Abstract
The ability of aminoguanidine (AG), an inhibitor of collagen crosslinking, to prevent changes in cardiac and vascular structure and function has been determined in the deoxycorticosterone acetate (DOCA)-salt hypertensive rat as a model of the cardiovascular remodelling observed in chronic human hypertension.
Uninephrectomized rats (UNX) administered DOCA (25 mg every fourth day s.c.) and 1% NaCl in drinking water for 28 days developed cardiovascular remodelling shown as systolic hypertension, left ventricular hypertrophy, increased thoracic aortic and left ventricular wall thickness, increased left ventricular inflammatory cell infiltration together with increased interstitial collagen and increased passive diastolic stiffness, impaired contractility, prolongation of the action potential duration and vascular dysfunction.
Treatment with AG (0.05–0.1% in drinking water; average 182±17 mg kg−1 day−1 in DOCA-salt rats) decreased blood pressure (DOCA-salt 176±4; +AG 144±5 mmHg; *P<0.05 vs DOCA-salt), decreased left ventricular wet weights (DOCA-salt 3.17±0.07; +AG 2.66±0.08 mg g−1 body wt*), reduced diastolic stiffness constant (DOCA-salt 30.1±1.2; +AG 24.3±1.2* (dimensionless)), improved cardiac contractility (DOCA-salt 1610±130; +AG 2370±100 mmHg s−1*) and vascular reactivity (3.4-fold increase in maximal contractile response to noradrenaline, 3.2-fold increase in maximal relaxation response to acetylcholine, twofold increase in maximal relaxation response to sodium nitroprusside) and prolonged the action potential duration at 50% repolarization without altering collagen content or inflammatory cell infiltration.
Thus, cardiovascular function in DOCA-salt hypertensive rats can be improved by AG independent of changes in collagen content. This suggests that collagen crosslinking is an important cause of cardiovascular dysfunction during cardiovascular remodelling in hypertension.
Keywords: Aminoguanidine, collagen crosslinking, DOCA-salt rats, hypertension, remodelling
Introduction
Cardiovascular remodelling in chronic hypertension involves ventricular hypertrophy, interstitial and perivascular fibrosis, electrical remodelling in the heart and endothelial dysfunction. The importance of the increased collagen deposition in cardiovascular dysfunction has long been recognized (Weber et al., 1993) with recent studies investigating the role of relevant mediators such as angiotensin II (Sun et al., 2004). Collagen crosslinking may be a more important determinant of the increased ventricular stiffness in hypertensive rats than collagen content (Norton et al., 1997); collagen crosslinking was increased in the deoxycorticosterone acetate (DOCA)-salt hypertensive rat (Ooshima & Midorikawa, 1977). An increased collagen crosslinking has been proposed as the major cause of the increased arterial wall stiffness and decreased myocardial compliance during ageing (Lakatta, 1993; Li et al., 1996; Cantini et al., 2001). The formation of crosslinks on long-lived matrix components follows non-enzymic reactions between glucose and proteins to form advanced glycation end-products (AGEs) (Brownlee, 1995). These products are markedly increased in ageing and diabetic rats (Brownlee, 1995). Increased AGE formation has also been shown in the aorta of stroke-prone spontaneously hypertensive rats (SHRs) (Mizutani et al., 2002). Further, methylglyoxal (the highly reactive dicarbonyl precursor of AGEs), AGEs, oxidized glutathione and oxidative stress were significantly higher in vascular smooth muscle cells from 12-week-old SHR compared with age-matched normotensive Wistar–Kyoto rats (Wu & Juurlink, 2002). In 5-week-old stroke-prone SHR, OPB-9195, an inhibitor of AGE formation, lowered systolic blood pressure and glycated albumin concentrations (Mizutani et al., 2002).
The nucleophilic hydrazine, aminoguanidine (AG), is an effective inhibitor of AGE formation thus slowing or preventing age-related aortic stiffening and cardiac hypertrophy in rats (Li et al., 1996; Corman et al., 1998). Further, AG is a selective inhibitor of inducible nitric oxide synthase (iNOS) (Nilsson, 1999) and may quench hydroxyl radicals and inhibit free radical formation, lipid peroxidation and oxidant-induced apoptosis (Giardino et al., 1998). In rats, AG ameliorated the neuropathic, ocular and cardiac complications of diabetes (Miyauchi et al., 1996; Norton et al., 1996; Swamy-Mruthinti et al., 1996), atherogenesis (Panagiotopoulos et al., 1998) and renal dysfunction in ageing rats (Reckelhoff et al., 1999). AG (pimegedine) has also been tested for the treatment of diabetic nephropathy in humans (Abdel-Rahman & Bolton, 2002).
The aim of the present study was to determine whether administration of AG attenuates the cardiac and vascular dysfunction in the DOCA-salt hypertensive rat, independent of changes in collagen content. This study has used a prevention protocol with oral administration of AG following initiation of cardiovascular remodelling in DOCA-salt hypertensive rats. We have used echocardiography to define cardiac structure in vivo and the isolated Langendorff heart to define function ex vivo. Histological methods were used to define infiltration of inflammatory cells and collagen deposition. Isolated thoracic aortic rings were used to examine endothelial dysfunction. Microelectrode studies on isolated left ventricular papillary muscles were used to determine changes in cardiac action potentials.
