Abstract
Two novel selective 5-HT6 receptor ligands E-6801 (6-chloro-N-(3-(2-(dimethylamino)ethyl)-1H-indol-5-yl)imidazo[2,1-b]thiazole-5-sulfonamide) and E-6837 (5-chloro-N-(3-(2-(dimethylamino)ethyl)-1H-indol-5-yl)naphthalene-2-sulfonamide) were investigated and compared to the putative 5-HT6 receptor antagonists SB-271046 (5-chloro-N-(4-methoxy-3-(piperazin-1-yl)phenyl)-3-methylbenzo[b]thiophene-2-sulfonamide) and Ro 04-06790 (N-(2,6-bis(methylamino)pyrimidin-4-yl)-4-aminobenzenesulfonamide) using a cAMP-mediated pathway.
Forskolin stimulation, to increase the magnitude of agonist cAMP responses, and site-directed mutagenesis of the 5-HT6 receptor, in order to yield constitutively active receptor, were applied.
5-HT (Emax, % over basal: 200), E-6801 (120) and E-6837 (23) induced cAMP formation at the rat 5-HT6 receptor. In the copresence of forskolin, cAMP responses were more potent and enhanced to 294 (5-HT, % over forskolin), 250 (E-6801) and 207 (E-6837), respectively. 5-HT-mediated cAMP formation was dose-dependently blocked by SB-271046 (pA2: 8.76±0.22) and Ro 04-6790 (pA2: 7.89±0.10) and not affected by the copresence of forskolin. Both E-6801 and E-6837 yielded partial antagonism of the 5-HT response in the absence of forskolin, whereas antagonism was either completely absent (E-6801) or attenuated (E-6837) in the copresence of forskolin. Intrinsic activity of these 5-HT6 receptor ligands at a constitutively active human S267K 5-HT6 receptor in Cos-7 cells indicated similar efficacy (Emax, % over basal) for 5-HT (97), E-6801 (91) and E-6837 (100), while Ro 04-6790 (-33) and SB-271046 (-39) were equi-efficacious inverse agonists.
The use of either forskolin or a constitutively active S267K 5-HT6 receptor enhances the resolution for monitoring the efficacy of 5-HT6 receptor ligands. E-6801 and E-6837 are potent partial agonists at the 5-HT6 receptor. Ro 04-6790 and SB-271046 appear to act as inverse agonists/antagonists.
Keywords: 5-HT6 receptor, ligand efficacy, constitutive activation, adenylyl cyclase, cyclic-AMP (cAMP), partial agonist, inverse agonist, HEK-293F and Cos-7 cells
Introduction
The 5-hydroxytryptamine6 (5-HT6) receptor is probably exclusively localized in the central nervous system (CNS), highest in limbic and cortical regions, and has been postulated to modulate CNS acetylcholine and glutamate function (Woolley et al., 2004). It may have a primary role in memory processes and learning, and 5-HT6 receptor ligands may be of benefit to improve cognitive function as suggested by a list of interesting studies (see Woolley et al., 2004). This receptor is also likely to play a role in obesity (Vickers & Dourish, 2004). Early studies demonstrated that chronic administration of 5-HT6 antisense oligonucleotides produced a significant reduction in food intake and body weight in rats (Bourson et al., 1995; Bentley et al, 1997). Furthermore, 5-HT6 receptor knockout mice are also resistant to weight gain when exposed to a high-fat diet (Caldirola, 2003). In addition, the 5-HT6 receptor has also been suggested to be involved in psychotic and affective disorders, anxiety and epilepsy (Woolley et al., 2004). The 5-HT6 receptor belongs to the G-protein-coupled receptor family and is coupled to the Gs-family of G proteins and has been demonstrated to increase cAMP formation in recombinant expression systems (Ruat et al., 1993; Kohen et al., 1996; Boess et al., 1997), cultured mouse striatal neurones (Sebben et al., 1994) and pig caudate membranes (Schoeffter & Waeber, 1994).
In order to elucidate functional roles of the 5-HT6 receptor, potent and selective ligands with defined properties are required. During the past few years, synthesis of novel ligands has been reported, introducing various new classes of compounds as potent and selective ligands for the 5-HT6 receptor subtype (Slassi et al., 2002; Davies et al., 2005; Holenz et al., 2006). Historically, the first 5-HT6-selective molecules were found by high-throughput-screening as, for example, the benzene-sulfonamide antagonists Ro 04-06790 (N-(2,6-bis(methylamino)pyrimidin-4-yl)-4-aminobenzenesulfonamide) and Ro 63-0563 (4-amino-N-(6-bromo-1H-indol-4-yl)benzenesulfonamide) (Sleight et al., 1998) and the phenyl-piperazine antagonist SB-271046 (5-chloro-N-(4-methoxy-3-(piperazin-1-yl)phenyl)-3-methylbenzo[b]thiophene-2-sulfonamide) (Bromidge et al., 1999). Later, by employing medicinal chemistry approaches starting from the endogenous ligand 5-HT, selectivity could be introduced by chemical modifications resulting in powerful agonists (i.e., EMDT (2-(2-ethyl-5-methoxy-1H-indol-3-yl)-N,N-dimethylethanamine) (Glennon et al., 2000) and WAY-181187/SAX-187 (Cole et al., 2005)) as well as antagonists (i.e. MS-245 (2-(5-methoxy-1-(phenylsulfonyl)-1H-indol-3-yl)-N,N-dimethylethanamine) (Russell et al., 2001), Ro 65-7199 (4-amino-N-(6-bromo-1H-indol-4-yl)benzenesulfonamide) (Bös et al., 2001), and LY-483518/SGS-518 (1-methyl-3-(1-methylpiperidin-4-yl)-1H-indol-5-yl 2,6-ifluorobenzenesulfonate) (Piñeiro-Núñez et al., 2005)). In parallel, optimization of the phenyl-piperazine motif resulted in antagonists, such as SB-357134 (N-(2,5-dibromo-3-fluorophenyl)-4-methoxy-3-(piperazin-1-yl)benzenesulfonamide) (Bromidge et al., 2001a), SB-399885 (N-(3,5-dichloro-2-methoxyphenyl)-4-methoxy-3-(piperazin-1-yl)benzenesulfonamide) (Hirst et al., 2003b) and SB-742457 (3-(phenylsulfonyl)-8-(piperazin-1-yl)quinoline) (Ahmed et al., 2003). Analyzing a variety of 5-HT6 reference compounds from a medicinal chemistry point of view, as described in a former publication (Holenz et al., 2005), we detected common structural motifs and converted this information into a hypothetical pharmacological framework model which allowed us to design and synthesis selective and high-affinity 3-aminoalkylindole sulfonamides, such as E-6801 (6-chloro-N-(3-(2-dimethylamino)ethyl)-1H-indol-5-yl)imidazo[2,1-b]thiazole-5-sulfonamide) and E-6837 (5-chloro-N-(3-(2-(dimethylamino)ethyl)-1H-indol-5-yl)naphthalene-2-sulfonamide). In terms of functionality, these ligands have been found to comprise antagonists, as well as agonists and partial agonists.
