Abstract
Cell-surface expression of CD40 in B-cell malignancies and multiple solid tumors has raised interest in its potential use as a target for antibody-based cancer therapy. SGN-40, a humanized monoclonal anti-CD40 antibody, mediates antibody-dependent cytotoxicity and inhibits B-cell tumor growth in vitro, properties of interest for the treatment of cancers, and is currently in Phase I clinical trials for B-cell malignancies. In this study, we determined in vivo activity and pharmacokinetics properties of SGN-40.
Effect of SGN-40 in xenograft model of CD40-expressing B-cell lymphoma in severe-combined immune deficiency mice and its in vivo pharmacokinetics properties in normal mice, rats and cynomolgus monkeys were studied.
Treatment with SGN-40 significantly increased the survival of mice xenografted with human B-cell lymphoma cell line. SGN-40 exhibited nearly 100% bioavailability in mice and it cleared faster when given at a low dose. In monkeys, clearance of SGN-40 was also much faster at low dose, suggesting nonlinear pharmacokinetics in these species. In rats, however, SGN-40 clearance at all tested doses was similar, suggesting that pharmacokinetics were linear in this dose range in rats. Administration of SGN-40 to monkeys also produced marked, dose-dependent, and persistent depletion of peripheral CD20+ B lymphocytes.
Data presented in this report suggest that SGN-40 is active in in vivo, and based upon interspecies scaling, SGN-40 clearance in humans is predicted to be similar to observed SGN-40 clearance in monkeys. These data suggest that SGN-40 has appropriate pharmacokinetic properties that support its clinical use.
Keywords: Ant i-CD40, B cell, animal models, pharmacodynamics, pharmacokinetics
Introduction
CD40 is a tumor necrosis factor (TNF) receptor superfamily member expressed as a type-I transmembrane protein (40 kDa) on both hematopoietic and nonhematopoietic tissues. CD40 is constitutively expressed on immature and mature B cells, dendritic cells, monocytes, macrophages, activated CD8 T cells and some endothelial cells (Grewal & Flavell, 1998). Expression of CD40 is upregulated upon activation of these cells. Ligand for CD40, CD40L (also known as CD154), a TNF superfamily member is preferentially expressed on activated T cells and platelets, and low-level expression is also detected on monocytes, B cells, dendritic cells and endothelial cells. CD40 along with its ligand, CD40L, regulates important biologic effects in the immune system. Thus, CD40/CD40L receptor–ligand interaction is important for the proliferation of normal B cells, monocytes, and dendritic cells; the production of T-dependent antibodies, the class switching of immunoglobulin; and the activation of antigen-presenting cells for upregulating costimulatory molecules. Mutations in the CD40L gene in humans that leads to the lack of functional expression of CD40L result in the hyper-IgM syndrome, a primary deficiency of immunoglobulin class switching (Van Kooten & Banchereau, 2000).
Interestingly, CD40 is also expressed at relatively high levels on neoplastic B cells, including B lymphoblastoid cells, Burkitt's lymphoma cells, B-acute lymphoblastic leukemia cells, non-Hodgkin's lymphoma (NHL) cells, multiple myeloma (MM) cells, and chronic lymphocytic leukemia cells (Banchereau et al., 1994; Pellat-Deceunynck et al., 1994; Wang et al., 1997). In solid tumors, CD40 expression occurs abundantly on primary biopsy specimens obtained from patients with epithelial tumors from many organs, including pancreas, lung, ovary, bladder, breast, colon, prostate, and head and neck (Stamenkovic et al., 1989; Vestal et al., 1997; Ottaiano et al., 2002). CD40 is also expressed on a number of cell lines derived from B-cell hematologic malignancies and solid tumors. Cell-surface expression of CD40 in B-cell malignancies and most solid tumors has raised interest in its potential use as a tumor target for antibody-based cancer therapy (Young et al., 1998; Schultze & Johnson, 1999; Tong & Stone, 2003; Eliopoulos & Young, 2004).
Although the role of CD40 signaling in immune system is well established, its role in cancers is less well understood (Aggarwal, 2003). In this respect, studies have presented evidence that CD40 receptor can send a productive antiapoptotic or proapoptotic signals to malignant B cells (Hollmann et al., 2002; Szocinski et al., 2002; Mathur et al., 2004). Binding of recombinant CD40 ligand (rCD40L) to CD40 induces apoptosis of malignant B-cell lines and CD40-expressing primary tumors, and inhibits growth of carcinomas (Funakoshi et al., 1994; Eliopoulos et al., 1996; Hess & Engelmann, 1996; Wingett et al., 1998; Hirano et al., 1999; Eliopoulos et al., 2000; Ghamande et al., 2001). rCD40L also improves survival and inhibits tumor growth in a xenograft tumor model comprising severe-combined immunodeficient (SCID) mice implanted with breast cancer cells (Funakoshi et al., 1994; Hirano et al., 1999). In addition, encouraging results were reported from a Phase I study of rCD40L in cancer patients with solid tumors and high-grade NHL (Vonderheide et al., 2001). Similarly, anti-CD40 antibodies have been shown to inhibit growth of tumor cells lines and induce cytotoxicity against primary tumor cells and malignant cell lines of B-cell origin (Funakoshi et al., 1994; Dilloo et al., 1997; Hayashi et al., 2003; Law et al., 2005). Furthermore, these antibodies prolonged survival of SCID mice xenotransplanted with human tumor cell lines of B-cell origin (Francisco et al., 2000). Conversely, both rCD40L and agonist anti-CD40 antibodies stimulated proliferation of some normal cells expressing CD40 (Grewal & Flavell, 1998; Van Kooten & Banchereau, 2000).
