Abstract
The peripheral effect of the ‘opioid-like' peptide nociceptin/orphanin FQ (N/OFQ) on joint blood flow was investigated in acutely inflamed rats. Sensory neuropeptide release from capsaicin-sensitive nerves and the involvement of synovial mast cells and leukocytes on these vasomotor responses were also studied.
Blood flow measurements of exposed knee joints were performed in urethane-anaesthetised rats (2 mg kg−1 intraperitoneal) using laser Doppler perfusion imaging. Topical administration of N/OFQ (10−13–10−8 mol) to acutely inflamed joints caused a dose-dependent increase in synovial perfusion with an ED50 of 4.0 × 10−10 mol. This vasodilatatory response was blocked by the selective NOP receptor antagonist [Phe1-(CH2-NH)-Gly2]-Nociceptin(1–13)-NH2 (10−9 mol) (P<0.0001).
Co-administration of N/OFQ with the neurokinin-1 (NK1) receptor antagonist [D-Arg1,D-Phe5,D-Trp7,9,Leu11]-Substance P (10−12 mol), the vasoactive intestinal peptide (VIP) receptor antagonist VIP6–28 (10−9 mol) or the calcitonin gene-related peptide (CGRP) receptor antagonist CGRP8–37 (10−9 mol) all blocked the hyperaemic effect of N/OFQ (P<0.0001).
Treatment of acutely inflamed knees with capsaicin (8-methyl-N-vanillyl-6-noneamide) to destroy unmyelinated joint afferents also inhibited N/OFQ vasomotor activity.
Stabilisation of synovial mast cells with disodium cromoglycate (cromolyn) ameliorated N/OFQ responses, whereas inactivation of circulating leukocytes with the pan-selectin inhibitor fucoidin completely blocked N/OFQ-induced hyperaemia in these joints.
These experiments show that in acutely inflamed knee joints, N/OFQ acts on NOP receptors located on synovial mast cells and leukocytes leading to the secondary release of proinflammatory mediators into the joint. These agents subsequently stimulate sensory neuropeptide release from capsaicin-sensitive nerves culminating in vasodilatation and increased articular blood flow.
Keywords: Arthritis, blood flow, capsaicin, knee joint nerves, mast cells, acute inflammation, neuropeptides, opioid peptides, leukocytes
Introduction
Opioids are a family of compounds that are related to opiates in structure and activity, but do not originate from the poppy plant Papaver somniferum. Originally, three opioid receptors were identified (termed μ, δ and κ), which are G protein-coupled receptors consisting of seven transmembrane-spanning domains. The genes encoding for these receptors have been sequenced and successfully cloned (Evans et al., 1992; Kieffer et al., 1992; Li et al., 1993; Wang et al., 1993). While screening genomic and cDNA libraries for putative opioid receptor subtypes, a novel receptor was uncovered that showed 65% homology to the δ-opioid receptor but did not bind any known opioid (Chen et al., 1994; Mollereau et al., 1994; Wang et al., 1994). This ‘opioid-like' receptor is called NOP and is now known to bind a heptadecapeptide called nociceptin/orphanin FQ (N/OFQ). Immunolocalisation studies have determined that N/OFQ is widely distributed throughout the central nervous system (Riedl et al., 1996; Schuligoi et al., 1997; Neal et al., 1999) as well as occurs in nerve terminals innervating peripheral tissues including the rat ankle joint (Ackermann et al., 2001).
The physiological function of N/OFQ is highly diverse encompassing actions such as pain modulation, locomotor coordination and memory organisation (Darland et al., 1998; Goda & Mutneja, 1998; Mogil & Pasternak, 2001). One of the confounding characteristics of N/OFQ is that the physiological effects of the peptide are complex and varied depending on factors such as mode of administration, dose, animal species and inflammatory status of the tissue (for review see Mogil & Pasternak, 2001). Studies in rats have shown that systemic administration of N/OFQ causes transient hypotension and bradycardia (Champion & Kadowitz, 1997; Giuliani et al., 1997; Bigoni et al., 1999), whereas Arndt et al. (1999) found that N/OFQ caused increased systemic blood pressure and pronounced tachycardia when injected into sheep. In vitro pharmacological studies on precontracted arterial rings demonstrated a vasorelaxant effect of N/OFQ (Gumusel et al., 1997; Champion et al., 1998b) indicating a peripheral role for the peptide in counteracting vasomotor tone. Conversely, in vivo blood flow experiments carried out on joints revealed a vasoconstrictor effect of N/OFQ, which proved to be sympathetically mediated (McDougall, 2003).
Neurogenic inflammation is a process in which capsaicin-sensitive afferent nerves fire in an antidromic direction to cause the peripheral release of sensory neuropeptides from nerve terminals (Jansco et al., 1967). In joints, antidromic electrical stimulation of sensory nerves results in protein extravasation and vasodilatation of articular blood vessels (Ferrell & Cant, 1986; 1987), whereas chemical activation of these nerves by local capsaicin treatment causes conspicuous synovial hyperaemia (Karimian et al., 1995; Varga et al., 2005). Sensory neuropeptides, which have been shown to be vasodilatatory in joints and are therefore considered neurogenic inflammatory mediators, include substance P (SP) (Lam & Ferrell, 1991; 1993; McDougall et al., 1995), calcitonin gene-related peptide (CGRP) (Ferrell et al., 1997; McMurdo et al., 1997) and vasoactive intestinal peptide (VIP) (McDougall & Barin, 2005). Evidence is beginning to accumulate that suggests that peripheral administration of N/OFQ causes secondary release of SP from afferent nerve terminals. Inoue et al. (1998) found that the pro-nociceptive effects of N/OFQ in the paw could be blocked by capsaicin depletion of SP-containing nerves or by disruption of the tachykinin-1 gene. Similarly, antagonism of SP-preferring NK1 receptors inhibited N/OFQ-mediated nerve sensitisation (McDougall et al., 2000; 2001) and urinary bladder contraction (Lecci et al., 2000). In contrast, others have found an inhibitory effect of N/OFQ on sensory neuropeptide release leading to a reduction in neurogenic inflammation locally within the tissue (Helyes et al., 1997; Nemeth et al., 1998). These conflicting data reiterate the complexity of N/OFQ involvement in controlling inflammation and nociception.
The aim of the present investigation was to determine the effect of N/OFQ on synovial blood flow during the acute phase of joint inflammation. Additional experiments were performed in the inflammatory model to see whether sensory neuropeptides, leukocytes and mast cells contribute to the vasoregulatory effects of this ‘opioid-like' peptide.
Methods
Animals and preparatory surgical procedures
Male Wistar rats (262–304 g) were housed in cages at room temperature under a 12 : 12-h (0700–1900) light–dark cycle, with rodent food and tap water available ad libitum. The animal handling and surgical procedures employed in this study adhered to the guidelines set out by the Canadian Council for Animal Care.
