Abstract
Voltage clamp was used to investigate the effects of N-[2-p-bromo-cinnamylamino)ethyl]-5-isoquinolinesulfonamide (H-89), a potent inhibitor of PKA, on transient outward K+ current (Ito) and inward rectifying K+ current (IK1) in rat cardiac muscle.
Initial experiments, performed using descending voltage ramps, showed that H-89 inhibited both the outward and inward ramp currents in a concentration-dependent manner at concentrations between 5 and 60 μmol l−1. A similar degree of inhibition was observed when Ito and IK1 were recorded using square wave depolarising and hyperpolarising voltage steps, respectively.
The IC50 was 35.8 μmol l−1 for Ito and 27.8 μmol l−1 for IK1 compared to 5.4 μmol l−1 for L-type Ca2+ current (ICa). The Hill coefficients for Ito, IK1 and ICa were −1.97, −1.60 and −1.21, respectively. In addition to inhibiting Ito amplitude, H-89 also accelerated the time to peak and the rate of voltage-dependent inactivation so that the time course of Ito was abbreviated.
Paired-pulse protocols were performed to study the effects of H-89 on steady-state activation and inactivation as well as recovery from voltage-dependent inactivation. H-89 produced a concentration-dependent rightward shift in voltage-dependent activation but had no significant effect on steady-state inactivation. Recovery from voltage-dependent inactivation was delayed, although this was only visible at the highest concentration (60 μmol l−1) used.
In experiments investigating the effects of elevated cyclic AMP, the β-adrenergic agonist isoprenaline and the phosphatase inhibitor calyculin A had no major effects on Ito or IK1.
Data suggest that the effects of H-89 on K+ currents are more complex than simple inhibition of PKA-mediated phosphorylation.
Keywords: K+ currents; H-89, myocytes; PKA; phosphorylation
Introduction
Depolarisation of rat cardiac muscle results from the influx of Na+ and Ca2+ through voltage gated ion channels that gives rise to the fast Na+ current (INa) and the slower L-type Ca2+ current (ICa), respectively. Repolarisation occurs due to K+ efflux through three main types of ionic currents: transient outward K+ current (Ito), responsible for the early repolarisation that occurs near the peak of the action potential; inward rectifying K+ current (IK1), responsible for maintaining the resting potential and a species-dependent steady-state current (Iss) that persists during sustained depolarisation. While increased phosphorylation of Na+ and Ca2+ channels, mediated by protein kinase A (PKA), is well known to underlie the response to β-adrenergic receptor stimulation (for reviews see Rossie, 1999; van der Heyden et al., 2005), the role of such phosphorylation in regulating potassium channels is controversial due to a significant body of contradictory data.
Stimulation of β-adrenergic receptors has been reported to increase IK1 in some studies (Gadsby, 1983; Dart & Leyland, 2001; Zitron et al., 2004) whereas others have reported a decrease (Tromba & Cohen, 1990; Koumi et al., 1995). The situation with respect to Ito is also unclear. While it is widely reported that regulation of Ito in response to catecholamines is mediated through stimulation of α1-adrenergic receptors coupled to protein kinase C (PKC), which results in a decreased Ito (for review see Oudit et al., 2001), some studies have also implicated PKA-mediated signalling in sustaining Ito (Xiao & Mcardle 1995; Gallego et al., 2005). In other species (e.g. guinea-pig) where the delayed rectifying potassium current (IK) is prevalent during sustained depolarisation, β-adrenergic receptor stimulation was, again, found to decrease current amplitude in some studies (Iijima et al., 1990; Karle et al., 2002) whereas others reported an increase (e.g. Freeman et al., 1992; Lei et al., 2000).
In view of these conflicting results, the aim of the present study was to investigate the effects of N-[2-p-bromo-cinnamylamino)ethyl]-5-isoquinolinesulfonamide (H-89), a potent and potentially selective inhibitor of PKA, on K+ currents to determine whether PKA-mediated phosphorylation played a role in sustaining these currents in rat cardiac muscle. H-89 has an in vitro Ki of 50 nmol l−1 for inhibiting PKA, which is about 10 times lower than that for inhibiting protein kinase G (PKG) and a hundred times lower than that for PKC (Chijiwa et al., 1990; Hidaka & Kobayashi, 1992). Given that in the accompanying paper we have already clearly demonstrated PKA-mediated inhibitory effects of H-89 on ICa, it was of interest to know whether there was any similarity between these and any effects on K+ currents. The actions of H-89 were assessed alongside those of calyculin A, an inhibitor of serine/threonine phosphatases PP1 and PP2 (Ishihara et al., 1989), and the β1-agonist isoprenaline. The main findings were that modulation of PKA-mediated phosphorylation with isoprenaline had no major effect on Ito or Iss and only slightly decreased IK1. Calyculin A had no effect on any of the three currents whereas H-89 inhibited all three but at concentrations higher than those required for ICa, suggesting that the action of H-89 on K+ currents was more complex and could be independent of direct inhibitory effects on PKA.
