Abstract
Splanchnic artery occlusion (SAO) followed by reperfusion causes endothelial injury and inflammation which contribute to the pathophysiology of shock. We investigated the effects of relaxin (RLX), known to afford protection against the deleterious effects of cardiac ischemia/reperfusion, given to rats subjected to splanchnic artery occlusion and reperfusion (SAO/R)-induced splanchnic injury.
RLX (30 ng kg−1, 15 min. before reperfusion) significantly reduced the drop of blood pressure and high mortality rate caused by SAO/R. RLX also reduced histopathological changes, leukocyte infiltration (myeloperoxidase) and expression of endothelial cell adhesion molecules in the ileum. RLX counteracted free radical-mediated tissue injury, as judged by significant decrease in the tissue levels of peroxidation and nitration products (malondialdehyde, nitrotyrosine), DNA damage markers (8-hydroxy-2′-deoxyguanosine, poly-ADP-ribosylated DNA) and consumption of tissue antioxidant enzymes (superoxide dismutase). As a result, RLX led to a reduction of ileal cell apoptosis (caspase 3, terminal deoxynucleotidyltransferase-mediated UTP end labeling). The effects of RLX appear specific, as inactivated RLX substituted for the bioactive hormone had no effects.
In conclusion, these results show that RLX exerts a clear-cut protective effect in SAO/R-induced splanchnic injury, likely due to endothelial protection, decreased leukocyte recruitment and hindrance of free radical-mediated tissue injury leading to cell death, lethal complications and high mortality rate. Thus, RLX could be used therapeutically in intestinal ischemia.
Keywords: Relaxin, splanchnic artery occlusion, oxygen-free radicals, apoptosis, endothelial cell adhesion molecules
Introduction
In humans, intestinal ischemia usually results from impaired perfusion of blood to the bowel due to a variety of causes, including cardiac insufficiency, sepsis, vaso- and cardiodepressant drugs and complications of long-lasting surgery (Stoney & Cunninghan, 1993; Mallick et al., 2004). Complications of intestinal ischemia may be severe, ranging from persistent bleeding and symptomatic intestinal strictures to bowel perforation and peritonitis. Thus, surgical resection of the affected segment is usually necessary to minimize adverse outcomes (Sreenarasimhaiah, 2005). The pathophysiology of intestinal ischemia and its sequelae has been widely investigated using animal models of splanchnic artery occlusion (SAO) followed by reperfusion, which result in severe circulatory shock, characterized by hypotension, hemoconcentration, intestinal injury and a high mortality rate (Lefer & Lefer, 1993; Zimmermann et al., 1993). An important component of SAO-induced shock is endothelial dysfunction (Altura et al., 1985; Carey et al., 1992; Zingarelli et al., 1992; Lefer & Lefer, 1993), yet induced by hypoxia during the ischemic phase (Harlan, 1985) and further exacerbated at reperfusion due to oxygen-derived free radicals generated from both endothelial cells (Ratych et al., 1987; Lefer & Lefer, 1993) and activated adherent leukocytes (Granger et al., 1981; McCord, 1981; Mullane et al., 1988; Bittermann et al., 1988). Endothelial dysfunction predisposes to vasospasm and increases adherence of platelets and neutrophils, which worsen the shock state. Normally, the endothelium inhibits adherence of leukocytes by producing antiadhesive factors, such as nitric oxide (NO) (Kubes et al., 1991; Laroux et al., 2000) and by expressing low levels of endothelial cell adhesion molecules (ECAMs), a generic term used to identify different types of surface glycoproteins based on their common function (Butcher, 1992; 1993; Sluiter et al., 1993). Inflammatory mediators generated during ischemia/reperfusion can stimulate the expression of ECAMs (Granger, 1977). These molecules include early-phase proteins, such as P-selectin that is held responsible for leukocyte rolling, as well as late-phase proteins, such as E-selectin, vascular cell adhesion molecule (VCAM)-1 and intercellular adhesion molecule 1 (ICAM-1), which are involved in firm adhesion of leukocytes to the vascular endothelium that allows leukocyte extravasation into the tissue (Lawrence & Springer, 1991; Butcher, 1992; Laroux et al., 2000). NO itself can reduce the expression of ECAMs, likely by interfering with the activation of the transcription factor NF-κB or by binding to the ECAM gene promoters (Peng et al., 1998; Spiecker & Liao, 1999). On the other hand, administration of NO synthesis inhibitors and targeted disruption of constitutive NO synthase (NOS) I and III genes cause an increased amount of leukocytes adherent to the vascular endothelium (Kubes et al., 1991; Lefer et al., 1999; Laroux et al., 2000).
At reperfusion, neutrophil activation at sites of injury results in a large production of superoxide anions (•O2−), which give a major contribution to tissue inflammation and damage by causing endothelial cell damage and increased microvascular permeability (Droy-Lefaix et al., 1991; Haglind et al., 1994; Xia et al., 1995), formation of chemotactic factors such as leukotriene B4 (Fantone & Ward 1982; Deitch et al., 1990), further neutrophil recruitment (Boughton-Smith et al., 1993; Salvemini et al., 1996; 1999a, 1999b), membrane lipid peroxidation and DNA damage (Dix et al., 1996). In addition, •O2− reacts with and inactivate NO, thereby reducing its anti-inflammatory and cytoprotective properties, including maintenance of blood vessel tone, decreased platelet and leukocyte reactivity, and increased generation of anti-inflammatory and cytoprotective prostacyclin via activation of constitutive cyclo-oxygenase (Salvemini et al., 1993). The reaction product of •O2− and NO is peroxynitrite (ONOO−), a potent cytotoxic and proinflammatory molecule which further enhances reperfusion-induced tissue injury (Crow & Beckman, 1995; Misko et al., 1998; Salvemini et al., 1999a, 1999b).
From the above grounds, it seems logical to assume that pharmacological strategies aimed at counteracting endothelial dysfunction could afford protection against ischemia/reperfusion-induced tissue damage. In this context, previous studies showed that the peptide hormone relaxin (RLX), best known for its effects on reproduction (Bani, 1997; Sherwood, 2004), is a potent cardiovascular effector (Bani, 1997; Dschietzig & Stangl, 2002; Samuel et al., 2003; Nistri & Bani, 2005). RLX promotes vasodilatation in many target organs by inducing upregulation of endogenous NO production by cells of the vascular wall (Bani et al., 1998a; Failli et al., 2002). RLX is also capable to reduce the expression of ECAMs and to blunt leukocyte activation, thereby exerting anti-inflammatory effects (Nistri et al., 2003; Masini et al., 2004). Of note, our previous studies showed that RLX, given either preventively before ischemia or also at reperfusion, affords a clear-cut protection against ischemia/reperfusion-induced damage in the heart (Masini et al., 1997; Bani et al., 1998b; Perna et al., 2005).
Aim of the current study is to investigate whether RLX could be useful as a therapeutic agent to blunt the adverse effects of ischemia/reperfusion in a well-assessed animal model of splanchnic artery occlusion and reperfusion (SAO/R) widely used for experimentation of potential new drugs for ischemia/reperfusion-induced splanchnic injury (Lefer & Lefer 1993; Zimmermann et al., 1993; Cuzzocrea et al., 2001).
Methods
Animals and reagents
Wistar albino rats (250–300 g; Charles River, Milan, Italy) were housed in a controlled environment and provided with standard rodent chow and water. Animal care was in compliance with Italian regulations on the use of animals for experimental and other scientific purposes (D.M. 116192), as well as with the current EC regulations (O.J. of E.C. L358/1 12/18/1986). Unless otherwise specified, all reagents and labware was from Sigma-Aldrich (Milano, Italy).
