Abstract
Inhibition of the Na+–K+–2Cl− cotransporter (NKCC1) with bumetanide reduced contractile responses to phenylephrine (PE) in male rat aortas (129±4% of 60 mM KCl-induced contraction control vs 108±7% bumetanide; PE 10−5 M; P<0.01) but did not change equivalent responses in female rat aortas. Removal of the endothelium blunted the effect of NKCC1 inhibition on the response to PE (10−5 M) in males, whereas in denuded aorta from female rats, bumetanide reduced this response (162±5% control vs 146±3% bumetanide; P<0.05).
NKCC1 basal activity did not show gender differences in intact aortic rings, but in the presence of PE, bumetanide-sensitive 86Rb+/K+ uptake increased more in male than female aortas (179±8 in males vs 158±5 nmol 86Rb+/K+ min−1 (g aorta)−1 in females; P<0.05). PE did not stimulate NKCC1 activity in denuded aorta from male rats. However, in female rats, PE increased NKCC1 activity similarly in both denuded (169±11 nmol 86Rb+/K+ min−1 (g aorta)−1) and intact aortas.
Ovariectomy increased the bumetanide-sensitive 86Rb+/K+ uptake increase elicited by PE (223±17 nmol 86Rb+/K+ min−1 (g aorta)−1) and hormone replacement with 17β-estradiol prevented this effect (159±29 nmol 86Rb+/K+ min−1 (g aorta)−1).
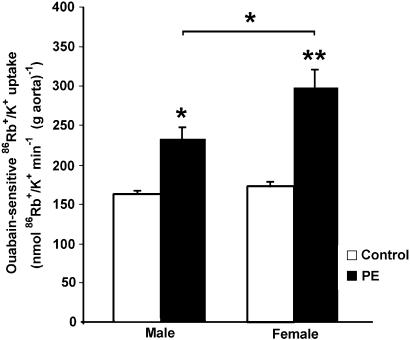
Na+,K+-ATPase basal activity, measured as ouabain-sensitive 86Rb+/K+ uptake, was similar in male and female rats, but the effect of PE was significantly less in intact male aortas (232±16 in males vs 296±25 nmol 86Rb+/K+ min−1 (g aorta)−1 in females; P<0.05).
Our results suggest that PE induced activation of NKCC1 and Na+,K+-ATPase in the rat aorta in a gender-dependent way.
Keywords: Phenylephrine, bumetanide, ouabain, vascular contraction, rat aorta
Introduction
Diverse ion channels and ion transporters are able to modulate the membrane potential and the tissue tension of smooth vascular muscle cells. Kreye et al. (1981) were the first to show the relevance of the Na+–K+–2Cl− (NKCC1) cotransporter in vascular smooth muscle contractility, and since then several studies have shown the possibility that the NKCC1 cotransporter intervenes in blood pressure regulation and normal vascular tone (Akar et al., 1999; Davis & Hill, 1999; Meyer et al., 2002). Phenylephrine (PE) stimulates the activity of the NKCC1 cotransporter (Akar et al., 1999), and its inhibition by bumetanide (a specific inhibitor of NKCC1) or furosemide reduces the contractile response to PE in rat aorta (Deth & Lynch, 1980; Lamb & Barna, 1998; Akar et al., 1999). Recently, Jiang et al. (2004) showed that blood pressure regulates the activity of the NKCC1 cotransporter in the rat aorta.
NKCC1 is an obligatory symport system, with an apparent stoichiometry of 1 : 1 : 2, sodium, potassium and chloride, respectively. Although the cotransporter is bidirectional in resting vascular smooth muscle cells, the sum of the electrochemical gradients for the three transported ion species determines net influx (O'Donnell & Owen, 1994). Stimulation of α-adrenoceptors induces a vasoconstrictor response by a mechanism that involves the release of Ca2+ from intracellular stores, calcium-dependent Cl− channel opening, membrane potential (Em) depolarization and voltage-dependent Ca2+ channel activation (Criddle et al., 1996). NKCC1 may potentiate vascular smooth muscle contraction by keeping [Cl−]i above the electrochemical equilibrium (Lamb & Barna, 1998; Chipperfield & Harper, 2000). More recently, Akar et al. (2001) postulated that the activation of the NKCC1 cotransporter by PE is the direct result of smooth muscle contraction through Ca2+-dependent activation of myosin light chain kinase.
Several studies have shown that gender differences in vasoconstrictor response are related to intracellular Ca2+ levels. For instance, Crews et al. (1999) showed that Ca2+ influx through voltage-dependent Ca2+ channels is less in female than in male rats. Barron et al. (2002) showed that in intact male rats, small physiological increases in [Na+]e enhance muscle contraction to PE by a mechanism involving Ca2+ entry, possibly via the reverse mode Na+–Ca2+ exchanger. This mechanism appears to be reduced in female rats by the presence of endogenous or exogenous estrogen. Also the substitution of chloride ions (Cl−) by thiocyanate ions in the extracellular medium of aortic rings caused a reduction in intracellular Ca2+ concentrations and a smaller or absent vasoconstrictor response in female rats (Standley et al., 1996). Therefore, transporters modulating intracellular chloride, such as NKCC1, could be implicated in gender-related differences to vasoconstrictors.
The Na+ pump of vascular smooth muscle cells plays a major role in the regulation of vascular tone (Bofill et al., 1994; Clausen & Nielsen, 1994; Therien & Blostein, 2000). Recently, the enhancement of acetylcholine (ACh)-induced relaxation observed in female rats may be in part explained by NO-dependent increased Na+,K+-ATPase activity in female vascular tissue (Palacios et al., 2004). An increase in Na+,K+-ATPase activity leads to hyperpolarization and relaxation of smooth muscle.
The purpose of the present study was to determine whether the specific gender differences in smooth muscle contraction are associated with changes in the function of NKCC1. PE-dependent contraction was measured in endothelium-intact and endothelium-denuded aortic rings of male and female rats in the presence and absence of bumetanide. In addition, the effect of PE on NKCC1 and Na+,K+-ATPase activity was studied in male and female rat aortas.
