by primers directed to exons 3 and 4 of the μ-opioid receptor (MOP) and HPRT. Double-stranded cDNA with the fluorescent dye SYBR Green intercalated was heated in 1°C increments, and the change in fluorescence measured. As the double-stranded cDNA melts, the SYBR Green dissociates and its fluorescence decreases. The rate of change of fluorescence/rate of change of temperature is plotted against temperature, the single peaks for both μ-opioid receptor and HPRT primers indicate that only a single species of double-stranded cDNA was present in each sample. (b) Raw data illustrating quantification of μ-opioid receptor cDNA. The amount of SYBR Green fluorescence after each cycle of PCR is plotted against cycle number. As more cDNA is made following each PCR cycle, there is increased fluorescence from the intercalated SYBR Green. Standard curves were generated with serial dilutions of HPRT-containing cDNA, and μ-opioid receptor cDNA levels were measured in 1 μg samples of pooled cDNA from the two TG of individual mice. This experiment illustrates quantification of μ-opioid receptor mRNA from two CMT and two vehicle animals. Each sample was run in quadruplicate, and runs have been averaged for graphical clarity. (c) A bar chart illustrating the levels of μ-opioid receptor cDNA in TG from vehicle and CMT TG, expressed as mean±s.e.m. of the percentage of HPRT cDNA from 10 CMT and 13 vehicle animals. There is no difference in the amount of cDNA between the two groups (P=0.81).
Discussion
The principle finding of this study is that sensory neurons from animals chronically treated with morphine develop tolerance to the effects of μ-opioid agonists. μ-Opioid receptor tolerance is accompanied by modest changes in the expression of calcium channels in putative nociceptors. C57 mice subjected to this CMT paradigm show a significant degree of tolerance to the antinociceptive effects of systemic morphine (Bagley et al., 2005a); however, the relative contribution of decreased μ-opioid receptor coupling and concomitant neural adaptations (Bagley et al., 2005b) to whole animal tolerance are unknown. The degree to which changes in sensory neurons contribute to systemic tolerance has also not been established, but the large reduction in the maximal effect of both the partial μ-opioid agonist morphine and the efficacious agonist DAMGO suggest that direct μ-opioid receptor inhibition of nociceptive transmission at the primary afferent/dorsal horn synapse is likely to be significantly compromised in CMT animals.
A reduction in the capacity of μ-opioid receptors to inhibit voltage-dependent ICa has also been observed in periaqueductal grey (PAG) neurons from morphine-treated mice (Bagley et al., 2005a) and locus coeruleus (LC) neurons from morphine-treated rats (Connor et al., 1999a). Similar to the study in PAG neurons (Bagley et al., 2005a), CMT produced a significant decrease in the maximum effect of morphine and DAMGO in trigeminal sensory neurons without a change in agonist potency, consistent with a situation where there are no spare receptors for coupling the agonists to ICa. Reduced μ-opioid receptor signalling to ion channels is found consistently in chronically morphine-treated animals (Christie et al., 1987; Ingram et al., 1998) and has also been observed in in vitro model systems (Kennedy & Henderson, 1991).
The reduction in the amount of P/Q and L-type ICa observed in the TG neurons from chronically morphine-treated mice is the first example of such an adaptation reported in an opioid-sensitive neuron. The ICa density of PAG or LC neurons is not changed by CMT (Connor et al., 1999a; Bagley et al., 2005a), and culturing SH-SY5Y neurons in morphine also did not change the properties of the ICa in those cells (Kennedy & Henderson, 1992). ICa are important in controlling the excitability of sensory neurons through direct depolarization (Blair & Bean, 2002) and Ca-entry-dependent activation of K channels (Akins & McCleskey, 1993), as well as providing the major source of the Ca required for neurotransmitter release; thus, CMT may alter sensory neuron excitability by mechanisms other than acute agonist/receptor-induced changes in ion channel function.