Surgical induction of spanchnic artery occlusion
The rats were anaesthetized with sodium pentobarbital (45 mg kg−1 i.p.). Following anaesthesia, catheters were placed in the carotid artery and jugular vein. Blood pressure was monitored continuously by a Maclab A/D converter (Ugo Basile, Varese, Italy), displayed and stored on a Macintosh personal computer. After midline laparotomy, the celiac and superior mesenteric arteries were isolated near their aortic origins. During this procedure, the intestinal tract was maintained at 37°C by placing it between gauze pads soaked with warmed 0.9% NaCl solution. Rats were allowed to equilibrate for 30 min before further surgical treatments. SAO was induced by clamping both the superior mesenteric artery and the celiac trunk, resulting in a total occlusion of these arteries for 45 min. After this period of occlusion, the clamps were removed. Sham-operated animals, subjected to the same surgical procedure except for arterial clamping, were used as controls (n=10).
Treatments
Rats undergoing SAO were divided in three experimental groups, 10 animals each. At 15 min before reperfusion, the rats were given an intravenous (i.v.) bolus of phosphate-buffered saline (PBS) containing the following substances: (i) highly purified porcine RLX, 30 ng kg−1 (SAO/R+RLX): the hormone (2500–3000 IU mg−1), prepared according to Sherwood & O'Byrne (1974), was generously donated by Dr O.D. Sherwood, University of Illinois, Urbana, IL, U.S.A.; (ii) inactivated RLX (iRLX), 30 ng kg−1 (SAO/R+iRLX): iRLX was obtained by blockade of functional arginine residues by reaction with cyclohexanedione (Büllesbach & Schwabe, 1988); (iii) no drugs added (SAO/R+vehicle). The administration protocol was designed to reproduce a possible therapeutical use of RLX, that is, the drug being administered between the onset of ischemic symptoms and the surgical procedures inducing reperfusion. The half-life of RLX has been assessed to be about 2 h (Chen et al., 1993), thus allowing us to reasonably assume that bioactive levels of the hormone were present in the blood of rats during the reperfusion period. Rats were killed at 60-min reperfusion, this time point being set based on the mean survival time. At laparotomy, an 8–10 cm-long ileal segment, 30 cm distal to the stomach was isolated, washed in saline and used for histological examination and for biochemical studies.
In another set of experiments, the different groups of rats (each group, n=20) were monitored for 6 h after SAO/R to evaluate survival time.
Light microscopy
For conventional histopathological examination, ileal tissue specimens were fixed in Dietric solution (14.25%. ethanol, 1.85% formaldehyde, 1% acetic acid) for 1 week at room temperature, dehydrated by graded ethanol and embedded in Paraplast (Sherwood Medical, Mahwah, NJ, U.S.A.). From each specimen, 7-μm thick sections were cut and stained with trichromic Van Gieson.
Measurement of myeloperoxidase activity
Myeloperoxidase (MPO) activity, an index of accumulation of inflammatory leukocytes, especially PMN, was determined as previously described (Mullane et al., 1985). Ileal fragments were homogenized in a solution containing 0.5% hexa-decyl-trimethyl-ammonium bromide dissolved in 10 mM potassium phosphate buffer (pH 7) and centrifuged for 30 min at 20,000 × g at 4°C. An aliquot of the supernatant was then allowed to react with a solution of tetramethylbenzidine (1.6 mM) and 0.1 mM H2O2. The rate of exchange in absorbance was measured by a spectrophotometer at 650 nm. MPO activity was defined as the enzyme amount degrading 1 μmol of peroxide min−1 at 37°C and was expressed in U g−1 of wet tissue.
Immunohistochemical analysis of P-selectin, ICAM-1, VCAM and E-selectin
P-selectin, ICAM-1, VCAM and E-selectin localization was detected in ileal sections by immunohistochemistry. Sections of Dietric-fixed, paraffin-embedded ileal fragments were dewaxed, rehydrated, permeabilized with 0.1% Triton X-100 in PBS for 20 min and incubated in 2% normal serum for 60 min. Sections were then incubated for 12 h at 4°C with antibodies directed to P-selectin (rabbit polyclonal, 1 : 500 in PBS; Pharmingen, San Diego, CA, U.S.A.), ICAM-1 (mouse monoclonal, 1 : 1000 in PBS; Santa Cruz/DBA, Milan, Italy), VCAM (mouse monoclonal, 1 : 500, Santa Cruz/DBA) and E-selectin (mouse monoclonal 1 : 1000, Santa Cruz/DBA). Labeling of antigen-antibody complexes was detected with appropriate peroxidase-conjugated secondary antisera, using diaminobenzidine as chromogen. Controls were sections incubated with nonimmune rabbit serum in the place of the primary antisera, as well as sections incubated without either the primary or the secondary antibody.
Measurement of malondialdehyde activity
Levels of malondialdehyde (MDA) in the ileal tissue was determined as an index of lipid peroxidation, as described by Ohkawa et al. (1979). Ileal fragments were homogenized in 1.15% KCl solution. An aliquot (100 μl) of the homogenate was added to a reaction mixture containing 200 μl of 8.1% SDS, 1500 μl of 20% acetic acid (pH 3.5), 1500 μl of 0.8% thiobarbituric acid and 700 μl of distilled water. Samples were then boiled for 1 h at 95°C and centrifuged at 3000 × g for 10 min. The absorbance of the supernatant was measured by spectrophotometry at 532 nm. Protein concentration was determined with the Bradford method. The values are expressed as nmol of thiobarbituric acid-reactive substances (MDA equivalents) mg−1 of protein, using a standard curve of 1,1,3,3-tetramethoxypropane.
Determination of 8-hydroxy-2′-deoxyguanosine
This was carried out on DNA isolated from ileal tissues, according to Lodovici et al. (2000). Briefly, samples were homogenized at 4°C, sonicated on ice (three times, 10 s) and diluted with 1 ml of 10 mM Tris-HCl buffer pH 8.0 containing 10 mM EDTA, 10 mM NaCl, 0.5% SDS. The samples were first incubated at 37°C for 60 min with RNAse (20 μg ml−1), then they were incubated at 37°C under argon in the presence of proteinase K (100 μg ml−1). At the end of incubation, the mixture was added with chloroform/isoamyl alcohol (10 : 2 v v−1) with 0.2 volumes of 10 M ammonium acetate and DNA was precipitated from the aqueous phase. DNA was solubilized in 100 μl of 20 mM acetate buffer pH 5.3 and denaturated at 90°C for 3 min. The total extracted DNA was supplemented with 10 IU of P1 nuclease in 10 μl and incubated for 60 min at 37°C with 5 IU of alkaline phosphatase in 0.4 M phosphate buffer, pH 8.8. All these procedures were performed under argon and the samples were protected from light. The hydrolyzed mixture was filtered by Amicon Micropure-EZ enzyme remover (Millipore, Milan, Italy) and 50 μl of samples were used for 8-hydroxy-2′-deaxyguanosine (8-OHdG) determination, using a Bioxytech®-EIA-kit following the manufacturer's protocol. Absorbance was read at 450 nm wavelength and the concentrations of 8-OHdG were determined by comparison with a standard curve. Values are expressed as ng μg−1 of proteins, determined with the Bradford method.
