Abstract
It is well known that most people who use psychoactive drugs started as teenagers. In spite of this, there has been little preclinical research on the effects of psychostimulants during adolescence. Recently, however, a number of laboratories have begun to focus on drug effects in adolescents as compared to adults. The data show that there are unique responses to drugs during this period of development. This review will focus on our current understanding of neurochemical and behavioral drug effects during adolescence.
Keywords: adolescence, nicotine, cocaine, amphetamine, cannabinoid, development
Introduction
Most research using animal models of drug abuse has focused on the effects of drugs on brain neurochemistry and behavior in adult, prenatal, or preweanling animals. Until recently, there has been little research focusing on the effects of drugs like psychostimulants or cannabinoids in adolescent rats. However, it is well known that a large number of people who use drugs started as teenagers. The average age of first use is 12–14 years of age, and there has been a recent increase in the use of multiple drugs by adolescents 1. In addition, illicit drug use by adolescents (eighth graders) nearly doubled between 1991 (11%) and 1995 (21%) 1. The CDC 2 reported that 9.9% of students report initial use of marijuana prior to age 13. Similarly, the mean age of first nicotine use is 12.5 years, and of first alcohol use is 12 years 3. Between 1997 and 1999 nearly 2 million new users of MDMA were between the ages of 12 and 17, and approximately 900,000 people in this age group tried cocaine for the first time, according to the National Household Survey on Drug Abuse 4.
It has been suggested that unique maturational changes occur in neurotransmitter systems and behavioral repertoires during late childhood/young adulthood and that these changes could affect a subject’s response in a way that is different from those that are juvenile or adult 5. In animals, levels of pre- and post-synaptic dopamine (DA) content and other neurochemical markers of transmitter activity in the striatum exhibit a gradual increase until the time of puberty, when adult levels are reached 6. It has been suggested that cocaine may have a greater addictive potential among adolescents than adults 7, perhaps because of the difference in neurochemical make-up throughout puberty. In fact, young humans around the time of puberty report negligible effects after snorting cocaine so they are encouraged to do more to see what happens 8.
It has long been questioned whether nicotine leads to cannabinoid use, and similarly, whether cannabinoid use leads to the use of other drugs, such as cocaine or amphetamine. Recent data suggest that the age of first marijuana use may be a critical factor in answering this question. An Australian twin study concluded that early marijuana plays a causal role in the use of other illicit drugs 9, although others have questioned the conclusions of this study based upon the fact that twins, while genetically equivalent, do not necessarily experience the identical environment for review see10.
The National Survey on Drug Use and Health showed that adults who used marijuana before age 15 were 6 times more likely to be dependent on an illicit drug than adults who first used marijuana at age 21 or older 11. In addition, of adults who first used marijuana before age 15, 62% reported lifetime cocaine use, 9% reported lifetime heroin use, and 54% reported nonmedical use of pharmacotherapeutics. By comparison, among marijuana users who reported first smoking the drug after age 20, some 16% used cocaine, 1% used heroin, and 21% used pharmacotherapeutics nonmedically in their lifetime. Among those who had never used marijuana, 0.6% reported lifetime cocaine use, 0.1% reported lifetime heroin use, and 5.1% reported lifetime nonmedical pharmacotherapeutic use. These data show that the earlier the first marijuana use, the more likely one is to use other illicit drugs. If the first use is earlier than age 15, there is about a four-fold greater likelihood of cocaine use, and a 9-fold greater likelihood of heroin use than if the initiation was at a later age. While these studies do not unequivocally show causality, the data suggest that there may be fundamental differences in the effects of marijuana in preadolescents and young adolescents compared to adults.
It also appears that early use of marijuana can lead to alterations in physical characteristics. MRI and PET studies show that first marijuana use prior to age 17 leads to smaller whole brain cortical gray matter and a larger percent of white matter 12. In addition, males who begin marijuana use before age 17 had higher cerebral blood flow than other males. After early onset, both males and females were smaller in height and weight, with the effect being greater in males. The authors of this study concluded that “Early adolescence may be a critical period for effects that are not present when exposure begins later” 12. In spite of this information, most of the animal studies that have been done with cannabinoids were done on adult animals. These findings, along with the most recent data from the NSDUH, clearly show that it is important to understand the effects of cannabinoids in the preadolescent and adolescent brain as well as in the adult brain.
The focus of this review will be on laboratory animal studies of psychostimulant drugs including cocaine, amphetamines and nicotine, and cannabinoid drugs administered during adolescence. An attempt will be made to include comparisons to similar studies in adult animals, and the focus will be on studies where direct comparisons were made. Unfortunately, not all studies include a direct comparison between adolescent and adult animals, thus it is not always clear whether the observed effects are specific to adolescence, or whether the adolescent animals respond in a similar manner to adults. Although alcohol is another drug often used by adolescence, there have been a number of recent reviews on animal models of alcohol use in adolescence e.g. 13; 14–16 and alcohol studies will therefore not be included in this review.
One of the biggest difficulties in trying to assimilate the available information is that different labs use different definitions of adolescence and of periadolescence. Since many studies suggest that the age of treatment is an important factor in determining the outcome of studies, this shift in the treatment period can alter the results. For the purposes of this review, the definition of Spear and Brake 17 that periadolescence is approximately the 10 day period prior to the onset of puberty (which occurs at about 40 days of age in the rat) will be used, and the period of treatment will be provided for each study. Adolescence encompasses a longer time period, and generally rats are considered to be adolescent until approximately day 60, at which point they are considered to be adults.
