Abstract
Pharmacological inhibitors of protein kinase A (PKA) and protein phosphatases 1/2A were used to determine whether basal L-type Ca2+ current (ICa) observed in the absence of exogenous β-adrenergic receptor stimulation is sustained by PKA-mediated phosphorylation. Amphotericin B was used to record whole-cell ICa in the perforated patch-clamp configuration.
Calyculin A and isoprenaline (both 1 μmol l−1) increased basal ICa (P<0.05), whereas H-89 inhibited ICa in a concentration-dependent manner with an IC50 ∼5 μmol l−1. H-89 also inhibited the response to 1.0 μmol l−1 isoprenaline, although relatively high concentrations (30 μmol l−1) were required to achieve complete suppression of the response.
Double-pulse protocols were used to study the effects of 10 μmol l−1 H-89 on time-dependent recovery of ICa from voltage-dependent inactivation as well as the steady-state gating of ICa. T0.5 (time for ICa to recover to 50% of the preinactivation amplitude) increased in the presence of H-89 (P<0.05) but was unaffected by calyculin A or isoprenaline.
Steady-state activation/inactivation properties of ICa were unaffected by 10 μmol l−1 H-89 or 1 μmol l−1 calyculin A, whereas isoprenaline caused a leftward shift in both curves so that V0.5 for activation and inactivation became more negative.
Data show that basal ICa is regulated by cAMP-PKA-mediated phosphorylation in the absence of externally applied β-receptor agonists and that relatively high concentrations of H-89 are required to fully suppress the response to β-adrenergic receptor stimulation, thereby limiting the value of H-89 as a useful tool in dissecting signalling pathways in intact myocytes.
Keywords: Cardiac myocytes, calcium current, protein kinase A, H-89, calyculin A
Introduction
β-adrenergic receptor stimulation is well known to increase ICa in cardiac muscle (Mcdonald et al., 1994). This effect is mediated by cyclic AMP-dependent protein kinase A (cAMP-PKA)-dependent phosphorylation of Ser1928 on the α1-subunit that constitutes the functional pore of the channel (De Jongh et al., 1996; Perets et al., 1996), although phosphorylation of the Ser478 and Ser479 on the β-subunit may also modulate the response (Bunemann et al., 1999). Increased phosphorylation of the α1-subunit alters the gating properties of the channel to promote a higher open probability (Yue et al., 1990) that results in an increase in the macroscopic ICa across the whole cell.
While the role of cAMP-PKA-mediated phosphorylation in the response to catecholamines is undisputed (Kamp & Hell, 2000), it remains unclear as to whether cAMP-PKA also sustains the basal ICa observed in the absence of β-adrenergic receptor stimulation. This is important in the context of heart failure where downregulation of β-adrenergic receptors blunts the response to catecholamines so that other means of phosphorylating the channel become potentially more significant. Constitutive PKA-mediated phosphorylation, independent of β-adrenergic receptor stimulation, could under such circumstances play an important role in sustaining the ICa and thus contraction, as well as regulating other PKA-dependent phosphorylation targets. In addition, protein phosphatases are colocalised with the channel (Davare et al., 2000) and can also tonically regulate the phosphorylation status of the channel to modulate ICa (Herzig & Neumann, 2000; Dubell et al., 2002; Dubell & Rogers, 2004). Constitutive mechanisms that determine the basal levels of channel phosphorylation are therefore important determinants of ICa regulation.
Kinase and phosphatase inhibitors are a useful tool in elucidating the contribution of different mechanisms that regulate protein phosphorylation and thus cellular function. However, their usefulness depends largely on their selectivity and potency. H-89 (N-[2-p-bromo-cinnamylamino)ethyl]-5-isoquinolinesulfonamide) is of particular interest in this regard because it is described as being highly selective for PKA over the other kinases; its in vitro Ki is 50 nmol l−1 for PKA, 500 nmol l−1 for protein kinase G and 5–50 μmol l−1 for PKC and Ca2+-calmodulin-dependent protein kinase (Chijiwa et al., 1990; Hidaka & Kobayashi, 1992). However, previous studies that have investigated the effects of H-89 have yielded some conflicting results: some have shown that H-89 decreased basal ICa (Hussain et al., 1999) and attenuated the response to isoprenaline (Mitarai et al., 2000), whereas others did not observe inhibition of basal ICa in cardiac myocytes (Yuan & Bers, 1995; Chen et al., 2002; Dubell & Rogers, 2004), concluding that constitutive PKA-mediated phosphorylation was not involved. Given these discrepancies, the present study examined the effects of H-89 in detail, alongside those of calyculin A (an inhibitor of protein phosphatases PP1/PP2A) to assess its potential usefulness in clarifying the role of constitutive PKA-mediated phosphorylation in regulating ICa.