The molecular mechanisms underlying the trigeminal sensory neuron adaptations to morphine treatment remain to be established. We observed no change in μ-opioid receptor mRNA in ganglia from CMT mice, but have been unable to establish whether CMT produces a change in μ-opioid receptor protein levels because of a lack of suitable antibody for Western blotting (EE Johnson & M Connor, unpublished observations). We are confident that we would have detected a change in μ-opioid receptor mRNA produced by CMT, should it have occurred, because we readily detected a significant reduction in μ-opioid receptor mRNA in TG from mice heterozygous for a deletion of the μ-opioid receptor. If there is no change in the amount of μ-opioid receptor, a reduction in coupling could be produced by a persistent post-translational modification such as receptor phosphorylation (Schulz et al., 2004; Johnson et al., 2005) or changes in the amount or activity of proteins that modify μ-opioid receptor signalling such as regulators of G-protein signalling (Garzon et al., 2005; Xie & Palmer, 2005) or G-protein receptor kinases (Terwilliger et al., 1994; Patel et al., 2002). The inhibition of ICa by Gi/Go-coupled GABAB receptors did not change in CMT neurons, which suggests that there was no nonspecific decrease in cellular G-protein activity or in the inhibitability of the calcium channels. This finding also suggests that the decrease in μ-opioid receptor coupling did not reflect a slowly reversing acute desensitization process, because in sensory neurons acute desensitization of ICa inhibition produced by μ-opioid receptor activation has repeatedly been shown to be heterologous with respect to GABAB receptors (Nomura et al., 1994; Samoriski & Gross, 2000).
Many neurotransmitter systems and signalling molecules have been implicated in the development of analgesic tolerance to morphine, but few of these have been directly shown to be altered in primary afferent neurons. Several studies have shown that chronic opioid treatment produces an apparent increase in the amount of calcitonin gene-related peptide or substance P in dorsal root ganglia or their central terminals, although the degree to which this represents a genuine neuroadaptation or is simply a reflection of ongoing μ-opioid receptor inhibition of transmitter release and accumulation of neuropeptide has not been firmly established (Menard et al., 1996; Ma et al., 2001; Gardell et al., 2002; King et al., 2005). The reduced ICa density observed in the present study could contribute to neuropeptide accumulation by reducing the amount of action potential-induced, calcium-dependent neurotransmitter release.
Although morphine has been shown to be an effective peripheral analgesic in animal models of trigeminal nociception (e.g. Eisenberg et al., 1996; Pelissier et al., 2002) and human trigeminal pain conditions (Likar et al., 1998; Dionne et al., 2001), there is no data of which we are aware that describes tolerance to these peripheral analgesic effects produced by either local or systemic CMT. However, tolerance does develop to antinociceptive effects of chronically administered morphine against electrical stimulation of the tooth pulp in rabbits (Polastron et al., 1994) and mechanical stimulation of the vibrissal pad of rats with a chronic constriction of the infraorbital nerve (Deseure et al., 2003). Although the antinociceptive effects of peripherally administered morphine are well recognized, the degree to which they contribute to the pain relief afforded by systemic opioids in trigeminal pain conditions is unknown.
In this study, we have shown that CMT with a high dose, sustained release preparation produces a substantial reduction in μ-opioid receptor signalling without an accompanying change in μ-opioid receptor mRNA levels. This result is in direct contrast to two other studies that examined μ-opioid receptor regulation in sensory neurons from chronically morphine-treated animals, which showed that intermittent morphine administration produced a significant downregulation of μ-opioid receptor mRNA (Meuser et al., 2003; Starowicz et al., 2005). Neither of these studies directly addressed μ-opioid receptor function in primary afferents, but together with our findings they indicate that the mechanisms underlying morphine-induced regulation of μ-opioid receptor activity may vary depending on how the drug is administered, and it would not be surprising if this also affected the nature of the neuronal adaptations to CMT. It remains an open question what proportion of clinical analgesic tolerance is produced by reduced opioid receptor function and how much arises because of adaptations produced by continued signalling by the remaining receptors, which means that a rational basis for developing therapies to counteract opioid analgesic tolerance is still to be established.