The animals were deeply anaesthetised by intraperitoneal injection of urethane (25% stock solution, 2 g kg−1), and the depth of surgical anaesthesia was confirmed by the absence of the hindpaw flexion withdrawal reflex. The trachea was exposed and cannulated to ensure unobstructed breathing, and in case of respiratory difficulties, the animal was artificially ventilated with 100% oxygen by a Harvard rodent ventilator (Harvard Apparatus, Holliston, MA, U.S.A.). Blood pressure was monitored continuously via a heparin-saline-filled cannula (Portex Fine Bore Tubing, 0.50 mm internal diameter, 1.00 mm outer diameter, SIMS Portex, Kent, U.K.) that was inserted into the right carotid artery. Rats were placed in dorsal recumbency on an electrically heated blanket (TR-100, Fine Science Tools Inc., Vancouver, Canada) to maintain their internal body temperature at 37°C as measured by a rectally inserted electronic thermometer. The skin over the knee was excised to expose the antero-medial aspect of the knee joint and relative changes in synovial blood flow to this region were measured using a Moor Laser Doppler Imager (LDI) (Moore Instrument Ltd, Axminster, U.K.). Rat knees were placed 30 cm under the laser head and the exposed knee joint was scanned to generate a two-dimensional map of joint blood flow as described previously (Karimian et al., 1995; McDougall & Barin, 2005). Scan resolution was set at 93 × 88 pixels with a scan speed of 4 ms pixel−1. Knee joint scans were taken before (control) and then at 0, 2, 4 and 6 min after topical application of drugs. At the completion of an experiment, the animal was killed by anaesthetic overdose (sodium pentobarbital, 240 mg intracardiac) and a dead scan taken of the knee. This ‘biological zero' value was subtracted from all collected images.
Acute inflammation model
Rats were anaesthetised by isoflurane (1.5–3% isoflurane; 100% O2 at 1 l min−1), and depth of anaesthesia was confirmed by abolition of the hindpaw flexion withdrawal reflex. When the animals were fully anaesthetised, their right knee was shaved and swabbed with 100% ethanol. A 0.2 ml of 2% kaolin was injected into the intra-articular space, a 0.1 ml bolus in the posterior compartment and a 0.1 ml bolus in the anterior compartment of the joint cavity. Manual extension/flexion of the knee joint was performed for 10 min. Subsequently, 0.2 ml of 2% carrageenan was similarly injected as described above and the joint was moved for 30 s to permit dispersion of the carrageenan throughout the joint. After 3 h, animals were prepared for blood flow measurements and pharmacological assessment of joint perfusion. This recovery time is adequate for the development of an inflammatory reaction to occur and was confirmed by an observable increase in knee diameter.
Experimental procedure
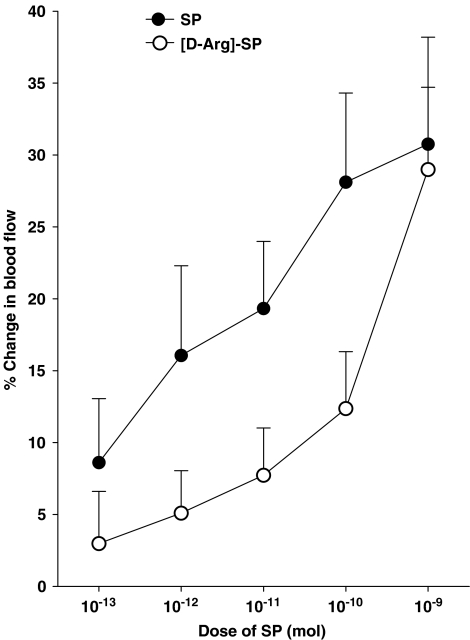
Animals with unilateral joint inflammation were randomly assigned to seven different groups. In one group of animals, a dose–response relationship to N/OFQ (10−13–10−8 mol) was determined in which the peptide was topically applied to the surface of the knee as a 0.1 ml bolus (37°C), with the order of dose being randomised between animals to discount any potential for tachyphylaxis. Between doses, the joint was washed with warmed (37°C) physiological saline (0.9% NaCl) to remove any residual peptide from the joint surface and to maintain tissue hydration. In subsequent animal groups, N/OFQ dose–response determination was repeated in the presence of selective sensory neuropeptide antagonists, which were also topically administered to the joint as a 0.1 ml bolus before each dose of N/OFQ. The inhibitors tested were the NOP receptor antagonist [Phe1-(CH2-NH)-Gly2]-Nociceptin(1–13)-NH2 (Noci1–13; 10−9 mol), the NK1 receptor antagonist [D-Arg1,D-Phe5,D-Trp7,9,Leu11]-Substance P ([D-Arg]-SP; 10−12 mol), the VPAC receptor antagonist VIP6–28 (10−9 mol) and the CGRP receptor antagonist CGRP8–37 (10−9 mol). With the exception of [D-Arg]-SP, these antagonists have previously been shown to block their respective receptors at the specified doses in the rat knee joint (McMurdo et al., 1997; McDougall, 2003; McDougall & Barin, 2005). NK1 receptor blockade with [D-Arg]-SP was confirmed in the rat knee by its ability to antagonise SP-mediated vasodilatation (see Figure 3).
Figure 3.
Vasomotor effect of SP on acutely inflamed knee joint blood flow and the inhibitory effect of 10−12 mol [D-Arg]-SP. Means±s.e.m. are indicated.
To further assess the role of sensory neuropeptides in N/OFQ responses, a separate group of six rats underwent capsaicin pretreatment to selectively destroy unmyelinated knee joint afferents. In these experiments, rats were deeply anaesthetised by intraperitoneal injection of diazepam (2.5 mg kg−1) and intramuscular injection of Hypnorm (0.2 ml kg−1), followed by an intramuscular injection of atropine (0.02–0.05 mg kg−1). A 0.2 ml of 1% capsaicin (vehicle consisting of 5% ethanol, 5% cremophor and saline) was injected into the right knee joint cavity, with 0.1 ml being introduced into the posterior and 0.1 ml into the anterior region. This approach has been shown to cause an 80% reduction in unmyelinated knee joint afferents (Ferrell et al., 1992). One week following capsaicin deafferentation, acute joint inflammation was induced and N/OFQ testing carried out as described.
To test whether synovial mast cells contribute to N/OFQ vasomotor responses in the acutely inflamed joint, a further group of rats were treated with the mast cell stabiliser disodium cromoglycate (cromolyn). In these experiments, a single dose of cromolyn (20 mg kg−1) was applied to the knee 5 min before N/OFQ dose–response determination. This treatment has previously been shown to stabilise connective tissue mast cells in the rat knee for over an hour, thereby preventing any vasoactive influence arising from the product of mast cell degranulation (McDougall & Barin, 2005).
