Abstract
We have previously described a Ca2+-permeable non-selective cation channel in freshly dispersed rabbit ear artery myocytes, which is activated by agents that deplete internal Ca2+ stores and also by protein kinase C (PKC). In the present study, we investigated the effect of calmodulin (CaM) on store-operated channels (SOCs) with electrophysiological techniques.
Bath application of the CaM inhibitor calmidazolium (CMZ) to quiescent cells produced transient activation of SOC activity in cell-attached patches. CMZ produced a dual effect on cyclopiazonic acid (CPA)-evoked SOCs by initially inducing an increase in mean open probability (NPo) and subsequently producing a marked inhibition of SOC activity. In contrast, SOCs activated by the cell-permeable Ca2+ chelator 1,2-bis (2-aminophenoxy)ethane-N-N,N′,N′-tetraacetic acid (BAPTA-AM) were inhibited by CMZ.
In inside-out patches where SOCs were activated by CPA or the PKC activator phorbol-12,13-dibutyrate (PDBu), bath application of CaM induced an initial inhibition followed by an increase in SOC activity. In contrast, CaM only enhanced BAPTA-AM-evoked SOC activity in inside-out patches.
Bath application of CaM to the cytoplasmic surface of quiescent inside-out patches evoked single channel currents with biophysical properties similar to SOCs.
The inhibitory action of CaM on PDBu-induced SOC activity was inhibited by the calmodulin-dependent kinase II (CaM kinase II) inhibitor autocamtide-related inhibitory peptide (AIP) but not by inhibitors of calcineurin or myosin light chain kinase (MLCK). In addition, CaM-evoked channel currents were inhibited by coapplication of purified CaM kinase II but not by inhibitors of CaM kinase II, calcineurin or MLCK.
With whole-cell and cell-attached recording, bath application of the CaM kinase II inhibitors KN93 and AIP evoked SOCs in unstimulated myocytes.
These results indicate that CaM has pronounced dual inhibitory and excitatory actions on SOCs with the inhibitory effect of CaM mediated by CaM kinase II. Moreover, the present work provides strong evidence that CaM has an important role in activating SOCs, possibly through a direct action on channel/associated proteins.
Keywords: Store-operated channels, calmodulin, CaM kinase II, vascular smooth muscle
Introduction
Store-operated channels (SOCs) are plasmalemmal Ca2+-permeable cation channels that are activated in response to depletion of intracellular Ca2+ stores. SOCs have been described in many cell types and these studies have shown that SOCs comprise a family of ion channels with different properties, which probably reflects different molecular entities (Parekh & Penner, 1997; Parekh & Putney, 2005). In smooth muscle, SOCs that have significantly different properties from those of calcium release-activated channels (Icrac) in non-excitable tissues have been described and these SOCs have been implicated in smooth muscle contraction, cell proliferation and gene expression (e.g. see McFadzean & Gibson, 2002; Sweeney et al., 2002; Albert & Large, 2003; Pulver et al., 2004; Parekh & Putney, 2005).
In vascular smooth muscle, there is evidence to suggest that there are two distinct types of SOCs that differ in their biophysical properties and activation mechanisms. In rabbit portal vein, we have described an SOC with a relatively high Ca2+ permeability (Albert & Large, 2002a) that requires a protein kinase C (PKC)-mediated process for channel opening (Albert & Large, 2002b) and whose channel activity is greatly potentiated by inositol 1,4,5-trisphosphate (Ins(1,4,5)P3) via a heparin-insensitive mechanism (Liu et al., 2005b). In contrast, aortic smooth muscle cells express a SOC that is poorly selective for Ca2+ ions (Trepakova et al., 2001) and is proposed to be activated via the production of a calcium influx factor (CIF) from depleted internal Ca2+ stores, which displaces calmodulin (CaM) from Ca2+-independent phospholipase A2 (iPLA2, Smani et al., 2004).
The Ca2+-binding protein CaM has an important regulatory effect on many Ca2+-permeable ion channels (Zhu, 2005) and therefore not surprisingly the role of CaM has been investigated on SOCs in many cell systems. However, these experiments have produced much conflicting data (Vaca, 1996; Cao & Chatton, 1998; Vazquez et al., 2000; Machaca, 2003; Litjens et al., 2004; Moreau et al., 2005) and thus there is no consistent picture on the role for CaM on SOCs. These discrepancies may be accounted for by the use of cell lines and cultured cells that may contain a variety of different Ca2+ entry pathways and underlying molecular entities (see Parekh & Putney (2005) for different types of SOCs). In addition, in most of these studies, SOCs were measured with Ca2+-sensitive dyes or occasionally whole-cell recording. Both of these techniques have serious drawbacks concerning contamination by multiple mechanisms/conductances contributing to the observed signals. Consequently, direct measurement of single channel activity has significant advantages over these indirect measurements of channel activity. In the present work, we have studied the effects of CaM on SOC activity recorded at the single channel level in freshly dispersed smooth muscle cells.
In a recent study with single channel recording in aortic myocytes, it was shown that CaM had an inhibitory effect on SOC activity by preventing activation of iPLA2 (Smani et al., 2004). In an earlier study, we had also suggested an inhibitory effect of CaM in SOCs in rabbit portal vein myocytes (Albert & Large, 2002a). As the SOCs in these preparations have significantly different biophysical properties and activation pathways, we have re-examined the role of CaM in rabbit portal vein myocytes. The present work reveals a different and novel mechanism involving CaM-dependent kinase II (CaM kinase II) underlying the inhibitory role of CaM and in addition we reveal a stimulatory role for CaM via a possible direct effect on channel proteins.
Methods
Cell isolation
New Zealand White rabbits (2–3 kg) were killed by an i.v. injection of sodium pentobarbitone (120 mg kg−1) and the portal vein was removed, dissected free of connective tissue and fat before being cut into strips and enzymatically dispersed as previously described (Liu et al., 2005a).
Electrophysiology
Whole-cell and single cation channel currents were recorded with a HEKA EPC-8 patch-clamp amplifier at room temperature using whole-cell recording, cell-attached and inside-out patch configurations of the patch-clamp technique (Hamill et al., 1981). Patch pipettes were manufactured from borosilicate glass and were fire polished, and we used pipettes with resistance of about 6 MΩ for whole-cell and between 10 and 15 MΩ when filled with patch pipette solution for cell-attached and inside-out patch recording. To reduce ‘line' noise, the recording chamber (vol. ca 150–200 μl) was perfused using two 10 ml syringes, one filled with external solution and the other used to drain the chamber, in a ‘push and pull' technique. The external solution could be exchanged twice within 30 s. Whole-cell currents were evoked by applying voltage ramps from −150 to +100 mV (0.5 V s−1) every 20–30 s from a holding potential of 0 mV and filtered at 1 kHz (−3 db, low pass 4-pole Bessel filter, HEKA EPC-8 patch-clamp amplifier) and sampled at 5 kHz (Digidata 1322A and pCLAMP 9.0 Software, Axon instruments, Inc., CA, U.S.A.). Control current-voltage (I/V) were measured about 1 min after whole-cell configuration was obtained and then at the peak of the response to applied agents. Experiments were continued only if the control whole-cell currents were stable. When recording single channel currents, the holding potential was routinely set at −80 mV and to evaluate I/V characteristics of single channel currents, the membrane potential was manually changed between −140 and 0 mV.