Methods
Isolation of ventricular myocytes
Male Wistar rats (250–350 g) were killed by a blow to the head followed by cervical dislocation in accordance with the Home Office Guidelines (Schedule 1, Animals Scientific Procedures Act, 1986). The heart was removed and cleared of blood by retrograde perfusion for 2–3 min with HEPES-Tyrodes containing 0.75 mmol l−1 CaCl2 (see Solutions and drugs for composition). This was followed by a 5 min perfusion with Ca2+-free HEPES-Tyrode containing 0.1 mmol l−1 EGTA (ethyleneglycol-bis(β-aminoethyl)-N,N,N′,N′-tetraacetic acid) and then with the enzyme solution containing 1.0 mg ml−1 collagenase (Type I; Worthington, Lakewood, Co, U.S.A.) and 0.05 mg ml−1 protease (Type XIV; Sigma, Poole, Dorset, U.K.) in low CaCl2 (50.0 μmol l−1) for 7 min. The left ventricle was removed, sliced into smaller chunks and agitated in the enzyme solution supplemented with 1.0% bovine serum albumin (Sigma, U.K.). Aliquots of myocytes were harvested at 5 min intervals by filtration of the digest through monofilament nylon cloth (Cadisch, U.K.) followed by gentle centrifugation of the filtrate (40 × g for 30 s). Pelleted myocytes were resuspended in enzyme-free isolation solution containing 0.75 mmol l−1 CaCl2 and maintained at 4°C in a Petri dish until used. The process was repeated 2–3 times to obtain a total of 3–4 batches of myocytes from each heart. Only cells that showed clearly defined striations and produced rapid contractions in response to field stimulation were used in this study. Myocyte isolation was carried out at 36±1°C.
Membrane currents
ICa was recorded in BAPTA AM (10 μmol l−1; 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis(acetoxymethyl ester)-loaded ventricular myocytes in the perforated patch-clamp configuration using amphotericin B (240 μg ml−1) as the pore-forming agent. Voltage clamp was performed using the Axopatch 200 B amplifier (Molecular Devices Corporation, Sunnyvale, CA, U.S.A.) linked to a Digidata 1200B A/D interface. ICa was filtered at 5 kHz, acquired using Clampex 8.2 and analysed with Clampfit 8.2 (Molecular Devices Corporation). Glass micropipettes were fabricated from filamented borosilicate capillaries (GC150TF, Harvard, MA, U.S.A.) to give tip resistances ∼1 MΩ when fire-polished and filled with a cesium-based pipette solution (see Solutions for composition). Current–voltage (I–V) curves for ICa were obtained by 5–10 mV steps from a holding potential of −40 to 60 mV. Conventional double-pulse protocols were used to obtain activation/inactivation curves: myocytes were depolarised from −40 to 60 mV for 400 ms in 5 mV increments, followed by a second test pulse to 0 mV. The currents obtained during the prepulse were corrected for the driving force and normalised to the peak current to obtain the activation curves, whereas ICa obtained during the second step (to 0 mV) was normalised and plotted against the prepulse potential to obtain the inactivation curves. A second series of double-pulse protocols was used to measure the time course of recovery from inactivation. Two successive pulses from –40 to 0 mV were separated by progressively increasing time intervals in 20 ms increments. ICa recorded during the second pulse were normalised and plotted against the interpulse interval. ICa were recorded at 35°C, unless stated otherwise.
Solutions
The composition of the physiological salt solution used during isolation procedure and storage of myocytes contained (mmol l−1): NaCl, 130; KCl, 5.4; MgCl2, 1.4; NaH2PO4, 0.4; HEPES, 10; glucose, 10; taurine, 20 and creatine, 10; and adjusted to pH 7.3 with 1 M NaOH. This was supplemented with either EGTA or Ca2+ as described above.
The external Tyrode solution used to superfuse cells during perforated patch-clamp experiments contained (mmol l−1) NaCl, 140; KCl, 5.4; MgCl2, 1; glucose, 10; HEPES, 10; CaCl2, 1; and adjusted to pH 7.35 with 1 M NaOH. Patch pipette solution contained (mmol l−1) Cs-glutamate, 120; KCl, 20; NaCl, 10; HEPES, 10, adjusted to pH 7.3 with 1 mol l−1 CsOH.
Data analysis and statistics
All data are expressed as mean±s.e.m. of n observations. Statistical comparisons were made (SPSS, vers. 11) using ANOVA and Student's t-test for independent samples. P<0.05 was deemed statistically significant.