Methods
DOCA-salt hypertensive rats
Male Wistar rats approximately 8 weeks old were obtained from the Central Animal Breeding House of The University of Queensland. All experimental protocols were approved by the Animal Experimentation Ethics Committee of The University of Queensland, under the guidelines of the National Health and Medical Research Council of Australia, which conform to the NIH Guidelines. Uninephrectomy was performed on all rats. Rats were anaesthetized with intraperitoneal injections of Zoletil® (tiletamine 25 mg kg−1 and zolazepam 25 mg kg−1) and xylazine (10 mg kg−1), a lateral abdominal incision was used to access the left kidney, the left renal vessels and ureter were ligated and the kidney removed. The incision site was surgically sutured. Uninephrectomized (UNX) rats were given either no further treatment (UNX rats) or 1% NaCl in the drinking water with subcutaneous injections of DOCA (25 mg in 0.4 ml of N,N-dimethyl formamide (DMF)) every fourth day (DOCA-salt rats). Rats were subsequently assigned to one of four groups: (i) UNX controls (UNX) (n=21); (ii) UNX controls receiving 0.1% AG in drinking water for 4 weeks (UNX+AG) (n=24); (iii) DOCA-salt hypertensive rats (DOCA) (n=29); and (iv) DOCA-salt hypertensive rats receiving 0.1% AG in drinking water for 1 week, then 0.05% AG in drinking water for 3 weeks (DOCA+AG) (n=31). All experiments were performed 4 weeks after uninephrectomy.
Assessment of physiological parameters
All rats for this study were housed separately. Food and water intake and body weights were measured daily for all rats. Daily AG consumption was calculated from the daily water intake. Systolic blood pressure was measured in selected rats lightly sedated with intraperitoneal Zoletil® (tiletamine 15 mg kg−1 with zolazepam 15 mg kg−1). A tail pulse transducer (MLT1010) and an inflatable tail cuff were used, connected via a Capto SP844 physiological pressure transducer (MLT844/D) to a PowerLab data acquisition unit (ADInstruments, Sydney, Australia).
Echocardiographic studies
Rats were anaesthetized with intraperitoneal injections of Zoletil® (tiletamine 25 mg kg−1 and zolazepam 25 mg kg−1) and xylazine (10 mg kg−1) to produce anaesthesia with minimal cardiovascular depression. Serial, non-invasive, in vivo echocardiographic images were obtained using the Hewlett Packard Sonos 5500 echocardiography machine (12 MHz neonatal transducer) with an image depth of 3 cm and using two focal zones (Brown et al., 2002). Left ventricular M-mode measurements at the level of the papillary muscles were used to obtain wall thicknesses. Suprasternal long axis views were used to obtain the internal diameters of the ascending aortic arch (Brown et al., 2002).
Isolated heart preparations
The diastolic stiffness constant was determined using a non-recirculating isolated Langendorff heart preparation (Brown et al., 1999). Briefly, rats were anaesthetized with sodium pentobarbitone (60 mg intraperitoneal) and heparin (200 IU kg−1) was administered via the femoral vein. After 2 min, the heart was rapidly excised and stunned in ice-cold crystalloid perfusate (modified Krebs–Henseleit bicarbonate buffer (KHB) containing (in mM) 119.1 NaCl, 4.75 KCl, 1.19 MgSO4, 1.19 KH2PO4, 25.0 NaHCO3, 11.0 glucose and 2.16 CaCl2). The aorta was isolated and cannulated via the dorsal root. Retrograde perfusion was initiated at 100 cm of constant pressure with KHB bubbled with carbogen (95% O2/5% CO2), giving a pH of 7.4 and the temperature maintained at 35±0.5°C. A water-filled latex balloon catheter was inserted in the left ventricle via the mitral orifice for measurement of left ventricular developed pressures. The catheter was connected via a three-way tap to a micrometer syringe and to a disposable pressure transducer (MLT844, ADInstruments) via a disposable clip-on dome (MLA844, ADInstruments) all connected to a MacLab system. The hearts were electrically paced at 250 beats min−1 by touching two electrodes to the surface of the right atrium. End-diastolic pressures were measured from 0 to 30 mmHg. Myocardial diastolic stiffness was calculated as the diastolic stiffness constant (κ, dimensionless), which is the slope of the linear relation between stress (σ, dyn cm−2) and tangent elastic modulus (E, dyn cm−2) (Brown et al., 1999). To assess contractile function, maximum +dP/dT (rate of contraction) and −dP/dT (rate of relaxation) were calculated at a diastolic pressure of 10 mmHg.
Histological studies of left ventricular collagen distribution, inflammatory cell infiltration and thoracic aortic wall thickness
Left ventricles (at mid-papillary level) and thoracic aortas were cut transversely, treated with Telly's fixative and Bouin's solution and stained with picrosirius red as previously described (Allan et al., 2005; Fenning et al., 2005). Thoracic aortic wall thicknesses were also measured using NIH-image software (National Institutes of Health, U.S.A.). For left ventricular inflammatory cell infiltration measurements, sections of 5 μm thickness were stained with haematoxylin and eosin. Inflammatory cells were identified by cell morphology and analyzed via a blinded experimental protocol using a Meopta binocular light microscope. Sections were examined for pathology as shown by the presence of inflammatory cells (for example, polymorphonuclear neutrophils (PMNs)), and graded on a scale of 0–4. For grading, a × 45 lens was used, whereas a × 100 lens was used to check cell/nucleus morphology. Sections were given a grade of 1 if no abnormalities were detected. A grade of 1 indicated the appearance of low numbers of PMNs in the interstitial spaces. A grade of 2 indicated the presence of PMNs in higher numbers and moderate infiltration to other areas of the interstitium. A grade of 3 was given to indicate a more prominent presence of PMNs in the interstitium and significant infiltration to other areas of the myocardium. A maximal grade of 4 indicated severe scarring of the myocardium and high levels of PMNs in the interstitium especially accumulating within the scar tissues and throughout the myocardium.