Methods
Animals
Male and female Sprague–Dawley rats (8–9 weeks of age, 180–250 g) were used. All rats were housed in groups of two or three in a temperature-controlled, light-cycled (08:00–20:00 hours) room with ad libitum access to water and standard rat chow (Champion, Santiago, Chile). The stages of the estrus cycle were determined by vaginal smear, and only females from the estrus stage were used. All procedures involving the use of animals were approved by the Institutional Animal Care and Use Committee. In some experiments, female rats were divided into three groups: control, ovariectomized (OVX) and ovariectomized plus estradiol (OVX+E2). Rats were anesthetized with ether and the ovaries were ligated and then removed. One week after the surgery, OVX rats were divided into two groups. One group received 17β-estradiol benzoate (20 μg/kg, every 48 h) in vegetal oil for 2 weeks by subcutaneous injection, and the control group received vehicle only. We selected the dose of hormone based on the ratio of (uterine weight : body weight × 10−2) in the three groups of rats (Palacios et al., 2004): estrus females (0.199±0.12), OVX (0.020±0.014) and OVX+E2 (0.204±0.014).
Isolation of aortic rings
Rats were killed by decapitation. The thoracic aorta was quickly excised and placed in cold (4°C) physiological Krebs–Ringer bicarbonate (KRB) buffer containing (in mM) 4.2 KCl, 1.19 KH2PO4, 120 NaCl, 25 Na2HCO3, 1.2 MgSO4, 1.3 CaCl2 and 5 D-glucose (pH 7.4). Rings (3–5 mm and 2–4 mg) were prepared after connective tissue was dissected from the aorta, taking special care to avoid endothelium damage. In some experiments, endothelium-denuded aortic rings were prepared by inserting a stainless steel wire into the lumen and gently rolling the ring on a filter paper soaked in KRB. Aortic rings were equilibrated for 40 min at 37°C in separate vials with 2 ml of KRB in a water-saturated atmosphere containing 95% O2–5% CO2 (Dubnoff incubator). After 40 min incubation in KRB, tissue samples were used for transport experiments.
Vascular reactivity experiments
The thoracic aorta was removed and placed in cold (4°C) physiological KRB buffer. Extreme care was taken during ring preparation to avoid stretching the tissue. In each experiment, two adjacent aortic rings were studied from the same animal in a paired manner. The method of Stallone et al. (1991) was followed for isometric tension measurements. The rings were mounted on two 25-gauge stainless steel wires; the lower one was attached to a stationary glass rod and the upper one was attached to a force–displacement transducer (Grass FT-03C). The transducer was connected to a Grass polygraph (model 7) for continuous recording of blood vessel tension. After the equilibration period, the aortic rings were stabilized by two successive near-maximal contractions with KCl (60 mM) for 10 min.
The role of NKCC1 in the vascular reactivity of female and male rat aortas in the presence and absence of bumetanide (10−5 M) was studied with PE concentration–response curves (10−9–10−5 M). Incubation with bumetanide was for 20 min. This protocol was repeated for different conditions: intact aortic rings, denuded aortic rings, incubated for 30 min with Nw-nitro-L-arginine (L-NNA 10−4 M; a nitric oxide synthase inhibitor) or indomethacin (prostaglandin synthesis inhibitor). To ensure that the resting tone of isolated aortas from male and female rats was similar, the resting tone of the blood vessels was assessed by relaxation with ACh (10−5 M) after the final contraction with PE.
NKCC1 and sodium pump activity: 86Rb+/K+ uptake into aortic rings
The NKCC1 and Na+,K+-ATPase activity in aortic rings was measured by bumetanide- and ouabain-sensitive 86Rb+/K+ uptake, respectively, according to Michea et al. (2001). The thoracic aorta was quickly excised and placed in cold (4°C) KRB. Aortic rings (3–5 mm long) were cut, weighed (2–4 mg per incubation sample) and equilibrated for 40 min in KRB (37°C). Then, triplicate samples were incubated in 2 ml of KRB containing 86Rb (0.1 mCi ml−1) in the presence or absence of bumetanide (10−5 M) or ouabain (10−3 M) for 20 min, as described previously (Bofill et al., 1994). Transferring the aortic rings into iced KRB stopped the reaction. The tissue was then quickly washed in cold buffer and gently blotted. Sample radioactivity was determined by Cerenkov radiation in a liquid scintillation counter in the presence of 0.1% Tween 20 (4 ml). The bumetanide- and ouabain-sensitive components of the 86Rb uptake, which are known to be an index of NKCC1 or Na+,K+-ATPase activity, respectively, were calculated by subtracting bumetanide- or ouabain-insensitive 86Rb uptake from the total 86Rb uptake. The results are expressed as nanomoles of 86Rb+/K+ min−1 (g aorta)−1.
Drugs
The following drugs were used in this study: L-phenylephrine hydrochloride (Sigma-Aldrich Co., St Louis, MO, U.S.A.), ACh chloride (Sigma-Aldrich Co., Munich, Germany), bumetanide (Sigma Co., U.S.A.), ouabain (Sigma-Aldrich Co., U.S.A.), L-NNA (Aldrich Chemical Co., Milwaukee, WI, U.S.A.), indomethacin (Rider, Santiago, Chile) and 17β-estradiol benzoate (Sigma Co., U.S.A.). Drugs were dissolved in distilled de-ionized water, except for bumetanide, which was dissolved in ethanol just before use. The final concentration of ethanol in solution was <0.05%. 17β-estradiol benzoate was dissolved in pure ethanol with sesame oil as vehicle. PE was prepared as a 10−3 M stock solution and stored at −20°C. Further dilutions were made in KRB solution. ACh as a stock solution in KRB (10−3 M) was prepared fresh before each experiment.
Statistical analysis
Values are expressed as means±s.e.m; n denotes the number of animals studied. Male and female groups were analyzed by gender (male vs female) and experimental treatment using a two-way analysis of variance (ANOVA) to detect significant differences, followed by Student–Newman–Keuls test to distinguish significant differences between the mean data from the male and female groups. Statistical evaluation of the contractile data was carried out by one-way ANOVA. The half-maximal concentration (EC50) of PE was determined from log–probit plots of the individual response vs concentrations, and data are shown as the average of the individual values. A P-value of <0.05 was considered statistically significant.
