Abstract
Spontaneous 7–10 Hz spike-wave discharges (SWDs) are the electroencephalographic hallmark of absence seizures, as can be observed in WAG/Rij as well as in GAERS, two commonly used well-validated genetic rat models of absence epilepsy. A local upregulation of sodium channels within the perioral region of the primary somatosensory cortex indicated an initiation site for SWDs in WAG/Rij rats, in line with a new theory that assumes that SWDs have a cortical focal origin in the perioral region of the somatosensory cortex. We tested whether bilateral microinfusion at this focal site of the sodium channel blocker phenytoin, which is known to aggravate SWDs after systemic administration, reduces SWDs in both models.
WAG/Rij rats and GAERS, chronically provided with cortical EEG electrodes and bilateral cortical cannula's, were used. The EEGs were recorded before and after or systemic or bilateral infusion of phenytoin.
Microinfusion of phenytoin at the perioral region of the somatosensory cortex produced an immediate cessation of seizure activity in WAG/Rij rats, while systemic injection produced an increase in both genetic models. Microinfusion of the same and higher concentrations of phenytoin in GAERS at the same stereotactic coordinates showed no effect. Phenytoin was effective in GAERS 2 mm more posteriorly.
The data suggest that both genetic models have a cortical area at which diametrically opposite effects of phenytoin can be found compared to systemic injections: a decrease after local microinfusion and aggravation after systemic administration, although the exact cortical location may be different. Moreover, a deficit in sodium channels might be an ethiological factor underlying an increased probability for the initiation of SWDs in the somatosensory cortex.
Keywords: Cortical focus theory, absence epilepsy, GAERS, WAG/Rij, phenytoin, spike-wave discharges, ethosuximide, genetic models, translation research
Introduction
Typical childhood absence epilepsy is a generalized nonconvulsive epilepsy characterized by a spontaneous interruption of consciousness accompanied by generalized bilateral synchronous spike-wave discharges (SWDs) with a frequency of 3–4 Hz in the electroencephalogram (EEG) in humans (Panayiotopoulos, 1997). Genetic absence epilepsy rats from Strasbourg (GAERS) and WAG/Rij (Wistar Albino Glaxo from Rijswijk) rats are considered as two valid genetic rodent models of absence epilepsy (Marescaux et al., 1992; Crunelli & Leresche, 2002; Coenen & van Luijtelaar, 2003; Depaulis & van Luijtelaar, 2006). All adult rats of both strains show SWDs (7–11 Hz) in their EEG with concomitant clinical signs during absence seizures (Depaulis & van Luijtelaar, 2006).
The thalamocortical circuit is thought to play an important role in the pathogenesis of absence epilepsy (Avoli & Gloor, 1982; Vergnes et al., 1987). The thalamic origin for SWDs, characteristic of absence epilepsy, has dominated earlier studies (Avanzini et al., 1992; 2000). Particularly, the contribution of the reticular thalamic nucleus (RTN) and the ventrobasal complex of the thalamus were crucial in eliciting and propagating SWDs (Avoli & Gloor, 1982; Gloor & Fariello, 1988). On the other hand, the role of the cortex in the generation of SWDs was proposed in previous studies (Steriade, 1974; Pinault et al., 1998; Seidenbecher et al., 1998; Steriade & Contreras, 1998). Recently, a part of the somatosensory cortex has been implicated as a site from which SWDs are initiated; from here they quickly spread over the cortex and to thalamic structures (Meeren et al., 2002a). Local microinjection of ethosuximide (ETX), a first choice antiabsence drug, into the perioral region of the primary somatosensory cortex (S1po) but not at other cortical sites suppressed SWDs in GAERS. However, microinfusion into the ventrobasal thalamus and RTN was not, or much less, effective in producing cessation of seizure activity (Manning et al., 2004).
Intrinsic excitability of the nervous system is dependent on the activity of voltage-gated sodium channels (VGSCs) (Porter & Rogawski, 1992). VGSC genes have a heterogeneous tissue distribution within the nervous system (Catterall, 2000; Goldin et al., 2000). In the WAG/Rij model of absence epilepsy mRNA and protein levels for sodium channels were upregulated in layer II–IV cortical neurons in the facial somatosensory cortex (Klein et al., 2004). In another study, unilateral local functional deactivation of the somatosensory cortex by lidocaine, acting by blockage of sodium channels, reduced SWDs in WAG/Rij rats (Sitnikova & van Luijtelaar, 2004). Phenytoin (PHT) exerts its anticonvulsant effect primarily by blocking voltage-VGSCs (Tunnicliff, 1996; Xie et al., 2001). It is an effective drug against partial and generalized convulsive epilepsies (Kwan et al., 2001; Deckers et al., 2003). However, PHT appears to be ineffective in generalized nonconvulsive epilepsy and even exacerbates SWDs in rats as well in man (Micheletti et al., 1985; Peeters et al., 1988; Genton, 2000). The mechanism of SWD aggravation by PHT is poorly understood (Osorio et al, 2000).
