Abstract
Interleukin-2 (IL-2) has proinflammatory properties that limit its therapeutic use. Its side effects are mainly explained by the induction of a vascular leakage syndrome. Cytokines, as TNF-α and IL-1β, and nitric oxide (NO) generated by IL-2-activated leukocytes play a role in this defect.
As the systemic release of these mediators inhibits neutrophil migration to a specific inflammatory site, we investigated now whether IL-2 administrated systemically inhibits the neutrophil recruitment to the inflamed peritoneum. The involvement of NO in the process was also addressed.
Using peritoneal neutrophils, we show that the intravenous treatment of the mice with IL-2 inhibits the neutrophil migration induced by carrageenin, LPS or fMLP. In confirmation, IL-2-treated mice showed a significant reduction in leukocyte rolling and adhesion in mesenteric microcirculation evaluated after carrageenin, LPS and fMLP injections. Aminoguanidine prevented the inhibitory effect of IL-2 on carrageenin-induced neutrophil migration, rolling and adhesion. In contrast, IL-2 failed to reduce the lung leukocyte infiltration induced by LPS. Therefore, IL-2 inhibition of neutrophil migration is organ specific.
Our results indicate that IL-2 administered systemically inhibits neutrophil recruitment to some inflammatory sites through a mechanism dependent on NO. The results also reinforce the needs to determine the mechanism by which patients treated with IL-2 show increased risks of infection.
Keywords: Interleukin-2, neutrophil migration, nitric oxide, vascular leakage syndrome
Introduction
The cytokine IL-2 is crucial for the maintenance of immune homeostasis and exhibits proinflammatory activities (Theze et al., 1996; Becknell & Caligiuri, 2005; Sinkovics & Horvath, 2005). IL-2 also stimulates the production of TNF-α, IFN-γ and GM-CSF (Wallays & Ceuppens, 1993; De Sanctis et al., 1997). Therapeutic use of IL-2 in oncology (renal cell carcinoma and melanoma) (Philip, 1998; Schrader et al., 2005) and in patients with HIV infection (Smith, 2001; David et al., 2001; 2002; Natarajan et al., 2002; Terao et al., 2005) is limited by its dose-dependent toxicity, which is associated with vascular leak syndrome (VLS). VLS is characterized by an increased vascular permeability and decreased microcirculatory perfusion, leading to interstitial edema and eventually to multiple organ failure within 2–24 h after IL-2 administration (Baluna & Vitetta, 1997; Carey et al., 1997; Locker et al., 1999). Increased circulating cytokines, such as TNF-α, IL-1, IL-6 and IFN-γ, are observed during rIL-2 infusion in human (Baluna & Vitetta, 1997), and monoclonal antibodies to TNF-α reduce VLS (Fraker et al., 1989). We and others have demonstrated that natural killer cells, lymphocytes and neutrophils are critical for IL-2-induced VLS (Lentsch et al., 1999; Assier et al., 2004). Moreover, studies demonstrated that IL-2-induced VLS is mediated by nitric oxide (NO), as inhibitors of NO synthase ameliorate VLS (Orucevic & Lala, 1996a, 1996b; Orucevic et al., 1997; Lentsch et al., 1998).
During the course of our studies on sepsis, we suggested that excessive systemic release of proinflammatory cytokines/chemokines, including TNF-α and IL-8, and concomitant increase in NO derived from inducible nitric oxide synthase (iNOS) leads to the paralysis of neutrophil migration to the infectious focus (Benjamim et al., 2000; 2002; Crosara-Alberto et al., 2002; Alves-Filho et al., 2005). Later, we demonstrated that the intravenous (i.v.) administration of TNF-α or IL-8 inhibits the neutrophil migration through the activation of L-arginine/NOS/NO mechanism (Tavares-Murta et al., 1998). Neutrophil paralysis and reduction of rolling/adhesion found in severe sepsis were not observed in iNOS-deficient mice nor in mice treated with aminoguanidine (amino), a selective iNOS inhibitor (Benjamim et al., 2000; 2002). Thus, although the production of cytokines, chemokines and NO in the infectious site is fundamental to control the infection, as they induce leukocyte migration and activation, the overproduction of these mediators systemically might be involved in the failure of neutrophil migration to the infection focus and in the deleterious pathophysiology found in sepsis.
During IL-2 therapy, an increased incidence and severity of bacterial infections is observed, especially in patients requiring i.v. catheters (Bock et al., 1990; Snydman et al., 1990). These findings prompted us to investigate whether IL-2 inhibits the neutrophil ability to migrate to inflammatory site. Accordingly, we studied whether IL-2 administrated systemically inhibits the neutrophil recruitment to an inflammatory site and the possibility that NO participates in the process. We also compared the ability of IL-2 to reduce neutrophil migration to the peritoneal cavity and neutrophil sequestration to the lung.
Methods
Mice
C57BL/6 mice (weight 20–22 g) obtained from the animal facilities of the School of Medicine of Ribeirão Preto were housed in cages in temperature-controlled rooms and received food and water ad libitum. All experiments were conduced in accordance with local ethical guidelines.
Drugs
The drugs used in this study were IL-2 (Aldesleukin; Chiron, Amsterdam, The Netherlands). Lipopolysacharide (LPS), N-formyl-methionyl-leucyl-phenylalanine (fMLP), carrageenin (Cg), amino, Evans Blue, 3,3′,5,5′-tetramethylbenzidine (TMB) and exadecyltrimethylammonium bromide (H-TAB) (Sigma, St Louis, MO, U.S.A.).
Determination of serum nitrite concentration
The nitrate concentration in serum samples was determined by enzymatic reduction of nitrate with nitrate reductase followed by nitrite determination using the Griess method (Green et al., 1982).