Results
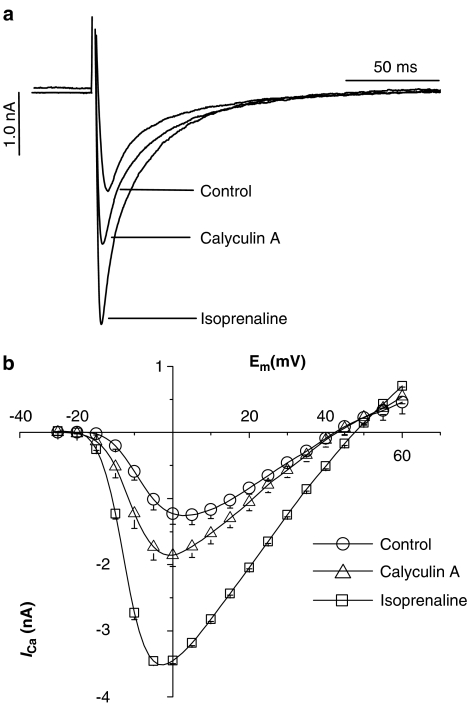
Figure 1 shows representative tracings of ICa recorded upon depolarisation from −40 to 0 mV (A) and the I–V curves (B) to demonstrate that β-adrenergic receptor stimulation with 1.0 μmol l−1 isoprenaline significantly increased ICa. Control ICa at 0 mV was −1.23±0.156 nA and increased by 181% to −3.45±0.04 nA. Similarly, 1.0 μmol l−1 calyculin A also increased ICa, although not to the same extent as isoprenaline: ICa increased by 52% to 1.87±0.17 nA (P<0.05). These data confirm that basal ICa can be modulated not only by β-stimulation but also by inhibition of constitutively active endogenous protein phosphatases PP1/2A in the absence of external stimulation.
Figure 1.
Effects of calyculin A and isoprenaline (both 1 μmol l−1) on ICa. (a) Representative tracings of ICa elicited by square depolarising pulses from −40 to 0 mV in the absence and presence of calyculin A and isoprenaline. (b) I–V curves for ICa obtained in response to 5 mV step increments from −40 to 60 mV. Each data point is the mean±s.e.m. of 8–10 different cells. Standard errors not visible are smaller than the symbols.
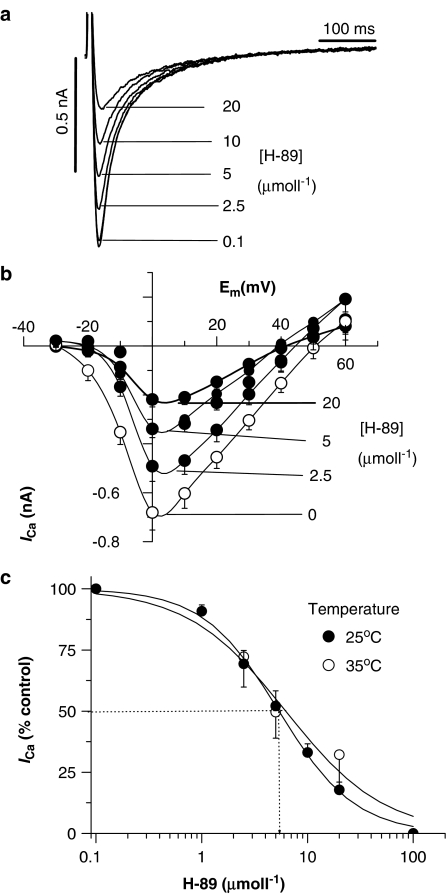
To investigate whether endogenous PKA also played a role in sustaining basal ICa, the PKA inhibitor H-89 was tested. Figure 2 shows original ICa recordings (A) in the absence and in the presence of a range of H-89 concentrations to show that ICa is inhibited in a concentration-dependent manner. Figure 2b shows the effects of a range of H-89 concentrations on the I–V relations for ICa to illustrate that the inhibitory effect of H-89 was similar at all the potentials tested and did not alter the bell-shaped voltage dependence of the I–V curve or the reversal potential for the outward flow of Ca2+. The IC50 was 5.4 μmol l−1 (Figure 2c; open symbols), a value similar to that described previously for the effects of H-89 on cell shortening in ferret ventricular myocytes (Hussain et al., 1999), as well as inhibition of tetanic Ca2+ transients in skeletal muscle (Blazev et al., 2001). Similar data were also obtained when ICa were recorded at room temperature, where the IC50 was 5.2 μmol l−1 (Figure 2c; closed symbols).
Figure 2.
Effects of H-89 on ICa in isolated rat ventricular myocytes. (a) Representative original traces of ICa at differing concentrations of H-89. (b) I–V curves of ICa in the absence and presence of different concentrations of H-89. (c) Concentration–effect curve for H-89 at 35 and 25°C. Each data point is the mean±s.e.m. from six to eight cells.