Single channel currents were initially recorded onto digital audiotape (DAT) using a Biologic DRA-200 digital tape-recorder (BioLogic Science Instruments, France) at a bandwidth of 5 kHz (−3 db, low pass 4-pole Bessel filter, HEKA EPC-8 patch-clamp amplifier) and a sample rate of 48 kHz. For offline analysis, single cation channel records were filtered at 100 Hz (−3 db, low pass 8-pole Bessel filter, Frequency Devices, model LP02, Scensys Ltd, Aylesbury, U.K.) and acquired using a Digidata 1322A and pCLAMP 9.0 Software (Axon instruments, Inc., CA, U.S.A.) at a sampling rate of 1 kHz. Data were captured with a Pentium III personal computer (Research Machines).
Single channel current amplitudes were calculated from idealised traces of at least 10 s in duration using the 50% threshold method with events lasting for >6.664 ms (2 × rise time for a 100 Hz (−3 db) low pass filter) being excluded from analysis (Colquhoun, 1987). Figure preparation was carried out using Origin software (version 6.0) where inward channel currents are shown as downward deflections. The number of channels in a patch was unknown and therefore open probability (NPo) was calculated using the equation: NPo=total open time of all channel levels in the patch/sample duration.
Solutions and drugs
The bathing solution used in whole-cell recording experiments was K+-free and contained (mM) the following: NaCl (126), CaCl2 (1.5), HEPES (10), glucose (11), DIDS (4,4 diisothiocyanostilbene-2,2-disulphonic acid) (0.1), niflumic acid (0.1) and nicardipine (0.005), pH to 7.2 with NaOH. The pipette solution used for whole-cell recording was also K+-free and contained (mM) the following: CsCl (18), caesium aspartate (108), MgCl (1.2), HEPES (10), glucose (11), BAPTA (10), CaCl2 (4.8) (free internal Ca2+ concentration approximately 100 nM as calculated using EQCAL software), Na2ATP (1), NaGTP (0.2), pH 7.2 with Tris. In cell-attached patch experiments, the membrane potential was set to approximately 0 mV by perfusing cells in a KCl external solution containing (mM) the following: KCl (126), CaCl2 (1.5), HEPES (10) and glucose (11), pH to 7.2 with 10 M NaOH. Nicardipine (5 μM) was also included to prevent smooth muscle cell contraction by blocking Ca2+ entry through voltage-dependent Ca2+ channels. The composition of the bathing solution used in inside-out experiments (intracellular solution) was the same as the pipette solution used for whole-cell recording, except that 1 mM BAPTA and 0.48 mM CaCl2 were included (free internal Ca2+ concentration approximately 100 nM). The pipette solution used for both cell-attached and inside-out recording (extracellular solution) was K+-free and contained (mM) the following: NaCl (126), CaCl2 (1.5), HEPES (10), glucose (11), TEA (10), 4-AP (5), iberiotoxin (0.0002), DIDS (0.1), niflumic acid (0.1) and nicardipine (0.005), pH to 7.2 with NaOH. Under these conditions, voltage-gated Ca2+ currents, K+ currents, swell-activated Cl− currents and Ca2+-activated conductances are abolished and nonselective cation currents can be recorded in isolation. The CaM inhibitory peptide Trp and its less-active analogue Tyr were kind gifts from Dr K. Torok (see Török et al. (1998) for peptide sequences). Autocamtide-related inhibitory peptide (AIP), KN-93, KN-92, cyclosporin A and cyclophillin A were purchased from Calbiochem (Nottingham, U.K.) and all other drugs, including bovine brain CaM and purified rat brain CaM kinase II, were from Sigma (Poole, U.K.). Agents were dissolved in distilled H2O, dimethyl sulphoxide (DMSO) or ethanol (EtOH) and all solutions containing cyclosporin A also contained 100 nM cyclophillin. Solutions of DMSO or EtOH (0.1%) used to dissolve agents had no effect on whole-cell or single channel currents. The values are the mean of n cells±s.e.m. Statistical analysis was carried out using paired (comparing effects of agents on the same cell) or unpaired (comparing effects of agents between cells) Student's t-test with the level of significance set at P<0.05.
Results
Agents that inhibit CaM transiently activate SOCs in rabbit portal vein myocytes
In freshly dispersed rabbit portal vein myocytes, we have previously described a Ca2+-permeable cation channel with a unitary conductance of approximately 2 pS that is activated by depletion of internal Ca2+ stores with agents such as cyclopiazonic acid (CPA) and 1,2-bis (2-aminophenoxy)ethane-N-N,N′,N′-tetraacetic acid (BAPTA-AM, Albert & Large, 2002a). This is in comparison to a 23 pS channel activated by noradrenaline in portal vein myocytes which cannot be evoked by Ca2+-store depletion (Wang & Large, 1991; Albert & Large, 2001).
We have shown that CaM antagonists activate single channel currents in rabbit portal vein myocytes, which have properties similar to channel currents induced by agents that deplete internal Ca2+ stores (Albert & Large, 2002a). These preliminary data suggested that CaM may have a pronounced inhibitory effect on SOC activity which we further investigated by studying the effect of two chemically distinct cell-permeable CaM antagonists, calmidazolium (CMZ) and the inhibitory peptide, Trp, on SOC activity using cell-attached patch and whole-cell recording configurations of the patch-clamp technique.
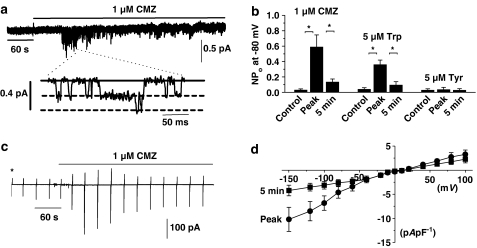
In eight out of 12 quiescent cell-attached patches, bath application of 1 μM CMZ activated SOCs after about 1 min, which had a mean unitary amplitude of −0.18±0.01 pA at −80 mV as previously reported (Figure 1a and Albert & Large, 2002a). However, the CMZ-evoked SOC activity subsequently significantly declined by about 80% after 5 min in the continued presence of the antagonist (P<0.05, Figure 1a and b).
Figure 1.