The purpose of this study is to examine and compare the effect on absence seizures of locally injected PHT into the cortical representation of the perioral region of GAERS and WAG/Rij rats in order to investigate whether GAERS and WAG/Rij rats share an area sensitive for sodium channels in the S1po. Local blockage of sodium channels is expected to result in a reduction of the SWDs, whereas systemic injection will increase SWDs.
Methods
Adult male WAG/Rij rats (>6 months old, n=7) weighing 370–450 g and GAERS (>4 months old, n=11) weighing 230–300 g were used in the experiments. Animals were maintained on a 12-h light/dark cycle (light out from 08:00 to 20:00) with unlimited access to food and water.
All the experiments were carried out with the approval of Marmara University Ethical Committee for Experimental Animals (61.2002.Mar) and RU-DEC Radboud University Nijmegen in accordance with the European Communities Council Directive of 24 November 1986 (86/609/EEC).
Surgery
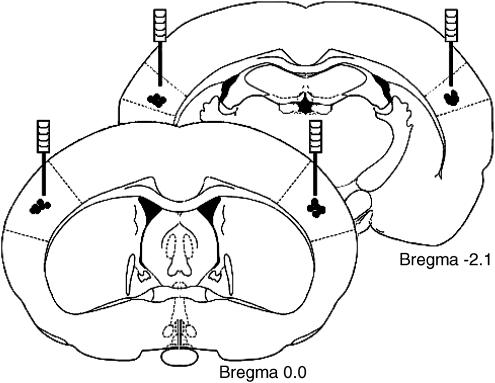
In WAG/Rij rats, a tripolar EEG electrode set (MS 333/2A, Plastics One, Roanoke, VI, U.S.A.) and two guide cannulae (C312G, Plastics One, Roanoke, VI, U.S.A.) were stereotaxically placed under isoflurane anesthesia (5% for induction and 2.5% for maintenance) after premedication with atropine sulfate (0.1 ml, intramuscularly (i.m.)). One of the poles of the recording electrode was stereotaxically placed on the frontal cortex (AP +2, ML −3.5 mm), another on the occipital cortex (AP −6, ML −4 mm) and the third (ground electrode) over the cerebellum (without specific coordinates). Stainless-steel guide cannulas were bilaterally implanted into the S1po (AP −0, ML ±5.5 mm: V −0.5 mm from the surface of the cortex) (Figure 1). These coordinates were originally described by Meeren et al. (2002a) and later by Klein et al. (2004) on the basis of their study of the expression of sodium channels in WAG/Rij rats (see Discussion). Ventral coordinates were 1 mm dorsal to the target area.
Figure 1.
Coronal sections of rat brain taken at 0.0 and −2.1 mm relative to bregma. Black spots indicate infusion cannula placement.
GAERS were anesthetized with ketamine (100 mg kg−1, intraperitoneally (i.p.)) and chlorpromazine (0.5 mg kg−1 (i.p.)). Animals were equipped with stainless-steel screws connected by insulated wires to a microconnector for EEG recordings and two guide cannulae (C312G, Plastics One, Roanoke, VI, U.S.A.). Cortical coordinates for EEG recordings were the same as for the WAG/Rij group. Stainless-steel guide cannulae were bilaterally implanted into S1po. Microinjections in GAERS were made at two different sites in S1po. In Group I (n=5), the same coordinates as for the WAG/Rij rats were used (AP −0, ML ±5.5 mm: V −0.5 mm from the surface of cortex) (Klein et al., 2004). In Group II (n=6), cannulas were bilaterally implanted into S1po at AP −2.1, ML±5.5 mm: V −1.3 mm from the surface of cortex (Figure 1), according to Manning et al. (2004). This is slightly more posterior and deeper than for the first group.
All coordinates were from the stereotaxic atlas of Paxinos & Watson (1998) with bregma as reference point. Electrode and cannula assemblies were attached to the skull with dental acrylic. Body temperature was kept at 37°C with a heating pad during surgery; following surgery, the rats were allowed to recover for at least 1 week.
Drugs
PHT and ETX were purchased from Sigma-Aldrich, the Netherlands.
Solutions
The concentrations of PHT for intracortical injections were equivalent to those used for systemic administration (Wang & Patsalos, 2003). The concentrations of ETX were obtained from the Manning et al. (2004) study.
PHT is a poorly soluble substance. Therefore, the pH of PHT and control solutions was adjusted with sodium hydroxide. The pH of the PHT solutions (720 μM and 3.6 mM) for intracortical injections was 9 and 9.8, respectively; the pH of the PHT solutions (40 and 80 mg kg−1) for i.p. injections was 10.5 and 11.5, respectively. Ringer solutions (intracortically) with pH of 6, 9, 9.8 and saline (i.p.) with pH of 7.4, 10.5 and 11.5 were used as control. No effect of different pH of these solutions on SWDs was observed. Therefore, in this study, all the results of Ringer and saline solutions were pooled and called ‘Ringer' and ‘Saline', respectively.
Experimental protocol
WAG/Rij rats
PHT was injected either i.p. (40 mg kg−1) (Peeters et al., 1988) or into S1po (720 μM per side). The same volume of saline (i.p.) or Ringer (intracortically) as for control solutions was injected. In half of the animals, randomly chosen, PHT was given first, and was followed by the control solution after a washout period of 2–3 days, and the reverse procedure was used in the other animals. The order of the i.p. and intracortical injections was also reversed in half of the animals.