We have previously characterized the binding and functional activities of the humanized SGN-40, which shows antitumor activities against human B-lymphoma cell lines (Law et al., 2005). SGN-40 is a humanized monoclonal antibody that selectively binds to human CD40. SGN-40 does not prevent CD40/CD40L interactions, yet sends a growth inhibitory signal to CD40-expressing neoplastic cells and induces apoptosis in several B-cell lymphoma cell lines. SGN-40 also mediates antibody-dependent cytotoxicity (ADCC) of B-cell lymphoma cell lines and primary MM tumor cells from patients in vitro (Hayashi et al., 2003; Tai et al., 2004). The ability of SGN-40 to induce apoptosis and inhibit cell growth in a wide variety of B-cell-driven cancer cell lines suggests that this molecule may be useful for treating hematological and other CD40-expressing malignancies. In this study, we demonstrate the in vivo activity of SGN-40 in a xenograft model of B-cell lymphoma and characterize the in vivo pharmacokinetics of SGN-40 in rodents and non-human primates.
Methods
Humanized anti-CD40 antibody
An engineered humanized version of a previously described murine monoclonal anti-CD40 (SGN-14; Francisco et al., 2000) designated as SGN-40 was used in this study. The structure of SGN-40 is based upon human IgG1(κ) framework sequences, consisting of two heavy chains and two light chains. The full-length antibody has a molecular weight of ∼150 kDa. The heavy-chain DNA codes for 443 amino acids (48,443 kDa), and the light-chain codes for 219 amino acids (24,001 kDa). SGN-40 binds the human CD40 receptor with a high affinity (Kd=∼1 nM) and high specificity.
Animal studies
All animal studies performed are consistent with current standards in research and procedures used were as humane as possible. Intuitional guidelines for animal care and welfare were strictly followed. All animal protocols were dully approved by institutional committees.
Xenograft studies in the mouse
In vivo activity of anti-CD40 on B-cell lymphomas was examined in xenograft model using SCID mice (Harlan, Indianapolis, IN, U.S.A.). In this model, the effect of the anti-CD40 antibody on the survival of mice xenotransplanted with a human NHL cell line was studied. SCID mice (10 per group) were intravenously (i.v.) inoculated with 1 × 106 Raji tumor cells 5 days before drug treatment. SGN-40 or control antibody was injected intraperitoneally (i.p.) at a dose of 4 mg−1 kg−1. One group of mice was left untreated. Mice were examined daily for survival for 103 days at which time experiment was terminated.
Pharmacokinetic studies in the mouse
SGN-40 was administered (1 or 10 mg kg−1; n=15 per group) as an i.v. bolus via the tail vein of male CD-1 mice (body wt=31±2 g (Charles River Laboratories, Raleigh, NC, U.S.A.; Table 1)). Serial blood samples (∼100 μl) were collected predose and between 10 min and 6 h postdose (n=3 mice per time point) via the orbital sinus under isoflurane anesthesia, or via cardiac puncture at killing. Blood was allowed to clot at room temperature; the serum was harvested and stored at −60 to −80°C until analyzed by enzyme-linked immunosorbent assay (ELISA) for total SGN-40 concentration.
Table 1.
Group assignments and dose levels
| Species | No of animals in group | Dose level (mg kg−1) | Dose concentration (mg ml−1) | Dose volume (ml) |
|---|---|---|---|---|
| CD-1 mouse | 15 | 1 | 0.3 | 0.1 |
| CD-1 mouse | 15 | 10 | 3.1 | 0.1 |
| Sprague–Dawley rat | 4 | 1 | 1 | 0.25 |
| Sprague–Dawley rat | 4 | 10 | 10 | 0.25 |
| Cynomolgus monkey | 4 | 1 | 1 | 1–2 |
| Cynomolgus monkey | 6 | 10 | 10 | 1–2 |
Pharmacokinetic studies in the rat
Micro-Renathane polyurethane cannulas (Braintree Scientific Inc., Braintree, MA, U.S.A.) were inserted into the femoral (0.84 mm o.d. × 0.36 mm i.d.) and jugular (1.02 mm o.d. × 0.64 mm i.d.) veins of male Sprague–Dawley rats (n=8; body wt=237±11 g; (Charles River Laboratories, Hollister, CA, U.S.A.) 72 h before dosing. A single i.v. bolus dose (1 or 10 mg kg−1; n=4 per group) of SGN-40 was given via the femoral vein (Table 1). Serial blood samples (∼200 μl) were taken predose and between 10 min and 35 days postdose from the jugular vein. Fluid volume was replaced with saline or heparinized saline when necessary as judged by the study monitor. Blood was processed to serum and stored at −60 to −80°C until analyzed for total SGN-40 concentration.
Pharmacokinetics/pharmacodynamics in the cynomolgus monkey
This study was conducted at Covance Laboratories Inc. (Vienna, VA, U.S.A.). Treatment of the animals was in accordance with regulations outlined in the USDA Animal Welfare act and the conditions specified in The Guide for Care and Use of Laboratory Animals (Institute of Laboratory Animal Research, 1996).