Transient activation of single channel currents and whole-cell cation conductance by CaM antagonists in rabbit portal vein myocytes. (a) Bath application of 1 μM CMZ induced SOCs in a cell-attached patch, which subsequently declined in activity after about 5 min. The inset illustrates channel openings on a faster time scale and shows that the patch contained at least two open levels. The bold line represents the closed level and the dashed lines the open levels. (b) Mean data of CMZ- and Trp-induced channel activity in cell-attached patches. The less-active analogue Tyr had little effect on channel activity. (c) Bath application of 1 μM CMZ induced a whole-cell cation conductance, which declined to about 40% of the maximum response after 5 min. *Denotes when the whole-cell configuration was obtained and the vertical deflections represent current responses to voltage ramps from −150 to +100 mV from a holding potential of 0 mV. (d) Mean I/V relationship of CMZ-evoked whole-cell cation conductance at peak and after 5 min (*P< 0.05).
Moreover, in seven out of eight cell-attached patches, bath application of the CaM inhibitory peptide Trp also transiently activated SOC activity with a mean unitary amplitude of −0.17±0.02 pA at −80 mV, whereas its less-active analogue Tyr had little effect on channel activity (Figure 1b). These data showing that CaM antagonists produced an initial stimulation of SOCs suggest that CaM has an inhibitory action on channel activity.
We further investigated this inhibitory effect of CaM on SOC activity by studying the effect of CMZ on whole-cell conductance. Figure 1c shows that bath application of 1 μM CMZ activated a whole-cell cation conductance in an unstimulated myocyte after about 1 min, which declined to approximately 60% of the mean peak amplitude after 5 min. In 12 out of 15 myocytes, CMZ activated whole-cell currents with a mean peak of −4.6±1.2 pA pF−1 at −80 mV, which significantly declined to a mean value of −2.6±0.7 pApF−1 after 5 min (P<0.01). Figure 1d illustrates mean I/V relationships of CMZ-induced whole-cell conductances at peak value and after 5 min when the current had declined, showing that the I/V relationships exhibited similar shapes with reversal potentials of about +20 mV.
The transient nature of the excitatory effects of the CaM antagonists may be due to channel run-down or suggests that CaM has an important additional role in sustaining channel activity and in future experiments, the latter hypothesis is supported. Thus, these data indicate an inhibitory effect of CaM on SOC activity and suggest that CaM may also be involved in maintaining SOC activity.
Effect of CMZ on SOCs evoked by store-depletion in cell-attached patches
To further elucidate whether CaM has both inhibitory and excitatory actions on SOC activity, we investigated the effect of CMZ on SOCs evoked in cell-attached patches by the agents CPA and BAPTA-AM, which induce SOC activity via depletion of intracellular Ca2+ stores.
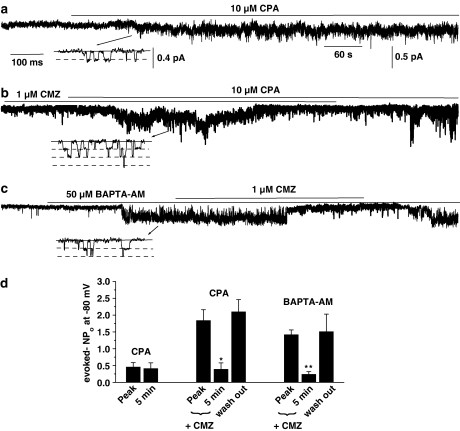
In six out of seven control cell-attached patches, bath application of 10 μM CPA evoked SOC activity, which had a mean unitary amplitude of −0.19±0.03 pA at −80 mV and which was sustained throughout the recording (Figure 2a and d). In six out of six patches, preincubation with 1 μM CMZ for 1–2 min (at which time SOCs were activated but not declining in activity), coapplication of 10 μM CPA evoked SOCs, which had a significantly increased mean peak NPo value compared to control (P<0.05), but subsequently there was a marked decline in activity by approximately 80% after 5 min, which did not occur in the absence of CMZ (P<0.05, Figure 2b and d). Moreover, Figure 2b and d show that the inhibitory effect of CMZ on CPA-evoked channel activity was reversed following wash out of this antagonist. These results support the previous data that CaM has an initial inhibitory effect and also the subsequent decrease in NPo indicated that CaM may have a role in SOC opening.
Figure 2.
Effect of CMZ on CPA- and BAPTA-AM-evoked SOC activity in cell-attached patches. (a) Bath application of 10 μM CPA induced SOC activity at −80 mV, which was sustained for over 8 min. (b) After incubation with 1 μM CMZ for 1–2 min, coapplication of 10 μM CPA initially evoked an increase in SOC activity, which markedly declined after 5 min. Note that following wash out of CMZ, the CPA-induced SOC activity returned to its peak value. (c) Bath application of 50 μM BAPTA-AM evoked SOC activity, which was reversibly inhibited by coapplication of 1 μM CMZ. The insets in (a), (b) and (c) show channel openings on a faster time scale. (d) Mean data showing the effects of 10 μM CPA (n=6), 1 μM CMZ+10 μM CPA (n=6) and 50 μM BAPTA-AM+1 μM CMZ (n=6) on SOC activity (*P<0.05, **P<0.01).
In six out of six patches, bath application of 50 μM BAPTA-AM evoked SOC activity with a mean unitary amplitude of −0.22±0.02 pA at −80 mV and the mean NPo value was reversibly inhibited by coapplication of 1 μM CMZ by about 85% after 5 min (Figure 3c and d). In contrast to the dual effects of CMZ on CPA-evoked SOC activity (Figure 3b and d), this CaM antagonist did not produce an initial enhancement in BAPTA-AM-evoked SOC activity (Figure 3c and d), which suggests that the inhibitory action of CaM on SOC activity is absent when BAPTA-AM is used to induce SOC activity in cell-attached patches (see Discussion).
Figure 3.
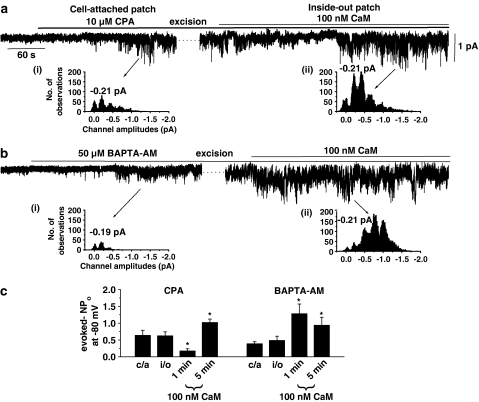
Effect on CaM on SOCs evoked by CPA and BAPTA-AM. (a) Bath application of 10 μM CPA induced channel currents in a cell-attached patch held at −80 mV, which were maintained after excision into the inside-out configuration at −80 mV. Coapplication of 100 nM CaM to the inside-out patch then produced an initial marked suppression of channel activity followed by a pronounced increase in activity (e.g. 5 min). (b) Following activation of SOCs by 50 μM BAPTA-AM in cell-attached mode and excision of the patch, coapplication of 100 nM CaM induced only a marked increase in channel activity in another inside-out patch. The insets (i) and (ii) in (a) and (b) show amplitude histograms and illustrate that the CaM-induced increases in channel activity were due to an increase in the activity of channel currents with a similar unitary amplitude. The peaks greater than the unitary amplitudes represent openings of more than one SOC in the patch. (c) Mean data showing the inhibitory and excitatory effects of CaM measured at 1 and 5 min on SOCs evoked by CPA and BAPTA-AM (c/a=cell-attached patch, i/o=inside-out patch, *P<0.05). Note that CaM only produced an excitatory effect on BAPTA-AM-induced SOCs.