Measurement of Mn-superoxide dismutase activities
Ileal samples were homogenized with 10 mM PBS, pH 7.4, sonicated on ice (three times, 20 s) and centrifuged at 100 × g for 10 min. Superoxide dismutase (SOD) activity was measured in the supernatants as described by Nishida et al. (2002), with minor modifications. The assay is based on the inhibition of nitro blue tetrazolium conversion by SOD into a blue tetrazolium salt, mediated by superoxide radicals, which are generated by xanthine oxidase. The reaction was performed in sodium carbonate buffer 50 mM, pH 10.1 containing 0.1 mM EDTA, 25 μM nitro blue tetrazolium (Sigma, Milan, Italy), 0.1 mM xanthine, 2 nM xanthine oxidase (Boehringer, Germany). The rate of reduction of nitro blue tetrazolium was followed at 560 nm with a Perkin-Elmer spectrophotometer. The amount required to inhibit the rate of reduction of nitro blue tetrazolium by 50% was defined as one unit of enzyme activity. For measurement of MnSOD, enzyme activity was inhibited performing the assay in the presence of 2 mM NaCN after preincubation for 30 min.
Immunohistochemistry for nitrotyrosine and poly-(ADP-ribose)
Tyrosine nitration, an index of nitrosylation of proteins by peroxynitrite and/or ROS, and nuclear poly-ADP-ribosylated DNA, an activity marker for poly-ADP-ribosyl-polymerase (PARP), an enzyme involved in DNA repair, were determined by immunohistochemistry as previously described (Cuzzocrea et al., 2001). Sections of Dietric-fixed, paraffin-embedded ileal fragments were dewaxed and rehydrated, endogenous peroxidase was quenched with 0.3% (v v−1) hydrogen peroxide in 60% (v v−1) methanol for 30 min. The sections were permeablized with 0.1% (w v−1) Triton X-100 in PBS for 20 min. Nonspecific adsorption was minimized by incubating the section in 2% (v v−1) normal goat serum in PBS for 20 min. Endogenous biotin or avidin binding sites were blocked by sequential incubation for 15 min with biotin and avidin (DBA, Milan, Italy), respectively. Sections were incubated overnight with anti nitrotyrosine (NT) rabbit polyclonal antibody (1 : 500 in PBS), or with anti-poly-ADP-ribose (PAR) goat polyclonal antibody (Jackson, Westgrave, PA, U.S.A.; 1 : 500 in PBS). Immune reaction was revealed by indirect immunoperoxidase method (Vectastain Elite kit, Vector, Burlingame, CA, U.S.A.), using 3,3′-diaminobenzidine as chromogen. As negative controls, sections incubated with only the primary or the secondary antisera were used. Negative controls for NT immunostaining were sections incubated with the primary antibody in the presence of excess NT (10 mM). Negative controls for PAR were sections incubated with only the primary or the secondary antibody. Quantitation of immunostaining was carried out by computer-aided densitometry on photomicrographs (n=5 per experimental group), using Optilab Graftek software on a Macintosh personal computer.
Determination of caspase-3 activity
The activity of caspase-3 was determined by use of a fluorescent substrate, according to Stennicke & Salvesen (1997). The Ac-Asp-Glu-Val-Asp-AMC (Ac-DEVD-AMC; Bachem, Germany) was used as a fluorescent substrate for caspase-3. Briefly, ileal fragments were homogenized with 10 mM N-2-hydroxyethylpiperazine-N-2-ethanesulfonic acid (HEPES), pH 7.4, containing 0.5% 3-[(3-cholamidopropyl)dimethylammonio]-1-propane-sulfonate (CHAPS), 42 mM KCl, 5 mM MgCl2, 1 mM dithiothreitol (DTT), 1 mM phenylmethylsulfonylfluoride (PMSF), 2 μg ml−1 leupeptin, and 1 μg ml−1 pepstatin A. The homogenates were then centrifuged at 10,000 × g for 10 min. Caspase-3 activity was determined in the supernatant by fluorometric assay using the substrate, Ac-DEVD-AMC. Values are expressed as mU mg−1 of proteins, determined with the Bradford method.
Terminal deoxynucleotidyltransferase-mediated UTP end labeling assay
Terminal deoxynucleotidyltransferase-mediated UTP end labelling (TUNEL) assay was conducted by using a TUNEL detection kit according to the manufacturer's instruction (Apotag, HRP kit; DBA, Milano, Italy). Briefly, sections of Dietric-fixed, paraffin-embedded ileal fragments were dewaxed, rehydrated and incubated with 15 mg ml−1 proteinase K for 15 min at room temperature and then washed with PBS. Endogenous peroxidase was inactivated by 3% H2O2 for 5 min at room temperature and then washed with PBS. Sections were immersed in terminal deoxynucleotidyltransferase (TdT) buffer containing deoxynucleotidyl transferase and biotinylated dUTP in TdT buffer, incubated in a humid atmosphere at 37°C for 90 min, and then washed with PBS. The sections were incubated at room temperature for 30 min with peroxidase-conjugated avidin and the signals were visualized with diaminobenzidine.
Statistical analysis
The reported data are expressed as the mean±s.e.m. of the reported number of animals per experimental group. Statistical comparison of differences between groups was carried out using either two-way ANOVA for two variable analysis or one-way ANOVA followed by Student–Newman–Keuls multiple comparison test for single variable analysis. A P-value ⩽0.05 was considered significant. Calculations were performed using the GraphPad Prism 2.0 statistical program (GraphPad Software, San Diego, CA, U.S.A.).
Results
Reperfusion of the ischemic splanchnic circulation led to a slight increase in mean arterial pressure which then decreased until death. The mean survival time was 67±4,7 min, whereas the sham-operated controls survived for the entire observation period (4 h).
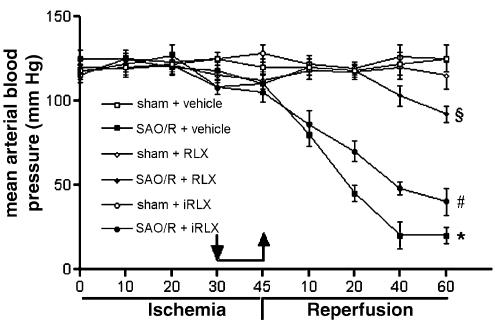
Bioactive RLX (30 ng kg−1), given i.v. 15 min before reperfusion, prevented the fall in blood pressure (Figure 1) seen during late reperfusion and increased the survival rate (88% survival at 4 h in RLX-treated rats vs 0% survival in vehicle-treated rats). iRLX was ineffective in preventing the fall in blood pressure and survival rate which was not different from vehicle-treated rats (Figure 1).
Figure 1.
Trend of mean arterial pressure in SAO/R rats. RLX, but not iRLX, prevents the fall in mean arterial pressure in SAO/R rats. Arrows point at start and withdrawal of RLX, iRLX or vehicle. Significance of differences (two-way ANOVA): *P<0.001 vs sham-operated animals; §P<0.001 vs SAO/R+vehicle; #P<0.001 vs SAO/R+RLX.