Sixteen (eight male and eight female) cynomolgus monkeys (Macaca fascicularis), 2 to 4 years of age, weighing between 1.5 and 1.9 kg (Covance Research Products, Denver, PA, U.S.A.) were used. Animals (n=4–6 per group) were assigned to SGN-40 treatment groups (1 or 10 mg kg−1) by sex and body weight (Table 1). Remaining animals (n=4 per group) received SGN-40 vehicle as control. Before dose administration, animals were not sedated but were temporarily restrained within their cages. Following i.v. bolus dosing (5 min infusion), serial samples of whole blood (∼0.75 ml) were collected by venipuncture from an available peripheral vein into tubes without anticoagulant. Intensive samples were collected after administration of the first dose (5 min postdose, and 7 days postdose) and the fifth dose (5 min predose, and 8 h postdose). Samples were also collected to monitor peak drug concentrations on study day 15 (5 min postdose) and trough drug concentrations on study days 7, 14, 21, and 28 (predose). Serum (∼0.5 ml) was harvested, placed on dry ice, and stored in a freezer at −60 to −80° C for later SGN-40 analysis.
FACS analyses
Approximately 2 ml blood was collected into vials containing sodium heparin as an anticoagulant and evaluated by fluorescence-activated cell sorting (FACS) to determine peripheral blood leukocyte populations (FAST Systems Inc., Gaithersburg, MD, U.S.A.). Lymphocytes were identified using monoclonal antibody markers recognizing surface CD45 and CD14. Total peripheral blood lymphocytes were identified as CD45+/CD14− cells within the light scatter gate. Lymphocyte phenotypes were further characterized using monoclonal antibodies to CD3, CD4, CD8, and CD20 as cell-surface markers. NK cells were identified as CD16+/CD3− cells.
SGN-40 ELISA
SGN-40 ELISA were performed by coating ELISA plates (Nunc, Neptune, NJ, U.S.A.) with CD40-IgG (produced at Genentech Inc., South Francisco, CA, U.S.A.) at 0.5 μg ml−1 for 12–72 h at 2 to 8°C. After blocking, appropriately diluted serum samples or SGN-40 standard was added to the plates. Captured SGN-40 was detected with protein L-horseradish peroxidase (HRP) (Pierce, Rockford, IL, U.S.A.). Color was developed using tetramethyl benzidine (TMB) (Kirkegaard & Perry Laboratories, Gaithersburg, MD, U.S.A.), and the reaction was stopped with 1 M phosphoric acid. Serum SGN-40 concentrations were interpolated from a four-parameter fit of the SGN-40 standard curve. The minimum quantifiable concentration or sensitivity of the assay was 8 ng ml−1 for CD-1 mice (1 : 20 minimum dilution of serum), 61.5 ng ml−1 for Sprague–Dawley rats (1 : 50 minimum serum dilution) and 20 ng ml−1 for cynomolgus monkeys (1 : 50 minimum serum dilution). Standard curves and control samples were observed to run reproducibly in the assay.
Assay for anti-SGN-40 antibodies
Plates coated with SGN-40 at 0.1 μg ml−1 were used for anti-SGN-40 ELISA. After blocking serum samples diluted at 1 : 100, and negative (naïve cynomolgus monkey serum pool) and positive (rabbit anti-SGN-40 antisera) controls were added to the plates and incubated 2 h. Biotinylated SGN-40 was added for 1 h, then streptavidin–HRP conjugate (Zymed Laboratories, South San Francisco, CA, U.S.A.) was added. Plates were developed as described above for SGN-40 ELISA. A multiplication factor of 1.2 was applied to the negative control mean OD response on any given plate to determine the cut-point of the assay. The SGN-40-specific antibody titer was determined by the log10 of the dilution at which the sample OD crosses the cut-point OD value. Samples at the minimum dilution with an OD value below the cut-point OD were determined to be SGN-40-specific antibody negative.
Pharmacokinetic analysis
Pharmacokinetic analysis was performed using the WinNonlin Professional Edition computer software, Version 3.2 (Pharsight Corp., Mountain View, CA, U.S.A.). A number of models and weighting factors were used to minimize the sum of squares residual value between the observed and model-predicted serum drug concentrations. Serum concentration–time profiles were fit using a two-compartment model with bolus input and first-order output. Calculations of rate constants and secondary parameters including AUC (area under the SGN-40 serum concentration–time curve), Cmax (model-predicted maximum serum SGN-40 concentration), Vss (estimated steady-state volume of distribution), and half-life have been described previously (Gibaldi & Perrier, 1982).
Statistical analysis
Group mean parameters for rats and cynomolgus monkeys were obtained by averaging parameter estimates from individual animals. The effect of SGN-40 dose on pharmacokinetic parameters in rats and monkeys was evaluated using a one-way analysis of variance (ANOVA) and Fisher's post hoc test (α=0.05). Pharmacokinetic parameters for mice were calculated by modeling group mean data and are therefore reported without measures of variance.
Results
Activity of SGN-40 against human NHL cells in xenograft model
The activity of the humanized anti-CD40 antibody (SGN-40) was evaluated using B-cell lymphoma mouse model. In this model, SCID mice were inoculated (i.v.) with Raji lymphoma cells 5 days before the treatment with either control antibody or with SGN-40 (4 mg kg−1). Mouse survival was monitored daily. Untreated and nine of 10 control mice did not survive beyond study day 40; however, nine of 10 mice treated with SGN-40 were alive at study day 40 and 50% of the SGN-40-treated mice were still alive at day 103 of the experiment (Figure 1). These data indicate that the humanized antibody, SGN-40, was effective in prolonging survival of SCID mice in this B-cell lymphoma models.
