Abstract
Although there is increasing evidence showing that boswellic acid might be a potential anticancer agent, the mechanisms involved in its action are unclear.
In the present study, we showed that acetyl-keto-β-boswellic acid (AKBA) inhibited cellular growth in several colon cancer cell lines. Cell cycle analysis by flow cytometry showed that cells were arrested at the G1 phase after AKBA treatment.
Further analysis showed that cyclin D1 and E, CDK 2 and 4 and phosphorylated Rb were decreased in AKBA-treated cells while p21 expression was increased.
The growth inhibitory effect of AKBA was dependent on p21 but not p53. HCT-116 p53−/− cells were sensitized to the apoptotic effect of AKBA, suggesting that p21 may have protected cells against apoptosis by inducing a G1 arrest.
In conclusion, we have demonstrated that AKBA inhibited cellular growth in colon cancer cells. These findings may have implications to the use of boswellic acids as potential anticancer agents in colon cancer.
Keywords: Acetyl-keto-β-boswellic acid, cyclin, cyclin-dependent kinase, p21, p53, colon cancer, cell cycle
Introduction
The anti-inflammatory and anticancer effect of the Boswellias serrata plant has been documented from ancient times. The active constituents of the plant that have been shown to have anti-inflammatory and anticancer properties are the boswellic acids, which are pentacyclic triterpenic acids found in the gum resin (Han, 1994a, 1994b).
Boswellic acids have been used for the treatment of Crohn disease, ulcerative colitis, bronchial asthma, endotoxin-induced hepatitis and arthritis based on their anti-inflammatory effects (Sharma et al., 1989; Safayhi et al., 1991; Gupta et al., 1997; 1998; 2001; Gerhardt et al., 2001; Kiela et al., 2005). In recent years, the anticancer effect of boswellic acids started to draw attention. Extracts from the gum resin of Boswellias trees have been used clinically as a palliative therapy for the treatment of brain tumors (Janssen et al., 2000; Winking et al., 2000; Streffer et al., 2001). Although their direct anti-tumor effect has not been documented in clinical trials, results from in vitro studies showed that boswellic acids inhibited cellular proliferation and induced apoptosis in brain tumor (Glaser et al., 1999; Winking et al., 2000; Park et al., 2002), leukemia (Han, 1994a; Shao et al., 1998; Hoernlein et al., 1999; Jing et al., 1999), melanoma, hepatoma and prostate cancer cell lines (Liu et al., 2002a; Zhao et al., 2003; Syrovets et al., 2005).
The mechanism by which boswellic acids inhibit cell growth is still unclear. It has been reported that boswellic acids could inhibit the activity of topoisomerase, thus interfering with the DNA synthesis and replication in cancer cells. Boswellic acids also inhibited the NF-κB signalling pathway in prostate cancer cells, thus inhibiting cell proliferation and inducing apoptosis (Syrovets et al., 2005).
Acetyl-keto-β-boswellic acid (AKBA) is a novel, orally active, selective and non-redox 5-lipoxygenase inhibitor (Hoernlein et al., 1999; Park et al., 2002). In a previous study, we found that AKBA-induced apoptosis via a caspase-8 dependent pathway and was the most potent inducer of apoptosis in colon cancer cells among the four types of boswellic acids tested (Liu et al., 2002b). Here, we report that AKBA also inhibits the proliferation of colon cancer cells at a lower dose. Colon cancer cells treated with AKBA were arrested at G1 phase. The growth arrest after AKBA treatment was accompanied by the downregulation of G1 phase cyclins and cyclin-dependent kinases (CDKs). We also found that the G1 phase arrest induced by AKBA was dependent upon the expression of CDK inhibitor p21.
Methods
Materials
HT-29, HCT-116 and LS174T cells were purchased from the American Type Culture Collection (Rockville, MD, U.S.A.). P53−/− and p21−/− HCT-116 cells and isogenic wild-type parental cells were gifts from Dr Bert. Vogelstein (Howard Hughes Medical Institute, Chevy Chase, MD, U.S.A.). Fetal bovine serum (FBS), modified McCoy 5A medium, RPMI-1640 medium, crystal violet, propidium iodide and 3H-thymidine were from Sigma Aldrich (St Louis, MO, U.S.A.). Acetyl-keto-β-boswellic acid (AKBA) was purchased from ChromaDax Incorporation (reagent grade, purity >90%, Santa Ana, CA, U.S.A.). Rabbit polyclonal cyclin D1, cyclin E, phosphorylated Rb (pRb, phosphorylated at serine 795), cyclin-dependent kinase 2 (CDK2), cyclin-dependent kinase 4 (CDK4) antibodies and mouse monoclonal p21 and p53 antibodies were obtained from Santa Cruz (Santa Cruz, CA, U.S.A.). Mouse polyclonal glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody was from Chemicon International (Temacula, CA, U.S.A.). Annexin V staining kit and Cell Proliferation Reagent 4-[3-(4-lodophenyl)-2-(4-nitrophenyl)-2H-5-tetrazoliol]-1,3-benzene disulfonate (WST-1) were purchased from Roche Diagnostics (Mannheim, Germany).
Cell culture
HT-29, HCT-116, p53−/− and p21−/− HCT-116 cells were cultured in modified McCoy 5A medium supplemented with 10% FBS (v v−1). LS174T cells were cultured in RPMI-1640 medium supplemented with 5% FBS. The cells were maintained at 37°C in an incubator containing 5% CO2. AKBA was dissolved in ethanol as a 40 mM stock and stored at −20°C. For drug treatment, the cells were harvested with 0.05% trypsin/0.02% EDTA at about 80% confluence and were subcultured. After incubation overnight for attachment, the cells were treated with AKBA in different concentrations. Control cells were treated with 0.2% ethanol.