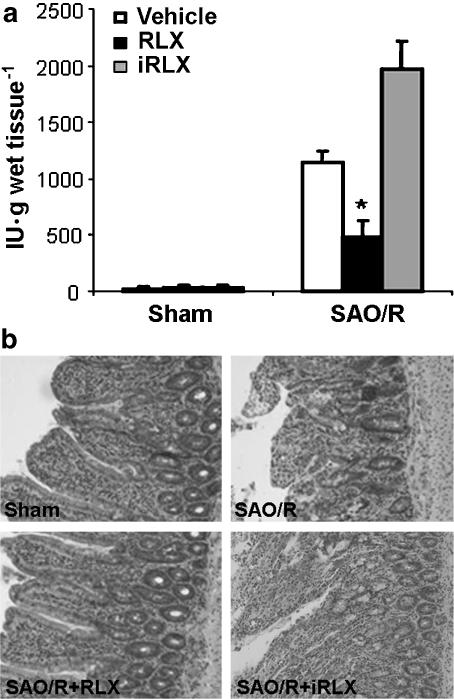
SAO/R also resulted in a marked infiltration of inflammatory leukocytes into the ileum, as shown by the increase in tissue MPO activity. Histopathological examination of ileal wall showed severe mucosal abnormalities, consisting of truncated, edematous villi, hemorrhage foci and loss of surface and gland epithelium. These events were triggered by reperfusion, as no changes were observed in preliminary experiments in which tissues were taken soon after ischemia (data not shown). Both MPO activity and histopathological abnormalities were prevented by pretreatment with RLX (Figure 2a and b). Conversely, iRLX was ineffective.
Figure 2.
(a) MPO activity is significantly increased in the ileum of SAO/R rats. RLX, but not iRLX, significantly reduces MPO activity. Significance of differences (one-way ANOVA): *P<0.01 vs SAO/R+vehicle and SAO/R+iRLX. (b) Representative histopathological features of the ileum in sham-operated rats and SAO/R rats treated with vehicle or RLX. Severe mucosal abnormalities, consisting of truncated, edematous villi, hemorrhage foci and loss of surface and gland epithelium, can be seen in SAO/R+vehicle rats. These abnormalities are absent in SAO/R+RLX rats.
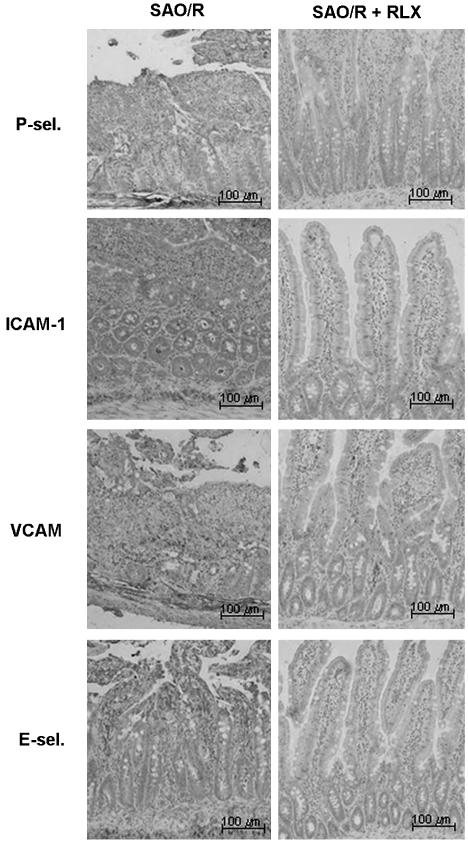
In the vehicle-treated rats subjected to SAO/R, leukocyte infiltration was accompanied by a rise in the local immunocytochemical expression of early-phase and late-phase ECAMs. In fact, examination of ileal sections from sham-operated rats showed faint immunostaining for ICAM-1 and no labeling for P-selectin, E-selectin and VCAM, indicating that, except ICAM-1, the other adhesion molecules were not constitutively expressed. In the vehicle-treated rats undergoing SAO/R, there was a strong immunostaining for all the adhesion molecules studied, especially in the blood vessels. Conversely, in the RLX-treated rats undergoing SAO/R, no appreciable immunostaining for P-selectin, E-selectin and VCAM was found, nor was any increase in the constitutive ICAM-1 immunocytochemical expression (Figure 3). No immunostaining was detected in the negative control sections (data not shown).
Figure 3.
Immunostaining for ECAMs of ileal tissue sections from SAO/R rats treated with vehicle or RLX. RLX reduces the overexpression of the adhesion molecules investigated.
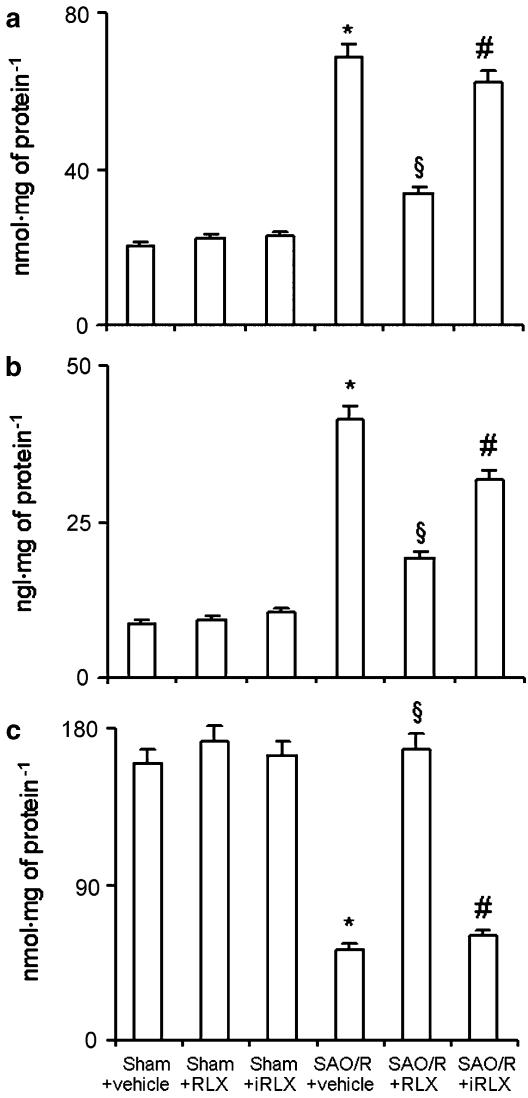
Local recruitment and activation of inflammatory cells in the vehicle-treated rats undergoing SAO/R led to a clear-cut increase in ileal tissue markers of oxidative stress, such as lipid peroxidation products (MDA), DNA damage (8-OHdG) and consumption of endogenous antioxidant enzymes, like MnSOD. RLX, but not iRLX, inhibited the increased ileum level of MDA and 8-OHdG and prevented the decrease of MnSOD content (Figure 4).
Figure 4.
MDA (a) and 8-OHdG (b) are increased, while MnSOD activity (c) is decreased in ileal tissue of SAO/R+vehicle rats. RLX, but not iRLX, significantly prevents the occurrence of the above changes. *P<0.01 vs sham-operated, §P<0.01 vs SAO/R+vehicle, #P<0.01 vs SAO/R+RLX.
Detection of nuclear poly-ADP-ribosylated DNA sites, a marker for PAR-polymerase (PARP) activity, an enzyme involved in DNA repair, in sections from SAO/R, vehicle-treated rat ileum showed an intense immunostaining mostly located at the epithelium and at the inflammatory infiltrate in the underlying mucosa. In the RLX-pretreated animals there was negligible PAR immunostaining (Figure 5). No immunostaining was detected in the negative control sections (data not shown).
Figure 5.