Protocols
Effects of the i.v. administration of IL-2 on neutrophil migration into the peritoneal cavity
Mice were pretreated i.v. with the vehicle (saline; 0.1 ml) or IL-2 (30–1000 ng mouse−1), and 15 min later, they were injected intraperitoneally (i.p.) with PBS (0.2 ml, control) or Cg (500 μg mouse−1). Mice were also pretreated i.v. with saline (0.1 ml) or IL-2 (300 ng mouse−1), and 15 min later, they were injected i.p. with PBS (0.2 ml), LPS (200 ng mouse−1) or fMLP (100 nmol mouse−1). To evaluate the neutrophil migration into the peritoneal cavities, the mice were killed 4 h after the inflammatory stimuli, and the cells were harvested with 3.0 ml of PBS containing 1 mM EDTA. Total counts were performed with a cell counter (Coulter AC T series analyzer; Coulter Corp., Miami, FL, U.S.A.), and differential cell counts were carried out on cytocentrifuge slides (Cytospin 3; Shandon Southern Products, Astmoore, U.K.) stained by the May–Grünwald–Giemsa (Rosenfeld) method. The results are expressed as the number of neutrophils per cavity.
Effects of the i.v. administration of IL-2 on leukocyte rolling and adhesion
The mice were pretreated i.v. with saline (0.1 ml) or IL-2 (300 ng), and 15 min later, they were injected i.p. with PBS (0.2 ml), Cg (500 μg), LPS (200 ng) or fMLP (100 nmol). The rolling and adhesion of leukocytes were evaluated 2 h later in the mesentery microcirculation by intravital microscopy, as described (Fortes et al., 1991; Benjamim et al., 2002). Briefly, mice were anesthetized with tribromoethanol (250 mg kg−1) and the mesenteric tissues were withdrawn for microscopic examination. The mice were maintained on a special board thermostatically controlled at 37°C, with a transparent platform on which the tissue to be transilluminated was placed. The preparation was kept moist and warm by irrigating the tissue with warmed (37°C) Ringer Locke's solution, pH 7.2–7.4, containing 1% gelatin. A 500-line television camera was incorporated onto a triocular Zeiss microscope to facilitate observation of the enlarged image ( × 3400) on the video screen. Images were recorded on a video recorder with a long-distance objective ( × 40) with a 0.65 numerical aperture. Vessels selected for study were third-order venules, defined according to their branch-order location within the microvascular network. These vessels correspond to postcapillary venules, with a diameter of 12–18 μm. Rolling leukocytes were defined as those white blood cells that moved at a lower velocity than erythrocytes in the same stream. The number of rolling leukocytes was determined at 10-min intervals. These leukocytes moved at a sufficiently slow pace as to be individually visible and were counted as they rolled past a 100 μm length of venule. A leukocyte was considered to be adherent onto the venular endothelium if it remained stationary for >30 s. The number of adherent cells was expressed as the number per 100 μm2 of venule.
Effect of inhibition of iNOS by amino on the inhibitory effects of IL-2 upon neutrophil migration, rolling and adhesion
The mice were pretreated subcutaneous (s.c.) with saline (0.2 ml) or amino (50 mg kg−1) (Benjamim et al., 2000; Secco et al., 2003), and 15 min later, they were injected with saline or IL-2 (300 ng) i.v. After 15 min, PBS or Cg (500 μg) was injected i.p. and neutrophil migration and leukocyte rolling and adhesion were determined as described above.
Effect of IL-2 injected i.v. on LPS-induced lung leukocyte sequestration
The leukocyte sequestration in the lung tissues was measured from total myeloperoxidase (MPO) activity as described previously with slight modification (Souza et al., 2001). Mice were injected i.v. with saline (0.1 ml) or IL-2 (300 ng), and 15 min later, they were injected i.v. again with LPS (200 ng). The mice were killed 4 h later and the lungs were isolated and immediately flushed with 5 ml of saline to wash the blood. Then, lung tissues (50–100 mg) were homogenized in two volumes of ice-cold buffer (0.1 M NaCl, 20 mM NaPO4, 15 mM Na EDTA), pH 4.7, and centrifuged at 3000 r.p.m. for 15 min. The pellets were then subjected to hypotonic lysis (900 μl of 0.2% NaCl solution followed 30 s later by the addition of an equal volume of a solution containing 1.6% NaCl and 5% glucose). After a further centrifugation, the pellets were resuspended in 200 μl of 50 mM NaPO4 buffer, pH 5.4, containing 0.5% H-TAB. The homogenates were then frozen and thawed twice and centrifuged again at 10,000 r.p.m. for 15 min at 4°C. MPO activity in the resuspended pellet was assayed by measuring the change in absorbance at 450 nm using TMB (1.6 mM) and H2O2 (0.5 mM). The results were reported as the number of neutrophils in 100 mg of lung tissue by comparing the absorbance of the tissue supernatants with the absorbance of mouse peritoneal neutrophils processed in the same way. To this end, neutrophil migration was induced in the peritoneum of mice by injecting Cg (300 μg mouse−1). A standard curve relating a known neutrophil numbers with the absorbance was obtained by processing purified neutrophils as described above and assaying for MPO activity.
Statistical analysis
The data are reported as the means±standard errors of the means (s.e.m.) of values obtained from two different experiments. The means of different treatments were compared by analysis of variance (ANOVA). If significance was determined, individual comparisons were subsequently tested with Bonferroni's t-test for unpaired values. A P-value of 0.05 or less was considered significant.
Results
IL-2 injected i.v. inhibits the neutrophil migration to the peritoneal cavity induced by Cg, LPS and fMLP
Firstly, we examined the effect of IL-2 on neutrophil migration to the peritoneal cavity induced by Cg (500 μg), LPS (200 ng) and fMLP (100 nM). As more neutrophils migrate to the peritoneal cavity 4 h after Cg, LPS or fMLP injections (data not shown), the experiments were carried on at this time.
