Abstract
This study provides a comprehensive evaluation of 5-HT3 receptor functional distribution in both the rat and mouse intestinal tract.
5-HT3A−S receptor splice variant mRNA was expressed throughout the intestine of the rat and mouse; the 5-HT3A−L variant being more common in the rat.
5-HT, m-CPB, 1-PBG and 2-methyl-5-hydroxytryptamine (2m5-HT) induced contraction in the jejunum, ileum, proximal colon and distal colon of the rat (pEC50 range: 2m5-HT, 5.86±0.40 to m-CPB, 7.47±0.27) and mouse (pEC50 range: 1-PBG, 5.34±0.06 to m-CPB, 6.49±0.14) in the presence of nontarget 5-HT receptor antagonists, methysergide (1 μM) and GR125487 (0.1 μM). The rank orders of potency in the four regions of the rat and mouse intestine were concordant with the accepted order and the responses to 5-HT were inhibited by ondansetron (0.1 μM).
5-HT3-induced contractions to 5-HT were reduced by tetrodotoxin (1 μM). Pargyline (10 μM) and fluoxetine (1 μM) potentiated responses in the rat jejunum. Atropine (0.1 μM) potentiated 5-HT3-induced responses in the rat jejunum (Emax 49–65%), but attenuated responses in most other regions of the rat and mouse (e.g. mouse ileum: Emax 57–26%). In the rat jejunum, L-NAME (100 μM) mimicked the effect of atropine, hexamethonium (100 μM) suppressed 5-HT3-induced responses, but tachykinin receptor antagonists were without effect.
It is concluded that functional 5-HT3 receptors are present in nerves along the length of the rat and mouse intestinal tract. The mouse proximal colon was found to discriminate 5-HT3 receptor agonist profiles better than any other region in the rat or mouse. The rat jejunum shows evidence of 5-HT uptake and inactivation processes as well as inhibitory nitrergic and nontachykinin excitatory pathways associated with the 5-HT3-induced response.
Keywords: Gastrointestinal, serotonin, 5-HT3 receptors, ondansetron, fluoxetine, pargyline, atropine, L-NAME, rat jejunum
Introduction
The 5-hydroxytryptamine 3 (5-HT3) receptor belongs to a superfamily of ligand-gated ion channels that include nicotinic, glycine and GABAA receptors (Maricq et al., 1991). The 5-HT3 receptor possesses molecular diversity, which may explain some of the species and tissue-specific differences in its pharmacological profiles. Two subunits have been fully characterised in rodents, where homomeric expression of the 5-HT3A subunit forms a functional channel, whereas coexpression of both subunits results in a channel with modified pharmacological and functional characteristics (Dubin et al., 1999). In addition, two splice variants (5-HT3A−L and 5-HT3A−S) of the 5-HT3A subunit have been discovered (Hope et al., 1993) and these may be responsible for differences in efficacy of specific agonists, as a result of altering the intracellular structure of the channel protein (Niemeyer & Lummis, 1998).
5-HT3 receptors are located on neurones in the brain and spinal cord, autonomic, sensory nerves and enteric nervous systems, heart, blood vessels, skin, and are relevant to various clinical conditions, both centrally and peripherally (Hoyer et al., 1994). Early studies found that activation of the ‘M receptor', now known as the 5-HT3 receptor, elicits contraction in isolated guinea-pig ileum via acetylcholine (ACh) release (Gaddum & Picarelli, 1957). More recent studies have established that 5-HT3 receptors are located on pre- and postganglionic autonomic neurones and those of the sensory and enteric nervous systems (Fozard, 1984; Peters et al., 1991; Glatzle et al., 2002), where they mediate effects in the control of intestinal motility (Sevcik et al., 1996; Tuladhar et al., 1997) and secretion (Furman & Watson, 1989; Nagakura et al., 1997). In the rat, 5-HT3 receptor immunoreactivity has been identified in various cell types of the stomach and intestine. These cell types are neurones of myenteric and submucosal plexuses, fibres in muscle layers and mucosa and ICC cells and some EC cells (Glatzle et al., 2002).
There have been several studies of the intestine, which have established the presence and the function of 5-HT3 receptors in various species and regions. For example, Tuladhar et al. (2000) reported that a neuronally located 5-HT3 receptor mediates a contractile response to 5-HT in the mouse ileum, but the receptor displayed a different operational profile to that previously reported for the guinea-pig ileum (Chahl, 1983). We also reported that 5-HT3 receptors mediate neurogenic contraction using the rat jejunum (McLean & Coupar, 1996). The most extensive study to investigate the role of 5-HT3 receptors in different species was by Yamano et al. (1997). These authors found that the 5-HT3 receptor agonists, 2-methyl-5-hydroxytryptamine (2m5-HT) and meta-chlorophenylbiguanide (m-CPB), caused concentration-dependent contractions in the isolated longitudinal muscle of the ferret ileum, but no effect was observed in either the rat or piglet ileum (Yamano et al., 1997).
Although 5-HT3 receptor antagonists have become clinically important agents in treating postoperative and chemotherapy-induced nausea and emesis (Alon et al., 1996; Tang et al., 1998; Watters et al., 2001) and in delaying large bowel transit and improving pain and discomfort associated with irritable bowel syndrome (Camilleri et al., 2000), less attention has been paid to 5-HT3 receptor agonists as potential therapeutic agents. Consequently, future studies in this area will require suitable models of intestinal function. Therefore, the aim of this study was to provide a comprehensive evaluation of 5-HT3 receptor functional distribution in both the rat and mouse intestine. In addition, potential species and regional differences in 5-HT uptake and inactivation mechanisms were investigated together with a pharmacological determination of tissue localisation.
Methods
Animals and housing conditions
The experiments were carried out on 8-week-old Hooded Wistar rats of either sex, weighing between 200 and 300 g and Swiss mice of either sex weighing between 25 and 35 g. The rats were housed in single-sex groups of five in a cage and allowed food and water ad libitum. The mice were housed in similar groupings. All cages were floored with sawdust and bedding was replaced on a regular basis. The room was maintained on a constant 12/12 h light/dark cycle at a temperature of 18–22°C. Experiments were carried out in accordance with the NHMRC Australian code of practice for the care and use of animals for scientific purposes (1997).
Preparation of tissues
Animals were asphyxiated using CO2 and segments of jejunum, ileum, proximal colon and distal colon were removed. The jejunum was excised approximately 4–5 cm distal to the ligament of Treitz in the rat and 3–5 cm in the mouse. The ileum was excised approximately 4–5 cm before the ileo-caecal junction in the rat and 3–5 cm in the mouse. Excision halfway between the distal end of the caecum and the rectum resulted in the proximal and distal colon segments. The lumen of these tissues were flushed with Krebs–Henseleit solution (in mM: NaCl, 118.1; KCl, 4.69; KH2PO4, 1.2; NaHCO3, 25.0; glucose, 11; MgSO4, 1.2; CaCl2, 2.5) to remove any intestinal debris. Each tissue was cut into 3.5–5.5 cm lengths and then mounted longitudinally in 10 ml organ baths containing Krebs–Henseleit solution, bubbled with 5% CO2 in O2 and maintained at 37°C. The tissues were equilibrated for 45–60 min followed by three repeated additions of ACh (1 μM). Two more doses of ACh (1 μM) were added to the bath to establish the integrity of the tissue at the end of the experiment. Ugo Basile (Comario, Italy) isotonic transducers connected to a Powerlab system (ADI Instruments, Castle Hill, NSW, Australia) were used to measure isotonic changes in length of each tissue.
