Abstract
This study investigated whether (a) cannabinoid CB1 receptor knockout (CB1−/−) mice displayed altered gastrointestinal transit and (b) cannabinoid CB1 and opioid receptors functionally interact in the regulation of gastrointestinal transit.
Gastrointestinal transit was assessed by the Whole Gastrointestinal Transit, measuring the excretion time of an intragastrically administered marker (whole intestine), and the Upper Gastrointestinal Transit, measuring the distance covered by the marker in the small intestine.
CB1−/− and homozygous CB1+/+ (CB1+/+) mice did not differ in both whole gut and small intestine transit. CB1−/− and CB1+/+ mice were equally responsive to the inhibitory effect of morphine (10 mg kg−1) and loperamide (3 mg kg−1) on whole gut transit.
Additionally, in CD1 mice the cannabinoid CB1 receptor antagonist, rimonabant (0–0.5 mg kg−1), failed to block the inhibitory effect of morphine (0–1.25 mg kg−1) and loperamide (0–0.5 mg kg−1) on transit in small and whole intestine. Similarly, the opioid receptor antagonists, naloxone (0–1 mg kg−1) and naltrexone (0–10 mg kg−1), failed to block the inhibitory effect of the cannabinoid WIN 55,212-2 (0–3 mg kg−1) on transit in small and whole intestine.
These results suggest that (a) compensatory mechanisms likely developed in CB1−/− mice to overcome the lack of inhibitory function of endocannabinoid system; (b) cannabinoid and opioid receptor systems did not interact in regulating gastrointestinal transit in mice.
Keywords: Cannabinoid CB1 receptor knockout (CB1−/−) mice; gastrointestinal motility; rimonabant; WIN 55,212-2; opioid receptor ligands; mice
Introduction
The involvement of the endocannabinoid system in the regulation of different gastrointestinal functions has been well established. For example, at a pharmacological level, cannabinoids have long been known to exert an inhibitory effect on gastric emptying and intestinal motility in laboratory animals and humans (see Izzo et al., 2001c; Pertwee, 2001; Duncan et al., 2005). More specifically, Δ9-tetrahydrocannabinol (Δ9-THC), the active ingredient of Cannabis sativa, the synthetic cannabinoids, WIN 55,212-2 and CP 55,940, and the endocannabinoid, anandamide, have been reported to slow the rate of gastric emptying and small intestinal transit in rodents (e.g., Cheser et al., 1973; Shook & Burks, 1989; Calignano et al., 1997; Colombo et al., 1998; Izzo et al., 1999a; 2001a; Capasso et al., 2001). Conversely, administration of the cannabinoid CB1 receptor antagonist, rimonabant (also known as SR 141716), beside counteracting the above-mentioned effects of cannabinoids, has been found to stimulate gastric emptying, small intestinal transit, defecation, and diarrhoea in rodents (e.g., Colombo et al., 1998; Izzo et al., 1999a, 1999b; Carai et al., 2004). More recently, a higher rate of episodes of diarrhoea was observed in rimonabant- than placebo-treated patients in two studies investigating the body weight-reducing effect of rimonabant (Després et al., 2005; Van Gaal et al., 2005).
The availability of mouse strains (Zimmer et al., 1999; Hungund et al., 2003) with global deletion of the gene encoding the cannabinoid CB1 receptor (CB1-knockout (CB1−/−) mice) provides a further tool for investigations on the function of the endocannabinoid system. Data generated to date with CB1−/− mice are generally consistent with those suggesting the involvement of the endocannabinoid system in the regulation of pain, body temperature, food and alcohol intake, body weight gain (Walker et al., 1999; Zimmer et al., 1999; Di Marzo et al., 2001; Hungund et al., 2003; Naassila et al., 2004). Pertaining to the gastrointestinal function, studies conducted to date found that the excitatory junction potential was higher in myenteric neuron/smooth muscle preparation from CB1−/− mice than homozygous CB1+/+ mice (Storr et al., 2004), and CB1−/− and CB1+/+ mice did not differ in motility in the upper portion of the intestine (Capasso et al., 2005). Study 1 of the present investigation was designed to provide further lines of evidence on the gastrointestinal function in CB1−/− and CB1+/+ mice. This study was conducted using two different procedures: the first assessed in vivo the transit in the whole intestine, whereas the second assessed ex vivo the transit in the small intestine.
Opioids and cannabinoids are known to share different pharmacological effects at the level of the gastrointestinal tract, including inhibition of gastric emptying, intestinal transit, and secretion (see Kurz & Sessler, 2003; Duncan et al., 2005). Additional studies have investigated whether the functional interaction existing between the opioid and the endocannabinoid systems in different organs, including the central nervous system (see Corchero et al., 2004; Cota et al., 2006), is also present in the gastrointestinal tract. Specifically, the studies conducted to date have found that: (a) the opioid receptor antagonist, naloxone, failed to block the inhibitory effect of Δ9-THC, WIN 55,212-2, and anandamide on transit in the mouse small intestine in ex vivo studies (Shook & Burks, 1989; Izzo et al., 1999b; 2001a); (b) rimonabant and naloxone did not alter the inhibitory effect of normorphine and CP 55,940, respectively, on evoked contractile responses in in vitro preparations of guinea-pig small intestine (Pertwee et al., 1996; Coutts & Pertwee, 1997); (c) naloxone did not block methanandamide-, WIN 55,212-2-, and CP 55,940-induced increase in peristaltic pressure threshold – a measure of propulsive motility – in guinea-pig isolated ileum (Heinemann et al., 1999; Izzo et al., 2000).
However, to our knowledge, to date no study has addressed the issue of the functional relationship between the opioid and endocannabinoid systems in the regulation of gastrointestinal motility in in vivo studies. A second aim of the present investigation was therefore to assess the capability of opioid and cannabinoid receptor antagonists to counteract the inhibitory action of cannabinoids and opioids, respectively, on the gastrointestinal motility in mice using the in vivo procedure of transit in the whole intestine (Study 2). In addition, the action of the two opioids, morphine, and loperamide (which is only peripherally active), on the gastrointestinal motility of CB1−/− mice was evaluated (Study 1).
Methods
The experimental procedures employed in the present study were in accordance with the European Communities Council Directive (86/609/EEC) and the subsequent Italian Law on the ‘Protection of animals used for experimental and other scientific reasons'.