Microelectrode studies
Isolated left ventricular papillary muscles were prepared for microelectrode studies as previously described (Fenning et al., 2005). After equilibration, action potential durations at 20, 50 and 90% of repolarization were recorded from the papillary muscle over a 30-min period, while continually being perfused with a drug-free Tyrode solution. Data were acquired, derived and analysed using Chart software (ADInstruments).
Isolated thoracic aortic rings
Thoracic aortic rings (approximately 4 mm in length) were suspended in an organ bath chamber with a resting tension of 10 mN and bathed in a modified Tyrode solution containing (in mM) 136.9 NaCl, 5.4 KCl, 1.05 MgCl2, 1.8 CaCl2, 22.6 NaHCO3, 0.42 NaH2PO4, 5.5 glucose, 0.28 ascorbic acid and 0.1 sodium ethylene diamine tetra-acetic acid (EDTA). The Tyrode solution was bubbled with carbogen (95% O2/5% CO2) and the temperature maintained at 35±0.5°C. Force of contraction was measured isometrically with Grass FT03C force transducers connected via amplifiers to a Macintosh computer via a MacLab system (Brown et al., 1991). Cumulative concentration-response curves were performed for noradrenaline and either acetylcholine or sodium nitroprusside in the presence of a submaximal (approximately 70%) contraction to noradrenaline.
Statistical analysis
All values are presented as mean±standard error of the mean (s.e.m.). The −log EC50 was determined from the concentration giving half-maximal responses in individual concentration–response curves. Statistical comparisons of the group means were made by one-way analysis of variance (ANOVA) with a Bonferroni post-test analysis for multiple groups or by paired or unpaired Student's t-test as appropriate for two-group comparison. P<0.05 was considered statistically significant.
Drugs
AG hemisulphate, DOCA, heparin, noradrenaline, acetylcholine and sodium nitroprusside were purchased from Sigma-Aldrich Chemical Company, St Louis, MO, U.S.A. Noradrenaline, acetylcholine and sodium nitroprusside were dissolved in distilled water; DOCA was dissolved in dimethylformamide with mild heating.
Results
Physiological parameters
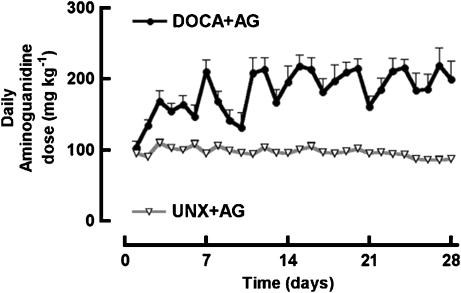
Daily AG doses remained relatively constant over the 4 weeks with an average daily dose for UNX+AG rats of 97±4 mg kg−1 day−1 (n=16–28), and an average daily dose for DOCA+AG rats of 182±17 mg kg−1 day−1 (n=16–28) (Figure 1). DOCA-salt rats developed hypertension; AG treatment significantly lowered systolic blood pressures in both UNX+AG and DOCA+AG groups (Table 1).
Figure 1.
Daily AG dose (mg kg−1) of UNX rats treated with AG and DOCA-salt rats treated with AG. Values are mean±s.e.m.; n=16–28 for all groups.
Table 1.
Physiological parameters
| Parameter | UNX 4 week | UNX+ AG | DOCA-salt 4 week | DOCA-salt+AG |
|---|---|---|---|---|
| Systolic blood pressure (mmHg) |
128±5 (12) |
117±3* (11) |
176±4* (11) |
144±5** (12) |
| Initial body weight (g) |
326±3 (17) |
338±8 (21) |
341±8 (17) |
344±6 (26) |
| Final body weight (g) |
431±8 (17) |
440±10 (19) |
354±10* (16) |
369±5 (21) |
| LV+septum weight (mg g−1 bodywt) |
1.97±0.04 (15) |
2.08±0.04 (12) |
3.17±0.07* (14) |
2.66±0.08* (10) |
| RV weight (mg g−1 bodywt) |
0.57±0.02 (15) |
0.53±0.02 (12) |
0.65±0.02* (14) |
0.56±0.03* (10) |
| Thoracic aortic wall thickness (μm) |
123±4 (6) |
121±5 (8) |
157±6* (7) |
145±5* (8) |
| LV interstitial inflammation (grade) |
0.8±0.4 (5) |
0.8±0.4 (5) |
2.8±0.4* (5) |
2.8±0.6* (5) |
| LV interstitial area of collagen (%) |
2.71±0.21 (7) |
4.06±0.77 (8) |
14.43±1.75* (8) |
10.96±1.42* (8) |
| Diastolic stiffness (κ) |
22.2±1.2 (12) |
19.4±0.9 (12) |
30.1±1.2* (12) |
24.3±1.2** (10) |
| +dP/dTmax (mmHg s−1) |
2080±70 (8) |
2340±130* (12) |
1610±130* (13) |
2370±100** (10) |
| −dP/dTmax (mmHg s−1) |
−1590±50 (9) |
−1550±60 (12) |
−1320±70* (12) |
−1480±40** (10) |
| LVPWd (mm) |
1.5±0.1 (15) |
1.7±0.1* (11) |
2.0±0.1* (14) |
2.0±0.1* (14) |
| LVIDd (mm) |
6.8±0.2 (17) |
7.1±0.3 (13) |
6.6±0.2 (15) |
6.6±0.2 (15) |
| Ejection fraction (%) |
91.0±1.5 (16) |
92.6±1.4 (13) |
93.8±0.9 (15) |
93.3±1.4 (15) |
| Ascending aortic arch diameter (mm) |
3.3±0.1 (7) |
3.6±0.1* (12) |
2.9±0.1* (5) |
3.3±0.1** (14) |
| APD20 (ms) |
6.8±1.1 (8) |
6.9±1.1 (4) |
10.1±1.5* (7) |
10.7±1.0* (4) |
| APD50 (ms) |
16.4±2.0 (8) |
22.8±4.9 (4) |
19.8±2.7 (7) |
42.4±6.2** (4) |
| APD90 (ms) | 34.4±3.5 (8) | 38.4±7.7 (4) | 59.1±10.3* (7) | 76.8±11.2* (4) |
All values shown represent the mean±s.e.m. (n-value); AG=aminoguanidine, LV=left ventricular, RV=right ventricular, LVPWd=left ventricular posterior wall thickness in diastole; LVIDd=left ventricular internal diameter in diastole; APD=action potential duration at 20, 50 or 90% of repolarization. Significance:
P<0.05 vs UNX rats;
P<0.05 vs DOCA-salt rats.