Cell viability and DNA synthesis assay
Cell viability was assayed by determination of the cleavage of tetrazodium salt (WST-1) to formazan by mitochondrial dehydrogenases. The amount of formazan formed is proportional to the viable cell numbers (Liu et al., 2002b). Briefly, cells were incubated with AKBA in different concentrations and WST-1 was then added at the end of drug treatment. After incubation with WST-1 for 0.5 h, the production of formazan was read at 405 nm against 620 nm as a background.
DNA synthesis was measured by 3H-thymidine incorporation. The cells were first incubated with AKBA in a six-well plate. At the end of drug treatment, 1 μCi of 3H-thymidine was added to each well (in 2 ml medium) and incubated with the cells for another 5 h. The cells were then washed with cold phosphate-buffered saline (PBS) three times and incubated with 10% trichloroacetic acid for 20 min at 4°C. After fixing with methanol for 10 min, the cells were lysed by addition of 800 μl lysis buffer containing 0.25 M sodium hydroxide and 0.1% sodium dodecyl sulfate. An aliquot of 100 μl of the lysate was taken for liquid scintillation counting.
Cell cycle analysis by flow cytometry
Cell cycle analysis using flow cytometry has been described previously (Liu et al., 2004). In brief, cells were treated with AKBA and trypsinized. After being resuspended in ice-cold 70% ethanol, they were stored at −20°C until analysis. The cellular DNA was stained with propidium iodide nuclear isolation medium (PBS containing 100 μg ml−1 propidium iodide, 0.6% Nonidet P-40 and 100 μg ml−1 RNase A) and flow cytometric analysis was performed in a BD Coulter flow cytometer. The cell cycle phase distribution was analyzed using the WINMDI software.
Apoptosis assay by annexin V staining
Apoptosis was also assayed by annexin V staining as described previously (Liu et al., 2004). Briefly, the cells were trypsinized after treatment with AKBA and washed once with cold PBS. The cells were resuspended in the staining solution containing annexin V and propidium iodide and incubated for 15 min at room temperature according to the manufacturer's instructions, followed by flow cytometric analysis. Cells with high annexin V but low propidium iodide staining were considered as apoptotic cells.
Western blot analysis
Cells were harvested with a rubber scraper and washed once with cold PBS. Proteins were extracted by suspending the cells in extraction buffer (50 mM Tris pH 7.0, 6 M urea, 1% (w v−1) SDS, 1% (v v−1) β-mercaptoethanol). Cells were sonicated for 20 s on ice, followed by centrifuge at 10,000 × g for 10 min at 4°C. Protein concentrations were quantified using bovine serum albumin as a standard.
Electrophoresis was performed using a 12% SDS/polyacrylamide gel except for pRb expression, where a 7% SDS/polyacrylamide gel was used. Proteins (30 μg) were loaded in each lane for Western blot analysis. The proteins were transferred to nitrocellulose membranes by electro-blotting. After washing and blocking, the membranes were incubated with primary antibodies in different concentrations for 1.5 h at room temperature, followed by incubation with secondary antibodies conjugated with horseradish peroxidase at room temperature for 1 h. After washing, the membrane was incubated with chemiluminescent substrate for 5 min and then exposed to Kodak Biomax MS film (Rochester, NY, U.S.A.).
To control for variations in loading, the expression of housekeeping gene GAPDH was also assayed by Western blot analysis. After incubation with primary and secondary antibodies, the membranes were stripped and reprobed with mouse anti-GAPDH polyclonal antibody (1 : 50,000 in PBS-T containing 5% non-fat milk) for 1 h, followed by incubation with goat anti-mouse secondary antibody conjugated with horseradish peroxidase (1 : 50,000) for 1 h.
Statistical analysis
Nonpaired Student's t-test was applied for the comparison of the mean values of two groups. One-way ANOVA was used to compare the multiple values in two groups and P<0.05 was considered as significant.
Results
AKBA inhibited cellular growth in a time- and dose-dependent manner in HCT-116 cells
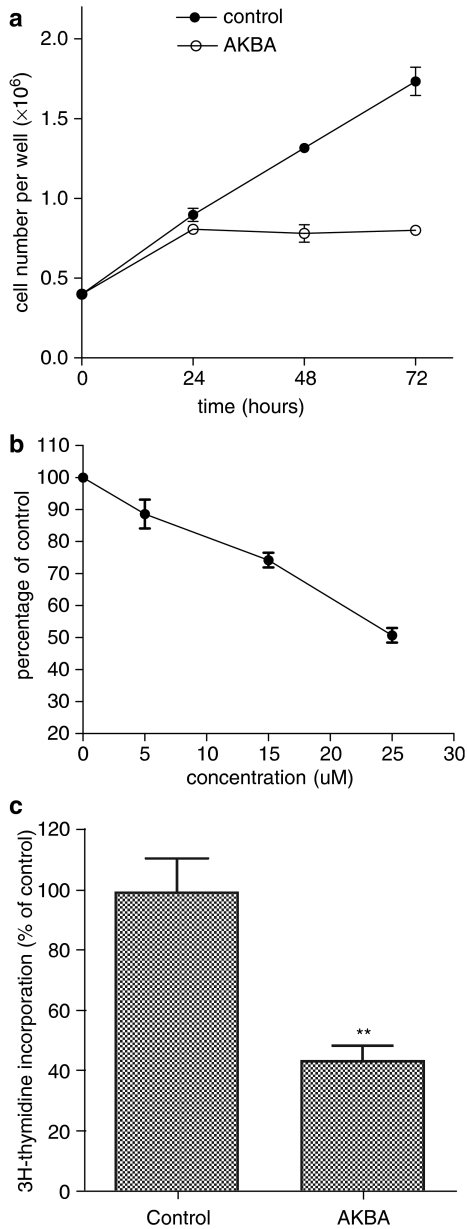
The changes in cellular growth after treatment with AKBA were assayed by monitoring cell numbers. AKBA inhibited the increase of cell numbers in HCT-116 cells as early as 24 h after drug treatment. There was no further increase in cell numbers 24 h after AKBA treatment, while the vehicle-treated controls increased in cell numbers linearly up to at least 72 h (Figure 1a, P<0.05). The effect of AKBA on cell viability was also determined. HCT-116 cells were treated with different concentrations of AKBA for 48 h. As shown in Figure 1b, cell viability was decreased in a dose dependent manner after AKBA treatment, compared to vehicle-treated controls. Cell viability was significantly reduced from as low as 5 μM of AKBA and reached 50% of controls with a concentration of 25 μM AKBA after 48 h of treatment.