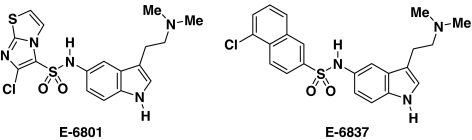
Little is known about most 5-HT6 receptor antagonists with regard to their intrinsic efficacy properties. For instance, are these compounds silent antagonists or do they possess either negative or positive efficacy? The degree and kind of efficacy may ultimately differentiate these compounds at the 5-HT6 receptor, in particular, when it is constitutively active or under the tonic control of 5-HT (Woolley et al., 2004). Recombinant expression systems have the advantage that they allow differentiation of efficacies of closely related compounds (i.e., Pauwels & Colpaert, 1995). In the present paper, we report a comparative study of the recently reported (Holenz et al., 2005) 3-aminoalkylindolyl sulfonamide derivatives: E-6801 and E-6837 (see Figure 1), and the presumed 5-HT6 receptor antagonists SB-271046 and Ro 04-6790 using a cAMP-mediated pathway as a functional read-out. Two experimental approaches were used to analyze the efficacy of these 5-HT6 compounds. First, the use of forskolin stimulation (Litosch et al., 1982) to increase the magnitude of agonist responses and second, site-directed mutagenesis of the 5-HT6 receptor in order to yield constitutively active receptor (Purohit et al., 2003). It appears that E-6801 and E-6837 are potent partial 5-HT6 receptor agonists, whereas SB-271047 and Ro 04-6790 possess negative efficacy and are as such inverse agonists/antagonists.
Figure 1.
Chemical structures of E-6801 and E-6837.
Methods
Construction of rat 5-HT6 receptor plasmid
PCR was performed with cDNA from Wistar rat striatum as a template using Expam High Fidelity polymerase (Roche). Flanking primers were: 5′- TGCAGTCACCATATCCCGTCTT-3′ and 5′- ACGGGGGAACTGAGTGGATGC-3′.
Sequential PCR conditions were: initial denaturation at 94°C for 2 min, 10 cycles at 94°C for 10 s, 65°C for 30 s and 72°C for 1½ min. Then, 20 cycles at 94°C for 15 s, 55°C for 30 s and 72°C for 1½ min. Finally, an elongation step was performed at 72°C for 7 min. PCR products were visualized on 0.8% agarose gel and visualized with ethidium bromide (10 μg ml−1) staining. PCR products were purified using Microcon PCR columns (Millipore, Billerica, MA, U.S.A.) following the manufacturer's instructions. The purified fragments were ligated into pCR3.1 vector (Invitrogen, Frederick, MD, U.S.A.) using T4 DNA ligase and after overnight incubation at 15°C, ligated fragments were introduced in competent Escherichia coli TOP-F10 cells. Fifty μl of cells were mixed with 0.4 μg of the ligation product and set in ice for 30 min. Mixture was incubated for 30 s at 42°C (heat shock) and replaced at 37°C for 1 h with additional 250 μl sodium bactotryptone (SOC) medium. Afterwards, cells were seeded into Luria-Bertani (LB)-agar medium with ampicillin (100 μg ml−1) and the culture was grown at 37°C overnight. Single colonies were selected and put into 3 ml LB with 100 μg ml−1 ampicillin and incubated at 37°C overnight. cDNA was obtained using a Qiaprep Spin Miniprep kit (Qiagen). Plasmids from selected colonies were sequenced and one corresponding to the original rat 5-HT6 receptor DNA sequence (Ruat et al., 1993) was taken for stable expression in HEK-293F cells.
Construction of human mutant S267 K 5-HT6 receptor plasmid
The S267K mutation was performed on native human 5-HT6 receptor cDNA (Kohen et al., 1996) and constructed by site-directed mutagenesis (Purohit et al., 2003). Two flanking primers (sense 5′-GGAAGGCCCTGAAGGCCAAGCTTACGCTGGGCATCCTGC-3′ and antisense 5′-GCAGGATGCCCAGCGTAAGCTTGGCCTTCAGGGCCTTCC-3′) were designed for mutagenesis of serine (AGC)-267 to lysine (AAG). PCR was performed using PFU Turbo DNA polymerase enzyme and the pCR3.1-mutant 5-HT6 fragment was transformed in DH5α cells. A HindIII restriction site was added by changing the wobble base of the adjacent leucine from CTG to CTT in order to verify its orientation and the sequence was confirmed by DNA sequencing.
Construction of HEK-293F cell line stably expressing rat 5-HT6 receptor
HEK-293F cells (Gibco) were grown in Dulbecco's modified Eagle's medium with GlutaMAX and pyruvate (DMEM: Gibco), and supplemented with 10% foetal bovine serum (Gibco), penicillin (50 U ml−1) and streptomycin (50 U ml−1) (Gibco). Cells were transfected with pcDNA3.1 containing 5-HT6 cDNA using FuGENE 6 Transfection Reagent (Roche) according to the manufacturer's protocol: 2 μl with 30 μg cDNA: 3 μl FuGENE per well of a six-well plate. At 24 h after transfection, cells were seeded by serial dilutions and plated in 384-well plates containing G418 (geneticin, Gibco) at 0.5 mg ml−1. Isolated single colonies of cells of the geneticin-resistant phenotype were expanded and assayed for their 5-HT-mediated cAMP response using a FlashPlate technique. One clone, number #5, was selected for further work. Stably transfected cells were always grown in the presence of 0.5 mg ml−1 geneticin except during the cAMP experiment.
Transient transfection of human wild-type and mutant S267 K 5-HT6 receptor in Cos-7 cells
Cos-7 cells were cultured in DMEM supplemented with 40 mM glutamine and 10% of foetal bovine serum. At 24 h before transfection, cells were seeded at a subconfluent state in 150-mm Petri dishes for cAMP assay by homogeneous time resolved fluorescense (HTRF). Cells were transfected with pCR3.1-vector containing either a wild-type or mutant h5-HT6 receptor cDNA using 150 μl of lipofectamine and 37.5 μg of plasmid DNA per plate. cAMP experiments was performed at 48 h after transfection.
Radioligand-binding assay
Expression of the 5-HT6 receptor was measured by radioligand-binding assay (Hirst et al., 2003a) in a 96-well plate with a total reaction volume of 200 μl, containing 100 μl of membrane suspension (25 μg protein well−1), 10 μl of [3H]-LSD (2.5–10.0 nM) in either the absence or presence of 90 μl of either buffer or methiothepin (5 μM) for total and nonspecific binding, respectively. Binding buffer contained 50 mM Tris-HCl, 10 mM MgCl2 and 0.5 mM EDTA at pH 7.4. Plates were incubated at 37°C for 60 min, filtered and plates were washed three times with ice-cold 50 mM Tris-HCL (pH 7.4). Filters were dried and counted at approximately 40% efficiency in a MicroBeta scintillation counter (Perkin-Elmer) using 25 μl per well of EcoScint liquid scintillation cocktail. To investigate binding properties of 5-HT6 ligands to h5-HT6 receptor, transfected HEK-293 membranes (35 μg protein assay−1) from Perkin-Elmer (Boston, MA, U.S.A.) and [3H]-LSD were used.
Measurement of cAMP responses by FlashPlate
Activation and inhibition of adenylyl cyclase activity was monitored by measuring levels of cAMP in 96-well plates by FlashPlate method (Perkin-Elmer). Briefly, HEK-293F cells expressing rat 5-HT6 receptor were grown to 80% confluency. At 2 h before the assay cells were kept on serum-free medium and subsequently dissociated with trypsin and centrifuged. The resulting pellet was resuspended (25,000 cells well−1) in buffer provided with the Perkin-Elmer kit and containing 1 mM 3-isobutyl-1- methyl-xanthine (IBMX) and 20 μM pargyline. Test compounds were added either in the absence or presence of forskolin (either 1, 3 or 10 μM) and different concentrations of 5-HT, and after 30 min the reaction was stopped by addition of 100 μl of detection mix solution per well containing [125I]-succinyl-cAMP tracer according to the supplier's instructions. When antagonists were used these were added 15 min before 5-HT. After 2 h incubation at room temperature, counting was performed using a MicroBeta scintillation counter (Perkin-Elmer).
