Abstract
We previously demonstrated that p-chloroamphetamine (PCA) intravenously (i.v.) evokes a specific patterned bursting response in the vas deferens nerve (VDN) of anaesthetised male rats that is associated with contraction of the vas deferens, and ejaculation and contraction of the bulbospongiosus muscles. The present study used selective 5-HT agonists to induce similar rhythmic bursting responses in the VDN in order to reveal the 5-HT receptor subtypes involved.
The 5-HT2C receptor agonist (1.0 mg kg−1 Ro600175 i.v.) evoked the characteristic bursting pattern responses in the VDN. The 5-HT1A receptor agonist (1.0 mg kg−1 8-OH-DPAT i.v.) failed to elicit any responses. However, 8-OH-DPAT coadministered in combination with Ro600175 induced a potentiation of the responses.
Responses were also evoked in rats with a mid-thoracic spinalisation, with a more predictable response being observed following the combination of agonists. This suggests an action of both agonists in the lumbosacral spinal cord.
Responses were blocked by 0.5 mg kg−1 SB206553 i.v. (5-HT2B/C receptor antagonist) or 0.5 mg kg−1 WAY100635 i.v. (5-HT1A receptor antagonist), but not 0.1 or 1.0 mg kg−1 SB269970 i.v. (5-HT7 receptor antagonist).
We suggest that activation of 5-HT2C and 5-HT1A receptor subtypes synergistically elicits contraction of the vas deferens through the activation of sympathetic preganglionic neurones in the spinal cord.
These data support the idea of a proejaculatory action of 5-HT2C receptors in the lumbosacral spinal cord, suggesting a descending 5-HT excitatory pathway in addition to a 5-HT inhibitory pathway. An excitatory action of 8-OH-DPAT at lumbosacral sites is also evident.
Keywords: 5-HT2C, 5-HT1A, ejaculation, Ro600175, 8-OH-DPAT, vas deferens
Introduction
We have previously described and characterised a series of responses associated with emission and ejaculation evoked by an intravenous administration of p-chloroamphetamine (PCA) (Stafford et al., 2003; 2006a). These in part consisted of a highly repeatable bursting pattern of intense sympathetic activity in the vas deferens nerve (VDN), a branch of the hypogastric nerve innervating the vas deferens. The responses were shown to be linked to emission and always associated with other ejaculatory events such as rhythmic contraction of the bulbospongiosus muscles and expulsion of seminal fluid, events occurring during sexual behaviour. These proejaculatory actions of PCA were not unexpected as several groups have reported that PCA given either intravenously (i.v.) or intraperitoneally can evoke the motor responses associated with sexual behaviour (Humphries et al., 1980; 1981; Rényi, 1985; Yonezawa et al., 2000). The novelty of our previous investigation was the characterisation of robust measurable responses in a sympathetic nerve that could be studied in an anaesthetised animal. In order to further investigate the central regulation of emission and ejaculation, we are interested in determining the neurotransmitters and receptor subtypes involved in generating this response. Here this model has now been used to address the question of how PCA could have induced the increased patterned activity in the VDN.
PCA interacts with 5-HT transporters, preventing reuptake of 5-HT into the axon terminal, and inhibits tryptophan hydroxylase, an enzyme used in the degradation of 5-HT (Gobbi et al., 2002), causing an acute rise in the extracellular concentration of 5-HT. It has been reported that at high concentrations PCA also causes the release of the catecholamines: noradrenaline and dopamine (Steranka & Sanders-Bush, 1977). We can therefore presume that the ejaculatory responses triggered by PCA may be as a result of activation of a certain receptor, or receptors, belonging to the 5-HT, dopaminergic or noradrenergic families.
However, several studies into the action of PCA-induced ejaculation have suggested its main influence is via a 5-HT effect (Humphries et al., 1980; Renyi, 1985; Yonezawa et al., 2000). Pretreatment of rats using p-chlorophenylalanine (PCPA), a 5-HT synthesis inhibitor, completely abolished ejaculations in both the conscious and urethane-anaesthetised rats (Humphries et al., 1980; Rényi, 1985; Yonezawa et al., 2000). Additionally, several 5-HT receptor antagonists (metitepine, methergoline, kentanserin and pirenperone) significantly reduced ejaculate weight (Rényi, 1985).
Our knowledge of the membrane receptor mediating the actions of 5-HT on the central pathways controlling ejaculation is presently unclear. On the one hand, a 5-HT2 receptor mediation is suggested by showing that 5-methoxy-dimethyltryptamine, a 5-HT2 receptor agonist evokes ejaculatory responses in the absence of PCA, with similar characteristics to the PCA-induced ejaculations (Renyi, 1986a, 1986b). 5-HT2 receptor activation has also been reported to cause seminal emission in ex-copula reflex tests (Mas et al., 1985). In addition to effects upon ejaculation, 5-HT2 receptors, in particular 5-HT2C receptors, have been implicated in the regulation and elicitation of erection in the rat (Steers & De Groat, 1989; Berendsen et al., 1990; Millan et al., 1997). Furthermore, it may be significant that m-chlorophenylpiperazine (a 5-HT2 agonist) induces responses in the cavernous nerve associated with erection (Steers & De Groat, 1989), which upon close inspection are very similar to the PCA responses in the VDN previously reported by us (Stafford et al., 2003; 2006a).
On the other hand, 5-HT1A receptor activation by 8-OH-DPAT significantly reduced ejaculatory latency during copulation and restored copulatory efficiency in castrated rats (Ahlenius et al., 1981). The facilitatory effects of 8-OH-DPAT on ejaculation in-copula were later shown to be blocked by pindolol (Ahlenius & Larsson, 1989), and by the more selective 5-HT1A receptor antagonists WAY100635 (Ahlenius & Larsson, 1998; Carro-Juárez & Rodríguez-Manzo, 2001) or NAD-299 (Hillegaart & Ahlenius, 1998). Administration of 8-OH-DPAT has also been shown to reverse coital reflex exhaustion ex-copula (Carro-Juárez & Rodríguez-Manzo, 2001) and decrease the ejaculation latency in ‘sluggish' ejaculators (Sura et al., 2001; Pattij et al., 2003). However, a 5-HT receptor effect of 8-OH-DPAT is not without question as it has been reported to increase dopaminergic transmission in the medial preoptic area of the brain by activation of D2-like receptors (Lorrain et al., 1998; Matuszewich et al., 1999; Clement et al., 2006), which we have recently shown can induce rhythmic activity in the VDN (Stafford & Coote, 2006b).