I. Psychostimulants
I.A. Behavioral effects
Pharmacologically, periadolescent rats exhibit a reduced responsiveness to catecholaminergic agonists such as apomorphine 18, clonidine 19, amphetamine 20, and cocaine 21 and show increased responsiveness to the catecholaminergic antagonist haloperidol 22; 23, compared to younger or older rats. Spear and Brake 17 suggest that this hyposensitivity to catecholaminergic drugs may be due to functional immaturity of presynaptic dopamine autoreceptors in mesolimbic brain regions during the periadolescent period. They suggest further the possibility that the development of inhibitory autoreceptors during development may temporarily decrease mesolimbic dopamine function, with activity levels returning to normal as the nervous system adapts to the presence of the autoreceptors17.
The attenuated behavioral response to catecholaminergic agonists, accentuated behavioral response to catecholaminergic antagonists, and general hyperactivity when observed in isolated test conditions are behaviors similar to adult rats with lesions of the VTA, rich in dopamine neurons projecting to the mesolimbic dopamine regions 17; 24.
The effects of repeated cocaine administration on locomotor activity and sensitization to the locomotor-activating effects of cocaine in periadolescent and adult rats have been examined. Periadolescent (PND 28–34) and adult (PND 60–66) rats were injected with cocaine or vehicle for seven days and locomotor activity was measured daily 25. Ten days later (PND 44 or 76), rats either were challenged with cocaine and locomotor activity was measured, or dopamine transporter and receptor and serotonin transporter binding were examined. Adult rats became sensitized to the locomotor-activating effects of cocaine during the seven-day treatment and remained sensitized 10 days later. In contrast, no sensitization developed to the effects of cocaine on locomotor activity during the treatment in adolescent rats, and a very small increase in activity was observed 10 days later 25. Table 1 shows a summary of data comparing the behavioral effects of drug administration in male rats during adolescence and adulthood. Similarly, in mice, there was a smaller degree of sensitization to cocaine administration during adolescence compared to adulthood 26. In a separate study, periadolescent rats showed a reduced sensitivity to the effects of acute cocaine administration on locomotor activity relative to adults when tested on days 1 and 3 of a four-day treatment period 21. Two days after the last treatment, a cocaine challenge injection was administered, and both periadolescent and adult rats showed sensitization to the locomotor activating effects of cocaine, although it seemed that the sensitization in the adolescents may have been due at least in part to conditioning effects, and not solely due to the pharmacological actions of the drug 21.
Table 1.
Behavioral effects of treatment with drugs during adolescence vs adulthood. Male data only.
| Treatment | Measure | Results | Reference |
|---|---|---|---|
| COCAINE | |||
| Cocaine 4 days, PND 34–39, 60–70 | Locomotor activity | Less sensitization in PA than AD | 21 |
| Cocaine PND 28–34, 60–66 | Locomotor activity | PA: no sens; AD: sens | 25 |
| AMPHETAMINE | |||
| Amp | Locomotor activity | PA: sens only after PND49 | 29 |
| Amp 30, 60 | CPP | PA: no amp CPP; AD: amp CPP | 30 |
| NICOTINE | |||
| Nic 7 days PND 28, 60 Test 1 or 30 days later | Locomotor activity | PA: no sens; AD: sens | 54; 55 |
| Nic 7 days, inj PND 28–34, 90-96, Test 3 days later | Locomotor activity | PA: no sens; AD: sens | 56 |
| Nic 10 days, PND 28, 70 | Locomotor activity, cue conditioning | PA: no sens, cue conditioning; AD: sens and cue conditioning | 57 |
| Nic continuous 21 days; PND 30, 60 | Locomotor activity | PA, AD: NC during tx | 89 |
| Nic continuous 12 days: PND 25, 55 | Locomotor activity | PA, AD: sens during tx | 58 |
| Nic continuous PND 30–47 | Grooming | PA: no change; AD: decreased | 59 |
| Nic acute or chronic | Anxiety | Nic produced anxiety in PA but not in AD | 63–65 |
| Nic 7 days PND 28, 60 Test 1 or 30 days later | Locomotor activity | PA: sens to cocaine days 1 and 30; AD: sens to cocaine day 1 only | 54 |
| Nic 7 days PND 28, 60 Test 1 or 30 days later | Locomotor activity | PA: sens to amp; AD: no change | 55 |
| Nic 10 days – test 5 weeks later | Nic S-A | Increased after tx as PA | 53 |
| Nic PND 42–60 | Self-administration | ↑ fentanyl S-A | 67 |
| Nic PND 28, 38, 90 | Nic CPP | CPP only at PND 28 | 62 |
| Nic PND 28, 58 Test PND 40, 70 | Nic CPP | CPP only in PA, not in AD | 61 |
| Nic PND 25–60 (mice) Tested 12 days later | Cocaine CPP | ↓ CPP | 68 |
| Nic PND 35–44, Test PND 80 | Cocaine CPP | ↑ CPP | 66 |
PA: periadolescent; AD: adult; sens: sensitization; Nic: nicotine; amp: amphetamine; CPP: conditioned place preference; S-A: self-administration; NC: no change
The behavioral effects of amphetamine in periadolescent and adult male rats also have been measured 27; 28. It was found that periadolescent rats were responsive to the activating effects of amphetamine in a linear dose-dependent fashion, but had a reduced response to the drug compared to the adult rats. In addition, it has been reported that sensitization to amphetamine occurred only if treatment was begun after postnatal day 49 29. While conditioned place preference to amphetamine developed in adult (PND >60) rats, there was no reliable conditioned place preference in adolescent (PND 30) rats trained with amphetamine 30. In addition, the adolescent rats appeared to be more impulsive than the adult rats, suggesting a pattern of greater impulsivity and lower reinforcing efficacy for amphetamine 30. Thus, it appears that periadolescent rats have a lower reinforcement efficacy than adult rats for amphetamine.
