Abstract
The aims of the present study were firstly, to characterize pharmacologically the subtypes of P1 purinoreceptors involved in the inhibitory effects induced by exogenous adenosine in longitudinal smooth muscle of mouse colon, and secondly, to examine differences in the function and distribution of these receptors between proximal and distal colon.
Adenosine (100 μM–3 mM) caused a concentration-dependent reduction of the amplitude of spontaneous contractions in the proximal colon, and muscular relaxation in the distal colon. In the proximal colon, adenosine effects were antagonized by a selective A1 receptor antagonist, 1,3-dipropyl-8-cyclopentylxanthine (DPCPX, 10 nM), but were not modified by 3,7-dimethyl-1-propargylxanthine (DMPX, 10 μM) or by 9-chloro-2-(2-furanyl)-5-((phenylacetyl)amino)- [1,2,4]triazolo[1,5-c]quinazoline (MRS 1220, 0.1 μM), selective A2 and A3 receptor antagonists, respectively. In the distal colon, adenosine effects were antagonized by DPCPX, DMPX, and by a selective A2B receptor antagonist, 8-[4-[((4-cyanophenyl)carbamoylmethyl)oxy]phenyl]-1,3-di(n-propyl) xanthine (MRS 1754, 10 μM), but not by 8-(3-chlorostyryl)-caffeine (CSC, 10 μM), a selective A2A receptor antagonist, or by MRS 1220.
Tetrodotoxin (TTX 1 μM), the nitric oxide (NO) synthase inhibitor, Nω-nitro-L-arginine methyl ester (L-NAME, 100 μM), or 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (10 μM), an inhibitor of soluble guanylyl cyclase, reduced adenosine effects only in distal colon. In addition, L-NAME induced a further reduction of adenosine relaxation in the presence of DPCPX, but not in the presence of MRS 1754.
From these results we conclude that, in the murine proximal colon, adenosine induces inhibitory effects via TTX-insensitive activation of A1 receptor. In the distal colon, adenosine activates both A1 and A2B receptors, the latter located on enteric inhibitory neurons releasing NO.
Keywords: Adenosine, mouse colon, mechanical activity, P1 purinoreceptors, adenosine A1 receptors, adenosine A2 receptors, adenosine A2B receptors, adenosine A3 receptors, nitrergic nerves
Introduction
Adenosine 5′-triphosphate (ATP) and related purine derivatives are known to act as neurotransmitters or neuromodulators in the central and peripheral nervous systems (Burnstock, 1997). ATP released from enteric nerves has been reported to mediate inhibition of muscular activity in different regions of the gastrointestinal tract from various animal species via activation of P2 receptors, mainly the P2Y family (Koh et al., 1997; Zagorodnyuk & Maggi, 1998; Xue et al., 1999; De Man et al., 2003a; Mulè & Serio, 2003; Serio et al., 2003b; Van Crombruggen & Lefebvre, 2004; Mulè et al., 2005). Adenosine, formed by the breakdown of ATP by ectoenzymes or released by enteric nerves, can influence gastrointestinal motility directly, by activating receptors located in smooth muscle (Serio et al., 1990; Nicholls et al., 1996; Kadowaki et al., 2000; Woods et al., 2003), or indirectly, by regulating the neurotransmitter release from enteric neurons (Tomaru et al., 1995; Moneta et al., 1997; Lee et al., 2001; Storr et al., 2002).
Adenosine receptors are designated as P1 receptors and further subdivided in A1, A2 (A2A and A2B) and A3 receptors by the relative order of potency of agonists and antagonists and by the coupled transduction mechanisms (Collis & Hourani, 1993; Ralevic & Burnstock, 1998). Their localization and function vary depending on the intestinal region or animal species. A1 receptors have been localized on excitatory nerve endings, reducing neurotransmitter release, in rat ileum and in guinea-pig ileum and distal colon (Kadowaki et al., 2000; Lee et al., 2001; Storr et al., 2002), and on smooth muscle, inducing direct muscular inhibition, in rat duodenum and ileum (Nicholls et al., 1996; Nicholls & Hourani, 1997). Moreover, A1 receptors have been shown to mediate also adenosine excitatory effects in several tissues (Bailey et al., 1992; Murthy et al., 1995; Shim et al., 2002). Postsynaptic inhibitory A2 receptors have been observed in rat colon (Bailey & Hourani, 1992), guinea-pig distal colon (Kadowaki et al., 2000) and possum duodenum (Woods et al., 2003). Lastly, postsynaptic inhibitory A3 receptors have been found in the possum duodenum (Woods et al., 2003).
Mice are becoming increasingly important subjects for investigating gastrointestinal motility because of the availability of mutants and the advent of gene-targeting technology. However, so far a full description of the functions of purines, and in particular of adenosine, in the mouse gastrointestinal tract is not yet available. Giaroni et al. (2002) reported that adenosine induced relaxation in all regions of the mouse gastrointestinal tract, suggesting the presence of inhibitory P1 receptors throughout the tract. In addition, postjunctional A1 receptors appear to be present in mouse jejunum (De Man et al., 2003a), and neuronal A1 receptors modulate cholinergic nerve activity in mouse ileum (De Man et al., 2003b). So far, there are no studies concerning the subtypes of adenosine receptors in mouse colon.