Acknowledgments
This work was supported by NH&MRC Project Grant No. 302002 to M.C. and Project Grant No. 302087 to B.C. We thank Professor MacDonald Christie for his support of this work. The study was designed by M.C.; E.E.J., B.C., I.N. and M.C. performed experiments and analysed data; MC wrote the first draft of the paper. All the authors have seen and approved the final manuscript. None of the authors have any conflicts of interest related to the present work.
Abbreviations
- CMT
chronic morphine treatment
- DAMGO
Tyr-D-Ala-Gly-N-Me-Phe-Gly-ol enkephalin
- DEPC
diethyl pyrocarbonate
- HBS
HEPES-buffered saline
- HPRT
hypoxanthine phosphoribosyltransferase
- ICa
voltage-gated calcium channel current
- LC
locus coeruleus
- PAG
periaqueductal grey
- RT–PCR
reverse transcription–polymerase chain reaction
- TG
trigeminal ganglion
References
- AKINS P.T., MCCLESKEY E.W. Characterization of potassium currents in adult rat sensory neurons and modulation by opioids and cyclic AMP. Neuroscience. 1993;56:759–769. doi: 10.1016/0306-4522(93)90372-m. [DOI] [PubMed] [Google Scholar]
- BAGLEY E.E., CHIENG B., CHRISTIE M.J., CONNOR M. Opioid tolerance in periaqueductal grey neurons isolated from mice chronically treated with morphine. Br. J. Pharmacol. 2005a;146:68–76. doi: 10.1038/sj.bjp.0706315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAGLEY E.E., GERKE M.B., VAUGHAN C.W., HACK S.P., CHRISTIE M.J. GABA transporter currents activated by protein kinase A excite midbrain neurons during opioid withdrawal. Neuron. 2005b;45:433–445. doi: 10.1016/j.neuron.2004.12.049. [DOI] [PubMed] [Google Scholar]
- BAILEY C.P., CONNOR M. Opioids: cellular mechanisms of tolerance and physical dependence. Curr. Opin. Pharmacol. 2005;5:60–68. doi: 10.1016/j.coph.2004.08.012. [DOI] [PubMed] [Google Scholar]
- BLAIR N.T., BEAN B.P. Roles of tetrodotoxin (TTX)-sensitive Na+ current, TTX-resistant Na+ current, and Ca2+ current in the action potentials of nociceptive sensory neurons. J. Neurosci. 2002;22:10277–10290. doi: 10.1523/JNEUROSCI.22-23-10277.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BORGLAND S.L., CONNOR M., CHRISTIE M.J. Nociceptin inhibits calcium channel currents in a subpopulation of small nociceptive trigeminal ganglion neurons in mouse. J. Physiol. (London) 2001;536:35–47. doi: 10.1111/j.1469-7793.2001.t01-1-00035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BORGLAND S.L., CONNOR M., RYAN R.M., BALL H., CHRISTIE M.J. Prostaglandin E2 inhibits calcium current in two subpopulations of acutely isolated mouse trigeminal sensory neurons. J. Physiol. (London) 2002;539:433–444. doi: 10.1113/jphysiol.2001.013322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUSTIN S.A., BENES V., NOLAN T., PFAFFL M.W. Quantitative real time RT–PCR – a perspective. J. Mol. Endocrinol. 2005;34:597–601. doi: 10.1677/jme.1.01755. [DOI] [PubMed] [Google Scholar]
- BUNTIN-MUSHOCK C., PHILLIP L., MORIYAMA K., PALMER P.P. Age-dependent opioid escalation in chronic pain patients. Anesth. Analg. 2005;100:1740–1745. doi: 10.1213/01.ANE.0000152191.29311.9B. [DOI] [PubMed] [Google Scholar]