Nonetheless, 5-HT appears to be able to initiate or facilitate copulation and ejaculation via two 5-HT receptor subtypes. How this might be is so far not explained. It may be that the 5-HT receptor subtypes are on different neurones in the central sympathetic network controlling ejaculation as such studies do not reveal where in the CNS PCA or 5-HT agonists and antagonists are acting. Additionally, the ejaculatory response to agonists may be secondary to other components of sexual processes and the methods used to date would not reveal this. This critisism can be overcome by the use of the preparation we have described (Stafford et al., 2006a) in which recordings are made from the VDN in anaesthetised rats with intact CNS and in which the lumbosacral spinal cord can be isolated by transection. Furthermore, the preparation provides predictable precisely measurable responses. Therefore, in this investigation, experiments were performed using selective 5-HT receptor agonists and antagonists that mimic the responses to PCA, with the aim of clarifying the roles of different receptor subtypes in the emission phase of ejaculation in rats.
Methods
The methods used here are similar to those previously described for PCA experiments (Stafford et al., 2003; 2006a, 2006c) and are briefly described here.
Animal preparation
In a total of 48 male Wistar rats, weighing 280–320 g, anaesthesia was induced using gaseous 5% enflurane, 95% oxygen. The right femoral vein was cannulated using 0.96 mm (outside diameter (o.d.)) polythene tubing. Urethane was then administered as required to invoke and sustain deep anaesthesia (1.75–2.25 g kg−1 i.v.). The right femoral artery was cannulated using 0.96 mm (o.d.) polythene tubing filled with heparinised saline (20 U ml−1). The arterial cannula was connected to a pressure transducer (Capto SP 844, ADInstruments, Oxfordshire, U.K.), Bridge Amp and Powerlab (ADInstruments) to monitor and record blood pressure and heart rate using Chart v4.1.1 (ADInstruments). Blood pressure and heart rate of rats (except spinal rats, see below) before drug adminstration were 114±4 mmHg and 416±4 beats min−1. The trachea was intubated using a shortened 6F (yellow) luer cannula (Portexn Ltd, Kent, U.K.) to maintain a clear airway. The animal was placed on a heating blanket (Harvard Instruments), and temperature monitored using a rectal probe, to maintain temperature at 37°C.
A ventral midline incision was made from the base of the sternum as far as the corpus penis, to access the abdomen. The abdomen was held open by securing the abdominal wall to a metal oval frame. The colon, ileum, seminal vesicles and bladder were secured aside in order to clearly access the VDN. The nerve was dissected away from the surrounding tissue and mounted onto 0.35 mm silver bipolar hook electrodes and the abdominal cavity filled with warm (37°C) liquid paraffin to provide electrical isolation and prevent the nerve from drying out.
VDN activity was recorded, amplified (Neurolog, Digitimer Ltd, Welwyn Garden City, U.K.), filtered at 50 Hz low frequency and 3 kHz high frequency (Neurolog, Digitimer Ltd) and displayed on an oscilloscope (Gould, Ilford, U.K.). The signal was also passed through a Powerlab 8SP system (ADInstruments) for analysis on a Power Mac G4 using Chart v4.1.1 (ADInstruments). Raw nerve activity was converted to firing frequency using a window discriminator in the Chart software set to remove electrical noise, which was confirmed by nerve crush at the end of experiment. All recordings were conducted with the rat in the supine position. Animals were left undisturbed for around 1 h before the administration of compounds.
Animal groups
The following three groups of animals were prepared: the first to investigate the effect of 5-HT2C and 5-HT1A receptor activation in the CNS-intact rat, the second to confirm the receptor subtypes using antagonists, and the third to investigate the CNS site of action of the agonists.
Group 1a: Effect of 5-HT2C receptor agonists
In four animals, an administration of 1.0 mg kg−1 Ro600175 i.v. was given while recording nerve activity in the VDN. A period of 1 h after the initial dose was left before a subsequent administration of the same dose.
Group 1b: Effect of 5-HT1A receptor agonist
In four animals, 1.0 mg kg−1 8-OH-DPAT i.v. was administered while recording VDN activity. A second administration of the same dose was given 1 h after the initial administration.
Group 1c: Effect of combination of receptor agonists
A cocktail containing an equal concentration of 8-OH-DPAT and Ro600175 was prepared. In 12 animals, this cocktail was administered i.v. via the femoral vein. Doses (1.0 ml kg−1) containing either 0.1, 0.5 or 1.0 mg ml−1 of each agonist were administered to four separate animals while recording VDN activity. A period of 1 h after the initial dose was left before a subsequent administration of the combination of agonists at the same dose.
Group 2: Preadministration of antagonists
In each of four animals (12 in total), 0.5 mg kg−1 SB206553 (a 5-HT2B/C antagonist), 0.1 mg kg−1 WAY100635 (a 5-HT1A antagonist) or 0.1 mg kg−1 SB269970 (a 5-HT7 antagonist) was administered i.v., 2 min before the administration of the combination of 0.5 mg kg−1 8-OH-DPAT and 0.5 mg kg−1 Ro600175. Two hours later, the agonist combination was administered again but without further administration of the antagonists to test for recovery. The exception was rats given SB269970, in which the second cocktail dose was preceded by a higher dose of 1.0 mg kg−1 SB269970. VDN activity was recorded as for the other groups.