Results
Tension development in response to PE in the presence and absence of bumetanide in aortas from male and female rats: role of NKCC1
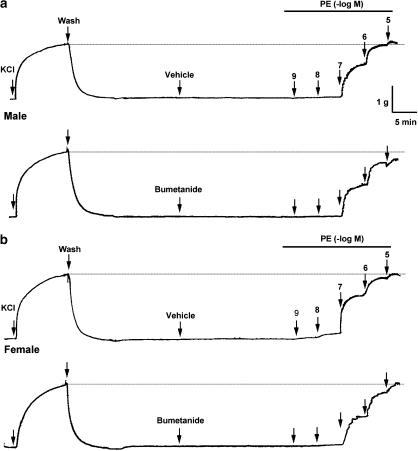
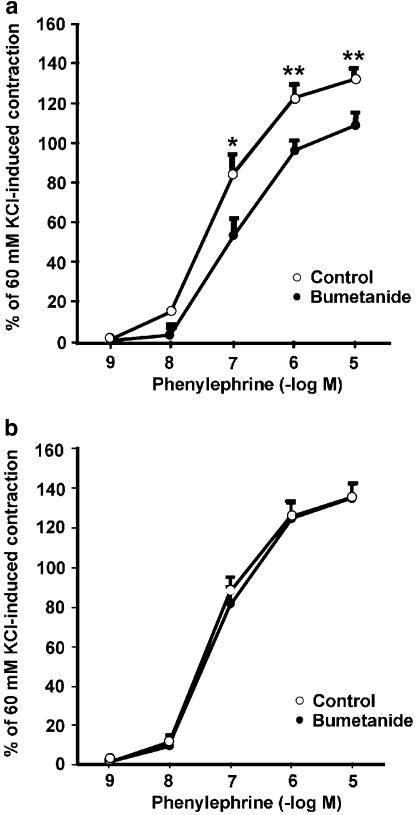
Figure 1 shows recordings of a typical response of aortic rings contracted with 60 mM KCl and then with PE (10−9–10−5 M). The recordings represent PE concentration–response curves in intact aortic rings from male and female rats in the estrus stage of their cycle. Bumetanide diminished the PE-induced contraction only in male rat aortas. As shown in Figure 2, the contractile response to PE (10−7–10−5 M) in intact aortas from male rats was significantly reduced in the presence of bumetanide with respect to control. At the maximal concentration of PE (10−5 M), values in male aortas were 129±4% of 60 mM KCl-induced contraction in control rings and 108±7% in bumetanide-treated rings (P<0.01) (Figure 2a). The sensitivity (EC50) to PE did not vary significantly in the presence of bumetanide (96.2±2.1 nM control vs 99.8±4.7 nM with bumetanide). In contrast, the participation of NKCC1 in the contractile response to PE in female rat aortas was not observed. The response to PE in intact arteries from female rats in the estrus stage of the cycle did not show significant changes after bumetanide treatment (Figure 2b). The results were similar when testing aortic rings obtained from male and female rats of higher body weight (340±4 g in male and 263±13 g in female; data not shown).
Figure 1.
Original trace showing the time course of the concentration–response curves to PE in intact aortic rings from male (a) and female (b) rats in the presence of bumetanide for 20 min or vehicle ethanol (0.02%). After an equilibration period and before PE, the aortic rings were stabilized by two successive near-maximal contractions with 60 mM KCl.
Figure 2.
PE concentration–response curves in endothelium-intact aortas from male (a) and female (b) rats in the presence or absence of 10 μM bumetanide. Contractile responses to PE are expressed as the percentage of 60 mM KCl-induced contraction after the equilibration period. Asterisks indicate statistically significant differences at intermediate (*P<0.05) and maximal (**P<0.01) concentrations of PE. Each data point represents the mean±s.e. of five independent experiments; points without error bars have s.e. smaller than symbol size.
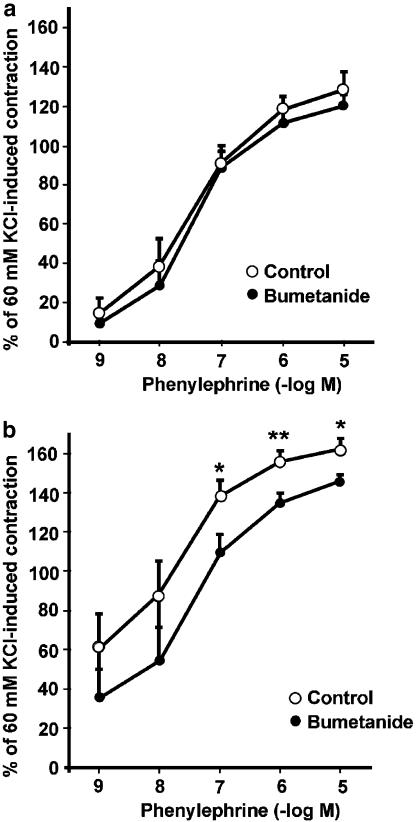
To evaluate the role of the endothelium on NKCC1 in the contractile response to PE, the experiments were repeated under the same conditions but after endothelium removal. Figure 3a shows that bumetanide did not modify the contractile response to PE in endothelium-denuded aortas from male rats. However, after removal of the endothelium from female rat aortas, bumetanide reduced the contractile response to PE. The mean response with the maximal concentration of PE (10−5 M) was 162±5% control vs 146±3% in the presence of bumetanide (P<0.05) (Figure 3b). In endothelium-denuded aortas from female rats, sensitivity (EC50) to PE was not significantly different in the presence of bumetanide (94.0±0.8 nM control vs 101.0±4.4 nM with bumetanide). In female rat aortas, the maximal contraction in response to PE (10−5 M) was significantly higher in endothelium-denuded aortas than in intact aortic rings (162±5% endothelium-denuded aortas vs 135±7% intact aortas; P<0.01) (Figures 2b and 3b).