Construction of concentration–response curves
Noncumulative concentration–effect curves to 5-HT, 2m5-HT, 1-phenylbiguanide (1-PBG) and m-CPB were constructed in the presence of methysergide (1 μM), to inhibit 5-HT1,2,5,6,7 receptors (Hoyer et al., 1994) and GR125487 (0.1 μM), to inhibit 5-HT4 receptors (Gale et al., 1994). Curves were established by adding increasing concentrations of agonist for <1 min at 10 min intervals, and washing after each addition. This procedure has been shown previously to avoid the development of tachyphylaxis to 5-HT in the rat jejunum (McLean & Coupar, 1996). The antagonists were incubated with the tissue for 1 h before the start of the experiment and re-applied after each wash. Each tissue was used for only one discrete concentration–response curve to prevent desensitisation and the relative effect of each agonist was measured as a percentage of the maximal contraction induced by ACh (1 μM).
The effect of ondansetron (0.1 μM), fluoxetine (1 μM), pargyline (10 μM), atropine (0.1 μM) and tetrodotoxin (TTX; 1 μM) on 5-HT-induced contractions, in all four regions of the rat and mouse intestine, were investigated in separate experiments in the presence of methysergide (1 μM) and GR125487 (0.1 μM). Hexamethonium (100 μM), L-NAME (100 μM), SR140333, SR48968 and SR142801 (each at 0.1 μM) were used for further studies of the rat jejunum.
Experiments were controlled by using a four-organ bath setup allowing the four different regions from each animal to be examined at the same time. Paired segments from the same region were used for determining apparent pKB values. Four adjacent segments of rat jejunum, one acting as control, were used to study the effects of the drugs.
Analysis of results
All results are expressed as means±s.e.m. The potencies of all agonists are expressed as pEC50 values and efficacies are expressed as Emax values in relation to individual maxima, where 100% indicates the same response as the internal standard ACh. These values were estimated from monophasic nonlinear regression plots of concentration–response curves.
Monophasic concentration–effect curves were analysed by fitting a four-parameter (variable slope) logistic equation to the data to obtain location and slope parameters. The equation is:
where Top and Bottom are the maximum and minimum responses, respectively, EC50 is the midpoint potency value and n is the Hill slope factor. Biphasic curves were analysed by fitting a modified four-parameter logistic equation:
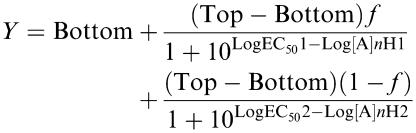 |
where Top and Bottom are the maximum and minimum responses, respectively, A is the agonist concentration, LogEC501 and LogEC502 are the midpoint potency parameters for the two phases, respectively, nH1 and nH2 are their corresponding Hill slopes and f is the fraction of the curve with the most potent phase.
Student's unpaired t-test was used to identify significant differences between EC50 and Emax values of agonists in each region. Differences between concentration–response curves of different agonists and of agonists in the presence and absence of ondansetron (0.1 μM), fluoxetine (1 μM), pargyline (10 μM), atropine (0.1 μM), L-NAME (100 μM), hexamethonium (100 μM) and TTX (1 μM) were calculated using two way analysis of variance (ANOVA). The number of tissues from different animals used to derive means is expressed by n.
Apparent pKB values were calculated from the relationship:
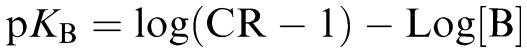 |
where CR is the ratio of the concentration of agonist producing equivalent responses in the presence and absence of antagonist (B) (Furchgott, 1972). All statistical analysis and graphics were performed using GraphPad Prism 4.0 (GraphPad Software, San Deigo, CA, U.S.A.).
cDNA preparation and PCR amplification of intestinal tissue
The intraluminal contents were flushed out using RNAse-free Krebs–Henseleit solution. The tissues were placed in microcentrifuge tubes containing RNAlater™ (Ambion, Austin, TX, U.S.A.) then immediately frozen in liquid nitrogen and stored at −80°C. Frozen jejunum, ileum, proximal colon and distal colon tissues from six Hooded Wistar rats and six Swiss mice, of either sex, were homogenised with a pestle and mortar in liquid nitrogen for total RNA extraction and reverse transcription as described (Liu et al., 2004). The quality of cDNA produced was assessed by amplifying cDNA for the house-keeping gene, glyceraldehyde-3-phosphate dehydrogenase (G3PDH) using the following primers (forward: ACCACAGTCCATGCCATCC; reverse: TCCACCACCCTGTTGCTGTA) and the PCR products were separated on 2% agarose gels. The cDNA specific for the 5-HT3A receptor subunit were detected using PCR amplification with primers (for the rat, forward 5′-GATAAGCCTCGCTGAGACCA-3′; reverse 5′- CAGCCTGTCCAGCACATATC-3′ and for the mouse, forward: 5′-GATAAGCCTCGCTGAGACCA-3′; reverse 5′- CGCATCTCATCCCGCTTCTC-3′) designed to coamplify the 5-HT3A−S and 5-HT3A−L splice variants. A standard PCR mixture contained cDNA (or negative RT reaction), 1 × PCR buffer, 0.1 μM primers, 200 μM dNTPs and 0.5 U Taq DNA polymerase (Qiagen, Doncaster, VIC, Australia), 1 × Q-solution per 25 μl reaction. All tubes were denatured at 94°C for 5 min and then 35 cycles of amplification were performed (60 s denaturation at 94°C, 60 s annealing at 60°C and 60 s extension at 72°C) with a final extension at 72°C for 8 min in a FTS-960 DNA Thermal sequencer (Corbett Research, NSW, Australia). A 1 μl aliquot of these PCR products was used as the new template for a second round of amplification using the same primers and conditions. The products of the PCR amplification were expected to be 420 and 405 bp in the rat and 374 and 356 bp in the mouse corresponding to 5-HT3A−L and 5-HT3A−S transcripts, respectively. The identity of the products was confirmed by restriction digest analysis. The re-amplified PCR products were then separated on 2% agarose gels.
Drugs
5-HT creatinine sulphate complex (Sigma, Castle Hill, NSW, Australia), m-CPB hydrochloride (Tocris, Ellisville, U.S.A.), 2-methyl-5-HT hydrochloride (Tocris), 1-PBG (Tocris), methysergide hydrogen maleate (Sandoz, Basle, Switzerland), GR125487 (1-{2[(methylsulfonyl)amino]ethyl}piperidin-4-yl) methyl 5-fluoro-2-methoxy-1H-indole-3-carboxylate) (Glaxo, Hertfordshire, U.K.), atropine sulphate (Sigma), fluoxetine hydrochloride (Sigma), pargyline hydrochloride (Sigma), ondansetron hydrochloride (Glaxo, Melbourne, Australia), hexamethonium bromide (Sigma), L-NAME (Nω-nitro-L-arginine methyl ester hydrochloride) (Sigma) and ACh chloride (Sigma) were dissolved in distilled water. TTX (Sigma) was dissolved in citrate buffer at pH 4.5. SR140333, SR48968 and SR142801 (Sanofi Recherche, Montpellier, France) were dissolved in 100 mM dimethylsulphoxide. The concentration of this solvent constituted less than 0.1% of the total bath concentration and when tested did not appear to affect the tone of the tissue.