Figure 1.
AKBA inhibited growth of HCT-116 cells. (a) Changes in cell numbers after treatment with AKBA. HCT-116 ls were treated with 20 μM AKBA for 24, 48 and 72 h and cell numbers was counted using a hemacytometer; (b) changes in cellular viability after treatment with different concentrations of AKBA. HCT-116 cells were treated with 0, 5, 15 and 25 μM AKBA for 48 h. Cell viability was assayed using WST-1; (c) Changes in DNA synthesis after treatment with AKBA. HCT-116 cells were treated with 20 μM AKBA for 48 h. DNA synthesis was assayed using the 3H-thymidine incorporation assay. Control cells were treated with 0.2% ethanol. Results are expressed as mean±s.d. and are representative of at least three separate experiments. **P<0.01 compared to control.
The growth inhibitory effect of AKBA was further confirmed by the 3H-thymidine incorporation assay. As shown in Figure 1c, cellular 3H-thymidine incorporation rate was decreased to about 45% of the control cells after treatment with 20 μM AKBA for 48 h (Figure 1c, P<0.01).
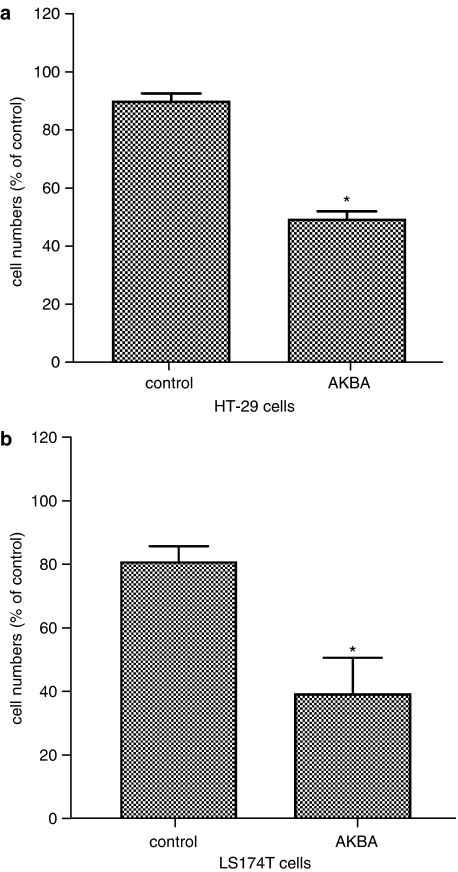
AKBA also inhibited cellular growth in other colon cancer cell lines
To determine whether AKBA has growth inhibitory effect in other colon cancer cells, HT-29 and LS174T cells were treated with 30 and 20 μM AKBA, respectively, for 72 h. The treatment resulted in a reduction of cell numbers to between 40 and 50% vehicle-treated controls in both HT-29 and LS174T cells (Figure 2a and b, P<0.05).
Figure 2.
AKBA inhibited growth of HT-29 and LS174T cells. HT-29 and LS174T cells were treated with 30 and 20 μM AKBA for 48 h, respectively. Control cells were treated with 0.2% ethanol. Cell numbers were counted using a hemacytometer. (a) Changes in cell numbers after treatment with AKBA in HT-29 cells; (b) changes in cell numbers after treatment with AKBA in LS174T cells. Results are shown as mean±s.d. *P<0.05 compared to control.
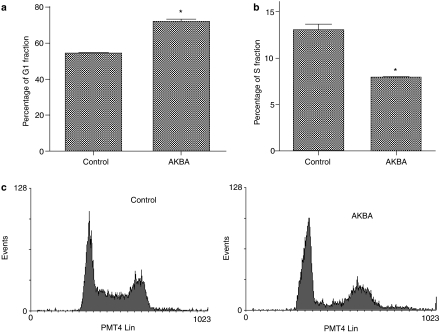
Colon cancer cells were arrested at G1 phase after treatment with AKBA
To determine the cell-cycle phase in which cells were arrested after treatment with AKBA, cellular DNA was stained by propidium iodide and analyzed by flow cytometry. Results showed that cells were arrested at the G1 phase after treatment with AKBA. The cells in G1 phase was increased from 55% to about 75% after AKBA treatment, while cells in the S phase was decreased from 13 to 8% (Figure 3a and b, P<0.05). The percentage of G2/M phase cells did not change significantly after treatment with AKBA compared to the control. As shown in Figure 3c, the percentage of sub-G1 cells did not change significantly in AKBA-treated cells, indicating that AKBA did not trigger extensive apoptosis at the dose used.
Figure 3.
AKBA caused a G1 arrest in HCT-116 cells. HCT1-116 cells were treated with 20 μM AKBA for 48 h. DNA was stained with propidium iodide and analyzed by flow cytometry. (a) Percentage of G1 phase cells in AKBA treated and control cells; (b) percentage of S phase cells in AKBA treated and control cells; (c) representative flow cytometric histogram in AKBA treated and control cells. Experiments were performed in triplicates. Results are expressed as mean±s.d. and are representative of at least three separate experiments. *P<0.05 compared to control.