Study 1: CB1−/− mice
Animals
Heterozygous CB1+/− mice were generated as described previously (Steiner et al., 1999; Zimmer et al., 1999) and were kindly provided by Dr Andreas Zimmer (NIH, Bethesda, MD, U.S.A.). Homozygous CB1−/− and homozygous CB1+/+ mice were produced by heterozygous intermatings and were backcrossed five times onto C57BL/6J background mice. The mice were bred at the Nathan Kline Institute animal facility. Thirteen- to 15-week-old mice were used for the studies.
Male mice weighing 25–28 g at the time of the experiments were used. Mice were singly housed in standard plastic cages (33.2 × 15.0 × 13.0 (h) cm) with wood chip bedding under a 12/12 h artificial light–dark cycle (lights on at 07:00 h), at a constant temperature of 22±2°C and relative humidity of approximately 60%. Tap water and standard laboratory rodent chow (Mucedola, Settimo Milanese, MI, Italy) were provided ad libitum; however, mice were deprived of food (a) 3 h before the start of the Whole Gastrointestinal Transit experiments, and (b) 12 h before the start of the Upper Gastrointestinal Transit experiments. These fasting times were chosen on the basis of literature data (Whole Gastrointestinal Transit: e.g. Croci & Bianchetti, 1992; Izzo et al., 1999a; Upper Gastrointestinal Transit: e.g. Izzo et al., 1999b; Capasso et al., 2005).
Whole Gastrointestinal Transit
The present procedure, set up and validated by Nagakura et al. (1996), assesses the motility of the whole intestine in rodents. This procedure includes the intragastric administration of a given amount of a nonabsorbable, coloured marker. Time to excretion of the first coloured fecal bolus is recorded as ‘time of whole gut transit' and is used as an index of peristaltic motility in the whole intestine.
In the present study, carmine (Sigma-Aldrich, Milan, Italy) was used as a marker. Carmine was suspended in water (6%, w v−1) containing 0.5% methylcellulose, and administered intragastrically (3.8-mm long gavage) at the dose of 0.3 ml mouse−1. Thirty minutes before carmine administration, CB1−/− and CB1+/+ mice were treated intraperitoneally with (R)-(+)-WIN 55,212-2 (WIN 55,212-2) (0, 0.3, 1, and 3 mg kg−1; mesylate salt; Sigma-RBI, Milan, Italy; suspended in 12.5 ml kg−1 saline with 0.1% Tween 80), morphine (0, 1, 3, and 5.6 mg kg−1; hydrochloride; Salars, Como, Italy; dissolved in 12.5 ml kg−1 bidistilled water), and loperamide (0, 0.1, 1, and 3 mg kg−1; hydrochloride, Sigma-Aldrich, Milan, Italy; suspended in 12.5 ml kg−1 saline with 0.1% Tween 80) in three separate experiments. In each experiment, groups of n=6–8 mice were used. Immediately after the carmine administration, mice were left undisturbed until the first red bolus was excreted. A thin layer of bedding covered the cage floor in order to facilitate recognition of red boluses. Data were statistically analysed by separate two-way (mouse strain; drug dose) analyses of variance (ANOVA), followed by the Newman–Keuls test for post hoc comparisons.
Upper Gastrointestinal Transit
The present procedure evaluates the motility of the upper gut (stomach and small intestine) (Nagakura et al., 1996; Carai et al., 2004). This procedure is based on the evidence that a nonabsorbable marker travels approximately 50% along the small intestine when infused intragastrically, 20 min before the killing, in undrugged mice.
Carmine (used as coloured marker) was suspended as described above and administered intragastrically (0.3 ml mouse−1) to n=10 CB1−/− and CB1+/+ mice. Twenty minutes after the carmine administration, mice were killed by cervical dislocation and intestines were removed from pylorus to cecum. The distance covered by the head of carmine was measured and expressed as percent of the total length of the small intestine. Data were statistically analysed by the unpaired, two-tailed Mann–Whitney test.
Study 2: CD1 mice
Animals
Male CD1 mice (Charles River, Calco, Italy), weighing 30–35 g, were used. Mice were singly housed under environmental conditions identical to those described above. Tap water and standard laboratory rodent chow were provided ad libitum; however, mice were deprived of food (a) 3 h before the start of the Whole Gastrointestinal Transit experiments, and (b) 12 h before the start of the Upper Gastrointestinal Transit experiments. These fasting times were chosen on the basis of literature data (Whole Gastrointestinal Transit: e.g., Croci & Bianchetti, 1992; Izzo et al., 1999a; Upper Gastrointestinal Transit: e.g. Izzo et al., 1999b; Capasso et al., 2005).
Whole Gastrointestinal Transit
Six separate experiments were conducted. In the first three experiments, a dose of rimonabant (1 mg kg−1; Sanofi-Aventis, Montpellier, France; suspended in 12.5 ml kg−1 saline with 0.1% Tween 80), chosen so as not to alter the time of whole gut transit per se, or vehicle were administered intraperitoneally 15 min before the intraperitoneal administration of WIN 55,212-2 (0 and 3 mg kg−1), morphine (0 and 10 mg kg−1), and loperamide (0 and 3 mg kg−1). Doses of WIN 55,212-2, morphine, and loperamide were chosen on the basis on preliminary dose–response experiments as those maximally effective per se. In the other three experiments, a dose of naltrexone (10 mg kg−1; hydrochloride; Sigma-Aldrich, Milan, Italy; dissolved in 12.5 ml kg−1 saline), chosen as to not alter the time of whole gut transit per se, or vehicle were administered intraperitoneally 15 min before the intraperitoneal administration of morphine (0 and 10 mg kg−1), loperamide (0 and 3 mg kg−1), and WIN 55,212-2 (0 and 3 mg kg−1). In each experiment, groups of n=10 mice were used.
Thirty minutes after WIN 55,212-2, morphine, or loperamide administration, mice were treated intragastrically with 0.3 ml mouse−1 carmine (prepared as described above). After carmine administration, mice were left undisturbed until the first red bolus was excreted. Data were statistically analysed by separate one-way ANOVAs, followed by the Newman–Keuls test for post hoc comparisons.