There were no significant alterations in the spontaneous motor activity of adult rats that had been treated with MDMA as adolescents 31. However, in adolescent mice treated daily with MDMA, cocaine-induced conditioned place preference was not altered initially but was increased in response to a cocaine challenge two weeks later 32. Similarly in rats treated with MDMA during adolescence, cocaine conditioned place preference was increased 31 days later, when the rats were adults 33. Similarly, it has been shown that MDMA administration to adult rats led to increased cocaine self-administration 34. Thus, it may be that MDMA has similar effects on subsequent cocaine administration in both adult and adolescent rats, although currently there do not appear to be studies where direct age comparisons were made.
I.B. Neurochemical effects
There are considerable neurochemical changes occurring during adolescence. In weeks 1–7 after birth (days 1–49) there are significant developmental changes in the dopamine system 35. During this period, there is a 2-fold reduction in dopamine turnover, but no change in the density of dopamine uptake sites. During the period of about 21–28 days of age, dopamine D2 receptors and GTP inhibition of adenylyl cyclase activity appear. In addition, dopamine D1 receptors are several fold higher in adolescents than adults and undergo pruning prior to adulthood 36; 37. Thus the dopamine system in the periadolescent brain is in a state of flux and is quite different from that seen in postnatal, or preweanling or adult brains.
Serotonin transporter density in the frontal cortex decreased from periadolescence (PND 25) until late adulthood, while norepinephrine transporters in the frontal cortex were highest at PND 25, decreased by puberty (PND 50), and then remained stable until old age 38. In the striatum and midbrain, both serotonin and norepinephrine transporter levels remained constant from weaning (PND 25) through old age. In contrast, dopamine transporter density in the striatum increased from day 25–50 and then decreased continuously until old age, whereas densities remained constant in the midbrain 38.
After seven days of cocaine injections, at which time adult rats were sensitized to the locomotor-activating effects of cocaine, there were significant increases in dopamine transporter density in the caudate putamen, and in serotonin transporter densities in the ventromedial caudate putamen, nucleus accumbens shell, and the olfactory tubercle compared to vehicle-treated adult rats 25. In contrast, in periadolescent rats that did not show sensitization to cocaine either during or after the treatment, there was no effect of cocaine on either dopamine or serotonin transporter densities. Table 2 shows a summary of the neurochemical effects of drug administration to male rats during adolescence and adulthood.
Table 2.
Neurochemical effects of treatment with drugs during adolescence vs adulthood. Male data only.
| Treatment | Results | Reference |
|---|---|---|
| COCAINE | ||
| Cocaine PND 28–34, 60–66 Test 10 days later | PA: NC; AD: ↑ DAT, ↑ SERT | 25 |
| Cocaine PND 35, 63, Test 15 min later (mice) | AD had higher plasma coc levels than PA | 39 |
| Cocaine binge PND 28, 42, 65 Test 1.5 h later | AD had higher plasma coc levels than PA, no diff in brain levels | 40 |
| AMPHETAMINE | ||
| Amp PND 35 | DA release is lower in PA than AD | 27 |
| Amp PND 35 | PA more sensitive than AD to coc, nom | 27 |
| Amp PND 35, 60 | No diff in c-fos in PA vs AD | 47 |
| Amp 3 days, PND 33–43, >70 | PA: ↑ amp stim DA release; AD: NC | 28 |
| M-A – 4 inj × 1 day, Test 7 days later | PA: NC; AD: ↓ DAT, TH, DA uptake; 5-HT: PA, AD: ↓ TPH | 44 |
| M-A – 4 days, PND 40,60 | PA: NC; AD: ↓ DA, ↓ TH | 45; 46 |
| MDMA PND 40, 70, Test 7 days later | ↓ 5-HT, DA in both PA and AD | 90 |
| MDMA acute – PND 28 | ↓ 5-HT | 51 |
| MDMA 2 inj × 3 days – PND 39 – 41; Test 12 days later | NC in 5-HT or SERT | 33 |
| NICOTINE | ||
| Nic from PND 28–34, 60–66 daily inj | PA: ↑ DAT, ↓ SERT, NC nAChR; AD: ↑ nAChR, NC DAT, SERT | 25 |
| Nic from PND 34–43, 60–69 daily inj | PA: ↑ α5, α6, β2; AD: NC | 53 |
| Nic 2 wks, PND 30 PA only | Initial ↑ DA turnover in STR, PND 50–60 ↓ DA turnover | 74 |
| Nic from PND 30–47 or 30–37 continuous | PA, AD: ↑ nAChR during tx; some persistence 30 days later | 70–72 |
| Nic continuous, PND 30–47.5, PA only | ↑ SERT PND 75; ↑ 5HT2 PND 45, 60 | 77 |
| Nic PND 30–47.5, PA only | PA: ↓ M2 muscarinic, ↑ basal and FSK-stim AC | 78 |
| Nic PND 30, 70 | Baseline arc and c-fos: PA>AD; > ↑ in arc in PFC in PA than AD after nic | 76 |
| Nic 4 days PND 31, 41, 56 | Nic acutely did not ↑ DA in PA; tolerance to ↑ DA in AD | 75 |
| Nic 7 days, AD | NC CB recs or mRNA | 80 |
| Nic from PND 28–34 daily inj | PA: ↑ CB recs in mPFC, hippo; AD: NC | |
| Nic 7 days, inj PND 28–34, 90–96, Test 3 days later | AD: tolerance to elevation in corticosterone; PA: NC | 56 |
PA: periadolescent; AD: adult; sens: sensitization; Nic: nicotine; amp: amphetamine; CPP: conditioned place preference; S-A: self-administration; NC: no change; DAT: dopamine transporter; SERT: serotonin transporter; nAChR: nicotinic acetylcholine receptor; DA: dopamine; 5-HT: serotonin; coc: cocaine; nom: nomifensine; TPH: tryptophan hydroxylase; STR: striatum; mPFC: medial prefrontal cortex; CB recs: cannabinoid receptors
An important question about the different behavioral effects of cocaine in adult and adolescent rodents is the question of whether differential pharmacokinetics can account for the differences in behavior. There are a few studies that have begun to address this question. It has been reported that plasma levels of cocaine subsequent to an acute injection are higher in adult than in adolescent mice 39 and rats 40 after injection with cocaine. The brain levels of cocaine, however, were not different in adults and adolescents in either rats 40 or in CD-1 mice 39. There do appear to be some strain differences, in that there were higher brain levels of cocaine in C57/BL6 adult compared to adolescent mice 39. In both studies, the brain levels of cocaine did not correlate with locomotor activity, suggesting that different pharmacokinetic properties of cocaine in adult and adolescent rodents did not account entirely for the differential behavioral effects of the drug in the two age groups. This is in contrast to studies in adult mice showing that the concentration of cocaine in brain and locomotor activity levels are highly correlated 41–43. In contrast to the similarity between brain levels of cocaine in adult and adolescent animals, methamphetamine levels in striatum are reported to be approximately twice as high in adult as in adolescent rats in response to a challenge injection compared to adults 44.