Group 3: Spinal animals
In 16 anaesthetised rats, following the initial placement of cannulae, a laminectomy and mid-thoracic (T8/9) complete spinal cord section was performed. Separation was ensured by placing small (∼1 mm) cotton wool balls between the cut ends. Blood pressure and heart rate during this period was 91±4 mmHg and 385±12 beats min−1. Two or more hours later, recordings of VDN activity were commenced and the experiment was then carried out as for the CNS-intact animals measuring VDN activity after a single dose of either 1.0 mg kg−1 8-OH-DPAT (n=4 rats), 1.0 mg kg−1 Ro600175 (n=4 rats), the combination of 0.5 mg kg−1 8-OH-DPAT and 0.5 mg kg−1 Ro600175 (n=4 rats) or the combination of 1.0 mg kg−1 8-OH-DPAT and 1.0 mg kg−1 Ro600175 (n=4 rats). A subsequent administration at the same dose was given 1 h after the initial administration.
All experiments conformed to the U.K. Animals Scientific Procedures Act 1986. Rats were killed at the end of the experiments using an overdose of anaesthetic.
Compounds
All compounds were obtained from Tocris (Bristol, U.K.), dissolved in physiological saline (0.9% (w v−1)) and administered i.v. into the femoral vein at a volume of 1.0 ml kg−1 and washed in with 0.1 ml saline.
Statistical analysis
Data are presented as mean±s.e.m. Comparisons were made at the 5% significance level using: a one-way ANOVA with Bonferroni's post hoc test for dose comparisons of group 1 animals, a paired t-test for comparisons between first and second administrations in all groups, and an unpaired t-test for other comparisons. Where no responses were obtained in any of the animals, comparisons of numbers of responses were made using a one-sample t-test to a hypothetical value of zero.
Results
Group 1a: Effect of 5-HT2C receptor agonist
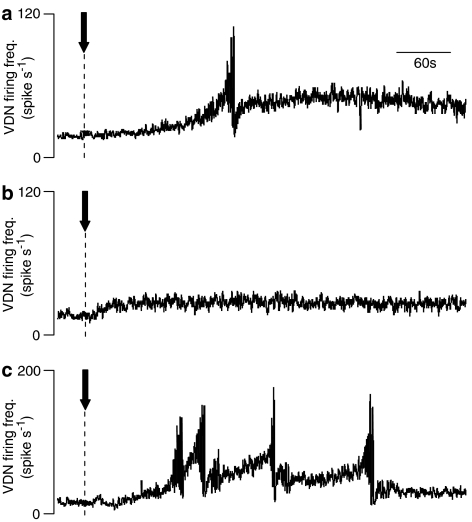
Following the initial preparation, the animal was left undisturbed for up to 1 h during which the baseline VDN activity remained stable. Administration of Ro600175 (1.0 mg kg−1 i.v.) caused a slow increase in baseline activity after a delay of 1–2 min, which culminated in a sequence of up to six intense synchronised bursts of activity, before decreasing rapidly to a tonic plateau level of 36±22 spikes s−1 above baseline (Figure 1a). In two rats, burst pattern responses in the VDN occurred following the first administration of Ro600175, while in one of these two rats and a third rat a response was observed following the second administration given 1 h after the first.
Figure 1.
Effects of 8-OH-DPAT and Ro600175 on VDN activity in CNS-intact rats. Traces show VDN firing frequency following administration of: (a) 1.0 mg kg−1 Ro600175 alone, (b) 1.0 mg kg−1 8-OH-DPAT alone and (c) 1.0 8-OH-DPAT and 1.0 mg kg−1 Ro600175 in combination. Within each response, one shown in (a) and four in (c), there are repetitive intense bursts of increased activity. Traces show recordings from separate rats. Compounds administered at time indicated by arrows.
The injection of the 5-HT2C receptor agonist caused an immediate rapid and transient increase in blood pressure of 33±4 mmHg and heart rate of 37±8 beats min−1, which were not associated with any alterations in VDN activity.
Group 1b: Effect of 5-HT1A receptor agonist
In four rats, administration of 8-OH-DPAT (1.0 mg kg−1 i.v.) increased tonic activity in VDN to around 13±3 spikes s−1 above baseline. However, in three animals neither a first or second administration of the agonist resulted in a typical sequence of synchronised bursting activity like that observed with Ro600175 (Figure 1b). In the fourth rat, two such responses were elicited but only after a second dose of 8-OH-DPAT.
The administration of 8-OH-DPAT caused a rapid and transient decrease of blood pressure of 34±4 mmHg and increase of heart rate of 67±20 beats min−1. These changes were not associated with any alterations in VDN activity.
Group 1c: Effect of combination of receptor agonists
Combining the agonists in a ‘cocktail' in doses of 0.1, 0.5 or 1.0 mg kg−1 for each agonist resulted in robust generation of repeated burst pattern responses (Figure 1c), so that the number of responses per drug treatment was markedly increased (Figure 2a). The mean number of responses to a cocktail of 1.0 mg kg−1 of each agonist was 5.0±0.9, which was significantly increased (P<0.001) compared to the effect of each agonist alone. Rhythmic contractions of the striated pelvic muscles (bulbospongiosus) could be observed simultaneously with responses in the VDN. Forceful emission of fluid from the penis was also seen.
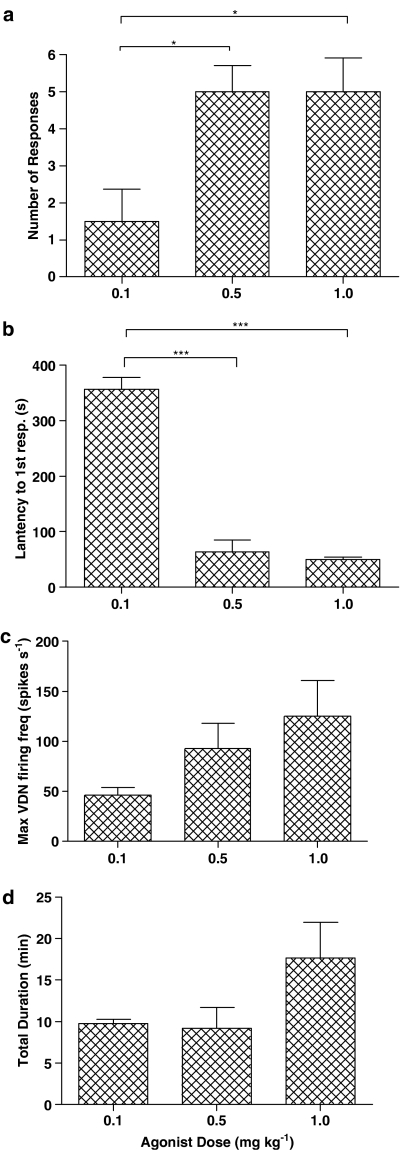
Figure 2.