Figure 3.
Concentration–response curves for PE in endothelium-denuded aortic rings from male (a) and female (b) rats in the presence or absence of 10 μM bumetanide. Contractile responses to PE are expressed as the percentage of 60 mM KCl-induced contraction, as in Figure 2. Asterisks indicate statistically significant differences at 10−7 and 10−5 M (*P<0.05) and 10−6 M (**P<0.01) concentrations of PE. Each data point represents the mean±s.e. of five experiments.
Role of NKCC1 in the contractile response to PE in the presence of L-NNA
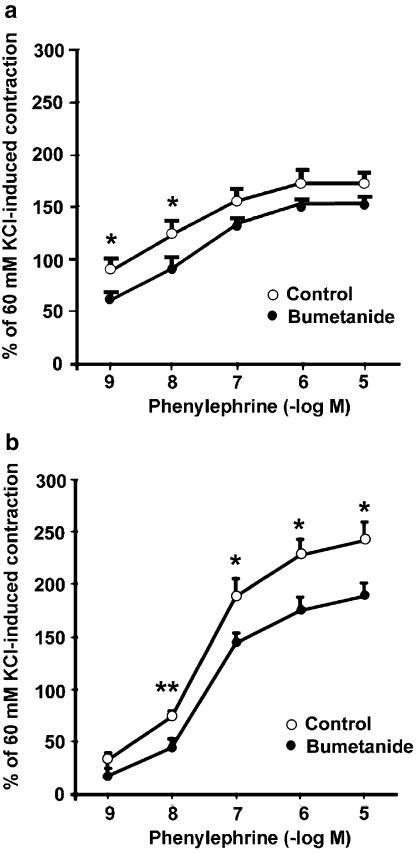
In order to gain insight into the potential role of NO in NKCC1 modulation in relation to the PE contractile response, experiments were carried out in intact arteries incubated with a nitric oxide synthase inhibitor (L-NNA, 100 μM) alone and in the presence of bumetanide. As shown in Figure 4a, bumetanide significantly reduced the vasoconstrictor response to PE (10−9 and 10−8 M; P<0.05) in male rat aortas in the presence of L-NNA. The EC50 for PE did not change in the presence of bumetanide.
Figure 4.
PE concentration–response curves in endothelium-intact aortas from male (a) and female (b) rats, preincubated (30 min) with 100 μM L-NNA and in the presence or absence of 10 μM bumetanide. Contractile responses to PE are expressed as the percentage of 60 mM KCl-induced contraction. Bumetanide significantly decreased the sensitivity to PE at the concentrations indicated by asterisks, *P<0.05 and **P<0.01. Each data point represents the mean±s.e. of five experiments.
On the other hand, in female rat aortic rings (Figure 4b), bumetanide significantly reduced the vasoconstrictor response to PE (10−8–10−5 M; P<0.05) in the presence of L-NNA. At the maximal concentration of PE (10−5 M), the female intact aortas averaged 242±17% control vs 189±13% with bumetanide (P<0.05) (Figure 4b). Also, the EC50 to PE was significantly different in the presence of L-NNA (control 12.5±1.59 nM vs 7.64±1.62 nM with bumetanide; P<0.05).
Role of NKCC1 in the contractile response to PE in the presence of indomethacin
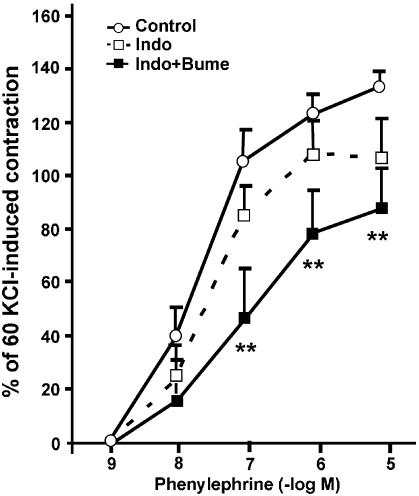
To provide some information as to the possible nature of the endothelial factor involved in the modulation of NKCC1 on the contractile response to PE in male rat aortas, the vascular rings were incubated with indomethacin (10 μM) for 30 min before the addition of PE. Indomethacin enhanced the effect of bumetanide in male intact aortic rings, whereas no significant changes were observed in the presence of indomethacin alone (Figure 5). The EC50 for PE was significantly different in the presence of indomethacin plus bumetanide (control 30.2±10.8 nM vs 129.3±50.7 nM indomethacin plus bumetanide; P<0.05).
Figure 5.
Concentration–response curves for PE in endothelium-intact aortic rings from male Sprague–Dawley rats, in the presence of 10 μM indomethacin (Indo), Indo plus 10 μM bumetanide (Bume) or vehicle control. Each data point represents the mean±s.e. of four experiments. Asterisks indicate statistically significant differences (**P<0.01) and points without error bars have s.e. smaller than symbol size.
Effect of PE on NKCC1 functional activity
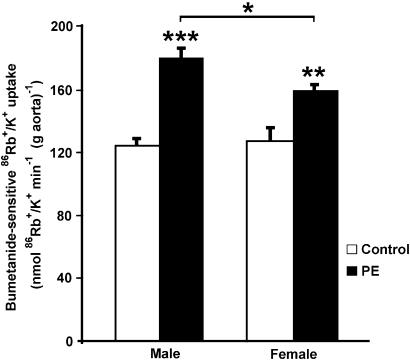
To investigate the effect of PE on NKCC1 functional activity in intact aortic rings from male and female rats, bumetanide-sensitive 86Rb+/K+ uptake was determined in the presence of 10−6 M PE. In previous transport experiments, using PE concentrations from 10−9 to 10−5 M, we have shown that 10−6 M PE was the lowest concentration that stimulated bumetanide-sensitive 86Rb uptake. In addition, 10−6 M PE was chosen after analyzing the vascular reactivity experiments presented above. NKCC1 basal activity did not show any gender differences (Figure 6). However, a higher PE-stimulated NKCC1 activity was observed in male compared with female intact aortic rings (179±8 in male vs 158±5 nmol 86Rb+/K+ min−1 (g aorta)−1 in female; P<0.05).