Methods
Cell isolation
Male Wistar rats (250–350 g) were killed by a blow to the head followed by cervical dislocation in accordance with the Home Office Guidelines (Schedule 1, Animals Scientific Procedures Act, 1986). The heart was removed and cleared of blood by retrograde perfusion for 2–3 min with HEPES-Tyrodes containing 0.75 mmol l−1 CaCl2 (see solutions and drugs for composition). This was followed by perfusion for 5 min with Ca2+-free HEPES-Tyrode containing 0.1 mmol l−1 ethyleneglycol-bis(β-aminoethyl)-N,N,N′,N′-tetraacetic acid and then with the enzyme solution containing 1.0 mg ml−1 collagenase (Type I; Worthington, Lakewood, U.S.A.) and 0.05 mg ml−1 protease (Type XIV; Sigma, Poole, Dorset, U.K.) in low CaCl2 (50.0 μmol l−1) for 6–7 min. The left ventricle was removed, sliced into smaller chunks and agitated in the enzyme solution supplemented with 1.0% bovine serum albumin (Sigma, U.K.). Aliquots of myocytes were harvested at 5 min intervals by filtration of the digest through monofilament nylon cloth (Cadisch, U.K.) followed by gentle centrifugation of the filtrate (40 g for 30 s). Pelleted myocytes were resuspended in enzyme-free isolation solution containing 0.75 mmol l−1 CaCl2 and maintained at 4°C in a Petri dish until used. The process was repeated 2–3 times to obtain a total of 3–4 batches of myocytes from each heart. Only cells that showed clearly defined striations and produced rapid contractions in response to field stimulation were used in this study. Myocyte isolation was carried out at 36±1°C.
Recording membrane currents
Myocytes were preloaded with the Ca2+ chelator BAPTA by incubating the cells with the membrane-permeable form BAPTA-AM (10 μmol l−1; (1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis(acetoxymethyl ester)). Myocytes were voltage clamped in the perforated-patch clamp configuration using amphotericin B (240 μg ml−1) as the pore-forming agent and an Axopatch 200 B amplifier (Molecular Devices Corporation, Sunnyvale, CA, U.S.A.) linked to a 1401 CED A/D interface (Cambridge, U.K.) or a TTL1 interface (Molecular Devices Corporation, U.S.A.). Currents were filtered at 5 kHz and acquired using Clampex 6.0 (Molecular Devices Corporation, U.S.A.) or Vclamp provided free of charge by Dr Dempster (Strathclyde, U.K.). Glass micropipettes were fabricated from filamented borosilicate capillaries (GC150TF, Harvard) to give tip resistances ∼1–2 MΩ after fire polishing.
Initial experiments were performed using voltage ramps where the myocyte was held at −40 mV, depolarised to 80 mV and then repolarised to −120 mV over 2 s. These experiments were followed by more detailed experiments where current–voltage (I–V) curves were obtained using conventional square wave steps (10 mV) from a holding potential of –40 mV. In the case of Ito myocytes were depolarised to 80 mV, whereas for IK1 they were hyperpolarised to −120 mV. Conventional double-pulse protocols were used to obtain activation/inactivation curves for Ito: myocytes were first depolarised in 10 mV steps from a holding potential of –40 mV for 500 ms and then by a second 500 ms pulse to 80 mV 20 ms later. The currents obtained during the prepulse were corrected for the driving force using G=I/(Vm−Vrev) and normalised to the peak current to obtain the activation curves, whereas currents obtained during the second step (to 80 mV) were normalised and plotted against the prepulse potential to obtain the inactivation curves. A second series of double-pulse protocols was used to measure the time course of recovery from inactivation. Two successive pulses from –40 to 80 mV were separated by progressively increasing time intervals in 20 ms increments. Currents recorded during the second pulse were normalised and plotted against the interpulse interval. Activation and inactivation curves were fitted with the Boltzmann equation to determine half maximal voltage (V0.5). Means were compared using one way ANOVA and student's t-test for unpaired observations. P<0.05 was taken to be significant.
Solutions and drugs
The solution used for isolation and storage of myocytes contained (mmol l−1): NaCl, 130; KCl, 5.4; MgCl2, 1.4; NaHPO4, 0.4; Creatine, 10; Taurine, 20; glucose, 10; HEPES, 10; titrated to pH 7.3 with NaOH. The extracellular solution used during current recording contained (mmol l−1): NaCl, 140; KCl, 5.4; MgCl2, 1.0, CaCl2, 1.0; glucose, 10.0 and HEPES, 10.0 titrated to pH 7.35. When recording K+ currents, the pipette solution contained (mmol l−1): Kglutamate 120.0; KCl, 20.0; NaCl, 10.0 and HEPES, 10.0 titrated to pH 7.2 with KOH. CdCl2 (1.0 mmol l−1) was added to the external solution to inhibit ICa. The latter were recorded using solutions described in the accompanying paper. All recordings were made at room temperature. All chemicals were purchased from VWR International, Sigma, or Alexis Corporation (Nottingham, U.K.).