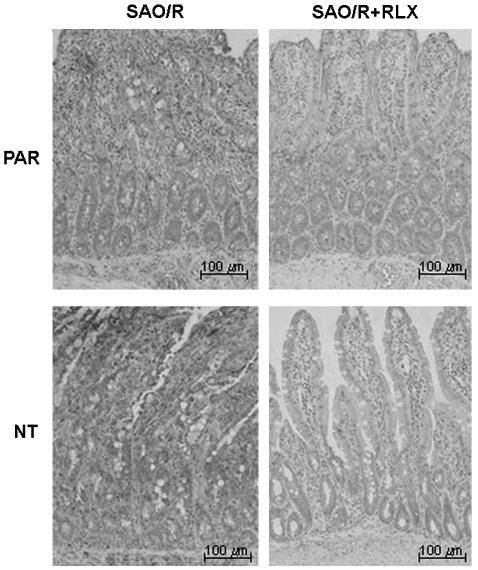
Immunocytochemical detection of Poly-ADP-ribosylated DNA (PAR) and NT in ileal tissue sections from SAO/R rats treated with vehicle or RLX. RLX reduces the expression of both PAR and NT.
In sections from ileal tissue specimens taken after SAO/R, we observed a strong immune reaction for NT, located mainly in submucosal vessels. Pretreatment with RLX, but not with iRLX, reduced the degree of NT immunostaining. Labeling was nearly absent in sections from the sham-operated rats (Figure 6). Specificity of the immune reaction for NT was confirmed by the almost complete inhibition of the labeling upon pretreatment of the primary antibody with excess NT (data not shown).
Figure 6.
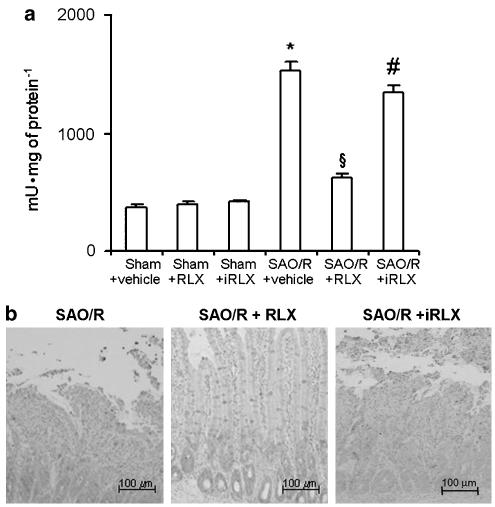
(a) Caspase-3 activity is increased in ileal tissue of SAO/R+vehicle rats. This effect is prevented by RLX, but not by iRLX. *P<0.01 vs sham-operated animals, P<0.01 vs SAO/R+vehicle, #P<0.01 vs SAO/R+RLX. (b) TUNEL-positive cells in ileal tissue sections from sham-operated rats and SAO/R rats treated with vehicle or RLX. Numerous apoptotic cells can be seen in the ileum of SAO/R+vehicle as well as in the SAO/R+iRLX rats. In contrast, only few apoptotic cells can be detected at the villum tips in the samples from SAO/R+RLX rats.
Inflammation-induced damage of cells in the ileal wall was assessed by analysing the occurrence of apoptosis through the measure of the activity of caspase-3, a key enzyme in early apoptotic cascade, and by detection of fragmented chromosomal DNA by TUNEL assay (Figure 6a and b). In the rats undergoing SAO/R, as compared with the sham-operated animals, caspase-3 activity was significantly increased and TUNEL-positive nuclear staining was markedly enhanced. Pretreatment with RLX inhibited caspase-3 activation and reduced the amount of TUNEL-positive nuclei, whereas iRLX had no effect.
Discussion
The current findings show that RLX, given i.v. to rats subjected to SAO-induced, prolonged ileal ischemia 15 min before reperfusion, affords protection against reperfusion-induced tissue injury. Compared with rats given PBS or iRLX, which underwent overt SAO/R-induced shock and severe ileal inflammation and damage, administration of bioactive RLX resulted in a marked, statistically significant reduction of the accumulation of inflammatory leukocytes into the ileum (tissue MPO activity), likely due to the observed decrease in the expression of early-phase P-selectin and late-phase E-selectin, VCAM and ICAM-1 by the ileal endothelium, as well as to a direct inhibitory effect of RLX on circulating neutrophils (Masini et al., 2004).
The marked reduction of the local inflammatory infiltrate caused by RLX treatment could likely account for the decrease in ileal tissue markers of oxidative stress, such as lipid peroxidation products (MDA), DNA damage (8-OHdG) and consumption of the endogenous antioxidant MnSOD (Crow & Beckman, 1995; Dix et al., 1996; Misko et al., 1998; Salvemini et al., 1999a, 1999b), observed in the RLX-treated rats. This also fits well with the observed reduction of nuclear poly-ADP-ribosylated DNA sites and of NT immunostaining in the RLX-treated animals as compared with those given PBS or iRLX, these markers being generated by interaction of free radicals produced by oxidative stress with DNA and proteins, respectively (Inoue & Kawanishi, 1995; Salgo et al., 1995; Eisserich et al., 1998). In turn, PARP activation at reperfusion can paradoxically worsen cell damage and endothelial dysfunction (Szabó & Dawson, 1998): thus, prevention of PARP activation may have an additional cytoprotective effect in the SAO/R ileum, as reported previously (Cuzzocrea et al., 1997; Szabó et al., 1997).
The RLX-dependent protection of ileal tissue from the adverse effects of inflammation and oxidative stress results in a clear-cut, statistically significant reduction of cell apoptosis, as shown by decreased caspase-3 activity and TUNEL-positive cells. Overall, all these beneficial effects of RLX on SAO/R ileum may account for the striking increase in the percentage of rats that survived until term reperfusion (4 h).
The present findings extend the current knowledge on the unique property of RLX to blunt ischemia/reperfusion-induced damage, previously investigated in animal models of myocardial infarction (Masini et al., 1997; Bani et al., 1998b; Perna et al., 2005). This property appears to depend on multiple, synergistic actions on vascular endothelial and smooth muscle cells (Bani et al., 1998a; Failli et al., 2002; Nistri et al., 2003) as well as on inflammatory cells playing a key role in reperfusion-induced tissue injury, such as neutrophils (Masini et al., 2004), likely mediated by a mechanism involving stimulation of NO production by the target cells (Nistri & Bani, 2005). In this context, it is worth noting that defective endogenous NO contributes substantially to SAO/R injury (Kanwar et al., 1994), whereas pharmacological increase of NO bioavailability by administration of NO donors, such as SNAP (Gauthier et al., 1994) or FK409 (Kalia et al. 2002), or of the NOS substrate L-arginine (Ward et al., 2000) has been shown to improve SAO/R-induced shock, especially by reducing endothelial dysfunction and leukocyte adhesion and recruitment. Compared with classical NO upregulating molecules, RLX could have additional, NO-independent beneficial effects: for instance, RLX has been recently ascribed anti-inflammatory properties by a glucocorticoid-like, plasma membrane receptor-independent action (Dschietzig et al., 2004).
At present, no definite medical therapy for intestinal ischemia exists, as several drugs have been anecdotally proven but not carefully studied. Many substances, including antioxidants and free radical scavengers, NO donors, cyclooxygenase and lipooxygenase blockers, leukocyte inhibitors and antichemotaxis agents, anti-ECAM monoclonal antibodies, etc., have shown protective effects in experimental models, but none of them is currently in clinical use (Mallick et al., 2004). On the other hand, numerous, recent experimental studies indicate ischemic preconditioning as the most promising strategy against intestinal ischemia, appearing to increase the tolerance of the intestine to reperfusion injury (Mallick et al., 2004). Research focused on the application of novel drugs that can mimic the effects of ischemic preconditioning to manipulate the cellular events during SAO/R of the intestine is required: in this context, RLX has a broad range of beneficial actions, such as leukocyte and platelet inhibition, endothelial protection and ECAM downregulation, which could effectively reproduce many of the effects of ischemic preconditioning. This issue affords RLX – or putative synthetic RLX agonists – the nomination among the novel drugs to be tested for possible therapeutic use in intestinal ischemia.