Figure 1.
In vivo antitumor activity of SGN-40. Effects of SGN-40 on survival of mice xenografted with human B-cell lymphoma line. SCID mice (n=10 per group) were inoculated (i.v.) with 106 Raji tumor cells, and 5 days later, mice were treated with anti-CD40 antibody or control antibody via i.p. injection at a single 4 mg kg−1 dose. Experiment was terminated 103 days after the treatment.
Characterization of SGN-40 pharmacokinetics
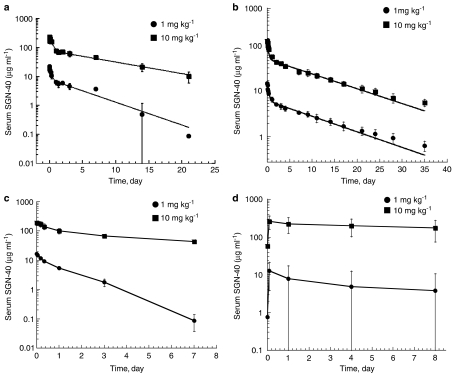
In order to determine pharmacokinetic properties of SGN-40, studies were conducted in mouse, rat, and cynomolgus monkeys. Animals used and their group assignment and SGN-40 dose levels are given in Table 1. The resulting serum concentration–time profiles are presented in Figure 2; corresponding pharmacokinetic parameters are presented in Table 2.
Figure 2.
Serum concentration–time profiles following i.v. bolus dosing with SGN-40 in (a) CD-1 mice (n=15 group), (b) Sprague–Dawley rats (n=4 per group), and (c) cynomolgus monkeys (n=4–6 per group). Data were collected from 5 min to 7 days following the first dose administration and mean data (±s.d.) are presented. (d) Cynomolgus monkeys (n=4–6 per group) were injected with SGN-40 (i.v.) once a week for 5 weeks and following the fifth dose administration of SGN-40, serum was collected from 5 min to 8 h postdose and mean data (±s.d.) are shown. Serum was analyzed for SGN-40 by ELISA.
Table 2.
Calculated pharmacokinetic parametersa
| Mouse | Rat | Monkeyb | ||||
|---|---|---|---|---|---|---|
| 1 mg kg−1 | 10 mg kg−1 | 1 mg kg−1 | 10 mg kg−1 | 1 mg kg−1 | 10 mg kg−1 | |
| BW (kg) | 0.031±0.002 | 0.032±0.002 | 0.239±0.015 | 0.235±0.008 | 1.68±0.17 | 1.67±0.08 |
| AUC (μg day−1 ml−1) | 58.2 | 961 | 88.1±12 | 845±110 | 17.9±3.2 | 947±170 |
| Cmaxc (ml day−1 kg−1) | 21 | 211 | 17.9±1.4 | 173±20 | 16.9±1.2 | 199±24 |
| CL (ml day−1 kg−1) | 17.2 | 10.4 | 12.0±1.5 | 12.8±2.3 | 57.7±13 | 10.8±1.8 |
| β1/2d | 5.34 | 8.23 | 8.74±1.4 | 8.57±1.5 | 1.15±0.30 | 6.77±1.7 |
| Vss (ml kg−1) | 124 | 115 | 145±17 | 148±13 | 87.1±11 | 96.7±15 |
Parameters calculated using compartmental analysis.
Parameters calculated from SGN-40 profiles after the first dose administration (days 1–7).
Cmax is the model-predicted maximum serum concentration of SGN-40.
For rats and monkeys values are given as mean (±s.d.).
SGN-40 pharmacokinetics following a single dose to rodents
Because of limitations on blood sampling in mice, these data were pooled; each mouse contributed a portion of the total serum–time profile (Figure 2a). Serum concentration data for SGN-40 versus time data from rats were modeled individually (Figure 2b). SGN-40 pharmacokinetics was similar in mice and rats and was characterized using a two-compartment model that provided a good fit to the observed data. Following dosing, SGN-40 distributed in a volume ∼2 to 3 times greater than serum volume, suggesting SGN-40 distributed beyond the vascular space. SGN-40 elimination from serum was biphasic, entering a prolonged terminal elimination phase ∼2–3 days after dosing. In mice, SGN-40 cleared ∼70% faster at the low dose (1 mg kg−1) compared with the high dose (10 mg kg−1). In the rat, however, SGN-40 clearance and half-lives (∼9 days) were similar across the same 10-fold dose range, suggesting that pharmacokinetics were linear over this range.
SGN-40 pharmacokinetics following a single dose to cynomolgus monkeys
The disposition of multiple SGN-40 doses (1–10 mg kg−1) in cynomolgus monkeys was studied; however, only data collected after the first dose (0–7 days) were detailed enough to give reasonable pharmacokinetic parameter estimates (Figure 2c and Table 2). Results from pharmacokinetic samples collected after day 7 are shown in Figure 3 and confirm predicted drug exposure and the accumulation of drug in serum.
Figure 3.
(a) Peak and trough SGN-40 concentrations in the serum of cynomolgus monkeys throughout a multiple-dose study. Peak SGN-40 levels were measured 5 min after the first, third, and fifth SGN-40 dose administrations. (b) Trough SGN-40 concentrations in serum of cynomolgus monkeys throughout a multiple-dose study. Trough SGN-40 levels were measured before second, third, forth, and fifth dose.