Results
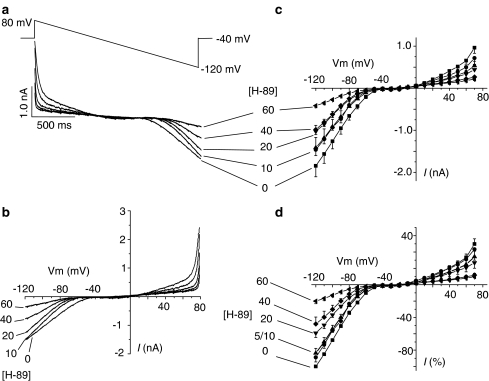
Initial experiments to assess the effects of H-89 on K+ currents were performed using voltage ramps where the cell was held at −40 mV and then depolarised to 80 mV, followed by a 2 s repolarising ramp to −120 mV (Figure 1a, top record). The initial depolarisation elicited an outward current that decreased within ∼500 ms following the onset of repolarisation. As the membrane potential became more negative during the ramp (i.e. below −40 mV), the outward current disappeared and was replaced by an inward current. Previous studies have shown that the outward current recorded under these conditions (i.e. in Cs+-free, K+-rich internal and external solutions and in myocytes where [Ca2+]i is buffered with BAPTA) is sensitive to inhibition by 4-aminopyridine (4-AP), and is therefore characterised as the Ca2+-independent and 4-AP-sensitive component of Ito (Tamargo et al., 2004). The inward current, on the other hand, is sensitive to inhibition by 1.0 mmol l−1 Ba2+ and therefore characterised as IK1 (Chorvatova & Hussain, 2003). Figure 1a also shows that increasing concentrations of H-89 inhibited both Ito and IK1, in a concentration-dependent manner. Current tracings shown in Figure 1a were replotted against the ramp voltage to generate the I–V curves (Figure 1b). Current amplitudes were then quantified at a range of voltages and are shown as absolute values (Figure 1c) and also as percent of the maximum inward current observed at −120 mV (Figure 1d). Although data in Figure 1 clearly show the inhibitory effects of H-89 on Ito and IK1, ramp protocols do not provide information on the time-dependence of currents. A further series of experiments were therefore performed using voltage steps to investigate these effects in more detail.
Figure 1.
Effects of H-89 on potassium currents recorded during hyperpolarising voltage ramps. (a) Voltage ramps and the original recordings of ramp currents in the absence and presence of different concentrations of H-89. (b) Membrane currents replotted against the ramp voltage in the absence and presence of ascending concentrations of H-89. (c) Quantitative analysis of ramp currents at selected voltages in the absence and presence of H-89. (d) Percent inhibition by H-89 relative to the maximal inward current at −120 mV. Each data point represents mean±s.e.m. of five separate observations. P<0.05 for outward currents above 40 mV in the presence of 40 and 60 μmol l−1 H-89 and also for inward currents below −50 mV for H-89 concentrations of 20, 40 and 60 μmol l−1.
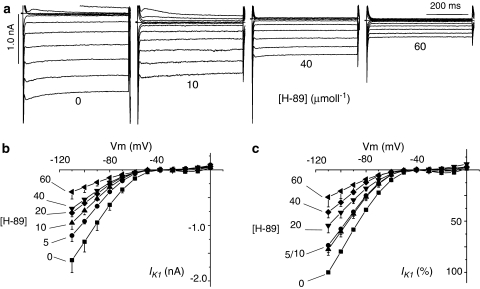
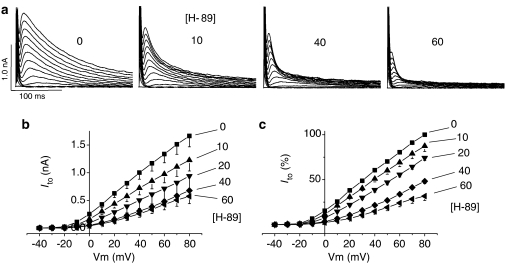
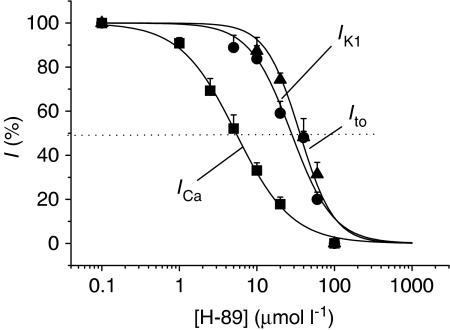
Figure 2a shows original recordings of currents elicited by voltage steps between 0 and −120 mV from a holding potential of −40 mV, in the absence and presence of 10, 40 and 60 μmol l−1 H-89. Hyperpolarisation elicited a large noninactivating and inwardly rectified current that was sensitive to inhibition by Ba2+ (not shown) and therefore described as IK1. Figure 2b and c show IK1 quantitatively, to demonstrate that the steady-state current amplitude was inhibited by the application of 5–60 μmol l−1 H-89 to the external bathing solution. In contrast to the data in Figure 2, when Vm was depolarised from −40 mV, an outward current was activated, but only transiently (Ito), and the amplitude of this was also inhibited by 5–60 μmol l−1 H-89 (Figure 3). Percent inhibition of IK1 and Ito by H-89 was plotted as concentration–effect curves (Figure 4), which were fitted with the Hill equation using Microcal Origin (version 6.0) to determine the IC50 of H-89 on K+ currents for comparison to the IC50 value for ICa demonstrated in the accompanying paper. Figure 4 shows that the IC50 of H-89 was 27.8 μmol l−1 for IK1 and 35.8 μmol l−1 for Ito compared to 5.4 μmol l−1 for ICa, suggesting that a kinase other than PKA may mediate the effects of H-89 on K+ currents. The Hill coefficient was −1.60 (r2=0.96) for IK1, −1.97 for Ito (r2=0.97) and −1.21 (r2=0.99) for ICa, suggesting that multiple processes may be involved in the effects of H-89 on K+ currents whereas the effects on ICa may be dependent solely on PKA.