- CHRISTIE M.J., WILLIAMS J.T., NORTH R.A. Cellular mechanisms of opioid tolerance: studies in single brain neurons. Mol Pharmacol. 1987;32:633–638. [PubMed] [Google Scholar]
- CONNOR M., BORGLAND S.L., CHRISTIE M.J. Continued morphine modulation of calcium channel currents in acutely isolated locus coeruleus neurons from morphine-dependent rats. Br. J. Pharmacol. 1999a;128:1561–1569. doi: 10.1038/sj.bjp.0702922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONNOR M., SCHULLER A., PINTAR J.E., CHRISTIE M.J. Opioid receptor modulation of calcium channel current in periaqueductal grey neurons form C57B16/J mice and mutant mice lacking MOR-1. Br. J. Pharmacol. 1999b;126:1553–1558. doi: 10.1038/sj.bjp.0702457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DESEURE K., KOEK W., ADRIAENSEN H., COLPAERT F.C. Continuous administration of the 5-hydroxytryptamine1A agonist (3-chloro-4-fluoro-phenyl)-[4-fluoro-4-{[(5-methyl-pyridin-2-ylmethyl)-amino]-methyl}piperidin-1-yl]-methadone (F 13640) attenuates allodynia-like behavior in a rat model of trigeminal neuropathic pain. J. Pharmacol. Exp. Ther. 2003;306:505–514. doi: 10.1124/jpet.103.050286. [DOI] [PubMed] [Google Scholar]
- DE WAARD M., HERING J., WEISS N., FELTZ A. How do G proteins directly control neuronal Ca2+ channel function. Trends Pharmacol. Sci. 2005;26:427–436. doi: 10.1016/j.tips.2005.06.008. [DOI] [PubMed] [Google Scholar]
- DIONNE R.A., LEPINSKI A.M., GORDON S.M., JABER L., BRAHIM J.S., HARGREAVES K.M. Analgesic effects of peripherally administered opioids in clinical models of acute and chronic inflammation. Clin. Pharmacol. Ther. 2001;70:66–73. doi: 10.1067/mcp.2001.116443. [DOI] [PubMed] [Google Scholar]
- EISENBERG E., VOS B.P., STRASSMAN A.M. The peripheral antinociceptive effect of morphine in a rat model of facial pain. Neuroscience. 1996;72:519–525. doi: 10.1016/0306-4522(95)00565-x. [DOI] [PubMed] [Google Scholar]
- GARDELL L.R., WANG R., BURGESS S.E., OSSIPOV M.H., VANDERAH T.W., MALAN T.P., LAI J., PORRECA F. Sustained morphine exposure induces a spinal dynorphin-dependent enhancement of excitatory transmitter release from primary afferent fibers. J. Neurosci. 2002;22:6747–6755. doi: 10.1523/JNEUROSCI.22-15-06747.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARZON J., RODRIGUEZ-MUNOZ M., DE LA TORRE-MADRID E., SANCHEZ-BLASQUEZ P. Effector antagonism by the regulators of G protein signalling (RGS) proteins causes desensitization of mu-opioid receptors in the CNS. Psychopharmacology. 2005;180:1–11. doi: 10.1007/s00213-005-2248-9. [DOI] [PubMed] [Google Scholar]
- GENDRON L., LUCIDO A.L., MENNICKEN F., O'DONNELL D., VINCENT J.-P., STROH T., BEAUDET A. Morphine and pain-related stimuli enhance cellular availability of somatic δ-opioid receptors in rat dorsal root ganglia. J. Neurosci. 2006;26:953–962. doi: 10.1523/JNEUROSCI.3598-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GU G., KONDO I., HUA X.-Y., YAKSH T.L. Resting and evoked spinal substance P release during chronic intrathecal morphine infusion: parallels with tolerance and dependence. J. Pharmacol. Exp. Ther. 2005;314:1362–1369. doi: 10.1124/jpet.105.087718. [DOI] [PubMed] [Google Scholar]