Number of burst pattern responses elicited following administration of 5-HT receptor agonists and antagonists. (a) Effect of 1.0 mg kg−1 Ro600175, 1.0 mg kg−1 8-OH-DPAT or combination of both at 1.0 mg kg−1 each in the CNS-intact animal. (b) Effect of combination of agonists (0.5 mg kg−1 of each agonist) in the absence of antagonists (none) compared to the effect of agonist combination following preadministration of 0.1 mg kg−1 WAY100635, 0.5 SB206553 or 0.1 mg kg−1 SB269970. (c) Effect of spinal transection at T8/9 (open columns) on number of responses evoked by the combination at two doses (0.5 mg kg−1 or 1.0 mg kg−1) or single dose of either agonist at 1.0 mg kg−1 compared to the CNS-intact animal (filled columns). ***P<0.001 using the unpaired Student's t-test. n=4 for all groups.
The number of burst pattern responses was dose-dependent, significantly increasing from 1.5±0.9 to 5.0±0.7 and 5.0±0.9, respectively (P=0.0233; Figure 3a). Furthermore, the latency from administration to the responses decreased from 357±22 s at the lowest dose to 64±22 and 50±4 s, respectively at the higher doses (P<0.0001; Figure 3b). Also, postdrug VDN firing frequency increased above baseline from 5.1±0.6 to 21.2±8.5 and 50.8±20.8 spikes s−1 following each dose of cocktail however these increases did not reach statistical significance (P=0.0901). In addition to this, the average maximum burst frequency during ejaculatory-like responses also increased from 46.5±7.6 to 92.9±25.1 and 125.1±35.8 spikes s−1, but despite the clear trend they were not significant (P=0.3398; Figure 3c). Other measured variables of the responses remained unchanged between doses as follows: number of bursts of high-frequency activity within responses (3.0±0.1 bursts response−1; P=0.7454), frequency of bursts within responses (0.44±0.01 bursts s−1; P=0.7530) and total duration of the responses (P=0.2184; Figure 3d). A second administration of the agonist cocktail (at the same dose) evoked further responses, which remained unchanged from the first administration.
Figure 3.
Effect of agonist combination on characteristics of burst pattern responses. Effect of increasing doses of agonists on: (a) number of burst pattern responses after a single administration, (b) latency to initiation of burst pattern responses, measured as the time from administration to the first burst of the initial response, (c) mean maximum VDN firing frequency during synchronous bursts and (d) total duration of responses, measured as the time from administration to the end of the final PCA response. *P<0.05, **P<0.01, ***P<0.001. n=4 for all groups.
Blood pressure fluctuated (rises and falls) by approximately 10 mmHg over a period of up to 60 s immediately following the administration of the cocktail. Thereafter, it returned to baseline and remained stable during the period when bursts of activity appeared in the VDN.
Group 2: Preadministration of antagonists
5-HT2B/C antagonist
The combination of 8-OH-DPAT and Ro600175 (both at 0.5 mg kg−1) failed to elicit any burst pattern responses in the four animals that were pretreated with 0.5 mg kg−1 SB206553 (5-HT2B/C antagonist; Figure 2c; P=0.0058 compared to agonist combination alone). Responses returned following a subsequent administration of the agonist combination given 2 h later.
5-HT1A antagonist
Burst pattern responses were observed following the combination of agonists (0.5 mg kg−1 8-OH-DPAT and 0.5 mg kg−1 Ro600175) in three out of four animals pretreated with WAY100635 (0.1 mg kg−1; 5-HT1A antagonist). This reduced the mean number of burst pattern responses to 1.0±0.4 (Figure 2c; P=0.0027), compared to the animals given the cocktail alone.
5-HT7 antagonist
Several burst pattern responses were observed following the combination of agonists (0.5 mg kg−1 8-OH-DPAT and 0.5 mg kg−1 Ro600175) in all four animals pretreated with SB269970 (0.1 mg kg−1; 5-HT7 antagonist). A subsequent higher dose of SB269970 (1.0 mg kg−1) also failed to prevent the elicitation of burst pattern responses following a second dose of the cocktail. The mean number of responses was 5.75±0.85 after the low dose and 4.75±1.11 after the high dose of SB269970, which were not significantly different compared to the first or second administration of combination alone (P=0.5239 and P=0.0683 respectively).
Group 3: Spinal animals
In four rats given Ro600175 (1.0 mg kg−1 i.v.), VDN tonic activity showed an increase of 31±17 spikes s−1, but only in one rat was there a single burst pattern response, which occurred after the first administration (Figure 2b).
8-OH-DPAT (1.0 mg kg−1 i.v.) failed to elicit any increase in VDN activity or bursting pattern responses in any of the four spinal rats (Figure 2b).
In the group of four spinal rats given the middle dose of combination of the two agonists (0.5 mg kg−1 i.v. for both), there was an increase in baseline tonic activity. This was measured at 37±3 spikes s−1 following the first dose and 31±9 spikes s−1 after the second dose. However, burst pattern responses were only elicited by the combination of agonists in one of these rats. In this animal, one burst pattern response occurred after the first dose and two such responses after a second dose. Thus, significantly fewer responses were observed in the spinal rat than the CNS-intact rat at this dose (P=0.0007; Figure 2b).
In a further four spinal rats, the effect of a higher dose of Ro600175 and 8-OH-DPAT (both at 1.0 mg kg−1 i.v.) was studied. Burst pattern responses were observed after both doses of agonists in three out of four rats. This gave a mean of 2.0±1.1 responses following the first dose and 2.8±1.4 following the second (Figure 2b). This was not significantly different to the number obtained from the CNS-intact rats (P=0.0781).