Figure 2.
Effects of H-89 on IK1 during hyperpolarising voltage steps. (a) Original current tracings illustrating the effects of 0, 10, 40 and 60 μmol l−1 H-89. (b) Effects of H-89 on Ik1 shown as absolute values in nA. (c) Percent inhibition relative to maximal inward current at −120 mV. Each data point is mean±s.e.m. of at least five separate determinations. P<0.05 for Vm values below −50 mV in the presence of all concentrations of H-89 used except for −60 mV in 5 μmol l−1 H-89.
Figure 3.
Effects of H-89 on Ito recorded during depolarising voltage steps. (a) Sample tracings of Ito at different test potentials in the presence of 0, 10, 40 and 60 μmol l−1 H-89. (b) I–V curves showing the effects of H-89 on Ito shown as absolute values. (c) Percent Ito in the absence and presence of ascending concentrations of H-89. Each data point represents mean±s.e.m. of between 4 and 14 observations for each point. P<0.05 for all potentials above 0 mV in the presence of 20, 40 and 60 μmol l−1 H-89.
Figure 4.
Comparison of concentration–effect curves for the effects of H-89 on Ito, IK1 and ICa in isolated rat ventricular myocytes. Data on IK1 and Ito was obtained voltage steps (Figures 2 and 3), whereas data for ICa are replotted from the accompanying paper. Each point is mean±s.e.m. of at least five separate observations.
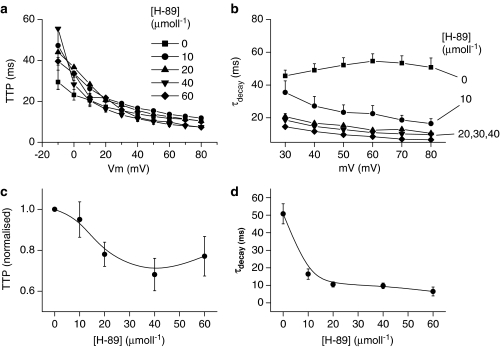
In addition to the inhibition of current amplitude (above), original tracings in Figure 3a show that H-89 also altered the time course of Ito. The time to peak (TTP) of Ito is voltage dependent and decreases as the voltage steps become more positive, both in the absence and presence of H-89 (Figures 3a and 5a). At voltages less than 10 mV when Ito is small in amplitude, TTP is also slow but is highly variable, giving rise to the relatively large standard errors (Figure 5a), which make quantitative comparisons at these voltages less reliable. However, at higher voltages (e.g. 80 mV), where Ito amplitude is much larger, TTP was 10.4±1.3 ms (n=11) under control conditions and decreased to 68.1±7.9% (P<0.05) of this value in the presence of 40 μmol l−1 H-89 (Figure 5c). At 60 μmol l−1H-89, TTP decreased even further so that the peak of Ito merged with the membrane capacitance transients that had not been cancelled (tracings Figure 3a), thereby, making quantification of TTP at this concentration less accurate. However, despite this limitation of the present study, Figures 3a, 5a and c clearly show that there was a general trend towards an accelerated TTP in the presence of H-89 and that this trend appeared to be concentration dependent.
Figure 5.
Effects of H-89 on the time course of Ito. (a) TTP. (b) Time constant (τ) to describe the decay of Ito. (c) TTP normalised to that at 80 mV in the absence of H-89. (d) Concentration dependence of τ for Ito recorded upon depolarisation from −40 to 80 mV. Each data point represents mean±s.e.m. of between 4 and 11 myocytes. TTP was significantly different (P<0.05) at 60–80 mV for all concentrations of H-89 tested. τdecay values above 40 mV were all significantly different (P<0.05).