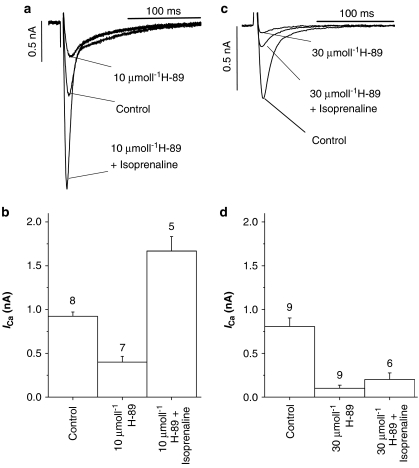
To establish whether the inhibitory effect of H-89 could be attributed to the inhibition of PKA, data in Figure 3 show the effects of 1 μmol l−1 isoprenaline in the presence of H-89. During these experiments, myocytes were first exposed to either 10 or 30 μmol l−1 H-89 until a steady-state level of ICa was achieved (typically 5–8 min). The solutions were then switched to H-89 plus isoprenaline. Figure 3a shows ICa tracings illustrating the effect of isoprenaline in the presence of 10 μmol l−1 H-89, a concentration that is almost double the IC50 value (see Figure 2). Although the response to isoprenaline was attenuated, it was not abolished: ICa increased by 93% in the presence of 10 μmol l−1 H-89 plus isoprenaline (Figure 3b). However, in the presence of 30 μmol l−1 H-89, the response to isoprenaline was almost completely blocked and ICa amplitude remained close to the values observed in the presence of 30 μmol l−1 H-89 alone (i.e. 23% of control; Figure 3d) and was not significantly different from this value. These data show that while 10 μmol l−1 H-89 did attenuate the effects of β-adrenergic receptor stimulation, relatively high concentrations (30 μmol l−1) were required to fully suppress the isoprenaline-induced increase in ICa.
Figure 3.
Effects of H-89 on the response to isoprenaline. The response to isoprenaline was determined following equilibration of myocytes with either 10 (a and b) or 30 μmol l−1 (c and d) H-89. The number above each bar is the number of separate myocytes tested. All the bars shown were significantly different from one another, except the effects of 30 μmol l−1 H-89 vs 30 μmol l−1 H-89 plus isoprenaline.
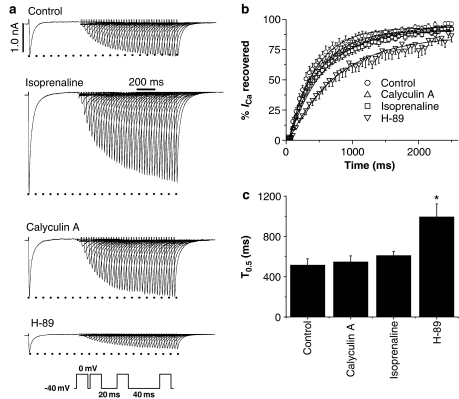
To gain further insights into mechanisms by which H-89 might act on the L-type Ca2+ channels, double-pulse protocols were used to investigate the effects of H-89, calyculin A and isoprenaline on time-dependent recovery of ICa from voltage-dependent inactivation. Original tracings in Figure 4a illustrate that under control conditions, ICa amplitude during the second test-pulse was small when the interpulse interval was short (e.g. 20 ms for the first pulse) and that ICa increased as the rest period was progressively lengthened such that at long interpulse intervals ICa recovered to the same amplitude as the ICa observed during the prepulse. A similar recovery of ICa from voltage-dependent inactivation was observed in the presence of calyculin A but not in the presence of H-89. This is shown quantitatively in Figure 4b and c, where ICa amplitude determined during the second test pulse was normalised to that in the pre–pulse and plotted against time before fitting with the Boltzmann function to determine T0.5 (the time taken for ICa to recover to 50% of the ICa amplitude observed during the pre–pulse). Mean (±s.e.m.) %ICa recovered is shown in Figure 4b alongside the effects of calyculin A, isoprenaline and H-89. T0.5 values are shown in Figure 4c to illustrate that the time course of recovery from voltage-dependent inactivation was significantly slowed in the presence of H-89 (P<0.05), but was not significantly different in the presence of calyculin A or isoprenaline (both 1 μmol l−1).
Figure 4.
Effects of H-89, calyculin A and isoprenaline on recovery of ICa from voltage-dependent inactivation. (a) The inset in the bottom part of the figure shows the double-pulse protocol during which myocytes were depolarised from −40 to 0 mV with a progressively increasing interpulse interval (20 ms increments). The other panels in (a) show representative tracings illustrating the recovery of ICa in control conditions and in the presence of calyculin A (1 μmol l−1), isoprenaline (1 μmol l−1) and H-89 (10 μmol l−1). (b) Mean (±s.e.m.) time course of ICa recovery fitted with the Boltzmann equation. (c) Mean (±s.e.m.) T0.5 under control conditions (n=6) and in the presence of calyculin A (n=6), isoprenaline (n=4) and H-89 (n=9). *P<0.05.