Figure 6.
Effect of PE on bumetanide-sensitive 86Rb+/K+ uptake in male and female intact aortic rings. Endothelium-intact aortic rings from male and female rats were preincubated with or without 10 μM bumetanide. Bumetanide-sensitive 86Rb+/K+ uptake was calculated by subtracting uptake obtained in the presence of bumetanide from uptake obtained in the absence of bumetanide. Rings were incubated for 20 min with 10−6 M PE (black bars) or vehicle (control, open bars). Results are the mean±s.e. of five experiments with each point assayed in triplicate; *P<0.05, **P<0.01 and ***P<0.001.
Effect of PE on NKCC1 in OVX rats
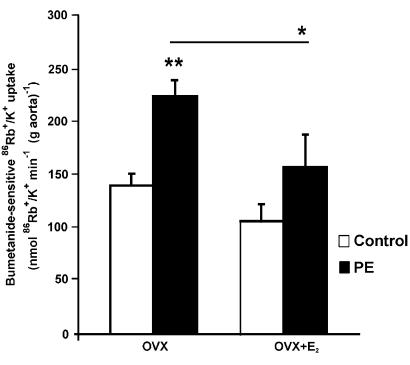
To test whether estrogens are involved in the gender difference of the action of PE (10−6 M) on the vascular NKCC1 cotransporter, we measured its functional activity in aortic rings from OVX rats. As shown in Figure 7 and 15 days after ovariectomy, there was an increased stimulation of bumetanide-sensitive 86Rb+/K+ uptake elicited by PE (223±17 nmol 86Rb+/K+ min−1 (g aorta)−1 in OVX). Interestingly, when OVX rats received estradiol replacement therapy, the effect of PE on bumetanide-sensitive 86Rb+/K+ uptake was prevented (159±29 nmol 86Rb+/K+ min−1 (g aorta)−1 in OVX+E2).
Figure 7.
Effect of ovariectomy and hormone replacement on PE-mediated stimulation of NKCC1 activity in female aortas. OVX rats were kept with (OVX+E2) or without (OVX) hormone replacement therapy as indicated in Methods. Bumetanide-sensitive 86Rb+/K+ uptake by endothelium-intact aortic rings was measured under basal conditions (open bars) or in the presence of 10−6 M PE (filled bars). Results are the mean±s.e. of five experiments with each point assayed in triplicate. E2, 17β-estradiol. *P<0.05 and **P<0.01.
Effect of endothelium on PE-stimulated NKCC1 activity
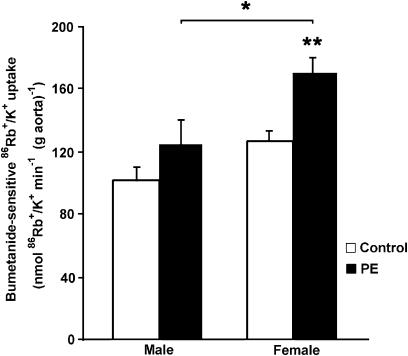
Removal of the endothelium inhibited the stimulatory action of PE (10−6 M) on NKCC1 activity only in aortas from male rats (124±16 nmol 86Rb+/K+ min−1 (g aorta)−1 in males; P<0.05; Figure 8). NKCC1 functional activity was increased to a similar extent in the presence of PE in female rats with both endothelium-intact and endothelium-denuded aortic rings (169±11 nmol 86Rb+/K+ min−1 (g aorta)−1). The lower bumetanide-sensitive uptake values observed in endothelium-denuded rings were not owing to 86Rb+/K+ uptake by endothelial cells, because in control experiments, we found that ablating the endothelium immediately after the uptake experiments (in ice-cold KRB) did not decrease the 86Rb counts, compared with the parallel intact rings, in agreement with previously described results (Hermsmeyer & Harder, 1986; Hishikawa et al., 1995; Goecke et al., 1998).
Figure 8.
Effect of endothelium on PE-stimulated NKCC1 activity in male and female rats. Endothelium-denuded aortic rings from male and female rats were preincubated with or without 10 μM bumetanide. Rings were incubated for 20 min with 10−6 M of PE (black bars) or vehicle (control, open bars). Results are the mean±s.e. of five experiments with each point assayed in triplicate; *P<0.05 and **P<0.01.
Effect of ACh on NKCC1 functional activity in intact aortic rings of male rats
Our results suggest that the endothelium affects NKCC1 activity in male rats (Figures 6 and 8). We evaluated the effect of the endothelium on NKCC1 activity by determining bumetanide-sensitive 86Rb+/K+ uptake in aortic rings with an intact endothelium in the presence of ACh (10−6 M). ACh did not increase NKCC1 function in the aortas obtained from male rats (control 156±27 vs 147±22 nmol 86Rb+/K+ min−1 (g aorta)−1 with ACh).
Effect of PE on the functional activity of Na+,K+-ATPase
Na+,K+-ATPase is responsible for the electrochemical gradient of sodium and potassium ions. In vascular smooth muscle cells, Na+,K+-ATPase plays a major role in the regulation of vascular tone (Blaustein, 1977; Clausen & Nielsen, 1994). An increase in Na+ pump activity may induce smooth muscle relaxation by increasing Na+/Ca2+ exchange and reducing Ca2+ influx through membrane potential-dependent Ca2+ channels (Clausen & Nielsen, 1994). To assess the role of the Na+ pump in the gender differences observed above, we tested Na+,K+-ATPase functional activity in the absence and presence of PE (10−6 M) in intact aortic rings from male and female rats. As shown in Figure 9, Na+,K+-ATPase basal activity was similar in male and female rats. However, although PE stimulated the activity of Na+,K+-ATPase in both male and female rats, significant gender differences were observed in the presence of PE (232±16 in male vs 296±25 nmol 86Rb+/K+ min−1 (g aorta)−1 in female aortic rings; P<0.05).
Figure 9.