- HAMILL O.P., MARTY A., NEHER E., SAKMANN B., SIGWORTH F.J. Improved patch-clamp techniques for high resolution current recording from cells and cell free membrane patches. Pflugers Archiv. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- HONORE P., CATHELINE G., LE GUEN S., BESSON J.-M. Chronic treatment with systemic morphine induced tolerance to the systemic and peripheral antinociceptive effects of morphine on both carrageenan induced mechanical hyperalgesia and spinal c-Fos expression. Pain. 1997;71:99–108. doi: 10.1016/s0304-3959(97)03345-9. [DOI] [PubMed] [Google Scholar]
- INGRAM S.L., VAUGHAN C.W., BAGLEY E.E., CONNOR M., CHRISTIE MJ. Enhanced opioid efficacy in opioid dependence is due to an additional signal transduction pathway. J. Neurosci. 1998;18:10269–10276. doi: 10.1523/JNEUROSCI.18-24-10269.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSON E.E., CHRISTIE M.J., CONNOR M. The role of opioid receptor phosphorylation and trafficking in adaptations to persistent opioid treatment. Neurosignals. 2005;14:290–302. doi: 10.1159/000093044. [DOI] [PubMed] [Google Scholar]
- KENNEDY C., HENDERSON G. Opioid receptor inhibition of calcium current: development of homologous tolerance in single SH-SY5Y cells after chronic exposure to morphine in vitro. Mol. Pharmacol. 1991;49:937–944. [PubMed] [Google Scholar]
- KENNEDY C., HENDERSON G. Chronic exposure to morphine does not induce dependence at the level of the calcium channel current in human SH-SY5Y cells. Neuroscience. 1992;49:937–944. doi: 10.1016/0306-4522(92)90369-d. [DOI] [PubMed] [Google Scholar]
- KING T., GARDELL L.R., WANG R., VARDANYAN A., OSSIPOV M.H., MALAN T.P., VANDERAH T.W., HUNT S.P., HRUBY V.J., LAI J., PORRECA F. Role of NK-1 neurotransmission in opioid-induced hyperalgesia. Pain. 2005;116:276–288. doi: 10.1016/j.pain.2005.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIKAR R., SITTL R., GRAGGER K., PIPAM W., BLATNIG H., BRESCHOU C., SCHALK H.V., STEIN C., SCHAFER M. Peripheral morphine analgesia in dental surgery. Pain. 1998;75:145–150. doi: 10.1016/s0304-3959(98)00036-0. [DOI] [PubMed] [Google Scholar]
- MA W., ZHENG W.-H., POWELL K., JHAMANDAS K., QUIRION R. Chronic morphine exposure increases the phosphorylation of MAP kinases and the transcription factor CREB in dorsal root ganglion neurons: an in vitro and in vivo study. Eur. J. Neurosci. 2001;14:1091–1104. doi: 10.1046/j.0953-816x.2001.01731.x. [DOI] [PubMed] [Google Scholar]
- MCQUAY H. Opioids in pain management. The Lancet. 1999;353:2229–2232. doi: 10.1016/S0140-6736(99)03528-X. [DOI] [PubMed] [Google Scholar]
- MENARD D.P., VAN ROSSUM D., KAR S., ST PIERRE S., SUTAK M., JHAMANDAS K., QUIRION R. A calcitonin gene-related peptide receptor antagonist prevents the development of tolerance to spinal morphine analgesia. J. Neurosci. 1996;16:2342–2351. doi: 10.1523/JNEUROSCI.16-07-02342.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEUSER T., GIESECKE T., GABRIEL A., HORSCH M., SABATOWSKI R., HESCHLER J., GROND S., PALMER P.P. Mu-opioid receptor mRNA regulation during morphine tolerance in the rat peripheral nervous system. Anesth. Analg. 2003;97:1458–1463. doi: 10.1213/01.ANE.0000081721.75663.87. [DOI] [PubMed] [Google Scholar]