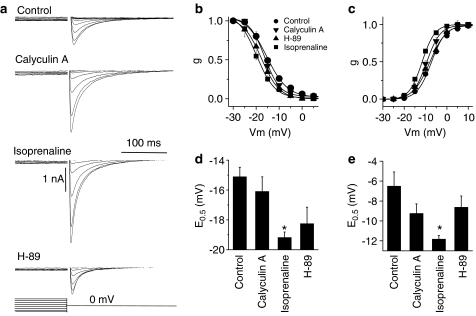
To investigate the effects of the three compounds on channel availability, a second series of double-pulse protocols were performed to obtain the steady-state activation and inactivation curves for ICa. In these experiments, a 400 ms pulse to potentials between −40 and 60 mV was followed by a second pulse to 0 mV. ICa obtained at each potential were converted to conductance (g) using the following equation: g=ICa/(Em–Erev) to account for potential-dependent differences in the driving force for Ca2+ entry. The conductance was then normalised to the maximal value to generate the activation curves, which were then fitted with the Boltzmann function to obtain E0.5 (Vm at which half-maximal conductance is obtained). In order to generate the inactivation curves, ICa amplitudes during the second step to 0 mV were first converted to conductance (as above) and then plotted against the prepulse potential before fitting with the Boltzmann equation to obtain E0.5 for inactivation. Figure 5a shows ICa during the second step to 0 mV after the pre-test potential was varied between −40 to 60 mV in 5 mV increments. Maximal ICa during the second step were obtained when the pre-test potential did not activate ICa during the first few steps (e.g. −30 mV). ICa then progressively declined as the prepulse voltage was stepped to more positive values. Figure 5b shows the inactivation curves alongside the E0.5 (Figure 5d) in control and in the presence of calyculin A, isoprenaline and H-89. Similarly, Figure 5c shows the activation curves and E0.5 for activation of ICa (Figure 5e). Only isoprenaline significantly decreased E0.5 for activation and inactivation by shifting the curves to more negative Vm values. Although calyculin A and H-89 both decreased E0.5 for both activation and inactivation, these effects did not reach statistical significance (Figure 5d and e).
Figure 5.
Effects of cayculin A, isoprenaline and H-89 on voltage-dependent activation and inactivation of ICa. Myocytes were stimulated using a double-pulse protocol where the voltage of the first pulse was increased in 5 mV step increments (400 ms) from −40 to 60 mV followed by a second step to 0 mV after 2 ms. ICa recorded were converted to conductance (see text). (a) ICa during the second pulse to 0 mV in control conditions and in the presence of 1 μmol l−1 calyculin A, 1 μmol l−1 isoprenaline and 10 μmol l−1 H-89. In each case, the largest amplitude ICa was recorded during the first step in the protocol. (b) Steady-state inactivation curves. (c) Steady-state activation curves. (d) Mean (±s.e.m.) voltage at half-maximal conductance for inactivation. (e) Mean (±s.e.m.) voltage for half-maximal conductance for activation. Both activation and inactivation curves were fitted with the Boltzmann function. Mean data are from 6–8 separate myocytes. *P<0.05.
Discussion
A range of protein kinase and phosphatase inhibitors has previously been used to investigate the identity of the protein kinase(s) and phosphatases that sustain the basal ICa (e.g. Hartzell et al., 1995; Yuan & Bers, 1995; Hirayama & Hartzell, 1997; Dubell et al., 2002; Dubell & Rogers, 2004). Such tools can be an invaluable adjunct to other techniques, such as biochemical measurements of phosphorylation, in deciphering the cellular mechanisms of function and even the interactions between different pathways. However, for this to be possible, it is necessary to establish the selectivity and potency of the compounds used in the setting in which they are tested. In the present study, we evaluated the PKA inhibitor H-89 to assess the role of PKA-mediated phosphorylation in maintaining basal ICa in the absence of β-adrenergic receptor stimulation, as well as its role in the response to such stimulation.
The main findings were that H-89 produced a concentration-dependent inhibition of ICa with an IC50 of ∼5.0 μmol l−1, irrespective of the experimental temperature. H-89 also blocked the response to β-adrenergic receptor stimulation with 1 μmol l−1 isoprenaline, although concentrations as high as 30 μmol l−1 were required to achieve this. Secondly, basal ICa could be increased by inhibition of PP1/PP2A by calyculin A, although the response was smaller than that to isoprenaline. The overall interpretation of these data seems fairly straightforward–that basal ICa is regulated by constitutive PKA-mediated phosphorylation, which also appears to be modulated by protein phosphatases PP1/PP2A. However, as noted in the introduction, some previous studies were unable to demonstrate such inhibition of basal ICa with H-89, leading to the conclusion that PKA-mediated phosphorylation was unlikely to be involved. Data from the present study (Figure 3) can explain this apparent discrepancy.
Previous studies, rather cautiously, used relatively low concentrations (1 μmol l−1) of H-89, presumably to avoid nonspecific effects on other kinases (Yuan & Bers, 1994; Dubell & Rogers, 2004). Such low concentrations were probably selected on the basis that the in vitro Ki for H-89 is 50 nmol l−1. However, concentration–effect curves in the present study clearly show that the IC50 for H-89 in rat ventricular myocytes is ∼5 μmol l−1 and that 1 μmol l−1 produces only a small effect (<10% inhibition). It seems reasonable, therefore, to suggest that the low concentrations used in some of the previous studies were simply too low to produce any significant inhibition of ICa. It is difficult, however, to explain why 10 μmol l−1 H-89 was ineffective in ferret ventricular myocytes (Yuan & Bers, 1995), in which we have previously shown that the IC50 for inhibiting cell shortening was also ∼5.0 μmol l−1 (Hussain et al., 1999). Furthermore, similar concentrations of H-89 have also been shown to be effective in inhibiting Ca2+ transients during tetanic contractions in skeletal muscle (Blazev et al., 2001), and even lower H-89 concentrations (Ki 76.4 nmol l−1) could inhibit ICa recorded from cloned channels in HEK-293 cells (Perez-Reyes et al., 1994). One possible reason for these apparent discrepancies could be that the in vitro Ki determined using purified kinases (Chijiwa et al., 1990) is not directly applicable to intact cardiac myocytes, where higher concentrations are required, perhaps due to inadequate equilibration even after a steady state is reached. Differences in the Ki values could also arise if different channel types are studied for example, cloned vs native Ca2+ channels (Perez-Reyes et al., 1994). It is therefore essential that the effective concentrations be determined empirically in the test system under study.