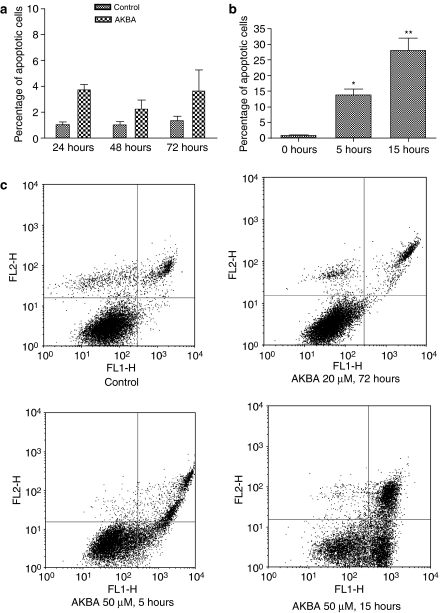
AKBA inhibited cellular growth without causing significant apoptosis at a lower dose
To confirm that AKBA did not trigger significant apoptosis at the dose used in the present study, apoptosis was also assayed by annexin V and propidium iodide staining. The percentage of apoptotic cells was increased only slightly after treatment with 20 μM AKBA for 24, 48 and 72 h in HCT-116 cells (Figure 4a and c). The cells were also treated with 50 μM AKBA as a positive control. As shown in Figure 4b and c, 50 μM AKBA induced significant apoptosis from as early as 5 h after drug treatment. At 15 h after treatment with 50 μM AKBA, the percentage of apoptotic cells was increased from 2% to about 30% (P<0.01 compared to vehicle treated control).
Figure 4.
Apoptosis assayed by annexin V and propidium iodide staining. (a) Percentage of apoptotic HCT-116 cells after treatment with 20 μM AKBA for 24, 48 and 72 h; (b) percentage of apoptotic HCT-116 cells after treatment with 50 μM AKBA for 5 and 15 h; (c) representative density plots of flow cytometric data. Results are expressed as mean±s.d. and are representative of at least three separate experiments. *P<0.05 compared to control. **P<0.01 compared to control.
Expression of G1 phase cyclins and CDKs was downregulated after treatment with AKBA
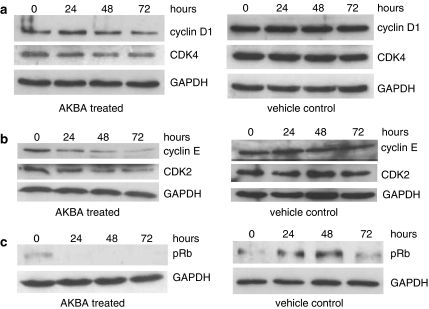
The expression of cyclins and CDKs involved in G1–S phase transition was assayed by Western blot to determine the mechanisms by which AKBA inhibited cell growth. The expression of cyclin D1 was downregulated 48 h after AKBA treatment while the expression of CDK4 was downregulated from as early as 24 h after AKBA treatment. At 72 h after drug treatment, the expression of cyclin D1 and CKD4 were decreased by 60 and 50%, respectively. The expression of cyclin D1 and CDK4 was unchanged in vehicle treated control cells (Figure 5a).
Figure 5.
AKBA regulated the expression of G1 phase cyclins and CKDs in HCT-116 cells. HCT-116 cells were treated with 20 μM AKBA for 0, 24, 48 and 72 h. The expression of cyclin D1, cyclin E, CDK2, CDK4 and phophorylated Rb (pRb) were assayed by Western blot analysis. GAPDH was used as a housekeeping control. (a) Expression of cyclin D1 and CDK4 after treatment with AKBA; (b) expression of cyclin E and CDK2 after treatment with AKBA; (c) expression of pRb after treatment with AKBA. Results shown are representative of at least three separate experiments.
Similarly, the expression of cyclin E and CDK2 was also downregulated significantly in AKBA treated cells. As shown in Figure 5b, the expression of cyclin E was decreased by 30, 50 and 80%, 24, 48 and 72 h after AKBA treatment, respectively, while the expression of CDK2 was decreased by 30, 50 and 60% after 24, 48 and 72 h drug treatment, respectively. Again, the expression of cyclin E and CDK2 was unchanged in vehicle treated control cells.
The downregulation of G1 phase cyclins and CDKs was accompanied by a significant reduction in phosphorylated Rb (pRb). At 24 h after AKBA treatment, the level of pRb was almost undetectable by Western blot analysis, while the level of phosphorylated Rb protein was maintained in vehicle treated control cells (Figure 5c).
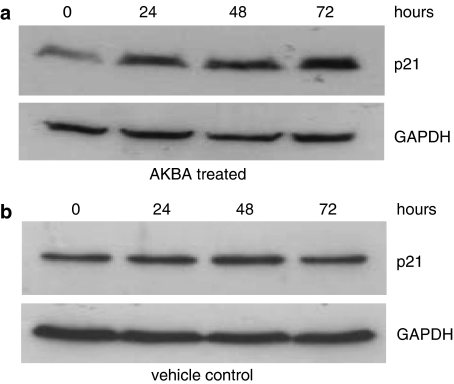
The expression of CDK inhibitor p21 was upregulated in AKBA-treated cells
In addition to the cyclins and CDKs, CDK inhibitors such as p21 are also important factors in the control of the G1–S transition. The expression of p21 was also determined in this study. As shown in Figure 6, the expression of p21 was increased after treatment with AKBA. The upregulation of p21 expression occurred from as early as 24 h after AKBA treatment and was increased between four and –five fold, 48 and 72 h after drug treatment (Figure 6a). As shown in Figure 6b, the expression of p21 did not change in vehicle-treated control cells at all the time points.
Figure 6.
Expression of p21 in AKBA treated cells. HCT-116 cells were treated with 20 μM AKBA for 0, 24, 48 and 72 h. The expression of p21 was assayed by Western blot analysis. GAPDH was used as a housekeeping control. (a) Expression of p21 in HCT-116 cells after treatment with AKBA; (b) expression of p21 in HCT-116 cells treated with vehicle control. Results shown are representative of at least three separate experiments.