The effects of methamphetamine on dopamine uptake and binding to the dopamine transporter were different in periadolescent and adult rats 44. Periadolescent (PND 40) or adult (PND 90) rats were administered four injections of methamphetamine in a single day and dopamine transporter density, tyrosine hydroxylase, and dopamine uptake were measured seven days later. All three measures were significantly decreased in the rats treated as adults, and none were changed in the periadolescent rats. Similarly, daily injections of methamphetamine for four days decreased the number of tyrosine hydroxylase-positive terminals in adult (PND 60), but not adolescent (PND 40) rats 45, and reduced striatal dopamine in adults only 46. In contrast, tryptophan hydroxylase activity (a measure of serotonin function) was significantly reduced in both adult and adolescent rats 44.
Treatment with amphetamine for 3 days during periadolescence (PND 33–43) or adulthood (PND >70) led to sensitization of amphetamine-stimulated dopamine release in adolescents only 28. There were no differences between adolescent (PND 35) and adult (PND 60) rats in the expression of c-fos in response to an acute injection of amphetamine 47. After chronic administration for seven days, however, both cocaine and amphetamine upregulated DeltaFosB in adolescent, but not adult mice 48.
One drug that has been studied more extensively than most in periadolescent rats is MDMA. In adult rats, a repeated injection regimen (4 injections in a single day) of MDMA led to a 50% decrease in striatal serotonin and significant reductions in locomotor activity seven days later 49. MDMA administered on PND 40 or PND 70 acutely increased dopamine levels in the caudate putamen, but had no effect on dopamine on PND 10 50. Thus, this effect appears to develop during adolescence.
Administration of MDMA on PND 40 or PND 70 led to decreased serotonin levels in multiple brain regions, including the frontal cortex and striatum 50. A similar effect was reported in rats at PND 35, who exhibited long-term reductions in serotonin, while rats treated on PND 14, 21 or 28 did not have effects subsequent to an acute reduction 51. Rats treated repeatedly with MDMA (7.5 mg/kg ip twice daily for 3 days) starting at PND 39 had no changes in serotonin levels or cortical serotonin transporter density 12 days later 33. There was, however, increased serotonin uptake in whole brain synaptosomes of adult rats treated with a repeated high dose of MDMA (20 mg/kg ip, twice daily for four days) for at least 21 days after the end of the treatment 52. These studies suggest that the adolescent rat is less susceptible than the adult rat to the neurotoxic effects of MDMA.
II. Nicotine
II.A. Behavior
Studies have shown that adolescent rats have a unique response to nicotine compared with adult rats, although the results have varied. Nicotine injections for 10 days during adolescence produced an increase in nicotine self-administration five weeks after treatment ended, at which time the rats were adults 53. During treatment with nicotine, locomotor sensitization did not develop in male 10 adolescent rats in response to repeated nicotine treatment, although sensitization was observed in adult (PND 60–66) male and female rats, and adolescent (PND 28–34) female rats 54; 55. The same result was seen when adolescent (PND 28–34) male rats were compared to older adult (PND 90–96) male rats and were tested three days after the seven-day treatment period 56. Similarly, it has been reported that both sensitization and nicotine cue-conditioning occurred in adult but not adolescent male rats during a 10-day treatment period with nicotine 57. In contrast, it has also been reported that sensitization occurred in both adults and adolescents over a 12-day period of continuous infusion of nicotine 58. In addition, during continuous infusion of nicotine during PNDs 30–47, female, but not male, rats showed decreased grooming, the opposite of that seen in adult rats 59.
Nicotine self-administration rates are higher in adolescent (PND 50–62 at start) female rats than in adult (PND 84–90 at start) female rats and this persists into adulthood if administration is begun during adolescence 60. In addition, adolescent male rats exhibited conditioned place preference to nicotine at (training began on PND 28 and testing on PND 40), in contrast to adult male rats (PND 58–70), who did not develop a significant conditioned place preference to nicotine 61. Similarly, it has been shown that rats only developed a conditioned place preference to nicotine when treated early in adolescence (PND 28) but not in later adolescence (PND 38) or adulthood (PND 90) 62. These findings suggest that adolescence, especially early adolescence, may be a time of increased vulnerability to the reinforcing effects of nicotine.