CaM produces both inhibition and excitation of SOC activity in inside-out patches
To investigate directly the effect of CaM on SOC activity, we used an experimental protocol previously used to study the effect of Ins(1,4,5)P3 on these channel currents (Liu et al., 2005b). SOC activity was activated in cell-attached patches by bath application of CPA or the cell-permeable Ca2+ chelator BAPTA-AM and then patches were excised into the inside-out configuration and, after exchanging the cell-attached bathing solution with an inside-out bathing solution (see Methods), CaM was applied directly to the intracellular surface of the plasma membrane.
Figure 3a shows that bath application of 10 μM CPA activated SOC activity in a cell-attached patch, which was maintained after excision into the inside-out configuration. Subsequent coapplication of 100 nM CaM (in the presence of 100 nM free Ca2+, see Methods) produced a biphasic effect on SOC activity with an initial suppression of channel activity followed by an increase in channel activity, which had a similar unitary amplitude as those channel currents evoked in the cell-attached configuration (Figure 3a(i) and (ii)). The latter result indicates that increased channel activity is due to SOC stimulation and not another conductance. Figure 3c shows the mean data of this biphasic effect of CaM on CPA-evoked SOC activity, illustrating that in six out of seven inside-out patches CaM initially reduced the mean peak NPo value by about 70% after 1 min, but after 5 min in the continued presence of CaM, the channel activity had increased by approximately two-fold.
In contrast, in seven out of eight cells when SOCs were evoked by 50 μM BAPTA-AM in the cell-attached configuration, subsequent application of 100 nM CaM to the inside-out patch induced only a pronounced increase in SOC activity (Figure 2b), which also had a similar unitary amplitude as those channel currents in cell-attached patches (Figure 2b(i) and (ii)) and the initial inhibitory effect of CaM seen when CPA was used to evoke SOCs was not observed (Figure 2b and c).
These results suggest that CaM has a dual effect on SOCs in portal vein myocytes by producing both inhibition and facilitation/excitation of channel activity, but the dual effect is seen only with CPA-induced SOC activity and not when BAPTA-AM was used to evoke SOC opening.
Effect of CaM on PDBu-evoked and constitutively active SOC activity in inside-out patches
Agents that activate PKC induce channel currents in inside-out patches with properties similar to SOCs evoked by store-depletion, which indicated that PKC has an important role in inducing SOC activity via a store-independent mechanism (Albert & Large, 2002b). Therefore, to investigate whether the effects of CaM on SOC activity were mediated by interactions with store-dependent or -independent mechanisms, we studied the effects of CaM on SOCs evoked by the PKC activator phorbol-12,13-dibutyrate (PDBu) in inside-out patches.
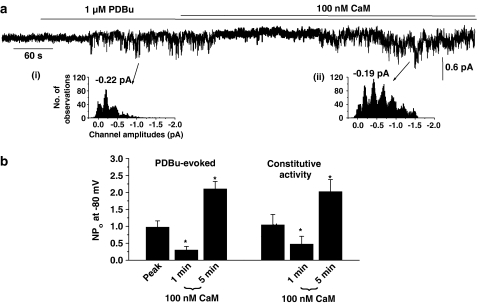
In eight out of 11 inside-out patches, bath application of 1 μM PDBu activated channel currents with a mean unitary amplitude of −0.20±0.02 pA at −80 mV and the subsequent application of 100 nM CaM produced an initial inhibition of channel activity followed by a marked enhancement in activity, which had a similar unitary amplitude to PDBu-evoked channel currents recorded in the absence of CaM (Figure 4a and b). Figure 4b shows that the mean data and application of CaM initially produced a significant inhibition of PDBu-evoked SOC activity by about 70% after 1 min (P<0.05), which was then followed by a pronounced increase in channel activity by approximately two-fold when measured after 5 min (P<0.05). A similar inhibition followed by excitation of PDBu-evoked SOC activity was also observed following bath application of 1 μM CaM (data not shown). These data are qualitatively and quantitatively similar to the results observed with CPA in the previous section.
Figure 4.
Effect of CaM on PDBu-evoked and constitutively active SOC activity in inside-out patches. (a) Bath application of 1 μM PDBu evoked channel activity that was initially inhibited and then increased by coapplication of 100 nM CaM. The insets (i) and (ii) show that the channel currents had similar unitary amplitudes. The multiple peaks represent openings of more than one SOC channel in the patch. (b) Mean data showing that CaM produced a dual effect on PBDu-evoked (n=8) and spontaneous SOC activity (n=5) composed of an initial inhibition of channel activity after 1 min followed by an increase in activity after 5 min (*P<0.05).
In portal vein, SOCs may be constitutively active in cell-attached and inside-out patches (Albert & Large, 2002a; Liu et al., 2005b) and therefore we investigated the effect of CaM on spontaneous activity in inside-out patches. Figure 4b shows that bath application of 100 nM CaM induced a dual effect on constitutive SOC activity with CaM producing an initial inhibition of about 60% after 1 min followed by marked excitation of approximately two-fold after 5 min.
These data show that the inhibitory and excitatory effects of CaM on SOC activity are present when SOCs are evoked by store-independent mechanisms (e.g. by PDBu) in inside-out patches, and as it is probable that internal Ca2+ stores are not present in this configuration it is likely that the inhibitory and excitatory effects of CaM do not involve internal Ca2+ stores.
CaM activates SOCs in inside-out patches
We next investigated whether the excitatory effect of CaM on SOCs was produced by CaM either potentiating channel opening or whether it may be able to open channels directly by applying CaM directly onto the cytoplasmic surface of quiescent inside-out patches.