A final group of seven rats were used to examine leukocyte involvement in N/OFQ vasomodulation. The pan-selectin blocker fucoidin (10 mg kg−1 intravenous, 50 μl bolus) was administered via a jugular vein cannulation (Portex Fine Bore Tubing, 0.40 mm internal diameter, 0.80 mm outer diameter, SIMS Portex, Kent, U.K.) to kaolin/carrageenan-inflamed animals 10 min before N/OFQ dose–response determination. A top-up dose of fucoidin was given every 30 min, which is sufficient to inhibit leukocyte function in arthritic joints (Gal et al., 2005).
Blood flow image analysis and statistics
Perfusion images were analysed with MoorLDI Processing software (Moor Instruments Ltd, Axminister, U.K.). The mean perfusion to the antero-medial aspect of the joint capsule was calculated and expressed in arbitrary perfusion units (PU). Blood flow changes in response to drug activity were calculated as the per cent change in PU between control and test images. Statistical tests were carried out using GraphPad Prism software (GraphPad Software, San Diego, U.S.A.). Dose–response relationships were determined by one-factor analysis of variance (ANOVA), whereas a two-factor ANOVA with a Bonferroni post hoc test was used to test for differences between experimental groups. Raw perfusion values measured before and following administration of neuropeptide antagonists alone were tested with a paired Student's t-test. Data are expressed as means±s.e.m. for n observations. A P-value less than 0.05 was considered statistically significant.
Drugs and reagents
All drugs and reagents were supplied by Sigma-Aldrich, Ontario, Canada. N/OFQ and all antagonists were dissolved in 0.9% saline and diluted to their final concentrations before being aliquoted and stored at −20°C until the day of experimentation.
Results
Effect of N/OFQ on acutely inflamed knee joint blood flow
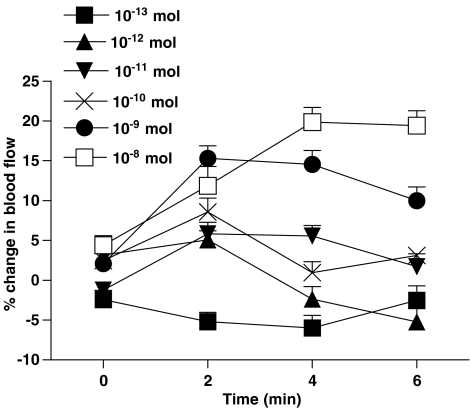
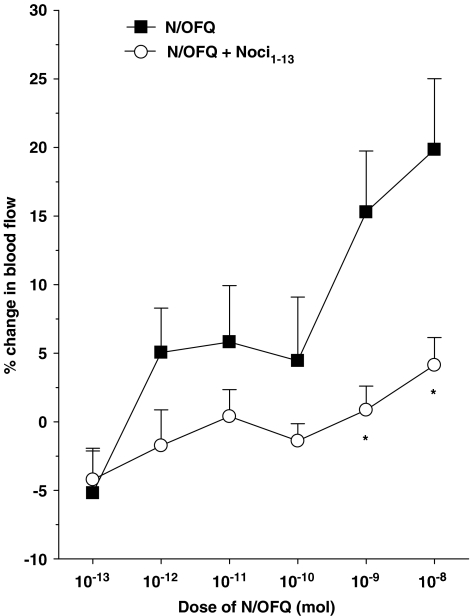
Topical application of N/OFQ onto the surface of acutely inflamed rat knee joints induced a progressive increase in articular blood flow indicative of synovial vasodilatation (Figure 1). For the 10−13–10−9 mol doses, the maximal effect occurred at the 2-min time point after N/OFQ administration, whereas the maximum response after application of the 10−8 mol dose occurred slightly later at 4 min. These time points were chosen to construct a dose–response curve to the neuropeptide (Figure 2), and a one-factor ANOVA confirmed that the vasodilator effect of N/OFQ was dose-dependent across the chosen dose range (P<0.005; n=8). The ED50 for this effect was calculated to be 4.0 × 10−10 mol. Co-administration of the selective NOP receptor antagonist Noci1–13 (Figure 2) significantly attenuated N/OFQ-mediated vasodilatation in the inflamed knee (P<0.0001, two-factor ANOVA; n=7–8).
Figure 1.
Time course of N/OFQ effects on synovial blood flow in acutely inflamed rat knee joints. Peak vasodilatation tended to occur 2 min after topical application of N/OFQ, although a maximal response time of 4 min was noted with the 10−8 mol dose. Data are means±s.e.m.
Figure 2.
Dose–response curves of N/OFQ either alone or in the presence of the NOP receptor antagonist Noci1–13 (10−9 mol). The 4-min time point was used for the 10−8 mol dose of N/OFQ and 2 min was used for all other doses. *P<0.05 Bonferroni post hoc test; n=7 and 8. Data are shown as means±s.e.m.
Influence of sensory neuropeptide antagonists on N/OFQ vasoactivity
To test the effectiveness of [D-Arg]-SP in blocking NK1 receptors in acutely inflamed knee joints, initial experiments were undertaken to confirm the antagonistic effect of the drug on SP vasomotor responses. Topical application of SP caused a significant dose-dependent vasodilatation in the joint capsule (Figure 3) and this effect was significantly attenuated by [D-Arg]-SP (P<0.01 two-factor ANOVA, n=12–14). Thus, [D-Arg]-SP is an effective antagonist of synovial NK1 receptors in this experimental model.
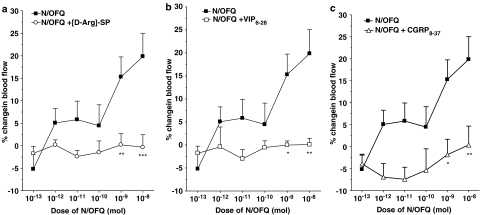
In Figure 4, the vasodilator effect of N/OFQ in acutely inflamed knee joints is completely blocked by co-administration of [D-Arg]-SP, VIP6–28 and CGRP8–37. Two-factor ANOVAs confirmed that the inhibitory effect of these sensory neuropeptide antagonists on N/OFQ vasoactivity was statistically significant (P<0.0001 for all groups, n=8 and 9).
Figure 4.
Dose–response relationship to N/OFQ either alone or in the presence of the NK1 receptor antagonist [D-Arg]-SP (10−12 mol) (a), the VPAC receptor antagonist VIP6–28 (10−9 mol) (b) or the CGRP receptor antagonist CGRP8–37 (10−9 mol) (c). *P<0.05, **P<0.01, ***P<0.001 Bonferroni post hoc test; n=8 and 9. Data are shown as means±s.e.m.