The aims of our study were to characterize the subtypes of P1 purinoreceptors, A1, A2 (A2A and A2B) or A3 receptors, involved in the inhibitory effects induced by adenosine in the longitudinal smooth muscle of mouse colon, and to examine differences in the function and distribution of the receptors between proximal and distal colon. In addition, possible interactions with the nitric oxide (NO) system, the main inhibitory system in murine intestine, have been evaluated.
Methods
Animals
All animal procedures complied with the regulations of the Ministero della Sanità (Italy), for animal welfare. Adult male mice of the C57BL/10SnJ strain (27±0.3 g body weight; 15 weeks old) were obtained from Charles River Laboratories (Calco-Lecco, Italy) and were maintained in a light (12 h dark/12 h light) and temperature (23°C) controlled environment with free access to food and water. Tissues were taken from animals killed by cervical dislocation. The abdomen was immediately opened, the colon was rapidly removed, and placed in a dissecting dish filled with oxygenated Krebs solution and its contents gently flushed out. Then, segments (about 12 mm in length) were obtained either from proximal (immediately distal to the caecum) or distal (about 5 mm proximal to the anus) colon and suspended in 10 ml organ baths containing oxygenated (95% O2 and 5% CO2) Krebs solution maintained at 37°C.
Recording of mechanical activity
The distal end of each segment was tied to an organ holder and the proximal end was secured with a silk thread to an isometric force transducer (FORT 10, Ugo Basile, Biological Research Apparatus, Comerio VA, Italy). Mechanical activity was digitized on a A/D converter, visualized, recorded, and analyzed on a personal computer using the PowerLab/400 system (Ugo Basile, Italy). Atropine (1 μM) and guanethidine (1 μM) were added to the Krebs solution at the beginning of the experiment to establish non adrenergic, non cholinergic (NANC) conditions. Longitudinal preparations were subjected to an initial tension of 200 mg and were allowed to equilibrate for at least 30 min. Spontaneous contractions of varying amplitude developed in all preparations. At the end of the equilibration period, the preparations were challenged with isoprotenerol (0.1 μM) to check that they were able to relax. Proximal and distal colon were relaxed by isoprotenerol equally (in proximal colon the relaxation was 193.1±9.0 mg, while in distal colon it was 225.4±19.5 mg; n=8, P>0.05).
Experimental protocols
After the equilibration time, concentration-dependent curves for adenosine were constructed by noncumulative addition of the drug before and after the different drugs used. Adenosine was applied for approximately 3 min at 20 min intervals. All the antagonists were allowed to maintain contact with the tissue for at least 30 min before repeating the doses of the agonist. The interval between the two assays was at least 1 h. Each preparation was tested with a single antagonist, except when otherwise stated. Time control experiments showed that a second curve to the agonist was reproducible. Concentrations of the drugs used were determined from the literature.
Solution and drugs
The composition of the Krebs solution was (mM): NaCl 119; KCl 4.5; MgSO4 2.5; NaHCO3 25; KH2PO4 1.2, CaCl2 2.5, glucose 11.1. The following drugs were used: adenosine, atropine sulfate, 8-(3-chlorostyryl)-caffeine (CSC), 9-chloro-2-(2-furanyl)-5-((phenylacetyl) amino)-[1,2,4]triazolo[1,5-c]quinazoline (MRS 1220), 8-[4-[((4-cyanophenyl) carbamoylmethyl) oxy] phenyl]-1,3-di (n-propyl)xanthine (MRS 1754), 3,7-dimethyl-1-propargylxanthine (DMPX), 1,3-dipropyl-8-cyclopentylxanthine (DPCPX), guanethidine monosulfate, isoprotenerol, Nω-nitro-L-arginine methyl ester (L-NAME), 1H-[1,2,4] oxadiazolo [4,3-a]quinoxalin-1-one (ODQ), 4-[[4-formyl-5-hydroxy-6-methyl-3-[(phosphonooxy) methyl]-2-pyridinyl]azo]-1,3-benzenedisulfonic acid tetrasodium salt (PPADS), sodium nitroprusside (SNP), theophylline, tetrodotoxin (TTX) (Sigma-Aldrich Inc., St Louis, U.S.A.). Adenosine, CSC, DPCPX, ODQ, MRS 1220, MRS 1754 were dissolved in dimethyl sulfoxide and further diluted in Krebs solution; DMPX was dissolved in ethanol. All the other drugs were dissolved in distilled water. The maximal final concentration of ethanol and dimethyl sulfoxide in the organ bath was 0.5%, which did not affect the contractility of the colonic segments.
The working solutions were prepared fresh on the day of the experiment by diluting the stock solutions in Krebs solution and were added to the organ bath.
Statistical analysis
All data are given as means±s.e.m.: ‘n' in the results section refers to the number of animal preparations on which observations were made. In proximal colon, inhibitory effects induced by adenosine were estimated as the decrease in the amplitude of the spontaneous contraction and reported as a percentage of the maximal effect induced by 3 mM adenosine, corresponding to total suppression of spontaneous contractions. In distal colon, inhibitory effects induced by adenosine were estimated as the decrease in basal tone and reported as a percentage of the maximal effect induced by 3 mM adenosine, corresponding to the maximal relaxation obtained. Adenosine responses in the absence or in the presence of the different antagonists were fitted to sigmoid curves (Prism 4.0, GraphPAD, San Diego, CA, U.S.A.), and EC25 values with 95% confidence limits (CLs) were determined from these curves. Statistical analysis was performed by means of Student's t-test or by means of analysis of variance followed by Bonferroni's test, when appropriate. A probability value of <0.05 was regarded as significant.