Measurement of cAMP responses by Homogeneous Time Resolved Fluorescense
After overnight serum-free medium incubation, cAMP measurements on Cos-7 cells that transiently expressed either human wild-type or mutant 5-HT6 receptor were performed by HTRF (Gabriel et al., 2003). Cell suspension (20,000 cells per well) was added in 96-well culture plate in incubation buffer composed of Ham's F12 medium plus 1 mM IBMX and 20 μM pargyline. Forty μl of cell suspension and 10 μl of either compound in the absence or presence of forskolin or vehicle was added to each well at indicated concentrations for 30 min at 37°C. The reaction was stopped with 25 μl of cryptate and 25 μl of XL-665. Plates were incubated for 1 h at room temperature and read at 665/620 nm using a RubyStar Plate reader (BMG LabTech).
Materials
pCR3.1 plasmid and other reagents for molecular biology experiments were purchased from either Invitrogen (Frederick, MD, U.S.A.), Qiagen (Germantown, MD, U.S.A.) or Roche (Penzberg, Germany) as indicated above. Site-directed mutagenesis kit was obtained from Stratagene (La Jolla, CA, U.S.A.). Cell culture media and reagents were purchased from Gibco (Paislay, U.K.). Adenylyl cyclase activation FlashPlate kit was supplied by Perkin-Elmer Life Science (Brussels, Belgium). HTRF cAMP kit was purchased from CisBio (Bagnols, France). [3H]-LSD was purchased from NEN (Boston, MA, U.S.A.). 5-hydroxytryptamine, dimethyl sulfoxide (DMSO), 3-isobutyl-1-methyl-xanthine (IBMX), forskolin and pargyline were obtained from Sigma (Poole, U.K.). Methiothepin was obtained from Tocris (Bristol, U.K.). SB-271046 and Ro 04-0670 were prepared intramuros. E-6801 and E-6837 are described in WO 2003/042175 A1 (Merce-Vidal et al., 2003). Stock compound solutions were prepared in DMSO and diluted with phosphate buffer solution (PBS) not exceeding 2% of DMSO at final concentration.
Data analysis
cAMP data are reported as mean±s.e.m. of at least four independent experiments, each of which was performed in duplicate. cAMP data are presented in pmol 106 cells−1. The response to modulate basal cAMP formation (Emax) by compounds was determined from the maximal stimulation or inhibition value that corresponded to a plateau value. The concentration of compound that produced a half-maximal response is represented by a pEC50 value and was calculated by nonlinear regression using XLfit (IDBS) and GraphPad Prism Version 3 programs. In experiments using antagonists, concentration ratios were calculated and used to obtain estimates of apparent pA2 values using the following equation: apparent pA2 = log (concentration ratio−1) – log (antagonist concentration). The concentration dependence of each compound in the absence or presence of forskolin was analyzed by a one-way ANOVA statistical analysis. To analyze possible differences between either Emax or pEC50 values of compounds in the absence and presence of forskolin, Tukey's test within a two-way (forskolin and compound) ANOVA statistical analysis was performed using SAS program (SAS Institute Inc., Cary, NC, U.S.A.). A similar approach was used to estimate differences between either Emax or pEC50 values at the wild-type and mutant S267K 5-HT6 receptor.
Results
Intrinsic activity of 5-HT6 receptor ligands at rat 5-HT6 receptor
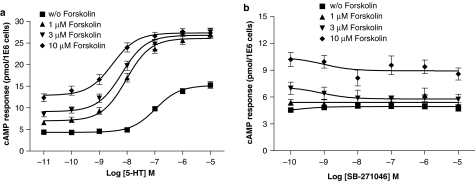
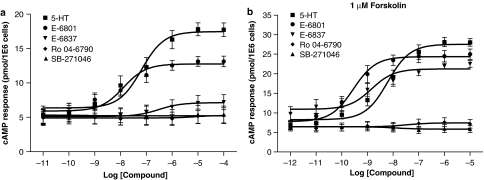
In contrast to nontransfected HEK-293F cells, 5-HT dose-dependently (pEC50: 6.98±0.08) induced cAMP formation in HEK-293F cells stably transfected with a rat 5-HT6 receptor in accordance with its pKi value (6.96±0.18). Coincubation with 1 μM forskolin enhanced not only basal cAMP formation, but also both the amplitude and potency of 5-HT (Figure 2a). Higher concentrations of forskolin did not affect the amplitude of 5-HT response (Figure 2a, Table 1). The ligand SB-271046 was free of intrinsic activity up to 10 μM and not affected by the copresence of forskolin (Figure 1b). Similar observations were made with Ro 04-6790 (Table 2). The 3-aminoalkylindolyl sulfonamide derivative E-6837 displayed a trend for intrinsic activity which was significantly enhanced in the copresence of forskolin (Figure 3) although its maximal effect was less than 5-HT (Table 2). A similar forskolin-effect was observed with the 3-aminoalkylindolyl sulfonamide derivative E-6801. It displayed potent partial agonism in the absence of forskolin and a pEC50 value similar to its pKi value as was observed for 5-HT (Figure 3 and Table 2). Interestingly, E-6801 displayed an Emax value indistinguishable to 5-HT when measured in the copresence of forskolin.
Figure 2.
Effect of forskolin on 5-HT and SB-271046-mediated cAMP formation in stably transfected HEK-293F/rat 5-HT6 cells. HEK-293F cells were stably transfected with rat 5-HT6 receptor as described in Methods. cAMP formation was determined after a 2 h serum-free incubation in the presence of either 5-HT or SB-271046 in the absence or copresence of indicated concentrations of forskolin using FlashPlate technology. pEC50 and Emax values related to 5-HT are summarized in Table 1. Data points correspond to mean values±s.e.m. from eight to ten independent experiments performed in duplicate. Specific [3H]-LSD binding (2.5–10 nM) to membranes of this cell line indicated 0.40±0.01 pmol mg protein−1. a: 5-HT, b: SB-271046.
Table 1.
Effect of forskolin on basal, Emax and pEC50 values of 5-HT-induced cAMP formation in stably transfected HEK-293F/rat 5-HT6 cells
| cAMP formation | |||
|---|---|---|---|
| Basal | 5-HT | ||
| (pmol 106 cells−1) | Emax (pmol 106 cells−1) | pEC50 | |
| w/o Forskolin | 4.6±0.4 | 15.2±0.4 | 6.98±0.08 |
| 1 μM Forskolin | 6.4±0.4a | 26.1±0.5b | 8.06±0.08c |
| 3 μM Forskolin | 8.8±0.7 | 26.8±0.5 | 8.22±0.09 |
| 10 μM Forskolin | 12.7±0.8 | 27.3±0.6 | 8.60±0.13d,e |
Emax and pEC50 values were derived from cAMP-mediated 5-HT response curves as illustrated in Figure 1a. Data correspond to mean±s.e.m. values of eight to ten independent experiments performed in duplicate. a: P<0.05 versus basal cAMP level w/o forskolin; b: P<0.001 versus Emax 5-HT w/o forskolin; c: P<0.001 versus pEC50 5-HT w/o forskolin; d: P<0.001 versus pEC50 5-HT 1 μM forskolin; e: P<0.05 versus Emax 5-HT 3 μM forskolin.
Table 2.