Mice injected i.p. with the inflammatory stimuli in the absence of IL-2 presented after 4 h a marked neutrophil migration in the peritoneal cavity. The i.v. pretreatment of the mice with IL-2 dose-dependently (30–1000 ng mouse−1) inhibited the neutrophil migration induced by Cg (Figure 1a). Moreover, IL-2 at 300 ng also inhibited the neutrophil migration induced by LPS and fMLP (Figure 1b).
Figure 1.
IL-2 injected i.v. inhibits the neutrophil migration to the peritoneal cavity induced by Cg, LPS and fMLP. (a) The mice were pretreated i.v. with saline (Sal, 0.1 ml) or IL-2 (30–1000 ng 0.1 ml−1), and 15 min later, they were injected i.p. with Cg (500 μg 0.2 ml−1). (b) The mice were pretreated i.v. with saline (Sal, 0.1 ml) or IL-2 (300 ng 0.1 ml−1), and 15 min later, they were injected i.p. with LPS (200 ng 0.2 ml−1) or fMLP (100 nmol 0.2 ml−1). The neutrophil migration was evaluated 4 h later. First bars in both panels represent the neutrophil migration induced by PBS injected i.p. The results are means±s.e.m. of neutrophils per cavity (n=5). *P<0.05 compared with PBS-injected group; #P<0.05 compared with Sal-treated group. Analysis of variation followed by Bonferroni t-test.
IL-2 injected i.v. inhibits the leukocyte rolling and adhesion induced by Cg, LPS and fMLP
As neutrophil migration depends on leukocyte rolling and adhesion on the endothelial surface, we investigated these parameters in mesenteric vessels in control and IL-2-pretreated mice injected with Cg (Figure 2), LPS (Figure 3) and fMLP (Figure 4). As the neutrophil migration to the peritoneal cavity is optimal 4 h after the inflammatory stimuli, we evaluated the rolling and adhesion after 2 h, in order to observe the maximum effects. The mice were pretreated with saline (0.1 ml, i.v.) or IL-2 (300 ng), and 10 min later, they were injected i.p. with Cg (500 μg), LPS (200 ng) or fMLP (100 nmol).
Figure 2.
IL-2 injected i.v. inhibits the leukocyte rolling and adhesion induced by Cg. The mice were pretreated i.v. with saline (Sal, 0.1 ml) or IL-2 (300 ng 0.1 ml−1), and 15 min later, they were injected i.p. with Cg (500 μg 0.2 ml−1). The rolling and adhesion of leukocyte were evaluated 2 h after in the mesentery microcirculation by intravital microscopy (n=5: 2 venules mice−1). First bars in both panels represent the rolling and adhesion of leukocyte induced by PBS injected i.p, respectively. The results are means±s.e.m. of rolling or adhered leukocyte. *P<0.05 compared with PBS-injected i.p. groups. #P<0.05 compared with Sal-treated groups. Analysis of variation followed by Bonferroni t-test.
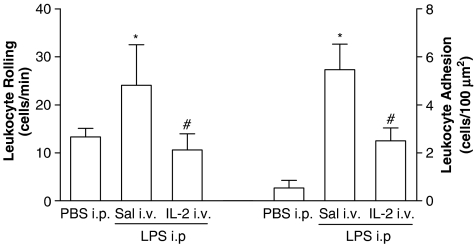
Figure 3.
IL-2 injected i.v. inhibits the leukocyte rolling and adhesion induced by LPS. The mice were pretreated i.v. with saline (Sal, 0.1 ml) or IL-2 (300 ng 0.1 ml−1), and 15 min later, they were injected i.p. with LPS (200 ng 0.2 ml−1). The rolling and adhesion of leukocyte were evaluated 2 h after in the mesentery microcirculation by intravital microscopy (n=5; 2 venules mice−1). First bars in both panels represent the rolling and adhesion of leukocyte induced by PBS injected i.p, respectively. The results are means±s.e.m. of rolling or adhered leukocyte. *P<0.05 compared with PBS-injected i.p. groups. #P<0.05 compared with Sal-treated groups. Analysis of variation followed by Bonferroni t-test.
Figure 4.
IL-2 injected i.v. inhibits the leukocyte rolling and adhesion induced by fMLP. The mice were pretreated i.v. with saline (Sal, 0.1 ml) or IL-2 (300 ng 0.1 ml−1), and 15 min later, they were injected i.p. with fMLP (100 nmol 0.2 ml−1). The rolling and adhesion of leukocyte were evaluated 2 h after in the mesentery microcirculation by intravital microscopy (n=5; 2 venules mice−1). First bars in both panels represent the rolling and adhesion of leukocyte induced by PBS injected i.p, respectively. The results are means±s.e.m. of rolling or adhered leukocyte. *P<0.05 compared with PBS-injected i.p. groups. #P<0.05 compared with Sal-treated groups. Analysis of variation followed by Bonferroni t-test.
Confirming the findings with neutrophil migration, the control mice injected i.p. with Cg, LPS or fMLP in the absence of IL-2 presented a marked leukocyte rolling and adhesion onto the mesenteric microcirculation. On the other hand, IL-2-pretreated mice showed a significant reduction in leukocyte rolling and consequently adhesion onto the mesenteric microcirculation evaluated 2 h after Cg, LPS and fMLP. Thus, inhibition by IL-2 of neutrophil migration correlates with the reduction of leukocyte rolling and adhesion to the microcirculation endothelium.