In contrast to the TTP, under control conditions inactivation of Ito did not appear to be voltage dependent and could be fitted monoexponentially to determine the inactivation time constant (τdecay, Figure 5b and d). In the presence of H-89, inactivation was markedly accelerated at all concentrations of H-89 and required two time constants to describe the decay accurately, although the fast time constant appeared to be the most important and is therefore shown in Figure 5b and d. It is noteworthy that the effect of H-89 on inactivation of Ito was not concentration dependent and 10 μmol l−1 produced almost the maximal effect.
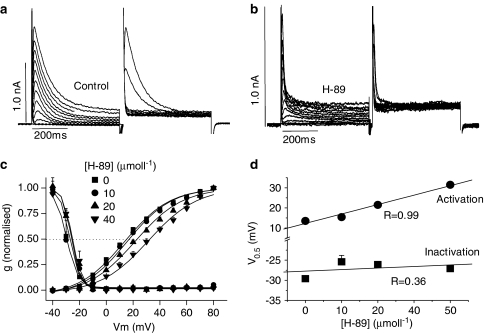
Given that Ito activates and inactivates faster in the presence of H-89 (above), double-pulse protocols were used to study the effects of H-89 on steady-state activation and inactivation as well as recovery from voltage-dependent inactivation. Figure 6a and b shows original tracings, obtained in the absence and presence of H-89, during a paired-pulse protocol where the prepulse voltage was stepped to different values while keeping the second voltage step constant to 80 mV. Currents were converted to conductance to account for changes in the driving force, normalised and fitted with the Boltzman function to determine the V0.5 for activation and inactivation (see Methods). Figure 6c and d show that while there was no effect of H-89 on steady-state inactivation, V0.5 for activation was significantly increased in the presence of H-89 in a concentration-dependent manner: approximately by 5 mV for every 10 μmol l−1 increase in H-89 (Figure 6d).
Figure 6.
Effects of H-89 on steady-state activation and inactivation of Ito. (a) Ito recorded during paired-pulse stimulation in control conditions. (b) Ito in the presence of H-89. (c) Steady-state activation and inactivation curves in the presence of 0, 10, 20 and 40 μmol l−1 H-89. (d) Half-maximal voltage (V0.5) for activation and inactivation, obtained using the Boltzman function, in the absence and presence of different concentrations of H-89. Each data point is mean±s.e.m. of 4–12 observations. V0.5 for activation was significantly different at 40 and 60 μmol l−1 H-89 (P<0.05).
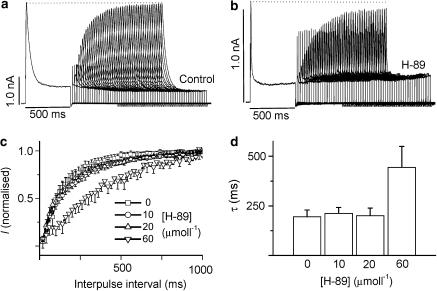
Figure 7 shows the effects of H-89 on the time course of recovery from inactivation. In this case, paired pulses from −40 to 80 mV were separated by progressively increasing time intervals in 20 ms increments. Figure 7a shows that while Ito recovered fully within the duration of the maximal interpulse interval (1 s) in the absence of H-89, recovery was significantly delayed in the presence of 60 μmol l−1 H-89 but not at the lower concentrations of the drug.
Figure 7.
Effects of H-89 on the time course of recovery of Ito from voltage-dependent inactivation. (a) Original current tracings illustrating the recovery of Ito under control conditions. (b) Tracings showing recovery of Ito in the presence of 60 μmol l−1 H-89. (c) Mean (±s.e.m.) time course of Ito recovery in 0, 10, 20 and 60 μmol l−1 H-89. (d) Mean (±s.e.m.) time constant (τ) for recovery of Ito. P<0.05 only for τ at 60 μmol l−1 H-89 (n=at least 6).
Given that the literature on the role of PKA-mediated phosphorylation in unclear (see Introduction) and H-89 inhibited IK1, Ito and Iss at concentrations much higher than those effective for ICa (Figure 4), it was of interest to know whether elevation of cyclic AMP with isoprenaline or inhibition of protein phosphatase 1/2A (PP1/2A) with calyculin A could modulate K+ currents in a similar manner to that described for ICa in the accompanying paper. Figure 8 shows that increasing phosphorylation levels through either of these pathways had no significant effect on IK1 or Ito, whether the recordings were made using voltage ramps or steps.
Figure 8.
Effects of β1-agonist isoprenaline and protein phosphatase inhibitor calyculin A on Ito and IK1. (a) Inward and outward currents recorded during voltage ramps. (b) IK1 during hyperpolarising voltage steps. (c) Ito during depolarising voltage steps. Each point is mean±s.e.m. of at least six observations. Invisible error bars are smaller than the symbols.