Emax and pEC50 values of several 5-HT6 receptor ligands for inducing cAMP formation in stably transfected HEK-293F/rat 5-HT6 cells in either absence or copresence of forskolin, and corresponding pKi values
| cAMP formation | |||||
|---|---|---|---|---|---|
| Forskolin (1 μm) | w/o | + | 5-HT6 receptor binding | ||
| Compound | Emax (pmol 106 cells−1) | pEC50 | Emax (pmol 106 cells−1) | pEC50 | pKi |
| 5-HT | 17.4±0.8 | 7.29±0.21 | 27.6±0.7a | 8.23±0.12b,c | 6.96±0.18 |
| E-6801 | 12.7±0.5 | 8.02±0.23 | 24.5±0.6a | 9.58±0.15b,c | 8.46±0.13 |
| E-6837 | 7.1±0.7 | 6.52±0.91d | 21.5±0.7a,e | 8.85±0.23b | 9.13±0.17 |
| Ro 04-6790 | 5.3±1.3 | <5 | 7.3±0.6 | <5 | 6.91±0.04 |
| SB-271046 | 5.2±0.5 | <5 | 5.9±0.5 | <5 | 8.68±0.09 |
| Basal | 5.8±0.4 | — | 7.0±0.4 | — | |
Emax and pEC50 values were derived from cAMP-mediated agonist curves as illustrated in Figure 3. Data correspond to mean±s.e.m. values of five to six independent experiments performed in duplicate. pKi values for h5-HT6 receptor were obtained as described in Methods. a: P<0.001 versus Emax w/o forskolin; b: P<0.001 versus pEC50 w/o forskolin; c: P<0.01 and d: P<0.001 versus pKi value; e: P<0.05 versus Emax 5-HT with forskolin.
Figure 3.
5-HT6 receptor ligand-mediated cAMP formation in stably transfected HEK-293F/rat 5-HT6 cells in either the absence or copresence of forskolin. HEK-293F cells were stably transfected with rat 5-HT6 receptor as described in Methods. cAMP formation was determined after a 2 h serum-free incubation in the presence of indicated ligands in either the absence (a) or presence (b) of 1 μM forskolin using FlashPlate technology. Mean dose–response curves±s.e.m. are shown from five to six independent experiments performed in duplicate. Mean Emax and pEC50 values±s.e.m. are summarized in Table 2.
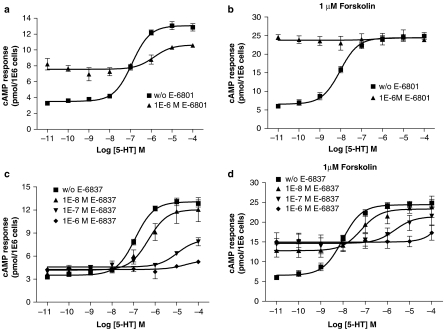
Antagonist activity of 5-HT6 receptor ligands at rat 5-HT6 receptor
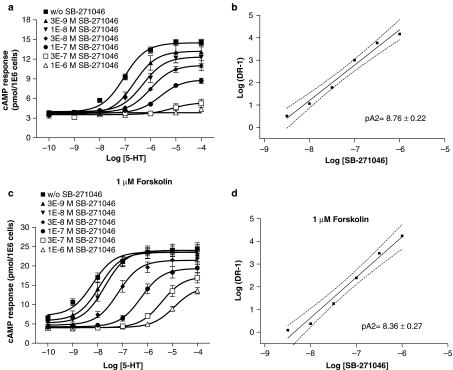
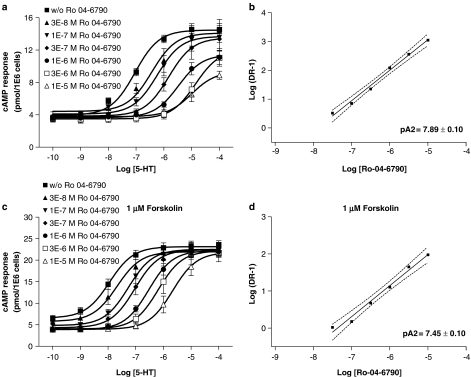
E-6801 (1 μM) displayed partial antagonism of the 5-HT response in the absence of forskolin as illustrated in Figure 4a. Otherwise, the combination of 5-HT and E-6801 in the copresence of 1 μM forskolin maintained maximal cAMP production (Figure 4b). E-6837 (1 μM) almost fully blocked the 5-HT response in the absence of forskolin (Figure 4c). Antagonism of 5-HT response by E-6837 (1 μM) was attenuated in the copresence of 1 μM forskolin (Figure 4d). SB-271046 and Ro 04-6790 antagonized in a dose-dependently manner the 5-HT response, both in the absence and presence of forskolin (Figures 5 and 6). The antagonist effects, as illustrated in Figure 5a and c, to a lesser extent 6a, appear not to be surmountable for the compounds SB-271046 and Ro 04-6790 at the highest agonist concentration. Otherwise, Ro 04-6790 behaved as a competitive antagonist in the copresence of forskolin (Figure 6c). pA2 values derived from Schild plot analysis indicate SB-271046 is a more potent antagonist than Ro 04-6790, and pA2 values are not significantly affected by the copresence of forskolin. A trend for both Ro 04-6790 and SB-271046 to attenuate cAMP values was apparent in the copresence of forskolin at low concentrations of 5-HT (Figures 5c and 6c) notwithstanding the lack of inhibition of basal receptor activity by SB-271046 in the copresence of 1 μM forskolin (Figure 2b).
Figure 4.
Antagonism of 5-HT-mediated cAMP formation by E-6801 and E-6837 in stably transfected HEK-293F/rat 5-HT6 cells in either the absence or copresence of forskolin. HEK-293F cells were stably transfected with rat 5-HT6 receptor as described in Methods. Antagonism of 5-HT-dose-dependent cAMP formation was determined after a 2 h serum-free incubation in the presence of 1 μM E-6801 or increasing concentrations of E-6837 in either the absence or copresence of 1 μM forskolin using FlashPlate technology. Mean 5-HT dose–response curves±s.e.m. are shown from four to seven independent experiments performed in duplicate. a, c: without forskolin; b, d: with 1 μM forskolin; a, b: with E-6801; c, d: with E-6837.
Figure 5.
Antagonism of 5-HT-mediated cAMP formation by SB-271046 in stably transfected HEK-293F/rat 5-HT6 cells in either the absence or copresence of forskolin. HEK-293F cells were stably transfected with rat 5-HT6 receptor as described in Methods. Antagonism of 5-HT-dose-dependent cAMP formation was determined after a 2 h serum-free incubation in the presence of increasing concentrations of SB-271046 in either the absence (a, b) or copresence of 1 μM forskolin (c, d) using FlashPlate technology. Mean 5-HT dose–response curves±s.e.m. are shown from five to ten independent experiments performed in duplicate. Schild plots (b, d) were constructed using mean values±s.e.m. The gradient of the best-fit straight line was determined by linear regression. Slope (95% confidence intervals) was between 1.28–1.87 (b) and 1.42 to 2.14 (d).
Figure 6.
Antagonism of 5-HT-mediated cAMP formation by Ro 04-6790 in stably transfected HEK-293F/rat 5-HT6 cells in either the absence or copresence of forskolin. HEK-293F cells were stably transfected with rat 5-HT6 receptor as described in Methods. Antagonism of 5-HT-dose-dependent cAMP formation was determined after a 2 h serum-free incubation in the presence of increasing concentrations of Ro 04-6790 in either the absence (a, b) or copresence of 1 μM forskolin (c, d) using FlashPlate technology. Mean 5-HT dose-response curves±s.e.m. are shown from five to ten independent experiments performed in duplicate. Schild plots (b, d) were constructed using mean values±s.e.m. The gradient of the best-fit straight line was determined by linear regression. Slope (95% confidence intervals) was between 0.92–1.19 (b) and 0.49–0.99 (d).