The characteristics of the responses (i.e. number of synchronous bursts, frequency of synchronous bursts, maximum VDN firing frequency, latency to and duration of responses) elicited in the spinal rats were not significantly different to those observed in the CNS-intact rats at the same dose.
Discussion
These experiments on anaesthetised rats have shown that the 5-HT2C receptor agonist Ro600175 given intravenously can elicit burst pattern responses in the sympathetic nerve to the VDN. These intense bursts of nerve activity have all the characteristics of responses in the VDN that are elicited by PCA (i.v.), which we have previously shown are owing to an action in the central nervous system and strongly associated with contractions of the vas deferens, ejection of a seminal plug and rhythmic contraction of the bulbospongiosus muscles (Stafford et al., 2003; 2006a). It therefore seems reasonable to conclude that a 5-HT pathway in the CNS has an excitatory effect on sympathetic circuits in the CNS that are concerned with emission mediated by 5-HT2C-like receptors. As this effect is still present in the spinal rat, although less robust, there is a strong likelihood that the 5-HT2C agonist was mimicking that action of supraspinal 5-HT terminals synapsing directly on sympathetic preganglionic neurones projecting to the vas deferens and/or the spinal neuronal network controlling their excitability. A direct effect on the sympathetic neurones is supported as there is abundant evidence from extracellular and intracellular recordings that sympathetic preganglionic neurones are directly excited by 5-HT and this is via a 5-HT2-like receptor (McCall et al., 1987; Coote 1988; Ramage, 1988; Clement & McCall, 1990). However, the lowered probability of evoking the response in the spinal animal suggests this alone may not be sufficient to cause a relatively synchronised intense activation of the VDN neuronal population.
In the rat with intact CNS, the burst pattern response in the VDN became more robust, being more predictable and more numerous, when Ro600175 was combined with the 5-HT1A receptor agonist 8-OH-DPAT. This is not too unlike the effect on ejaculation of 8-OH-DPAT observed in-copula, which shows that activation of 5-HT1A receptors enhances the triggering of ejaculation induced by afferent stimulation (Ahlenius et al., 1981). Thus, together these studies support the idea that activation of 5-HT1A receptors can increase the excitability of neuronal networks involved in emission and ejaculation. The clear increase in predictability of evoking a burst pattern response in the spinal animal when 8-OH-DPAT was combined with Ro600175 suggests that part of this facilitatory action is occurring on intraspinal networks.
The effects of 5-HT2 receptor activation upon ejaculation have previously been disputed, with reports of inhibition (Foreman et al., 1989; Watson & Gorzalka, 1991) and facilitation of ejaculation (Mas et al., 1985). Ro600175 is a centrally active 5-HT2C agonist with over 150-fold selectivity over 5-HT2A and 5-HT2B receptors (pKi=8.8, 6.0 and 5.8, respectively) and over a thousand-fold selectivity over other 5-HT receptor subtypes (Martin et al., 1998). The robustness of the responses to Ro600175 at a dose of 1.0 mg kg−1 strongly indicates that 5-HT2C receptors were activated rather than any other 5-HT receptor subtype. This was confirmed by using SB206553, a centrally active 5-HT2B/C antagonist (Kennett et al., 1996), which completely abolishes the PCA-like responses. This present report is the first detailing the use of selective 5-HT2C receptor activation to investigate the control of emission. The results clearly demonstrate the participation of 5-HT2C receptors in an ex-copula model. Furthermore, it is evident that Ro600175 has an action upon lumbosacral 5-HT2C receptors as the response still occurs in the spinal animal.
Bancila et al. (1999) conducted immunocytochemical studies into the location of 5-HT2C receptors within the spinal cord, with particular reference to neurones involved in the control of penile erection. A high level of 5-HT2C-labelled neurones were identified in the dorsal horn neurones of L5-S1, in particular in areas that receive primary afferents from dorsal penile nerve. This suggests that activation of these receptors could modulate the transmission of sensory information from these primary afferents. It is possible that a proportion of the receptors identified by Bancila et al. (1999) also influence the processes involved in ejaculation in addition to erection, although in our study we did not test the ejaculatory response to sensory afferent stimulation.
8-OH-DPAT is a 5-HT1A receptor agonist with moderate affinity for 5-HT7 receptors (Middlemiss & Fozard, 1983; Plassat et al., 1993). In the experiments presented here antagonism of the responses was achieved using a moderate dose of WAY100635, a selective 5-HT1A antagonist, at a similar dose to that which has previously been demonstrated to antagonise 8-OH-DPAT-induced facilitation of ejaculation (Ahlenius & Larsson, 1997). The antagonism by WAY 100635 would appear to diminish the possibility that the effect of 8-OH-DPAT was mediated by supraspinal dopamine D2-like receptors, which we have recently shown can initiate the responses in the VDN (Stafford & Coote, 2006b). However, we cannot rule out the possibility for some of the enhancement by 8-OH-DPAT being mediated via supraspinal D2 receptors, as when it is given intracerebroventricularly, it elicits rhythmic contractions of the bulbospongiosus muscles, a marker of the expulsion phase of ejaculation (Clement et al., 2006).
It is possible that activation of 5-HT7 receptors contributes to the excitatory action of both 8-OH-DPAT and Ro600175, as both compounds do have affinity for 5-HT7 receptors (pKi=6.6 and 5.6, respectively). However, the selectivity of the agonists and antagonists used in these studies suggests that the effects are primarily mediated through 5-HT1A and 5-HT2C receptors. Nonetheless, the involvement of 5-HT7 receptors was directly investigated by using a preadministration of SB269970, a 5-HT7 antagonist. This compound failed to prevent the elicitation, or reduce the number, of PCA-like responses at the two doses tested, suggesting that 5-HT7 receptors are not involved in the generation of these responses. Therefore, we conclude that the effects of 8-OH-DPAT are primarily mediated via 5-HT1A receptors.