Upper Gastrointestinal Transit
Six separate experiments were conducted. In the first three experiments, a dose of rimonabant (0.5 mg kg−1), chosen so as not to alter the distance travelled by the head of the marker per se, or vehicle were administered intraperitoneally 15 min before the intraperitoneal administration of WIN 55,212-2 (0 and 0.5 mg kg−1), morphine (0 and 1.25 mg kg−1), and loperamide (0 and 0.5 mg kg−1). Doses of WIN 55,212-2, morphine, and loperamide were chosen on the basis on preliminary dose–response experiments as those maximally effective per se. In the other three experiments, a dose of naloxone (1 mg kg−1; hydrochloride; Salars, Como, Italy; dissolved in 12.5 ml kg−1 saline), chosen as to not alter the distance travelled by the head of the marker per se, or vehicle were administered intraperitoneally 15 min before the intraperitoneal administration of WIN 55,212-2 (0 and 0.5 mg kg−1), morphine (0 and 1.25 mg kg−1), and loperamide (0 and 0.5 mg kg−1). In each experiment, groups of n=10 mice were used.
Twenty minutes after WIN 55,212-2, morphine, or loperamide administration, mice were treated intragastrically with 0.3 ml mouse−1 carmine. Twenty minutes later, mice were killed and intestines were removed as described above. Data were statistically analysed by separate one-way ANOVAs, followed by the Newman–Keuls test for post hoc comparisons.
Results
Study 1: CB1−/− mice
Whole Gastrointestinal Transit
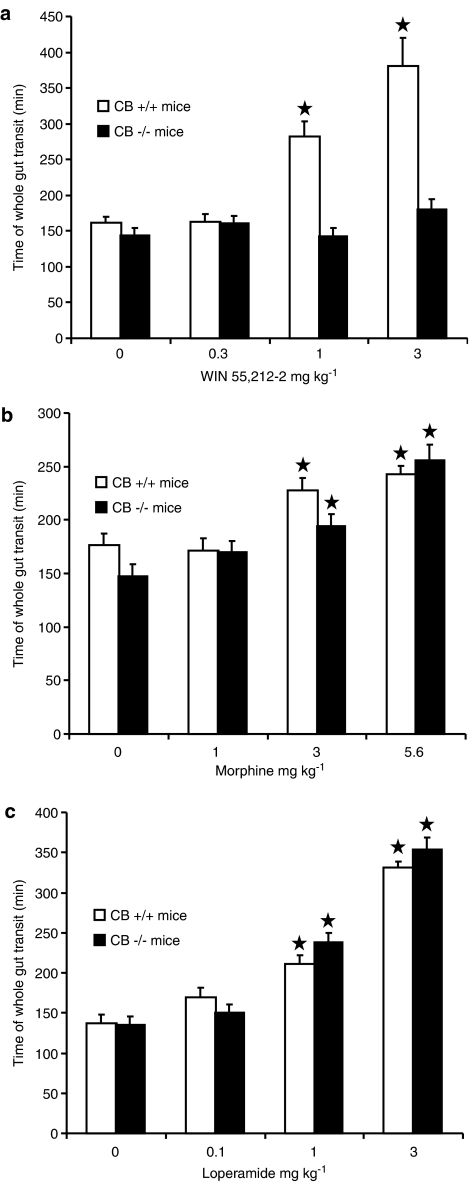
In the experiment testing WIN 55,212-2, ANOVA revealed significant effects of both factors, mouse strain (F(1,56)=36.21, P<0.0001) and drug dose (F(3,56)=15.67, P<0.0001), as well as of their interaction (F(3,56)=10.51, P<0.0001). Time of whole gut transit did not differ between vehicle-treated CB1−/− and CB1+/+ mice (Figure 1a). The administration of WIN 55,212-2 resulted in a dose-dependent, significant increase in the time of whole gut transit in CB1+/+ mice, whereas it was completely ineffective in CB1−/− mice (Figure 1a).
Figure 1.
Effect of the acute, intraperitoneal administration of different doses of the cannabinoid, WIN 55,212-2 (a), morphine (b), and loperamide (c) on time of whole gut transit in propulsive activity in CB1−/− and CB1+/+ mice. Time of whole gut transit indicated the time elapsing between the intragastric administration of the nonabsorbable coloured marker, carmine, and excretion of the first red fecal bolus. Each bar is the mean±s.e.m. of n=6–8 mice. *P<0.05 with respect to vehicle-treated mice of the same strain (Newman–Keuls test).
In the experiment testing morphine, ANOVA revealed a significant effect of drug dose (F(3,53)=28.53, P<0.0001), but not of mouse strain (F(1,53)=2.74, P>0.05) and interaction (F(3,53)=2.20, P>0.05). Time of whole gut transit did not differ between vehicle-treated CB1−/− and CB1+/+ mice (Figure 1b). The administration of morphine resulted in a dose-dependent, significant increase in the time of whole gut transit, which was of comparable magnitude in CB1−/− and CB1+/+ mice (Figure 1b).
In the experiment testing loperamide, ANOVA revealed a significant effect of drug dose (F(3,51)=85.60, P<0.0001), but not of mouse strain (F(1,51)=0.54, P>0.05) and interaction (F(3,51)=1.16, P>0.05). Again, no difference in time of whole gut transit was observed between vehicle-treated CB1−/− and CB1+/+ mice (Figure 1c). The administration of loperamide induced a dose-dependent and significant, although comparable between CB1−/− and CB1+/+ mice, increase in the time of whole gut transit (Figure 1c).
Upper Gastrointestinal Transit
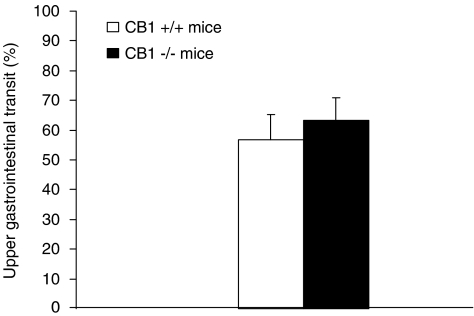
The distance travelled by the head of the marker, a measure of motility in the small intestine, did not significantly differ between undrugged CB1−/− and CB1+/+ mice (P>0.05, Mann–Whitney test) (Figure 2).
Figure 2.