Results
Isolated segments of mouse colon displayed spontaneous activity consisting of phasic contractions with an amplitude of 401.7±60.8 mg and a frequency of 5.54±0.77 c.p.m. in the proximal region (n=32), and with an amplitude of 254.6±22.4 mg and a frequency of 5.3±0.55 c.p.m. in the distal region (n=36).
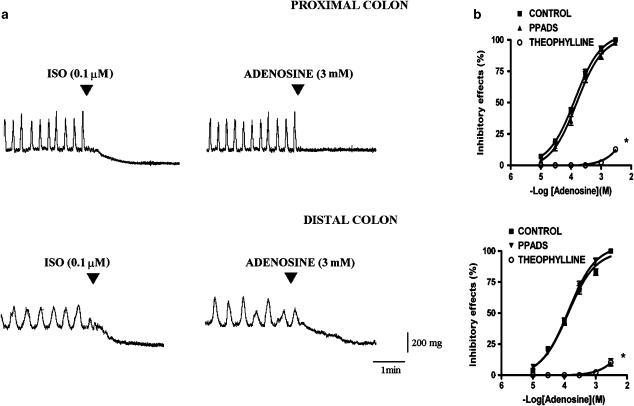
Adenosine (100 μM–3 mM) caused concentration-dependent inhibitory effects on longitudinal muscle of both proximal and distal colon. In proximal colon, the response to adenosine consisted of a reduction of the amplitude of spontaneous contractions, up to their complete disappearance at a concentration of 3 mM (Figure 1). In distal colon, adenosine caused a concentration-dependent relaxation (Figure 1). The actual amplitude of the relaxation observed in the distal colon in response to 3 mM of adenosine was 231.2±18.9 mg (n=27).
Figure 1.
(a) Original recordings showing the inhibitory responses evoked by isoprotenerol or adenosine in proximal and in distal longitudinal colonic muscular preparations. (b) Concentration–response curves to adenosine in proximal and in distal colon in the absence or in the presence of theophylline (0.1 mM, n=4) or PPADS (50 μM, n=4). Data are means±s.e.m. and are expressed as percentages of the maximal effect induced by 3 mM adenosine. The values for the control curves are the means of the control data obtained before each treatment (n=8). *P<0.05 when the concentration–response curves were compared to those obtained in the respective control condition.
In both proximal and in distal colon, theophylline (0.1 mM), a nonselective P1 receptor blocker, significantly antagonized the inhibitory effects induced by adenosine (Figure 1). On the contrary, PPADS (50 μM), a nonselective P2 purinoceptor antagonist, had no effect on the inhibitory effects of adenosine (Figure 1, Table 1), indicating that adenosine exerts its effects by acting at P1 receptors.
Table 1.
EC25 values for adenosine-induced inhibitory effects before and after different pharmacological treatments
| |
Proximal colon |
Distal colon |
||||
|---|---|---|---|---|---|---|
| EC25 (mM) | 95% CLs (mM) | n | EC25 95% (mM) | CLs (mM) | n | |
| Control |
0.044 |
0.038–0.050 |
4 |
0.040 |
0.035–0.044 |
4 |
| PPADS |
0.058 |
0.048–0.072 |
4 |
0.043 |
0.036–0.052 |
4 |
| |
|
|
|
|
|
|
| Control |
0.045 |
0.031–0.068 |
5 |
0.043 |
0.031–0.060 |
5 |
| DPCPX |
0.336* |
0.256–0.440 |
5 |
0.069* |
0.039–0.120 |
5 |
| |
|
|
|
|
|
|
| Control |
0.040 |
0.033–0.070 |
6 |
0.045 |
0.031–0.075 |
6 |
| DMPX |
0.051 |
0.034–0.075 |
6 |
0.920* |
0.650–1.297 |
6 |
| |
|
|
|
|
|
|
| Control |
— |
— |
|
0.042 |
0.026–0.098 |
4 |
| DPCPX+DMPX |
— |
— |
|
3.365§ |
2.786–4.055 |
4 |
| |
|
|
|
|
|
|
| Control |
— |
— |
|
0.046 |
0.028–0.074 |
5 |
| CSC |
— |
— |
|
0.059 |
0.027–0.130 |
5 |
| |
|
|
|
|
|
|
| Control |
— |
— |
|
0.044 |
0.030–0.071 |
5 |
| MRS 1754 |
— |
— |
|
0.596* |
0.354–0.881 |
5 |
| |
|
|
|
|
|
|
| Control |
0.041 |
0.031–0.075 |
4 |
0.043 |
0.030–0.077 |
4 |
| MRS 1220 |
0.046 |
0.034–0.064 |
4 |
0.051 |
0.032–0.079 |
4 |
| |
|
|
|
|
|
|
| Control |
0.035 |
0.027–0.046 |
6 |
0.042 |
0.031–0.076 |
6 |
| TTX |
0.049 |
0.027–0.090 |
6 |
0.780* |
0.052–1.112 |
6 |
| |
|
|
|
|
|
|
| Control |
— |
— |
|
0.050 |
0.032–0.089 |
4 |
| DPCPX |
— |
— |
|
0.084* |
0.047–0.150 |
4 |
| DPCPX+TTX |
— |
— |
|
1.343§ |
0.960–1.875 |
4 |
| |
|
|
|
|
|
|
| Control |
— |
— |
|
0.051 |
0.029–0.108 |
5 |
| MRS 1754 |
— |
— |
|
0.483* |
0.260–0.898 |
5 |
| MRS 1754+TTX |
— |
— |
|
0.583* |
0.335–1.106 |
5 |
| |
|
|
|
|
|
|
| Control |
0.049 |
0.030–0.078 |
6 |
0.044 |
0.029–0.066 |
6 |
|
L-NAME |
0.067 |
0.040–0.111 |
6 |
0.710* |
0.590–0.850 |
6 |
| |
|
|
|
|
|
|
| Control |
— |
— |
|
0.034 |
0.024–0.048 |
4 |
| DPCPX |
— |
— |
|
0.073* |
0.034–0.156 |
4 |
| DPCPX+L-NAME |
— |
— |
|
1.076§ |
0.863–1.346 |
4 |
| |
|
|
|
|
|
|
| Control |
— |
— |
|
0.047 |
0.026–0.085 |
4 |
| MRS 1754 |
— |
|
|
0.454* |
0.221–0.665 |
4 |
| MRS 1754+L-NAME |
— |
— |
|
0.501* |
0.356–0.704 |
4 |
| |
|
|
|
|
|
|
| Control |
— |
— |
|
0.044 |
0.027–0.075 |
4 |
| ODQ | — | — | 0.530* | 0.480–0.620 | 4 | |
P<0.05 when compared to the respective control.