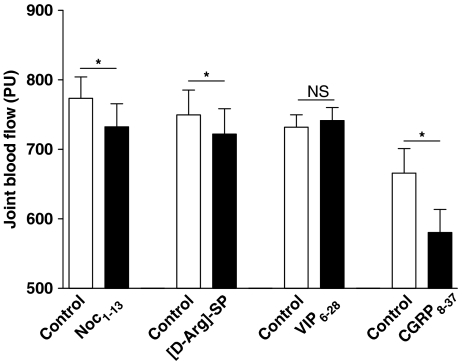
Experiments were also undertaken to examine the effect of the neuropeptide antagonists on joint basal blood flow in acutely inflamed knees (Figure 5). With the exception of VIP6–28, all of the sensory neuropeptide antagonists tested reduced synovial perfusion compared to preadministered control levels. The perfusion values for the different treatments were 773±82.0 PU (control) and 732±88.0 PU (Noci1–13; n=7), 749±113.1 PU (control) and 722±115.4 PU ([D-Arg]-SP; n=10), 732±50.2 PU (control), 741±56.0 PU (VIP6–28; n=9), 666±99.3 PU (control) and 580±94.2 PU (CGRP8–37; n=8).
Figure 5.
Effect of the sensory neuropeptide antagonists Noci1–13 (10−9 mol: NOP receptor), [D-Arg]-SP (10−12 mol: NK1 receptor), CGRP8–37 (10−9 mol: CGRP receptor) and VIP6–28 (10−9 mol: VPAC receptor antagonist) on acutely inflamed knee joint basal blood flow. *P<0.05 two-tailed paired Student's t-test, n=7–10. NS: not significantly different. Data are shown as means±s.e.m.
N/OFQ responses following capsaicin pretreatment of acutely inflamed knees
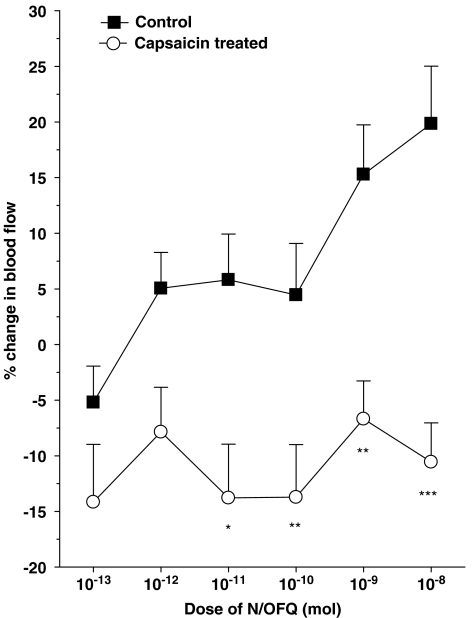
To test the involvement of joint afferents in N/OFQ activity, unmyelinated sensory nerves were destroyed by capsaicin treatment 1 week before inflammation induction and N/OFQ application. In these capsaicin-treated animals, the vasodilator effect of N/OFQ was completely abolished across the entire dose range (two-factor ANOVA, P<0.001, n=6–8; Figure 6).
Figure 6.
Effect of capsaicin treatment on N/OFQ-induced hyperaemia in acutely inflamed knees. *P<0.05, **P<0.01, ***P<0.001 Bonferroni post hoc test; n=6–8. Means±s.e.m. are shown for all data.
Role of synovial mast cells and leukocytes in N/OFQ-mediated vasodilatation
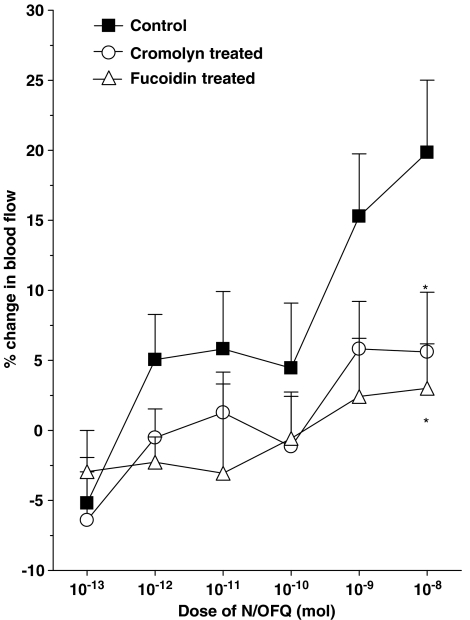
Further experiments were carried out to investigate whether synovial mast cells and leukocytes are involved in the vasoactive effects of N/OFQ. Stabilisation of synovial mast cells by topical cromolyn treatment (20 mg kg−1) significantly attenuated (P<0.0005 two-factor ANOVA, n=8–9) the dilator effect of N/OFQ in acutely inflamed knees (Figure 7).
Figure 7.
N/OFQ responses in control, mast cell stabilised (cromolyn treated) and leukocyte impaired (fucoidin treated) knee joints. *P<0.05 Bonferroni post hoc test; n=5–9. Data are shown as means±s.e.m.
In separate experiments, impairment of leukocyte function with the pan-selectin inhibitor fucoidin completely blocked N/OFQ-induced vasodilatation (P<0.001 two-factor ANOVA, n=5–9). The inhibitory effect of fucoidin on N/OFQ responses is shown in Figure 7.
Blood pressure
Blood pressure was monitored throughout all experiments (Table 1). None of the drugs investigated in this study had any significant effect on mean arterial blood pressure (P>0.05), confirming that all vasomotor changes were localised to the scanned joint and not influenced by centrally mediated baroreflex events. Therefore, the observed changes in blood flow described here were local and solely owing to the influence of the drugs.
Table 1.
MAP in response to topical application of N/OFQ, Noc1–13, [D-Arg1]-SP, VIP6–28, CGRP8–37 and cromolyn. Fucoidin was administered i.v.
| Drug | MAP (mmHg) | N-values |
|---|---|---|
|
N/OFQ (mol) | ||
| Control |
95±10 |
8 |
| 10−13 |
93±8 |
8 |
| 10−12 |
93±10 |
8 |
| 10−11 |
94±8 |
8 |
| 10−10 |
100±8 |
7 |
| 10−9 |
95±8 |
8 |
| 10−8 |
93±9 |
8 |
| | ||
|
Noci1–13 (mol) | ||
| Control |
105±3 |
6 |
| 10−9 |
100±3 |
6 |
| | ||
|
[D-Arg]-SP (mol) | ||
| Control |
107±4 |
10 |
| 10−12 |
106±4 |
10 |
| | ||
|
VIP6–28 (mol) | ||
| Control |
89±7 |
9 |
| 10−9 |
89±7 |
9 |
| | ||
|
CGRP8–37 (mol) | ||
| Control |
96±6 |
9 |
| 10−9 |
95±5 |
9 |
| | ||
|
Cromolyn (20 mg kg−1) | ||
| Control |
96±5 |
7 |
| Cromolyn |
93±4 |
7 |
| | ||
|
Fucoidin (10 mg kg−1) | ||
| Control |
96±4 |
7 |
| Fucoidin | 92±3 | 7 |
CGRP, calcitonin gene-related peptide; [D-Arg1]-SP, [D-Arg1,D-Phe5,D-Trp7,9,Leu11]-Substance P; i.v., intravenous; MAP, mean arterial blood pressure; N/OFQ, nociceptin/orphanin FQ; Noc1–13, [Phe1-(CH2-NH)-Gly2]-Nociceptin(1–13)-NH2; VIP, vasoactive intestinal peptide.