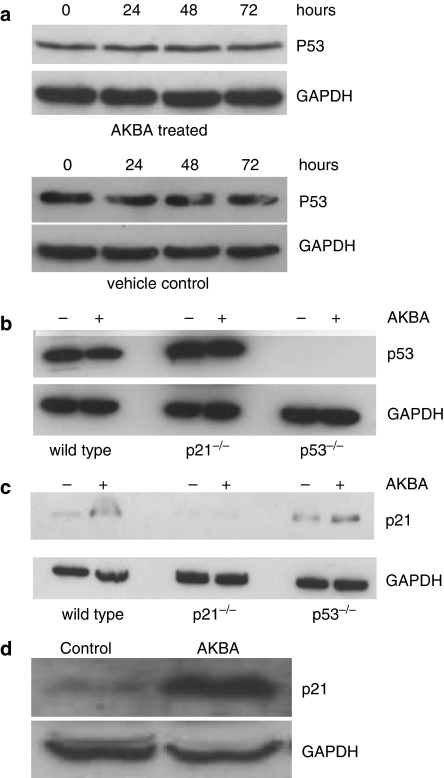
The upregulation of p21 was not dependent on p53
P53 is one of the most common transactivators of p21. In the present study, we also investigated whether the upregulation of p21induced by AKBA was due to p53 activation. We found that the expression of p53 in HCT-116 wild-type (Figure 7a and b) and p21−/− cells (Figure 7b) was unchanged after AKBA treatment.
Figure 7.
Expression of p21 and p53 in colon cancer cells after AKBA treatment. (a) Expression of p53 in HCT-116 cells treated with AKBA and vehicle control. HCT-116 cells were treated with 20 μM AKBA for 0, 24, 48 and 72 h. Expression of p53 was assayed by Western blot analysis; (b) expression of p53 in parental, p21−/− and p53−/− HCT-116 cells after treatment with AKBA. Cells were treated with 20 μM AKBA for 48 h. Expression of p53 was assayed by Western blot analysis. Control cells were treated with 0.2% ethanol; (c) changes in p21 expression in parental, p21−/− and p53−/− HCT-116 cells after treatment with AKBA. Cells were treated with 20 μM AKBA for 48 h. Expression of p21 was assayed by Western blot analysis; (d) changes in p21 expression in HT-29 cells after treatment with AKBA. HT-29 cells were treated with 30 μM AKBA for 72 h. Expression of p21 was assayed by Western blot analysis. Control cells were treated with 0.2% ethanol. Results shown are representative of three separate experiments. Expression of GAPDH was used as a housekeeping control.
To further study whether p21 upregulation induced by AKBA was dependent on p53, the regulation of p21expression was also studied in p53−/− HCT-116 cells. At 48 h after AKBA treatment, the expression of p21 was increased about 2.5-fold in p53−/− HCT-116 cells compared to the vehicle-treated control (Figure 7c). Furthermore, the expression of p21 was also determined in HT-29 colorectal cancer cells in which p53 was mutated. Results showed that AKBA treatment resulted in the upregulation of p21 in these cells as well. The expression of p21 was increased eight-fold compared to the vehicle-treated control 72 h after AKBA treatment (Figure 7d).
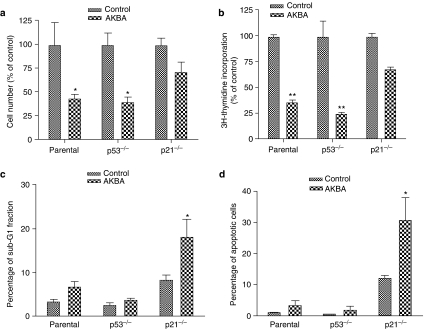
The growth inhibitory effect of AKBA was dependent on p21, not p53
To investigate whether the growth inhibitory effect of AKBA was dependent on p21, the cellular response to AKBA was studied in wild-type, p53−/− and p21−/− HCT-116 cells. The total cell numbers were decreased about 45% compared to vehicle-treated control in both wild type and p53−/− HCT-116 cells 48 h after treatment with 20 μM AKBA while in p21−/− HCT-116 cells, the cell number was decreased only 25% compared to control after treatment with the same concentration of AKBA (Figure 8a). Similar results were observed in DNA synthesis rate as assayed by 3H-thymidine incorporation. As shown in Figure 8b, the 3H-thymidine incorporation rate was decreased about 65 and 75% compared to vehicle-treated control cells in both wild type and p53−/− HCT-116 cells (P<0.01), while in p21−/− HCT-116 cells, the 3H-thymidine incorporation rate was only decreased about 30% compared to the vehicle-treated control.
Figure 8.
Effect of AKBA on growth and apoptosis in parental, p21−/− and p53−/− HCT-116 cells. Cells were treated with 20 μM AKBA for 48 h. Control cells were treated with 0.1% ethanol. The number of cells was counted using a hemacytometer. DNA synthesis was assayed by 3H-thymidine incorporation. Apoptosis was assayed by both cell cycle analysis and annexin V staining. (a) Changes in cell numbers after treatment with AKBA in parental, p21−/− and p53−/− HCT-116 cells; (b) changes in DNA synthesis in parental, p21−/− and p53−/− HCT-116 cells after treatment with AKBA; (c) changes in sub-G1 fraction after treatment with AKBA in parental, p21−/− and p53−/− HCT-116 cells; (d) changes in apoptotic rate assayed by annexin V and propidium iodide double staining in parental, p21−/− and p53−/− HCT-116 cells. Results shown are representative of three separate experiments. *P<0.05 compared to control. **P<0.01 compared to control.