P<0.05 when compared to DPCPX alone.
As P1 purinoreceptors are divided into A1, A2 (A2A and A2B) and A3 subclasses, we tested the effects of selective antagonists on adenosine-induced inhibitory effects, to characterize the receptor subtype(s) involved in the observed response. DPCPX (10 nM), a selective A1 receptor antagonist, which per se did not modify spontaneous activity, significantly reduced the adenosine induced effects in both portions of colon, indicating the presence of A1 receptors in both regions (Figure 2, Table 1). The A2 receptor antagonist, DMPX (10 μM), did not modify the spontaneous mechanical activity and had no effect on the inhibition induced by adenosine in proximal colon, while it significantly antagonized the relaxation in the distal portion. Application of DPCPX and DMPX, together, to samples of distal colon showed additive effects (Figure 2). MRS 1220 (0.1 μM), a selective A3 receptor antagonist, failed to modify the effects of adenosine in both portions (Figure 2, Table 1). In distal colon, in order to discriminate between A2A and A2B receptor subtypes we tested the effects of CSC or MRS 1754, which are selective A2A and A2B receptor antagonists, respectively, on the adenosine-induced relaxation. Relaxation was not modified by CSC (10 μM), but it was significantly antagonized by MRS 1754 (10 μM) (Figure 3). However, neither drug modified the spontaneous mechanical activity.
Figure 2.
Concentration–response curves to adenosine before and after DPCPX (10 nM, n=5), DMPX (10 μM, n=6), MRS 1220 (0.1 μM, n=4) in proximal colon or DPCPX plus DMPX (n=4) in distal colon. Data are means±s.e.m. and are expressed as percentages of the maximal effect induced by 3 mM adenosine. The values for the control curves are the means of the control data obtained before each treatment (proximal colon: n=15; distal colon: n=19). *P<0.05 when the concentration–response curves were compared to those obtained in the respective control condition. §P<0.05 when the concentration–response curves were compared to DPCPX alone.
Figure 3.
Concentration–response curves to adenosine before or after CSC (10 μM, n=5) or MRS 11754 (10 μM, n=5) in distal colon. Data are means±s.e.m. and are expressed as percentages of the maximal effect induced by 3 mM adenosine. The values for the control curve are the means of the control data obtained before each treatment (n=10). *P<0.05 when the concentration–response curve was compared to that obtained in the control condition.
Tetrodotoxin (TTX; 1 μM), which blocks voltage-gated Na+ channels in neurons, did not prevent the inhibitory effects of adenosine in proximal colon, while in distal colon, TTX partially reduced the relaxation evoked by adenosine. In distal colon, TTX caused a further inhibition of the response to adenosine remaining in the presence of DPCPX, but it was without effect on the response resistant to MRS 1754 (Figure 4, Table 1).
Figure 4.
(a, b) Concentration–response curves to adenosine before (n=6) and after treatment with TTX (1 μM, n=6) in proximal and distal colon. (c) Concentration–response curves to adenosine before (n=4) and after DPCPX (10 nM, n=4) or DPCPX plus TTX (n=4) in distal colon. (d) Concentration–response curves to adenosine before (n=5) and after MRS 1754 (10 μM, n=5) or MRS 1754 plus TTX (n=5) in distal colon. Data are means±s.e.m. and are expressed as percentages of the maximal effect induced by 3 mM adenosine. *P<0.05 when the concentration–response curves were compared to those obtained in the respective control condition. §P< 0.05 when the concentration–response curves were compared to DPCPX alone.