Discussion
Pharmacological inhibitors and/or activators can be an invaluable tool in dissecting signalling pathways that regulate cell function. However, this depends largely on the potency and selectivity of the compound in question for a given target. In this study, we assessed the value of using PKA inhibitor H-89 in studying the role of PKA-mediated phosphorylation in regulating basal K+ currents in rat ventricular myocytes. The main finding was that although H-89 inhibited IK1, Ito and Iss, the concentrations required for such inhibition were higher than those effective at inhibiting PKA-mediated effects on ICa (Figure 4). This suggests that PKA is probably not involved in regulating basal K+ currents and that the effects of H-89 are unlikely to be due to a simple inhibition of PKA, a conclusion also supported by data in Figure 8 where increasing cyclic AMP by stimulating β-adrenergic receptors with isoprenaline, or by inhibiting PP1/2A using calyculin A, failed to alter K+ current amplitude. However, in the absence of further work, some other possibilities cannot yet be completely dismissed. For example, PKA associated with K+ currents may be compartmentalised and therefore less accessible to H-89, thereby requiring higher concentrations than the PKA associated with the L-type Ca2+ channels. The lack of effect of isoprenaline and calyculin A on Ito and IK1 could be explained by maximal basal phosphorylation of K+ currents even in the absence of externally applied phosphorylating agents. In addition, the possibility that more than one protein kinase is involved in the case of K+ currents cannot be excluded. This is supported by the observation that the Hill coefficients for Ito and IK1 are closer to two, implying multiple binding sites, possibly synergistic effects through a combination of different kinases.
If the inhibitory effects of H-89 on Ito and IK1 amplitude are indeed independent of PKA-mediated phosphorylation, then the mechanism(s) by which H-89 produces inhibition remain to be determined. One possibility is that another kinase, for example, PKG or PKC, might be involved, especially at the high concentrations used to inhibit K+ currents. Previous studies have shown that PKG and PKC can be inhibited by H-89 in vitro, although the Ki is 10 or 100 times higher then that for PKA, respectively (Chijiwa et al., 1990; Hidaka & Kobayashi, 1992), although these determinations were made using purified bovine PKA and porcine lung PKG. In addition, given that the molecular structures of Ito and IK1 differ significantly and yet the IC50 values of H-89 are relatively close to one another, it is possible that the observed effect(s) of H-89 are independent of kinase(s) involvement altogether and are due to nonspecific actions for example, by binding of H-89 at the pore region of the channels.
In addition to inhibition of IK1 and Iss, the inhibitory effect of H-89 on Ito amplitude is also accompanied by accelerated kinetics of activation and inactivation, increased V0.5 for steady-state activation (but not inactivation) as well as delayed recovery from voltage-dependent inactivation. The effects of H-89 on Ito are therefore complex and probably due to multiple actions. The observation that 10 μmol l−1 H-89 produced near maximal effect on the decay of Ito (Figure 5d) could be due to the involvement of PKA in voltage-dependent inactivation of Ito, in addition to PKG or other mechanisms that regulate amplitude of the current.
The inhibition of Ito amplitude, irrespective of whether this is mediated through changes in phosphorylation of the channel or by some other nonspecific means, is likely to result either from a decrease in the open probability or a decrease in the unitary conductance of the channel, or a combination of both. These effects could potentially occur independently of the effects on the kinetics of Ito (i.e. the accelerated TTP and the τdecay of voltage-dependent inactivation), which probably reflect faster channel gating where the channel protein undergoes transitions between the activated-open (conducting) state and the inactivated-closed (nonconducting) state more rapidly. Abbreviation of the overall time course of the macroscopic current can be explained by a shorter duration of the bursts of openings at the single channel level.
To explain the effects of H-89 on steady-state activation and the recovery from voltage-dependent inactivation it is necessary to consider the activation/inactivation mechanisms in molecular terms. Activation of voltage-gated ion channels such as Ito depends heavily on the voltage sensor, which is located on one of the six membrane spanning segments (segment 4; S4) of the α-subunit (Snyders, 1999). Depolarisation of the membrane causes a physical movement of S4, which induces a further conformational change that opens the channel. Inactivation occurs through either N- or C-type inactivation, although a third type (V-type) has now also been postulated (for reviews see Oudit et al., 2001; Patel & Campbell, 2005). It seems from Figure 5c and d that V0.5 for steady-state activation, but not inactivation, was increased by H-89, in a concentration-dependent manner, implying that the voltage sensor is less effective at opening the channel in the presence of H-89. This could be due to direct or indirect effects of H-89 on S4 itself (e.g. modification of the positively charged basic residues located in this region) or the subsequent conformational changes that are responsible for opening the channel. Given that the channel is more rapidly inactivated (Figure 5), it is possible that the inactivated conformation in the presence of H-89 is more stable and more difficult to reactivate. This may explain why recovery from inactivation is also delayed (Figure 7), as is the case for ICa presented in the accompanying paper, although this becomes apparent only at the very high concentrations in each case that is at concentrations of H-89 where the currents are almost completely inhibited.