Intrinsic activity of 5-HT6 receptor ligands at human wild-type and S267K 5-HT6 receptor
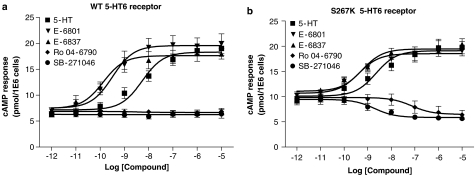
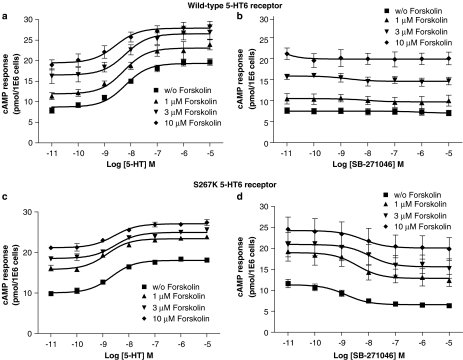
Basal cAMP formation was enhanced by 38±4% at the S267K mutant 5-HT6 receptor transiently expressed in Cos-7 cells as compared to nontransfected and wild-type human 5-HT6 receptor expressed in Cos-7 cells. Furthermore, the expression level of the mutant 5-HT6 receptor (0.52±0.01 pmol mg protein−1) was lower than wild-type 5-HT6 receptor (1.08±0.01 pmol mg protein−1). Such decreased expression is common for a constitutively active receptor, especially when mutated (S267 K) in or close to its B261BXXB265 motif in the junction of the third intracellular loop with the sixth transmembrane domain (Pauwels & Wurch, 1998). Interestingly, SB-271046 and Ro-046790 displayed negative intrinsic activity at the mutant S267K 5-HT6 receptor (Figure 7b) with potencies in agreement with their pKi values (Tables 2). In contrast, SB-271046 and Ro 04-6790 appeared silent at the human wild-type 5-HT6 receptor (Figure 7a). E-6801 and E-6837 displayed both at wild-type and mutant 5-HT6 receptor positive efficacy with an Emax value indistinguishable from 5-HT (Figure 7, Table 3). Copresence of forskolin (1–10 μM) altered neither the 5-HT nor the SB-271046 potency at either wild-type or mutant 5-HT6 receptors in Cos-7 cells (Figure 8, Table 4).
Figure 7.
5-HT6 receptor ligand-mediated cAMP formation in Cos-7 cells transiently transfected with either human wild-type or S267K 5-HT6 receptor. Cos-7 cells were transiently transfected with either human wild-type 5-HT6 (a) or S267K 5-HT6 receptor (b) as described in Methods. cAMP formation was determined after a 24 h serum-free incubation in the presence of indicated ligands using HTRF. Mean dose–response curves±s.e.m. are shown from five to ten independent experiments performed in duplicate. Mean Emax and pEC50 values±s.e.m. are summarized in Table 3. Specific [3H]LSD binding (2.5–10 nM) to membranes of the wild-type 5-HT6 and S267K 5-HT6 receptor cell lines indicated 1.08±0.01 and 0.52±0.01 pmol mg protein−1, respectively.
Table 3.
Emax and pEC50 values of several 5-HT6 receptor ligands for inducing cAMP formation in transiently transfected COS-7 cells with either human wild-type or S267K mutant 5-HT6 receptor
| cAMP formation | ||||
|---|---|---|---|---|
| 5-HT6 receptor | Wild-type | S267K | ||
| Compound | Emax (pmol 106 cells−1) | pEC50 | Emax (pmol 106 cells−1) | pEC50 |
| 5-HT | 18.3±0.6 | 8.31±0.16 | 19.1±0.3 | 8.73±0.25 |
| E-6801 | 19.6±0.6 | 9.50±0.21 | 18.5±0.3 | 9.40±0.14 |
| E-6837 | 17.6±0.5 | 9.89±0.21 | 19.4±0.4 | 9.31±0.18 |
| SB-271046 | 6.3±0.2 | <5 | 5.9±0.4a | 8.69±0.36 |
| Ro 04-6790 | 6.7±0.3 | <5 | 6.5±0.7b | 7.13±0.78 |
| Basal | 6.8±0.2 | — | 9.7±0.4c | — |
Emax and pEC50 values were derived from cAMP-mediated agonist/inverse agonist curves as illustrated in Figure 7. Data correspond to mean±s.e.m. values of five to ten independent experiments performed in duplicate. a: P<0.001 versus basal value of mutant 5-HT6 receptor; b P<0.01 versus basal value of mutant 5-HT6 receptor; c: P<0.001 versus basal value of wild-type 5-HT6 receptor.
Figure 8.
Effect of forskolin on 5-HT and SB-271046-mediated cAMP formation in Cos-7 cells transiently transfected with either human wild-type or S267K 5-HT6 receptor. Cos-7 cells were transiently transfected with either human wild-type 5-HT6 (a, b) or S267K 5-HT6 receptor (c, d) as described in Methods. cAMP formation was determined after a 24 h serum-free incubation in the presence of 5-HT (a, c) or SB-271046 (b, d) in either the absence or copresence of indicated concentrations of forskolin using HTRF. pEC50 and Emax values related to 5-HT are summarized in Table 4. pEC50 values of SB-271046 at S267K 5-HT6 receptor: 8.85±0.26, 8.42±0.64, 7.97±0.88 and 8.22±1.29 in, respectively, the absence and presence of 1, 3 and 10 μM forskolin. Data points correspond to mean values±s.e.m. from four to eight independent experiments performed in duplicate.
Table 4.
Effect of forskolin on basal, Emax and pEC50 values of 5-HT-induced cAMP formation in transiently transfected COS-7 cells with either human wild-type or S267K mutant 5-HT6 receptor
| cAMP formation | ||||||
|---|---|---|---|---|---|---|
| 5-HT6 receptor | Wild-type | S267K | ||||
| Basal (pmol 106 cells−1) | 5-HT Emax (pmol 106 cells−1) | pEC50 | Basal (pmol 106 cells−1) | 5-HT Emax (pmol 106 cells−1) | pEC50 | |
| w/o Forskolin | 7.8±0.3 | 19.5±0.8 | 8.21±0.13 | 10.4±0.5a | 18.1±0.2 | 8.60±0.07 |
| 1 μM Forskolin | 11.6±0.4b | 23.1±1.5 | 8.25±0.17 | 18.8±0.7c | 23.6±0.4d | 8.86±0.04e |
| 3 μM Forskolin | 16.1±0.9 | 26.6±0.9f | 8.21±0.10 | 21.5±2.2 | 24.9±0.1 | 8.62±0.04 |
| 10 μM Forskolin | 20.9±0.9 | 27.9±0.7 | 8.57±0.11 | 24.4±2.0 | 27.1±0.6 | 8.58±0.17 |
Emax and pEC50 values were derived from cAMP-mediated 5-HT response curves as illustrated in Figure 8. Data correspond to mean±s.e.m. values of four to eight independent experiments performed in duplicate. a: P<0.05 versus basal value wild-type 5-HT6 receptor; b: P<0.001 versus basal w/o forskolin wild type 5-HT6 receptor; c: P<0.01 versus basal value w/o forskolin mutant 5-HT6 receptor; d: P<0.001 versus Emax 5-HT w/o forskolin mutant 5-HT6 receptor; e: P<0.01 versus pEC50 wild-type 5-HT6 receptor; f: P<0.01 versus Emax 5-HT w/o forskolin wild type 5-HT6 receptor.