Distance travelled in the small intestine by the head of the nonabsorbable coloured marker, carmine, administered intragastrically to undrugged CB1−/− and CB1+/+ mice 20 min before the killing. Distance travelled was expressed as percent of total length of the small intestine. Each bar is the mean±s.e.m. of n=10 mice.
Study 2: CD1 mice
Whole Gastrointestinal Transit
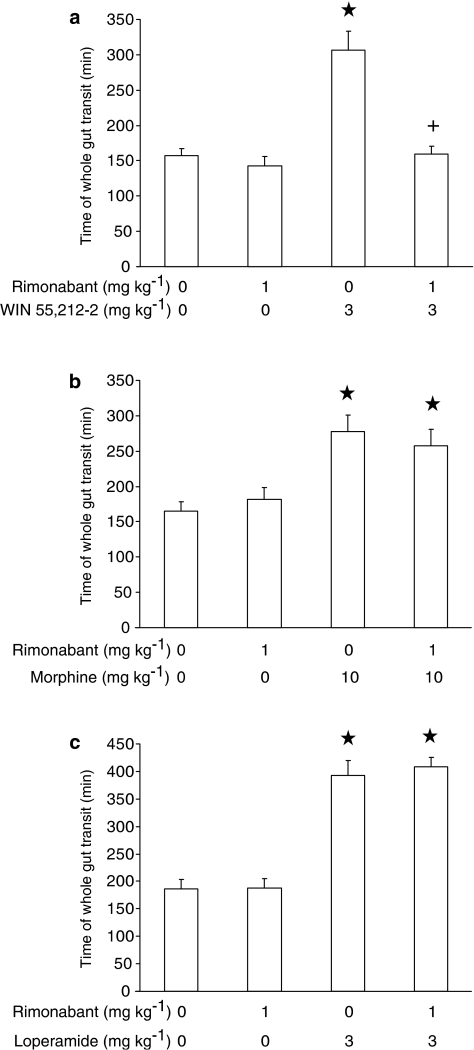
In the experiment testing rimonabant and WIN 55,212-2, ANOVA revealed significant differences among mouse groups (F(3,33)=19.37, P<0.0001). As expected, the administration of 1 mg kg−1 rimonabant – devoid of any effect when given alone – resulted in a complete blockade of WIN 55,212-2-induced increase in time of whole gut transit (Figure 3a).
Figure 3.
Effect of the acute, intraperitoneal administration of rimonabant on the increase in time of whole gut transit induced by the acute, intraperitoneal administration of WIN 55,212-2 (a), morphine (b), and loperamide (c) in CD1 mice. Time of whole gut transit indicated the time elapsing between the intragastric administration of the nonabsorbable coloured marker, carmine, and excretion of the first red fecal bolus. Each bar is the mean±s.e.m. of n=10 mice. *P<0.05 with respect to: 0 mg kg−1 rimonabant plus 0 mg kg−1 WIN 55,212-2-treated mice (Newman–Keuls test) in (a); 0 mg kg−1 rimonabant plus 0 mg kg−1 morphine-treated mice (Newman–Keuls test) in (b); 0 mg kg−1 rimonabant plus 0 mg kg−1 loperamide-treated mice (Newman–Keuls test) in (c). +: P<0.05 with respect to 0 mg kg−1 rimonabant plus 3 mg kg−1 WIN 55,212-2-treated mice (Newman–Keuls test) in (a).
In the experiment testing rimonabant and morphine, ANOVA revealed significant differences among mouse groups (F(3,36)=7.55, P<0.0005). However, rimonabant administration did not prevent morphine-induced increase in time of whole gut transit (Figure 3b).
In the experiment testing rimonabant and loperamide, ANOVA revealed significant differences among mouse groups (F(3,36)=40.02, P<0.0001). Pretreatment with rimonabant did not affect loperamide-induced increase in time of whole gut transit (Figure 3c).
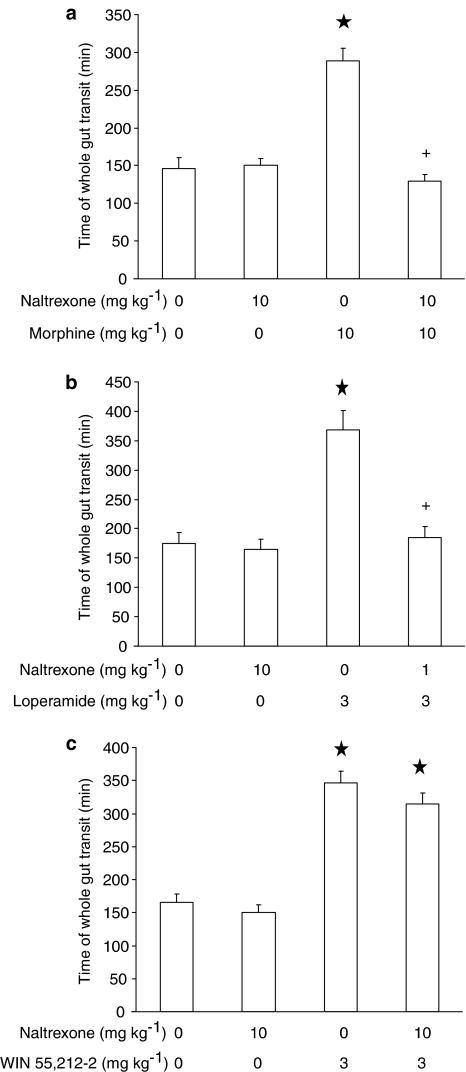
In the experiment testing naltrexone and morphine, ANOVA revealed significant differences among mouse groups (F(3,36)=32.60, P<0.0001). As expected, administration of 10 mg kg−1 naltrexone – which did not affect time of whole gut transit when given alone – produced a complete blockade of the increasing effect of morphine on time of whole gut transit (Figure 4a).
Figure 4.