Results
Expression of 5-HT3A receptor mRNA in the intestine
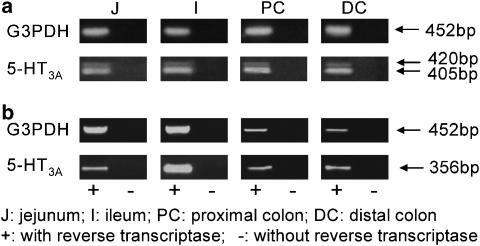
The 5-HT3A−S receptor splice variant was detected in all gastrointestinal tract tissues in the rat and mouse by RT—PCR double amplification (see Figure 1). The identity of the products was confirmed by restriction digest analysis. The 5-HT3A−L splice variant, in contrast, was present (albeit considerably less) in all regions of the rat (Figure 1a; n=6), but was virtually undetectable in all regions of the mouse (Figure 1b). Specifically, 5-HT3A−L was only observed in the jejunum (one out of six mice) and ileum (two out of seven mice) of the mouse. The G3PDH levels were comparable between all the rat intestinal regions and in the mouse the G3PDH levels were comparable within the small intestine and within the large intestine, indicating that similar amounts of mRNA were present.
Figure 1.
A representative example of the RT–PCR analysis of the 5-HT3A receptor isoform mRNA expression in the different intestinal regions of the rat (a) and mouse (b). A double PCR amplification was undertaken to amplify cDNA fragments of 405 and 420 bp in the rat and 356 and 374 bp in the mouse representing the isoforms 5-HT3A−S and 5-HT3A−L, respectively. Control amplifications of the G3PDH gene (452 bp) are from the same RNA samples with (plus) or without (minus) reverse transcriptase (RT) to monitor for RNA amount and DNA contamination.
Conditions for studying functional 5-HT3 receptor activity
Responses to Ach
The responses to 5-HT and 5-HT3 receptor agonists in the rat and mouse jejunum, ileum, proximal and distal colon are expressed as a percentage of the maximum response to ACh (1 μM). ACh caused the same response in each region when measured in terms of shortening per unit length in the resting state. Consequently, this method of expressing results was adopted, because it has the advantage of showing that any differences in maximal contractions to 5-HT3 receptor agonists would be due to factors related to 5-HT3 receptors rather than nonspecific differences in the ability of the regions to contract per se. However, between-species comparison showed that ACh contracted all regions of the rat intestine to a greater extent than the regions in the mouse (two way ANOVA, P<0.0001, n=12 rats and 12 mice). Individual values for the rat jejunum, ileum, proximal colon and distal colon were as follows: 34.65±2.07, 33.31±2.11, 34.06±1.72 and 34.99±2.26%, respectively. Corresponding values for the mouse were as follows: 24.56±1.48, 23.15±1.56, 24.14±1.27 and 24.46±1.09% (n=12 each mean).
Responses to 5-HT
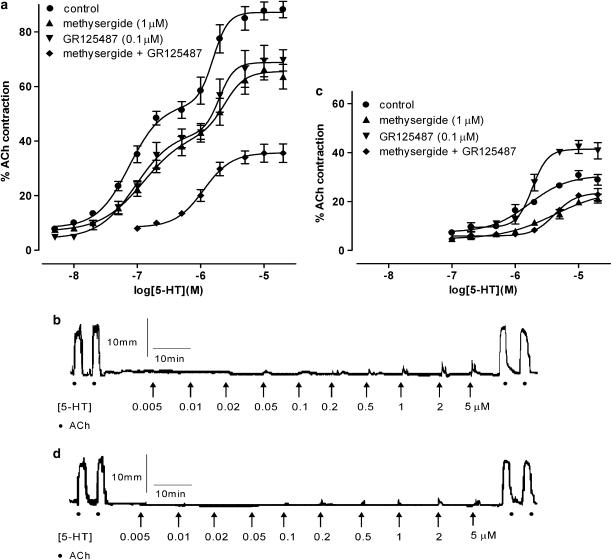
The rat jejunum and ileum were used to establish the conditions for studying the 5-HT3-induced responses by adding antagonists to the bathing solution to block nontarget 5-HT receptors. The nontarget 5-HT receptors are the 5-HT2A (Briejer et al., 1997) and 5-HT4 receptors of the rat ileum (Tuladhar et al., 1996) and colon (Kadowaki et al., 2002) and the ‘atypical' 5-HT7 receptor of the rat jejunum (McLean & Coupar, 1996; Liu et al., 2004). Therefore, we investigated the effects of methysergide, which is an antagonist at 5-HT1,2,5,6,7 receptors (Hoyer et al., 1994) and GR125487, which is a 5-HT4 receptor antagonist (Gale et al., 1994) using the rat jejunum and ileum. The concentration–response curve to 5-HT alone was biphasic in rat jejunum (Figure 2a) with an Emax value of 87.17±1.87% (n=8) compared to ACh (100%). The fraction of the first curve was 0.57 with a pEC50 of 7.13±0.09 (n=8), whereas the pEC50 of the second phase was 5.8±0.07 (n=8). Previously, we had shown that the first phase is due to the activation of an ‘atypical' 5-HT7 receptor population in the longitudinal muscle demonstrated by the presence of mRNA and functional properties (McLean & Coupar, 1996; Hemedah et al., 1999; Liu et al., 2004), whereas the second phase, shown at concentrations of 5-HT above 1 μM, is due to activation of neuronal 5-HT3 receptors (McLean & Coupar, 1996). Responses to 5-HT were reduced by methysergide (1 μM) and GR125487 (0.1 μM) alone, but the combination of the antagonists at the same concentrations limited the 5-HT response to a monophasic curve with an Emax value of 35.72±1.6 and pEC50 of 5.95±0.07 (n=10, Figure 2a). A typical trace of the 5-HT3-induced responses in the presence of the nontarget receptor antagonists, in the rat jejunum, is shown in Figure 2b.
Figure 2.
Concentration–response curves and typical traces for the rat small intestine. Concentration–response curves of the rat jeunum (a; n=8–10) and ileum (c; n=6–10) to 5-HT in the absence or presence of methysergide (1 μM), GR125487 (0.1 μM) or both. Each point represents the mean±s.e.m. Typical trace showing contractile responses as a result of discrete additions of acetylcholine 1 μM and 5-HT (0.005–20 μM) in the presence of methysergide (1 μM) and GR125487 (0.1 μM) in the (b) rat jejunum and (d) rat ileum.
In contrast to the rat jejunum, the responses of the rat ileum to 5-HT were monophasic with Emax and pEC50 values of 30.67±2.23 and 5.75±0.11 (n=6), respectively (Figure 2c). Methysergide (1 μM) caused a significant inhibition of this curve, but GR125487 (0.1 μM) significantly potentiated the responses to 5-HT giving an Emax value of 41.39±1.40 (two way ANOVA, F1,130=100.14, P<0.0001, n=6). However, the combination of both antagonists at the same concentrations as used in the jejunum resulted in significantly reduced responses to 5-HT with an Emax of 23.28±1.30 and pEC50 of 5.36±0.06 (two way ANOVA, F1,104=69.74, P<0.0001, n=10, Figure 2c). A typical trace of the 5-HT3-induced responses in the presence of the nontarget receptor antagonists, in the rat ileum, is shown in Figure 2d.
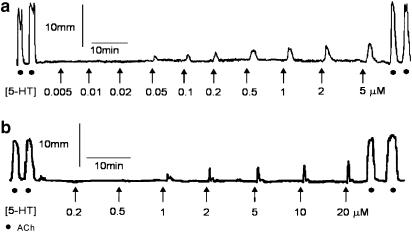
Methysergide and GR125487 were included in the bathing solution in all further functional experiments to inhibit 5-HT1,2,5,6,7 receptors (Hoyer et al., 1994) and 5-HT4 receptors (Gale et al., 1994), respectively. Representative traces of 5-HT3-induced responses in the rat and mouse distal colon are shown in Figure 3.