Structural parameters
DOCA-salt rats exhibited marked cardiovascular remodelling, including left and right ventricular hypertrophy, increased left ventricular wall thickness, increased thoracic aortic wall thickness and decreased ascending aortic arch diameter but without dilatation of the left ventricular chamber (Table 1). Furthermore, DOCA-salt rats showed increased left ventricular interstitial inflammatory cell infiltration together with left ventricular interstitial fibrosis (Table 1). Treatment with AG in the DOCA-salt rats attenuated the increase in left and right ventricular wet weights and prevented the decrease in ascending aortic arch diameter (Table 1). AG did not significantly alter inflammatory cell infiltration or left ventricular fibrosis (Table 1).
Functional parameters
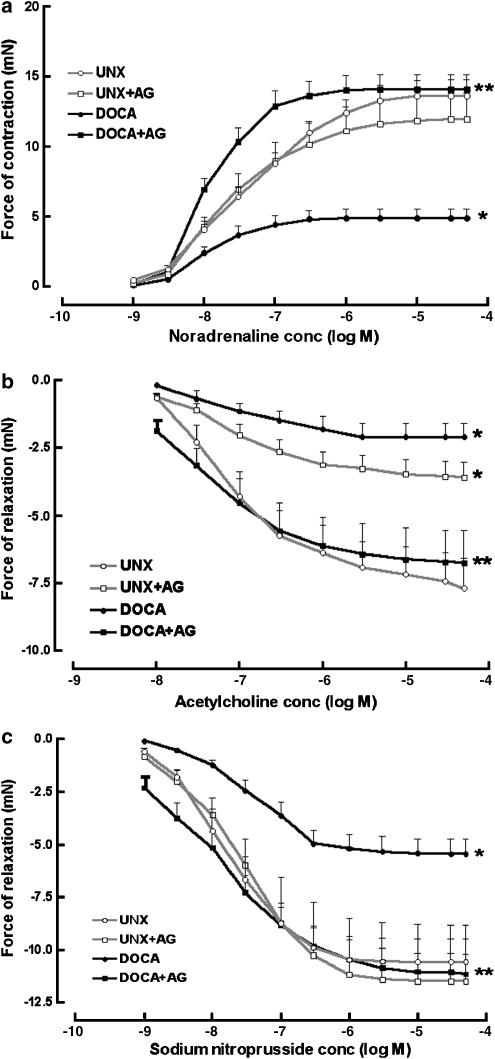
DOCA-salt hypertensive rats exhibited significantly increased passive diastolic stiffness (Table 1), reduced maximal rates of contraction and relaxation together with prolongation of the action potential durations at 20 and 90% of repolarization (Table 1). Vascular dysfunction was also evident in the DOCA-salt rats as shown by reduced maximal contractile responses to noradrenaline and reduced maximal endothelium-dependent relaxation to acetylcholine and endothelium-independent relaxation to sodium nitroprusside (Figure 2). Treatment with AG in the DOCA-salt rats prevented the increased diastolic stiffness and improved rates of contraction and relaxation (Table 1). In addition, AG increased action potential durations at 50% of repolarization but did not affect action potential durations at 20 and 90% of repolarization (Table 1). Finally, AG treatment prevented the decreased maximal vascular contractile and relaxant responses in the DOCA-salt hypertensive rats (Figure 2).
Figure 2.
Concentration–response curves to noradrenaline (a) for UNX rats (−log EC50 7.4±0.1, n=9), UNX rats treated with AG (−log EC50 7.7±0.1, n=7), DOCA-salt rats (−log EC50 7.8±0.1, n=9) and DOCA-salt rats treated with AG (−log EC50 8.0±0.1, n=7). Concentration–response curves to acetylcholine (b) for UNX rats (−log EC50 7.0±0.1, n=9), UNX rats treated with AG (−log EC50 7.1±0.2, n=7), DOCA-salt rats (−log EC50 7.0±0.1, n=9) and DOCA-salt rats treated with AG (−log EC50 7.6±0.2 n=7). Concentration-response curves to sodium nitroprusside (c) for UNX rats (−log EC50 7.8±0.1, n=9), UNX rats treated with AG (−log EC50 7.7±0.1, n=7), DOCA-salt rats (−log EC50 7.4±0.1, n=9) and DOCA-salt rats treated with AG (−log EC50 8.0±0.2, n=7). Values are mean±s.e.m.; Significance: *P<0.05 vs UNX rats; **P<0.05 vs DOCA-salt rats.