The second aspect to the inhibitory action of H-89 on ICa was the concentration required to inhibit the response to β-adrenergic receptor stimulation. A previous study showed that the response to 0.1 μmol l−1 isoprenaline could be blocked by 10 μmol l−1 H-89 (Mitarai et al., 2000). In the present study, 10 μmol l−1 H-89 attenuated the response to 1 μmol l−1 isoprenaline, but a much higher concentration (30 μmol l−1) was required to produce complete inhibition. The most likely explanation for this observation is that a much greater concentration of the free catalytic subunit of PKA becomes available in the presence of 1.0 μmol l−1 isoprenaline. Consequently, much higher concentrations of H-89 are required for effective inhibition. This explanation is plausible given that the proposed mechanism of action of H-89 is thought to involve competitive binding at the ATP binding site on the catalytic subunit rather than interactions with the cAMP binding site on the regulatory subunit. [ATP] in myocytes is normally in the millimolar range and therefore not a limiting factor (Elliott et al., 1989). This mechanism of action has been postulated from binding studies performed to assess the degree of competition between binding of ATP and H-89 to PKA (Hidaka & Kobayashi, 1992).
L-type Ca2+ channels are subject to Ca2+-dependent inactivation as well as voltage-dependent inactivation, which has several components (Hadley & Lederer, 1991; Findlay, 2002a). Ca2+-dependent inactivation occurs as Ca2+ enters the cell across the channel pore, as well as due to the Ca2+ released from the sarcoplasmic reticulum. Voltage-dependent inactivation, although not particularly well characterised in molecular terms, is likely to involve conformational changes in the channel complex to impede or obstruct the flow of Ca2+ across the channel pore. The inactivation mechanism(s) may also be modulated by the ancillary subunits as well as the phosphorylation status of any number of these components (Mitarai et al., 2000; Findlay, 2002b; 2004).
β-Adrenergic stimulation is well known to alter the steady-state activation and inactivation of L-type Ca2+ channels and the E0.5 for activation and inactivation of ICa is typically shifted to the left (Chen et al., 2002). This implies that the channel is more susceptible to activation and inactivation when phosphorylated, and that lower voltages would be required to activate or inactivate the channel. A logical extension of this argument is that dephosphorylation, for example with H-89, may be expected to have the opposite effect and shift the activation/inactivation curves to the right. Data in Figure 5 show that while the expected leftward shift in the activation/inactivation curves was observed in response to isoprenaline, H-89 and calyculin A had no significant effect in either direction, suggesting that the voltage sensor was unlikely to be affected. In fact, although the effects of H-89 were not significant, the general trend was for the E0.5 values to be decreased in the presence of H-89 (i.e. in the same direction as in response to isoprenaline). Similar data were also reported by Yuan & Bers (1994; 1995), where the E0.5 values were significantly shifted in the negative direction. These data suggest that the effects of H-89 may be more complex than simple inhibition of PKA.
In addition, in paired-pulse experiments performed to examine whether there was an effect on the overall time course of recovery of ICa from inactivation, H-89 (10 μmol l−1) was found to slow the time-dependent recovery of ICa from inactivation (Figure 4), whereas isoprenaline and calyculin A had no significant effect. Whether this effect of H-89 was due to decreased PKA-mediated phosphorylation is difficult to say given that isoprenaline and calyculin did not have the opposite effect. It is interesting to note, however, that this effect of H-89 on recovery from inactivation was previously observed in studies where ICa amplitude was largely unaffected (Yuan & Bers, 1994; 1995). The fact that the two effects can occur independently suggests that different mechanisms could be involved simultaneously. Inhibition of ICa could, for instance, occur in response to the effects of H-89 on PKA-mediated phosphorylation, whereas the slowed recovery from inactivation could occur owing to nonspecific actions on other targets. That H-89 has the potential to produce additional non-PKA-related effects is supported by several lines of evidence. First, we have previously shown that H-89 could inhibit SR Ca2+ uptake in SR vesicles independently of the effect on phosphorylation of phospholamban, probably due to a direct effect on the SR Ca2+ ATPase (Hussain et al., 1999). Secondly, in the accompanying paper, we show that H-89 can also inhibit K+ currents that are not sensitive to modulation by β-adrenergic receptor stimulation. It is therefore necessary to consider the concentrations of H-89 used carefully before implicating the involvement or exclusion of PKA-mediated phosphorylation.