Figure 3.
Typical trace showing contractile responses as a result of discrete additions of acetylcholine 1 μM and 5-HT (0.005–20 μM) in the presence of methysergide (1 μM) and GR125487 (0.1 μM) in the (a) rat distal colon and (b) mouse distal colon.
Effect of ondansetron on 5-HT-induced contractions
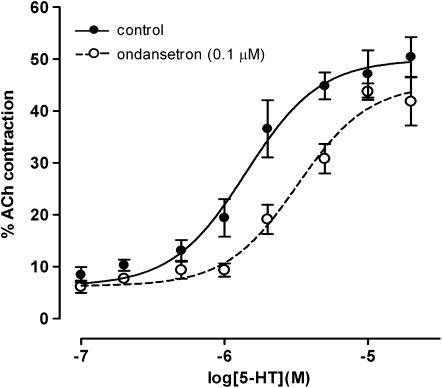
This series of experiments established that a 0.1 μM concentration of the 5-HT3 receptor antagonist ondansetron caused significant rightward shifts to the concentration–effect curves to 5-HT in the intestinal regions of both rat and mouse. The apparent pKB values were not significantly different between the species or regions (two way ANOVA, P>0.05, n=5 rats, n=5 mice; Table 1). Figure 4 shows a representative concentration–response curve of the effect of ondansetron on the rat jejunum using control and ondansetron-paired tissues performed in the presence of methysergide (1 μM) and GR125487 (0.1 μM)).
Table 1.
Apparent pKB values of ondansetron (0.1 μM) in the rat and mouse intestinal tract in the presence of methysergide (1 μM) and GR125487 (0.1 μM)
| Regions | Rata | Mousea |
|---|---|---|
| Jejunum |
7.45±0.14 |
7.27±0.18 |
| Ileum |
6.95±0.11 |
6.88±0.17 |
| Proximal colon |
7.14±0.28 |
7.27±0.12 |
| Distal colon | 6.92±0.16 | 7.41±0.13 |
Values are the means±s.e.m. (n=5).
Figure 4.
Concentration–response curves to 5-HT in the rat jejunum (n=5) in the absence and presence of ondansetron (0.1 μM). All experiments routinely contained methysergide (1 μM) and GR125487 (0.1 μM). Each point represents the mean±s.e.m.
Regional effects of 5-HT3 receptor agonist in the rat and mouse
Agonist profiles
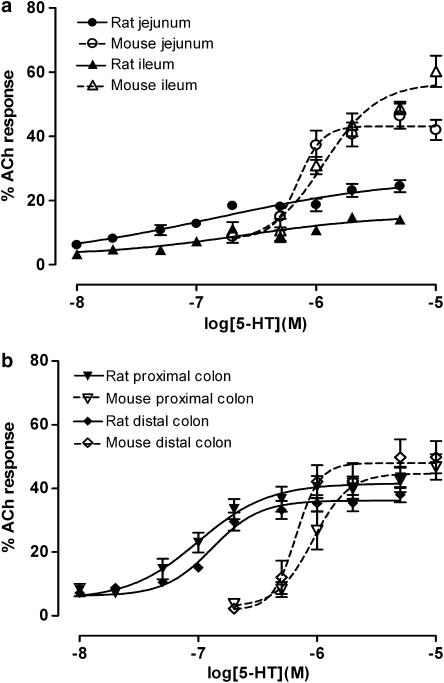
In addition to 5-HT, contractile responses were also observed with m-CPB, 2m5-HT and 1-PBG, in all regions of the rat and mouse intestine. It was shown that differences in efficacy were more evident between the regions than between the species. However, efficacies were higher in the mouse small intestine compared to those recorded in the rat (Tables 2 and 3). These differences are clearly seen in responses to 5-HT in the small (Figure 5a) and large intestine (Figure 5b) of the rat and mouse.
Table 2.
Rank order of potencies of 5-HT3 receptor agonists in the rat and mouse intestinal tract in the presence of methysergide (1 μM) and GR125487 (0.1 μM)
| Region | Rat | Mouse |
|---|---|---|
| Jejunum |
m-CPB⩾5-HT⩾2m5-HT⩾1-PBG |
m-CPB⩾5-HT⩾2m5-HT⩾1-PBG |
| Ileum |
m-CPB>5-HT⩾1-PBG⩾2m5-HT |
m-CPB>5-HT⩾2m5-HT⩾1-PBG |
| Proximal colon |
m-CPB⩾1-PBG⩾2m5-HT⩾5-HT |
m-CPB>5-HT>2m5-HT>1-PBG |
| Distal colon | m-CPB>5-HT>1-PBG⩾2m5-HT | m-CPB⩾5-HT>2m5-HT⩾1-PBG |
Table 3.
pEC50 and Emax values of 5-HT3 receptor agonists in the rat and mouse intestinal tract in the presence of methysergide (1 μM) and GR125487 (0.1 μM)
| Rat regions | mCPBa | 5-HT | 2m5-HT | 1-PBG |
|---|---|---|---|---|
|
Jejunum |
|
|
|
|
| pEC50 |
7.42±0.25 |
6.86±0.28 |
6.48±0.54 |
6.08±0.31 |
| Emax |
20.07±1.52 |
24.5±1.9 |
24.3±7.78 |
38.29±9.03 |
| |
|
|
|
|
|
Ileum |
|
|
|
|
| pEC50 |
7.07±0.17 |
6.47±0.39 |
6.07±0.68 |
6.28±0.42 |
| Emax |
16.1±1.05 |
14.0±1.1 |
12.35±4.94 |
18.1±4.37 |
| |
|
|
|
|
|
Proximal colon |
|
|
|
|
| pEC50 |
7.47±0.27 |
6.99±0.08 |
7.27±0.22 |
7.33±0.23 |
| Emax |
36.79±3.42 |
41.57±1.85 |
29.19±2.63 |
33.01±2.79 |
| |
|
|
|
|
|
Distal colon |
|
|
|
|
| pEC50 |
7.45±0.37 |
6.85±0.05 |
5.86±0.40 |
6.46±0.23 |
| Emax |
25.73±2.24 |
36.38±0.99 |
35.35±7.91 |
35.05±4.59 |
| |
|
|
|
|
| Mouse regions |
|
|
|
|
|
Jejunum |
|
|
|
|
| pEC50 |
6.29±0.32 |
6.16±0.07 |
5.98±0.15 |
5.67±0.19 |
| Emax |
44.0±2.12 |
43.86±1.96 |
54.74±3.93 |
58.42±9.81 |
| |
|
|
|
|
|
Ileum |
|
|
|
|
| pEC50 |
6.19±0.07 |
5.94±0.08 |
5.69±0.19 |
5.67±0.17 |
| Emax |
49.16±2.25 |
56.64±2.94 |
52.76±9.79 |
51.30±7.35 |
| |
|
|
|
|
|
Proximal colon |
|
|
|
|
| pEC50 |
6.34±0.10 |
6.05±0.07 |
5.57±0.17 |
5.34±0.06 |
| Emax |
21.35±2.06 |
44.94±2.25 |
22.64±3.07 |
38.94±3.43 |
| |
|
|
|
|
|
Distal colon |
|
|
|
|
| pEC50 |
6.49±0.14 |
6.22±0.16 |
5.93±0.09 |
5.87±0.10 |
| Emax | 43.46±3.61 | 39.55±3.01 | 29.31±2.57 | 46.1±3.85 |
Data are presented as mean±s.e.m. (n=10). pEC50 values are expressed relative to individual maxima and Emax relative to 1 μM ACh (100%).
Figure 5.