- NOMURA K., REUVENY E., NARAHASHI T. Opioid inhibition and desensitization of calcium channel currents in rat dorsal root ganglion neurons. J. Pharmacol. Exp. Ther. 1994;270:466–474. [PubMed] [Google Scholar]
- OSSIPOV M.H., LAI J., KING T., VANDERAH T.W., MALAN T.P., HRUBY V.J., PORECCA F. Antinociceptive and nociceptive actions of opioids. J. Neurobiol. 2004;61:125–148. doi: 10.1002/neu.20091. [DOI] [PubMed] [Google Scholar]
- PATEL M.B., PATERL C.N., RAJASHEKARA V., YOBURN B.C. Opioid agonists differentially regulate μ-opioid receptor trafficking proteins in vivo. Mol. Pharmacol. 2002;62:1464–1470. doi: 10.1124/mol.62.6.1464. [DOI] [PubMed] [Google Scholar]
- PELISSIER T., PAJOT J., DALLEL R. The orofacial capsaicin test in rats: effects of different capsaicin concentrations and morphine. Pain. 2002;96:81–87. doi: 10.1016/s0304-3959(01)00432-8. [DOI] [PubMed] [Google Scholar]
- POLASTRON J., MEUNIER J.-C., JAUZAC P. Chronic morphine induces tolerance and desensitization of μ-opioid receptor but not down-regulation in rabbit. Eur. J. Pharmacol. 1994;266:139–146. doi: 10.1016/0922-4106(94)90103-1. [DOI] [PubMed] [Google Scholar]
- ROBERTS L.A., CHRISTIE M.J., CONNOR M. Anandamide is a partial agonist at native vanilloid receptors in acutely isolated mouse trigeminal sensory neurons. Br. J. Pharmacol. 2002;137:421–428. doi: 10.1038/sj.bjp.0704904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMORISKI G.M., GROSS R.A. Functional compartmentalization of opioid desensitization in primary sensory neurons. J. Pharmacol. Exp. Ther. 2000;294:500–509. [PubMed] [Google Scholar]
- SCHULLER A.G.P., KING M.A., ZHANG J., BOLAN E., PAN Y.-X., MORGAN D.J., CHANG A., CZICK M.E., UNTERWALD E.M., PASTERNAK G.W., PINTAR J.E. Retention of heroin and morphine-6β-glucuronide analgesia in anew strain of mice lacking exon 1 one MOR-1. Nat. Neurosci. 1999;2:151–156. doi: 10.1038/5706. [DOI] [PubMed] [Google Scholar]
- SCHULZ S., MAYER D., PFEIFFER M., STUMM R., KOCH T., HOLLT V. Morphine induces terminal μ-opioid receptor desensitization by sustained phosphorylation of serine-375. EMBO J. 2004;23:3282–3289. doi: 10.1038/sj.emboj.7600334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STAROWICZ K., OBARA I., PRZEWLOCKI R., PRZEWLOCKA B. Inhibition of morphine tolerance by spinal melanocortin receptor blockade. Pain. 2005;117:401–411. doi: 10.1016/j.pain.2005.07.003. [DOI] [PubMed] [Google Scholar]
- TERWILLIGER R.Z., ORTIZ J., GUITART X., NESTLER E.J. Chronic morphine administration increases b-adrenergic kinase (BARK) levels in the rat locus coeruleus. J. Neurochem. 1994;63:1983–1986. doi: 10.1046/j.1471-4159.1994.63051983.x. [DOI] [PubMed] [Google Scholar]
- WILLIAMS J.T., CHRISTIE M.J., MANZONI O. Cellular and synaptic adaptations mediating opioid dependence. Physiol. Rev. 2001;81:299–343. doi: 10.1152/physrev.2001.81.1.299. [DOI] [PubMed] [Google Scholar]
- XIE G.-X., PALMER P.P. RGS proteins: new players in the field of opioid signaling and tolerance mechanisms. Anesth. Analg. 2005;100:1034–1042. doi: 10.1213/01.ANE.0000147711.51122.4B. [DOI] [PubMed] [Google Scholar]