Discussion
Long-lived proteins such as collagen undergo continual non-enzymatic crosslinking with AGEs during ageing (Lakatta, 1993; Corman et al., 1998) as well as in diabetes (Brownlee, 1995; Norton et al., 1996) and hypertension (Mizutani et al., 2002; Wu & Juurlink, 2002). AG inhibits the formation of collagen crosslinking by reacting with the reactive glucose–protein Amadori product and forming an unreactive substituted Amadori product that cannot form AGEs (Brownlee et al., 1986; Edelstein & Brownlee, 1992). Increased collagen crosslinking has been shown to correlate with increased myocardial and vascular stiffness (Corman et al., 1998; Badenhorst et al., 2003). Thus, inhibition of collagen crosslinking and AGE formation should decrease AGE-induced crosslinking and decrease stiffness. AG has additional actions that may be of relevance in the prevention of cardiovascular disease. It is a selective inhibitor of the endotoxin-induced or cytokine-induced iNOS isoform (Griffiths et al., 1993). By inhibiting iNOS, AG may also decrease the formation of peroxynitrite (Hong et al., 2000), a pro-oxidant and an effective contributor to endothelial dysfunction (Kojda & Harrison, 1999). AG may also act as an antioxidant in the DOCA-salt rat, a model of increased superoxide production (Somers et al., 2000; Wu et al., 2001), by quenching hydroxyl radicals and inhibiting free radical formation (Giardino et al., 1998). Further, AGEs binding to their receptor RAGE trigger a cascade of events leading to intracellular generation of free radicals, oxidative stress and activation of the transcription factor NF-κB (Yan et al., 1994; Lander et al., 1997); inhibition of AGE formation may prevent this cascade.
One possible mechanism of action of AG in DOCA-salt rat hearts is as an iNOS inhibitor (Nilsson, 1999). Inflammatory cell infiltration has been demonstrated in the left ventricles of DOCA-salt rats (Fujisawa et al., 2001; Ammarguellat et al., 2002) and iNOS has been implicated in inflammation (Salvemini et al., 1995) and myocardial dysfunction (Salvemini et al., 1995; Oyama et al., 1998). However, it is important to note that as increased iNOS activity is characteristic of inflammation, a selective reduction in iNOS activity should also result in a reduction in inflammation. In this study, AG did not reduce inflammatory cell infiltration into the myocardium, suggesting that iNOS inhibition by AG is unlikely to be a major mechanism in the cardiac responses in the current study. The reduced response in thoracic aorta from UNX rats to acetylcholine could be owing to inhibition of NO synthase in endothelial cells by AG; however, this does not seem to apply to aorta from DOCA-salt rats where the endothelium is damaged.
The dosage of AG used for this study was based on previous studies using 0.1% in the drinking water (Li et al., 1996; Reckelhoff et al., 1999). As dose depends on water intake, this protocol produced different doses to DOCA-salt and control (uninephrectomized) rats. However, a wide range of AG doses has been reported (25 mg kg−1 (Brownlee et al., 1986), 100 mg kg−1 (Kochakian et al., 1996), 250 mg kg−1 (Bucala et al., 1991) and 7.35 mmol kg−1 (which as a free base equates to approximately 545 mg kg−1) (Norton et al., 1996)), all shown to be effective in inhibiting the formation of AGEs and crosslinking. Thus, it is likely that the average AG dose administered in this study would be effective in inhibiting collagen crosslinking.
Most experimental studies have investigated the role of increased collagen crosslinking in ageing and diabetes, rather than in hypertension. An increased crosslinking was shown in DOCA-salt hypertensive rats (Ooshima & Midorikawa, 1977) and in SHRs (Badenhorst et al., 2003) but not in aortic-banded pressure overload hypertrophy (Badenhorst et al., 2003). Methylglyoxal, the precursor of AGEs, and AGE formation was increased in the vascular tissues of SHRs (Mizutani et al., 2002; Wu & Juurlink, 2002); inhibition of AGE formation lowered systolic blood pressure and glycated albumin concentrations (Mizutani et al., 2002). As far as we are aware, this is the first study to show that a compound known to reduce collagen crosslinking can improve cardiac function in hypertensive rats.
Endothelial dysfunction is characteristic of the DOCA-salt rat (Kirchner et al., 1993; Somers et al., 2000). Numerous studies have shown favourable effects of AG on the vasculature of ageing and diabetic rats by decreasing crosslinking, reducing reactive oxygen species and inhibiting quenching of nitric oxide (Bucala et al., 1991; Li et al., 1996; Corman et al., 1998). This study shows that this improvement can also be demonstrated in the DOCA-salt hypertensive rat. Changes in blood pressure as in the DOCA-salt hypertensive rat may lead to vascular remodelling to maintain the wall stress, according to the LaPlace relationship. An analysis of wall thicknesses at the operating pressure in treated and untreated rats, rather than in vitro in an isolated vessel, may allow estimation of changes in wall stress during treatment.
Action potential prolongation in hypertrophied DOCA-salt hearts is probably caused by a reduction in the transient outward potassium current (Ito) (Momtaz et al., 1996). However, AG appeared to have minimal effects on regulating potassium currents in our study. We have shown prolongation of the APD50 in DOCA-salt rats treated with AG. Prolongation of APD50 is believed to be primarily owing to an increased L-type Ca2+ inward current (ICa) (Hart, 1994). AG has been shown to reverse the depression of the L-type calcium current in transplanted myocytes (Ziolo et al., 2001). Thus, this could be one mechanism by which AG treatment selectively prolonged APD50 in these rats.