Concentration–response curves to 5-HT in the rat and mouse (a) jejunum and ileum (n=10) and (b) in the proximal and distal colon (n=10). All experiments routinely contained methysergide (1 μM) and GR125487 (0.1 μM). Each point represents the mean±s.e.m.
The rank order of potency (Table 2) based on individual pEC50 values (Table 3) of 5-HT and the 5-HT3 receptor agonists was determined at each of the four regions of the rat and mouse intestinal tract. The orders were similar between the regions of the mouse intestine, but slight differences were apparent in the orders of potency between the regions of the rat intestine. The potencies of the agonists were generally greater in the regions of the rat intestine than those of the mouse.
Mediation and modulation of 5-HT3-induced responses in regions of the rat and mouse
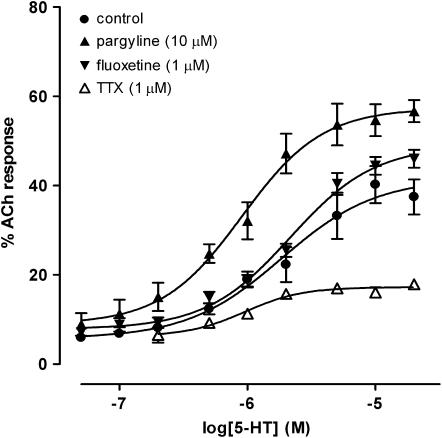
The neuronal blocker TTX (1 μM) caused a significant reduction (P<0.05, n=3, two way ANOVA) of all concentration–effect curves to 5-HT in the rat and mouse intestine. A representative example of the effect of TTX in the rat jejunum is shown in Figure 6.
Figure 6.
Concentration–response curves to 5-HT in the absence (control, n=6) and presence of TTX (1 μM; n=3), pargyline (10 μM; n=6) and fluoxetine (1 μM; n=6) in the rat jejunum. All experiments routinely contained methysergide (1 μM) and GR125487 (0.1 μM). Each point represents the mean±s.e.m.
The contractile responses of the rat jejunum to 5-HT were significantly increased (F1,110=67.93, P<0.05, n=6; two way ANOVA) by the monoamine-oxidase inhibitor (MAO-I) pargyline (10 μM, Figure 6). The Emax value of 5-HT was increased from 41.57±4.90% in the absence of pargyline to 57.34±3.04% in its presence. All other regions in the rat and mouse, including the mouse jejunum, were not significantly affected by pargyline (P>0.05, n=6; two way ANOVA).
The 5-HT3-induced responses of the rat jejunum were also significantly increased (F1,110=70.52, P<0.05, n=6; two way ANOVA) by the selective serotonin reuptake inhibitor (SSRI) fluoxetine (1 μM). The effect was shown as an increase of the Emax, value of 5-HT from 41.6±4.90% in the absence of fluoxetine to 48.93±2.54% in the presence of fluoxetine. However, fluoxetine did not alter the pEC50 of 5-HT (P>0.05). Fluoxetine did not significantly affect the Emax or pEC50 values for 5-HT in the other regions of the rat and mouse intestine including the mouse jejunum.
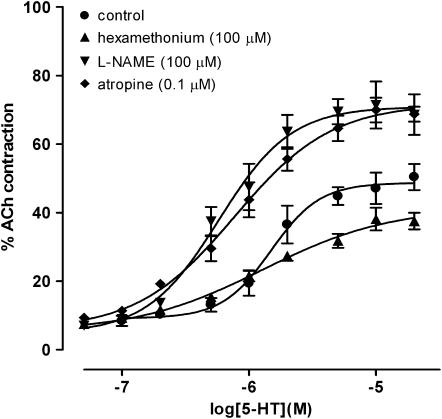
The muscarinic receptor antagonist atropine (0.1 μM) induced a significant increase in the Emax value of 5-HT from 48.69±2.41 to 64.72±3.17%, in the rat jejunum (F1,126=88.01, P<0.001, n=6; two way ANOVA; Figure 7). This effect was greater with 1 μM of atropine where the Emax value was increased to 71.81±2.70. Conversely, atropine significantly reduced the Emax values of 5-HT in the rat ileum from 15.59±1.89 to 6.14±0.32% and proximal colon from 41.35±1.99 to 32.88±2.71% (P<0.05, n=6 and 3, respectively; two way ANOVA). The concentration–effect curve to 5-HT was unaltered by atropine in the rat distal colon (data not shown). Atropine significantly reduced the Emax values of 5-HT in the mouse jejunum, from 43.15±1.71 to 36.04±3.76%, and ileum, from 56.65±2.86 to 26.28±3.79% (P<0.05, n=3; two way ANOVA), but did not alter the concentration–effect curves of 5-HT in the mouse proximal and distal colon (data not shown).
Figure 7.
Concentration–response curves to 5-HT in the absence (control, n=6) or presence of atropine (0.1 μM; n=6), hexamethonium (100 μM; n=6) and L-NAME (100 μM; n=5) in the rat jejunum. All experiments routinely contained methysergide (1 μM) and GR125487 (0.1 μM). Each point represents the mean±s.e.m.
Rat jejunum
Additional experiments were carried out on the rat jejunum to gain more information on the mechanism/mediator of the 5-HT3-induced contractile response to 5-HT. In these experiments, the nACh receptor antagonist hexamethonium (100 μM) suppressed the concentration–response curve to 5-HT with a resultant Emax of 41.56±3.19% from a value of 48.69±2.41% (P<0.01; n=6; two way ANOVA; Figure 7).
The NOS inhibitor L-NAME (100 μM) had the opposite effect to hexamethonium, but was similar to atropine, in that it caused a significant potentiation of 5-HT over its concentration range resulting in a significantly larger Emax value of 70.93±2.72% (F1,99=85.66, P<0.001; n=5; two way ANOVA; Figure 7).
The tachykinin receptor antagonists, SR140333, SR48968 and SR142801, were also used to investigate the potential involvement of tachykinin NK1, NK2 and NK3 receptors, respectively, in the 5-HT3-induced contractile responses to 5-HT (graphs not shown). The antagonists did not significantly alter either the Emax or pEC50 values for 5-HT compared to the control curve (two way ANOVA, P>0.05, n=5). This was despite the fact that the concentrations and incubation times used are considered to be effective (Croci et al., 1995; Johnson et al., 1998).
Discussion
This study has established the presence of functional 5-HT3 receptor populations in various regions of the rat and mouse intestinal tract. The receptor was identified by the presence of mRNA and characterised functionally on the basis of rank order of potency and susceptibility to the 5-HT3 receptor antagonist ondansetron. As differences were found in the potencies and efficacies of the agonists between species and between the regions in the same species, the 5-HT uptake, metabolism and neuronal systems were examined as potential sources of the variation.
First, conditions for studying 5-HT3-induced contractile activity in rat jejunum and ileum were established. ACh was selected as the internal standard, which was found to elicit larger contractions in the regions of the rat intestine than the mouse. This indicates that there is a species difference in the signal transduction system for ACh or a nonspecific mechanical property that limits the maximum contraction of muscle preparations in the two species investigated. This is an interesting physiological observation that warrants further investigation.
The biphasic curve to 5-HT in the jejunum was reduced by methysergide and also GR125487, but the curve was further reduced and converted to a monophasic curve when the antagonists were present together. The pEC50 (5.95) of this curve was similar to the second phase in the absence of the antagonists (5.8) and was in close agreement with our previous estimate of 6.1 (McLean & Coupar, 1996).