Acknowledgments
This work was supported by funds from the University of Florence, Italy, to D. Bani.
Abbreviations
- ECAMs
endothelial cell adhesion molecules
- ICAM-1
intercellular adhesion molecule 1
- MDA
malondialdehyde
- MPO
myeloperoxidase
- NT
nitrotyrosine
- 8-OHdG
8-hydroxy-2′-deoxyguanosine
- PAR
poly-ADP-ribose
- PARP
poly-ADP-ribosyl-polymerase
- RLX
relaxin
- SAO/R
splanchnic artery occlusion and reperfusion
- SOD
superoxide dismutase
- VCAM
vascular cell adhesion molecule
References
- ALTURA B.M., GEBREWOLDA A., BURTON R.W. Reactive hyperaemic responses of single arterioles are attenuated markedly after intestinal ischemia, endotoxemia and traumatic shock: possible role of endothelial cells. Microcirc. Endoth. Lymp. 1985;2:3–14. [PubMed] [Google Scholar]
- BANI D. Relaxin: a pleiotropic hormone. Gen. Pharmacol. 1997;28:13–22. doi: 10.1016/s0306-3623(96)00171-1. [DOI] [PubMed] [Google Scholar]
- BANI D., FAILLI P., BELLO M.G., THIEMERMANN C., BANI SACCHI T., BIGAZZI M., MASINI E. Relaxin activates the L-arginine-nitric oxide pathway in vascular smooth muscle cells in culture. Hypertension. 1998a;31:1240–1247. doi: 10.1161/01.hyp.31.6.1240. [DOI] [PubMed] [Google Scholar]
- BANI D., MASINI E., BELLO M.G., BIGAZZI M., BANI SACCHI T. Relaxin protects against myocardial injury caused by ischemia and reperfusion in rat heart. Am. J. Pathol. 1998b;152:1367–1376. [PMC free article] [PubMed] [Google Scholar]
- BITTERMANN H., AOKI N., LEFER A.M. Anti-shock effects of human superoxide dismutase in splanchnic occlusion shock. Proc. Soc. Exp. Biol. Med. 1988;188:256–271. doi: 10.3181/00379727-188-42734. [DOI] [PubMed] [Google Scholar]
- BOUGHTON-SMITH N.K., EVANS S.M., LASZLO F., WHITTLE B.J., MONCADA S. The induction of nitric oxide synthase and intestinal vascular permeability by endotoxin in the rat. Br. J. Pharmacol. 1993;110:1189–1195. doi: 10.1111/j.1476-5381.1993.tb13940.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BÜLLESBACH E.E., SCHWABE C. On the receptor binding sites of relaxins. Int. J. Pept. Protein. Res. 1988;32:361–367. doi: 10.1111/j.1399-3011.1988.tb01271.x. [DOI] [PubMed] [Google Scholar]
- BUTCHER E.C. Leukocyte-endothelial cell adhesion as an active, multi-step process: a combinatorial mechanism for specificity and diversity in leukocyte targeting. Adv. Exp. Med. Biol. 1992;323:181–194. doi: 10.1007/978-1-4615-3396-2_23. [DOI] [PubMed] [Google Scholar]
- BUTCHER E.C. Specificity of leukocyte-endothelial interactions and diapedesis: physiologic and therapeutic implications of an active decision process. Res. Immunol. 1993;144:695–698. doi: 10.1016/s0923-2494(93)80053-2. [DOI] [PubMed] [Google Scholar]
- CAREY C., SIEGFRIED M.R., MA X.L., WEYRICH A.S., LEFER A.M. Antishock and endothelial protective actions of a NO donor in mesenteric and reperfusion. Circ. Shock. 1992;38:209–216. [PubMed] [Google Scholar]
- CHEN S.A., PERLMAN A.J., SPANSKI N., PETERSON C.M., SANDERS S.W., JAFFE R., MARTIN M., YALCINKAYA T., CEFALO R.C., CHESCHEIR N.C., MENARD M.K., MORDENTI J. The pharmacokinetics of recombinant human relaxin in nonpregnant women after intravenous, intravaginal, and intracervical administration. Pharm. Res. 1993;10:834–838. doi: 10.1023/a:1018901009062. [DOI] [PubMed] [Google Scholar]
- CROW J.P., BECKMAN J.S. The role of peroxynitrite in nitric oxide-mediated toxicity. Curr. Top Microbiol. Immunol. 1995;196:57–73. doi: 10.1007/978-3-642-79130-7_7. [DOI] [PubMed] [Google Scholar]
- CUZZOCREA S., MAZZON E., DUGO L., CAPUTI A.P., ASTON K., RILEY D.P., SALVEMINI D. Protective effects of a new stable, highly active SOD mimetic, M40401 in splanchnic artery occlusion and reperfusion. Br. J. Pharmacol. 2001;132:19–29. doi: 10.1038/sj.bjp.0703775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CUZZOCREA S., ZINGARELLI B., COSTANTINO G., SZABÓ A., SALZMAN A.L., CAPUTI A.P., SZABÓ C. Beneficial effects of 3-aminobenzamide, an inhibitor of poly (ADP-ribose) polymerase in a rat model of splanchnic artery occlusion and reperfusion. Br. J. Pharmacol. 1997;121:1065–1074. doi: 10.1038/sj.bjp.0701234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEITCH E.A., BRIDGES W., BERG R., SPECIAN R.D., GRANGER N. Hemorrhagic Shock-induced bacterial translocation: the role of neutrophils and hydroxyl radicals. J. Trauma. 1990;30:942–951. doi: 10.1097/00005373-199008000-00002. [DOI] [PubMed] [Google Scholar]
- DIX T.A., HESS K.M., MEDINA M.A., SULLIVAN R.W., TILLY S.L., WEBB T.L.L. Mechanism of site-selective DNA nicking by the hydrodioxyl (perhydroxyl) radical. Biochemistry. 1996;35:4578–4583. doi: 10.1021/bi952010w. [DOI] [PubMed] [Google Scholar]
- DROY-LEFAIX M.T., DROUET Y., GERAUD G., HOSFOD D., BRAQUET P. Superoxide dismutase (SOD) and the PAF-antagonist (BN 52021) reduce small intestinal damage induced by ischemia-reperfusion. Free Rad. Res. Commun. 1991;12-13:725–735. doi: 10.3109/10715769109145852. [DOI] [PubMed] [Google Scholar]
- DSCHIETZIG T., STANGL K. Relaxin: a pregnancy hormone as a central player of body fluid and circulation homeostasis. Cell Mol Life Sci. 2002;59:1–13. doi: 10.1007/s00018-003-2169-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DSCHIETZIG T., BARTSCH C., STANGL V., BAUMANN G., STANGL K. Identification of the pregnancy hormone relaxin as glucocorticoid receptor agonist. FASEB J. 2004;13:1536–1538. doi: 10.1096/fj.03-1120fje. [DOI] [PubMed] [Google Scholar]
- EISSERICH J.P., HRISTOVA M., CROSS C.E., JONES A.D., FREEMAN B.A., HALLIWELL B., VAN DER VLIET A. Formation of nitric oxide-derived inflammatory oxidants by myeloperoxidase in neutrophils. Nature. 1998;391:393–397. doi: 10.1038/34923. [DOI] [PubMed] [Google Scholar]