GAERS
PHT (40 and 80 mg kg−1) and ETX (100 mg kg−1) were injected i.p. (Micheletti et al., 1985). The same volume of saline as of control solution was injected (i.p.). PHT (720 μM and 3.6 mM per side), ETX (40 and 400 mM per side) and Ringer solution (500 nl per side) were bilaterally injected into the S1po in both groups. The dose of 80 mg kg−1 of PHT (i.p.) was administered in GAERS since 40 mg kg−1 (i.p.) was not sufficient to aggravate SWDs. In line with this, two concentrations (low and high) of PHT were administered intracortically in the GAERS groups.
Intracortical injections were made with a Hamilton syringe at a flow rate of 500 nl min−1.
EEG
The EEG signals were amplified, filtered between 1 and 100 Hz, digitized at 200 samples s−1 and stored on a hard disk for offline analyses. The EEG was continuously recorded in freely moving rats for 2 h before and 2 h after each injection. SWDs (type I) (van Luijtelaar & Coenen, 1986; Midzianovskaia et al., 2001) were detected automatically using software developed by P.L.C. van den Broek (NICI, University of Nijmegen, The Netherlands) in WAG/Rij rats and manually in GAERS. Numbers and cumulative total durations of SWDs over 20-min time periods were calculated. The mean duration of SWDs is the ratio of cumulative total duration to the number of SWDs.
Histological verification
To determine cannula placement for all experiments, the animals were decapitated and the brains were placed in a formaline/sucrose mixture. Thick frozen sections (40 μm) were cut on a cryostat and stained with thionine. Only the animals with correct cannula placements were included in the study.
Statistical analysis
The results were expressed as mean±s.e.m. Data were statistically evaluated by repeated measures analysis of variance, if appropriate, followed by post hoc Bonferroni tests. The level of statistical significance was considered to be P<0.05.
Results
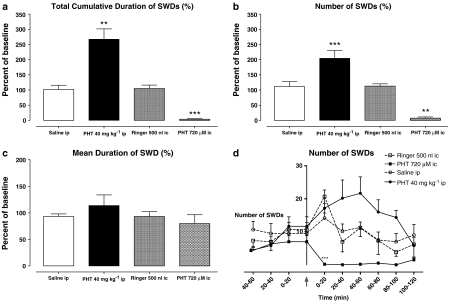
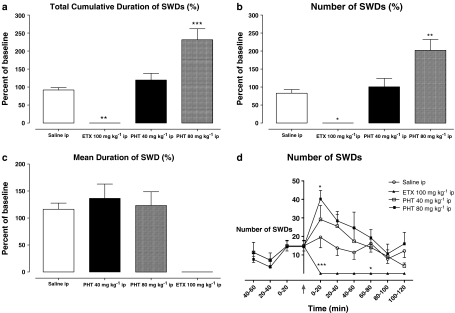
I.p. injection of PHT significantly increased the total cumulative duration and number of SWDs both in WAG/Rij rats and GAERS (Figures 2a, b and 3a, b). The effect of PHT after i.p. administration started within the first 20 min in both groups. In GAERS, it reached a maximum during the first 20 min after the injection, but in WAG/Rij rats, it did not reach a maximum until 40–60 min (Figure 2d). The dose of PHT required to enhance the cumulative duration and number of SWDs in GAERS (80 mg kg−1) was double that needed for WAG/Rij rats (40 mg kg−1). I.p. injection of ETX completely abolished SWDs in GAERS (Figure 3d).
Figure 2.
Effect of PHT on total cumulative duration (a), number (b) and mean duration (c) of SWDs throughout 2 h and the number of SWDs per 20 min intervals (d) before and after i.p. and intracortical (i.c.) injections in WAG/Rij rats (n=7). **P<0.01; ***P<0.001.
Figure 3.
Effect of PHT and ETX on total cumulative duration (a), number (b) and mean duration (c) of SWDs throughout 2 h and the number of SWDs per 20 min intervals (d) before and after i.p. injections of PHT and ETX in GAERS (n=6). *P<0.05; **P<0.01; ***P<0.001.
In contrast to the above, intracortical administration of PHT suppressed the occurrence of SWDs in WAG/Rij rats immediately after the injection (Figure 2d). No SWDs were observed for the first 100 min, but some animals showed several SWDs 100–120 min after local administration. The mean duration of these late complexes was similar to those observed after a control injection (Figure 2c).