Values are expressed as means±s.e.m.
Discussion
Opioids and endogenous opioid peptides are quintessential modulators of pain and nociception, but are less recognised for their cardiovascular influences. Information regarding the most recent member of the opioid receptor family, NOP, and the role of its endogenous ligand N/OFQ in the control of vasomotor tone and inflammation is only just starting to emerge. The present investigation found that in acutely inflamed knee joints, local application of N/OFQ caused a dose-dependent increase in synovial blood flow, which was blocked by the selective NOP receptor antagonist [Phe1-(CH2-NH)-Gly2]-Nociceptin(1–13)-NH2 (Noci1–13). This finding is in contrast to what has recently been reported in normal knee joints where N/OFQ caused a sympathetically mediated vasoconstriction of articular blood vessels (McDougall, 2003). It appears, therefore, that the effect of N/OFQ on joint vasomotor tone depends upon whether the tissue is inflamed or not. The reason for this plasticity change following inflammation is unclear but may involve the influence of various immunocytes as well as alterations to second messenger systems, N/OFQ levels or NOP receptor density. Carrageenan inflammation causes a rapid increase in prepronociceptin expression in capsaicin-sensitive primary afferent neurones (Andoh et al., 1997; Itoh et al., 2001) and the resulting rise in N/OFQ may be necessary for vasodilatation to occur. Note from the normal knee joint blood flow study that although N/OFQ predominantly caused vasoconstriction, a high dose of the opioid peptide did produce a conspicuous vasodilatatory response (McDougall, 2003). Thus, in inflamed tissues, the elevation of constitutive N/OFQ levels transforms the normally vasoconstrictive effect of exogenous N/OFQ application into a vasodilatatory response. Inflammation-induced shifts in N/OFQ function have been demonstrated in other physiological processes such as pain perception. For example, peripheral administration of N/OFQ to normal animals sensitises knee joint primary afferents but has a desensitising effect following the application of a painful mechanical stimulus to acutely inflamed knees (McDougall et al., 2000). Similarly, it has been shown that the modulatory effect of N/OFQ on pain behaviour is altered in acutely inflamed animals when compared to normal non-inflamed controls (Xu et al., 1999; Carpenter et al., 2000). Clearly, the inflammatory status of a tissue appears to play a major role in determining the physiological outcome of N/OFQ activity.
Sensory neuropeptide antagonists and N/OFQ responses
Inoue et al. (1998) have reported that N/OFQ can elicit SP release from sensory nerve terminals to cause peripheral sensitisation and hyperalgesia. Similarly, the sensitising effect of N/OFQ on peripheral nociceptors and its involvement in central nociceptive processing can be blocked by treatment with selective NK1 receptor antagonists (Inoue et al., 1999; McDougall et al., 2001). The data presented here corroborate these findings by showing that in addition to blocking SP vasodilator effects, the NK1 receptor antagonist [D-Arg1,D-Phe5,D-Trp7,9,Leu11]-Substance P also abolished the vasodilatatory actions of N/OFQ in the acutely inflamed knee. Additional experiments extended this concept further by clearly showing that pharmacological blockade of other sensory neuropeptide receptors (viz. VPAC and CGRP receptors) also attenuated N/OFQ vasoactivity. Thus, the dilator effect of N/OFQ in the kaolin/carrageenan arthritis model is dependent upon the activation of sensory neuropeptide receptors located in the joint. Destruction of articular unmyelinated afferent nerve fibres by capsaicin treatment also significantly inhibited N/OFQ influences on synovial blood flow, confirming that the vasomotor effects of the opioid are neurally mediated. Taken together, these observations suggest that in acutely inflamed knees, N/OFQ acts on NOP receptors to cause the secondary release of proinflammatory neuropeptides from capsaicin-sensitive afferent nerves to produce vasorelaxation and ultimately increased blood flow. The capsaicin result also confirms that N/OFQ is not acting directly on the vascular endothelium to cause the release of endothelially derived relaxing factors such as nitric oxide. This potential mechanism of N/OFQ action has uniformly been repudiated in other studies (Champion et al., 1998a; Arndt et al., 1999).
In contrast to the present findings, a number of studies describe an inhibitory effect by N/OFQ on neurogenic inflammation. In rat skin, for example, vasodilatation and protein extravasation produced by antidromic stimulation of afferent C fibres was attenuated by systemic administration of N/OFQ (Helyes et al., 1997; Häbler et al., 1999) and the proposed reason for this inhibitory effect was a reduction in SP and CGRP release from sensory nerve endings. One explanation for the discrepancy between these results and the data presented here could be the route of administration of the opioid peptide. With systemic administration, N/OFQ has the propensity to alter centrally regulated vasomotor control as well as having direct effects on the heart and other organs. In the present experiments, however, N/OFQ was restricted to the periphery and only influenced articular blood vessels, as mean arterial pressure was unaffected by topical administration of the opioid peptide (see Table 1).
A supplementary finding of this study was that, with the exception of the VPAC receptor antagonist VIP6–28, synovial blood flow was significantly reduced by topical application of selective neuropeptide antagonists to acutely inflamed knees. The inability of VIP6–28 to alter joint perfusion in the kaolin/carrageenan model has previously been reported (McDougall & Barin, 2005). Thus, N/OFQ, SP and CGRP are all released locally into the joint to contribute to the hyperaemia commonly associated with acute synovitis.
Essential role of leukocytes and synovial mast cells in N/OFQ-induced vasodilatation
Further experiments were undertaken to determine whether N/OFQ was stimulating neuropeptide-containing nerves directly or if inflammatory cells were somehow involved in the process. It has recently been shown that N/OFQ has the potential to act as an immunomodulator by controlling the activity of various inflammatory cells. Following kaolin/carrageenan injection, there is a significant accumulation of leukocytes and immunocytes at the site of inflammation and this cellular infiltration is in part owing to the chemoattractant effect of N/OFQ on polymorphonuclear leukocytes (Halford et al., 1995; Serhan et al., 2001; Fiset et al., 2003). Further activation of NOP receptors on the surface of leukocytes promotes cellular degranulation prompting the suggestion that N/OFQ may stimulate inflammatory neuropeptide release from primary afferent nerves via the secondary release of leukocyte mediators (Fiset et al., 2003). This hypothesis is supported by the present study, which found that inactivation of circulating leukocytes with the pan-selectin inhibitor fucoidin completely blocked N/OFQ-mediated vasodilatation in kaolin/carrageenan-treated knees. Thus, N/OFQ does not appear to activate peptidergic nerves directly, but seems to stimulate neuropeptide release via the products of leukocyte degranulation. The identity of the leukocyte mediators and the mechanism by which they stimulate neuropeptide release from capsaicin-sensitive nerves into the joint requires further investigation.