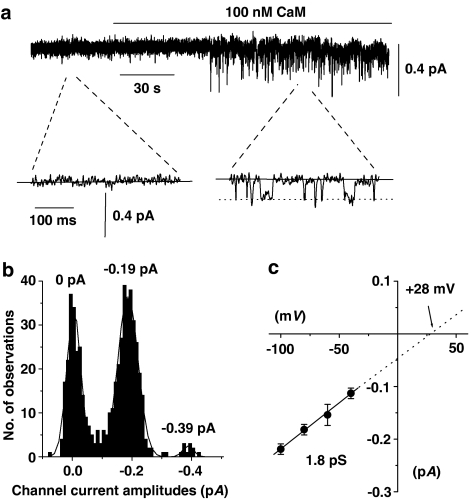
In over 80% of all inside-out patches tested, bath application of 100 nM CaM (in the presence of 100 nM free Ca2+) activated sustained channel currents, which had a mean unitary amplitude of −0.21±0.01 pA and a mean peak NPo value of 0.672±0.098 at −80 mV (n=38, Figure 5a). Figure 5b illustrates that the amplitude histogram of the CaM-evoked channel currents shown in Figure 5a could be fitted with three Gaussian curves with peaks of 0 pA, −0.19 pA and −0.39 pA, which represented the closed and two open levels with the larger open level representing more than one channel in the patch. Figure 5c shows that the mean pooled I/V relationship of the CaM-evoked channel currents was linear between −100 mV and −40 mV, which represented an unitary conductance of 1.8 pS and had an extrapolated Er of +28 mV.
Figure 5.
Effect of CaM on inside-out patches from unstimulated myocytes. (a) At a holding potential of −80 mV, bath application of 100 nM CaM evoked channel currents, which the inset shows had unitary amplitudes of about −0.2 pA. (b) Amplitude histogram of CaM-induced channel currents shown in (a). These amplitudes could be fitted with three Gaussian curves with peaks of 0, −0.19 and −0.39 pA corresponding to the closed and two open levels with the larger open level representing more than one SOC in the patch. (c) Mean pooled I/V relationship of CaM-induced channel currents showing a unitary conductance of 1.8 pS and an extrapolated Er of +28 mV (each point from at least n=4).
The properties of CaM-activated channel currents are similar to channel currents evoked by agents that deplete intracellular Ca2+ stores (Albert & Large, 2002a), which suggests that CaM activates the same SOCs. Moreover, activation of channel currents by CaM in inside-out patches suggests that CaM may induce channel opening via a mechanism at, or close to, the channel proteins.
Effect of agents that inhibit CaM-dependent mechanisms on the effects of CaM on PDBu-activated SOC activity in inside-out patches
It is well established that when CaM is bound to Ca2+, the resulting Ca2+–CaM complex activates a number of enzymes involved in phosphorylation processes such as CaM kinase II, myosin light chain kinase (MLCK) and the phosphatase calcineurin, which are known to modulate ion channel activity by altering the phosphorylation state of channel proteins (e.g. Braun & Schulman, 1995; Rusnak & Mertz, 2000; Kamm & Stull, 2001). Therefore, we investigated the effect of established agents that are known to inhibit these Ca2+–CaM-dependent enzymes on CaM-evoked changes in SOC activity, which were evoked by PDBu in inside-out patches.
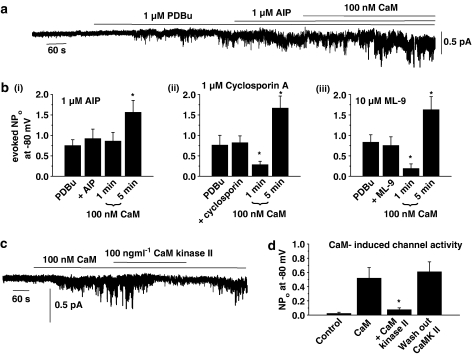
Figure 6a and b(i) show that SOC activity induced by PDBu was not altered by 1 μM AIP, a selective CaM kinase II inhibitor, but that subsequent application of 100 nM CaM in the presence of AIP and PDBu did not produce the usual initial inhibitory effect but only induced a significant increase in SOC activity after 5 min (cf. Figure 4a and b). Figure 6b(i) shows the mean data that application of CaM did not reduce mean peak NPo values of PDBu-induced channel activity after 1 min, but significantly increased channel activity by about two-fold after 5 min in the presence of AIP (P<0.05, cf. Figure 4b), indicating that CaM kinase II may mediate the inhibitory effect of CaM on PDBu-evoked SOC activity.
Figure 6.
Effects of inhibitors of CaM-dependent enzymes and purified CaM kinase II on CaM-induced changes in SOC activity in inside-out patches. (a) Preincubation with 1 μM AIP prevented CaM-induced inhibition of PDBu-evoked SOC activity, whereas the excitatory effect of CaM on channel activity was not affected. (b) (i) Mean data showing that AIP prevents CaM-induced inhibition of SOC activity, but not channel excitation (n=6). (ii) and (iii) show that preincubation with cyclosporin A (n=6) and ML-9 (n=6) had no effect on CaM-evoked changes in SOC activity. (c) Bath application of 100 ngml−1 CaM kinase II reversibly inhibited channel activity evoked by 100 nM CaM in an inside-out patch held at −80 mV. (d) Mean data showing CaM kinase II significantly reduced CaM-evoked channel activity (n=5, *P<0.05).
Figure 6b(ii) and (iii) show the effect of preincubating with 1 μM cyclosporin A, a calcineurin inhibitor, and 10 μM ML-9, an MLCK inhibitor, did not effect PDBu-evoked SOC activity in inside-out patches or prevent CaM from producing a significant initial inhibition (1 min) or excitation (5 min) of PDBu-evoked SOC activity (P<0.05, cf. Figure 6b (i)).
To further confirm that CaM kinase II is involved in inhibiting SOCs, we investigated the effect of purified CaM kinase II on channel activity induced by CaM in the presence of 100 nM free Ca2+. In five out of five inside-out patches, bath application of 100 ng ml−1 CaM kinase II reversibly inhibited channel activity evoked by 100 nM CaM at −80 mV (Figure 6c) and Figure 6d shows that CaM kinase II significantly inhibited the mean peak NPo value of CaM-induced channel activity by about 85% (P<0.05). Moreover, bath application of 1 μM AIP (n=6), 1 μM cyclosporin (n=6) and 10 μM ML-9 (n=6) had no effect on CaM-induced stimulation of channel activity.
These results provide strong evidence that the inhibitory effect of CaM on SOC activity is mediated by a CaM kinase II-dependent process and not by calcineurin or MLCK. Moreover, these data also suggest that the excitatory effect of CaM on SOC activity is not mediated by any of these three CaM-dependent enzymes, which further supports a direct excitatory effect of CaM at, or close to, the channel proteins.
Effect of agents that inhibit CaM kinase II on whole-cell cation conductances and single channel currents
To investigate the role of constitutive CaM kinase II activity on SOCs, we investigated the effect of known inhibitors of CaM kinase II on quiescent cells using whole-cell recording and cell-attached patch configurations.