Interaction with the NO pathway
The inhibitory response to adenosine could be related to activation of the NO pathway in the colon. We therefore tested the effects of adenosine in the presence of drugs which interfere with nitrergic transmission. The NO synthase inhibitor, L-NAME (100 μM), significantly reduced adenosine effects in the distal colon, but did not affect responses to adenosine in the proximal colon (Figure 5, Table 1). A selective inhibitor of NO-stimulated soluble guanylyl cyclase, ODQ (10 μM), also induced a reduction of adenosine-induced inhibitory effects in distal colon (Figure 5, Table 1). Moreover, in this tissue, L-NAME reduced the response to adenosine remaining in the presence of DPCPX, but it was without any effects on the response after MRS 1754 (Figure 5, Table 1). The NO donor, sodium nitroprusside (SNP; 100 μM), induced a muscular relaxation that was insensitive to pretreatment with theophylline (0.1 mM), DPCPX (10 nM) or DMPX (10 μM) (data not illustrated).
Figure 5.
(a) Concentration–response curves to adenosine before (n=6) and after L-NAME (100 μM, n=6) in proximal colon. (b) Concentration–response curves to adenosine before (n=10, the values are the means of the control data obtained before each treatment) and after L-NAME (100 μM, n=6) or ODQ (1 μM, n=4) in distal colon. (c) Concentration–response curves to adenosine before (n=4) and after DPCPX (10 nM, n=4) or DPCPX plus L-NAME (n=4), in distal colon. (d) Concentration–response curves to adenosine before (n=4) and after MRS 1754 (10 μM, n=4) or MRS 1754 plus L-NAME (n=4), in distal colon. Data are means±s.e.m. and are expressed as percentages of the maximal effect induced by 3 mM adenosine. *P<0.05 when the concentration–response curves were compared to those obtained in the respective control condition. §P<0.05 when the concentration–response curves were compared to DPCPX alone.
Discussion
The results of the present study show that exogenous adenosine exerted an inhibitory effect on muscular contractility in mouse colon in conditions where both cholinergic and catecholaminergic transmission were blocked (NANC conditions). In proximal colon, adenosine effects were due to TTX-insensitive activation of A1 purinoceptors, whereas in distal colon, adenosine activated A1 and A2B purinoceptors, the latter located on enteric inhibitory nerves, most probably generating NO.
Adenosine is considered as an inhibitory neurotransmitter of the enteric nervous system in different animal species (Ralevic & Burnstock, 1998). Adenosine can influence gastrointestinal motility directly, by activating receptors located on smooth muscle (Serio et al., 1990; Nicholls et al., 1996; Kadowaki et al., 2000; Woods et al., 2003), or indirectly, by regulating neurotransmitter release from enteric neurons. Giaroni et al. (2002) suggested the presence of P1 receptors in mouse colon, but the receptors subtypes were not investigated. Our studies confirm that in mouse colon, adenosine induces inhibitory effects and, as our experiments have been performed in the presence of atropine and guanethidine (NANC conditions), they are not the consequence of a modulation of cholinergic or catecholaminergic transmission. The response of the tissue was observed in a range of adenosine concentrations similar to that observed by Giaroni et al. (2006) in mouse intestine, but quite high when compared to other animal preparations. This may reflect a species specificity in sensitivity of intestinal smooth muscle to adenosine. Interestingly, the responses to adenosine differ between proximal and distal colon. In proximal colon, adenosine plays a modulator role, causing only a decrease in the amplitude of the spontaneous contractions, although the proximal colon was able to relax in response to isoprotenerol. Indeed, in distal colon adenosine induces muscular relaxation. It may be that these different responses are related to the different motor patterns of the proximal and distal colon: the former functions as reservoir, whereas the latter acts as a propulsive conduit (Camilleri & Ford, 1998).
The observation that adenosine effects were prevented by theophylline, a nonselective P1 purinoceptor antagonist, but not by PPADS, the nonselective P2 purinoreceptor antagonist, indicated that adenosine was acting on P1 purinoceptors. However, theophylline is known also as a nonselective inhibitor of cyclic nucleotide phosphodiesterase, so the marked reduction of the adenosine effects could be also due to an interference with the intracellular signalling pathways induced by activation of adenosine receptors.
The next step was to analyze the effects induced by more selective adenosine receptor antagonists in order to distinguish between the different receptor subtypes. Adenosine acts through different G-protein-coupled P1 purinoreceptor subtypes classified as A1, A2 (further subdivided into A2A and A2B), and A3 receptors (Collis & Hourani, 1993; Ralevic & Burnstock, 1998). A1 receptors are known to mediate mainly adenosine excitatory effects (Bailey et al., 1992; Murthy et al., 1995; Shim et al., 2002), although A1 relaxant receptors have been reported in rat duodenum and ileum (Nicholls et al., 1992; Nicholls & Hourani, 1997). Our results suggest the presence of relaxant A1 adenosine receptors also in mouse colon because DPCPX, a selective A1 receptor antagonist, at the concentration we used (Coupar, 1999) inhibited the response to adenosine in both regions. In distal colon, the relaxation remaining in the presence of DPCPX was antagonized by DMPX, a selective A2 receptor antagonist (Fredholm et al., 1994; De Man et al., 2003b), suggesting that, in this part of the colon, both A1 and A2 adenosine receptors were present and mediated the relaxation. This finding is in agreement with the general idea that A2 receptors mediate adenosine-induced muscular relaxation (Bailey & Hourani, 1992; Nicholls et al., 1996; Kadowaki et al., 2000; Woods et al., 2003). Furthermore, our experiments indicated the presence of functional A2B receptors involved in the adenosine-induced relaxation in murine distal colon, as this relaxation was antagonized by MRS 1754, a selective A2B receptor antagonist, but not by CSC, a selective A2A receptor antagonist. Postjunctional inhibitory A2B receptors have been demonstrated in other animal preparations, such rat ileum and colon (Bailey & Hourani, 1992; Nicholls et al., 1996), or guinea-pig colon (Kadowaki et al., 2000). In addition, data from our experiments would not support a role for A3 receptors in the inhibitory effects induced by adenosine in mouse colon, as suggested in the possum duodenum (Woods et al., 2003). Lastly, the observation that none of the adenosine receptor antagonists used had any effect on the spontaneous activity rules out an involvement of endogenous adenosine in the maintenance of the basal muscular activity in murine colon, again in contrast to results from possum duodenum (Woods et al., 2003).