Inhibition of NO production prevents the suppression by IL-2 of Cg-induced rolling, adhesion and neutrophil migration
Previous studies from our group (Tavares-Murta et al., 1998; Benjamim et al., 2000; Secco et al., 2003) demonstrated that NO downmodulates the neutrophil migration into inflammatory sites. This led us to investigate the participation of NO in the inhibitory effect of IL-2 on neutrophil migration induced by Cg. The mice were injected with vehicle (0.2 ml s.c.) or amino (50 mg kg−1), a selective inhibitor of inducible NO synthase (Misko et al., 1993), and 15 min later, they were injected with saline (0.1 ml) or IL-2 (300 ng) i.v. After 15 min, Cg (500 μg) was injected i.p. and neutrophil migration was determined 6 h later. Amino prevented inhibition by IL-2 of neutrophil migration to inflammatory site induced by Cg (Figure 5a). In order to evaluate the role of NO on the IL-2-induced reduction of leukocyte rolling and adhesion, the mice were injected with saline or amino and 15 min later with saline (0.1 ml) or IL-2 (300 ng) i.v. After 15 min, Cg (500 μg) was injected i.p. and the rolling and adhesion of leukocyte were evaluated 2 h later in the mesentery microcirculation by intravital microscopy. Inhibition of NO production also prevented the reduction of leukocyte rolling and adhesion induced by Cg in IL-2-pretreated mice (Figure 5b). These data suggest that the inhibitory effects of IL-2 on neutrophil migration are mediated by NO, via the induction of iNOS, although we could not exclude the possibility that others isofoms have been affected by amino at dose used. To confirm these findings, we determined whether IL-2 treatment induces NO production. Thus, we assayed nitrite concentration in serum from saline- (0.1 ml) or IL-2- (300 ng) treated mice and observed that IL-2 caused a significant elevation of nitrite levels 6 h after administration (saline: 26.20±2.80 and IL-2: 58.70±5.80).
Figure 5.
Inhibition of NO production prevents the suppression by IL-2 of Cg-induced rolling, adhesion and neutrophil migration. (a) The mice were injected s.c. with saline (0.2 ml) or amino (50 mg kg−1), and 15 min later, they were injected i.v. with saline (Sal) or IL-2 (300 ng 0.1 ml−1). After 15 min, Cg (500 μg mouse−1) was injected i.p. and neutrophil migration was determined 6 h later. The results are means±s.e.m. of neutrophils per cavity (n=5). *P<0.05 compared with Sal-injected i.v. group; #P<0.05 compared with IL-2 i.v. group. (b) The mice were injected s.c. with saline or amino (50 mg kg−1), and 15 min later, they were injected i.v. with saline or IL-2 (300 ng 0.1 ml−1). After 15 min, Cg (500 μg mouse−1) was injected i.p. and the rolling and adhesion of leukocyte were evaluated 2 h later in the mesentery microcirculation by intravital microscopy (n=5; 2 venules mice−1). The results are means±s.e.m. of rolling or adhered leukocyte. *P<0.05 compared with Sal-injected i.v. group; #P<0.05 compared with IL-2 i.v. group. Analysis of variation followed by Bonferroni t-test.
IL-2 injected i.v. fails to inhibit LPS-induced lung neutrophil sequestration
In order to determine if the inhibition of LPS-induced lung vascular protein leakage induced by IL-2 correlates with the reduction of neutrophil migration, we evaluated the neutrophil sequestration to the lungs through MPO activity. The mice received i.v. saline (0.1 ml) or IL-2 (300 ng), and 15 min later, LPS (200 ng) was injected. The mice were killed 4 h after LPS and MPO activity per mg of lung tissue was determined. Despite the inhibitory effects of IL-2 on neutrophil migration in the systemic circulation, this cytokine failed to reduce the lung neutrophil infiltration induced by LPS (Figure 6).
Figure 6.
IL-2 injected i.v. fails to inhibit LPS-induced lung neutrophil sequestration. The mice were pretreated i.v. with saline (Sal, 0.1 ml) or IL-2 (30–1000 ng 0.1 ml−1), and 15 min later, LPS (200 ng mice−1) was also injected i.v. The mice were killed 4 h after LPS administration and MPO activity was determined. The first bar represents the lung neutrophil sequestration in mice injected i.v. with PBS. The results are means±s.e.m. of neutrophils in 100 mg of lung tissue (n=5). *P<0.05 compared with PBS injected i.p. Analysis of variation followed by Bonferroni t-test.
Discussion
In the current study, we observed that the administration of IL-2 to mice inhibits neutrophil migration to the peritoneal cavity, through a mechanism mediated by NO, irrespective of the triggering agent.
We demonstrated that the systemic administration of IL-2 inhibited dose-dependently peritoneal neutrophil migration induced by Cg, as well as neutrophil migration induced by LPS and fMLP. Furthermore, IL-2-treated mice showed a reduced rolling and adhesion induced by Cg, LPS and fMLP. These inhibitory effects of IL-2 on neutrophil migration seem to be mediated by NO, as elevated serum nitrite levels were observed after IL-2 treatment and amino, a selective inhibitor of inducible NO synthase (Misko et al., 1993), prevented inhibition by the systemic administration of IL-2 of neutrophil migration to the inflammatory site induced by Cg. It is important to mention that there was no significant difference between white blood cell counts (monocyte, lymphocyte and neutrophil) from animals after treatment with any of the reagents used (data not shown), suggesting that the changes observed were not the result of alterations in circulating leukocyte counts.