Effect of PE on ouabain-sensitive 86Rb+/K+ uptake by male and female intact aortic rings. Aortic rings from male and female rats were preincubated with or without 1 mM ouabain. Ouabain-sensitive 86Rb+/K+ uptake was calculated by subtracting uptake obtained in the presence of ouabain from uptake obtained in the absence of ouabain. Rings were incubated (20 min) with 10−6 MPE (black bars) or vehicle (control, open bars). Results are the mean±s.e. of six experiments with each point assayed in triplicate; *P<0.05 and **P<0.01.
Discussion
It is known that NKCC1 is involved in vascular contractility (Kreye et al., 1981; Lamb & Barna, 1998; Akar et al., 1999; Jiang et al., 2004). Bumetanide has a vasodilatory effect in arteries pre-contracted by vasoactive substances, like catecholamines (Lamb & Barna, 1998), vasopressin, angiotensin and endothelin (Akar et al., 1999). Furthermore, Jiang et al. (2004) postulated that the NKCC1 cotransporter affected vascular tone in vivo.
Gender differences in the vascular contraction in the presence of bumetanide
Bumetanide treatment revealed gender differences in vascular NKCC1 function and contraction in response to PE. A reduction in the contractile response to PE in male rat aortas and no changes in female rat aortas were observed in the presence of bumetanide. In male aortas, the endothelium was a positive modulator of NKCC1, as endothelium removal blunted the involvement of NKCC1 in the contractile response to PE. In agreement with contraction studies, the absence of the endothelium also attenuated the PE-induced increase in bumetanide-sensitive 86Rb+ uptake, which is normally observed in endothelium-intact aortic rings. These results are also in agreement with previous studies, showing that the endothelium stimulates Na+–K+–2Cl−-dependent 86Rb+/K+ uptake in male aortas (Goecke et al., 1998; Michea et al., 2001). Potential endothelial factors stimulating NKCC1 function in response to PE were analyzed. ACh 10−6 M had no effect on bumetanide-sensitive 86Rb+ uptake. In addition, inhibition of NO synthase did not affect NKCC1 function. Interestingly, incubation with indomethacin increased the effect of bumetanide on the contractile response to PE. These results suggest that endothelial prostanoids could be involved in the enhancement of NKCC1 function in the vascular wall from male rats in response to PE. Mtabaji et al. (1976) demonstrated that bumetanide decreased the response of the rat mesenteric vascular bed to norepinephrine by inhibiting prostaglandin synthesis. In addition, it has been proposed that all venodilation is caused by decreased activity of NKCC1 (Greenberg et al., 1994) and/or prostaglandin release (Pickkers et al., 1997).
In contrast to the effect of bumetanide on the response of aortic rings obtained from male rats, the lack of variation in the contractile response in the presence of bumetanide observed in intact aortic rings from female rats would suggest that NKCC1 has little relevance or is not activated by PE in aortas from female rats. Alternatively, there may be vasodilatory mechanisms masking or counterbalancing the increase in NKCC1 activity. This hypothesis is supported by the observation that, in female rat aortas denuded of endothelium, bumetanide reduced the PE-induced contraction. The contractile response to PE in the presence of L-NNA was increased and, in the presence of the NO synthase inhibitor, bumetanide reduced the contractile response of endothelium-intact female aortic rings. Moreover, PE increased NKCC1 activity, measured as bumetanide-sensitive 86Rb+ uptake, to the same extent in female endothelium-intact and endothelium-denuded rat aortic rings. This is in agreement with the previous finding that female rat endothelium releases a greater amount of endothelial relaxing factors, such as NO, than male rat endothelium (Hishikawa et al., 1995; Andersen et al., 1999; Stallone et al., 2001; Cid et al., 2002).
Gender differences in the NKCC1 response to PE: role of estradiol
NKCC1 basal activity, measured as bumetanide-sensitive 86Rb+ uptake of intact aortic rings, did not show any gender differences. However, PE-stimulated NKCC1 activity was significantly less in female rat arteries. Ovariectomy increased PE-stimulated activity and we found that estradiol administration to OVX rats (15 days) prevented the stimulation of the bumetanide-sensitive 86Rb+/K+ uptake elicited by PE. These data suggest that vascular wall smooth muscle of female rat aortas could have lower [Na+]i and [Cl−]i in response to PE, secondary to estradiol. Gender differences in vascular activity may be modulated by actions of estradiol on chloride handling and other anions in vascular smooth muscle, which may be linked to transport of Ca2+ across the vascular muscle cell membrane (Zhang et al., 1991).
Relationship between NKCC1 and Na+,K+-ATPase activities
NKCC1 activity could be affected by Na+ pump activity. Indeed, pump inhibition by ouabain treatment leads to an increase in intracellular Ca2+ by depolarization-induced opening of voltage-dependent Ca2+ channels and by inhibition of Ca2+ efflux by the Na+/Ca2+ exchanger (Rhoden & Douglas, 1995). Although the results on cultured cells do not necessarily provide a guide to the intact tissue, Smith & Smith (1987) demonstrated that in cultured smooth muscle cells, the NKCC1 is activated by an increase in intracellular Ca2+ and inhibited by calmodulin antagonists. Therefore, in the present study, we analyzed the functional relationship between Na+,K+-ATPase and NKCC1 activities in the absence and presence of PE. Basal activities of NKCC1 and Na+,K+-ATPase were similar when comparing male and female intact aortas. In addition, there were no significant gender differences in the relative amounts of NKCC1 protein measured by Western blot (unpublished observations). In a previous study, we established that female aortas have greater amounts of α2 Na+ pump catalytic subunit protein than male aortas (Palacios et al., 2004). As the present results show, PE produced a greater effect on Na+,K+-ATPase activity in female rats than in male rats, which could be secondary to the greater abundance of Na+ catalytic subunits previously reported. Similarly, direct comparison of hearts obtained from spontaneously hypertensive rats of both genders showed increased activity of Na+,K+-ATPase in female hearts (Vlkovicova et al., 2005).