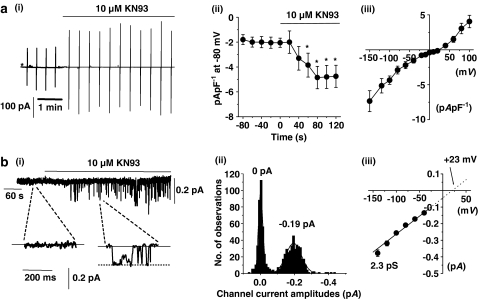
Figure 7a(i), (ii) and (iii) illustrate that bath application of the CaM kinase II inhibitor KN93 (10 μM) induced whole-cell cation conductances in five out of eight unstimulated cells, which had a mean peak amplitude of −2.9±0.6 pA pF−1 at −80 mV and a mean I/V relationship with an Er of about +25 mV. In five out of seven quiescent cell-attached patches, bath application of 10 μM KN93 induced channel activity (e.g. Figure 7b(i)), which had a mean unitary amplitude of −0.22±0.01 pA and a mean peak NPo value of 0.274±0.144 at −80 mV. Figure 7b(ii) illustrates that the amplitude histogram of KN93-evoked channel currents shown in Figure 7b(i) could be fitted by two Gaussian curves with peaks of 0 and −0.19 pA, indicating closed and open levels and Figure 7b(iii) shows that the mean pooled I/V relationship of KN93-evoked channel currents was linear between −140 and −40 mV with a unitary conductance of 2.3 pS and an extrapolated Er of about +23 mV. In control experiments, 10 μM KN92, an analogue of KN93, which has no effect on CaM kinase II, had no effect on whole-cell conductances (n=4) and did not activate channel currents in cell-attached patches (n=6, data not shown). An important observation is that in contrast to the effects of CaM inhibitors (cf. Figure 1), the KN93-induced whole-cell and single channels currents did not decline and were maintained throughout the duration of the recordings.
Figure 7.
Effect of KN93, a CaM kinase inhibitor, on whole-cell cation conductances and single channel currents. (a) (i) Bath application of 10 μM KN93 induced a whole-cell cation conductance (same experimental protocol as Figure 1a, *denotes when the whole-cell configuration was obtained) and (ii) shows the mean time course (P<0.05) and (iii) the mean I/V relationship of the KN93-evoked whole-cell current (n=5). (b) (i) Bath application of 10 μM KN93 induced channel currents in a cell-attached patch held at −80 mV. (ii) Amplitude histogram of KN93-evoked channel currents shown in (i). (iii) Mean pooled I/V relationship of KN93-induced channel currents (each point at least n=4).
Bath application of 1 μM AIP also activated single channel currents in cell-attached patches, which had a mean unitary amplitude of −0.21±0.01 pA and a mean peak NPo value of 0.259±0.103 (n=8) at −80 mV and the mean pooled I/V relationship showed that these AIP-evoked channel currents had an unitary conductance of 2.1 pS and an extrapolated Er of +26 mV.
The properties of KN93-activated whole-cell and single channel currents and AIP-activated channel currents are similar to the properties of SOCs (Albert & Large, 2002a) and indicate that a constitutively active CaM kinase II-dependent mechanism has a pronounced inhibitory influence on SOCs in unstimulated portal vein myocytes.
Discussion
The present study shows that CaM has dual excitatory and inhibitory actions on a biophysically identified SOC recorded at the single channel level in freshly dispersed rabbit portal vein smooth muscle cells. Previously, an inhibitory action of CaM was reported in this preparation (Albert & Large, 2002a) and the present work provides evidence that this inhibitory mechanism involves CaM kinase II which contrasts markedly from the hypothesis regarding the inhibitory effect of CaM on iPLA2 in aortic myocytes (Smani et al., 2004) and is in direct contrast to other studies that have implicated CaM kinase II in potentiating SOCs in cultured cells (Vazquez et al., 2000; Machaca, 2003). Also to our knowledge, this is the first study to show that CaM activates SOCs directly in inside-out patches, indicating that this may be an endogenous ligand for the channel, although the precise mechanism remains to be elucidated. In previous studies on cultured cells, CaM has been shown to facilitate Ca2+-entry after internal Ca2+ stores had been depleted (Vaca, 1996; Cao & Chatton, 1998; Vazquez et al., 2000; Machaca, 2003; Litjens et al., 2004; Moreau et al., 2005).
Mechanism underlying the inhibitory effect of CaM
The inhibitory effects of CaM were observed when CaM was applied to the cytoplasmic surface of inside-out patches in which SOCs had been activated by PDBu or CPA. With the latter method, channels were stimulated by CPA in the cell-attached mode before patch excision. This inhibitory action of CaM was blocked by the CaM kinase II inhibitor AIP, but was not affected by inhibitors of MLCK or calcineurin. Moreover in quiescient cells, another chemically distinct CaM kinase II inhibitor KN93 (but not the inactive analogue KN92) activated SOCs recorded in the whole-cell recording configuration and KN93 and AIP induced SOC activity in cell-attached patches. A notable result was that purified CaM kinase II also inhibited channel activity evoked by CaM. Together, these data suggest that the inhibitory effects of CaM are mediated by CaM kinase II. It is not known how CaM kinase II inhibits channel opening but since its inhibitory action is present in isolated inside-out patches it may be envisaged that CaM kinase II activity may lead to phosphorylation of channel/associated proteins and subsequent reduction in probability of channel opening. Importantly, the results also strongly indicate that CaM kinase II exerts a physiologically potent constitutive inhibitory action on SOCs when prevents channel opening through a constitutive active excitatory pathway. Thus, application of inhibitors of CaM kinase II to quiescent cells removes this inhibitory action leading to channel activation. In a previous study, we reported a similar constitutive inhibitory action of protein kinase A (PKA) on SOCs in portal vein myocytes (Liu et al., 2005a), which together with the current study suggests that SOCs are under complex control from inhibitory as well as excitatory pathways in unstimulated myocytes.
It is clearly evident that this mechanism of CaM mediating the inhibitory effect of CaM in portal vein myocytes is quite different from the mechanism involving iPLA2 in aortic myocytes (Smani et al., 2004). This represents a further difference between the properties of SOCs in these two vascular preparations (see Introduction).
This proposed inhibitory mechanism of Ca2+/CaM-dependent kinase II on SOC activity may provide an explanation regarding the lack of an inhibitory effect of CaM on SOCs which were stimulated by BAPTA-AM. In these experiments, BAPTA-AM was applied to evoke SOCs when using cell-attached recording and it would be predicted that BAPTA-AM would decrease the intracellular Ca2+ concentration ([Ca2+]i). This might cause dissociation of CaM kinase II from the plasma membrane before detaching the isolated patch so that when CaM is added subsequently to the cytoplasmic membrane of the inside-out patch, no CaM kinase II is present to mediate the inhibitory response and only the excitatory effect of CaM is evident. In the experiments with CPA, [Ca2+]i is unlikely to decrease and may even rise, owing to its action of inhibiting Ca2+-ATPase on the sarcoplasmic reticulum, before the patch is excised. Thus [Ca2+]i may remain sufficiently high for CaM kinase II to remain bound to the membrane during cell-attached recording and after formation of the excised inside-out patch. In this case, subsequent addition of CaM will produce an inhibitory action as well as an excitatory effect. In experiments with PDBu, inside-out patches were formed in 100 nM [Ca2+]i, which may also allow CaM kinase II to remain attached to the membrane following excision from the cell-attached configuration. This explanation raises the interesting possibility that BAPTA-AM induces SOC activity by removing the inhibitory effect of CaM kinase II by lowering [Ca2+]i in addition to depleting internal Ca2+ stores.