TTX was unable to reduce adenosine effects in proximal colon indicating that, in this region, the inhibitory response to adenosine is independent of neuronal action potentials, and suggesting that the A1 adenosine inhibitory receptors may be localized postjunctionally. However, it cannot be excluded that prejunctional A1 receptors may directly induce neurotransmitter release from nerve terminals.
On the other hand, in distal colon, TTX reduced the effects of adenosine, suggesting that in this region some receptors are localized on enteric nerves, where adenosine would act causing neurotransmitter release. The observation that TTX was able to further antagonize adenosine effects persisting after A1 receptor blockade suggests the presence of functional A1 receptors that, as it was observed in proximal colon, would be more likely to be postjunctional. Indeed, TTX did not modify adenosine relaxation in the presence of the A2B receptor antagonist indicating that the A2B receptors were located presynaptically on inhibitory neurons. Although the presence of neuronal A1 receptors has been reported in the gastrointestinal tract (Kadowaki et al., 2000; Lee et al., 2001; Storr et al., 2002; De Man et al., 2003b), to our knowledge this is the first report of A2 receptors located on enteric inhibitory neurons. However, prejunctional facilitatory A2 receptors have been shown on cholinergic nerves in guinea-pig and rat ileum (Tomaru et al., 1995; Duarte-Araujo et al., 2004).
As NO is the main mediator released by inhibitory NANC nerves in various regions of the gastrointestinal tract from different animal species, including mouse (Olsson & Holmgren, 2001; Serio et al., 2003a; Ueno et al., 2004) and an interaction between NO and the purinergic system has been reported in gastrointestinal smooth muscle preparation of rodents (De Luca et al., 1999; Giaroni et al., 2002; Van Crombruggen & Lefebvre, 2004), we tested the possibility that nitrergic inhibitory nerves may be involved in the inhibition in mouse colon, evoked by adenosine. Inhibition of NO biosynthesis with L-NAME decreased the response to adenosine only in distal colon and not in proximal colon, suggesting that, in distal colon, adenosine acts also through NO production to induce the inhibitory response. This suggestion was further supported by the observation that ODQ, which is a potent and selective inhibitor of NO-stimulated soluble guanylyl cyclase, significantly reduced the effect of adenosine in distal colon. In this tissue, L-NAME was still able to antagonise the response to adenosine after block of A1 receptors but not after block of A2B receptors, indicating that adenosine was modulating NO release via A2B receptors. Lastly, our study indicated that, in mouse distal colon, NO would not facilitate the release of adenosine from inhibitory nerve terminals. This conclusion is supported by our finding that the inhibitory effects induced by exogenous NO (derived from the NO donor, SNP) were not antagonized by the different adenosine receptor antagonists.
In conclusion, we provide evidence that exogenous adenosine acts as inhibitory modulator of the contractility of mouse longitudinal smooth muscle of proximal and distal colon by activating P1 purinoceptors. In proximal colon, adenosine reduces spontaneous muscular activity via a mechanism not dependent on neuronal action potentials, activating exclusively A1 receptors. In distal colon, adenosine induces muscular relaxation via activation of A1 and A2B receptors, the latter located on the enteric inhibitory neurons which release NO.
Acknowledgments
This work was supported by a grant from Ministero dell'Università e della Ricerca Scientifica – Italy.