Discussion
Recombinant expression systems are well known for their capacity to differentiate between closely related compounds with respect to their intrinsic efficacy properties (i.e., Pauwels & Colpaert, 1995). Although there is no doubt that these model systems are useful to differentiate between functional properties of compounds, some caution should be taken to extrapolate findings from such recombinant systems to in vivo integrated systems. Indeed, a compound with partial agonist properties in a recombinant expression system may demonstrate antagonist activity in in vivo integrated systems (Hoyer & Boddeke, 1993). Partial agonism will be observed probably in most systems for such compounds although full agonism can be seen in very efficiently coupled systems and silent, competitive antagonism in very poorly coupled receptor systems. Therefore, a molecular pharmacological characterization of new ligands can only be based on several observations as made in different expression systems and using different read-outs. In the present report we have analyzed the ability of several compounds to modulate the Gs-coupled-cAMP pathway linked to the 5-HT6 receptor. Two experimental conditions, copresence of forskolin and/or a constitutively active mutant 5-HT6 receptor, were applied to enhance the resolution to monitor either positive or negative efficacy. In the copresence of forskolin, agonist features such as Emax and pEC50 values of 5-HT, E-6801 and E-6837 were significantly enhanced at the rat 5-HT6 receptor. Modulation of adenylyl cyclase activation by forskolin as mediated by wild-type β2-adrenergic receptors in adipocytes (Litosch et al., 1982) and constitutively active Gs-coupled receptors, such as histamine H2 and 5-HT7 receptors in HEK-293 cells, has previously been reported (Alewijnse et al., 1997; Krobert & Levy, 2002). Moreover, the increased forskolin-mediated cAMP response was inhibited by either H2 receptor inverse agonists or 5-HT7 receptor inverse agonists. We did not observe agonist-independent rat 5-HT6 receptor activation in HEK-293F cells, neither in the absence nor in the copresence of forskolin. Constitutive activation of the human 5-HT6 receptor was, however, observed by mutation of its Ser267 to a Lys in accordance with previous observations (Teitler et al., 2002). Copresence of forskolin did not modify the potency of agonist/inverse agonist-dependent cAMP formation in Cos-7 cells. Therefore, the effect of forskolin seems to be mainly determined by the host cell type and likely the receptor subtype. The S267K mutant receptor expressed in Cos-7 cells described here provides a sensitive system to monitor both inverse agonism and agonism in 5-HT6 receptor ligands.
Several major conclusions can be drawn with regard to the 5-HT6 receptor ligands investigated in this report. Firstly, SB-271046 behaved as a 5-HT6 antagonist at the human 5-HT6 receptor in accordance with the report of Routledge et al. (2000). This compound was reported as virtually free of intrinsic activity but it was measured under presumably silent 5-HT6 receptor conditions. The present study demonstrates SB-271046 displays negative efficacy at a constitutively active human S267K 5-HT6 receptor. Similar negative efficacy has been previously reported at this constitutively active mutant receptor for the atypical antipsychotic clozapine (Teitler et al., 2002; Purohit et al., 2003; 2005) and typical antipsychotic fluphenazine (Purohit et al., 2005). This strongly suggests SB-271046 is an inverse agonist and this property, although its physiological role is not well-defined (see Kenakin, 2004), may be of importance under both acute and chronic constitutively active 5-HT6 receptor conditions. In these instances in which negative efficacy is expressed, there may be conditions in which this is a useful property (i.e., reduce pathologically-induced constitutive activity) or an undesired property (tolerance to antagonism). Ro 04-6790 also behaved as a 5-HT6 receptor inverse agonist/antagonist. Sleight et al. (1998) reported this compound had no effect on basal cAMP accumulation in HeLa cells stably expressing human 5-HT6 receptor, suggesting Ro 04-6790 is neither an agonist nor an inverse agonist. The present study suggests that Ro 04-6790 is an inverse agonist/antagonist. Secondly, partial to full agonism was observed both with E-6801 and E-6837. Full and highly potent agonism was observed for E-6801 and E-6837 in the Cos-7 expression systems, both at human wild-type and mutant S267 K 5-HT6 receptors. Nonetheless, it is unlikely E-6801 and E-6837 display similar efficacy as 5-HT when taking their pKi/pEC50 values into account. Indeed, 5-HT illustrated a larger pKi/pEC50 ratio (22 and 58 for wild-type and S267 K 5-HT6 receptor, respectively) as compared to 11 and 9 (E-6801) and 6 and 2 (E-6837), respectively. Agonism by E-6837 was also observed at the rat 5-HT6 receptor but principally in the copresence of forskolin. E-6801 was a potent partial agonist at rat 5-HT6 receptor in the absence of forskolin. Moreover, it could be converted in the copresence of forskolin into a highly efficacious agonist with similar efficacy to 5-HT. Therefore, we can postulate the agonist response by E-6801 at the rat 5-HT6 receptor resembles more closely that of 5-HT than E-6837. This also fits with corresponding pKi/pEC50 ratios: 18, 13 and 0.5 for 5-HT, E-6801 and E-6837, respectively. Other examples of reported 5-HT6 receptor partial agonists are: SB-331711 (5-chloro-3-methyl-N-(4-(piperazin-1-yl)quinolin-6-yl)benzo[b]thiophene-2-sulfonamide) and related 4-piperazinyl quinolines (Bromidge et al., 2001b), and several similar substituted piperazine analogs (Bromidge et al., 2001b) producing some stimulation of basal adenylyl cyclase. It seems that each of the investigated 5-HT6 compounds actually possesses intrinsic activity. However, the ‘observable' magnitude of this effect is variable and mainly dependent on the experimental model system. Nonobservance of efficacy does not necessarily imply absence of efficacy. The experimental conditions must be appropriate for the effect to be monitored. The challenge is still to find a neutral, silent 5-HT6 receptor antagonist in order to learn more about the advantages/disadvantages under physiological and pathological CNS conditions of either a 5-HT6 neutral antagonist versus either a partial agonist or inverse agonist/antagonist. Interestingly, the predominance of inverse agonism agrees with theoretical predictions which indicate that neutral antagonists are the minority species in pharmacological space (Kenakin, 2004). The model systems described here will be useful to identify truly silent 5-HT6 receptor antagonists as they can exclude any ligand with efficacy, either positive or negative.
In conclusion, the use of either forskolin or a constitutively active S267K 5-HT6 receptor enhances the resolution to analyze the efficacy of 5-HT6 compounds. SB-271046 and Ro 04-6790 exhibit an inverse agonist/antagonist profile, whereas the novel 5-HT6 receptor ligands E-6801 and E-6837 are potent partial agonists at the 5-HT6 receptor in vitro.
Acknowledgments
In honor of the memory of our dear friend and colleague Dr Gonzalo Romero (26th of September 1964, Sevilla), who had a great sense of humour and love of life, who died much too soon on the 9th of July 2006. We sincerely thank Professor P. Strange for critical reading of the manuscript. We acknowledge Dr J. Giraldo for support with the statistical analysis. We also thank A. Dordal for providing pKi values and X. Monroy for sequence analysis of rat 5-HT6 receptor.