A two-compartment model provided a good fit to observed data following the first SGN-40 dose. The disposition of SGN-40 was similar in male and female monkeys receiving the same dose of SGN-40. Serum drug concentrations as high as 198 μg ml−1 were seen 5 min after dosing. SGN-40 pharmacokinetics was bi-phasic. Its clearance was dose-dependent, being five- to six-fold faster after the lower dose of SGN-40 compared with the higher dose (57.7±13 versus 10.9±1.8 ml day−1 kg−1, respectively; P=0.0001). This was demonstrated by the greater than dose-proportional increase in AUC and Cmax over the 10-fold dose range, and a markedly faster elimination half-life in the lower dose group compared with that of the higher dose group (1.15±0.30 versus 6.94±1.7 days, respectively; P=0.0002). These observations suggest that the pharmacokinetics of SGN-40 is nonlinear in cynomolgus monkeys across the dose range studied.
In contrast with observations following the first dose, there was a high degree of interanimal variability in drug concentrations after the last SGN-40 dose. SGN-40 serum concentrations were ∼10- to 100-fold lower in 75% of the low-dose and 25% of the high-dose monkeys on dosing day 29, compared with day 1 (data not shown). This rapid elimination suggested that antibodies form against the human framework of SGN-40 (cynomolgus anti-human antibodies; CAHA), which was subsequently confirmed. When data from these animals were removed, serum SGN-40 concentration–time profiles were similar to those after the first high-dose administration (Figure 2d).
To evaluate the accumulation of SGN-40 in serum following multiple dose administrations, intermittent peak and trough levels of SGN-40 were monitored over the course of the study. As shown in Figure 3a and b, there was no significant accumulation of serum SGN-40 between study days 14 and 29. However, the increased variability in peak and trough concentrations seen in individual animals over the course of the study might also reflect formation of antibodies against SGN-40.
Impact of CAHA on SGN-40 pharmacokinetics
Pharmacokinetic results from this multiple-dose study suggest that several monkeys may have formed antibodies against SGN-40. In this study, three of four monkeys dosed weekly with 1 mg kg−1 and one of eight monkeys dosed weekly with 10 mg kg−1 SGN-40 showed that serum drug levels dropped markedly faster after their fifth SGN-40 dose compared with after their first dose. An ELISA analysis showed that antidrug antibodies were present only in those monkeys with a faster drug clearance after their fifth dose.
Pharmacodynamic effects of SGN-40 in cynomolgus monkeys
The cynomolgus monkey was chosen as an appropriate species in which to assess pharmacodynamic effects of SGN-40 because SGN-40 binds CD40+ cells in cynomolgus monkey peripheral blood (data not shown). Following dose administration, a substantial and persistent decrease in peripheral blood CD20+ cells to ∼10% of baseline levels was observed (Figure 4a). There was a transient decrease in peripheral blood T-cells (both CD3+/CD4+ and CD3+/CD8+) and NK cells; however, similar lymphocyte decreases were observed in control groups (Figure 4b and c).
Figure 4.
Pharmacodynamic response of lymphocyte and mononuclear cell counts to SGN-40 either in cynomolgus monkeys. Blood was analyzed by FACS. Lymphocytes were identified using monoclonal antibody markers recognizing surface-cell CD45 and CD14. Total peripheral blood lymphocytes were identified as CD45+/CD14− cells within the light-scatter gate. (a) Effect of SGN-40 on B-lymphocytes as characterized using monoclonal antibodies to CD20 cell-surface marker. (b) Pharmacodynamic response of CD3+/CD4+ lymphocyte counts to SGN-40 in cynomolgus monkeys. Lymphocyte phenotypes were characterized using monoclonal antibodies to CD3 and CD4 cell-surface markers. (c) Pharmacodynamic response of CD3+/CD8+ lymphocyte counts to SGN-40 in cynomolgus monkeys. Lymphocyte phenotypes were further characterized using monoclonal antibodies to CD3 and CD8 cell-surface markers. Data represent mean values (±s.d.).
Dosing strategy for Phase I interspecies scaling
Allometric scaling was used to provide estimates of SGN-40 clearance in humans. The relationship between SGN-40 clearance and body weight is shown in Figure 5. First, a linear plot was obtained by logarithmic transformation of both axes. The resulting linear relationship is described by equation 1); parameter (P) was estimated by simple linear regression of the transformed data:
In this equation, A is the coefficient (y-axis intercept), B is body weight, and α is the power function (slope) (Ings, 1990). Regression analysis showed that clearance is strongly correlated with body weight across species. The regression-derived clearance estimates for a 70 kg human given a single i.v. bolus dose of SGN-40 (12 ml day−1 kg−1) is also presented in Figure 5 (dashed line).
Figure 5.
Interspecies scaling for prediction of SGN-40 clearance in humans. Observed SGN-40 clearance in CD-1 mice, Sprague–Dawley rats, and cynomolgus monkeys after i.v. dosing. Regression analysis estimates SGN-40 clearance as 11.52 × body wt1.01. Predicted SGN-40 clearance for a 70 kg human based on regression analysis of SGN-40 clearance across species is given.