Effect of the acute, intraperitoneal administration of naltrexone on the increase in time of whole gut transit induced by the acute, intraperitoneal administration of morphine (a), loperamide (b), and WIN 55,212-2 (c) in CD1 mice. Time of whole gut transit indicated the time elapsing between the intragastric administration of the nonabsorbable coloured marker, carmine, and excretion of the first red fecal bolus. Each bar is the mean±s.e.m. of n=10 mice. *P<0.05 with respect to: 0 mg kg−1 naltrexone plus 0 mg kg−1 morphine-treated mice (Newman–Keuls test) in (a); 0 mg kg−1 naltrexone plus 0 mg kg−1 loperamide-treated mice (Newman–Keuls test) in (b); 0 mg kg−1 naltrexone plus 0 mg kg−1 WIN 55,212-2-treated mice (Newman–Keuls test) in (c). +: P<0.05 with respect to: 0 mg kg−1 naltrexone plus 10 mg kg−1 morphine-treated mice (Newman–Keuls test) in (a); 0 mg kg−1 naltrexone plus 3 mg kg−1 loperamide-treated mice (Newman–Keuls test) in (b).
In the experiment testing naltrexone and loperamide, ANOVA revealed significant differences among mouse groups (F(3,36)=18.26, P<0.0001). Naltrexone administration completely prevented loperamide-induced increase in time of whole gut transit (Figure 4b).
In the experiment testing naltrexone and WIN 55,212-2, ANOVA revealed significant differences among mouse groups (F(3,36)=46.62, P<0.0001). However, treatment with naltrexone failed to counteract the increase in time of whole gut transit produced by WIN 55,212-2 (Figure 4c).
Upper Gastrointestinal Transit
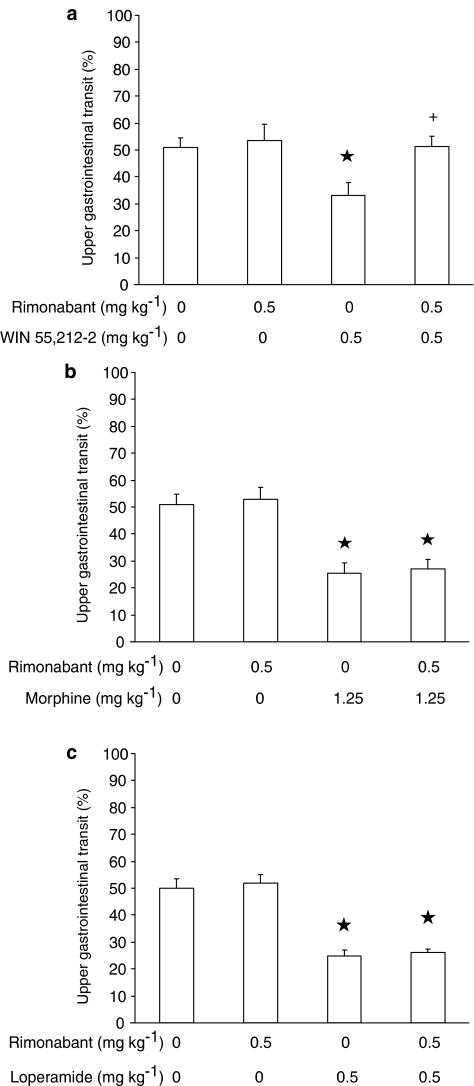
In the experiment testing rimonabant and WIN 55,212-2, ANOVA revealed significant differences among mouse groups (F(3,36)=3.71, P<0.05). As predicted, 0.5 mg kg−1 rimonabant – which was ineffective per se – completely blocked WIN 55,212-2-induced decrease in distance travelled by the head of the marker (Figure 5a).
Figure 5.
Effect of the acute, intraperitoneal administration of rimonabant on the decrease, induced by the acute, intraperitoneal administration of WIN 55,212-2 (a), morphine (b), and loperamide (c), in the distance travelled in the small intestine by the head of the nonabsorbable coloured marker, carmine, in CD1 mice. Mice were killed 20 min after carmine administration. Distance travelled was expressed as percent of total length of the small intestine. Each bar is the mean±s.e.m. of n=10 mice. *P<0.05 with respect to: 0 mg kg−1 rimonabant plus 0 mg kg−1 WIN 55,212-2-treated mice (Newman–Keuls test) in (a); 0 mg kg−1 rimonabant plus 0 mg kg−1 morphine-treated mice (Newman–Keuls test) in (b); 0 mg kg−1 rimonabant plus 0 mg kg−1 loperamide-treated mice (Newman–Keuls test) in (c). +: P<0.05 with respect to 0 mg kg−1 rimonabant plus 0.5 mg kg−1 WIN 55,212-2-treated mice (Newman–Keuls test) in (a).
In the experiment testing rimonabant and morphine, ANOVA revealed significant differences among mouse groups (F(3,36)=13.11, P<0.00001). However, rimonabant administration did not exert any effect on morphine-induced decrease in distance travelled by the head of the marker (Figure 5b).
In the experiment testing rimonabant and loperamide, ANOVA revealed significant differences among mouse groups (F(3,36)=31.22, P<0.000001). Pretreatment with rimonabant did not affect loperamide-induced decrease in distance travelled by the head of the marker (Figure 5c).
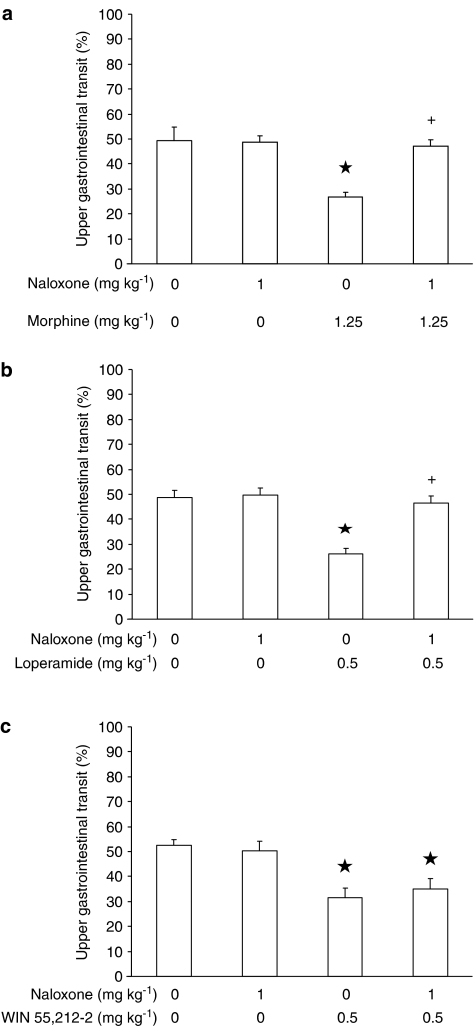
In the experiment testing naloxone and morphine, ANOVA revealed significant differences among mouse groups (F(3,36)=8.94, P<0.0005). As expected, the administration of 1 mg kg−1 naloxone – which was ineffective per se – produced a complete blockade of the decreasing effect of morphine on distance travelled by the head of the marker (Figure 6a).