- FAILLI P., NISTRI S., QUATTRONE S., MAZZETTI L., BIGAZZI M., BANI SACCHI T., BANI D. Relaxin up-regulates inducible nitric oxide synthase expression and nitric oxide generation in rat coronary endothelial cells. FASEB J. 2002;16:252–254. doi: 10.1096/fj.01-0569fje. [DOI] [PubMed] [Google Scholar]
- FANTONE J.C., WARD P.A. A review: role of oxygen-derived free radicals and metabolites in leukocyte-dependent inflammatory reactions. Am. J. Pathol. 1982;107:395–418. [PMC free article] [PubMed] [Google Scholar]
- GAUTHIER T.W., DAVENPECK K.L., LEFER A.M. Nitric oxide attenuates leukocyte-endothelial interaction via P-selectin in splanchnic ischemia-reperfusion. Am. J. Physiol. 1994;267:562–568. doi: 10.1152/ajpgi.1994.267.4.G562. [DOI] [PubMed] [Google Scholar]
- GRANGER D.N. Cell adhesion and migration. II. Leukocyte-endothelial cell adhesion in the digestive system. Am. J. Physiol. 1977;273:G982–G986. doi: 10.1152/ajpgi.1997.273.5.G982. [DOI] [PubMed] [Google Scholar]
- GRANGER D.N., RUTILI G., MCCORD J.M. Superoxide radicals in feline intestinal ischemia. Gastroenterology. 1981;81:22–23. [PubMed] [Google Scholar]
- HAGLIND E., XIA G., RYLANDER R. Effects of antioxidants and PAF receptor antagonist in intestinal shock in the rat. Circ. Shock. 1994;42:83–91. [PubMed] [Google Scholar]
- HARLAN J.M. Leukocyte-endothelial interactions. Blood. 1985;65:513–525. [PubMed] [Google Scholar]
- INOUE S., KAWANISHI S. Oxidative DNA damage induced by simultaneous generation of nitric oxide and superoxide. FEBS Lett. 1995;371:86–88. doi: 10.1016/0014-5793(95)00873-8. [DOI] [PubMed] [Google Scholar]
- KALIA N., BROWN N.J., HOPKINSON K., STEPHENSON T.J., WOOD R.F., POCKLEY A.G. FK409 inhibits both local and remote organ damage after intestinal ischaemia. J. Pathol. 2002;197:595–602. doi: 10.1002/path.1136. [DOI] [PubMed] [Google Scholar]
- KANWAR S., TEPPERMAN B.L., PAYNE D., SUTHERLAND L.R., KUBES P. Time course of nitric oxide production and epithelial dysfunction during ischemia/reperfusion of the feline small intestine. Circ. Shock. 1994;42:135–140. [PubMed] [Google Scholar]
- KUBES P., SUZUKI M., GRANGER D.N. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc. Natl. Acad. Sci. U.S.A. 1991;88:4651–4655. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAROUX F.S., LEFER D.J., KAWACHI S., SCALIA R., COCKRELL A.S., GRAY L., VAN DER HEYDE H., HOFFMANN J.M., GRISHAM M.B. Role of nitric oxide in the regulation of acute and chronic inflammation. Antiox. Redox. Signal. 2000;2:391–396. doi: 10.1089/15230860050192161. [DOI] [PubMed] [Google Scholar]
- LAWRENCE M.B., SPRINGER T.A. Leukocytes roll on a selectin at physiologic flow rates: distinction from and prerequisite for adhesion through integrins. Cell. 1991;65:859–873. doi: 10.1016/0092-8674(91)90393-d. [DOI] [PubMed] [Google Scholar]
- LEFER A.M., LEFER D.J. Pharmacology of the endothelium in ischemia-reperfusion and circulatory shock. Ann. Rev. Pharmacol. Toxicol. 1993;33:71–90. doi: 10.1146/annurev.pa.33.040193.000443. [DOI] [PubMed] [Google Scholar]
- LEFER D.J., JONES S.P., GIROD W.G., BAINES A., GRISHAM M.B., COCKRELL A.S., HUANG P.L., SCALIA R. Leukocyte-endothelial cell interactions in nitric oxide synthase-deficient mice. Am. J. Physiol. 1999;276:H1943–H1950. doi: 10.1152/ajpheart.1999.276.6.H1943. [DOI] [PubMed] [Google Scholar]
- LODOVICI M., CASALINI C., CARIEGGI R., MICHELACCI L., DOLARA P. Levels of 8-hydroxydeoxyguanosine as a marker of DNA damage in human leukocytes. Free Rad. Biol. Med. 2000;28:13–17. doi: 10.1016/s0891-5849(99)00194-x. [DOI] [PubMed] [Google Scholar]
- MALLICK I.H., YANG W., WINSLET M.C., SEIFALIAN A.M. Ischemia-reperfusion injury of the intestine and protective strategies against injury. Dig. Dis. Sci. 2004;49:1359–1377. doi: 10.1023/b:ddas.0000042232.98927.91. [DOI] [PubMed] [Google Scholar]
- MASINI E., BANI D., BELLO M.G., BIGAZZI M., MANNAIONI P.F., BANI SACCHI T. Relaxin counteracts myocardial damage induced by ischemia-reperfusion in isolated guinea pig hearts: evidence for an involvement of nitric oxide. Endocrinology. 1997;138:4713–4720. doi: 10.1210/endo.138.11.5520. [DOI] [PubMed] [Google Scholar]
- MASINI E., NISTRI S., VANNACCI A., BANI SACCHI T., NOVELLI A., BANI D. Relaxin inhibits the activation of human neutrophils. Involvement of the nitric oxide pathway. Endocrinology. 2004;145:1106–1112. doi: 10.1210/en.2003-0833. [DOI] [PubMed] [Google Scholar]
- McCORD J.M. Oxygen-derived free radicals in post ischemic tissue injury. N. Engl. J. Med. 1981;312:159–163. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- MISKO T.P., HIGHKIN M.K., VEENHUIZEN A.W., MANNING P.T., STERN M.K., CURRIE M.G., SALVEMINI D. Characterization of the cytoprotective action of peroxynitrite decomposition catalysts. J. Biol. Chem. 1998;273:15646–15653. doi: 10.1074/jbc.273.25.15646. [DOI] [PubMed] [Google Scholar]
- MULLANE K.M., KRAEMER R., SMITH B. Myeloperoxidase activity as a quantitative assessment of neutrophil infiltration into ischemic myocardium. J. Pharmacol. Methods. 1985;14:157–167. doi: 10.1016/0160-5402(85)90029-4. [DOI] [PubMed] [Google Scholar]
- MULLANE K.M., WESTLIN W., KRAEMER R. Activated neutrophils release mediators that may contribute to myocardial injury and dysfunction associated with ischemia and reperfusion. Ann. NY Acad. Sci. 1988;524:103–121. doi: 10.1111/j.1749-6632.1988.tb38534.x. [DOI] [PubMed] [Google Scholar]
- NISHIDA S., TERAMOTO K., KIMOTO-KINOSHITA S., TOHDA Y., NAKAJIMA S., TOMURA T.T., IRIMAJIRI K. Changes of Cu,Zn-superoxide dismutase activity of guinea pig lung in experimental asthma. Free Radical. Res. 2002;36:601–606. doi: 10.1080/10715760210872. [DOI] [PubMed] [Google Scholar]
- NISTRI S., BANI D. Relaxin in vascular physiology and pathophysiology: possible implications in ischemic brain disease. Curr. Neurovasc. Res. 2005;2:225–234. doi: 10.2174/1567202054368362. [DOI] [PubMed] [Google Scholar]