Abbreviations
- E-6801
6-chloro-N-(3-(2-(dimethylamino)ethyl)-1H-indol-5-yl)imidazo[2,1-b]thiazole-5-sulfonamide
- E-6837
5-chloro-N-(3-(2-(dimethylamino)ethyl)-1H-indol-5-yl)naphthalene-2-sulfonamide
- EMDT
2-(2-ethyl-5-methoxy-1H-indol-3-yl)-N,N-dimethylethanamine
- LY-483518/SGS-518
1-methyl-3-(1-methylpiperidin-4-yl)-1H-indol-5-yl 2,6-ifluorobenzenesulfonate
- MS-245
2-(5-methoxy-1-(phenylsulfonyl)-1H-indol-3-yl)-N,N-dimethylethanamine
- Ro 04-06790
N-(2,6-bis(methylamino)pyrimidin-4-yl)-4-aminobenzenesulfonamide
- Ro 63-0563
N-(2,6-bis(methylamino)pyridin-4-yl)-4-aminobenzenesulfonamide
- Ro 65-7199
4-amino-N-(6-bromo-1H-indol-4-yl)benzenesulfonamide
- SB-271046
5-chloro-N-(4-methoxy-3-(piperazin-1-yl)phenyl)-3-methylbenzo[b]thiophene-2-sulfonamide
- SB-331711
5-chloro-3-methyl-N-(4-(piperazin-1-yl)quinolin-6-yl)benzo[b]thiophene-2-sulfonamide
- SB-357134
N-(2,5-dibromo-3-fluorophenyl)-4-methoxy-3-(piperazin-1-yl)benzenesulfonamide
- SB-399885
N-(3,5-dichloro-2-methoxyphenyl)-4-methoxy-3-(piperazin-1-yl)benzenesulfonamide
- SB-742457
3-(phenylsulfonyl)-8-(piperazin-1-yl)quinoline
- WAY-181187/SAX187
2-(1-(6-chloroimidazo[2,1-b]thiazol-5-ylsulfonyl)-1H-indol-3-yl)ethanamine
References
- AHMED M., JOHNSON C.N., JONES M.C., MACDONALD G.J., MOSS S.F., THOMPSON M., WADE C.E., WITTY D. 2003. Patent no. WO 2003/080580, Glaxo Group Limited
- ALEWIJNSE A.E., SMIT M.J., RODRIGUEZ-PENA M.S., VERZIJL D., TIMMERMAN H., LEURS R. Modulation of forskolin-mediated adenylyl cyclase activation by constitutively active G(S)-coupled receptors. FEBS Lett. 1997;419:171–174. doi: 10.1016/s0014-5793(97)01440-3. [DOI] [PubMed] [Google Scholar]
- BENTLEY J.C., MARSDEN C.A., SLEIGHT A.J., FONE K.C.F.5-HT6 antisense oligonucleotide i.c.v. affects rat performance in the water maze and feeding 1997. British Association for Psychopharmacology Meeting, Cambridge, 13–17th July 1997, P255
- BOESS F.G., MONSMA F.J., JR, CAROLO C., MEYER V., RUDLER A., ZWINGELSTEIN C., SLEIGHT A.J. Functional and radioligand binding characterization of rat 5-HT6 receptors stably expressed in HEK293 cells. Neuropharmacology. 1997;36:713–720. doi: 10.1016/s0028-3908(97)00019-1. [DOI] [PubMed] [Google Scholar]
- BÖS M., SLEIGHT A.J., GODEL T., MARTIN J.R., RIEMER C., STADLER H. 5-HT6 receptor antagonists: lead optimisation and biological evaluation of N-aryl and N-heteroaryl 4-amino-benzene sulfonamides. Eur. J. Med. Chem. 2001;36:165–178. doi: 10.1016/s0223-5234(00)01209-5. [DOI] [PubMed] [Google Scholar]
- BOURSON A., BORRONI E., MONSMA F.J., SLEIGHT A.J. Determination of the role of the 5-HT6 receptor in the rat brain: a study using antisense oligonucleotides. J. Pharmacol. Exp. Ther. 1995;274:173–180. [PubMed] [Google Scholar]
- BROMIDGE S.M., BROWN A.M., CLARKE S.E., DODGSON K., GAGER T., GRASSAM H.L., JEFFREY P.M., JOINER G.F., KING F.D., MIDDLEMISS D.N., MOSS S.F., NEWMAN H., RILEY G., ROUTLEDGE C., WYMAN P. 5-Chloro-N-(4-methoxy-3-piperazin-1-yl-phenyl)-3-methyl-2-benzothiophenesulfon- amide (SB-271046): a potent, selective, and orally bioavailable 5-HT6 receptor antagonist. J. Med. Chem. 1999;42:202–205. doi: 10.1021/jm980532e. [DOI] [PubMed] [Google Scholar]
- BROMIDGE S.M., CLARKE S.E., GAGER T., GRIFFITH K., JEFFREY P., JENNINGS A.J., JOINER G.F., KING F.D., LOVELL P.J., MOSS S.F., NEWMAN H., RILEY G., ROGERS D., ROUTLEDGE C., SERAFINOWSKA H., SMITH D.R. Phenyl benzenesulfonamides are novel and selective 5-HT6 antagonists: identification of N-(2,5-dibromo-3-fluorophenyl)-4-methoxy-3-piperazin-1-ylbenzenesulfonamide (SB-357134) Bioorg. Med. Chem. Lett. 2001a;11:55–58. doi: 10.1016/s0960-894x(00)00597-7. [DOI] [PubMed] [Google Scholar]
- BROMIDGE S.M., GRIFFITH K., HEIGHMAN T.D., JENNINGS A., KING F.D., MOSS S.F., NEWMAN H., RILEY G., ROUTLEDGE C., SERAFINOWSKA H.T., THOMAS D.R. Novel (4-piperazin-1-ylquinolin-6-yl) arrylsulfonamides with high affinity and selectivity for the 5-HT6 receptor. Bioorg. Med. Chem. Lett. 2001b;11:2843–2846. doi: 10.1016/s0960-894x(01)00558-3. [DOI] [PubMed] [Google Scholar]
- CALDIROLA P.5-HT6 receptor antagonism. A novel mechanism for the management of obesity SMi Conf Obesity and Related Disorders 2003(February 17–February 18), London, U.K
- COLE D.C., STOCK J.R., LENNOX W.J., BERNOTAS R.C., ELLINGBOE J.W., LEUNG L., SMITH D., ZHANG G., LI P., QIAN L., DAWSON L.A., BOIKESS S., ROSENZWEIG-LIPSON S., BEYER C.E., SCHECHTER L.E.Discovery of a potent, selective and orally active 5-HT6 receptor agonist, WAY-181187 230th ACS National Meet 2005(August 28–September 1), Washington, U.S.A
- DAVIES S.L., SILVESTRE J.S., GUITART X. Drug discovery targets: 5-HT6 receptor. Drug Future. 2005;30:479–495. [Google Scholar]
- GABRIEL D., VERNIER M., PFEIFER M.J., DASEN B., TENAILLON L., BOUHELAL R. High throughput screening technologies for direct cyclic AMP measurement. Assay Drug Dev. Technol. 2003;1:291–303. doi: 10.1089/15406580360545107. [DOI] [PubMed] [Google Scholar]
- GLENNON R.A., LEE M., RANGISETTY J.B., DUKAT M., ROTH B.L., SAVAGE J.E., MCBRIDE A., RAUSER L., HUFEISEN L., LEE D.K.H. 2-Substituted tryptamines: Agents with selectivity for 5-HT6 serotonin receptors. J. Med. Chem. 2000;43:1011–1018. doi: 10.1021/jm990550b. [DOI] [PubMed] [Google Scholar]
- HIRST W.D., ABRAHAMSEN B., BLANEY F.E., CALVER A.R., ALOJ L., PRICE G.W., MEDHURST A.D. Differences in the central nervous system distribution and pharmacology of the mouse 5-hydroxytryptamine-6 receptor compared with rat and human receptors investigated by radioligand binding, site-directed mutagenesis, and molecular modeling. Mol Pharmacol. 2003a;64:1295–1308. doi: 10.1124/mol.64.6.1295. [DOI] [PubMed] [Google Scholar]
- HIRST W.D., MOSS S.F., BROMIDGE S.M.Characterisation of SB-399885, a potent and selective 5-HT6 receptor antagonist 33rd Annual Meet Soc Neurosci 2003b(November 8–November 12), Abstract 576.7, New Orleans, U.S.A
- HOLENZ J., MERCE R., DIAZ J.L., GUITART X., CODONY X., DORDAL A., ROMERO G., TORRENS A., MAS J., ANDALUZ B., HERNANDEZ S., MONROY X., SANCHEZ E., HERNANDEZ E., PEREZ R., CUBI R., SANFELIU O., BUSCHMANN H. Medicinal chemistry driven approaches toward novel and selective serotonin 5-HT6 receptor ligands. J. Med. Chem. 2005;48:1781–1795. doi: 10.1021/jm049615n. [DOI] [PubMed] [Google Scholar]