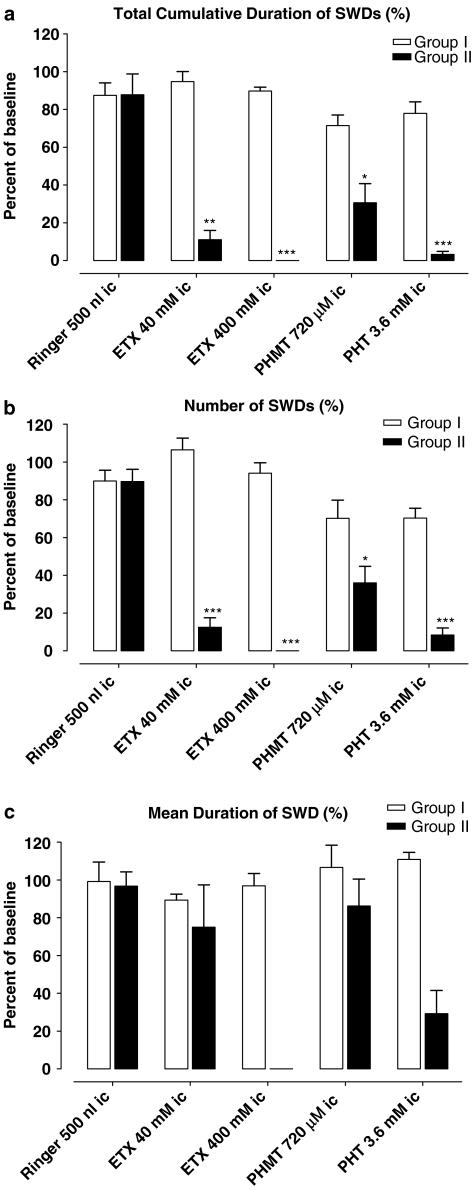
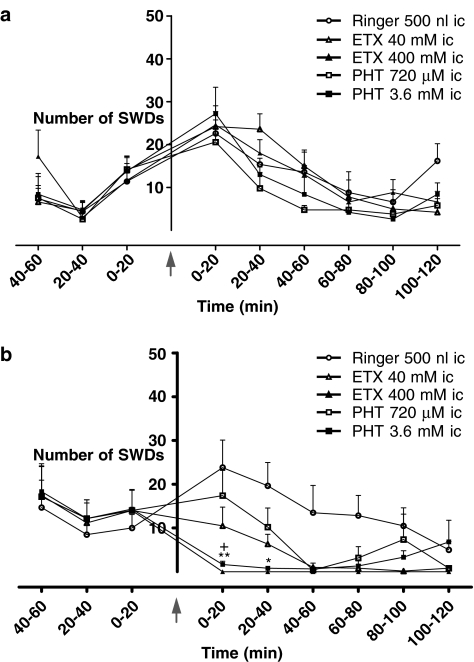
As mentioned above, GAERS were divided into two groups; Group I had the same coordinates for intracortical injections as the WAG/Rij rats, and Group II had coordinates in which ETX was previously found to be effective (Manning et al., 2004). Although the coordinates were the same as for the WAG/Rij rats, the effect of PHT after intracortical administration in Group I was completely different. No significant changes in duration and number of SWDs were found after two different concentrations of PHT in Group I (Figures 4 and 5a), the concentrations given were effective in WAG/Rij rats. As for the injection of PHT, no effect on SWDs was seen after intracortical injections of ETX in this group. In order to examine whether intracortical injections of PHT was ineffective in GAERS in contrast to WAG/Rij rats or whether the injection site was relevant, intracortical injection of ETX in two different concentrations were given.
Figure 4.
Effect of PHT and ETX on total cumulative duration (a), number (b) and mean duration (c) of SWDs after intracortical (i.c.) injections in Group I (n=5) and Group II (n=6) of GAERS. *P<0.05.
Figure 5.
Number of SWDs per 20 min before and after intracortical (i.c.) injections of PHT and ETX in Group I (a, n=5) and Group II (b, n=6) of GAERS. *P<0.05.
Group II differed from Group I in its responses to intracortical injections of either PHT or ETX. The concentration of 40 mM of ETX significantly decreased the total cumulative duration and the number of SWDs, whereas a higher concentration (400 mM) of ETX completely abolished SWDs (Figure 5b). Both concentrations of PHT also diminished the total cumulative duration and the number of SWDs, a higher concentration (3.6 mM) was more effective (Figure 4a–c). None of the injections caused significant changes in the mean durations of SWDs as well (Figure 4c).
Discussion
In this study, we have demonstrated that microinjection of PHT (720 μM) into the somatosensory cortex of WAG/Rij rats produces an immediate cessation of SWDs, whereas systemic administration (40 mg kg−1) induces diametrically opposite effects: it increases the number and cumulative duration of SWDs. In GAERS, intracortical injections of PHT and ETX are effective only at a different site within the somatosensory cortex. Thus, in Group I neither PHT nor ETX produces significant changes in both cumulative duration and number of SWDs. However, microinjection of ETX (40 and 400 mM) completely abolishes SWDs, and PHT (720 μM, 3.6 mM) significantly decreases the number of SWDs in rats of Group II. Systemic injections of PHT aggravate SWDs in WAG/Rij rats, injection of the same dose of PHT is ineffective in GAERS; however, a higher dose (80 mg kg−1), indeed, aggravates absence activity.