5-HT produced a monophasic curve in the ileum, which was moved to the right by the combined antagonists giving a pEC50 of 5.36. Although methysergide alone inhibited responses to 5-HT, GR125487 alone caused a significant potentiation. This result supports the finding of Tuladhar et al. (1996), who reported that 5-HT relaxed the rat terminal ileum in the presence of methysergide and atropine and that the response was blocked by 5-HT4 receptor antagonists such as GR113808.
The residual concentration–effect curves to 5-HT in the combined presence of methysergide and GR125487 were confirmed to be due to 5-HT3 receptors, because ondansetron caused rightward and parallel displacements in all regions from the rat and mouse. The apparent pKB values ranged from 6.88 (mouse ileum) to 7.45 (rat jejunum), but none of the values were significantly different from each other. Although consistent, the apparent pKB values are relatively low compared to literature values from the rat and mouse, but similar to guinea-pig values (Hoyer et al., 1994; Tuladhar et al., 2000).
Receptor characterisation invariably relies on establishing agonist activity profiles. We selected 2m5-HT and m-CPB for study, because they are well-established 5-HT3-selective receptor agonists (Alexander et al., 2004) and 1-PBG has agonist activity in some 5-HT3 receptor preparations (Hoyer et al., 1994). The absolute potency values can be useful in helping to define receptor subclasses. In the case of 5-HT3 receptors, the potencies of 2m5-HT and m-CPB are similar, with pEC50 values in the range of 5.3–5.5 and 5.4–5.7, respectively (Alexander et al., 2004). The potency values of these agonists were shown to fall close to these limits in the regions of the mouse intestine (Table 3). However, the pEC50 values obtained from all regions of the rat intestine were higher. This difference in potencies between the species is not surprising, as similar species differences have been evident for several years. For example, 1-PBG, m-CPB, 2m5-HT and 5-HT were all found to have higher binding affinities in the rat ileum than in the rabbit ileum (Kilpatrick et al., 1991).
Another useful criterion for receptor characterisation is rank order of potency, which has been established as: m-CPB>5-HT>2m5-HT⩾1-PBG (Hoyer et al., 1994) for the 5-HT3 receptor. The similar potencies of these agonists tends to limit the usefulness of potency order. Nevertheless, the rank order of potency of the agonists in the four regions of the mouse intestine were found to be the same and identical to the accepted order. Until our study, the only other characterisation of the 5-HT3 receptor in the mouse intestine was by Tuladhar et al. (2000). They showed the rank order of potencies, with pEC50 values in parenthesis, in the mouse ileum was: m-CPB (5.81)>5-HT (5.47)>1-PBG (5.05)⩾2m5-HT (5.00) in the presence of methysergide. This order is concurrent with our order for the mouse ileum (Table 2). The pEC50 values are also in reasonable agreement. In addition, our results from the rat intestine showed the expected order in the jejunum, but minor differences, which were statistically nonsignificant, occurred in the other three regions.
5-HT3-preferring agents have previously been shown to be partial agonists. This is confirmed in the present study by the wide range of efficacy (Emax) values, where there was variation in efficacy according to region and between the same region of the two species. These variations are not due to the differences in the abilities of the regions to contract, because the reference contracting agent, ACh, produced the same maximal response within each species. In general, 5-HT and 1-PBG showed the highest efficacies. However, responses to 5-HT itself were small in the rat jejunum and ileum compared to these regions in the mouse.
A factor responsible for the variation in 5-HT3-induced responses may be the presence of long and short splice variants and/or the combination of different 5-HT3 subunits. There is mounting evidence that these molecular properties do influence operational properties. For instance, the efficacy of 2m5-HT appeared to be greater at the 5-HT3A−L receptor splice variant, whereas the efficacy of m-CPB was greater at the 5-HT3A−S receptor variant (Niemeyer & Lummis, 1998). This was attributed to conformational differences of the receptor protein structure between the different splice variants. We found the shorter splice variant was expressed in all regions in both species, whereas the 5-HT3A−L variant was expressed at lower levels and only consistently evident in the rat.
Another possible reason for the low efficacy of 5-HT in the rat jejunum and ileum is that uptake and/or inactivation processes are particularly active in these regions. The series of experiments using the MAO-I, pargyline, and the SSRI, fluoxetine, provides some supportive evidence for this suggestion, at least in the rat jejunum. Pargyline increased the responses to 5-HT over its concentration range including the Emax value and fluoxetine increased the Emax of 5-HT. However, pargyline and fluoxetine did not produce either of these effects in the rat ileum or any other regions of the rat or mouse intestine revealing another species difference. Whether it relates to differences in the functional roles of 5-HT (e.g. mixing) in the upper small intestine remains to be established.
5-HT3 receptors are almost exclusively localised to central and peripheral nerves. The first function to be identified in the enteric nervous system was neurogenic contraction in the guinea-pig ileum (Buchheit et al., 1985). 5-HT3 receptors are mainly located on cholinergic nerves in the mouse ileum (Tuladhar et al., 2000), but the receptor is associated with noncholinergic nerves in the rat jejunum (McLean & Coupar, 1996). However, it has been reported that 5-HT-induced contraction of the rat proximal colon is resistant to both atropine and TTX (Gelal & Guven, 1998). Therefore, we investigated whether cholinergic or noncholinergic nerves are involved in 5-HT3-induced contractions in regions of the rat and mouse intestinal tract. The results showed that the responses to 5-HT in all regions of the rat and mouse intestine are TTX-sensitive.
The use of atropine revealed surprisingly different cholinergic influences on the 5-HT3-induced responses to 5-HT. The most commonly seen effect of atropine was inhibition (rat and mouse ileum, mouse jejunum, rat proximal colon), followed by no significant effect (rat and mouse distal colon, mouse proximal colon), followed by augmentation of 5-HT3-induced responses, including an increase in the Emax, in the rat jejunum. This latter result is particularly interesting, as it suggests the enteric cholinergic nerves are inhibitory to the excitatory 5-HT3 pathway in the rat jejunum. In further experiments on the rat jejunum, we showed that L-NAME mimicked the effect of atropine, suggesting that NO is the inhibitory mACh receptor mediator. This conclusion is concordant with previous findings, which have established that nNOS is distributed in NANC nerve fibres of the rat jejunum myenteric plexus (Chen et al., 2002) and that their stimulation leads to relaxation, which is partially sensitive to the NOS inhibitor L-NOARG (Niioka et al., 1997). Further, the M1 receptor agonist McN-A-343 has been shown to relax the rat jejunum and that its effect is abolished by L-NOARG but not hexamethonium (Olgart & Iversen, 1999). Our study extends this knowledge of neurotransmission in the rat jejunum by providing evidence that 5-HT activates inhibitory nitrergic nerves by stimulating 5-HT3 receptors and that this atropine-sensitive system is probably confined to the rat jejunum.
As the result with atropine discounted ACh as the excitatory transmitter in the rat jejunum we investigated whether the tachykinins might satisfy this role. This possibility was considered because the rat ileum expresses functional NK1, NK2 and NK3 receptors in the longitudinal muscle, where their activation by selective agonists causes tonic and phasic contractions (Willis et al., 1993). Moreover, the 5-HT3 receptor agonist, 2m5-HT, releases SP in the guinea-pig ileum (Ramirez et al., 1994). However, a role for tachykinins in mediating 5-HT3-induced contraction was ruled out on the basis that the NK1, NK2 and NK3 receptor antagonists, SR140333, SR48968 and SR142801, respectively, did not alter the 5-HT3 component of the concentration–response curve to 5-HT.