Impaired neutrophil migration is also observed in severe sepsis, which is associated with increased numbers of bacteria in the peritoneal exudate and blood and to high mortality (Benjamim et al., 2000; Crosara-Alberto et al., 2002; Alves-Filho et al., 2005; 2006). Moreover, reduction of neutrophil chemotaxis was also observed in patients that underwent severe sepsis (Tavares-Murta et al., 2002). The mechanism of this reduction seems to be owing to an excessive systemic release of proinflammatory chemokines/cytokines, including TNF-α and IL-8 and to a concomitant increase in NO derived from iNOS. Similarly to what was observed in mice treated with IL-2 (present study), the neutrophil paralysis found in severe sepsis was neither observed in iNOS-deficient mice nor in mice treated with amino (Benjamim et al., 2000; 2002; Crosara-Alberto et al., 2002). Furthermore, in both human or experimental sepsis or in human receiving IL-2, an increase in systemic levels of the cytokines TNF-α, IFN-γ, IL-1β and IL-6 (Carey et al., 1997; Cavaillon et al., 2003) is described. In this context, it is remarkable that different inflammatory cytokines exert anti-inflammatory effects when administered systemically against their own in situ effects and/or against the effects of unrelated inflammatory agents (Tavares-Murta et al., 1998; Hurst et al., 2001; Lokuta & Huttenlocher, 2005). Accordingly, neutrophil influx into the peritoneal cavity was also observed after the i.p. injection of IL-2 in mice (Stevens & Piazza, 1990).
Although the mechanisms by which NO attenuates neutrophil accumulation are not fully elucidated, evidences suggest that NO modulates the leukocyte–endothelial cell interaction. Both mice treated with IL-2 or those undergoing severe sepsis showed a reduced leukocyte rolling and adhesion, and the pharmacological (use of amino) or genetic (use of iNOS−/−) tools prevent both phenomena. Supporting these results, inhibitors of NOS increase neutrophil adhesion to endothelial cells, whereas NO donors decrease both adhesion and leukocyte transmigration to inflammatory sites (Gauthier et al., 1994; Tavares-Murta et al., 1998; Sato et al., 1999; Benjamim et al., 2002; Secco et al., 2003). Moreover, these parameters are also increased in iNOS−/− mice (Benjamim et al., 2002; Secco et al., 2003). Finally, the expression of the cell adhesion molecules CD11b/CD18, L-, P-, E-selectin, ICAM-1 and VCAM-1 among others are downregulated by NO donors and upregulated by NOS inhibitors (Gauthier et al., 1994; Armstead et al., 1997; Spiecker et al., 1998; Sato et al., 1999).
It is important to discuss the absence of IL-2 inhibition of neutrophil sequestration to the lung. IL-2-treated mice showed a reduced neutrophil migration to the inflammatory focus, but displayed a significant sequestration of these cells in the lungs, an event largely accounting a role in IL-2-induced pulmonary VLS (Assier et al., 2004). Similarly, during severe sepsis, despite the failure of neutrophil migration to the infectious focus, a marked neutrophil sequestration in lungs is noted (Mercer-Jones et al., 1997; Sato et al., 1998; Razavi et al., 2004; Alves-Filho et al., 2006). There are experimental evidences showing that neutrophil migration to the pulmonary or systemic circulation is fundamentally different. Margination of neutrophils is essential for their migration in the systemic circulation and is mediated by selectins (E-, L- and P-selectin) and their respective ligands (Luster et al., 2005). However, in pulmonary vasculature, the small diameter of the capillary network (average 5–6 μm) does not allow the neutrophil rolling process. The neutrophils, whose diameter is of 7–8 μm, must deform to an elongated shape to pass through the lung capillaries. These processes contribute to the neutrophil firm adhesion process (Gebb et al., 1995). Furthermore, studies have also shown that, although ICAM-1 has a low constitutive level expression in peripheral tissues, it is 30-fold higher in lung tissue (Panes et al., 1995; Doerschuk, 2000), allowing the firm adhesion of neutrophils previously activated in vivo, owing to the presence of the circulating inflammatory mediators. Thus, in the systemic circulation, the firm adhesion of neutrophils to the endothelial cells of the microcirculation requires the induction of both integrins and ICAMs, which together mediates the process. In contrast, in the pulmonary tissue, owing to the high basal expression level of ICAM-1, the induction of adhesion molecules is not required for the lung neutrophil sequestration.
Overall, our results indicate that IL-2 administered systemically inhibits neutrophil recruitment to the inflammatory site through an NO-dependent mechanism. As neutrophils are the first cells to migrate to a site of infection, the clinical use of IL-2 may reduce the host ability to restrict the infection locally. These findings may provide an interesting possibility to explain the increased incidence and severity of bacterial infections during IL-2 therapy (Bock et al., 1990; Snydman et al., 1990). In agreement with this hypothesis, the systemic administration of IL-2 by intermittent or continuous administration is associated to marked changes in neutrophil functions (Jablons et al., 1990). Thus, the understating of the mechanism by which IL-2 inhibits the neutrophil migration might help to prevent the side effects of the IL-2 therapy.
Acknowledgments
We are grateful to Fabíola Leslie Mestriner, Ana Kátia dos Santos and Diva Amabile Montanha de Sousa for technical assistance. This work was supported by a Grant from the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Conselho Nacional de Pesquisa e Desenvolvimento Tecnológico (CNPq) and Programa de Núcleos de Excelência (PRONEX).