The decrease in the number of SWDs after microinjections of PHT is in agreement with a recent study in which ‘local functional deactivation of the somatosensory cortex' by lidocaine reduced SWDs in WAG/Rij rats (Sitnikova & van Luijtelaar, 2004). Lidocaine is acting via blockage of sodium channels (Shankaran & Quastel, 1972; Castañeda-Castellanos et al., 2002). On the other hand, exacerbation of absence activity both in animals and humans by systemic administration of carbamazepine, another sodium channel-blocker, was shown in previous studies (Micheletti et al., 1985; Peeters et al., 1988; Genton, 2000). There are no data demonstrating effects of systemic administration of lidocaine and intracortical injection of carbamazepine on absence seizures. Klein et al. (2004) found that only certain types of sodium channel were upregulated in the cortex of WAG/Rij rats. There may be a preferential effect of PHT on different subtypes of Na+ channels (Song et al., 1996). The different selectivity of sodium channels in the cortex and other brain areas may underlie the distinct response after systemic and cortical injections. It is clear that, from a functional point of view, sodium channels are not all the same: different channels can have different physiological characteristics, and can play different roles in the physiology of excitable cells (Waxman et al., 2000).
Opposite effects after local and systemic injections of certain drugs were found earlier: the γ-aminobutyric acid (GABA)A agonist muscimol decreases SWDs when injected in the RTN, whereas systemic injections of GABA-mimetics such as tiagabine dose-dependently increase SWDs (Coenen et al., 1995; Danober et al., 1998). In line with this is that benzodiazepines reduce absence seizures by selective augmentation of function of GABAA receptors in the RTN, but not the thalamus itself (Coulter, 1997).
In contrast to this, other mechanisms of action of PHT may also be involved in this dual effect on SWDs. CBZ is thought to aggravate absence seizures acting on the ventrobasal complex of the thalamus, and that activation of GABAA receptors is important in the mechanism of this effect (O'Brien et al., 2005). Granger et al. (1995) showed that PHT and carbamazepine potentiate GABA-induced Cl− currents in human embryonic kidney cells and in cultured rat cortical neurons.
Another finding in this study is that the cortical focus of absence seizures in GAERS and WAG/Rij rats seems to have different localizations within the somatosensory cortex. The cortical focus for SWDs in WAG/Rij rats was determined by signal analysis of field potentials (Meeren et al., 2002a), and Klein et al. (2004) showed that the mRNA and protein levels for some sodium channels were upregulated only at this site. Injection of PHT into this cortical site in WAG/Rij rats dramatically reduced SWDs; however, this was ineffective in GAERS. Even injection of ETX into the same site caused no effect on SWDs in GAERS. However, both PHT and ETX suppressed SWDs in a distinct site within the somatosensory cortex in GAERS. Based on these findings, it is thought that the cortical focus for absence seizures in GAERS might be different from WAG/Rij rats. However, a similar signal analytical approach of cortical propagation of SWDs and studies aimed at establishing the expression of mRNA and protein levels of sodium channels in specific cortical site in GAERS has not been taken place.
In our study, injections of PHT and ETX at two distinct sites of the somatosensory cortex in GAERS produced different responses. Similarly, it has been shown that ETX injected into the somatosensory cortex of GAERS reduced SWDs, whereas injection into the motor cortex produced no effect (Manning et al., 2004). Distinct parts of RTN, important component of thalamocortical circuit involved in generating or sustaining SWDs, seem to have different effect in the regulation of SWDs (Meeren et al., 2002b). These regional functional differences might also be present in the somatosensory cortex. However, whether the two different cortical zones in the two models also represent functional differences need to be established. The body weight of the two strains was different and body weight and age might have contributed to the differences between the strains in the localization of the cortical focal zone. In WAG/Rij rats, functional studies were carried out and its outcomes demonstrate that the perioral region contains the focus (Meeren et al., 2002a).
Dose and concentration of PHT for systemic and cortical injections to exert effect on SWDs tended to be different (no ED50's were statistically evaluated) in GAERS and WAG/Rij rats. This could be explained by different intensities of baseline absence activity in GAERS and WAG/Rij rats, a higher incidence and longer mean duration in GAERS than in WAG/Rij rats (Depaulis & van Luijtelaar, 2006). Therefore, it is possible that in GAERS, higher doses/concentrations of PHT are required to get a similar effect as observed in WAG/Rij.
Other differences between the two models are the age of onset of SWDs, which is earlier in GAERS than in WAG/Rij rats (Danober et al., 1998; Coenen & van Luijtelaar, 2003). In another study, the distribution of D2-like DA receptors in GAERS and WAG/Rij rats was found to be different (Birioukova et al., 2005). Differences in the kindling process between GAERS and WAG/Rij rats were shown in our recent study (Aker et al., 2006). Finally, genes controlling SWDs were located at chromosomes 5 and 9 in WAG/Rij rats and at chromosome 4, 7 and 8 in GAERS (Gauthier et al., 2004). It seems that although these two genetic models share a lot of properties of absence epilepsy such as a cortical focus, they are not identical in all respects.
Acknowledgments
A part of this study was supported by the CISP (Faculty of Social Sciences, Radboud University Nijmegen). We thank Ray Guillery for his critical review in the preparation of the manuscript.