Complete antagonism of the generation of VDN burst responses is not achieved using WAY100635. This result is expected as administration of Ro600175 alone, in the absence of any antagonist, induces several responses. This suggests that the few VDN responses evoked by Ro600175 observed following pretreatment with WAY100635 are primarily a result of activation of 5-HT2C receptors. Furthermore, the results presented here demonstrate that activation of 5-HT1A receptors by administration of 8-OH-DPAT does not normally induce emission responses in VDN, but rather causes a synergistic facilitation of the excitatory effects of 5-HT2C activation by Ro600175 as described above. Indeed, antagonism of 5-HT2C receptors completely prevented the elicitation of any VDN burst responses. These data indicate the existence of an excitatory descending pathway acting on postsynaptic 5-HT2C receptors. Activation of such lumbosacral receptors causes an excitement of the neuronal network and if the trigger threshold is met, activation of a pattern generator for emission and possibly ejaculation.
Fewer responses were observed in the spinal animal than in the CNS-intact animal using the same dose of the combination of agonists. This may be owing to actions of one or both agonists at supraspinal sites, which are lost following spinal transection, in addition to the proposed lumbosacral actions. An alternative explanation is that a supraspinal excitatory reflex loop is lost following spinal transection, as previously suggested by Hubscher & Johnson (1999; 2000), and is well established to occur in the micturition reflex (de Groat et al., 1981).
We suggest that in the CNS-intact rat 8-OH-DPAT primarily suppresses the previously documented tonic serotonergic inhibitory pathway (Marson & McKenna, 1992; 1994) by activation of somatodendritic 5-HT1A autoreceptors. There is substantial evidence from previous investigations to support this. In the spinal animal, this tonic descending inhibition is no longer present as the pathways have been disrupted, so it would be expected that 8-OH-DPAT would no longer exert its facilitatory effects to the same degree. However, 8-OH-DPAT did have a facilitatory action in combination with Ro600175 in the spinal rat. This surprising result may be difficult to explain with current understanding. One possible explanation to consider is a 5-HT1A-mediated inhibition of glycine interneurones, which, at least in spinal cord slices in vitro, keep up a constant barrage of inhibitory post-synaptic potentials (IPSPs) on the membrane of sympathetic preganglionic neurones (Miyazaki et al., 1989; Lewis & Coote, 1990). An alternative explanation may be that, at the concentrations used, 8-OH-DPAT activates postsynaptic D2-like receptors in the spinal cord, although the effect of spinal dopamine receptor activation on sexual reflexes is currently unclear.
Summary
The anaesthetised rat model established previously by us to measure ejaculatory-like neural responses in a nerve to the vas deferens has been used to examine the activation of specific 5-HT receptors. The main findings of these studies are that activation of lumbosacral 5-HT2C receptors in both intact and spinal rats can evoke emission associated with ejaculatory-like responses indicative of sexual responses in the male rat. This suggests a descending excitatory role for 5-HT systems in addition to the well-documented inhibitory pathways. This 5-HT2C-mediated response is synergistically potentiated by coactivation of 5-HT1A receptors in CNS-intact rats. The new findings also suggest that part of this enhancement is at the level of a lumbosacral pattern generator.
Acknowledgments
The work presented in this paper was funded by the Biotechnology and Biological Sciences Research Council (BBSRC) and Pfizer Ltd.
Abbreviations
- o.d.
outside diameter
- PCA
p-chloroamphetamine
- PCPA
p-chlorophenylalanine
- VDN
vas deferens nerve
References
- AHLENIUS S., LARSSON K. Antagonism by pindolol, but not betaxolol, of 8-OH-DPAT-induced facilitation of male rat sexual behavior. J. Neural Transm. 1989;77:163–170. doi: 10.1007/BF01248929. [DOI] [PubMed] [Google Scholar]
- AHLENIUS S., LARSSON K. Specific involvement of central 5-HT1a receptors in the mediation of male rat ejaculatory behavior. Neurochem. Res. 1997;22:1065–1070. doi: 10.1023/a:1022443413745. [DOI] [PubMed] [Google Scholar]
- AHLENIUS S., LARSSON K. Evidence for an involvement of 5-HT1b receptors in the inhibition of male rat ejaculatory behavior produced by 5-HTP. Psychopharmacology (Berlin) 1998;137:374–382. doi: 10.1007/s002130050633. [DOI] [PubMed] [Google Scholar]
- AHLENIUS S., LARSSON K., SVENSSON L., HJORTH S., CARLSSON A., LINDBERG P., WIKSTROM H., SANCHEZ D., ARVIDSSON L.E., HACKSELL U., NILSSON J.L. Effects of a new type of 5-HT receptor agonist on male rat sexual behavior. Pharmacol. Biochem. Behav. 1981;15:785–792. doi: 10.1016/0091-3057(81)90023-x. [DOI] [PubMed] [Google Scholar]