Figure 6.
Effect of the acute, intraperitoneal administration of naloxone on the decrease, induced by the acute, intraperitoneal administration of morphine (a), loperamide (b), and WIN 55,212-2 (c), in the distance travelled in the small intestine by the head of the nonabsorbable coloured marker, carmine, in CD1 mice. Mice were killed 20 min after carmine administration. Distance travelled was expressed as percent of total length of the small intestine. Each bar is the mean±s.e.m. of n=10 mice. *P<0.05 with respect to: 0 mg kg−1 naloxone plus 0 mg kg−1 morphine-treated mice (Newman–Keuls test) in (a); 0 mg kg−1 naloxone plus 0 mg kg−1 loperamide-treated mice (Newman–Keuls test) in (b); 0 mg kg−1 naloxone plus 0 mg kg−1 WIN 55,212-2-treated mice (Newman–Keuls test) in (c). +: P<0.05 with respect to: 0 mg kg−1 naloxone plus 1.25 mg kg−1 morphine-treated mice (Newman–Keuls test) in (a); 0 mg kg−1 naloxone plus 0.5 mg kg−1 loperamide-treated mice (Newman–Keuls test) in (b).
In the experiment testing naloxone and loperamide, ANOVA revealed significant differences among mouse groups (F(3,36)=15.47, P<0.00005). Naloxone administration completely prevented loperamide-induced distance travelled by the head of the marker (Figure 6b).
Finally, in the experiment testing naloxone and WIN 55,212-2, ANOVA revealed significant differences among mouse groups (F(3,36)=7.05, P<0.001). However, treatment with naloxone did not counteract the decrease in distance travelled by the head of the marker produced by WIN 55,212-2 (Figure 6c).
Discussion
The results of Study 1 indicate that gastrointestinal transit did not differ between CB1−/− and CB1+/+ mice. Indeed, vehicle-treated CB1−/− and CB1+/+ mice displayed comparable values of time of whole gut transit in each of the three experiments performed using the Whole Gastrointestinal Transit test, a method that evaluates propulsive activity in the physiological condition. Indeed, in each of the above experiments, average time of excretion of the first coloured fecal bolus, after intragastric administration of the marker, ranged between 135 and 150 min in CB1−/− mice and between 135 and 175 min in CB1+/+ mice. Similar results have been collected in the experiment testing CB1−/− and CB1+/+ mice in the Upper Gastrointestinal Transit procedure, where distance travelled by the head of the coloured marker in the small intestine was comparatively similar between the two mouse strains 20 min after intragastric administration. The latter results closely resemble those recently collected by Capasso et al. (2005), indicating similar peristaltic activity in the small intestine of CB1−/− and CB1+/+ mice. Taken together, these results suggest that the genetic deletion of the cannabinoid CB1 receptor did not modify gastrointestinal transit in mice. It is likely that other neurotransmitter systems may have compensated for the lack of activity of the cannabinoid CB1 receptor system (Capasso et al., 2005). The apparent discrepancy between the pharmacological studies (which strongly suggest the involvement of the CB1 receptor system in the control of gastrointestinal motility; e.g., Cheser et al., 1973; Shook & Burks, 1989; Pertwee et al., 1996; Coutts & Pertwee, 1997; Colombo et al., 1998; Croci et al., 1998; Ross et al., 1998; Izzo et al., 1999a; 2001b) and the genetic studies (Capasso et al., 2005; present study) lead to hypothesise that compensatory mechanism(s) involving other neurotransmitter system(s) may have developed in CB1−/− mice (Capasso et al., 2005).
Accumulating lines of experimental evidence suggest that the cannabinoid and opioid receptor systems functionally interact with each other to modulate different processes, including nociception, motor behaviour, appetite and food intake, as well as drug-related reward (see Corchero et al., 2004; Cota et al., 2006). Accordingly, (a) cannabinoid CB1 and opioid receptors are colocalised in different organs, including – with particular interest with regard to the aims of the present investigation – the gastrointestinal tract, where cannabinoid CB1 and opioid receptors are located in neurons of myenteric and submucosal plexuses (Kulkarni-Narla & Brown, 2001), and (b) cannabinoid CB1 and opioid receptors share the same intracellular, signal transduction mechanisms and effector (see Law & Loh, 1999; Howlett et al., 2002). The present investigation evaluated whether this functional interaction between cannabinoid CB1 and opioid receptors would also extend to gastrointestinal transit. To this aim, pharmacological experiments were conducted to test the ability of cannabinoid CB1 and opioid receptor antagonists to block the in vivo inhibitory effect of opioids and cannabinoids, respectively, on gastrointestinal transit.
The results of the experiments included in Study 2 indicate how rimonabant, given at a dose which completely abolished the effect of WIN 55,212-2, failed to prevent the inhibitory action of morphine and loperamide on transit in the small and whole intestine in CD1 mice. Similarly, naloxone and naltrexone, injected at doses that completely blocked the effect of morphine and loperamide, did not alter the inhibitory action of WIN 55,212-2 on transit in the small and whole intestine in CD1 mice. These results suggest the lack of functional interactions between the cannabinoid CB1 and opioid receptors in the regulation of gastrointestinal motility. This conclusion (a) is consistent with previous observations on the inability of cannabinoid CB1 and opioid receptor antagonists to counteract different effects of opioids and cannabinoids, respectively, at the level of the gastrointestinal tract (see the Introduction for references), and (b) extends to the whole intestine observations previously made only in the small intestine (Shook & Burks, 1989; Izzo et al., 1999b; 2001a).
Accordingly, CB1−/− mice – which were, as expected, totally unresponsive to WIN 55,212-2 – displayed a completely unaltered sensitivity to the inhibitory effect of morphine and loperamide on gastrointestinal transit. Indeed, time of whole gut transit in CB1−/− mice treated with morphine or loperamide did not differ from that recorded in wild-type mice receiving the same drug treatment.