Excitatory response of CaM
With both whole-cell and cell-attached recording, CaM antagonists initially activated SOCs, owing to removal of the inhibitory action of CaM kinase II, but subsequently SOC activity was greatly reduced. This latter effect suggests that CaM has a facilitatory action on SOCs. Indeed, application of CaM on its own to quiescent inside-out patches evoked channel opening and the activity of SOCs evoked by CPA, BAPTA-AM and PDBu was increased by CaM. In addition, inhibitors of CaM kinase II, MLCK or calcineurin did not antagonise this excitatory effect of CaM on SOC activity. These data show that CaM is an activator of SOCs and that this action is mediated by another CaM-mediated signalling molecule and possibly by a direct action of CaM on the ion channel. Moreover, data from isolated inside-out patches suggest that CaM activates SOCs in portal vein myocytes independently of store-depletion.
Stimulation of SOCs was observed with 0.1–1 μM CaM, which is well within the estimated intracellular concentration of CaM (1–10 μM, Saimi & Kung, 2002). Also, CaM evoked SOCs with 100 nM [Ca2+]i, which is expected to be close to the resting [Ca2+]i in smooth muscle cells and therefore it would be predicted that CaM should have an important role in physiological activation of SOCs. Support for this proposal was provided by the demonstration that CMZ inhibited greatly channel activity activated by CPA and BAPTA-AM, although the initial response to CPA was increased in the presence of CMZ (probably owing to removal of the inhibitory effect of CaM kinase II, see above).
Three further points need to be considered. First, the CaM kinase II inhibitors produced only sustained activation of SOCs in cell-attached and whole-cell recording, whereas CaM antagonists produced initial excitation followed by substantial reduction of SOC activity. These data highlight the inhibitory effects of CaM kinase II and the dual effects of CaM. Secondly, the observation that CaM antagonists produce increased SOC activity before reducing channel opening can be interpreted by a higher affinity of CaM for its excitatory site than by CaM kinase II. Thirdly, the molecular identity of SOCs in portal vein myocytes is unknown, although it is likely that the channel proteins belong to the canonical transient receptor potential (TRPC) family of ion channels (especially TRPC1, Xu & Beech (2001) and see Albert & Large, 2006). It is therefore interesting to note that all the members of TRPC subfamily contain CaM-binding domains (Zhu, 2005) and therefore it is possible that CaM may activate SOCs directly to produce channel opening and the data with inside-out patches support this hypothesis.
Multiple activation of SOCs
Our previous data have shown that SOCs in portal vein myocytes are activated by multiple stimuli such as agents that deplete intracellular Ca2+ stores (Albert & Large, 2002a), noradrenaline (Albert & Large, 2002b), phorbol esters (Albert & Large, 2002b), a purified PKC fragment (Liu et al., 2005b) and inhibitors of PKA (Liu et al., 2005a) via store-dependent and -independent pathways. Moreover, the present work also shows that CaM kinase II inhibitors and CaM activate the same channels. In addition, we have also shown that SOCs in portal vein may be constitutively active (Albert & Large, 2002a). Our conclusion from these data is that the term SOC does not correctly define these ion channels and in keeping with terminology used for non-selective cation channels such as receptor-operated channels (ROCs) and SOCs, it would be more appropriate to define these channels as multi-operated channels or MOCs.
Conclusions
The present work demonstrates that CaM has dual excitatory and inhibitory effects on SOCs in portal vein myocytes. The inhibitory action appears to be mediated via CaM kinase II and the excitatory effect may be a direct action on the ion channel or a closely associated molecule. In addition, it is suggested that the balance between excitatory and inhibitory effects of CaM has important physiological consequences.
Acknowledgments
This work was funded by The British Heart foundation and The Wellcome Trust. Trp and Tyr peptides were a kind gift from Dr K Török.
Abbreviations
- AIP
autocamtide-related inhibitory peptide
- BAPTA-AM
1,2-bis (2-aminophenoxy)ethane-N-N,N′,N′-tetraacetic acid
- [Ca2+]i
intracellular Ca2+ concentration
- CaM
calmodulin
- CaM kinase II
calmodulin-dependent kinase II
- CIF
calcium influx factor
- CMZ
calmidazolium
- CPA
cyclopiazonic acid
- DIDS
4,4 diisothiocyanostilbene-2,2-disulphonic acid
- DMSO
dimethyl sulphoxide
- EtOH
ethanol
- Ins(1,4,5)P3
inositol 1,4,5-trisphosphate
- Icrac
calcium release-activated channels
- iPLA2
Ca2+-independent phospholipase A2
- I/V
current/voltage relationship
- MLCK
myosin light chain kinase
- NPo
open probability
- PDBu
phorbol-12,13-dibutyrate
- PKA
protein kinase A
- PKC
protein kinase C
- SOCs
store-operated channels
- TRPC
canonical transient receptor potential
References
- ALBERT A.P., LARGE W.A. Comparison of spontaneous and noradrenaline-evoked non-selective cation channels in rabbit portal vein myocytes. J. Physiol. 2001;530:457–468. doi: 10.1111/j.1469-7793.2001.0457k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALBERT A.P., LARGE W.A. A Ca2+-permeable non-selective cation channel activated by depletion of internal Ca2+ stores in single rabbit portal vein myocytes. J. Physiol. 2002a;538:717–728. doi: 10.1113/jphysiol.2001.013101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALBERT A.P., LARGE W.A. Activation of store-operated channels by noradrenaline via protein kinase C in rabbit portal vein myocytes. J. Physiol. 2002b;544:113–125. doi: 10.1113/jphysiol.2002.022574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALBERT A.P., LARGE W.A. Store-operated Ca2+-permeable non-selective cation channels in smooth muscle cells. Cell Calcium. 2003;33:345–356. doi: 10.1016/s0143-4160(03)00048-4. [DOI] [PubMed] [Google Scholar]