- HOLENZ J., PAUWELS P.J., DIAZ J.L., MERCE R., CODONY X., BUSCHMANN H. Medicinal chemistry strategies to 5-HT6 receptor ligands as potential cognitive enhancers and antiobesity agents. Drug Discovery Today. 2006;11:283–299. doi: 10.1016/j.drudis.2006.02.004. [DOI] [PubMed] [Google Scholar]
- HOYER D., BODDEKE H.W. Partial agonists, full agonists, antagonists: dilemmas of definition. Trends Pharmacol. Sci. 1993;14:270–275. doi: 10.1016/0165-6147(93)90129-8. [DOI] [PubMed] [Google Scholar]
- KENAKIN T. Efficacy as a vector: the relative prevalence and paucity of inverse agonism. Mol. Pharmacol. 2004;65:2–11. doi: 10.1124/mol.65.1.2. [DOI] [PubMed] [Google Scholar]
- KOHEN R., METCALF M.A., KHAN N., DRUCK T., HUEBNER K., LACHOWICZ J.E., MELTZER H.Y., SIBLEY D.R., ROTH B.L., HAMBLIN M.W. Cloning, characterization, and chromosomal localization of a human 5-HT6 serotonin receptor. J. Neurochem. 1996;66:47–56. doi: 10.1046/j.1471-4159.1996.66010047.x. [DOI] [PubMed] [Google Scholar]
- KROBERT K.A., LEVY F.O. The human 5-HT7 serotonin receptor splice variants: constitutive activity and inverse agonist effects. Br. J. Pharmacol. 2002;135:1563–1571. doi: 10.1038/sj.bjp.0704588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LITOSCH I., HUDSON T.H., MILLS I., LI S.-Y., FAIN J.N. Forskolin as an activator of cyclic AMP accumulation and lipolysis in rat adipocytes. Mol. Pharmacol. 1982;22:109–115. [PubMed] [Google Scholar]
- MERCE-VIDAL R., ANDALUZ-MATARO B., FRIGOLA-CONSTANSA J.Preparation of N-(1H-indol-5-yl) sulfonamide derivatives with 5-HT6 receptor antagonist activity, their preparation, and their application as medicaments for CNS diseases 2003. WO 2003/042175 A1, Laboratorios Dr. Esteve S.A
- PAUWELS P.J., COLPAERT F.C. Differentiation between partial and silent 5-HT1D beta receptor antagonists using rat C6-glial and Chinese hamster ovary cell lines permanently transfected with a cloned human 5-HT1D beta receptor gene. Biochem. Pharmacol. 1995;50:1651–1658. doi: 10.1016/0006-2952(95)02059-4. [DOI] [PubMed] [Google Scholar]
- PAUWELS P.J., WURCH T. Amino acid domains involved in constitutive activation of G-protein-coupled receptors. Mol. Neurobiol. 1998;17:109–135. doi: 10.1007/BF02802027. [DOI] [PubMed] [Google Scholar]
- PIÑEIRO-NÚÑEZ M.M., BAUZON D.D., BYMASTER F.P., CHEN Z., CHERNET M.P., CLAY M.P., CRILE R., DELAPP N.W., DENNY C.P., FALCONE J.F., FLAUGH M.E., HEINZ L.J., KIEFER A.D., JR, KOCH D.J., KRUSHINSKI J.H., JR, LEANDER J.D., LINDSTROM T.D., LIU B., MCKINZIE D.L., NELSON D.L., PHEBUS L.A., ROCCO V.P., SCHAUS J.M., WOLFF M.C., WARD J.S.Discovery and SAR studies of 2,6-difluorobenzenesulfonic acid 1-methyl-3-(1-methylpiperidin-4-yl)-1H-indol-5-yl ester, a novel and potent 5-HT6 antagonist for the treatment of cognitive deficit 229th ACS Natl. Meet 2005(Mach 13–March 17), San Diego, U.S.A
- PUROHIT A., HERRICK-DAVIS K., TEITLER M. Creation, expression, and characterization of a constitutively active mutant of the human serotonin 5-HT6 receptor. Synapse. 2003;47:218–224. doi: 10.1002/syn.10157. [DOI] [PubMed] [Google Scholar]
- PUROHIT A., SMITH C., HERRICK-DAVIS K., TEITLER M. Stable expression of constitutively activated mutant h5HT6 and h5HT7 serotonin receptors: inverse agonist activity of antipsychotic drugs. Psychopharmacology. 2005;179:461–469. doi: 10.1007/s00213-004-2057-6. [DOI] [PubMed] [Google Scholar]
- ROUTLEDGE C., BROMIDGE S.M., MOSS S.F., PRICE G.W., HIRST W., NEWMAN H., RILEY G., GAGER T., STEAN T., UPTON N., CLARKE S.E., BROWN A.M., MIDDLEMISS D.N. Characterization of SB-271046: a potent, selective and orally active 5-HT6 receptor antagonist. Br. J. Pharmacol. 2000;130:1606–1612. doi: 10.1038/sj.bjp.0703457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUAT M., TRAIFFORT E., ARRANG J.M., TARDIVEL-LACOMBE J., DIAZ J., LEURS R., SCHWARTZ J.C. A novel rat serotonin (5-HT6) receptor: molecular cloning, localization and stimulation of cAMP accumulation. Biochem. Biophys. Res. Commun. 1993;193:268–276. doi: 10.1006/bbrc.1993.1619. [DOI] [PubMed] [Google Scholar]
- RUSSELL M.G.N., BAKER R.J., BARDEN L., BEER M.S., BRISTOW L., BROUGHTON H.B., KNOWLES M., MCALLISTER G., PATEL S., CASTRO J.L. N-Arylsulfonylindole Derivatives as Serotonin 5-HT6 Receptor Ligands. J. Med. Chem. 2001;44:3881–3895. doi: 10.1021/jm010943m. [DOI] [PubMed] [Google Scholar]
- SCHOEFFTER P., WAEBER C. 5-Hydroxytryptamine receptors with a 5-HT6 receptor-like profile stimulating adenylyl cyclase activity in pig caudate membranes. Naunyn-Schmiedeberg's Arch. Pharmacol. 1994;350:356–360. doi: 10.1007/BF00178951. [DOI] [PubMed] [Google Scholar]
- SEBBEN M., ANSANAY H., BOCKAERT J., DUMUIS A. 5-HT6 receptors positively coupled to adenylyl cyclase in striatal neurones in culture. Neuroreport. 1994;5:2553–2557. doi: 10.1097/00001756-199412000-00037. [DOI] [PubMed] [Google Scholar]
- SLASSI A., ISAAC M., O'BRIEN A. Recent progress in 5-HT6 receptor antagonists for the treatment of CNS diseases. Expert Opin. Ther. Patents. 2002;12:513–527. [Google Scholar]
- SLEIGHT A.J., BOESS F.G., BOS M., LEVET-TRAFIT B., RIEMER C., BOURSON A. Characterization of Ro 04-6790 and Ro 63-0563: potent and selective antagonists at human and rat 5-HT6 receptors. Br. J. Pharmacol. 1998;124:556–562. doi: 10.1038/sj.bjp.0701851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TEITLER M., HERRICK-DAVIS K., PUROHIT A. Constitutive activity of G-protein coupled receptors: emphasis on serotonin receptors. Curr. Top. Med. Chem. 2002;2:529–538. doi: 10.2174/1568026023393859. [DOI] [PubMed] [Google Scholar]
- VICKERS S.P., DOURISH C.T. Serotonin receptor ligands and the treatment of obesity. Curr. Opin. Invest. Drugs. 2004;5:377–388. [PubMed] [Google Scholar]
- WOOLLEY M.L., MARSDEN C.A., FONE K.C. 5-ht6 receptors. Curr. Drug. Targets CNS Neurol. Disord. 2004;3:59–79. doi: 10.2174/1568007043482561. [DOI] [PubMed] [Google Scholar]