Nicotine increased anxiety acutely 63, and this persisted ten days after the end of a five-day transdermal nicotine patch 64 in adolescent rats, and increased anxiety in mid-adolescent mice when administered via drinking water for 12 days 53. In contrast to the increased anxiety on an elevated plus maze test in adolescent rats (PND 30), nicotine appeared to have an anxiogenic effect in adult rats (PND 60) 65. Thus, it appears that nicotine has the opposite effect on anxiety in adolescent and adult rats.
Nicotine exposure in adolescence also has been shown to alter the subsequent behavioral responses to other classes of drugs. Nicotine treatment for seven days produced cross-sensitization to cocaine 54 and amphetamine 55 one day after treatment. These effects persisted into adulthood and were evident in response to a challenge 30 days later on PND 65 55. These effects were not seen in the adult rats or in the adolescent female rats. Similarly, nicotine administration during PNDs 35–44 led to increased cocaine CPP during adulthood (PND 80) 66. The cross-sensitization in response to adolescent nicotine was not evident for psychostimulants only, in that treatment with nicotine for 19 days during adolescence (PND 42–60) let to increased fentanyl self-administration during adulthood in male rats, but had no effect in female rats 67. It is not known whether this effect is specific to the adolescent period, since the effect of nicotine on opioid self-administration does not appear to have been studied in adult rats. The increased fentanyl self-administration in males only does not appear to be related to alterations in corticosterone or adrenocorticotropin hormone, since these hormones were increased subsequent to nicotine treatment in both male and female rats 67.
In another study, nicotine was administered twice daily to mice on PNDs 25–60 and cocaine conditioned place preference studies were begun 12 days later on PND 72 68. The group that had been treated with nicotine exhibited less cocaine conditioning than the group treated with saline. A more recent study showed that there was a dose-effect relationship between the dose of nicotine used during the treatment period and the effect on cocaine reinforcement 69. In this study, mice were treated with nicotine on PNDs 25–57 and testing began 28 days later. In contrast, in rats, treatment with nicotine on PNDs 35–44 produced increased cocaine conditioned place preference on PND 80, compared to animals treated with water 66. Thus it appears that treatment with nicotine during adolescence decreased the reinforcing effects of cocaine in mice and increased it in rats.
II.B. Neurochemistry
Daily administration of nicotine from PND 28–34 produced no significant differences in nicotinic acetylcholine receptor (nAChR) densities, as measured by autoradiography, in the rostral or caudal caudate putamen or nucleus accumbens core or shell one day later compared to vehicle controls 25. Adult rats pretreated with nicotine for seven days, however, had significantly greater nAChR densities in the rostral caudate putamen and the nucleus accumbens core and shell compared to vehicle controls. Nicotine did not produce any significant differences in the more caudal regions of the caudate putamen in either the periadolescent or adult rats 25. Although nACh receptors do not appear to be upregulated in the adolescent rats treated with nicotine, there is evidence that there is an increased gene expression of the α5, α6, and β2 nAChR subunits subsequent to 10 days (PND34–43) of nicotine administration 53. These changes were not seen in adult rats treated with nicotine (PND 60–69).
In contrast to once-daily administration of nicotine, during continuous 70–72 or twice-daily 71 administration of nicotine beginning on PND 30 there was an increase in nAChR density in the midbrain, cerebral cortex and hippocampus that persisted after treatment ended. The effect was greater in males than in females, although, as the authors point out, this could be due to the lack of control for dose 70. Since the drug was administered via osmotic minipump, and the males gained more weight than the females over the treatment period, their effective dose/body weight was approximately half the starting dose toward the end of the treatment period than at the beginning. In addition, the female rats were receiving approximately 20% more drug/body weight than the males. When rats were treated as adults starting on PND 90, there were also significant increases in nACh receptor density in the same brain regions, and they remained elevated for a period of time after treatment ended, although for less time than in the adolescents 70. Again, since dose was not corrected for body weight, and the adults gained less weight over time than the adolescents, their dose remained fairly constant over the treatment period, in contrast to the diminishing dose in the adolescents. There was also a small decrease in choline acetyltransferase activity in the midbrain of adolescent male rats treated with nicotine, although this was not studied in the adults 73.
Daily injection of nicotine from PND 28–34 produced an increase in dopamine transporter densities and a decrease in serotonin transporter densities in periadolescent rats, but no change in dopamine D1 or D2 receptor densities on PND 35 25. In adult rats pretreated with nicotine, there were no changes in dopamine transporter, dopamine D1 or D2 receptor, or serotonin transporter densities.
It has been shown that treatment with nicotine during the periadolescent phase produces an initial (3–10 days after the treatment) decrease in dopamine turnover in the midbrain, followed by a later activation of these pathways (30 days after the treatment) 74. Dopamine turnover was altered in adolescent males and females by day 45 of a 2-week continuous infusion of nicotine that began at PND 30, and some of these changes persisted into adulthood 74. Acutely, nicotine did not increase DA levels in the nucleus accumbens of adolescent (PND 35, 45) rats but did increase extracellular DA in adult (PND 60) rats 75. After 4 days of nicotine administration, tolerance developed to the effect of the drug on DA release in the adults. This finding is interesting, but may be confounded by the necessary use of anesthetic to implant the microdialysis probes on the day before testing. It is not known whether the ketamine/xylazine mixture has different effects in the adult and adolescent rats that may interfere with the dopamine and/or nACh systems.