In conclusion, our study documented that PE stimulates the activities of the NKCC1 cotransporter and Na+,K+-ATPase in a gender-dependent way that may contribute to gender differences in vascular tone. The vascular endothelium from male rats in the presence of adrenergic agonists could release endothelial factors (prostaglandins) that increase NKCC1 activity, enhancing the contractile response. The role of vascular NKCC1 in PE-induced contraction of female arteries is less relevant, probably because of a greater amount of vasodilator factors produced by the endothelium (Hishikawa et al., 1995; Andersen et al., 1999; Stallone et al., 2001; Cid et al., 2002).
Acknowledgments
We thank Dr Elisa T. Marusic (Universidad de los Andes, School of Medicine) for her helpful comments on the manuscript. This work was supported by grants from FONDECYT 1050690 and Fondo Interno de Investigación Científica at Universidad de Los Andes Med. 001-03.
Abbreviations
- KRB
Krebs–Ringer bicarbonate
- L-NNA
Nw-nitro-L-arginine
- NKCC1
Na+–K+–2Cl−
- OVX
ovariectomized
- OVX+E2
ovariectomized plus estradiol
- PE
phenylephrine
References
- AKAR F., JIANG G., PAUL R.J., O'NEILL W.C. Contractile regulation of the Na(+)–K(+)–2Cl(−) cotransporter in vascular smooth muscle. Am. J. Physiol. 2001;281:C579–C584. doi: 10.1152/ajpcell.2001.281.2.C579. [DOI] [PubMed] [Google Scholar]
- AKAR F., SKINNER E., KLEIN J.D., JENA M., PAUL R.J., O'NEILL W.C. Vasoconstrictors and nitrovasodilators reciprocally regulate the Na+–K+–2Cl− cotransporter in rat aorta. Am. J. Physiol. 1999;276:C1383–C1390. doi: 10.1152/ajpcell.1999.276.6.C1383. [DOI] [PubMed] [Google Scholar]
- ANDERSEN H.L., WEIS J.U., FJALLAND B., KORSGAARD N. Effect of acute and long-term treatment with 17-beta-estradiol on the vasomotor responses in the rat aorta. Br. J. Pharmacol. 1999;126:159–168. doi: 10.1038/sj.bjp.0702289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARRON L.A., GREEN G.M., KHALIL R.A. Gender differences in vascular smooth muscle reactivity to increases in extracellular sodium salt. Hypertension. 2002;39:425–432. doi: 10.1161/hy02t2.102779. [DOI] [PubMed] [Google Scholar]
- BLAUSTEIN M.P. Sodium ions, calcium ions, blood pressure regulation, and hypertension: a reassessment and a hypothesis. Am. J. Physiol. 1977;232:C165–C173. doi: 10.1152/ajpcell.1977.232.5.C165. [DOI] [PubMed] [Google Scholar]
- BOFILL P., GOECHE I.A., BONILLA S., ALVO M., MARUSIC E.T. Tissue-specific modulation of Na+,K+-ATPase alpha-subunit gene expression in uremic rats. Kidney Int. 1994;45:672–678. doi: 10.1038/ki.1994.90. [DOI] [PubMed] [Google Scholar]
- CHIPPERFIELD A.R., HARPER A.A. Chloride in smooth muscle. Prog. Biophys. Mol. Biol. 2000;74:175–221. doi: 10.1016/s0079-6107(00)00024-9. [DOI] [PubMed] [Google Scholar]
- CID M.C., SCHNAPER H.W., KLEINMAN H.K. Estrogens and the vascular endothelium. Ann. NY Acad. Sci. 2002;966:143–157. doi: 10.1111/j.1749-6632.2002.tb04211.x. [DOI] [PubMed] [Google Scholar]
- CLAUSEN T., NIELSEN O.B. The Na+,K+-pump and muscle contractility. Acta Physiol. Scand. 1994;152:365–373. doi: 10.1111/j.1748-1716.1994.tb09818.x. [DOI] [PubMed] [Google Scholar]
- CREWS J.K., MURPHY J.G., KHALIL R.A. Gender differences in Ca(2+) entry mechanisms of vasoconstriction in Wistar–Kyoto and spontaneously hypertensive rats. Hypertension. 1999;34:931–936. doi: 10.1161/01.hyp.34.4.931. [DOI] [PubMed] [Google Scholar]
- CRIDDLE D.N., DE MOURA R.S., GREENWOOD I.A., LARGE W.A. Effect of niflumic acid on noradrenaline-induced contractions of the rat aorta. Br. J. Pharmacol. 1996;118:1065–1071. doi: 10.1111/j.1476-5381.1996.tb15507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS M.J., HILL M.A. Signaling mechanisms underlying the vascular myogenic response. Physiol. Rev. 1999;79:387–423. doi: 10.1152/physrev.1999.79.2.387. [DOI] [PubMed] [Google Scholar]
- DETH R.C., LYNCH C.J. The binding of 3H-ouabain to Na+–K+ ATPase sites in arterial smooth muscle. Pharmacology. 1980;21:29–37. doi: 10.1159/000137412. [DOI] [PubMed] [Google Scholar]
- GREENBERG S., MCGOWAN C., XIE J., SUMMER W.R. Selective pulmonary and venous smooth muscle relaxation by furosemide: a comparison with morphine. J. Pharmacol. Exp. Ther. 1994;270:1077–1085. [PubMed] [Google Scholar]
- GOECKE A., KUSANOVIC J.P., SERRANO M., CHARLIN T., ZUNIGA A., MARUSIC E.T. Increased Na,K,Cl cotransporter and Na, K-ATPase activity of vascular tissue in two-kidney Goldblatt hypertension. Biol. Res. 1998;31:263–271. [PubMed] [Google Scholar]