- BANCILA M., VERGE D., RAMPIN O., BACKSTROM J.R., SANDERS-BUSH E., MCKENNA K.E., MARSON L., CALAS A., GIULIANO F. 5-Hydroxytryptamine2c receptors on spinal neurons controlling penile erection in the rat. Neuroscience. 1999;92:1523–1537. doi: 10.1016/s0306-4522(99)00082-2. [DOI] [PubMed] [Google Scholar]
- BERENDSEN H.H., BROEKKAMP C.L., VAN DELFT A.M. Antagonism of 8-OH-DPAT-induced behaviour in rats. Eur. J. Pharmacol. 1990;187:97–103. doi: 10.1016/0014-2999(90)90344-6. [DOI] [PubMed] [Google Scholar]
- CARRO-JUAREZ M., RODRIGUEZ-MANZO G. Exhaustion of the coital reflex in spinal male rats is reversed by the serotonergic agonist 8-OH-DPAT. Behav. Brain Res. 2001;118:161–168. doi: 10.1016/s0166-4328(00)00327-2. [DOI] [PubMed] [Google Scholar]
- CLEMENT M.E., MCCALL R.B. Studies on the site and mechanism of the sympathoexcitatory action of 5-HT2 agonists. Brain Res. 1990;515:299–302. doi: 10.1016/0006-8993(90)90610-n. [DOI] [PubMed] [Google Scholar]
- CLEMENT P., BERNABE J., KIA H.K., ALEXANDRE L., GIULIANO F. D2-like receptors mediate the expulsion phase of ejaculation by 8-hydroxy-2-(di-N-propylamino)tetralin in rats. J. Pharmacol. Exp. Therap. 2006;316:830–834. doi: 10.1124/jpet.105.092411. [DOI] [PubMed] [Google Scholar]
- COOTE J.H. The organisation of cardiovascular neurons in the spinal cord. Rev. Physiol. Biochem. Pharmacol. 1988;110:147–285. doi: 10.1007/BFb0027531. [DOI] [PubMed] [Google Scholar]
- DE GROAT W.C., NADELHAFT I., MILNE R.J., BOOTH A.M., MORGAN C., THOR K. Organization of the sacral parasympathetic reflex pathways to the urinary bladder and large intestine. J. Auton. Nerv. Syst. 1981;3:135–160. doi: 10.1016/0165-1838(81)90059-x. [DOI] [PubMed] [Google Scholar]
- FOREMAN M.M., HALL J.L., LOVE R.L. The role of the 5-HT2 receptor in the regulation of sexual performance of male rats. Life Sci. 1989;45:1263–1270. doi: 10.1016/0024-3205(89)90128-8. [DOI] [PubMed] [Google Scholar]
- GOBBI M., MOIA M., PIRONA L., CEGLIA I., REYES-PARADA M., SCORZA C., MENNINI T. P-methylthioamphetamine and 1-(m-chlorophenyl)piperazine, two non-neurotoxic 5-HT releasers in vivo, differ from neurotoxic amphetamine derivatives in their mode of action at 5-HT nerve endings in vitro. J. Neurochem. 2002;82:1435–1443. doi: 10.1046/j.1471-4159.2002.01073.x. [DOI] [PubMed] [Google Scholar]
- HILLEGAART V., AHLENIUS S. Facilitation and inhibition of male rat ejaculatory behaviour by the respective 5-HT1a and 5-HT1b receptor agonists 8-OH-DPAT and anpirtoline, as evidenced by use of the corresponding new and selective receptor antagonists nad-299 and nas-181. Br. J. Pharmacol. 1998;125:1733–1743. doi: 10.1038/sj.bjp.0702239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBSCHER C.H., JOHNSON R.D. Effects of acute and chronic midthoracic spinal cord injury on neural circuits for male sexual function. I. Ascending pathways. J. Neurophysiol. 1999;82:1381–1389. doi: 10.1152/jn.1999.82.3.1381. [DOI] [PubMed] [Google Scholar]
- HUBSCHER C.H., JOHNSON R.D. Effects of acute and chronic midthoracic spinal cord injury on neural circuits for male sexual function. Ii. Descending pathways. J. Neurophysiol. 2000;83:2508–2518. doi: 10.1152/jn.2000.83.5.2508. [DOI] [PubMed] [Google Scholar]
- HUMPHRIES C.R., O'BRIEN M., PAXINOS G. PCA: effects on ejaculation, thermoregulation, salivation, and irritability in rats. Pharmacol. Biochem. Behav. 1980;12:851–854. doi: 10.1016/0091-3057(80)90443-8. [DOI] [PubMed] [Google Scholar]
- HUMPHRIES C.R., PAXINOS G., O'BRIEN M. Mechanisms of PCA-induced hypothermia, ejaculation, salivation and irritability in rats. Pharmacol. Biochem. Behav. 1981;15:197–200. doi: 10.1016/0091-3057(81)90177-5. [DOI] [PubMed] [Google Scholar]
- KENNETT G.A., WOOD M.D., BRIGHT F., CILIA J., PIPER D.C., GAGER T., THOMAS D., BAXTER G.S., FORBES I.T., HAM P., BLACKBURN T.P. In vitro and in vivo profile of SB 206553, a potent 5-HT2c/5-HT2b receptor antagonist with anxiolytic-like properties. Br. J. Pharmacol. 1996;117:427–434. doi: 10.1111/j.1476-5381.1996.tb15208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LORRAIN D.S., MATUSZEWICH L., HULL E.M. 8-OH-DPAT influences extracellular levels of serotonin and dopamine in the medial preoptic area of male rats. Brain Res. 1998;790:217–223. doi: 10.1016/s0006-8993(98)00065-1. [DOI] [PubMed] [Google Scholar]
- LEWIS D.I., COOTE J.H. The influence of 5-hydroxytryptamine agonists and antagonists on identified sympathetic preganglionic neurones in the rat, in vivo. Br. J. Pharmacol. 1990;99:667–672. doi: 10.1111/j.1476-5381.1990.tb12987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARSON L., MCKENNA K.E. A role for 5-hydroxytryptamine in descending inhibition of spinal sexual reflexes. Exp. Brain Res. 1992;88:313–320. doi: 10.1007/BF02259106. [DOI] [PubMed] [Google Scholar]
- MARSON L., MCKENNA K.E. Serotonergic neurotoxic lesions facilitate male sexual reflexes. Pharmacol. Biochem. Behav. 1994;47:883–888. doi: 10.1016/0091-3057(94)90292-5. [DOI] [PubMed] [Google Scholar]
- MARTIN J.R., BOS M., JENCK F., MOREAU J., MUTEL V., SLEIGHT A.J., WICHMANN J., ANDREWS J.S., BERENDSEN H.H., BROEKKAMP C.L., RUIGT G.S., KOHLER C., DELFT A.M. 5-HT2c receptor agonists: pharmacological characteristics and therapeutic potential. J. Pharmacol. Exp. Ther. 1998;286:913–924. [PubMed] [Google Scholar]