In this study, the mouse proximal colon was found to discriminate 5-HT3 receptor agonist profiles better than any other region in the rat or mouse (Table 2). This was because contractions, compared to the internal standard, were generally higher in the mouse than rat. In addition, functional data on the mouse digestive tract are particularly relevant as the full genome of the mouse is known and knockout mice are available. The rat jejunum was also found to have a potential use for investigating uptake and metabolic inhibitors compared to other regions in the rat and mouse. 5-HT was found to mediate its effect via 5-HT3 receptors by releasing an unknown transmitter. Both these points are interesting and useful for investigating the other transmitters released from nerves expressing 5-HT3 receptors and they could throw more light on the 5-HT3 receptor functions and regional effects.
Acknowledgments
We thank Drs N Tochon-Danguy and YY Tan for helpful discussions and technical assistance. The project was supported by the NHMRC Australia and N. Chetty by NHMRC Australia and a scholarship from the Faculty of Pharmacy, Monash University.
Abbreviations
- ACh
acetylcholine
- G3PDH
glyceraldehyde-3-phosphate dehydrogenase
- GR125487
1-{2[(methylsulfonyl) amino]ethyl}piperidin-4-yl) methyl 5-fluoro-2-methoxy-1H-indole-3-carboxylate
- 5-HT
5-hydroxytryptamine
- L-NAME
Nω-Nitro-L-arginine methyl ester hydrochloride
- MAO-I
monoamine oxidase inhibitor
- m-CPB
meta-chlorophenylbiguanide
- 2m5-HT
2-methyl-5-hydroxytryptamine
- 1-PBG
1-phenylbiguanide
- RT–PCR
reverse transcription–polymerase chain reaction
- SR140333
(S)-1-[2-[3-(3,4-dichlorphenyl)-1 (3-isopropoxy-phenylacetyl)piperidin-3yl]ethyl]-4-phenyl-1 azaniabicyclo[2.2.2] octane chloride
- SR142801
[(3)-(N)-(1-(3-(1-benzoyl-3-(3,4-dichlorophenyl)piperidin-3-yl)propyl)-4-phenylpiperidin-4-yl)-N-methylacetamide]
- SR48968
((S)-N-methyl-N[4-(4-acetyl-amino-4-phenylpiperi-dino)-2-(3,4-dichloro-phenyl)butyl]benzamide
- SSRI
selective serotonin reuptake inhibitor
- TTX
tetrodotoxin
References
- ALEXANDER S.P., MATHIE A., PETERS J.A. Guide to receptors and channels, 1st edition. Br. J. Pharmacol. 2004;141 (Suppl 1):S1–S126. doi: 10.1038/sj.bjp.0705672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALON E., KOCIAN R., NETT P.C., KOECHLI O.R., BAETTIG U., GRIMAUDO V. Tropisetron for the prevention of postoperative nausea and vomiting in women undergoing gynecologic surgery. Anesth. Analg. 1996;82:338–341. doi: 10.1097/00000539-199602000-00022. [DOI] [PubMed] [Google Scholar]
- BRIEJER M.R., MATHIS C., SCHUURKES J.A. 5-HT receptor types in the rat ileum longitudinal muscle: focus on 5-HT2 receptors mediating contraction. Neurogastroenterol. Motil. 1997;9:231–237. doi: 10.1046/j.1365-2982.1997.d01-62.x. [DOI] [PubMed] [Google Scholar]
- BUCHHEIT K.H., ENGEL G., MUTSCHLER E., RICHARDSON B. Study of the contractile effect of 5-hydroxytryptamine (5-HT) in the isolated longitudinal muscle strip from guinea-pig ileum. Evidence for two distinct release mechanisms. Naunyn. Schmiedeberg's Arch. Pharmacol. 1985;329:36–41. doi: 10.1007/BF00695189. [DOI] [PubMed] [Google Scholar]
- CAMILLERI M., NORTHCUTT A.R., KONG S., DUKES G.E., MCSORLEY D., MANGEL A.W. Efficacy and safety of alosetron in women with irritable bowel syndrome: a randomised, placebo-controlled trial. Lancet. 2000;355:1035–1040. doi: 10.1016/S0140-6736(00)02033-X. [DOI] [PubMed] [Google Scholar]
- CHAHL L.A. Substance P mediates atropine-sensitive response of guinea-pig ileum to serotonin. Eur. J. Pharmacol. 1983;87:485–489. doi: 10.1016/0014-2999(83)90090-0. [DOI] [PubMed] [Google Scholar]
- CHEN Y.M., QIAN Z.M., ZHANG J., CHANG Y.Z., DUAN X.L. Distribution of constitutive nitric oxide synthase in the jejunum of adult rat. World J. Gastroenterol. 2002;8:537–539. doi: 10.3748/wjg.v8.i3.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CROCI T., EMONDS-ALT X., LE FUR G., MANARA L. In vitro characterization of the non-peptide tachykinin NK1 and NK2-receptor antagonists, SR140333 and SR48968 in different rat and guinea-pig intestinal segments. Life Sci. 1995;56:267–275. doi: 10.1016/0024-3205(94)00921-x. [DOI] [PubMed] [Google Scholar]
- DUBIN A.E., HUVAR R., D'ANDREA M.R., PYATI J., ZHU J.Y., JOY K.C., WILSON S.J., GALINDO J.E., GLASS C.A., LUO L., JACKSON M.R., LOVENBERG T.W., ERLANDER M.G. The pharmacological and functional characteristics of the serotonin 5-HT3A receptor are specifically modified by a 5-HT3B receptor subunit. J. Biol. Chem. 1999;274:30799–30810. doi: 10.1074/jbc.274.43.30799. [DOI] [PubMed] [Google Scholar]
- FOZARD J.R. Neuronal 5-HT receptors in the periphery. Neuropharmacology. 1984;23:1473–1486. doi: 10.1016/0028-3908(84)90091-1. [DOI] [PubMed] [Google Scholar]
- FURCHGOTT R.F.The classification of adrenoreceptors (adrenergic receptors). An evaluation from the standpoint of receptor theory Handbook of Experimental Pharmacology: Catecholamines 1972Berlin, Heidelberg, New York: Springer-Verlag; 283–335.eds. Blaschko, H.R. & Muscholl, E., pp [Google Scholar]
- FURMAN B.L., WATSON N.G.5-hydroxytryptamine and peripheral secretory mechanisms The Peripheral Actions of 5-Hydroxytryptamine 1989New York: Oxford University Press; 274–300.ed. Fozard, J.R., pp [Google Scholar]
- GADDUM J.H., PICARELLI Z.P. Two kinds of tryptamine receptor. Br. J. Pharmacol. Chemother. 1957;12:323–328. doi: 10.1111/j.1476-5381.1957.tb00142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GALE J.D., GROSSMAN C.J., DARTON J., BUNCE K.T., WHITE-HEAD J.W.F., KNIGHT J., PARKHOUSE T.J., OXFORD A.W., HUMPHREY P.P.A. GR125487: A selective and high affnity 5-HT4 receptor antagonist. Br. J. Pharmacol. 1994;113:120P. doi: 10.1111/j.1476-5381.1994.tb14064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GELAL A., GUVEN H. Characterization of 5-HT receptors in rat proximal colon. Gen. Pharmacol. 1998;30:343–346. doi: 10.1016/s0306-3623(97)00096-7. [DOI] [PubMed] [Google Scholar]
- GLATZLE J., STERNINI C., ROBIN C., ZITTEL T.T., WONG H., REEVE J.R., Jr, RAYBOULD H.E. Expression of 5-HT3 receptors in the rat gastrointestinal tract. Gastroenterology. 2002;123:217–226. doi: 10.1053/gast.2002.34245. [DOI] [PubMed] [Google Scholar]