Abbreviations
- CSC
8-(3-chlorostyryl)-caffeine
- DMPX
3,7-dimethyl-1-propargylxanthine
- DPCPX
1,3-dipropyl-8-cyclopentylxanthine
- L-NAME
Nω-nitro-L-arginine methyl ester
- MRS 1754
8-[4-[((4-cyanophenyl) carbamoylmethyl) oxy] phenyl]-1,3-di (n-propyl)xanthine
- MRS 1220
9-chloro-2-(2-furanyl)-5-((phenylacetyl)amino)-[1,2,4]triazolo [1,5-c]quinazoline
- NO
nitric oxide
- ODQ
1H-[1,2,4] oxadiazolo [4,3-a]quinoxalin-1-one
- PPADS
4-[[4-formyl-5-hydroxy-6-methyl-3-[(phosphonooxy) methyl]-2-pyridinyl]azo]-1,3-benzenedisulfonic acid
- SNP
sodium nitroprusside
- TTX
tetrodotoxin
References
- BAILEY S.J., HOURANI S.M.O. Effects of purines on the longitudinal muscle of the rat colon. Br. J. Pharmacol. 1992;105:885–892. doi: 10.1111/j.1476-5381.1992.tb09073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAILEY S.J., HICKMAN D., HOURANI S.M.O. Characterisation of the P1-purinoceptors mediating contraction of the rat colon muscularis mucosae. Br. J. Pharmacol. 1992;105:400–404. doi: 10.1111/j.1476-5381.1992.tb14265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURNSTOCK G. The past, present and future of purine nucleotides as signalling molecules. Neuropharmacology. 1997;36:1127–1139. doi: 10.1016/s0028-3908(97)00125-1. [DOI] [PubMed] [Google Scholar]
- CAMILLERI M., FORD M.J. Review article: colonic sensorimotor physiology in health, and its alteration in constipation and diarrhoeal disorders. Aliment. Pharmacol. Ther. 1998;12:287–302. doi: 10.1046/j.1365-2036.1998.00305.x. [DOI] [PubMed] [Google Scholar]
- COLLIS M.G., HOURANI S.M. Adenosine receptor subtypes. Trends Pharmacol. Sci. 1993;14:360–366. doi: 10.1016/0165-6147(93)90094-z. [DOI] [PubMed] [Google Scholar]
- COUPAR I.M. Characterization and tissue location of the neural adenosine receptor in the rat ileum. Br. J. Pharmacol. 1999;126:1269–1275. doi: 10.1038/sj.bjp.0702411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE LUCA A., LI C.G., RAND M.J. Nitrergic and purinergic mechanisms and their interactions for relaxation of the rat internal anal sphincter. J. Auton. Pharmacol. 1999;19:29–37. doi: 10.1046/j.1365-2680.1999.00114.x. [DOI] [PubMed] [Google Scholar]
- DE MAN J.G., DE WINTER B.Y., SEERDEN T.C., DE SCHEPPER H.U., HERMAN A.G., PELCKMANS P.A. Functional evidence that ATP or a related purine is an inhibitory NANC neurotransmitter in the mouse jejunum: study on the identity of P2X and P2Y purinoceptors involved. Br. J. Pharmacol. 2003a;140:1108–1116. doi: 10.1038/sj.bjp.0705536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE MAN J.G., SEERDEN T.C., DE WINTER B.Y., VAN MARCK E.A., HERMAN A.G., PELCKMANS P.A. Alteration of the purinergic modulation of enteric neurotransmission in the mouse ileum during chronic intestinal inflammation. Br. J. Pharmacol. 2003b;139:172–184. doi: 10.1038/sj.bjp.0705218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUARTE-ARAUJO M., NASCIMENTO C., ALEXANDRINA TIMOTEO M., MAGALHAES-CARDOSO T., CORREIA-DE-SA P. Dual effects of adenosine on acetylcholine release from myenteric motoneurons are mediated by junctional facilitatory A(2A) and extrajunctional inhibitory A(1) receptors. Br. J. Pharmacol. 2004;141:925–934. doi: 10.1038/sj.bjp.0705697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREDHOLM B.B., ABBRACCHIO M.P., BURNSTOCK G., DALY J.W., HARDEN T.K., JACOBSON K.A., LEFF P., WILLIAMS M. Nomenclature and classification of purinoceptors. Pharmacol Rev. 1994;46:143–156. [PMC free article] [PubMed] [Google Scholar]
- GIARONI C., KNIGHT G.E., RUAN H.Z., GLASS R., BARDINI M., LECCHINI S., FRIGO G., BURNSTOCK G. P2 receptors in the murine gastrointestinal tract. Neuropharmacology. 2002;43:1313–1323. doi: 10.1016/s0028-3908(02)00294-0. [DOI] [PubMed] [Google Scholar]
- GIARONI C., KNIGHT G.E., ZANETTI E., CHIARAVALLI A.M., LECCHINI S., FRIGO G., BURNSTOCK G. Postnatal development of P2 receptors in the murine gastrointestinal tract. Neuropharmacology. 2006;50:690–704. doi: 10.1016/j.neuropharm.2005.11.015. [DOI] [PubMed] [Google Scholar]
- KADOWAKI M., TAKEDA M., TOKITA K., HANAOKA K., TOMOI M. Molecular identification and pharmacological characterization of adenosine receptors in the guinea-pig colon. Br. J. Pharmacol. 2000;129:871–876. doi: 10.1038/sj.bjp.0703123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOH S.D., DICK G.M., SANDERS K.M. Small-conductance Ca2+-dependent K+ channels activated by ATP in murine colonic smooth muscle. Am. J. Physiol. 1997;273:C2010–C2021. doi: 10.1152/ajpcell.1997.273.6.C2010. [DOI] [PubMed] [Google Scholar]
- LEE J.J., TALUBMOOK C., PARSONS M.E. Activation of presynaptic A1-receptors by endogenous adenosine inhibits acetylcholine release in the guinea-pig ileum. J. Auton. Pharmacol. 2001;21:29–38. doi: 10.1046/j.1365-2680.2001.00201.x. [DOI] [PubMed] [Google Scholar]