Abbreviations
- EEG
electroencephalogram
- ETX
ethosuximide
- GAERS
genetic absence epilepsy rats from Strasbourg
- PHT
phenytoin
- RTN
reticular thalamic nucleus
- SWD
spike-wave discharge
- WAG/Rij
Wistar Albino Glaxo from Rijswijk
References
- AKER R.G., YANANLI H.R., GURBANOVA A.A., OZKAYNAKCI A.E., ATES N., VAN LUIJTELAAR G., ONAT F.Y. Amygdala kindling in the WAG/Rij rat model of absence epilepsy. Epilepsia. 2006;47:33–40. doi: 10.1111/j.1528-1167.2006.00367.x. [DOI] [PubMed] [Google Scholar]
- AVANZINI G., DE CURTIS M., MARESCAUX C., PANZICA F., SPREAFICO R., VERGNES M. Role of the thalamic reticular nucleus in the generation of rhythmic thalamo–cortical activities subserving spike and waves. J. Neural. Transm. 1992;35 (Suppl):85–95. doi: 10.1007/978-3-7091-9206-1_6. [DOI] [PubMed] [Google Scholar]
- AVANZINI G., PANZICA F., DE CURTIS M. The role of the thalamus in vigilance and epileptogenic mechanisms. Clin. Neurophysiol. 2000;111 (Suppl 2):S19–S26. doi: 10.1016/s1388-2457(00)00398-9. [DOI] [PubMed] [Google Scholar]
- AVOLI M., GLOOR P. Interaction of cortex and thalamus in spike and wave discharges of feline generalized penicillin epilepsy. Exp. Neurol. 1982;76:196–217. doi: 10.1016/0014-4886(82)90112-1. [DOI] [PubMed] [Google Scholar]
- BIRIOUKOVA L.M., MIDZYANOVSKAYA I.S., LENSU S., TUOMISTO L., VAN LUIJTELAAR G. Distribution of D1-like and D2-like dopamine receptors in the brain of genetic epileptic WAG/Rij rats. Epilepsy Res. 2005;63:89–96. doi: 10.1016/j.eplepsyres.2004.12.001. [DOI] [PubMed] [Google Scholar]
- CASTAÑEDA-CASTELLANOS D.R., NIKONOROV I., KALLEN R.G., RECIO-PINTO E. Lidocaine stabilizes the open state of CNS voltage-dependent sodium channels. Molecular Brain Research. 2002;99:102–113. doi: 10.1016/s0169-328x(01)00340-0. [DOI] [PubMed] [Google Scholar]
- CATTERALL W.A. From ionic currents to molecular mechanisms: the structure and function of voltage-gated sodium channels. Neuron. 2000;26:13–25. doi: 10.1016/s0896-6273(00)81133-2. [DOI] [PubMed] [Google Scholar]
- COENEN A., VAN LUIJTELAAR G. Genetic animal models for absence epilepsy: a review of the WAG/Rij strain of rats. Behav. Genet. 2003;33:635–655. doi: 10.1023/a:1026179013847. [DOI] [PubMed] [Google Scholar]
- COENEN A.M., BLEZER E.H., VAN LUIJTELAAR E.L. Effects of the GABA-uptake inhibitor tiagabine on electroencephalogram, spike-wave discharges and behaviour of rats. Epilepsy Res. 1995;21:89–94. doi: 10.1016/0920-1211(95)00015-3. [DOI] [PubMed] [Google Scholar]
- COULTER D.A. Antiepileptic drug cellular mechanisms of action: where does lamotrigine fit in. J. Child Neurol. 1997;12 (Suppl 1):S2–S9. doi: 10.1177/0883073897012001031. [DOI] [PubMed] [Google Scholar]
- CRUNELLI V., LERESCHE N. Childhood absence epilepsy: genes, channels, neurons and networks. Nat. Rev. Neurosci. 2002;3:371–382. doi: 10.1038/nrn811. [DOI] [PubMed] [Google Scholar]
- DANOBER L., DERANSART C., DEPAULIS A., VERGNES M., MARESCAUX C. Pathophysiological mechanisms of genetic absence epilepsy in the rat. Progr. Neurobiol. 1998;55:27–57. doi: 10.1016/s0301-0082(97)00091-9. [DOI] [PubMed] [Google Scholar]
- DECKERS C.L.P., GENTON P., SILLS G.J., SCHMIDT D. Current limitations of antiepileptic drug therapy: a conference review. Epilepsy Res. 2003;53:1–17. doi: 10.1016/s0920-1211(02)00257-7. [DOI] [PubMed] [Google Scholar]
- DEPAULIS A., VAN LUIJTELAAR G.Genetic models of Absence epilepsy in the rat Models of Seizures and Epilepsy 2006San Diego, CA: Elsevier Ac Press; 233–248.ed. Pitkanen, A., Schwartzkroin, P.A. & Moshe, S.L., pp [Google Scholar]
- GAUTHIER D., VAN LUIJTELAAR G., BIHOREAU M.T., WILDER S.P., GODFREY R.F., VOSSEN J., COENEN A., COX R.D. Chromosomal mapping of genetic loci controlling absence epilepsy phenotypes in the WAG/Rij rat. Epilepsia. 2004;45:908–915. doi: 10.1111/j.0013-9580.2004.13104.x. [DOI] [PubMed] [Google Scholar]