- HEMEDAH M., COUPAR I.M., MITCHELSON F.J. [3H]-Mesulergine labels 5-HT7 sites in rat brain and guinea-pig ileum but not rat jejunum. Br. J. Pharmacol. 1999;126:179–188. doi: 10.1038/sj.bjp.0702293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOPE A.G., DOWNIE D.L., SUTHERLAND L., LAMBERT J.J., PETERS J.A., BURCHELL B. Cloning and functional expression of an apparent splice variant of the murine 5-HT3 receptor A subunit. Eur. J. Pharmacol. 1993;245:187–192. doi: 10.1016/0922-4106(93)90128-v. [DOI] [PubMed] [Google Scholar]
- HOYER D., CLARKE D.E., FOZARD J.R., HARTIG P.R., MARTIN G.R., MYLECHARANE E.J., SAXENA P.R., HUMPHREY P.P.A. International union of pharmacology classification of receptors for 5-hydroxytryptamine (serotonin) Pharmacol. Rev. 1994;46:157–203. [PubMed] [Google Scholar]
- JOHNSON P.J., BORNSTEIN J.C., BURCHER E. Roles of neuronal NK1 and NK3 receptors in synaptic transmission during motility reflexes in the guinea-pig ileum. Br. J. Pharmacol. 1998;124:1375–1384. doi: 10.1038/sj.bjp.0701967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KADOWAKI M., WANG X.O., SHIMATANI H., YONEDA S., TAKAKI M. 5-HT4 receptor enhances the propulsive power of the peristaltic reflex in the rat distal colon. Auton. Neurosci. 2002;99:62–65. doi: 10.1016/s1566-0702(02)00063-2. [DOI] [PubMed] [Google Scholar]
- KILPATRICK G.J., BARNES N.M., CHENG H.H.K., COSTALL B., NAYLOR R.J., TYERS M.B. The parmacological characterization of 5-HT3 receptor binding sites in rabbit ileum and rat brain. Neurochem. Int. 1991;19:389–396. [Google Scholar]
- LIU H., IRVING H.R., TAN Y.Y., MENG L., CHETTY N., COUPAR I.M. Influences of gender and region on responses to 5-HT in the rat small intestine. Pharmacology. 2004;72:220–224. doi: 10.1159/000080376. [DOI] [PubMed] [Google Scholar]
- MARICQ A.V., PETERSON A.S., BRAKE A.J., MYERS R.M., JULIUS D. Primary structure and functional expression of the 5-HT3 receptor, a serotonin-gated ion channel. Science. 1991;254:432–437. doi: 10.1126/science.1718042. [DOI] [PubMed] [Google Scholar]
- MCLEAN P.G., COUPAR I.M. Characterisation of a postjunctional 5-ht7-like and a prejunctional 5-HT3 receptor mediating contraction of rat isolated jejunum. Eur. J. Pharmacol. 1996;312:215–225. doi: 10.1016/0014-2999(96)00456-6. [DOI] [PubMed] [Google Scholar]
- NAGAKURA Y., KONTOH A., TOKITA K., TOMOI M., SHIMOMURA K., KADOWAKI M. Combined blockade of 5-HT3 and 5-HT4 serotonin receptors inhibits colonic functions in conscious rats and mice. J. Pharmacol. Exp. Ther. 1997;281:284–290. [PubMed] [Google Scholar]
- NIEMEYER M.I., LUMMIS S.C. Different efficacy of specific agonists at 5-HT3 receptor splice variants: the role of the extra six amino acid segment. Br. J. Pharmacol. 1998;123:661–666. doi: 10.1038/sj.bjp.0701657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIIOKA S., TAKEUCHI T., KISHI M., ISHII T., NISHIO H., TAKEWAKI T., HATA F. Nonadrenergic, noncholinergic relaxation in longitudinal muscle of rat jejunum. Jpn. J. Pharmacol. 1997;73:155–161. doi: 10.1254/jjp.73.155. [DOI] [PubMed] [Google Scholar]
- OLGART C., IVERSEN H.H. Nitric oxide-dependent relaxation induced by M1 muscarinic receptor activation in the rat small intestine. Br. J. Pharmacol. 1999;127:309–313. doi: 10.1038/sj.bjp.0702529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETERS J.A., MALONE H.M., LAMBERT J.J.Characterisation of 5 HT3 receptor-mediated electrical responses in nodose ganglion neurones and clonal neuroblastoma cells maintained in culture Serotonin: Molecular Biology, Receptors and Functional Effects 1991Basel: Birkhauser Verlag; 84–94.eds. Fozard, J.R. & Saxena, P.R., pp [Google Scholar]
- RAMIREZ M.J., CENARRUZABEITIA E., DEL RIO J., LASHERAS B. Involvement of neurokinins in the non-cholinergic response to activation of 5-HT3 and 5-HT4 receptors in guinea-pig ileum. Br. J. Pharmacol. 1994;111:419–424. doi: 10.1111/j.1476-5381.1994.tb14751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEVCIK J., RUZICKA V., SLANSKY J., MASEK K. Which type of 5-hydroxytryptamine receptor mediates relaxation of the longitudinal muscle of guinea pig proximal colon in vitro. Methods Find. Exp. Clin. Pharmacol. 1996;18:421–430. [PubMed] [Google Scholar]
- TANG J., D'ANGELO R., WHITE P.F., SCUDERI P.E. The efficacy of RS-25259, a long-acting selective 5-HT3 receptor antagonist, for preventing postoperative nausea and vomiting after hysterectomy procedures. Anesth. Analg. 1998;87:462–467. doi: 10.1097/00000539-199808000-00043. [DOI] [PubMed] [Google Scholar]
- TULADHAR B.R., COSTALL B., NAYLOR R.J. Pharmacological characterization of the 5-hydroxytryptamine receptor mediating relaxation in the rat isolated ileum. Br. J. Pharmacol. 1996;119:303–310. doi: 10.1111/j.1476-5381.1996.tb15986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TULADHAR B.R., KAISAR M., NAYLOR R.J. Evidence for a 5-HT3 receptor involvement in the facilitation of peristalsis on mucosal application of 5-HT in the guinea pig isolated ileum. Br. J. Pharmacol. 1997;122:1174–1178. doi: 10.1038/sj.bjp.0701503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TULADHAR B.R., WOMACK M.D., NAYLOR R.J. Pharmacological characterization of the 5-HT receptor-mediated contraction in the mouse isolated ileum. Br. J. Pharmacol. 2000;131:1716–1722. doi: 10.1038/sj.bjp.0703747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATTERS J., RILEY M., PEDLEY I., WHITEHEAD A., OVEREND M., GOSS I., ALLGAR V. The development of a protocol for the use of 5-HT3 antagonists in chemotherapy-induced nausea and vomiting. Clin. Oncol. (R. Coll. Radiol.) 2001;13:422–426. doi: 10.1053/clon.2001.9305. [DOI] [PubMed] [Google Scholar]
- WILLIS S., ALLESCHER H.D., SCHUSDZIARRA V., REGOLI D., DRAPEAU G., CLASSEN M. Differential effects of selective neurokinin agonists on phasic and tonic activity in rat ileal longitudinal muscle. Neuropeptides. 1993;25:315–323. doi: 10.1016/0143-4179(93)90049-g. [DOI] [PubMed] [Google Scholar]
- YAMANO M., ITO H., MIYATA K. Species differences in the 5-hydroxytryptamine-induced contraction in the isolated distal ileum. Jpn. J. Pharmacol. 1997;74:267–274. doi: 10.1254/jjp.74.267. [DOI] [PubMed] [Google Scholar]