Discussion and conclusions
Interest in SGN-40 as an anticancer therapy stems from its ability to induce apoptosis and inhibit growth of a wide variety of B-cell-derived cancer cell lines, whereas having little effect on normal (nontumor) cells. Results with rodent xenograft models show that murine anti-CD40 antibody has substantial antitumor activity against human tumor cell lines of non-Hodgkin's lymphoma (Francisco et al., 2000). From these studies, a humanized version of a murine anti-CD40, SGN-40, was developed to study its potential therapeutic use in humans. SGN-40 mediates ADCC against tumor cell lines and primary MM cells (Hayashi et al., 2003; Law et al., 2005). In addition, SGN-40 also has direct signaling properties as it downmodulates expression of the IL-6 receptor on primary MM cells, which results in impaired IL-6-induced survival effects on MM cells (Tai et al., 2004). SGN-40 activity against B-cell lymphoma is similar to that of the murine parent anti-CD40 antibody and confirms our previously published data with other lymphoma models (Law et al., 2005).
In our studies, we demonstrate clear evidence of the antitumor activity of SGN-40, in xenograft models of B-cell lymphomas. The ability of SGN-40 to mediate ADCC, and induce apoptosis and inhibit cell growth in a wide variety of B-cell-driven cancer cell lines in vitro suggests that the in vivo antitumor mechanism of SGN-40 is likely to occur via ADCC and/or direct cytotoxicity to tumor cells. As for as activity of SGN-40 is concerned, multiple doses of SGN-40 given to cynomolgus monkeys resulted in transient decreases in peripheral blood T cells and NK cells, but these decreases were similar to those observed in vehicle controls. However, SGN-40 caused marked, dose-related, and persistent depletion of peripheral CD20+ lymphocytes. Although these changes are not unexpected considering the central role that CD40 plays in the immune system, future studies are needed to determine if CD20+ cells return to normal levels after cessation of SGN-40 treatment. Nevertheless, these data suggest that SGN-40 is active in vivo in cynomolgus monkeys.
After dosing in mice, SGN-40 exhibited nearly 100% bioavailability. SGN-40 elimination from serum was relatively slow in all species studied. In the mouse and the cynomolgus monkey, SGN-40 clearance was nonlinear between 1 and 10 mg kg−1 dose levels, suggesting the presence of a saturable clearance mechanism. However, at high doses, the in vivo disposition of SGN-40 is compatible with a clinical application. Additionally, all doses of SGN-40 in monkeys resulted in a marked pharmacodynamic response (CD20+ depletion). To guide clinical dose selection, SGN-40 disposition across species and the B cell depleting activity of SGN-40 was evaluated in non-human primates. Based on interspecies scaling, SGN-40 clearance in humans is predicted to be similar to observed SGN-40 clearance in cynomolgus monkeys: clearance for a 70 kg human is expected to be 841 ml day−1 or 12 ml day−1 kg−1. If linear pharmacokinetics in humans is assumed, we predict that doses of 0.2–11 mg kg−1 will result in drug exposures similar to those seen in cynomolgus monkeys (1 and 10 mg kg−1, respectively), and could result in similar reductions in peripheral B cells if the pharmacokinetic/pharmacodynamic relationship holds from primates to humans.
In several monkeys, SGN-40 clearance was increased following multiple-dose administration, suggesting the formation of antibodies directed against SGN-40. An antibody assay confirmed the presence of antidrug antibodies in all monkeys with time-dependent changes in drug clearance. These antibodies were likely directed against the human framework of SGN-40 (CAHA); however, the epitope characterization was not performed on these samples. Because SGN-40 is a fully humanized IgG1, we anticipate that the incidence of anti-SGN-40 antibodies will be reduced in humans. However, it will be important to monitor for antidrug antibodies in the clinic to determine any potential impact on drug exposure and safety.
In summary, these studies have characterized the pharmacokinetics and pharmacodynamic effects of SGN-40 in rodents and non-human primates and suggest that intravenous administration of SGN-40 has pharmacokinetic properties compatible with a clinical application. Once the Phase I clinical studies with SGN40 are complete, it will be interesting to compare them to these nonclinical findings.
Acknowledgments
We thank Ann Bricarello, Michael DuVall, Gabrielle Hatami, Mark Hartmangruber, Marie McKeon, Paul Pisacane, and Ketevan Siradze for their assistance with these studies and this article and Christopher Dant for assistance with manuscript preparation. Sean K. Kelley, Thomas Gelzleichter, Dong Xie, Wyne P. Lee, Walter C. Darbonne, and Ferhan Qureshi are paid employees of Genentech Inc. and are receiving salary and stock compensation from Genentech Inc. Kim Kissler, Ezogelin Oflazoglu, and Iqbal S. Grewal are paid employees of Seattle Genetics Inc. and are receiving salary and stock compensation from Seattle Genetics Inc. Iqbal S. Grewal has received salary support and stock compensation from Genentech in the past.
Abbreviations
- ADCC
antibody-dependent cellular cytotoxicity
- ANOVA
one-way analysis of variance
- AUC
area under the SGN-40 serum concentration–time curve
- BW
body weight
- CAHA
cynomolgus anti-human antibodies
- Cmax
model-predicted maximum serum SGN-40 concentration
- ELISA
enzyme-linked immunosorbent assay
- FACS
fluorescence-activated cell sorting
- i.p.
intraperitonealy
- i.v.