In summary, data in the present study demonstrate that the PKA-inhibitor H-89 has inhibitory effects on IK1, Ito and Iss at concentrations higher than those effective at inhibiting basal ICa sustained by cyclic AMP-PKA (cyclic AMP-dependent protein kinase A)-mediated signalling (accompanying paper). It is therefore likely that the effects of H-89 on K+ currents are at least partly mediated by pathways not involving PKA-mediated phosphorylation. The nature of such PKA-independent inhibitory actions of H-89 is unknown and may involve other kinases or even other direct/indirect nonspecific effects unrelated to channel phosphorylation. This is particularly likely given that the three K+ currents investigated in this study have markedly different structures at the molecular level and also because previous studies have already documented some other nonspecific effects of H-89 (Hussain et al., 1999; Blasev et al., 2001; Choi et al., 2001), which can only therefore be used as an invaluable tool if these other effects are carefully avoided.
Abbreviations
- 4-AP
4-aminopyridine
- BAPTA-AM
1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid-acetoxymethyl ester
- cyclic AMP-PKA
cyclic AMP-dependent protein kinase A
- EGTA
ethyleneglycol-bis(β-aminoethyl)-N,N,N′,N′-tetraacetic acid
- H-89
N-[2-p-bromo-cinnamylamino)ethyl]-5-isoquinolinesulfonamide
- ICa
L-type Ca2+ current
- IK
delayed rectifier
- IK1
inward rectifying K+ current
- IKss
steady-state K+ current
- INa
Na+ current
- Ito
transient outward K+ current
- I–V
current–voltage
- PKA
protein kinase A
- PKC
protein kinase C
- PKG
protein kinase G
- PP1/2A
protein phosphatase 1/2A
- Ser/Thr
serine/threonine
- TTP
time to peak
- T0.5
time to recover to 50% of the pre-inactivation amplitude
- V0.5
half maximal voltage
References
- BLASEV R., HUSSAIN M., BAKKER A.J., HEAD S.I., LAMB G.D. Effects of the PKA inhibitor H-89 on excitation-contraction coupling in skinned and intact skeletal muscle fibres. J. Muscle Res. Cell. Motil. 2001;22:277–286. doi: 10.1023/a:1012289526618. [DOI] [PubMed] [Google Scholar]
- CHIJIWA T., MISHIMA A., HAGIWARA M., SANO M., HAYASHI K., INOUE T., NAITO K., TOSHIOKA T., HIDAKA H. Inhibition of forskolin-induced neurite outgrowth and protein phosphorylation by a newly synthesized selective inhibitor of cyclic AMP-dependent protein kinase, N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide (H-89), of PC12D pheochromocytoma cells. J. Biol. Chem. 1990;265:5267–5272. [PubMed] [Google Scholar]
- CHOI J., CHOI B.H., HAHN S.J., YOON S.H., MIN D.S., JO Y., KIM M. Inhibition of Kv1.3 channels by H-89 (N--[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide) independent of protein kinase A. Biochem. Pharmacol. 2001;61:1029–1032. doi: 10.1016/s0006-2952(01)00556-1. [DOI] [PubMed] [Google Scholar]
- CHORVATOVA A., HUSSAIN M. Effects of caffeine on potassium currents in isolated rat ventricular myocytes. Pflugers Arch. 2003;446:422–428. doi: 10.1007/s00424-003-1031-1. [DOI] [PubMed] [Google Scholar]
- DART C., LEYLAND M.L. Targeting of an A kinase-anchoring protein, AKAP79, to an inwardly rectifying potassium channel, Kir2.1. J. Biol. Chem. 2001;276:20499–20505. doi: 10.1074/jbc.M101425200. [DOI] [PubMed] [Google Scholar]
- FREEMAN L.C., KWOK W., KASS R.S. Phosphorylation-independent regulation of cardiac IK by guanine nucleotides and isoproterenol. Am. J. Physiol. 1992;262:H1298–H1302. doi: 10.1152/ajpheart.1992.262.4.H1298. [DOI] [PubMed] [Google Scholar]
- GADSBY D.C. Beta-adrenoceptor agonists increase membrane K+ conductance in cardiac purkinje fibres. Nature. 1983;306:691–693. doi: 10.1038/306691a0. [DOI] [PubMed] [Google Scholar]
- GALLEGO M., SETIEN R., PUEBLA L., BOYANO-ADANEZ MDEL C., ARILLA E., CASIS O. alpha1-adrenoceptors stimulate a Gαs protein and reduce the transient outward K+ current via a cAMP/PKA-mediated pathway in the rat heart. Am. J. Physiol. 2005;288:C577–C585. doi: 10.1152/ajpcell.00124.2004. [DOI] [PubMed] [Google Scholar]