Further studies indicated that the slight decrease in cell number and DNA synthesis after AKBA treatment in p21−/− cells could be accounted for, at least in large part, by an increase in apoptosis in these cells. The percentage of apoptotic cells increased significantly in p21−/− HCT-116 cells compared to the parental and p53−/− cells after 48 h of treatment with 20 μM AKBA (Figure 8c, P<0.05). The percentage of apoptotic cells was also determined by annexin V and propidium iodide double staining. As shown in Figure 8d, the percentage of apoptotic cells in the wild type and p53−/− HCT-116 cells was only increased slightly after treatment with 20 μM AKBA, while it was increased from 12 to 30% in p21−/− HCT-116 cells treated with the same concentration of AKBA (P<0.05).
The percentage of apoptotic cells in the vehicle ethanol-treated p21−/− control cells was also increased compared to the parental counterpart, mainly due to the increased cytotoxicity of ethanol in the absence of p21.
Discussion
There is an increasing interest in the use of boswellic acid as a potential anticancer agent in colon cancer (Huang et al., 2000; Liu et al., 2002b). Our previous data had shown that boswellic acid induced apoptosis in a caspase-8 dependent pathway in colon cancer cells. The present data showed that at a lower dose, boswellic acid can also inhibit the growth of colon cancer cells. The growth inhibitory effect of AKBA might, at least in part, explain the chemo-preventive properties of boswellic acid observed in mice where it inhibited the formation of azoxymethane-induced aberrant crypt foci (Huang et al., 2000).
We showed that AKBA exerted its growth inhibitory effect by inhibiting cell proliferation, evidenced by a decrease in 3H-thymidine incorporation in cells treated with AKBA. We further showed that AKBA treatment arrested the cancer cells at the G1 phase of the cells cycle. The G1 phase of the cell cycle is tightly regulated by the synthesis and degradation of cyclins, cyclin-dependent kinases (CDK) and CDK inhibitors (McGowan, 2003). The G1 phase regulators include cyclin D, cyclin E, CDK2 and CDK4. The synthesis of the D-type cyclins begins at the G0/G1 transition. Cyclin D then activates CDK4 and drives the cell from early G1 to late G1, largely by the regulation of the tumor suppressor retinoblastoma (Rb) protein. (Morris & Dyson, 2001). The initial phosphorylation of Rb leads to transcription of cyclin E. The increase in cyclin E levels lead to the activation of CDK2 which then completes the phosphorylation of Rb. Complete phosphorylation of Rb releases E2F from inhibition by Rb to activate genes required to drive cells through the S transition (Payton & Coats, 2002). The present study showed that AKBA significantly downregulated the expression of G1 phase cyclins (cyclin D1 and cyclin E) and CDKs (CDKs 2 and 4) in colon cancer cells. The downregulation of these proteins was consistent with the G1 arrest, we observed after AKBA treatment. We speculate that the downregulation of the cyclins and CDKs resulted in a decrease in phosphorylation of Rb which prevented the activation of E2F-dependent genes required for cell cycle.
The finding that AKBA regulated the expression of multiple G1 cyclins, CDKs and Rb phosphorylation might have clinical implications. Mutations in cell cycle regulators are a common feature of human tumors (Temple et al., 1980; Caputi et al., 2005). Further, components of the cell cycle have frequently been investigated as targets for cancer therapy and a variety of inhibitors of the CDKs have been studied (Malumbres & Barbacid, 2001).
Concomitant with the downregulation of cyclins and CDKs, AKBA also caused an upregulation of the CDK inhibitor p21, which also played an important role in control of cell cycle progression by opposing the activities of CDKs (Hunter & Pines, 1994; Harper & Elledge, 1996). p21 has been shown to be dysregulated in cancers and is hypothesized to function as a tumor suppressor. It is often inversely correlated to tumor proliferation and progression and several oncogenes have been shown to repress the expression of p21 to promote tumor growth (Gartel & Radhakrishnan, 2005). Our results showing the upregulation of p21 by AKBA suggests that the G1 arrest may be mediated at least in part by the upregulation of p21.
Interestingly, although the cycle arrest induced by AKBA was dependent on p21, it was independent of p53. The significant reduction in cell numbers and cell proliferation (evident by a reduction in 3H-thymidine incorporation) was also observed in the p53−/− HCT-116 cells.
The mechanism by which AKBA increased p21 levels is currently unclear. Although p53 has been shown to be a potent transactivator of p21 gene expression, our results indicate that the upregulation of p21 by AKBA was likely mediated by a p53-independent mechanism. This was derived from several lines of evidences. Firstly, the upregulation of p21 in HCT-116 cells was not associated with an upregulation of p53. Secondly, p21 expression was also upregulated in p53−/− HCT-116 cells. Finally, p21 was also upregulated in HT-29 colon cancer cells where p53 was mutated. A variety of transcription factors that are induced by a number of different signaling pathways activate p21 transcription by p53-independent mechanisms, including Sp1, Sp3, Ap2, STATs, C/EBPα, C/EBPβ and the bHLH proteins BETA2 and MyoD. In addition, p21 expression may also be regulated post-transcriptionally by both ubiquitin-dependent and -independent proteasome-mediated degradation (Gartel & Tyner, 2002). The mechanism by which AKBA regulates p21 expression requires further investigation.
The functional significance of the upregulation of p21 induced by AKBA is unclear. Whereas induction of p21 expression may lead cell cycle arrest and decreased cell proliferation (Gartel & Radhakrishnan, 2005), p21 may also function as an antiapoptotic factor. This was evidenced by the increase in apoptosis observed in p21 knockout cells compared to wild type HCT-116 cells. It is likely that HCT-116 cells lacking p21 were unable to arrest cells at G1, thus resulting in a greater percentage of cells being driven to the apoptotic pathway. Similar results have been reported recently where CDK inhibitors, like p21, have been shown to influence the sensitivity to proapoptotic signals in DNA damage-induced cancer cells (Le et al., 2005). We speculate that p21 functions as a switch between apoptosis and growth arrest in the cellular response to AKBA treatment. In the presence of wild-type functional p21, AKBA causes an upregulation of p21 and growth arrest. In the absence of p21, cancer cells are sensitized to apoptotic signals.