- MAS M., ZAHRADNIK M.A., MARTINO V., DAVIDSON J.M. Stimulation of spinal serotonergic receptors facilitates seminal emission and suppresses penile erectile reflexes. Brain Res. 1985;342:128–134. doi: 10.1016/0006-8993(85)91360-5. [DOI] [PubMed] [Google Scholar]
- MATUSZEWICH L., LORRAIN D.S., TRUJILLO R., DOMINGUEZ J., PUTNAM S.K., HULL E.M. Partial antagonism of 8-OH-DPAT's effects on male sexual behaviour with a D2 but not a 5-HT1A, antagonist. Brain Res. 1999;820:55–62. doi: 10.1016/s0006-8993(98)01331-6. [DOI] [PubMed] [Google Scholar]
- MCCALL R.B., PATEL B.N., HARRIS L.T. Effects of serotonin1 and serotonin2 receptor agonists and antagonists on blood pressure, heart rate and sympathetic nerve activity. J. Pharmacol. Exp. Ther. 1987;242:1152–1159. [PubMed] [Google Scholar]
- MIDDLEMISS D.N., FOZARD J.R. 8-Hydroxy-2-(di-n-propylamino)-tetralin discriminates between subtypes of the 5-HT1 recognition site. Eur. J. Pharmacol. 1983;90:151–153. doi: 10.1016/0014-2999(83)90230-3. [DOI] [PubMed] [Google Scholar]
- MILLAN M.J., PEGLION J.L., LAVIELLE G., PERRIN-MONNEYRON S. 5-HT2c receptors mediate penile erections in rats: actions of novel and selective agonists and antagonists. Eur. J. Pharmacol. 1997;325:9–12. doi: 10.1016/s0014-2999(97)89962-1. [DOI] [PubMed] [Google Scholar]
- MIYAZAKI T., COOTE J.H., DUN N.J. Excitatory and inhibitory effects of epinephrine on neonatal rat sympathetic preganglionic neurons in vitro. Brain Res. 1989;497:108–116. doi: 10.1016/0006-8993(89)90976-1. [DOI] [PubMed] [Google Scholar]
- PATTIJ T., VEENING J.G., WALDINGER M.D., OLIVIER B., VAN DER GRAAF P.H. Developing preclinical models for studying ejaculation disorders. 2003.
- PLASSAT J.L., AMLAIKY N., HEN R. Molecular cloning of a mammalian serotonin receptor that activates adenylate cyclase. Mol. Pharmacol. 1993;44:229–236. [PubMed] [Google Scholar]
- RAMAGE A.G. Examination of the effects of some 5-ht2 receptor antagonists on central sympathetic outflow and blood pressure in anaesthetised cats. Naunyn Schmiedebergs Arch. Pharmacol. 1988;338:601–607. doi: 10.1007/BF00165623. [DOI] [PubMed] [Google Scholar]
- RENYI L. Ejaculations induced by p-chloroamphetamine in the rat. Neuropharmacology. 1985;24:697–704. doi: 10.1016/0028-3908(85)90001-2. [DOI] [PubMed] [Google Scholar]
- RENYI L. The effect of selective 5-hydroxytryptamine uptake inhibitors on 5-methoxy-n,n-dimethyltryptamine-induced ejaculation in the rat. Br. J. Pharmacol. 1986a;87:639–648. doi: 10.1111/j.1476-5381.1986.tb14580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RENYI L. The effects of monoamine oxidase inhibitors on the ejaculatory response induced by 5-methoxy-n,n-dimethyltryptamine in the rat. Br. J. Pharmacol. 1986b;88:827–835. doi: 10.1111/j.1476-5381.1986.tb16256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STAFFORD S.A., BOWERY N.G., COOTE J.H. p-Chloroamphetamine-induced activity in the vas deferens and associated sympathetic nerve activity in the anaesthetised rat. J. Physiol. 2003;522P:C83. [Google Scholar]
- STAFFORD S.A., BOWERY N.G., TANG K., COOTE J.H. Activation by p-Chloroamphetamine (PCA) of the spinal ejaculatory pattern generator in anaesthetized male rats. Neuroscience. 2006a;140:1031–1040. doi: 10.1016/j.neuroscience.2006.02.039. [DOI] [PubMed] [Google Scholar]
- STAFFORD S.A., COOTE J.H.Activation of D2-like receptors induces sympathetic climatic-like responses in male and female anaesthetized rats Br. J. Pharmacol. 2006b(in press) [DOI] [PMC free article] [PubMed]
- STAFFORD S.A., TANG K., COOTE J.H. Sympathetic genital responses induced by p-Chloroamphetamine (PCA) in anaesthetized female rats. Neuroscience. 2006c;138:725–732. doi: 10.1016/j.neuroscience.2005.11.044. [DOI] [PubMed] [Google Scholar]
- STEERS W.D., DE GROAT W.C. Effects of m-chlorophenylpiperazine on penile and bladder function in rats. Am. J. Physiol. 1989;257:R1441–R1449. doi: 10.1152/ajpregu.1989.257.6.R1441. [DOI] [PubMed] [Google Scholar]
- STERANKA L., SANDERS-BUSH E. Temporal effects of p-chloroamphetamine on catecholamine synthesis. Eur. J. Pharmacol. 1977;45:83–86. doi: 10.1016/0014-2999(77)90062-0. [DOI] [PubMed] [Google Scholar]
- SURA A., OVERSTREET D.H., MARSON L. Selectively bred male rat lines differ in naive and experienced sexual behavior. Physiol. Behav. 2001;72:13–20. doi: 10.1016/s0031-9384(00)00300-0. [DOI] [PubMed] [Google Scholar]
- WATSON N.V., GORZALKA B.B. Doi-induced inhibition of copulatory behavior in male rats: reversal by 5-HT2 antagonists. Pharmacol. Biochem. Behav. 1991;39:605–612. doi: 10.1016/0091-3057(91)90135-o. [DOI] [PubMed] [Google Scholar]
- YONEZAWA A., WATANABE C., ANDO R., FURUTA S., SAKURADA S., YOSHIMURA H., IWANAGA T., KIMURA Y. Characterization of p-chloroamphetamine-induced penile erection and ejaculation in anesthetized rats. Life Sci. 2000;67:3031–3039. doi: 10.1016/s0024-3205(00)00895-x. [DOI] [PubMed] [Google Scholar]