- HIDAKA H., KOBAYASHI R. Pharmacology of protein kinase inhibitors. Annu. Rev. Pharmacol. Toxicol. 1992;32:377–397. doi: 10.1146/annurev.pa.32.040192.002113. [DOI] [PubMed] [Google Scholar]
- HUSSAIN M., DRAGO G.A., BHOGAL M., COLYER J., ORCHARD C.H. Effects of the protein kinase A inhibitor H-89 on Ca2+ regulation in isolated ferret ventricular myocytes. Pflugers Arch. 1999;437:529–537. doi: 10.1007/s004240050814. [DOI] [PubMed] [Google Scholar]
- IIJIMA T., IMAGAWA J., TAIRA N. Differential modulation by beta adrenoceptors of inward calcium and delayed rectifier potassium current in single ventricular cells of guinea pig heart. J. Pharmacol. Exp. Ther. 1990;254:142–146. [PubMed] [Google Scholar]
- ISHIHARA H., MARTIN B.L., BRAUTIGAN D.L., KARAKI H., OZAKI H., KATO Y., FUSETANI N., WATABE S., HASHIMOTO K., UEMURA D. Calyculin A and okadaic acid: inhibitors of protein phosphatase activity. Biochem. Biophys. Res. Commun. 1989;159:871–877. doi: 10.1016/0006-291x(89)92189-x. [DOI] [PubMed] [Google Scholar]
- KARLE C.A., ZITRON E., ZHANG W., WENDT-NORDAHL G., KATHOFER S., THOMAS D., GUT B., SCHOLZ E., VAHL C.F., KATUS H.A., KIEHN J. Human cardiac inwardly-rectifying K+ channel Kir(2.1b) is inhibited by direct protein kinase C-dependent regulation in human isolated cardiomyocytes and in an expression system. Circulation. 2002;106:1493–1499. doi: 10.1161/01.cir.0000029747.53262.5c. [DOI] [PubMed] [Google Scholar]
- KOUMI S., WASSERSTROM J.A., TEN EICK R.E. Beta-adrenergic and cholinergic modulation of inward rectifier K+ channel function and phosphorylation in guinea-pig ventricle. J. Physiol. 1995;486:661–678. doi: 10.1113/jphysiol.1995.sp020842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEI M., BROWN H.F., TERRAR D.A. Modulation of delayed rectifier potassium current, IK, by isoprenaline in rabbit isolated pacemaker cells. Exp. Physiol. 2000;85:27–35. [PubMed] [Google Scholar]
- OUDIT Y., KASSIRI Z., SAH R., RAMIREZ R.J., ZOBEL C., BACKX P.H. The molecular physiology of the cardiac transient outward potassium current (Ito) in normal and diseased myocardium. J. Mol. Cell. Cardiol. 2001;33:851–872. doi: 10.1006/jmcc.2001.1376. [DOI] [PubMed] [Google Scholar]
- PATEL S.P., CAMPBELL D.L. Transient outward current “Ito” phenotypes in the mammalian left ventricle: underlying molecular, cellular and biophysical mechanisms. J. Physiol. 2005;569:7–39. doi: 10.1113/jphysiol.2005.086223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSSIE S. Regulation of voltage-sensitive sodium and calcium channels by phosphorylation. Adv. Sec. Mess. Phospho. Res. 1999;33:23–48. doi: 10.1016/s1040-7952(99)80004-4. [DOI] [PubMed] [Google Scholar]
- SNYDERS D.J. Structure and function of potassium channels. Cardiovasc. Res. 1999;42:377–390. doi: 10.1016/s0008-6363(99)00071-1. [DOI] [PubMed] [Google Scholar]
- TAMARGO J., CABALLERO R., GOMEZ R., VALENZUELA C., DELPON E. Pharmacology of cardiac potassium channels. Cardiovasc. Res. 2004;62:9–33. doi: 10.1016/j.cardiores.2003.12.026. [DOI] [PubMed] [Google Scholar]
- TROMBA C., COHEN I.S. A novel action of isoproterenol to inactivate a cardiac K+ current is not blocked by beta and alpha adrenergic blockers. Biophys. J. 1990;58:791–795. doi: 10.1016/S0006-3495(90)82422-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN DER HEYDEN M.A., WIJNHOVEN T.J., OPTHOF T. Molecular aspects of adrenergic modulation of cardiac L-type Ca2+ channels. Cardiovasc. Res. 2005;65:28–39. doi: 10.1016/j.cardiores.2004.09.028. [DOI] [PubMed] [Google Scholar]
- XIAO Y.F., MCARDLE J.T. Effects of 2,3-butanedione monoxime on blood pressure, myocardial Ca2+ currents, and action potentials of rats. Am. J. Hypertens. 1995;12:1232–1240. doi: 10.1016/0895-7061(95)00251-0. [DOI] [PubMed] [Google Scholar]
- ZITRON E., KIESECKER C., LUCK S., KATHOFER S., THOMAS D., KREYE V.A., KIEHN J., KATUS H.A., SCHOELS W., KARLE C.A. Human cardiac inwardly rectifying current IKir2.2 is upregulated by activation of protein kinase A. Cardiovasc. Res. 2004;63:520–527. doi: 10.1016/j.cardiores.2004.02.015. [DOI] [PubMed] [Google Scholar]