Abbreviations
- Cg
carrageenin
- FMLP
N-formyl-methionyl-leucyl-phenylalanine
- IFN-γ
interferon gamma
- iNOS
inducible nitric oxide synthase
- LPS
lipopolysacharide
- MPO
myeloperoxidase
- NO
nitric oxide
- TNF-α
tumor necrosis factor alpha
- VLS
vascular leak syndrome
References
- ALVES-FILHO J.C., BENJAMIM C., TAVARES-MURTA B.M., CUNHA F.Q. Failure of neutrophil migration toward infectious focus in severe sepsis: a critical event for the outcome of this syndrome. Mem. Inst. Oswaldo Cruz. 2005;100 (Suppl. 1):223–226. doi: 10.1590/s0074-02762005000900038. [DOI] [PubMed] [Google Scholar]
- ALVES-FILHO J.C., DE FREITAS A., RUSSO M., CUNHA F.Q. Toll-like receptor 4 signaling leads to neutrophil migration impairment in polymicrobial sepsis. Crit. Care Med. 2006;34:461–470. doi: 10.1097/01.ccm.0000198527.71819.e1. [DOI] [PubMed] [Google Scholar]
- ARMSTEAD V.E., MINCHENKO A.G., SCHUHL R.A., HAYWARD R., NOSSULI T.O., LEFER A.M. Regulation of P-selectin expression in human endothelial cells by nitric oxide. Am. J. Physiol. 1997;273:H740–H746. doi: 10.1152/ajpheart.1997.273.2.H740. [DOI] [PubMed] [Google Scholar]
- ASSIER E., JULLIEN V., LEFORT J., MOREAU J.L., DI SANTO J.P., VARGAFTIG B.B., LAPA E SILVA J.R., THEZE J. NK cells and polymorphonuclear neutrophils are both critical for IL-2-induced pulmonary vascular leak syndrome. J. Immunol. 2004;172:7661–7668. doi: 10.4049/jimmunol.172.12.7661. [DOI] [PubMed] [Google Scholar]
- BALUNA R., VITETTA E.S. Vascular leak syndrome: a side effect of immunotherapy. Immunopharmacology. 1997;37:117–132. doi: 10.1016/s0162-3109(97)00041-6. [DOI] [PubMed] [Google Scholar]
- BECKNELL B., CALIGIURI M.A. Interleukin-2, interleukin-15, and their roles in human natural killer cells. Adv. Immunol. 2005;86:209–239. doi: 10.1016/S0065-2776(04)86006-1. [DOI] [PubMed] [Google Scholar]
- BENJAMIM C.F., FERREIRA S.H., CUNHA F.Q. Role of nitric oxide in the failure of neutrophil migration in sepsis. J. Infect. Dis. 2000;182:214–223. doi: 10.1086/315682. [DOI] [PubMed] [Google Scholar]
- BENJAMIM C.F., SILVA J.S., FORTES Z.B., OLIVEIRA M.A., FERREIRA S.H., CUNHA F.Q. Inhibition of leukocyte rolling by nitric oxide during sepsis leads to reduced migration of active microbicidal neutrophils. Infect. Immun. 2002;70:3602–3610. doi: 10.1128/IAI.70.7.3602-3610.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOCK S.N., LEE R.E., FISHER B., RUBIN J.T., SCHWARTZENTRUBER D.J., WEI J.P., CALLENDER D.P., YANG J.C., LOTZE M.T., PIZZO P.A. A prospective randomized trial evaluating prophylactic antibiotics to prevent triple-lumen catheter-related sepsis in patients treated with immunotherapy. J. Clin. Oncol. 1990;8:161–169. doi: 10.1200/JCO.1990.8.1.161. [DOI] [PubMed] [Google Scholar]
- CAREY P.D., WAKEFIELD C.H., GUILLOU P.J. Neutrophil activation, vascular leak toxicity, and cytolysis during interleukin-2 infusion in human cancer. Surgery. 1997;122:918–926. doi: 10.1016/s0039-6060(97)90333-0. [DOI] [PubMed] [Google Scholar]
- CAVAILLON J.M., ADIB-CONQUY M., FITTING C., ADRIE C., PAYEN D. Cytokine cascade in sepsis. Scand. J. Infect. Dis. 2003;35:535–544. doi: 10.1080/00365540310015935. [DOI] [PubMed] [Google Scholar]
- CROSARA-ALBERTO D.P., DARINI A.L., INOUE R.Y., SILVA J.S., FERREIRA S.H., CUNHA F.Q. Involvement of NO in the failure of neutrophil migration in sepsis induced by Staphylococcus aureus. Br. J. Pharmacol. 2002;136:645–658. doi: 10.1038/sj.bjp.0704734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVID D., KELLER H., NAIT-IGHIL L., TREILHOU M.P., JOUSSEMET M., DUPONT B., GACHOT B., MARAL J., THEZE J. Involvement of Bcl-2 and IL-2R in HIV-positive patients whose CD4 cell counts fail to increase rapidly with highly active antiretroviral therapy. AIDS. 2002;16:1093–1101. doi: 10.1097/00002030-200205240-00002. [DOI] [PubMed] [Google Scholar]
- DAVID D., NAIT-IGHIL L., DUPONT B., MARAL J., GACHOT B., THEZE J. Rapid effect of interleukin-2 therapy in human immunodeficiency virus-infected patients whose CD4 cell counts increase only slightly in response to combined antiretroviral treatment. J. Infect. Dis. 2001;183:730–735. doi: 10.1086/318824. [DOI] [PubMed] [Google Scholar]
- DE SANCTIS J.B., BLANCA I., BIANCO N.E. Secretion of cytokines by natural killer cells primed with interleukin-2 and stimulated with different lipoproteins. Immunology. 1997;90:526–533. doi: 10.1046/j.1365-2567.1997.00174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOERSCHUK C.M. Leukocyte trafficking in alveoli and airway passages. Respir. Res. 2000;1:136–140. doi: 10.1186/rr24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FORTES Z.B., FARSKY S.P., OLIVEIRA M.A., GARCIA-LEME J. Direct vital microscopic study of defective leukocyte–endothelial interaction in diabetes mellitus. Diabetes. 1991;40:1267–1273. doi: 10.2337/diab.40.10.1267. [DOI] [PubMed] [Google Scholar]