In summary, it is likely that inhibition of AGE-induced collagen crosslinking as well as its antioxidant properties contributed to the favourable effects of AG on hypertension, hypertrophy, cardiac contractility and vascular reactivity without necessarily reducing fibrosis, inflammatory cell infiltration or iNOS activity in the DOCA-salt rats. Furthermore, this study clearly supports the concept that improvement in function is not necessarily dependent on a reduced content of collagen but rather reduced collagen crosslinking. Indeed, if collagen crosslinking plays such an important role in hypertension, the use of selective AGE crosslink breakers such as ALT-711 (Vasan et al., 2003; Bakris et al., 2004) could be of significant therapeutic importance in reversing end-organ damage in hypertension. A recent study by Little et al. (2005) demonstrated that treatment with ALT-711 decreased left ventricular mass and improved left ventricular diastolic filling parameters and quality of life in patients with diastolic heart failure. Although experience with ALT-711 in clinical studies is still limited, this compound is a potential treatment for the symptoms of diastolic heart failure (Little et al., 2005; Redfield, 2005).
Acknowledgments
We acknowledge Dr Darryl Burstow and Dr Andrew Fenning for their assistance with the echocardiographic and electrophysiological measurements.
Abbreviations
- AGEs
advanced glycation end-products
- APD20,50,90
action potential duration at 20, 50 or 90% of repolarization
- DOCA
deoxycorticosterone acetate
- UNX
uninephrectomized
References
- ABDEL-RAHMAN E., BOLTON W.K. Pimagedine: a novel therapy for diabetic nephropathy. Exp. Opin. Invest. Drugs. 2002;11:565–574. doi: 10.1517/13543784.11.4.565. [DOI] [PubMed] [Google Scholar]
- ALLAN A., FENNING A., LEVICK S., HOEY A., BROWN L. Reversal of cardiac dysfunction by selective ET-A receptor antagonism. Br. J. Pharmacol. 2005;146:846–853. doi: 10.1038/sj.bjp.0706384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AMMARGUELLAT F.Z., GANNON P.O., AMIRI F., SCHIFFRIN E.L. Fibrosis, matrix metalloproteinases, and inflammation in the heart of DOCA-salt hypertensive rats: role of ETA receptors. Hypertension. 2002;39:679–684. doi: 10.1161/hy0202.103481. [DOI] [PubMed] [Google Scholar]
- BADENHORST D., MASEKO M., TSOTETSI O.J., NAIDOO A., BROOKSBANK R., NORTON G.R., WOODIWISS A.J. Cross-linking influences the impact of quantitative changes in myocardial collagen on cardiac stiffness and remodelling in hypertension in rats. Cardiovasc. Res. 2003;57:632–641. doi: 10.1016/s0008-6363(02)00733-2. [DOI] [PubMed] [Google Scholar]
- BAKRIS G.L., BANK A.J., KASS D.A., NEUTEWL J.M., PRESTON R.A., OPARIL S. Advanced glycation end-product cross-link breakers. A novel approach to cardiovascular pathologies related to the aging process. Am. J. Hypertens. 2004;17:23S–30S. doi: 10.1016/j.amjhyper.2004.08.022. [DOI] [PubMed] [Google Scholar]
- BROWN L., CRAGOE E.J., JR, ABEL K.C., MANLEY S.W., BOURKE J.R. Amiloride analogues induce responses in isolated rat cardiovascular tissues by inhibition of Na+/Ca2+ exchange. Naunyn-Schmiedeberg's Arch. Pharmacol. 1991;344:220–224. doi: 10.1007/BF00167222. [DOI] [PubMed] [Google Scholar]
- BROWN L., DUCE B., MIRIC G., SERNIA C. Reversal of cardiac fibrosis in deoxycorticosterone acetate-salt hypertensive rats by inhibition of the renin-angiotensin system. J. Am. Soc. Nephrol. 1999;10:S143–S148. [PubMed] [Google Scholar]
- BROWN L., FENNING A., CHAN V., LOCH D., WILSON K., ANDERSON B., BURSTOW D. Echocardiographic assessment of cardiac structure and function in rats. Heart Lung Circ. 2002;11:167–173. doi: 10.1046/j.1444-2892.2002.00148.x. [DOI] [PubMed] [Google Scholar]
- BROWNLEE M. Advanced protein glycosylation in diabetes and aging. Annu. Rev. Med. 1995;46:223–234. doi: 10.1146/annurev.med.46.1.223. [DOI] [PubMed] [Google Scholar]
- BROWNLEE M., VLASSARA H., KOONEY A., ULRICH P., CERAMI A. Aminoguanidine prevents diabetes-induced arterial wall protein cross-linking. Science. 1986;232:1629–1632. doi: 10.1126/science.3487117. [DOI] [PubMed] [Google Scholar]
- BUCALA R., TRACEY K.J., CERAMI A. Advanced glycosylation products quench nitric oxide and mediate defective endothelium-dependent vasodilatation in experimental diabetes. J. Clin. Invest. 1991;97:432–438. doi: 10.1172/JCI115014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CANTINI C., KIEFFER P., CORMAN B., LIMIÑANA P., ATKINSON J., LARTAUD-IDJOUADIENE I. Aminoguanidine and aortic wall mechanics, structure, and composition in aged rats. Hypertension. 2001;38:943–948. doi: 10.1161/hy1001.096211. [DOI] [PubMed] [Google Scholar]