intravenously
- MM
multiple myeloma
- NHL
non-Hodgkin's lymphoma
- OD
optical density
- rCD40L
recombinant CD40 ligand
- SC
subcutaneously
- SCID
severe-combined immune deficiency
- SGN-14
murine monoclonal anti-human CD40 antibody
- SGN-40
humanized monoclonal anti-human CD40 antibody
- TNF
tumor necrosis factor
- Vss
estimated steady-state volume of distribution
References
- AGGARWAL B.B. Signalling pathways of the TNF superfamily: a double-edged sword. Nat. Rev. Immunol. 2003;3:745–756. doi: 10.1038/nri1184. [DOI] [PubMed] [Google Scholar]
- BANCHEREAU J., BAZAN F., BLANCHARD D., BRIERE F., GALIZZI J.P., VAN KOOTEN C., LIU Y.J., ROUSSET F., SAELAND S. The CD40 antigen and its ligand. Annu. Rev. Immunol. 1994;12:881–922. doi: 10.1146/annurev.iy.12.040194.004313. [DOI] [PubMed] [Google Scholar]
- DILLOO D., BROWN M., ROSKROW M., ZHONG W., HOLLADAY M., HOLDEN W., BRENNER M. CD40 ligand induces an antileukemia immune response in vivo. Blood. 1997;90:1927–1933. [PubMed] [Google Scholar]
- ELIOPOULOS A.G., DAVIES C., KNOX P.G., GALLAGHER N.J., AFFORD S.C., ADAMS D.H., YOUNG L.S. CD40 induces apoptosis in carcinoma cells through activation of cytotoxic ligands of the tumor necrosis factor superfamily. Mol. Cell. Biol. 2000;20:5503–5515. doi: 10.1128/mcb.20.15.5503-5515.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELIOPOULOS A.G., DAWSON C.W., MOSIALOS G., FLOETTMANN J.E., ROWE M., ARMITAGE R.J., DAWSON J., ZAPATA J.M., KERR D.J., WAKELAM M.J., REED J.C., KIEFF E., YOUNG L.S. CD40-induced growth inhibition in epithelial cells is mimicked by Epstein–Barr virus-encoded LMP1: involvement of TRAF3 as a common mediator. Oncogene. 1996;13:2243–2254. [PubMed] [Google Scholar]
- ELIOPOULOS A.G., YOUNG L.S. The role of the CD40 pathway in the pathogenesis and treatment of cancer. Curr. Opin. Pharmacol. 2004;4:360–367. doi: 10.1016/j.coph.2004.02.008. [DOI] [PubMed] [Google Scholar]
- FRANCISCO J.A., DONALDSON K.L., CHACE D., SIEGALL C.B., WAHL A.F. Agonistic properties and in vivo antitumor activity of the anti-CD40 antibody SGN-14. Cancer Res. 2000;60:3225–3231. [PubMed] [Google Scholar]
- FUNAKOSHI S., LONGO D.L., BECKWITH M., CONLEY D.K., TSARFATY G., TSARFATY I., ARMITAGE R.J., FANSLOW W.C., SPRIGGS M.K., MURPHY W.J. Inhibition of human B-cell lymphoma growth by CD40 stimulation. Blood. 1994;83:2787–2794. [PubMed] [Google Scholar]
- GHAMANDE S., HYLANDER B.L., OFLAZOGLU E., LELE S., FANSLOW W., REPASKY E.A. Recombinant CD40 ligand therapy has significant antitumor effects on CD40-positive ovarian tumor xenografts grown in SCID mice and demonstrates an augmented effect with cisplatin. Cancer Res. 2001;61:7556–7562. [PubMed] [Google Scholar]
- GIBALDI M., PERRIER D. Pharmacokinetics 1982New York: Markel Dekker Inc; 2nd edn., revised and expanded [Google Scholar]
- GREWAL I.S., FLAVELL R.A. CD40 and CD154 in cell-mediated immunity. Annu. Rev. Immunol. 1998;16:111–135. doi: 10.1146/annurev.immunol.16.1.111. [DOI] [PubMed] [Google Scholar]
- HAYASHI T., TREON S.P., HIDESHIMA T., TAI Y.T., AKIYAMA M., RICHARDSON P., CHAUHAN D., GREWAL I.S., ANDERSON K.C. Recombinant humanized anti-CD40 monoclonal antibody triggers autologous antibody-dependent cell-mediated cytotoxicity against multiple myeloma cells. Br. J. Haematol. 2003;121:592–596. doi: 10.1046/j.1365-2141.2003.04322.x. [DOI] [PubMed] [Google Scholar]
- HESS S., ENGELMANN H. A novel function of CD40: induction of cell death in transformed cells. J. Exp. Med. 1996;183:159–167. doi: 10.1084/jem.183.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRANO A., LONGO D.L., TAUB D.D., FERRIS D.K., YOUNG L.S., ELIOPOULOS A.G., AGATHANGGELOU A., CULLEN N., MACARTNEY J., FANSLOW W.C., MURPHY W.J. Inhibition of human breast carcinoma growth by a soluble recombinant human CD40 ligand. Blood. 1999;93:2999–3007. [PubMed] [Google Scholar]
- HOLLMANN A.C., GONG Q., OWENS T. CD40-mediated apoptosis in murine B-lymphoma lines containing mutated p53. Exp. Cell Res. 2002;280:201–211. doi: 10.1006/excr.2002.5640. [DOI] [PubMed] [Google Scholar]