- FRAKER D.L., LANGSTEIN H.N., NORTON J.A. Passive immunization against tumor necrosis factor partially abrogates interleukin 2 toxicity. J. Exp. Med. 1989;170:1015–1020. doi: 10.1084/jem.170.3.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAUTHIER T.W., DAVENPECK K.L., LEFER A.M. Nitric oxide attenuates leukocyte–endothelial interaction via P-selectin in splanchnic ischemia-reperfusion. Am. J. Physiol. 1994;267:G562–G568. doi: 10.1152/ajpgi.1994.267.4.G562. [DOI] [PubMed] [Google Scholar]
- GEBB S.A., GRAHAM J.A., HANGER C.C., GODBEY P.S., CAPEN R.L., DOERSCHUK C.M., WAGNER W.W., JR. Sites of leukocyte sequestration in the pulmonary microcirculation. J. Appl. Physiol. 1995;79:493–497. doi: 10.1152/jappl.1995.79.2.493. [DOI] [PubMed] [Google Scholar]
- GREEN L.C., WAGNER D.A., GLOGOWSKI J., SKIPPER P.L., WISHNOK J.S., TANNENBAUM S.R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- HURST S.M., WILKINSON T.S., MCLOUGHLIN R.M., JONES S., HORIUCHI S., YAMAMOTO N., ROSE-JOHN S., FULLER G.M., TOPLEY N., JONES S.A. Il-6 and its soluble receptor orchestrate a temporal switch in the pattern of leukocyte recruitment seen during acute inflammation. Immunity. 2001;14:705–714. doi: 10.1016/s1074-7613(01)00151-0. [DOI] [PubMed] [Google Scholar]
- JABLONS D., BOLTON E., MERTINS S., RUBIN M., PIZZO P., ROSENBERG S.A., LOTZE M.T. IL-2-based immunotherapy alters circulating neutrophil Fc receptor expression and chemotaxis. J. Immunol. 1990;144:3630–3636. [PubMed] [Google Scholar]
- LENTSCH A.B., EDWARDS M.J., SIMS D.E., MILLER F.N. N omega-nitro-L-arginine methyl ester inhibits inflammatory liver injury induced by interleukin-2. J. Leukocyte Biol. 1998;63:22–30. [PubMed] [Google Scholar]
- LENTSCH A.B., MILLER F.N., EDWARDS M.J. Mechanisms of leukocyte-mediated tissue injury induced by interleukin-2. Cancer Immunol. Immunother. 1999;47:243–248. doi: 10.1007/s002620050527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOCKER G.J., KAPIOTIS S., VEITL M., MADER R.M., STOISER B., KOFLER J., SIEDER A.E., RAINER H., STEGER G.G., MANNHALTER C., WAGNER O.F. Activation of endothelium by immunotherapy with interleukin-2 in patients with malignant disorders. Br. J. Haematol. 1999;105:912–919. doi: 10.1046/j.1365-2141.1999.01453.x. [DOI] [PubMed] [Google Scholar]
- LOKUTA M.A., HUTTENLOCHER A. TNF-alpha promotes a stop signal that inhibits neutrophil polarization and migration via a p38 MAPK pathway. J. Leukocyte Biol. 2005;78:210–219. doi: 10.1189/jlb.0205067. [DOI] [PubMed] [Google Scholar]
- LUSTER A.D., ALON R., VON ANDRIAN U.H. Immune cell migration in inflammation: present and future therapeutic targets. Nat. Immunol. 2005;6:1182–1190. doi: 10.1038/ni1275. [DOI] [PubMed] [Google Scholar]
- MERCER-JONES M.A., HEINZELMANN M., PEYTON J.C., WICKEL D., COOK M., CHEADLE W.G. Inhibition of neutrophil migration at the site of infection increases remote organ neutrophil sequestration and injury. Shock. 1997;8:193–199. doi: 10.1097/00024382-199709000-00007. [DOI] [PubMed] [Google Scholar]
- MISKO T.P., MOORE W.M., KASTEN T.P., NICKOLS G.A., CORBETT J.A., TILTON R.G., MCDANIEL M.L., WILLIAMSON J.R., CURRIE M.G. Selective inhibition of the inducible nitric oxide synthase by aminoguanidine. Eur. J. Pharmacol. 1993;233:119–125. doi: 10.1016/0014-2999(93)90357-n. [DOI] [PubMed] [Google Scholar]
- NATARAJAN V., LEMPICKI R.A., SERETI I., BADRALMAA Y., ADELSBERGER J.W., METCALF J.A., PRIETO D.A., STEVENS R., BASELER M.W., KOVACS J.A., LANE H.C. Increased peripheral expansion of naive CD4+ T cells in vivo after IL-2 treatment of patients with HIV infection. Proc. Natl. Acad. Sci. U.S.A. 2002;99:10712–10717. doi: 10.1073/pnas.162352399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORUCEVIC A., HEARN S., LALA P.K. The role of active inducible nitric oxide synthase expression in the pathogenesis of capillary leak syndrome resulting from interleukin-2 therapy in mice. Lab. Invest. 1997;76:53–65. [PubMed] [Google Scholar]
- ORUCEVIC A., LALA P.K. Effects of N(G)-Nitro-L-arginine methyl ester, an inhibitor of nitric oxide synthesis, on IL-2-induced LAK cell generation in vivo and in vitro in healthy and tumor-bearing mice. Cell Immunol. 1996a;169:125–132. doi: 10.1006/cimm.1996.0100. [DOI] [PubMed] [Google Scholar]