Given that basal PKA-mediated phosphorylation does appear to be involved in sustaining ICa, it is of interest to know the mechanisms responsible for PKA activity that exists in the absence of β-adrenergic receptor stimulation, that is, is there a basal level of activation of β-adrenergic receptors even in the absence of catecholamines? or are there separate constitutive mechanism(s) responsible for the tonic levels of PKA-mediated phosphorylation? These questions could be important in the context of heart failure, where alterations in basal phosphorylation of L-type Ca2+ channels, perhaps due to enhanced phosphatase activity (Chen et al., 2002; Neumann, 2002), could contribute to the pathogenesis of the disease.
Abbreviations
- cAMP-PKA
cyclic AMP-dependent protein kinase A
- EGTA
ethyleneglycol-bis(β-aminoethyl)-N,N,N′,N′-tetraacetic Acid
- H-89
N-[2-p-bromo-cinnamylamino)ethyl]-5-isoquinolinesulfonamide
- ICa
L-type Ca2+ current
- I–V
current–voltage
- PP1/2A
protein phosphatase 1/2A
- Ser
serine
- T0.5
time to recover to 50% of the preinactivation amplitude
References
- BLAZEV R., HUSSAIN M., BAKKER A.J., HEAD S.I., LAMB G.D. Effects of the PKA inhibitor H-89 on excitation–contraction coupling in skinned and intact skeletal muscle fibres. J. Muscle Res. Cell. Motil. 2001;22:277–286. doi: 10.1023/a:1012289526618. [DOI] [PubMed] [Google Scholar]
- BUNEMANN M., GERHARDSTEIN B.L., GAO T., HOSEY M.M. Functional regulation of L-type calcium channels via protein kinase A-mediated phosphorylation of the beta(2) subunit. J. Biol. Chem. 1999;274:33851–33854. doi: 10.1074/jbc.274.48.33851. [DOI] [PubMed] [Google Scholar]
- CHEN X., PIACENTINO V., III, FURUKAWA S., GOLDMAN B., MARGULIES K.B., HOUSER S.R. L-type Ca2+ channel density and regulation are altered in failing human ventricular myocytes and recover after support with mechanical assist devices. Circ. Res. 2002;91:517–524. doi: 10.1161/01.res.0000033988.13062.7c. [DOI] [PubMed] [Google Scholar]
- CHIJIWA T., MISHIMA A., HAGIWARA M., SANO M., HAYASHI K., INOUE T., NAITO K., TOSHIOKA T., HIDAKA H. Inhibition of forskolin-induced neurite outgrowth and protein phosphorylation by a newly synthesized selective inhibitor of cyclic AMP-dependent protein kinase, N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide (H-89), of PC12D pheochromocytoma cells. J. Biol. Chem. 1990;265:5267–5272. [PubMed] [Google Scholar]
- DAVARE M.A., HORNE M.C., HELL J.W. Protein phosphatase 2A is associated with class C L-type calcium channels (Cav1.2) and antagonizes channel phosphorylation by cAMP-dependent protein kinase. J. Biol. Chem. 2000;275:39710–39717. doi: 10.1074/jbc.M005462200. [DOI] [PubMed] [Google Scholar]
- DE JONGH K.S., MURPHY B.J., COLVIN A.A., HELL J.W., TAKAHASHI M., CATTERALL W.A. Specific phosphorylation of a site in the full-length form of the alpha 1 subunit of the cardiac L-type calcium channel by adenosine 3′,5′-cyclic monophosphate-dependent protein kinase. Biochemistry. 1996;35:10392–10402. doi: 10.1021/bi953023c. [DOI] [PubMed] [Google Scholar]
- DUBELL W.H., ROGERS T.B. Protein phosphatase 1 and an opposing protein kinase regulate steady-state L-type Ca2+ current in mouse cardiac myocytes. J. Physiol. 2004;1;556 (Part 1):79–93. doi: 10.1113/jphysiol.2003.059329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUBELL W.H., GIGENA M.S., GUATIMOSIM S., LONG X., LEDERER W.J., ROGERS T.B. Effects of PP1/PP2A inhibitor calyculin A on the E–C coupling cascade in murine ventricular myocytes. Am. J. Physiol. 2002;282:H38–H48. doi: 10.1152/ajpheart.00536.2001. [DOI] [PubMed] [Google Scholar]
- ELLIOTT A.C., SMITH G.L., ALLEN D.G. Simultaneous measurements of action potential duration and intracellular ATP in isolated ferret hearts exposed to cyanide. Circ. Res. 1989;64:583–591. doi: 10.1161/01.res.64.3.583. [DOI] [PubMed] [Google Scholar]