- ALBERT A.P., LARGE W.A. Signal transduction pathways and gating mechanisms of native TRP-like cation channels in vascular myocytes. J. Physiol. 2006;570:45–51. doi: 10.1113/jphysiol.2005.096875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRAUN A.P., SCHULMAN H. The multifunctional calcium/calmodulin-dependent protein kinase: from form to function. Ann. Rev. Physiol. 1995;57:417–445. doi: 10.1146/annurev.ph.57.030195.002221. [DOI] [PubMed] [Google Scholar]
- CAO Y., CHATTON J.Y. Involvement of calmodulin in the activation of store-operated Ca2+ entry in rat hepatocytes. FEBS Lett. 1998;424:33–36. doi: 10.1016/s0014-5793(98)00133-1. [DOI] [PubMed] [Google Scholar]
- COLQUHOUN D.Practical analysis of single channel records Microelectrode Techniques 1987Cambridge: The Company of Biologists; 83–104.ed. Standen N.B., Gray, P.T.A. & Whitaker, M.J., pp [Google Scholar]
- HAMILL O.P., MARTY A., NEHER E., SAKMANN B., SIGWORTH F.J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- KAMM K.E., STULL J.T. Dedicated myosin light chain kinases with diverse cellular functions. J. Biol. Chem. 2001;276:4527–4530. doi: 10.1074/jbc.R000028200. [DOI] [PubMed] [Google Scholar]
- LITJENS T., HARLAND M.L., ROBERTS M.L., BARRITT G.J., RYCHKOV G.Y. Fast Ca2+-dependent inactivation of the store-operated Ca2+ current (Isoc) in liver cells: a role of calmodulin. J. Physiol. 2004;558:85–97. doi: 10.1113/jphysiol.2004.065870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU M., ALBERT A.P., LARGE W.A. Facilitatory effect of Ins(1, 4, 5)P3 on store- operated Ca2+- permeable cation channels in rabbit portal vein myocytes. J. Physiol. 2005a;566:161–171. doi: 10.1113/jphysiol.2005.088260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU M., LARGE W.A., ALBERT A.P. Stimulation of β-adrenoceptors inhibits store-operated channel currents via a cAMP-dependent protein kinase mechanism in rabbit portal vein myocytes. J. Physiol. 2005b;562:395–406. doi: 10.1113/jphysiol.2004.077602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACHACA K. Ca2+-calmodulin dependent protein kinase II potentiates store-operated Ca2+ current. J. Biol. Chem. 2003;278:33730–33737. doi: 10.1074/jbc.M305023200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCFADZEAN I., GIBSON A. The developing relationship between receptor-operated and store-operated calcium channels in smooth muscle. Br. J. Pharmacol. 2002;135:1–13. doi: 10.1038/sj.bjp.0704468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOREAU B., STRAUBE S., FISHER R.J., PUTNEY J.W., Jr, PAREKH A.B. Ca2+-calmodulin-dependent facilitation and Ca2+ inactivation of Ca2+ release-activated Ca2+ channels. J. Biol. Chem. 2005;280:8776–8783. doi: 10.1074/jbc.M409619200. [DOI] [PubMed] [Google Scholar]
- PAREKH A.B., PENNER R. Store depletion and calcium influx. Physiol. Rev. 1997;77:901–930. doi: 10.1152/physrev.1997.77.4.901. [DOI] [PubMed] [Google Scholar]
- PAREKH A.B., PUTNEY J.W. Store-operated calcium channels. Physiol. Rev. 2005;85:757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- PULVER R.A., ROSE-CURTIS P., ROE M.W., WELLMAN LOUNSBURY K.M. Store-operated Ca2+ entry activates the CREB transcription factor in vascular smooth muscle. Circ. Res. 2004;28:1351–1358. doi: 10.1161/01.RES.0000127618.34500.FD. [DOI] [PubMed] [Google Scholar]
- RUSNAK F., MERTZ P. Calcineurin: form and function. Physiol. Rev. 2000;80:1483–1521. doi: 10.1152/physrev.2000.80.4.1483. [DOI] [PubMed] [Google Scholar]
- SAIMI Y., KUNG C. Calmodulin as an ion channel subunit. Annu. Rev. Physiol. 2002;64:289–311. doi: 10.1146/annurev.physiol.64.100301.111649. [DOI] [PubMed] [Google Scholar]
- SMANI T., ZAKHAROV S.I., CSUTORA P., LENO E., TREPAKOVA E.S., BOLOTINA V.M. A novel mechanism for the store-operated calcium influx pathway. Nat. Cell. Biol. 2004;6:113–120. doi: 10.1038/ncb1089. [DOI] [PubMed] [Google Scholar]
- SWEENEY M., YU Y., PLASTOSHYN O., ZHANG S., MCDANIEL S.S., YUAN J.X. Inhibition of endogenous TRP1 decreases capacitative Ca2+ entry and attenuates pulmonary artery smooth muscle cell proliferation. Am. J. Physiol. 2002;283:L144–L155. doi: 10.1152/ajplung.00412.2001. [DOI] [PubMed] [Google Scholar]
- TÖRÖK K., COWLEY D.J., BRANDMEIER B.D., HOWELL S., AITKEN A., TRENTHAM D.R. Inhibition of calmodulin-activated smooth-muscle myosin light-chain kinase by calmodulin-binding peptides and fluorescent (phosphodiesterase-activation) calmodulin derivatives. Biochemistry. 1998;37:6188–6198. doi: 10.1021/bi972773e. [DOI] [PubMed] [Google Scholar]
- TREPAKOVA E.S., GERICKE M., HIRAKAWA Y., WEISBROD R.M., COHEN R.A., BOLOTINA V.M. Properties of a native cation channel activated by Ca2+ store depletion in vascular smooth muscle cells. J. Biol. Chem. 2001;276:7782–7790. doi: 10.1074/jbc.M010104200. [DOI] [PubMed] [Google Scholar]
- VACA L. Calmodulin inhibits calcium influx current in vascular endothelium. FEBS Lett. 1996;390:289–293. doi: 10.1016/0014-5793(96)00675-8. [DOI] [PubMed] [Google Scholar]
- VAZQUEZ G., BOLAND d.e., BOLAND R.L. Involvement of calmodulin in 1-alpha, 25-dihydroxyvitamin D3 stimulation of store-operated Ca2+ influx in skeletal muscle cells. J. Biol. Chem. 2000;275:16134–16138. doi: 10.1074/jbc.C901008199. [DOI] [PubMed] [Google Scholar]
- WANG Q., LARGE W.A. Noradrenaline-evoked cation conductance recorded with the nystatin whole-cell method in rabbit portal vein cells. J. Physiol. 1991;435:21–39. doi: 10.1113/jphysiol.1991.sp018496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- XU S.-Z., BEECH D.J. TrpC1 is a membrane spanning subunit of store-operated Ca2+ channels in native vascular smooth muscle cells. Circ. Res. 2001;88:84–87. doi: 10.1161/01.res.88.1.84. [DOI] [PubMed] [Google Scholar]
- ZHU MX. Multiple roles of calmodulin and other Ca2+-binding proteins in the functional regulation of TRP channels. Pflugers Arch. 2005;451:105–115. doi: 10.1007/s00424-005-1427-1. [DOI] [PubMed] [Google Scholar]