- HERMSMEYER K., HARDER D. Membrane ATPase mechanism of K+-return relaxation in arterial muscles of stroke-prone SHR and WKY. Am. J. Physiol. 1986;250:C557–C562. doi: 10.1152/ajpcell.1986.250.4.C557. [DOI] [PubMed] [Google Scholar]
- HISHIKAWA K., NAKAKI T., MARUMO T., SUZUKI H., KATO R., SARUTA T. Up-regulation of nitric oxide synthase by estradiol in human aortic endothelial cells. FEBS Lett. 1995;360:291–293. doi: 10.1016/0014-5793(95)00124-r. [DOI] [PubMed] [Google Scholar]
- JIANG G., AKAR F., COBBS S.L., LOMASHVILLI K., LAKKIS R., GORDON F.J., SUTLIFF R.L., O'NEILL W.C. Blood pressure regulates the activity and function of the Na–K–2Cl cotransporter in vascular smooth muscle. Am. J. Physiol. 2004;286:H1552–H1557. doi: 10.1152/ajpheart.00695.2003. [DOI] [PubMed] [Google Scholar]
- KREYE V.A., BAUER P.K., VILLHAUER I. Evidence for furosemide-sensitive active chloride transport in vascular smooth muscle. Eur. J. Pharmacol. 1981;73:91–95. doi: 10.1016/0014-2999(81)90150-3. [DOI] [PubMed] [Google Scholar]
- LAMB F.S., BARNA T.J. Chloride ion currents contribute functionally to norepinephrine-induced vascular contraction. Am. J. Physiol. 1998;275:H151–H160. doi: 10.1152/ajpheart.1998.275.1.H151. [DOI] [PubMed] [Google Scholar]
- MTABAJI J.P., MANKU M.S., HORROBIN D.F. Vascular actions of furosemide and bumetanide on the rat superior mesenteric vascular bed: interactions with prolactin and prostaglandins. Can. J. Physiol. Pharmacol. 1976;54:357–366. doi: 10.1139/y76-050. [DOI] [PubMed] [Google Scholar]
- MEYER J.W., FLAGELLA M., SUTLIFF R.L., LORENZ J.N., NIEMAN M.L., WEBER C.S., PAUL R.J., SHULL G.E. Decreased blood pressure and vascular smooth muscle tone in mice lacking basolateral Na(+)–K(+)–2Cl(−) cotransporter. Am. J. Physiol. 2002;283:H1846–H1855. doi: 10.1152/ajpheart.00083.2002. [DOI] [PubMed] [Google Scholar]
- MICHEA L., IRRIBARRA V., GOECKE I.A., MARUSIC E.T. Reduced Na–K pump but increased Na–K–2Cl cotransporter in aorta of streptozotocin-induced diabetic rat. Am. J. Physiol. 2001;280:H851–H858. doi: 10.1152/ajpheart.2001.280.2.H851. [DOI] [PubMed] [Google Scholar]
- O'DONNELL M.E., OWEN N.E. Regulation of ion pumps and carriers in vascular smooth muscle. Physiol. Rev. 1994;74:683–721. doi: 10.1152/physrev.1994.74.3.683. [DOI] [PubMed] [Google Scholar]
- PALACIOS J., MARUSIC E.T., LOPEZ N.C., GONZALEZ M., MICHEA L. Estradiol-induced expression of Na+–K+-ATPase catalytic isoforms in rat arteries: gender differences in activity mediated by nitric oxide donors. Am. J. Physiol. 2004;286:H1793–H1800. doi: 10.1152/ajpheart.00990.2003. [DOI] [PubMed] [Google Scholar]
- PICKKERS P., DORMANS T.P., RUSSEL F.G.M., HUGHES A.D., THIEN T., SCHAPER N., SMITS P. Direct vascular effects of furosemide in humans. Circulation. 1997;96:1847–1852. doi: 10.1161/01.cir.96.6.1847. [DOI] [PubMed] [Google Scholar]
- RHODEN K.J., DOUGLAS J.S. Evidence of Na–K–Cl cotransport in airway smooth muscle. Am. J. Physiol. 1995;268:L551–L557. doi: 10.1152/ajplung.1995.268.4.L551. [DOI] [PubMed] [Google Scholar]
- SMITH J.B., SMITH L. Na+/K+/Cl− cotransport in cultured vascular smooth muscle cells: stimulation by angiotensin II and calcium ionophores, inhibition by cyclic AMP and calmodulin antagonists. J. Membr. Biol. 1987;99:51–63. doi: 10.1007/BF01870621. [DOI] [PubMed] [Google Scholar]
- STANDLEY P.R., ZHANG F., RAVI J., RAM J.L., SOWERS J.R. Effects of SCN substitution for Cl− on the tension, [Ca2+]i, and ionic currents in vascular smooth muscle. Life Sci. 1996;59:739–752. doi: 10.1016/0024-3205(96)00360-8. [DOI] [PubMed] [Google Scholar]
- STALLONE J.N., SALISBURY R.L., FULTON C.T. Androgen-receptor defect abolishes sex differences in nitric oxide and reactivity to vasopressin in rat aorta. J. Appl. Physiol. 2001;91:2602–2610. doi: 10.1152/jappl.2001.91.6.2602. [DOI] [PubMed] [Google Scholar]
- STALLONE J.N., CROFTON J.T., SHARE L. Sexual dimorphism in vasopressin-induced contraction of rat aorta. Am. J. Physiol. 1991;260:H453–H458. doi: 10.1152/ajpheart.1991.260.2.H453. [DOI] [PubMed] [Google Scholar]
- THERIEN A.G., BLOSTEIN R. Mechanisms of sodium pump regulation. Am. J. Physiol. 2000;279:C541–C566. doi: 10.1152/ajpcell.2000.279.3.C541. [DOI] [PubMed] [Google Scholar]
- VLKOVICOVA J., JAVORKOVA V., PECHANOVA O., VRBJAR N. Gender difference in functional properties of Na,K-ATPase in the heart of spontaneously hypertensive rats. Life Sci. 2005;76:971–982. doi: 10.1016/j.lfs.2004.10.013. [DOI] [PubMed] [Google Scholar]
- ZHANG A., ALTURA B.T., ALTURA B.M. Sexual dimorphism of vascular smooth muscle responsiveness is dependent on anions and estrogen. Steroids. 1991;56:524–526. doi: 10.1016/0039-128x(91)90118-f. [DOI] [PubMed] [Google Scholar]