The role of mast cells in N/OFQ responses remains equivocal. In normal rat knee joints, the hyperalgesic effect of N/OFQ was found to be mast cell-independent (McDougall & Larson, 2006), but this could be owing to the low number of synovial mast cells present in normal tissue. In the acutely inflamed joints described here, stabilisation of synovial mast cells with cromolyn abrogated N/OFQ increases in joint blood flow, suggesting that mast cells contribute to vasoregulation in these inflamed tissues. This finding is consistent with a previous study that found that N/OFQ stimulates histamine release from peritoneal mast cells (Kimura et al., 2000), which could then have the potential to initiate inflammatory changes such as protein extravasation and hyperaemia. It is entirely possible, therefore, that histamine and other mast cell agents liberated by N/OFQ could also trigger neuropeptide release from sensory nerve endings leading to neurogenic inflammation in the joint.
Based on the evidence described here and elsewhere, it appears that in acutely inflamed joints, the ‘opioid-like' peptide N/OFQ acts on NOP receptors located on synovial mast cells and leucokytes to cause cellular degranulation. The proinflammatory products of this process subsequently stimulate capsaicin-sensitive nerves leading to the release of SP, CGRP and VIP. These sensory neuropeptides then act on synovial blood vessels to elicit articular vasodilatation and increased synovial blood flow. Future studies examining the vasomotor effect of N/OFQ in clinically relevant chronic inflammatory models still need to be addressed.
Acknowledgments
The financial support of the Alberta Heritage Foundation for Medical Research (AHFMR), the Arthritis Society of Canada and the Canadian Institutes of Health Research (CIHR) are gratefully acknowledged. J.J. McD is an AHFMR Scholar and a CIHR/Arthritis Society of Canada New Investigator.
Abbreviations
- [D-Arg]-SP
[D-Arg1,D-Phe5,D-Trp7,9,Leu11]-Substance P
- CGRP
calcitonin gene-related peptide
- LDI
laser Doppler imager
- MAP
mean arterial pressure
- NK1
neurokinin-1
- N/OFQ
nociceptin/orphanin FQ
- Noci1–13
[Phe1-(CH2-NH)-Gly2]-Nociceptin(1–13)-NH2
- NOP
non-opioid
- PU
perfusion units
- SP
substance P
- VIP
vasoactive intestinal peptide
References
- ACKERMANN P.W., SPETEA M., NYLANDER I., PLOJ K., AHMED M., KREICBERGS A. An opioid system in connective tissue. A study of achilles tendon in the rat. J. Histochem. Cytochem. 2001;49:1387–1396. doi: 10.1177/002215540104901107. [DOI] [PubMed] [Google Scholar]
- ANDOH T., ITOH M., KURAISHI Y. Nociceptin gene expression in rat dorsal root ganglia induced by peripheral inflammation. Neuroreport. 1997;8:2793–2796. doi: 10.1097/00001756-199708180-00028. [DOI] [PubMed] [Google Scholar]
- ARNDT M.L., WU D., SOONG Y., SZETO H.H. Nociceptin/orphanin FQ increases blood pressure and heart rate via sympathetic activation in sheep. Peptides. 1999;20:465–470. doi: 10.1016/s0196-9781(99)00027-3. [DOI] [PubMed] [Google Scholar]
- BIGONI R., GIULIANI S., CALO G., RIZZI A., GUERRINI R., SALVADORI S., REGOLI D., MAGGI C.A. Characterization of nociceptin receptors in the periphery: in vitro and in vivo studies. Naunyn-Schmiedeberg's Arch. Pharmacol. 1999;359:160–167. doi: 10.1007/pl00005338. [DOI] [PubMed] [Google Scholar]
- CARPENTER K.J., VITHLANI M., DICKENSON A.H. Unaltered peripheral excitatory actions of nociceptin contrast with enhanced spinal inhibitory effects after carrageenan inflammation: an electrophysiological study in the rat. Pain. 2000;85:433–441. doi: 10.1016/S0304-3959(99)00301-2. [DOI] [PubMed] [Google Scholar]
- CHAMPION H.C., BIVALACQUA T.J., FRIEDMAN D.E., ZADINA J.E., KASTIN A.J., KADOWITZ P.J. Nitric oxide release mediates vasodilator responses to endomorphin 1 but not nociceptin/OFQ in the hindquarters vascular bed of the rat. Peptides. 1998a;19:1595–1602. doi: 10.1016/s0196-9781(98)00110-7. [DOI] [PubMed] [Google Scholar]
- CHAMPION H.C., KADOWITZ P.J. Nociceptin, an endogenous ligand for the ORL1 receptor, has novel hypotensive activity in the rat. Life Sci. 1997;60:PL241–PL245. doi: 10.1016/s0024-3205(97)00087-8. [DOI] [PubMed] [Google Scholar]
- CHAMPION H.C., PIERCE R.L., KADOWITZ P.J. Nociceptin, a novel endogenous ligand for the ORL1 receptor, dilates isolated resistance arteries from the rat. Regul. Peptides. 1998b;78:69–74. doi: 10.1016/s0167-0115(98)00117-7. [DOI] [PubMed] [Google Scholar]
- CHEN Y., FAN Y., LIU J., MESTEK A., TIAN M., KOZAK C.A., YU L. Molecular cloning, tissue distribution and chromosomal localization of a novel member of the opioid receptor gene family. FEBS Lett. 1994;347:279–283. doi: 10.1016/0014-5793(94)00560-5. [DOI] [PubMed] [Google Scholar]
- DARLAND T., HEINRICHER M.M., GRANDY D.K. Orphanin FQ/nociceptin: a role in pain and analgesia, but so much more. Trends Neurosci. 1998;21:215–221. doi: 10.1016/s0166-2236(97)01204-6. [DOI] [PubMed] [Google Scholar]
- EVANS C.J., KEITH D.E., JR, MORRISON H., MAGENDZO K., EDWARDS R.H. Cloning of a delta opioid receptor by functional expression. Science. 1992;258:1952–1955. doi: 10.1126/science.1335167. [DOI] [PubMed] [Google Scholar]
- FERRELL W.R., CANT R.Vasodilatation of articular blood vessels induced by antidromic electrical stimulation of joint C fibres Fine Afferent Nerve Fibres and Pain 1987Weinheim, FRG: VCH Verlagsgesellschaft; 187–192.Schmidt, R.F., Schaible, H.-G. & Vahle-Hinz, C. (ed) pp [Google Scholar]