- GENTON P. When antiepileptic drugs aggravate epilepsy. Brain Dev. 2000;22:75–80. doi: 10.1016/s0387-7604(99)00113-8. [DOI] [PubMed] [Google Scholar]
- GLOOR P., FARIELLO R.G. Generalized epilepsy: some of its cellular mechanisms differ from those of focal epilepsy. Trends Neurosci. 1988;11:63–68. doi: 10.1016/0166-2236(88)90166-x. [DOI] [PubMed] [Google Scholar]
- GOLDIN A.L., BARCHI R.L., CALDWELL J.H., HOFMANN F., HOWE J.R., HUNTER J.C., KALLEN R.G., MANDEL G., MEISLER M.H., NETTER Y.B., NODA M., TAMKUN M.M., WAXMAN S.G., WOOD J.N., CATTERALL W.A. Nomenclature of voltage-gated sodium channels. Neuron. 2000;28:365–568. doi: 10.1016/s0896-6273(00)00116-1. [DOI] [PubMed] [Google Scholar]
- GRANGER P., BITON B., FAURE C., VIGE X., DEPOORTERE H., GRAHAM D., LANGER S.Z., SCATTON B., AVENET P. Modulation of the γ-aminobutyric acid type A receptor by the antiepileptic drugs carbamazepine and phenytoin. Mol. Pharmacol. 1995;47:1189–1196. [PubMed] [Google Scholar]
- KLEIN J.P., KHERA D.S., NERSESYAN H., KIMCH E.Y., WAXMAN S.G., BLUMENFELD H. Dysregulation of sodium channel expression in cortical neurons in a rodent model of absence epilepsy. Brain Res. 2004;1000:102–109. doi: 10.1016/j.brainres.2003.11.051. [DOI] [PubMed] [Google Scholar]
- KWAN P., SILLS G.J., BRODIE M.J. The mechanism of action of commonly used antiepileptic drugs. Pharmacol. Ther. 2001;90:21–34. doi: 10.1016/s0163-7258(01)00122-x. [DOI] [PubMed] [Google Scholar]
- MANNING J.-P.A., RICHARDS D.A., LERESCHE N., CRUNELLI V., BOWERY N.G. Cortical-area specific block of genetically determined absence seizures by ethosuximide. Neuroscience. 2004;123:5–9. doi: 10.1016/j.neuroscience.2003.09.026. [DOI] [PubMed] [Google Scholar]
- MARESCAUX C., VERGNES M., DEPAULIS A. Genetic absence epilepsy in rats from Strasbourg. J. Neural. Transm. 1992;35 (Suppl):37–69. doi: 10.1007/978-3-7091-9206-1_4. [DOI] [PubMed] [Google Scholar]
- MEEREN H.K., PIJN J.P., VAN LUIJTELAAR E.L., COENEN A.M., LOPES DA SILVA F.H. Cortical focus drives widespread corticothalamic networks during spontaneous absence seizures in rats. J. Neurosci. 2002a;22:1480–1495. doi: 10.1523/JNEUROSCI.22-04-01480.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEEREN H.K.M., MÖDERSCHEİM T.A.E., VEENING J.G., COENEN A.M.L., VAN LUIJTELAAR E.L.J.M. Cortico-Thalamic Mechanisms Underlying Generalized Spike-Wave Discharges of Absence Epilepsy. Nijmegen: P. Nijmegen University Press; 2002b. Thalamic lesions: effects of generalized spike-wave discharges and other forms of low-frequency thalamocortical oscillations; pp. 40–67. [Google Scholar]
- MICHELETTI G., VERGNES M., MARESCAUX C., REIS J., DEPAULIS A., RUMBACH L., WARTER J.M. Antiepileptic drug evaluation in a new animal model: spontaneous petit mal epilepsy in the rat. Arzheim. Forsch./Drug Res. 1985;35:483–495. [PubMed] [Google Scholar]
- MIDZIANOVSKAIA I.S., KUZNETSOVA G.D., COENEN A.M., SPIRIDONOV A.M., VAN LUIJTELAAR E.L. Electrophysiological and pharmacological characteristics of two types of spike-wave discharges in WAG/Rij rats. Brain Res. 2001;911:62–70. doi: 10.1016/s0006-8993(01)02705-6. [DOI] [PubMed] [Google Scholar]
- O'BRIEN T.J., LIU L., WALLENGREN C., LOHMAN R.-J., MORRIS M.J. Carbamazepine aggravates absence seizures in GAERS rats by acting on the ventrobasal thalamus via a GABA-A mediated mechanism. Epilepsia. 2005;46 (Suppl 8):296. [Google Scholar]
- OSORIO I., REED R.C., PELTZER J.N. Refractory idiopathic absence status epilepticus: a probable paradoxical effect of phenytoin and carbamazepine. Epilepsia. 2000;41:887–894. doi: 10.1111/j.1528-1157.2000.tb00258.x. [DOI] [PubMed] [Google Scholar]
- PANAYIOTOPOULOS C.Absence epilepsies Epilepsy: A Comprehensive Textbook 1997Philadelphia: Lippincott-Raven Publishers; 2327–2346.ed. Engel, J. & Pedley, T.A., pp [Google Scholar]