- NISTRI S., CHIAPPINI L., SASSOLI C., BANI D. Relaxin inhibits lipopolysaccharide-induced adhesion of neutrophils to coronary endothelial cells by a nitric oxide-mediated mechanism. FASEB J. 2003;17:2109–2111. doi: 10.1096/fj.03-0216fje. [DOI] [PubMed] [Google Scholar]
- OHKAWA H., OHISHI N., YAGI K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- PENG H.B., SPIECKER M., LIAO J.K. Inducible nitric oxide: an autoregulatory feedback inhibitor of vascular inflammation. J. Immunol. 1998;161:1970–1976. [PubMed] [Google Scholar]
- PERNA A.M., MASINI E., NISTRI S., BRIGANTI V., CHIAPPINI L., STEFANO P., BIGAZZI M., PIERONI C., BANI SACCHI T., BANI D. Novel drug development opportunity for relaxin in acute myocardial infarction. Evidences from a swine model. FASEB J. 2005;19:1525–1527. doi: 10.1096/fj.04-3664fje. [DOI] [PubMed] [Google Scholar]
- RATYCH R.E., CHUKNYSKA R.S., BURKLEY G.B. The primary localisation of free radical generation after anoxia/reoxygenation in isolated endothelial cells. Surgery. 1987;102:122–131. [PubMed] [Google Scholar]
- SALGO M.G., BERMUDEZ E., SQUADRITO G., PRYOR W. DNA damage and oxidation of thiols peroxynitrite causes in rat thymocytes. Arch. Biochem. Biophys. 1995;322:500–505. doi: 10.1006/abbi.1995.1493. [DOI] [PubMed] [Google Scholar]
- SALVEMINI D., MISKO T.P., MASFERRER J., SEIBERT K., CURRIE M.G., NEEDLEMAN P. Nitric oxide activates cyclooxygenase enzymes. Proc. Natl. Acad. Sci. U.S.A. 1993;90:7240–7244. doi: 10.1073/pnas.90.15.7240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALVEMINI D., RILEY D.P., LENNON P.J., WANG Z.Q., CURRIE M.G., MACARTHUR H., MISKO T.P. Protective effects of a superoxide dismutase mimetic and peroxynitrite decomposition catalysts in endotoxin-induced intestinal damage. Br. J. Pharmacol. 1999a;127:685–692. doi: 10.1038/sj.bjp.0702604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALVEMINI D., WANG Z.Q., WYATT P., BOURDON D.M., MARINO M.H., MANNING P.T., CURRIE M.G. Nitric oxide: a key mediator in the early and late phase of carrageenan-induced rat paw inflammation. Br. J. Pharmacol. 1996;118:829–838. doi: 10.1111/j.1476-5381.1996.tb15475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALVEMINI D., WANG Z.Q., ZWEIER J.L., SAMOUILOV A., MACARTHUR H., MISKO T.P., CURRIE M.G., CUZZOCREA S., SIKORSKI J.A., RILEY D.P. A nonpeptidyl mimetic of superoxide dismutase with therapeutic activity in rats. Science. 1999b;286:304–306. doi: 10.1126/science.286.5438.304. [DOI] [PubMed] [Google Scholar]
- SAMUEL C., PARRY L.J., SUMMERS R.J. Physiological or pathological – a role for relaxin in the cardiovascular system. Curr. Opin. Pharmacol. 2003;3:152–158. doi: 10.1016/s1471-4892(03)00011-0. [DOI] [PubMed] [Google Scholar]
- SHERWOOD O.D. Relaxin's physiological roles and other diverse actions. Endocr. Rev. 2004;25:205–234. doi: 10.1210/er.2003-0013. [DOI] [PubMed] [Google Scholar]
- SHERWOOD O.D., O'BYRNE E.M. Purification and characterization of porcine relaxin. Arch. Biochem. Biophys. 1974;60:185–196. doi: 10.1016/s0003-9861(74)80025-1. [DOI] [PubMed] [Google Scholar]
- SLUITER W., PIETERSMA A., LAMERS J.M.J., KOSTER J.F. Leukocyte adhesion molecules on the vascular endothelium: their role in the pathogenesis of cardiovascular disease and the mechanism underlying their expression. J. Cardiovasc. Pharmacol. 1993;22 Suppl. 4:S37–S44. [PubMed] [Google Scholar]
- SPIECKER M., LIAO J.K. Assessing induction of I kappa B by nitric oxide. Methods Enzymol. 1999;300:374–388. doi: 10.1016/s0076-6879(99)00142-1. [DOI] [PubMed] [Google Scholar]
- SREENARASIMHAIAH J. Diagnosis and management of ischemic colitis. Curr. Gastroenterol. Rep. 2005;7:421–426. doi: 10.1007/s11894-005-0013-1. [DOI] [PubMed] [Google Scholar]
- STENNICKE H.R., SALVESEN G.S. Biochemical characteristics of caspases-3, -6, -7, and -8. J. Biol. Chem. 1997;272:25719–25723. doi: 10.1074/jbc.272.41.25719. [DOI] [PubMed] [Google Scholar]
- STONEY R.J., CUNNINGHAN C.G. Acute mesenteric ischemia. Surgery. 1993;114:489–490. [PubMed] [Google Scholar]
- SZABÓ C., CUZZOCREA S., ZINGARELLI B., O'CONNOR M., SALZMAN A.L. Endothelial dysfunction in a rat model of endotoxic shock. Importance of the activation of poly (ADP-ribose) polymerase by peroxynitrite. J. Clin. Invest. 1997;100:723–735. doi: 10.1172/JCI119585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SZABÓ C., DAWSON V.L. Role of poly(ADP-ribose) polymerase in inflammation and ischaemia-reperfusion. Trends Pharmacol. Sci. 1998;19:287–298. doi: 10.1016/s0165-6147(98)01193-6. [DOI] [PubMed] [Google Scholar]
- WARD D.T., LAWSON S.A., GALLAGHER C.M., CONNER W.C., SHEA-DONOHUE T. Sustained nitric oxide production viaL-arginine administration ameliorates effects of intestinal ischemia-reperfusion. J. Surg. Res. 2000;89:13–19. doi: 10.1006/jsre.1999.5795. [DOI] [PubMed] [Google Scholar]
- XIA Z.F., HOLLYOAK M., BARROW R.E., HE F., MULLER M.J., HERNDON D.N. Superoxide dismutase and leupeptin prevent delayed reperfusion injury in the rat small intestine during burn shock. J. Burn Care Rehab. 1995;16:111–117. doi: 10.1097/00004630-199503000-00004. [DOI] [PubMed] [Google Scholar]
- ZIMMERMANN B.J., ARNDT H., KUBES P., KURTEL H., GRANGER D.N.Reperfusion injury in the small intestine Pathophysiology of Shock, Sepsis and Organ Failure 1993Berlin, Germany: Springer-Verlag; 322–335.eds. SCHLAG, G., & REDL, H., pp [Google Scholar]
- ZINGARELLI B., SQUADRITO F., INOCULANO M.P., ALTAVILLA D., BUSSOLINO F., CAMPO G.M., CAPUTI A.P. Platelet activating factor in splanchnic artery occlusion shock. Eur. J. Pharmacol. 1992;222:13–19. doi: 10.1016/0014-2999(92)90456-e. [DOI] [PubMed] [Google Scholar]