- FERRELL W.R., LAM F., MONTGOMERY I. Differences in the axon composition of nerves supplying the rat knee joint following intra-articular injection of capsaicin. Neurosci. Lett. 1992;141:259–261. doi: 10.1016/0304-3940(92)90908-p. [DOI] [PubMed] [Google Scholar]
- FERRELL W.R., MCDOUGALL J.J., BRAY R.C. Spatial heterogeneity in the effects of calcitonin gene-related peptide (CGRP) on the microvasculature of ligaments in the rabbit knee joint. Br. J. Pharmacol. 1997;121:1397–1405. doi: 10.1038/sj.bjp.0701265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FERRELL W.R., RUSSELL N.J.W. Extravasation in the knee induced by antidromic stimulation of articular C fibre afferents of the anaesthetised cat. J. Physiol. 1986;379:407–416. doi: 10.1113/jphysiol.1986.sp016260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FISET M.E., GILBERT C., POUBELLE P.E., POULIOT M. Human neutrophils as a source of nociceptin: a novel link between pain and inflammation. Biochemistry. 2003;42:10498–10505. doi: 10.1021/bi0300635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAL I., BAJNOK E., SZANTO S., SARRAJ B., GLANT T.T., MIKECZ K. Visualization and in situ analysis of leukocyte trafficking into the ankle joint in a systemic murine model of rheumatoid arthritis. Arthritis Rheum. 2005;52:3269–3278. doi: 10.1002/art.21532. [DOI] [PubMed] [Google Scholar]
- GIULIANI S., TRAMONTANA M., LECCI A., MAGGI C.A. Effect of nociceptin on heart rate and blood pressure in anaesthetized rats. Eur. J. Pharmacol. 1997;333:177–179. doi: 10.1016/s0014-2999(97)01128-x. [DOI] [PubMed] [Google Scholar]
- GODA Y., MUTNEJA M. Memory mechanisms: the nociceptin connection. Curr. Biol. 1998;8:R889–R891. doi: 10.1016/s0960-9822(07)00553-2. [DOI] [PubMed] [Google Scholar]
- GUMUSEL B., HAO Q., HYMAN A., CHANG J.K., KAPUSTA D.R., LIPPTON H. Nociceptin: an endogenous agonist for central opioid like1 (ORL1) receptors possesses systemic vasorelaxant properties. Life Sci. 1997;60:PL141–PL145. doi: 10.1016/s0024-3205(96)00696-0. [DOI] [PubMed] [Google Scholar]
- HÄBLER H., TIMMERMANN L., STEGMANN J., JANIG W. Effects of nociceptin and nocistatin on antidromic vasodilatation in hairless skin of the rat hindlimb in vivo. Br. J. Pharmacol. 1999;127:1719–1727. doi: 10.1038/sj.bjp.0702712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALFORD W.P., GEBHARDT B.M., CARR D.J. Functional role and sequence analysis of a lymphocyte orphan opioid receptor. J. Neuroimmunol. 1995;59:91–101. doi: 10.1016/0165-5728(95)00030-6. [DOI] [PubMed] [Google Scholar]
- HELYES Z., NEMETH J., PINTER E., SZOLCSANYI J. Inhibition by nociceptin of neurogenic inflammation and the release of SP and CGRP from sensory nerve terminals. Br. J. Pharmacol. 1997;121:613–615. doi: 10.1038/sj.bjp.0701209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INOUE M., KOBAYASHI M., KOZAKI S., ZIMMER A., UEDA H. Nociceptin/orphanin FQ-induced nociceptive responses through substance P release from peripheral nerve endings in mice. Proc. Natl. Acad. Sci. U.S.A. 1998;95:10949–10953. doi: 10.1073/pnas.95.18.10949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INOUE M., SHIMOHIRA I., YOSHIDA A., ZIMMER A., TAKESHIMA H., SAKURADA T., UEDA H. Dose-related opposite modulation by nociceptin/orphanin FQ of substance P nociception in the nociceptors and spinal cord. J. Pharmacol. Exp. Ther. 1999;291:308–313. [PubMed] [Google Scholar]
- ITOH M., TAKASAKI I., ANDOH T., NOJIMA H., TOMINAGA M., KURAISHI Y. Induction by carrageenan inflammation of prepronociceptin mRNA in VR1-immunoreactive neurons in rat dorsal root ganglia. Neurosci. Res. 2001;40:227–233. doi: 10.1016/s0168-0102(01)00230-9. [DOI] [PubMed] [Google Scholar]
- JANSCO G., JANSCO-GABOR A., SZOLCSANJI J. Direct evidence for neurogenic inflammation and its prevention by denervation and pretreatment with capsaicin. Br. J. Pharmacol. 1967;31:138–151. doi: 10.1111/j.1476-5381.1967.tb01984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KARIMIAN S.M., MCDOUGALL J.J., FERRELL W.R. Neuropeptidergic and autonomic control of the vasculature of the rat knee joint revealed by laser Doppler perfusion imaging. Exp. Physiol. 1995;80:341–348. doi: 10.1113/expphysiol.1995.sp003851. [DOI] [PubMed] [Google Scholar]
- KIEFFER B.L., BEFORT K., GAVERIAUX-RUFF C., HIRTH C.G. The delta-opioid receptor: isolation of a cDNA by expression cloning and pharmacological characterization. Proc. Natl. Acad. Sci. U.S.A. 1992;89:12048–12052. doi: 10.1073/pnas.89.24.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIMURA T., KITAICHI K., HIRAMATSU K., YOSHIDA M., ITO Y., KUME H., YAMAKI K., SUZUKI R., TAKAGI K. Intradermal application of nociceptin increases vascular permeability in rats: the possible involvement of histamine release from mast cells. Eur. J. Pharmacol. 2000;407:327–332. doi: 10.1016/s0014-2999(00)00746-9. [DOI] [PubMed] [Google Scholar]
- LAM F.Y., FERRELL W.R. Specific neurokinin receptors mediate plasma extravasation in the rat knee joint. Br. J. Pharmacol. 1991;103:1263–1267. doi: 10.1111/j.1476-5381.1991.tb12334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAM F.Y., FERRELL W.R. Effects of interactions of naturally-occurring neuropeptides on blood flow in the rat knee joint. Br. J. Pharmacol. 1993;108:694–699. doi: 10.1111/j.1476-5381.1993.tb12863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LECCI A., GIULIANI S., TRAMONTANA M., MEINI S., SANTICIOLI P., MAGGI C.A. Tachykinin-mediated effect of nociceptin in the rat urinary bladder in vivo. Eur. J. Pharmacol. 2000;389:99–102. doi: 10.1016/s0014-2999(99)00802-x. [DOI] [PubMed] [Google Scholar]