In conclusion, the results of the present series of experiments suggest that (a) the global disruption of the cannabinoid CB1 receptor did not alter the basal and opiate-inhibited gastrointestinal motility in mice, and (b) the functional interaction observed between the cannabinoid and opioid receptor systems in different processes apparently did not extend to the regulation of gastrointestinal transit in mice.
Acknowledgments
We are grateful to Ms Anne Farmer for language editing of the manuscript. This research was supported in part by grants from NIH (AA13003 and AA015525).
Abbreviations
- CB1−/−
cannabinoid CB1 receptor knockout mice
- CB1+/+
cannabinoid CB1 receptor homozygous mice
References
- CALIGNANO A., LA RANA G., MAKRIYANNIS A., LIN S.Y., BELTRAMO M., PIOMELLI D. Inhibition of intestinal motility by anandamide, an endogenous cannabinoid. Eur. J. Pharmacol. 1997;340:R7–R8. [PubMed] [Google Scholar]
- CAPASSO R., IZZO A.A., FEZZA F., PINTO A., CAPASSO F., MASCOLO N., DI MARZO V. Inhibitory effect of palmitoylethanolamide on gastrointestinal motility in mice. Br. J. Pharmacol. 2001;134:945–950. doi: 10.1038/sj.bjp.0704339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAPASSO R., MATIAS I., LUTZ B., BORRELLI F., CAPASSO F., MORSICANO G., MASCOLO N., PETROSINO S., MONORY K., VALENTI M., DI MARZO V., IZZO A.A. Fatty acid amide hydrolase controls mouse intestinal motility in vivo. Gastroenterology. 2005;129:941–951. doi: 10.1053/j.gastro.2005.06.018. [DOI] [PubMed] [Google Scholar]
- CARAI M.A.M., COLOMBO G., GESSA G.L. Rapid tolerance to the intestinal prokinetic effect of cannabinoid CB1 receptor antagonist, SR 141716 (rimonabant) Eur. J. Pharmacol. 2004;494:221–224. doi: 10.1016/j.ejphar.2004.05.005. [DOI] [PubMed] [Google Scholar]
- CHESER G.B., DAHL C.J., EVERINGHAM M., JAKSON D.M., MARCHANT-WILLIAMS H., STARMER G.A. The effect of cannabinoids on intestinal motility and their antinociceptive effect in mice. Br. J. Pharmacol. 1973;49:588–594. doi: 10.1111/j.1476-5381.1973.tb08534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLOMBO G., AGABIO R., LOBINA C., REALI R., GESSA G.L. Cannabinoid modulation of intestinal propulsion in mice. Eur. J. Pharmacol. 1998;344:67–69. doi: 10.1016/s0014-2999(97)01555-0. [DOI] [PubMed] [Google Scholar]
- CORCHERO J., MANZANARES J., FUENTES J.A. Cannabinoid/opioid crosstalk in the central nervous system. Crit Rev Neurobiol. 2004;16:159–172. doi: 10.1615/critrevneurobiol.v16.i12.170. [DOI] [PubMed] [Google Scholar]
- COTA D., TSCHÖP M.H., HORVAT L., LEVINE A.S. Cannabinoids, opioids and eating behavior: the molecular face of hedonism. Brain Res. Rev. 2006;51:85–107. doi: 10.1016/j.brainresrev.2005.10.004. [DOI] [PubMed] [Google Scholar]
- COUTTS A.A., PERTWEE R. Inhibition by cannabinoid receptor agonists of acetylcholine release from the guinea-pig myenteric plexus. Br. J. Pharmacol. 1997;121:1557–1566. doi: 10.1038/sj.bjp.0701301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CROCI T., BIANCHETTI A. Stimulation of faecal excretion in rats by alpha 2-adrenergic antagonists. J. Pharm. Pharmacol. 1992;44:358–360. doi: 10.1111/j.2042-7158.1992.tb03621.x. [DOI] [PubMed] [Google Scholar]
- CROCI T., MANARA L., AUREGGI G., GUAGNINI F., RINALDI-CARMONA M., MAFFRAND J.P., LEFUR G., MUKENGE S., FERLA G. In vitro functional evidence of neuronal cannabinoid CB1 receptors in human ileum. Br. J. Pharmacol. 1998;125:1393–1395. doi: 10.1038/sj.bjp.0702190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DESPRÉS J.P., GOLAY A., SJOSTROM L., Rimonabant in Obesity-Lipids Study Group Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N. Engl. J. Med. 2005;353:2121–2134. doi: 10.1056/NEJMoa044537. [DOI] [PubMed] [Google Scholar]
- DI MARZO V., GOPARAJU S.K., WANG L., LIU J., BATKAI S., JARAI Z., FEZZA F., MIURA G.I., PALMITER R.D., SUGIURA T., KUNOS G. Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature. 2001;410:822–825. doi: 10.1038/35071088. [DOI] [PubMed] [Google Scholar]
- DUNCAN M., DAVISON J.S., SHARKEY A. Review article: endocannabinoids and their receptors in the enteric nervous system. Aliment. Pharmacol. Ther. 2005;22:667–683. doi: 10.1111/j.1365-2036.2005.02648.x. [DOI] [PubMed] [Google Scholar]
- HEINEMANN A., SHAHBAZIAN A., HOLZER P. Cannabinoid inhibition of guinea-pig intestinal peristalsis via inhibition of excitatory and activation of inhibitory neural pathways. Neuropharmacology. 1999;38:1289–1297. doi: 10.1016/s0028-3908(99)00056-8. [DOI] [PubMed] [Google Scholar]
- HOWLETT A.C., BARTH F., BONNER T.I., CABRAL G., CASELLAS P., DEVANE W.A., FELDER C.C., HERKENHAM M., MACKIE K., MARTIN B.R., MECHOULAM R., PERTWEE R.G. International union of pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol. Rev. 2002;54:161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- HUNGUND B.L., SZAKALL I., ADAM A., BASAVARAJAPPA B.S., VADASZ C. Cannabinoid CB1 receptor knockout mice exhibit markedly reduced voluntary alcohol consumption and lack alcohol-induced dopamine release in the nucleus accumbens. J. Neurochem. 2003;84:698–704. doi: 10.1046/j.1471-4159.2003.01576.x. [DOI] [PubMed] [Google Scholar]