- CORMAN B., DURIEZ M., POITEVIN P., HEUDES D., BRUNEVAL P., TEDGUI A., LEVY B.I. Aminoguanidine prevents age-related arterial stiffening and cardiac hypertrophy. Proc. Natl. Acad. Sci. U.S.A. 1998;95:1301–1306. doi: 10.1073/pnas.95.3.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDELSTEIN D., BROWNLEE M. Mechanistic studies of advanced glycosylation end product inhibition by aminoguanidine. Diabetes. 1992;41:26–29. doi: 10.2337/diab.41.1.26. [DOI] [PubMed] [Google Scholar]
- FENNING A., HARRISON G., ROSE'MEYER R., HOEY A., BROWN L. L-Arginine attenuates cardiovascular impairment in DOCA-salt hypertensive rats. Am. J. Physiol. 2005;289:H1408–H1416. doi: 10.1152/ajpheart.00140.2005. [DOI] [PubMed] [Google Scholar]
- FUJISAWA G., DILLEY R., FULLERTON M.J., FUNDER J.W. Experimental cardiac fibrosis: differential time course of response to mineralocorticoid-salt administration. Endocrinology. 2001;142:3625–3631. doi: 10.1210/endo.142.8.8339. [DOI] [PubMed] [Google Scholar]
- GIARDINO I., FARD A.K., HATCHELL D.L., BROWNLEE M. Aminoguanidine inhibits reactive oxygen species formation, lipid peroxidation and oxidant induced apoptosis. Diabetes. 1998;47:1114–1120. doi: 10.2337/diabetes.47.7.1114. [DOI] [PubMed] [Google Scholar]
- GRIFFITHS M.J.D., MESSENT M., MACALLISTER R.J., EVANS T.W. Aminoguanidine selectively inhibits inducible nitric oxide synthase. Br. J. Pharmacol. 1993;110:963–968. doi: 10.1111/j.1476-5381.1993.tb13907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HART G. Cellular electrophysiology in cardiac hypertrophy and failure. Cardiovasc. Res. 1994;28:933–946. doi: 10.1093/cvr/28.7.933. [DOI] [PubMed] [Google Scholar]
- HONG H., LOH S., YEN M. Suppression of the development of hypertension by the inhibitor of inducible nitric oxide synthase. Br. J. Pharmacol. 2000;131:631–637. doi: 10.1038/sj.bjp.0703603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIRCHNER K.A., SCANLON P.H., JR, DZIELAK D.J., HESTER R.L. Endothelium-derived relaxing factor responses in DOCA-salt hypertensive rats. Am. J. Physiol. 1993;265:R568–R572. doi: 10.1152/ajpregu.1993.265.3.R568. [DOI] [PubMed] [Google Scholar]
- KOCHAKIAN M., MANJULA B.N., EGAN J.J. Chronic dosing with aminoguanidine and novel glycosylation end product-formation inhibitors ameliorates cross-linking of tail tendon collagen in STZ-induced diabetic rats. Diabetes. 1996;45:1694–1700. doi: 10.2337/diab.45.12.1694. [DOI] [PubMed] [Google Scholar]
- KOJDA G., HARRISON D. Interactions between NO and reactive oxygen species: pathophysiological importance in atherosclerosis, hypertension, diabetes and heart failure. Cardiovasc. Res. 1999;43:562–571. doi: 10.1016/s0008-6363(99)00169-8. [DOI] [PubMed] [Google Scholar]
- LAKATTA E.G. Cardiovascular regulatory mechanisms in advanced age. Physiol. Rev. 1993;73:413–467. doi: 10.1152/physrev.1993.73.2.413. [DOI] [PubMed] [Google Scholar]
- LANDER H.M., TAURAS J.M., OGISTE J.S., HORI O., MOSS R.A., SCHMIDT A.M. Activation for the receptor for AGE triggers a p21 ras dependent mitogen activated protein kinase pathway regulated by oxidant stress. J. Biol. Chem. 1997;272:17810–17814. doi: 10.1074/jbc.272.28.17810. [DOI] [PubMed] [Google Scholar]
- LI Y., STEFFES M., DONNELLY T., LIU C., FUH H., BASGEN J., BUCALA R., VLASSARA H. Prevention of cardiovascular and renal pathology of aging by the advanced glycation inhibitor aminoguandine. Proc. Natl. Acad. Sci. U.S.A. 1996;93:3902–3907. doi: 10.1073/pnas.93.9.3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LITTLE W.C., ZILE M.R., KITZMAN D.W., HUNDLEY W.G., O'BRIEN T.X., DEGROOF R.C. The effect of alagebrium chloride (ALT-711), a novel glucose cross-link breaker, in the treatment of elderly patients with diastolic heart failure. J. Card. Fail. 2005;11:191–195. doi: 10.1016/j.cardfail.2004.09.010. [DOI] [PubMed] [Google Scholar]
- MIYAUCHI Y., SHILAMA H., TAKASU T., OKAMIYA H., UMEDA M., HIRASAKI E., OHHATA I., NAKAYAMA H., NAKAGAWA S. Slowing of peripheral nerve conduction was ameliorated by aminoguanidine in streptozotocin-induced diabetic rats. Eur. J. Endocrinol. 1996;134:467–473. doi: 10.1530/eje.0.1340467. [DOI] [PubMed] [Google Scholar]
- MIZUTANI K., IKEDA K., TSUDA K., YAMORI Y. Inhibitor for advanced glycation end products formation attenuates hypertension and oxidative damage in genetic hypertensive rats. J. Hypertens. 2002;20:1607–1614. doi: 10.1097/00004872-200208000-00024. [DOI] [PubMed] [Google Scholar]
- MOMTAZ A., COULOMBE A., RICHER P., MERCADIER J.-J., CORABOEUF E. Action potential and plateau ionic currents in moderately and severely DOCA-salt hypertrophied rat hearts. J. Mol. Cell Cardiol. 1996;28:2511–2522. doi: 10.1006/jmcc.1996.0243. [DOI] [PubMed] [Google Scholar]