- PAXINOS G., WATSON N. The Rat Brain in Stereotaxic Coordinates 1998San Diego, CA: Academic Press; 4th edn. [Google Scholar]
- PEETERS B.W.M.M., SPOOREN W.P.J.M., VAN LUIJTELAAR E.L.J.M., COENEN A.M.L. The WAG/Rij rat model for absence epilepsy: anticonvulsant drug evaluation. Neurosci. Res. Commun. 1988;2:93–97. [Google Scholar]
- PINAULT D., LERESCHE N., CHARPIER S., DENIAU J.M., MARESCAUX C., VERGNES M., CRUNELLI V. Intracellular recordings in thalamic neurones during spontaneous spike and wave discharges in rats with absence epilepsy. J. Physiol. 1998;509:449–456. doi: 10.1111/j.1469-7793.1998.449bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORTER R.J., ROGAWSKI M.A. New antiepileptic drugs: from serendipity to rational discovery. Epilepsia. 1992;33 (Suppl 1):S1–S6. doi: 10.1111/j.1528-1157.1992.tb05895.x. [DOI] [PubMed] [Google Scholar]
- SEIDENBECHER T., STAAK R., PAPE H.-C. Relations between cortical and thalamic cellular activities during absence seizures in rats. Eur. J. Neurosci. 1998;10:1103–1112. doi: 10.1046/j.1460-9568.1998.00123.x. [DOI] [PubMed] [Google Scholar]
- SHANKARAN R., QUASTEL J.H. Effects of anesthetics on sodium uptake into rat brain cortex in vitro. Biochem. Pharmacol. 1972;21:1763–1773. doi: 10.1016/0006-2952(72)90083-4. [DOI] [PubMed] [Google Scholar]
- SITNIKOVA E., VAN LUIJTELAAR G. Cortical control of generalized absence seizures: effect of lidocaine applied to the somatosensory cortex in WAG/Rij rats. Brain Res. 2004;1012:127–137. doi: 10.1016/j.brainres.2004.03.041. [DOI] [PubMed] [Google Scholar]
- SONG J.H., NAGATA K., HUANG C.S., YEH J.Z., NARAHASHI T. Differential block of two types of sodium channels by anticonvulsants. Neuroreport. 1996;7:3031–3036. doi: 10.1097/00001756-199611250-00047. [DOI] [PubMed] [Google Scholar]
- STERIADE M. Interneuronal epileptic discharges related to spike-and-wave cortical seizures in behaving monkeys. Electroencephalogr. Clin. Neurophysiol. 1974;37:247–263. doi: 10.1016/0013-4694(74)90028-5. [DOI] [PubMed] [Google Scholar]
- STERIADE M., CONTRERAS D. Spike-wave complexes and fast components of cortically generated seizures. I. Role of neocortex and thalamus. J. Neurophysiol. 1998;80:1439–1455. doi: 10.1152/jn.1998.80.3.1439. [DOI] [PubMed] [Google Scholar]
- TUNNICLIFF G. Basis of the antiseizure action of phenytoin. Gen. Pharmacol. 1996;27:1091–1097. doi: 10.1016/s0306-3623(96)00062-6. [DOI] [PubMed] [Google Scholar]
- VAN LUIJTELAAR E.L., COENEN A.M. Two types of electrocortical paroxysms in an inbred strain of rats. Neurosci. Lett. 1986;70:393–397. doi: 10.1016/0304-3940(86)90586-0. [DOI] [PubMed] [Google Scholar]
- VERGNES M., MARESCAUX C., DEPAULIS A., MICHELETTI G., WARTER J.M. Spontaneous spike and wave discharges in thalamus and cortex in a rat model of genetic petit mal-like seizures. Exp. Neurol. 1987;96:127–136. doi: 10.1016/0014-4886(87)90174-9. [DOI] [PubMed] [Google Scholar]
- WANG X., PATSALOS P. A comparison of central brain (cerebrospinal and extracellular fluids) and peripheral blood kinetics of phenytoin after intravenous phenytoin and fosphenytoin. Seizure. 2003;12:330–336. doi: 10.1016/s1059-1311(03)00099-2. [DOI] [PubMed] [Google Scholar]
- WAXMAN S.G., DIB-HAJJ S., CUMMINS T.R., BLACK J.A. Sodium channels and their genes: dynamic expression in the normal nervous system, dysregulation in disease states. Brain Res. 2000;886:5–14. doi: 10.1016/s0006-8993(00)02774-8. [DOI] [PubMed] [Google Scholar]
- XIE X., DALE T.J., JOHN V.H., CATER H.L., PEAKMAN T.C., CLARE J.J. Electrophysiological and pharmacological properties of the human brain type IIA Na+ channel expressed in a stable mammalian cell line. Pflugers Arch. 2001;441:425–433. doi: 10.1007/s004240000448. [DOI] [PubMed] [Google Scholar]