- IZZO A.A., CAPASSO R., PINTO L., DI CARLO G., MASCOLO N., CAPASSO F. Effect of vanilloid drugs on gastrointestinal transit in mice. Br. J. Pharmacol. 2001a;132:1411–1416. doi: 10.1038/sj.bjp.0703975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IZZO A.A., FEZZA F., CAPASSO R., BISOGNO T., PINTO L., IUVONE T., ESPOSITO G., MASCOLO N., DI MARZO V., CAPASSO F. Cannabinoid CB1-receptor mediated regulation of gastrointestinal motility in mice in a model of intestinal inflammation. Br. J. Pharmacol. 2001b;134:563–570. doi: 10.1038/sj.bjp.0704293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IZZO A.A., MASCOLO N., BORRELLI F., CAPASSO F. Defaecation, intestinal fluid accumulation and motility in rodents: implications of cannabinoid CB1 receptors. Naunyn-Schmiedeberg's Arch. Pharmacol. 1999a;359:65–70. doi: 10.1007/pl00005325. [DOI] [PubMed] [Google Scholar]
- IZZO A.A., MASCOLO N., CAPASSO F. The gastrointestinal pharmacology of cannabinoids. Curr. Opin. Pharmacol. 2001c;1:597–603. doi: 10.1016/s1471-4892(01)00102-3. [DOI] [PubMed] [Google Scholar]
- IZZO A.A., MASCOLO N., PINTO L., CAPASSO R., CAPASSO F. The role of cannabinoid receptors in intestinal motility, defaecation and diarrhoea in rats. Eur. J. Pharmacol. 1999b;384:37–42. doi: 10.1016/s0014-2999(99)00673-1. [DOI] [PubMed] [Google Scholar]
- IZZO A.A., MASCOLO N., TONINI M., CAPASSO F. Modulation of peristalsis by cannabinoid CB(1) ligands in the isolated guinea-pig ileum. Br. J. Pharmacol. 2000;129:984–990. doi: 10.1038/sj.bjp.0703116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KULKARNI-NARLA A., BROWN D.R. Opioid, cannabinoid and vanilloid receptor localization on porcine cultured myenteric neurons. Neurosci. Lett. 2001;308:153–156. doi: 10.1016/s0304-3940(01)01998-x. [DOI] [PubMed] [Google Scholar]
- KURZ A., SESSLER D. Opioid-induced bowel dysfunction: pathophysiology and potential new therapies. Drugs. 2003;63:649–671. doi: 10.2165/00003495-200363070-00003. [DOI] [PubMed] [Google Scholar]
- LAW P.Y., LOH H.H. Regulation of opioid receptor activities. J. Pharmacol. Exp. Ther. 1999;289:607–624. [PubMed] [Google Scholar]
- NAASSILA M., PIERREFICHE O., LEDENT C., DAOUST M. Decreased alcohol self-administration and increased alcohol sensitivity and withdrawal in CB1 receptor knockout mice. Neuropharmacology. 2004;46:243–253. doi: 10.1016/j.neuropharm.2003.09.002. [DOI] [PubMed] [Google Scholar]
- NAGAKURA Y., NAITOH Y., KAMATO T., YAMANO M., MIYATA K. Compounds possessing 5-HT3 receptor antagonistic activity inhibit intestinal propulsion in mice. Eur. J. Pharmacol. 1996;311:67–72. doi: 10.1016/0014-2999(96)00403-7. [DOI] [PubMed] [Google Scholar]
- PERTWEE R.G. Cannabinoids and the gastrointestinal tract. Gut. 2001;48:859–867. doi: 10.1136/gut.48.6.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERTWEE R.G., FERNANDO S.R., NASH J.E., COUTTS A.A. Further evidence for the presence of cannabinoid CB1 receptors in guinea-pig small intestine. Br. J. Pharmacol. 1996;118:2199–2205. doi: 10.1111/j.1476-5381.1996.tb15663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSS R.A., BROCKIE H.C., FERNANDO S.R., SAHA B., RAZDAN R.K., PERTWEE R.G. Comparison of cannabinoid binding sites in guinea-pig forebrain and small intestine. Br. J. Pharmacol. 1998;125:1345–1351. doi: 10.1038/sj.bjp.0702204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHOOK J.E., BURKS T.F. Psychoactive cannabinoids reduce gastrointestinal propulsion and motility in rodents. J. Pharmacol. Exp. Ther. 1989;249:444–449. [PubMed] [Google Scholar]
- STEINER H., BONNER T.I., ZIMMER A.M., KITAI S.T., ZIMMER A. Altered gene expression in striatal projection neurons in CB1 cannabinoid receptor knockout mice. Proc. Natl. Acad. Sci. U.S.A. 1999;96:5786–5790. doi: 10.1073/pnas.96.10.5786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STORR M., SIBAEV A., MARSICANO G., LUTZ B., SCHUSDZIARRA V., TIMMERMANS J.-P., ALLESCHER H.D. Cannabinoid receptor type 1 modulates excitatory and inhibitory neurotransmission in mouse colon. Am. J. Physiol. Gastrointest. Liver Physiol. 2004;286:G110–G117. doi: 10.1152/ajpgi.00148.2003. [DOI] [PubMed] [Google Scholar]
- VAN GAAL L.F., RISSANEN A.M., SCHEEN A.J., ZIEGLER O., ROSSNER S., RIO-Europe Study Group Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. Lancet. 2005;365:1389–1397. doi: 10.1016/S0140-6736(05)66374-X. [DOI] [PubMed] [Google Scholar]
- WALKER J.M., HUANG S.M., STRANGMAN N.M., TSOU K., SAÑUDO-PEÑA M.C. Pain modulation by release of the endogenous cannabinoid anandamide. Proc. Natl. Acad. Sci. U.S.A. 1999;96:12198–12203. doi: 10.1073/pnas.96.21.12198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZIMMER A., ZIMMER A.M., HOHMANN A.G., HERKENHAM M., BONNER T.I. Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc. Natl. Acad. Sci. U.S.A. 1999;96:5780–5785. doi: 10.1073/pnas.96.10.5780. [DOI] [PMC free article] [PubMed] [Google Scholar]