In summary, we have shown that AKBA has significant effects on cell cycle progress in colon cancer cells at lower doses than what had previously been used. Further, we show that the G1 phase arrest induced by AKBA was dependent on p21 but not p53 and was associated with a downregulation of G1 cyclins and CKDs.
Acknowledgments
This work was supported in part by the National University of Singapore Academic Research Fund R-185-000-079-112 and R-185-000-087-112.
Abbreviations
- AKBA
acetyl-keto-β-boswellic acid
- CDK
cyclin-dependent kinase
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- pRb
phosphorylated retinoblastoma protein
References
- CAPUTI M., RUSSO G., ESPOSITO V., MANCINI A., GIORDANO A. Role of cell-cycle regulators in lung cancer. J. Cell Physiol. 2005;205:319–327. doi: 10.1002/jcp.20424. [DOI] [PubMed] [Google Scholar]
- GARTEL A.L., RADHAKRISHNAN S.K. Lost in transcription: p21 repression, mechanisms, and consequences. Cancer Res. 2005;65:3980–3985. doi: 10.1158/0008-5472.CAN-04-3995. [DOI] [PubMed] [Google Scholar]
- GARTEL A.L., TYNER A.L. The role of the cyclin-dependent kinase inhibitor p21 in apoptosis. Mol. Cancer Ther. 2002;1:639–649. [PubMed] [Google Scholar]
- GERHARDT H., SEIFERT F., BUVARI P., VOGELSANG H., REPGES R. Therapy of active Crohn disease with Boswellia serrata extract H 15] Z. Gastroenterol. 2001;39:11–17. doi: 10.1055/s-2001-10708. [DOI] [PubMed] [Google Scholar]
- GLASER T., WINTER S., GROSCURTH P., SAFAYHI H., SAILER E.R., AMMON H.P., SCHABET M., WELLER M. Boswellic acids and malignant glioma: induction of apoptosis but no modulation of drug sensitivity. Br. J. Cancer. 1999;80:756–765. doi: 10.1038/sj.bjc.6690419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUPTA I., GUPTA V., PARIHAR A., GUPTA S., LUDTKE R., SAFAYHI H., AMMON H.P. Effects of Boswellia serrata gum resin in patients with bronchial asthma: results of a double-blind, placebo-controlled, 6-week clinical study. Eur. J. Med. Res. 1998;3:511–514. [PubMed] [Google Scholar]
- GUPTA I., PARIHAR A., MALHOTRA P., GUPTA S., LUDTKE R., SAFAYHI H., AMMON H.P. Effects of gum resin of Boswellia serrata in patients with chronic colitis. Planta. Med. 2001;67:391–395. doi: 10.1055/s-2001-15802. [DOI] [PubMed] [Google Scholar]
- GUPTA I., PARIHAR A., MALHOTRA P., SINGH G.B., LUDTKE R., SAFAYHI H., AMMON H.P. Effects of Boswellia serrata gum resin in patients with ulcerative colitis. Eur. J. Med. Res. 1997;2:37–43. [PubMed] [Google Scholar]
- HAN R. Highlight on the studies of anticancer drugs derived from plants in China. Stem. Cells. 1994a;12:53–63. doi: 10.1002/stem.5530120110. [DOI] [PubMed] [Google Scholar]
- HAN R. Recent progress in the study of anticancer drugs originating from plants and traditional medicines in China. Chin. Med. Sci. J. 1994b;9:61–69. [PubMed] [Google Scholar]
- HARPER J.W., ELLEDGE S.J. Cdk inhibitors in development and cancer. Curr. Opin. Genet. Dev. 1996;6:56–64. doi: 10.1016/s0959-437x(96)90011-8. [DOI] [PubMed] [Google Scholar]
- HOERNLEIN R.F., ORLIKOWSKY T., ZEHRER C., NIETHAMMER D., SAILER E.R., SIMMET T., DANNECKER G.E., AMMON H.P. Acetyl-11-keto-beta-boswellic acid induces apoptosis in HL-60 and CCRF-CEM cells and inhibits topoisomerase I. J. Pharmacol. Exp. Ther. 1999;288:613–619. [PubMed] [Google Scholar]
- HUANG M.T., BADMAEV V., DING Y., LIU Y., XIE J.G., HO C.T. Anti-tumor and anti-carcinogenic activities of triterpenoid, beta-boswellic acid. Biofactors. 2000;13:225–230. doi: 10.1002/biof.5520130135. [DOI] [PubMed] [Google Scholar]
- HUNTER T., PINES J. Cyclins and cancer. II: Cyclin D and CDK inhibitors come of age. Cell. 1994;79:573–582. doi: 10.1016/0092-8674(94)90543-6. [DOI] [PubMed] [Google Scholar]
- JANSSEN G., BODE U., BREU H., DOHRN B., ENGELBRECHT V., GOBEL U. Boswellic acids in the palliative therapy of children with progressive or relapsed brain tumors. Klin. Padiatr. 2000;212:189–195. doi: 10.1055/s-2000-9676. [DOI] [PubMed] [Google Scholar]
- JING Y., NAKAJO S., XIA L., NAKAYA K., FANG Q., WAXMAN S., HAN R. Boswellic acid acetate induces differentiation and apoptosis in leukemia cell lines. Leuk. Res. 1999;23:43–50. doi: 10.1016/s0145-2126(98)00096-4. [DOI] [PubMed] [Google Scholar]