- NILSSON B. Biological effects of aminoguanidine: an update. Inflamm. Res. 1999;48:509–515. doi: 10.1007/s000110050495. [DOI] [PubMed] [Google Scholar]
- NORTON G.R., CANDY G., WOODIWISS A.J. Aminoguanidine prevents the decreased myocardial compliance produced by streptozotocin-induced diabetes mellitus in rats. Circulation. 1996;93:1905–1912. doi: 10.1161/01.cir.93.10.1905. [DOI] [PubMed] [Google Scholar]
- NORTON G.R., TSOTETSI J., TRIFUNOVIC B., HARTFORD C., CANDY G.P., WOODIWISS A.J. Myocardial stiffness is attributed to alterations in cross-linked collagen rather than total collagen or phenotypes in spontaneously hypertensive rats. Circulation. 1997;96:1991–1998. doi: 10.1161/01.cir.96.6.1991. [DOI] [PubMed] [Google Scholar]
- OOSHIMA A., MIDORIKAWA O. Increased lysyl oxidase activity in blood vessels of hypertensive rats and effect of β-aminopropionitrile on arteriosclerosis. Jpn. Circ. J. 1977;41:1337–1340. doi: 10.1253/jcj.41.1337. [DOI] [PubMed] [Google Scholar]
- OYAMA J., SHIMOKAWA H., MOMII H., CHENG X., FUKUYAMA N., ARAI Y., EGASHIRA K., NAKAZAWA H., TAKESHITA A. Role of nitric oxide and peroxynitrite in the cytokine-induced sustained myocardial dysfunction in dogs in vivo. J. Clin. Invest. 1998;101:2207–2214. doi: 10.1172/JCI986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PANAGIOTOPOULOS S., O'BRIEN R.C., BUCCALA R., COOPER M.E., JERUMS G. Aminoguanidine has an anti-atherogenic effect in the cholesterol-fed rabbit. Atherosclerosis. 1998;136:125–131. doi: 10.1016/s0021-9150(97)00192-5. [DOI] [PubMed] [Google Scholar]
- RECKELHOFF J.F., HENNINGTON B.S., KANJI V., RACUSEN L.C., SCHMIDT A.M., YAN S.D., MORROW J., ROBERTS L.J., II, SALAHUDEEN A.K. Chronic aminoguanidine attenuates renal dysfunction and injury in aging rats. Am. J. Hypertens. 1999;12:492–498. doi: 10.1016/s0895-7061(98)00264-7. [DOI] [PubMed] [Google Scholar]
- REDFIELD M.M. Treating diastolic heart failure with AGE crosslink breakers: thinking outside the heart failure box. J. Card. Fail. 2005;11:196–199. doi: 10.1016/j.cardfail.2005.02.001. [DOI] [PubMed] [Google Scholar]
- SALVEMINI D., MANNING P.T., ZWEIFEL B.S., SEIBERT K., CONNOR J., CURRIE M.G., NEEDLEMAN P., MASFERRER J.L. Dual inhibition of nitric oxide and prostaglandin production contributes to the antiinflammatory properties of nitric oxide synthase inhibitors. J. Clin. Invest. 1995;96:301–308. doi: 10.1172/JCI118035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOMERS M.J., MAVROMATIS K., GALIS Z.S., HARRISON D.G. Vascular superoxide production and vasomotor function in hypertension induced by deoxycorticosterone acetate-salt. Circulation. 2000;101:1722–1728. doi: 10.1161/01.cir.101.14.1722. [DOI] [PubMed] [Google Scholar]
- SUN Y., ZHANG J., LU L., BEDIGIAN M.P., ROBINSON A.D., WEBER K.T. Tissue angiotensin II in the regulation of inflammatory fibrogenic components of repair in the rat heart. J. Lab. Clin. Med. 2004;143:41–51. doi: 10.1016/j.lab.2003.07.004. [DOI] [PubMed] [Google Scholar]
- SWAMY-MRUTHINTI S., GREEN K., ABRAHAM E.C. Inhibition of cataracts in moderately diabetic rats by aminoguanidine. Exp. Eye Res. 1996;62:505–510. doi: 10.1006/exer.1996.0061. [DOI] [PubMed] [Google Scholar]
- VASAN S., FOILES P., FOUNDS H. Therapeutic potential of breakers of advanced glycation end-product-protein cross-linkages. Arch. Biochem. Biophys. 2003;419:89–96. doi: 10.1016/j.abb.2003.08.016. [DOI] [PubMed] [Google Scholar]
- WEBER K.T., BRILLA C.G., JANICKI J.S. Myocardial fibrosis: functional significance and regulatory factors. Cardiovasc. Res. 1993;27:341–348. doi: 10.1093/cvr/27.3.341. [DOI] [PubMed] [Google Scholar]
- WU L., JUURLINK B.H.J. Increased methylglyoxal and oxidative stress in hypertensive rat vascular smooth muscle cells. Hypertension. 2002;39:809–814. doi: 10.1161/hy0302.105207. [DOI] [PubMed] [Google Scholar]
- WU R., MILLETTE E., WU L., DE CHAMPLAIN J. Enhanced superoxide anion formation in vascular tissues from spontaneously hypertensive and desoxycorticosterone acetate-salt hypertensive rats. J. Hypertens. 2001;19:741–748. doi: 10.1097/00004872-200104000-00011. [DOI] [PubMed] [Google Scholar]
- YAN S.D., SCHIMDT A.M., ANDERSON G.M. Enhanced cellular oxidant stress by the interaction of advanced glycation end products with their receptors/binding proteins. J. Biol. Chem. 1994;269:9889–9897. [PubMed] [Google Scholar]
- ZIOLO M.T., HARSHBARGER C.H., ROYCROFT K.E., SMITH J.M., ROMANO F.D., SONDGEROTH K.L., WAHLER G.M. Myocytes isolated from rejecting transplanted rat hearts exhibit a nitric oxide-mediated reduction in the calcium current. J. Mol. Cell Cardiol. 2001;33:1691–1699. doi: 10.1006/jmcc.2001.1420. [DOI] [PubMed] [Google Scholar]