- MONETA N.A., MCDONALD T.J., COOK M.A. Endogenous adenosine inhibits evoked substance P release from perifused networks of myenteric ganglia. Am. J. Physiol. 1997;272:G38–G45. doi: 10.1152/ajpgi.1997.272.1.G38. [DOI] [PubMed] [Google Scholar]
- MULÈ F., SERIO R. NANC inhibitory neurotransmission in mouse isolated stomach: involvement of nitric oxide, ATP and vasoactive intestinal polypeptide. Br. J. Pharmacol. 2003;140:431–437. doi: 10.1038/sj.bjp.0705431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MULÈ F., NACCARI D., SERIO R. Evidence for the presence of P2y and P2x receptors with different functions in mouse stomach. Eur. J. Pharmacol. 2005;513:135–140. doi: 10.1016/j.ejphar.2005.01.052. [DOI] [PubMed] [Google Scholar]
- MURTHY K.S., MCHENRY L, GRIDER J.R., MAKHLOUF G.M. Adenosine A1 and A2b receptors coupled to distinct interactive signaling pathways in intestinal muscle cells. J. Pharmacol. Exp. Ther. 1995;274:300–306. [PubMed] [Google Scholar]
- NICHOLLS J., HOURANI S.M. Characterization of adenosine receptors on rat ileum, ileal longitudinal muscle and muscularis mucosae. Eur. J. Pharmacol. 1997;338:143–150. doi: 10.1016/s0014-2999(97)81942-5. [DOI] [PubMed] [Google Scholar]
- NICHOLLS J., BROWNHILL V.R., HOURANI S.M.O. Characterization of P1-purinoceptors on rat isolated duodenum longitudinal muscle and muscularis mucosae. Br. J. Pharmacol. 1996;117:170–174. doi: 10.1111/j.1476-5381.1996.tb15170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NICHOLLS J., HOURANI S.M., KITCHEN I. Characterization of P1-purinoceptors on rat duodenum and urinary bladder. Br. J. Pharmacol. 1992;105:639–642. doi: 10.1111/j.1476-5381.1992.tb09032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OLSSON C., HOLMGREN S. The control of gut motility. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2001;128:481–503. doi: 10.1016/s1095-6433(00)00330-5. [DOI] [PubMed] [Google Scholar]
- RALEVIC V., BURNSTOCK G. Receptors for purines and pyrimidines. Pharmacol. Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- SERIO R., ALESSANDRO M., ZIZZO M.G., TAMBURELLO M.P., MULÈ F. Neurotransmitters involved in the fast inhibitory junction potentials in mouse distal colon. Eur. J. Pharmacol. 2003b;460:183–190. doi: 10.1016/s0014-2999(02)02923-0. [DOI] [PubMed] [Google Scholar]
- SERIO R., MULÈ F., ADAMO E.B., POSTORINO A. Evidence against purines being neurotransmitters of non-adrenergic, non-cholinergic nerves in rat duodenum. Eur. J. Pharmacol. 1990;182:487–495. doi: 10.1016/0014-2999(90)90046-9. [DOI] [PubMed] [Google Scholar]
- SERIO R., ZIZZO M.G., MULÈ F. Nitric oxide induces muscular relaxation via cyclic GMP-dependent and -independent mechanisms in the longitudinal muscle of the mouse duodenum. Nitric Oxide. 2003a;8:48–52. doi: 10.1016/s1089-8603(02)00144-1. [DOI] [PubMed] [Google Scholar]
- SHIM J.O., SHIN C.Y., LEE T.S., YANG S.J., AN J.Y., SONG H.J., KIM T.H., HUH I.H., SOHN U.D. Signal transduction mechanism via adenosine A1 receptor in the cat esophageal smooth muscle cells. Cell Signal. 2002;14:365–372. doi: 10.1016/s0898-6568(01)00270-4. [DOI] [PubMed] [Google Scholar]
- STORR M., THAMMER J., DUNKEL R., SCHUSDZIARRA V., ALLESCHER H.D. Modulatory effect of adenosine receptors on the ascending and descending neural reflex responses of rat ileum. BMC Neurosci. 2002;3:21. doi: 10.1186/1471-2202-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOMARU A., INA Y., KISHIBAYASHI N., KARASAWA A. Excitation and inhibition via adenosine receptors of the twitch response to electrical stimulation in isolated guinea pig ileum. Jpn. J. Pharmacol. 1995;69:429–433. doi: 10.1254/jjp.69.429. [DOI] [PubMed] [Google Scholar]
- UENO T., DUENES J.A., ZARROUG A.E., SARR M.G. Nitrergic mechanisms mediating inhibitory control of longitudinal smooth muscle contraction in mouse small intestine. J. Gastrointest. Surg. 2004;8:831–841. doi: 10.1016/j.gassur.2004.06.004. [DOI] [PubMed] [Google Scholar]
- VAN CROMBRUGGEN K., LEFEBVRE R.A. Nitrergic-purinergic interactions in rat distal colon motility. Neurogastroenterol. Motil. 2004;16:81–98. doi: 10.1046/j.1365-2982.2003.00454.x. [DOI] [PubMed] [Google Scholar]
- WOODS C.M., TOOULI J., SACCONE G.T. A2A and A3 receptors mediate the adenosine-induced relaxation in spontaneously active possum duodenum in vitro. Br. J. Pharmacol. 2003;138:1333–1339. doi: 10.1038/sj.bjp.0705165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- XUE L., FARRUGIA G.M., SARR G., SZURSZEWSKI J.H. ATP is a mediator of the fast inhibitory junction potential in human jejunal circular smooth muscle. Am. J. Physiol. 1999;276:G1373–G1379. doi: 10.1152/ajpgi.1999.276.6.G1373. [DOI] [PubMed] [Google Scholar]
- ZAGORODNYUK V., MAGGI C.A. Pharmacological evidence for the existence of multiple P2 receptors in the circular muscle of guinea-pig colon. Br. J. Pharmacol. 1998;123:122–128. doi: 10.1038/sj.bjp.0701558. [DOI] [PMC free article] [PubMed] [Google Scholar]