- FINDLAY I. Physiological modulation of inactivation in L-type Ca2+ channels: one switch. J. Physiol. 2004;554 (Part 2):275–283. doi: 10.1113/jphysiol.2003.047902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FINDLAY I. Voltage- and cation-dependent inactivation of L-type Ca2+ channel currents in guinea-pig ventricular myocytes. J. Physiol. 2002a;541 (Part 3):731–740. doi: 10.1113/jphysiol.2002.019729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FINDLAY I. beta-Adrenergic stimulation modulates Ca2+- and voltage-dependent inactivation of L-type Ca2+ channel currents in guinea-pig ventricular myocytes. J. Physiol. 2002b;541 (Part 3):741–751. doi: 10.1113/jphysiol.2002.019737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HADLEY R.W., LEDERER W.J. Ca2+ and voltage inactivate Ca2+ channels in guinea-pig ventricular myocytes through independent mechanisms. J. Physiol. 1991;444:257–268. doi: 10.1113/jphysiol.1991.sp018876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARTZELL H.C., HIRAYAMA Y., PETIT-JACQUES J. Effects of protein phosphatase and kinase inhibitors on the cardiac L-type Ca current suggest two sites are phosphorylated by protein kinase A and another protein kinase. J. Gen. Physiol. 1995;106:393–414. doi: 10.1085/jgp.106.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERZIG S., NEUMANN J. Effects of serine/threonine protein phosphatases on ion channels in excitable membranes. Physiol. Rev. 2000;80:173–210. doi: 10.1152/physrev.2000.80.1.173. [DOI] [PubMed] [Google Scholar]
- HIDAKA H., KOBAYASHI R. Pharmacology of protein kinase inhibitors. Ann. Rev. Pharmacol. Toxicol. 1992;32:377–397. doi: 10.1146/annurev.pa.32.040192.002113. [DOI] [PubMed] [Google Scholar]
- HIRAYAMA Y., HARTZELL H.C. Effects of protein phosphatase and kinase inhibitors on Ca2+ and Cl− currents in guinea pig ventricular myocytes. J. Pharmacol. Exp. Therap. 1997;52:725–734. doi: 10.1124/mol.52.4.725. [DOI] [PubMed] [Google Scholar]
- HUSSAIN M., DRAGO G.A., BHOGAL M., COLYER J., ORCHARD C.H. Effects of the protein kinase A inhibitor H-89 on Ca2+ regulation in isolated ferret ventricular myocyte. Pflugers Arch. 1999;437:529–537. doi: 10.1007/s004240050814. [DOI] [PubMed] [Google Scholar]
- KAMP T.J., HELL J.W. Regulation of L-type calcium channels by protein kinase A and protein kinase C. Circ. Res. 2000;87:1095–1102. doi: 10.1161/01.res.87.12.1095. [DOI] [PubMed] [Google Scholar]
- MCDONALD T.F., PELZER S., TRAUTWEIN W., PELZER D.J. Regulation and modulation of calcium channels in cardiac, skeletal, and smooth muscle cells. Physiol. Rev. 1994;74:365–507. doi: 10.1152/physrev.1994.74.2.365. [DOI] [PubMed] [Google Scholar]
- MITARAI S., KAIBARA M., YANO K., TANIYAMA K. Two distinct inactivation processes related to phosphorylation in cardiac L-type Ca2+ channel currents. Am. J. Physiol. 2000;279:C603–C610. doi: 10.1152/ajpcell.2000.279.3.C603. [DOI] [PubMed] [Google Scholar]
- NEUMANN J. Altered phosphatase activity in heart failure, influence on Ca2+ movement. Basic Res. Cardiol. 2002;97 (Suppl 2):I91–I95. doi: 10.1007/s003950200036. [DOI] [PubMed] [Google Scholar]
- PERETS T., BLUMENSTEIN Y., SHISTIK E., LOTAN I., DASCAL N. A potential site of functional modulation by protein kinase A in the cardiac Ca2+ channel alpha 1C subunit. FEBS Lett. 1996;384:189–192. doi: 10.1016/0014-5793(96)00303-1. [DOI] [PubMed] [Google Scholar]
- PEREZ-REYES E., YUAN W., WEI X., BERS D.M. Regulation of the cloned L-type cardiac calcium channel by cyclic-AMP-dependent protein kinase. FEBS Lett. 1994;342:119–123. doi: 10.1016/0014-5793(94)80484-2. [DOI] [PubMed] [Google Scholar]
- YUAN W., BERS D.M. Ca-dependent facilitation of cardiac Ca current is due to Ca-calmodulin-dependent protein kinase. Am. J. Physiol. 1994;267 (Part 2):H982–H993. doi: 10.1152/ajpheart.1994.267.3.H982. [DOI] [PubMed] [Google Scholar]
- YUAN W., BERS D.M. Protein kinase inhibitor H-89 reverses forskolin stimulation of cardiac L-type calcium current. Am. J. Physiol. 1995;268 (Part 1):C651–C659. doi: 10.1152/ajpcell.1995.268.3.C651. [DOI] [PubMed] [Google Scholar]
- YUE D.T., HERZIG S., MARBAN E. Beta-adrenergic stimulation of calcium channels occurs by potentiation of high-activity gating modes. Proc. Natl. Acad. Sci. USA. 1990;87:753–757. doi: 10.1073/pnas.87.2.753. [DOI] [PMC free article] [PubMed] [Google Scholar]