There also appear to be differences in the regulation of acetylcholine (ACh) release in periadolescent compared to adult rats 27. Both cocaine and nomifensine inhibit ACh release in vitro in striatal tissue from adult and periadolescent rats, with maximal inhibition occurring at lower doses in periadolescent rats (i.e. a sensitized effect). Thus, it is possible that there is an increased cholinergic tone that may mediate the decreased activity following dopaminergic agonists in the periadolescent group. In periadolescent rats, a more efficient regulation of cholinergic neurons by dopamine may lead to upregulation of post-synaptic striatal cholinergic receptors. Behavioral subsensitivity in periadolescent rats could be attributed to increased cholinergic transmission despite increased regulatory influence of striatal cholinergic interneurons by dopamine 27.
Nicotine administration during adolescence has differential effects on early gene expression than during adulthood. An acute injection of nicotine produced greater induction of arc in prefrontal cortex, and decreased induction of arc, c-fos and NGFI-B in the somatosensory cortex of adolescent than adult rats 76. This suggests that the early gene response to nicotine administration is different in adult and adolescent rats and may lead to a differential cascade of events.
It has also been shown that nicotine can alter the serotonergic system in adolescent rats. Continuous infusion with nicotine for 2 weeks (beginning at PND 30) in adolescent rats produced an increase in serotonin transporter binding in female rats by day 45 and in male rats at day 75 77, as well as a decrease in M2 muscarinic receptors 78. Both basal and forskolin-stimulated adenylyl cyclase activity were increased at the same time after this continuous regimen 78. Further, after the same treatment regimen, there was an increase in 5HT2 receptor binding in male rats aged 45 and 60 that was not apparent in female rats 79.
An acute administration of nicotine on PND 37 or 99 increased plasma corticosterone levels 56. After repeated nicotine injections (PND 28–34, 90–96) for seven days prior, however, there was tolerance to this effect in the adult but not the adolescent rats 56. Thus, it appeared that the adults adapted to the repeated nicotine administration, while the response of the adolescents did not change.
In adult rats, chronic nicotine (1 mg/kg base, s.c. daily for 7 days, killed 2 h later) had no effect on CB1 receptors in the cerebral cortex, caudate putamen, nucleus accumbens, globus pallidus, substantia nigra, hippocampus, or dentate gyrus 80. Similarly, there were no changes in CB1 receptor mRNA, studied using in situ hybridization, except for a small decrease in the septum. Chronic nicotine did, however, decrease levels of two endogenous ligands for cannabinoid receptors (arachidonoylethanolamide, AEA, and 2-arachidonoyl-glycerol, 2-AG) in the striatum and cerebral cortex, and increased these ligands in the brainstem 80. In contrast, chronic cocaine (15 mg/kg/twice daily for 10 days) had no effects on any of these measures 80. In studies from our laboratory, we found that chronic treatment with nicotine (0.4 mg/kg/day base for 7 days) also produced no effect on CB receptors in male adult rats. In contrast, however, there were significant increases in the medial prefrontal cortex, the dentate gyrus, and the CA3 region of the hippocampus in adolescent male rats after the same treatment. Thus, it appears that there are differential changes in the cannabinoid system after nicotine treatment at different developmental stages.
III. Cannabinoids
III.A. Behavior
In adult rats, acute administration of low doses of the cannabinoid agonist CP 55,940 produced decreases in locomotor activity, which persisted over two weeks of treatment 81. When challenged with cocaine one week later, there were no differences in cocaine-stimulated activity compared to rats pretreated with vehicle 81. Similarly, if CP 55,940 was co-administered with cocaine for two weeks, there were no differences in the development of sensitization compared to the effects of cocaine alone. Studies from our laboratory fully support the data obtained in the adult rats, but show different adaptations in adolescent rats. We found that seven days of treatment with the cannabinoid agonist WIN 55212-2 (5 mg/kg/day split into two daily doses) had no effect on the subsequent stimulation of locomotor activity by cocaine in adult rats (Fig. 1), as reported by Arnold et al. as seen by 81, but led to a significant increase in cocaine-stimulated activity in the adolescent rats. This finding is supported by a recent study by Ambrosio and colleagues showing that treatment with CP 55,940 during early adolescence led to an increase in cocaine self-administration once the rats became adults, and this effect was greater in female than in male rats 82. Other behavioral changes were also found in adults who had been treated with CP 55,940 as adolescents, with decreases seen in head dipping on a hole board test in male rats 83. There were no changes in corticosterone levels in response to CP 55,940 in any group, thus this could not account for differences seen in male and female rats.
Fig. 1.
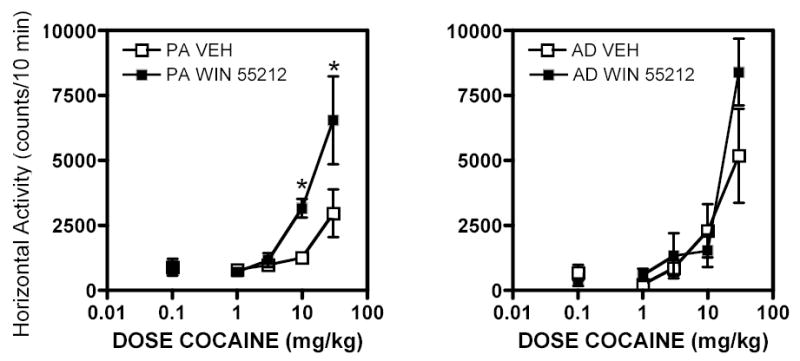
Effects of the cannabinoid agonist WIN 55212-2 during periadolescence (PA) and adulthood (AD). Rats were treated for 7 days with WIN 55212-2 or vehicle. On day 8, all rats were injected with vehicle (saline), followed by 1.0, 3.0, 10.0 and 30.0 mg/kg cocaine (i.p.) in a cumulative dosing regimen (actual injections of 1.0, 2.0, 7.0 and 10.0 mg/kg cocaine). Five min after each injection, locomotor activity was measured for a total of 10 min for vehicle and for each cumulative dose of cocaine.