- LI S., ZHU J., CHEN C., CHEN Y.W., DERIEL J.K., ASHBY B., LIU-CHEN L.Y. Molecular cloning and expression of a rat kappa opioid receptor. Biochem. J. 1993;295:629–633. doi: 10.1042/bj2950629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCDOUGALL J.J. Involvement of sympathetic efferents but not capsaicin-sensitive afferents in nociceptin-mediated dual control of rat synovial blood flow. Am. J. Physiol.: Regul. Integr. Comp. Physiol. 2003;284:R1477–R1485. doi: 10.1152/ajpregu.00733.2002. [DOI] [PubMed] [Google Scholar]
- MCDOUGALL J.J., BARIN A.K. The role of joint nerves and mast cells in the alteration of vasoactive intestinal peptide (VIP) sensitivity during inflammation progression in rats. Br. J. Pharmacol. 2005;145:104–113. doi: 10.1038/sj.bjp.0706169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCDOUGALL J.J., HANESCH U., PAWLAK M., SCHMIDT R.F. Participation of NK1 receptors in nociceptin-induced modulation of rat knee joint mechanosensitivity. Exp. Brain Res. 2001;137:249–253. doi: 10.1007/s002210000650. [DOI] [PubMed] [Google Scholar]
- MCDOUGALL J.J., KARIMIAN S.M., FERRELL W.R. Prolonged alteration of sympathetic vasoconstrictor and peptidergic vasodilator responses in rat knee joints by adjuvant-induced arthritis. Exp. Physiol. 1995;80:349–357. doi: 10.1113/expphysiol.1995.sp003852. [DOI] [PubMed] [Google Scholar]
- MCDOUGALL J.J., LARSON S.E.M. Nociceptin/orphanin FQ evokes knee joint pain in rats via a mast cell independent mechanism. Neurosci. Lett. 2006;398:135–138. doi: 10.1016/j.neulet.2005.12.066. [DOI] [PubMed] [Google Scholar]
- MCDOUGALL J.J., PAWLAK M., HANESCH U., SCHMIDT R.F. Peripheral modulation of rat knee joint afferent mechanosensitivity by nociceptin/orphanin FQ. Neurosci. Lett. 2000;288:123–126. doi: 10.1016/s0304-3940(00)01211-8. [DOI] [PubMed] [Google Scholar]
- MCMURDO L., LOCKHART J.C., FERRELL W.R. Modulation of synovial blood flow by the calcitonin gene-related peptide (CGRP) receptor antagonist, CGRP(8–37) Br. J. Pharmacol. 1997;121:1075–1080. doi: 10.1038/sj.bjp.0701237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOGIL J.S., PASTERNAK G.W. The molecular and behavioral pharmacology of the orphanin FQ/nociceptin peptide and receptor family. Pharmacol. Rev. 2001;53:381–415. [PubMed] [Google Scholar]
- MOLLEREAU C., PARMENTIER M., MAILLEUX P., BUTOUR J.L., MOISAND C., CHALON P., CAPUT D., VASSART G., MEUNIER J.C. ORL1, a novel member of the opioid receptor family. Cloning, functional expression and localization. FEBS Lett. 1994;341:33–38. doi: 10.1016/0014-5793(94)80235-1. [DOI] [PubMed] [Google Scholar]
- NEAL C.R., JR, MANSOUR A., REINSCHEID R., NOTHACKER H.P., CIVELLI O., WATSON S.J., JR Localization of orphanin FQ (nociceptin) peptide and messenger RNA in the central nervous system of the rat. J. Comp. Neurol. 1999;406:503–547. [PubMed] [Google Scholar]
- NEMETH J., HELYES Z., OROSZI G., THAN M., PINTER E., SZOLCSANYI J. Inhibition of nociceptin on sensory neuropeptide release and mast cell- mediated plasma extravasation in rats. Eur. J. Pharmacol. 1998;347:101–104. doi: 10.1016/s0014-2999(98)00216-7. [DOI] [PubMed] [Google Scholar]
- RIEDL M., SHUSTER S., VULCHANOVA L., WANG J., LOH H.H., ELDE R. Orphanin FQ/nociceptin-immunoreactive nerve fibers parallel those containing endogenous opioids in rat spinal cord. Neuroreport. 1996;7:1369–1372. doi: 10.1097/00001756-199605310-00007. [DOI] [PubMed] [Google Scholar]
- SCHULIGOI R., AMANN R., ANGELBERGER P., PESKAR B.A. Determination of nociceptin-like immunoreactivity in the rat dorsal spinal cord. Neurosci. Lett. 1997;224:136–138. doi: 10.1016/s0304-3940(97)13453-x. [DOI] [PubMed] [Google Scholar]
- SERHAN C.N., FIERRO I.M., CHIANG N., POULIOT M. Cutting edge: nociceptin stimulates neutrophil chemotaxis and recruitment: inhibition by aspirin-triggered-15-epi-lipoxin A4. J. Immunol. 2001;166:3650–3654. doi: 10.4049/jimmunol.166.6.3650. [DOI] [PubMed] [Google Scholar]
- VARGA A., NEMETH J., SZABO A., MCDOUGALL J.J., ZHANG C., ELEKES K., PINTER E., SZOLCSANYI J., HELYES Z. Effects of the novel TRPV1 receptor antagonist SB366791 in vitro and in vivo in the rat. Neurosci. Lett. 2005;398:135–138. doi: 10.1016/j.neulet.2005.05.015. [DOI] [PubMed] [Google Scholar]
- WANG J.B., IMAI Y., EPPLER C.M., GREGOR P., SPIVAK C.E., UHL G.R. mu opiate receptor: cDNA cloning and expression. Proc. Natl. Acad. Sci. U.S.A. 1993;90:10230–10234. doi: 10.1073/pnas.90.21.10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG J.B., JOHNSON P.S., IMAI Y., PERSICO A.M., OZENBERGER B.A., EPPLER C.M., UHL G.R. cDNA cloning of an orphan opiate receptor gene family member and its splice variant. FEBS Lett. 1994;348:75–79. doi: 10.1016/0014-5793(94)00557-5. [DOI] [PubMed] [Google Scholar]
- XU I.S., GRASS S., WIESENFELD-HALLIN Z., XU X.J. Effects of intrathecal orphanin FQ on a flexor reflex in the rat after inflammation or peripheral nerve section. Eur. J. Pharmacol. 1999;370:17–22. doi: 10.1016/s0014-2999(99)00111-9. [DOI] [PubMed] [Google Scholar]