- INGS R.M.J. Interspecies scaling and comparisons in drug development and toxicokinetics. Xenobiotica. 1990;20:1201–1231. doi: 10.3109/00498259009046839. [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Research, Commission on Life Sciences, National Research Council. Guide for the care and use of laboratory animals 1996Washington, DC, U.S.A.: National Academy Press; 140p [Google Scholar]
- LAW C.L., GORDON K.A., COLLIER J., KLUSSMAN K., MCEARCHERN J.A., CERVENY C.G., MIXAN B.J., LEE W.P., LIN Z., VALDEZ P., WAHL A.F., GREWAL I.S. Preclinical antilymphoma activity of a humanized anti-CD40 monoclonal antibody, SGN-40. Cancer Res. 2005;65:8331–8338. doi: 10.1158/0008-5472.CAN-05-0095. [DOI] [PubMed] [Google Scholar]
- MATHUR R.K., AWASTHI A., WADHONE P., RAMANAMURTHY B., SAHA B. Reciprocal CD40 signals through p38MAPK and ERK-1/2 induce counteracting immune responses. Nat. Med. 2004;10:540–544. doi: 10.1038/nm1045. [DOI] [PubMed] [Google Scholar]
- OTTAIANO A., PISANO C., DE CHIARA A., ASCIERTO P.A., BOTTI G., BARLETTA E., APICE G., GRIDELLI C., IAFFAIOLI V.R. CD40 activation as potential tool in malignant neoplasms. Tumori. 2002;88:361–366. doi: 10.1177/030089160208800502. [DOI] [PubMed] [Google Scholar]
- PELLAT-DECEUNYNCK C., BATAILLE R., ROBILLARD N., HAROUSSEAU J.L., RAPP M.J., JUGE-MORINEAU N., WIJDENES J., AMIOT M. Expression of CD28 and CD40 in human myeloma cells: a comparative study with normal plasma cells. Blood. 1994;84:2597–2603. [PubMed] [Google Scholar]
- SCHULTZE J., JOHNSON P. A stimulating new target for cancer immunotherapy. Lancet. 1999;354:1225–1227. doi: 10.1016/S0140-6736(99)90130-7. [DOI] [PubMed] [Google Scholar]
- STAMENKOVIC I., CLARK E.A., SEED B. A B-lymphocyte activation molecule related to the nerve growth factor receptor and induced by cytokines in carcinomas. EMBO J. 1989;8:1403–1410. doi: 10.1002/j.1460-2075.1989.tb03521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SZOCINSKI J.L., KHALED A.R., HIXON J., HALVERSON D., FUNAKOSHI S., FANSLOW W.C., BOYD A., TAUB D.D., DURUM S.K., SIEGALL C.B., LONGO D.L., MURPHY W.J. Activation-induced cell death of aggressive histology lymphomas by CD40 stimulation: induction of bax. Blood. 2002;100:217–223. doi: 10.1182/blood.v100.1.217. [DOI] [PubMed] [Google Scholar]
- TAI Y.T., LI X.F., CATLEY L., COFFEY R., BREITKREUTZ I., BAE J., SONG W., PODAR K., HIDESHIMA T., CHAUHAN D., SCHLOSSMAN R., RICHARDSON P., TREON S.P., GREWAL I.S., MUNSHI N.C., ANDERSON K.C. Mechanisms by which SGN-40, a humanized anti-CD40 antibody, induces cytotoxicity in human multiple myeloma cells: clinical implications. Cancer Res. 2004;64:2846–2852. doi: 10.1158/0008-5472.can-03-3630. [DOI] [PubMed] [Google Scholar]
- TONG A.W., STONE M.J. Prospects for CD40-directed experimental therapy of human cancer. Cancer Gene Ther. 2003;10:1–13. doi: 10.1038/sj.cgt.7700527. [DOI] [PubMed] [Google Scholar]
- VAN KOOTEN C., BANCHEREAU J. CD40–CD40 ligand. J. Leukocyte Biol. 2000;67:2–17. doi: 10.1002/jlb.67.1.2. [DOI] [PubMed] [Google Scholar]
- VESTAL R.E., WINGETT D.G., KNIGHT L.K.Expression of CD40 in breast, colon, lung, and ovarian tumors (1997) Proc. Am. Assoc. Cancer Res. 199738230(abstr 1550) [Google Scholar]
- VONDERHEIDE R.H., DUTCHER J.P., ANDERSON J.E., ECKHARDT S.G., STEPHANS K.F., RAZVILLAS B., GARL S., BUTINE M.D., PERRY V.P., ARMITAGE R.J., GHALIE R., CARON D.A., GRIBBEN J.G. Phase I study of recombinant human CD40 ligand in cancer patients. J. Clin. Oncol. 2001;19:3280–3287. doi: 10.1200/JCO.2001.19.13.3280. [DOI] [PubMed] [Google Scholar]
- WANG D., FREEMAN G.J., LEVINE H., RITZ J., ROBERTSON M.J. Role of the CD40 and CD95 (APO-1/Fas) antigens in the apoptosis of human B-cell malignancies. Br. J. Haematol. 1997;97:409–417. doi: 10.1046/j.1365-2141.1997.422688.x. [DOI] [PubMed] [Google Scholar]
- WINGETT D.G., VESTAL R.E., FORCIER K., HADJOKAS N., NIELSON C.P. CD40 is functionally expressed on human breast carcinomas: variable inducibility by cytokines and enhancement of Fas-mediated apoptosis. Breast Cancer Res. Treat. 1998;50:27–36. doi: 10.1023/a:1006012607452. [DOI] [PubMed] [Google Scholar]
- YOUNG L.S., ELIOPOULOS A.G., GALLAGHER N.J., DAWSON C.W. CD40 and epithelial cells: across the great divide (1998) Immunol. Today. 1998;19:502–506. doi: 10.1016/s0167-5699(98)01340-1. [DOI] [PubMed] [Google Scholar]