III.B. Neurochemistry
In addition to the behavioral differences between adult and adolescent rats in response to chronic cannabinoid agonist treatment, there appear to be differential alterations in receptor density. Cannabinoid receptor density ([3H]CP 55,940 autoradiography) was determined in brain sections from rats treated with WIN 55212-2 or vehicle for seven days during either adolescence or adulthood. There were significant decreases in CB1 receptors in the CA3 region of the hippocampus in adolescent male rats and in the DG and CA1 regions of the hippocampus in adult male rats. No significant effects of cannabinoid treatment were seen in the striatum, nucleus accumbens, or substantia nigra of either adolescent or adult male rats. These findings are consistent with a number of studies in adult rats where decreases in CB1 density were seen after treatment with Δ9-THC e.g.84; 85–87. In addition to these changes in the hippocampus, there were significant decreases in the Cg1 and Cg2 regions of the prefrontal cortex in adolescent male rats treated with WIN 55212-2. Thus, both adults and adolescents exhibited tolerance to the behavioral effects of repeated WIN 55212-2 and both had changes in CB1 receptors in the hippocampus, although the pattern of changes was different. In contrast, only the adolescent rats had receptor changes in the prefrontal cortex.
Other studies have shown that treatment with WIN 55212-2 for 3 days led to a decreased responsiveness of dopamine neurons to WIN 55212-2 two weeks later in both adult and adolescent rats 88. In rats treated during adolescence, however, there was also cross-tolerance to the increase in firing rate of ventral tegmental dopamine neurons in response to morphine, cocaine and amphetamine. This cross-tolerance was not evident in the rats treated as adults.
Conclusions
These studies show that there may be an increased vulnerability to the effects of drugs during adolescence. Nicotine in particular, appears to have more robust effects in adolescent than in adult rats. The overall findings of the nicotine studies to date suggest that nicotine during adolescence may lead to increased susceptibility to the subsequent effects of other psychostimulants. In general, these studies suggest that it is important to examine the effects of psychoactive drugs in the adolescent population and not to assume that drug effects will be the same as in adults. To fully understand the response of the developing brain to drugs of abuse, more studies need to be done where comparisons between adult and adolescent responses are compared. An additional caveat is that the effects during adolescence appear to vary greatly in males and females, thus, it is important to compare the two sexes. A greater understanding of the differences between adult and adolescent drug responses will aid in the development of appropriate age-specific treatments for substance abuse.
Acknowledgments
This work was supported by NIDA grants DA 13936 and DA 15119.
References
- 1.Fishman M, Bruner A, Adger H., Jr Substance abuse among children and adolescents. Pediatrics in Review. 1997;18:394–403. [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Tobacco use among middle and high school students--United States, 2002. MMWR Morb Mortal Weekly Report. 2003;52:1096–1098. [PubMed] [Google Scholar]
- 3.CASA, National Survey of American Attitudes on Substance Abuse VIII: Teens and Parents. 2003;
- 4.NHSDA, Summary of findings from the 2001 national household survey on drug abuse, Rockville, MD, Substance Abuse and Mental Health Services Administration. Office of Applied Studies., 2001.
- 5.Witt ED. Mechanisms of alcohol abuse and alcoholism in adolescents: a case for developing animal models. Behav Neural Biol. 1994;62:168–177. doi: 10.1016/s0163-1047(05)80015-9. [DOI] [PubMed] [Google Scholar]
- 6.Noisin EL, Thomas WE. Ontogeny of dopaminergic function in the rat midbrain tegmentum, corpus striatum and frontal cortex. Dev Brain Res. 1988;41:241–252. doi: 10.1016/0165-3806(88)90186-1. [DOI] [PubMed] [Google Scholar]
- 7.Estroff TW, Schwartz RH, Hoffmann NG. Adolescent cocaine abuse. Addictive potential, behavioral and psychiatric effects. Clinical Pediatrics. 1989;28:550–555. doi: 10.1177/000992288902801201. [DOI] [PubMed] [Google Scholar]
- 8.Weiss RD, Mirin SM, Bartel RL. Cocaine. American Psychiatric Press; Washington, London: 1994. [Google Scholar]
- 9.Lynskey MT, Heath AC, Bucholz KK, Slutske WS, Madden PA, Nelson EC, Statham DJ, Martin NG. Escalation of drug use in early-onset cannabis users vs co-twin controls. JAMA. 2003;289:427–433. doi: 10.1001/jama.289.4.427. [DOI] [PubMed] [Google Scholar]
- 10.Kandel DB. Does marijuana use cause the use of other drugs? JAMA. 2003;289:482–483. doi: 10.1001/jama.289.4.482. [DOI] [PubMed] [Google Scholar]
- 11.NSDUH, Results from the 2002 National Survey on Drug Use and Health. Substance Abuse and Mental Health Services Administration. Office of Applied Studies. Rockville, MD. 2002:1–3.