- ORUCEVIC A., LALA P.K. NG-nitro-L-arginine methyl ester, an inhibitor of nitric oxide synthesis, ameliorates interleukin 2-induced capillary leakage and reduces tumour growth in adenocarcinoma-bearing mice. Br. J. Cancer. 1996b;73:189–196. doi: 10.1038/bjc.1996.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PANES J., PERRY M.A., ANDERSON D.C., MANNING A., LEONE B., CEPINSKAS G., ROSENBLOOM C.L., MIYASAKA M., KVIETYS P.R., GRANGER D.N. Regional differences in constitutive and induced ICAM-1 expression in vivo. Am. J. Physiol. 1995;269:H1955–H1964. doi: 10.1152/ajpheart.1995.269.6.H1955. [DOI] [PubMed] [Google Scholar]
- PHILIP P.A. Interleukin-2 in the treatment of malignant melanoma. Expert. Opin. Invest. Drugs. 1998;7:361–371. doi: 10.1517/13543784.7.3.361. [DOI] [PubMed] [Google Scholar]
- RAZAVI H.M., WANG LE F., WEICKER S., ROHAN M., LAW C., MCCORMACK D.G., MEHTA S. Pulmonary neutrophil infiltration in murine sepsis: role of inducible nitric oxide synthase. Am. J. Respir. Crit. Care Med. 2004;170:227–233. doi: 10.1164/rccm.200306-846OC. [DOI] [PubMed] [Google Scholar]
- SATO Y., VAN EEDEN S.F., ENGLISH D., HOGG J.C. Pulmonary sequestration of polymorphonuclear leukocytes released from bone marrow in bacteremic infection. Am. J. Physiol. 1998;275:L255–L261. doi: 10.1152/ajplung.1998.275.2.L255. [DOI] [PubMed] [Google Scholar]
- SATO Y., WALLEY K.R., KLUT M.E., ENGLISH D., D'YACHKOVA Y., HOGG J.C., VAN EEDEN S.F. Nitric oxide reduces the sequestration of polymorphonuclear leukocytes in lung by changing deformability and CD18 expression. Am. J. Respir. Crit. Care Med. 1999;159:1469–1476. doi: 10.1164/ajrccm.159.5.9808063. [DOI] [PubMed] [Google Scholar]
- SCHRADER A.J., HEIDENREICH A., HEGELE A., OLBERT P., OHLMANN C.H., VARGA Z., VON KNOBLOCH R., HOFMANN R. Application of thalidomide/interleukin-2 in immunochemotherapy–refractory metastatic renal cell carcinoma. Anticancer Drugs. 2005;16:581–585. doi: 10.1097/00001813-200506000-00014. [DOI] [PubMed] [Google Scholar]
- SECCO D.D., PARON J.A., DE OLIVEIRA S.H., FERREIRA S.H., SILVA J.S., CUNHA FDE Q. Neutrophil migration in inflammation: nitric oxide inhibits rolling, adhesion and induces apoptosis. Nitric Oxide. 2003;9:153–164. doi: 10.1016/j.niox.2003.11.001. [DOI] [PubMed] [Google Scholar]
- SINKOVICS J.G., HORVATH J.C. Human natural killer cells: a comprehensive review. Int. J. Oncol. 2005;27:5–47. [PubMed] [Google Scholar]
- SMITH K.A. To cure chronic HIV infection, a new therapeutic strategy is needed. Curr. Opin. Immunol. 2001;13:617–624. doi: 10.1016/s0952-7915(00)00270-3. [DOI] [PubMed] [Google Scholar]
- SNYDMAN D.R., SULLIVAN B., GILL M., GOULD J.A., PARKINSON D.R., ATKINS M.B. Nosocomial sepsis associated with interleukin-2. Ann. Intern. Med. 1990;112:102–107. doi: 10.7326/0003-4819-112-2-102. [DOI] [PubMed] [Google Scholar]
- SOUZA D.G., CASSALI G.D., POOLE S., TEIXEIRA M.M. Effects of inhibition of PDE4 and TNF-alpha on local and remote injuries following ischaemia and reperfusion injury. Br. J. Pharmacol. 2001;134:985–994. doi: 10.1038/sj.bjp.0704336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPIECKER M., DARIUS H., KABOTH K., HUBNER F., LIAO J.K. Differential regulation of endothelial cell adhesion molecule expression by nitric oxide donors and antioxidants. J. Leukocyte Biol. 1998;63:732–739. [PubMed] [Google Scholar]
- STEVENS P., PIAZZA D.E. Interleukin-2 increases the oxidative activity and induces migration of murine polymorphonuclear leukocytes in vivo. Int. J. Immunopharmacol. 1990;12:605–611. doi: 10.1016/0192-0561(90)90097-7. [DOI] [PubMed] [Google Scholar]
- TAVARES-MURTA B.M., CUNHA F.Q., FERREIRA S.H. The intravenous administration of tumor necrosis factor alpha, interleukin 8 and macrophage-derived neutrophil chemotactic factor inhibits neutrophil migration by stimulating nitric oxide production. Br. J. Pharmacol. 1998;124:1369–1374. doi: 10.1038/sj.bjp.0701965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAVARES-MURTA B.M., ZAPAROLI M., FERREIRA R.B., SILVA-VERGARA M.L., OLIVEIRA C.H., MURTA E.F., FERREIRA S.H., CUNHA F.Q. Failure of neutrophil chemotactic function in septic patients. Crit. Care Med. 2002;30:1056–1061. doi: 10.1097/00003246-200205000-00017. [DOI] [PubMed] [Google Scholar]
- TERAO S., SHIRAKAWA T., GODA K., KAMIDONO S., FUJISAWA M., GOTOH A. Recombinant interleukin-2 enhanced the antitumor effect of ADV/RSV-HSV-tk/ACV therapy in a murine bladder cancer model. Anticancer Res. 2005;25:2757–2760. [PubMed] [Google Scholar]
- THEZE J., ALZARI P.M., BERTOGLIO J. Interleukin 2 and its receptors: recent advances and new immunological functions. Immunol. Today. 1996;17:481–486. doi: 10.1016/0167-5699(96)10057-c. [DOI] [PubMed] [Google Scholar]
- WALLAYS G., CEUPPENS J.L. Human T lymphocyte activation by pokeweed mitogen induces production of TNF-alpha and GM-CSF and helper signaling by IL-1 and IL-6 results in IL-2-dependent T cell growth. Eur. Cytokine Network. 1993;4:269–277. [PubMed] [Google Scholar]