- KIELA P.R., MIDURA A.J., KUSCUOGLU N., JOLAD S.D., SOLYOM A.M., BESSELSEN D.G., TIMMERMANN B.N., GHISHAN F.K. Effects of Boswellia serrata in mouse models of chemically induced colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2005;288:G798–G808. doi: 10.1152/ajpgi.00433.2004. [DOI] [PubMed] [Google Scholar]
- LE H.V., MINN A.J., MASSAGUE J. Cyclin-dependent kinase inhibitors uncouple cell cycle progression from mitochondrial apoptotic functions in DNA-damaged cancer cells. J. Biol. Chem. 2005;280:32018–32025. doi: 10.1074/jbc.M504689200. [DOI] [PubMed] [Google Scholar]
- LIU J.J., NILSSON A., OREDSSON S., BADMAEV V., DUAN R.D. Keto- and acetyl-keto-boswellic acids inhibit proliferation and induce apoptosis in Hep G2 cells via a caspase-8 dependent pathway. Int. J. Mol. Med. 2002a;10:501–505. [PubMed] [Google Scholar]
- LIU J.J., NILSSON A., OREDSSON S., BADMAEV V., ZHAO W.Z., DUAN R.D. Boswellic acids trigger apoptosis via a pathway dependent on caspase-8 activation but independent on Fas/Fas ligand interaction in colon cancer HT-29 cells. Carcinogenesis. 2002b;23:2087–2093. doi: 10.1093/carcin/23.12.2087. [DOI] [PubMed] [Google Scholar]
- LIU J.J., ZHANG J., RAMANAN S., JULIAN J., CARSON D.D., HOOI S.C. Heparin/heparan sulfate interacting protein plays a role in apoptosis induced by anticancer drugs. Carcinogenesis. 2004;25:873–879. doi: 10.1093/carcin/bgh081. [DOI] [PubMed] [Google Scholar]
- MALUMBRES M., BARBACID M. To cycle or not to cycle: a critical decision in cancer. Nat. Rev. Cancer. 2001;1:222–231. doi: 10.1038/35106065. [DOI] [PubMed] [Google Scholar]
- MCGOWAN C.H. Regulation of the eukaryotic cell cycle. Prog. Cell. Cycle Res. 2003;5:1–4. [PubMed] [Google Scholar]
- MORRIS E.J., DYSON N.J. Retinoblastoma protein partners. Adv. Cancer Res. 2001;82:1–54. doi: 10.1016/s0065-230x(01)82001-7. [DOI] [PubMed] [Google Scholar]
- PARK Y.S., LEE J.H., HARWALKAR J.A., BONDAR J., SAFAYHI H., GOLUBIC M. Acetyl-11-keto-beta-boswellic acid (AKBA) is cytotoxic for meningioma cells and inhibits phosphorylation of the extracellular-signal regulated kinase 1 and 2. Adv. Exp. Med. Biol. 2002;507:387–393. doi: 10.1007/978-1-4615-0193-0_60. [DOI] [PubMed] [Google Scholar]
- PAYTON M., COATS S. Cyclin E2, the cycle continues. Int. J. Biochem. Cell. Biol. 2002;34:315–320. doi: 10.1016/s1357-2725(01)00137-6. [DOI] [PubMed] [Google Scholar]
- SAFAYHI H., MACK T., AMMON H.P. Protection by boswellic acids against galactosamine/endotoxin-induced hepatitis in mice. Biochem. Pharmacol. 1991;41:1536–1537. doi: 10.1016/0006-2952(91)90575-p. [DOI] [PubMed] [Google Scholar]
- SHAO Y., HO C.T., CHIN C.K., BADMAEV V., MA W., HUANG M.T. Inhibitory activity of boswellic acids from Boswellia serrata against human leukemia HL-60 cells in culture. Planta. Med. 1998;64:328–331. doi: 10.1055/s-2006-957444. [DOI] [PubMed] [Google Scholar]
- SHARMA M.L., BANI S., SINGH G.B. Anti-arthritic activity of boswellic acids in bovine serum albumin (BSA)-induced arthritis. Int. J. Immunopharmacol. 1989;11:647–652. doi: 10.1016/0192-0561(89)90150-1. [DOI] [PubMed] [Google Scholar]
- STREFFER J.R., BITZER M., SCHABET M., DICHGANS J., WELLER M. Response of radiochemotherapy-associated cerebral edema to a phytotherapeutic agent, H15. Neurology. 2001;56:1219–1221. doi: 10.1212/wnl.56.9.1219. [DOI] [PubMed] [Google Scholar]
- SYROVETS T., GSCHWEND J.E., BUCHELE B., LAUMONNIER Y., ZUGMAIER W., GENZE F., SIMMET T. Inhibition of IkappaB kinase activity by acetyl-boswellic acids promotes apoptosis in androgen-independent PC-3 prostate cancer cells in vitro and in vivo. J. Biol. Chem. 2005;280:6170–6180. doi: 10.1074/jbc.M409477200. [DOI] [PubMed] [Google Scholar]
- TEMPLE W.J., SUGARBAKER E.V., THORNTHWAITE J.T., HENSLEY G.T., KETCHAM A.S. Correlation of cell cycle analysis with Duke's staging in colon cancer patients. J. Surg. Res. 1980;28:314–318. doi: 10.1016/0022-4804(80)90091-8. [DOI] [PubMed] [Google Scholar]
- WINKING M., SARIKAYA S., RAHMANIAN A., JODICKE A., BOKER D.K. Boswellic acids inhibit glioma growth: a new treatment option. J. Neurooncol. 2000;46:97–103. doi: 10.1023/a:1006387010528. [DOI] [PubMed] [Google Scholar]
- ZHAO W., ENTSCHLADEN F., LIU H., NIGGEMANN B., FANG Q., ZAENKER K.S., HAN R. Boswellic acid acetate induces differentiation and apoptosis in highly metastatic melanoma and fibrosarcoma cells. Cancer Detect. Prev. 2003;27:67–75. doi: 10.1016/s0361-090x(02)00170-8. [DOI] [PubMed] [Google Scholar]