- 12.Wilson W, Matthew R, Turkington T, Hawk T, Coleman RE, Provenzale J. Brain morphological changes and early marijuana use: a magnetic resonance and positron emission tomography study. J Addictive Dis. 2000;19:1–22. doi: 10.1300/J069v19n01_01. [DOI] [PubMed] [Google Scholar]
- 13.Spear NE, Molina JC. Fetal or infantile exposure to ethanol promotes ethanol ingestion in adolescence and adulthood: a theoretical review. Alcohol Clin Exp Res. 2005;29:909–929. doi: 10.1097/01.alc.0000171046.78556.66. [DOI] [PubMed] [Google Scholar]
- 14.Spear LP, Varlinskaya EI. Adolescence. Alcohol sensitivity, tolerance, and intake. Recent Dev Alcohol. 2005;17:143–159. [PubMed] [Google Scholar]
- 15.McBride WJ, Bell RL, Rodd ZA, Strother WN, Murphy JM. Adolescent alcohol drinking and its long-range consequences. Studies with animal models. Recent Dev Alcohol. 2005;17:123–142. doi: 10.1007/0-306-48626-1_6. [DOI] [PubMed] [Google Scholar]
- 16.Spear L. Modeling adolescent development and alcohol use in animals. Alcohol Res Health. 2000;24:115–123. [PMC free article] [PubMed] [Google Scholar]
- 17.Spear LP, Brake SC. Periadolescence: age-development behavior and psychopharmacological responsivity in rats. Dev Psychobio. 1983;16:83–109. doi: 10.1002/dev.420160203. [DOI] [PubMed] [Google Scholar]
- 18.Reinstein DK, McClearn D, Isaacson RL. The development of responsiveness to dopaminergic agonists. Brain Res. 1978;150:216–223. doi: 10.1016/0006-8993(78)90670-4. [DOI] [PubMed] [Google Scholar]
- 19.Reinstein DK, Isaacson RL. Clonidine sensitivity in the developing rat. Brain Res. 1977;135:378–382. doi: 10.1016/0006-8993(77)91042-3. [DOI] [PubMed] [Google Scholar]
- 20.Lanier LP, Isaacson RL. Early developmental changes in the locomotor response to amphetamine and their relation to hippocampal function. Brain Res. 1977;126:567–575. doi: 10.1016/0006-8993(77)90610-2. [DOI] [PubMed] [Google Scholar]
- 21.Laviola G, Wood RD, Kuhn C, Francis R, Spear LP. Cocaine sensitization in periadolescent and adult rats. J Pharmacol Exp Ther. 1995;275:345–357. [PubMed] [Google Scholar]
- 22.Shalaby IA, Spear LP. Psychopharmacological effects of low and high doses of apomorphine during ontogeny. Eur J Pharmacol. 1980;67:451–459. doi: 10.1016/0014-2999(80)90186-7. [DOI] [PubMed] [Google Scholar]
- 23.Spear LP, Shalaby IA, Brick J. Chronic administration of haloperidol during development: behavioral and psychopharmacological effects. Psychopharmacol. 1980;70:47–58. doi: 10.1007/BF00432369. [DOI] [PubMed] [Google Scholar]
- 24.Bowman BP, Kuhn CM. Age-related differences in the chronic and acute response to cocaine in the rat. Dev Psychobio. 1996;29:597–611. doi: 10.1002/(SICI)1098-2302(199611)29:7<597::AID-DEV4>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 25.Collins SL, Izenwasser S. Cocaine differentially alters behavior and neurochemistry in periadolescent versus adult rats. Dev Brain Res. 2002;138:27–34. doi: 10.1016/s0165-3806(02)00471-6. [DOI] [PubMed] [Google Scholar]
- 26.Niculescu M, Ehrlich ME, Unterwald EM. Age-specific behavioral responses to psychostimulants in mice. Pharmacol Biochem Behav. 2005;82:280–288. doi: 10.1016/j.pbb.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 27.Bolanos CA, Glatt SJ, Jackson D. Subsensitivity to dopaminergic drugs in periadolescent rats: a behavioral and neurochemical analysis. Dev Brain Res. 1998;111:25–33. doi: 10.1016/s0165-3806(98)00116-3. [DOI] [PubMed] [Google Scholar]
- 28.Laviola G, Pascucci T, Pieretti S. Striatal dopamine sensitization to D-amphetamine in periadolescent but not in adult rats. Pharmacol Biochem Behav. 2001;68:115–124. doi: 10.1016/s0091-3057(00)00430-5. [DOI] [PubMed] [Google Scholar]
- 29.Kolta MG, Scalzo FM, Ali SF, Holson RR. Ontogeny of the enhanced behavioral response to amphetamine in amphetamine-pretreated rats. Psychopharmacol. 1990;100:377–382. doi: 10.1007/BF02244610. [DOI] [PubMed] [Google Scholar]
- 30.Adriani W, Laviola G. Elevated levels of impulsivity and reduced place conditioning with d-amphetamine: two behavioral features of adolescence in mice. Behav Neurosci. 2003;117:695–703. doi: 10.1037/0735-7044.117.4.695. [DOI] [PubMed] [Google Scholar]
- 31.Piper BJ, Fraiman JB, Meyer JS. Repeated MDMA (“Ecstasy”) exposure in adolescent male rats alters temperature regulation, spontaneous motor activity, attention, and serotonin transporter binding. Dev Psychobio. 2005;47:145–157. doi: 10.1002/dev.20085. [DOI] [PubMed] [Google Scholar]
- 32.Achat-Mendes C, Ali SF, Itzhak Y. Differential effects of amphetamines-induced neurotoxicity on appetitive and aversive Pavlovian conditioning in mice. Neuropsychopharmacol. 2005;30:1128–1137. doi: 10.1038/sj.npp.1300675. [DOI] [PubMed] [Google Scholar]
- 33.Fone KCF, Beckett SRG, Topham IA, Swettenham J, Ball M, Maddocks L. Long-term changes in social interaction and reward following repeaeted MDMA administrations to adoloscent rats without accompanying serotonergic neurotoxicity. Psychopharmacol. 2002;159:437–444. doi: 10.1007/s00213-001-0931-z. [DOI] [PubMed] [Google Scholar]
- 34.Fletcher PJ, Robinson SR, Slippoy DL. Pre-exposure to (±) 2,4-methylenedioxymethamphetamine (MDMA) facilitates acquisition of intravenous cocaine self-administration in rats. Neuropsychopharmacol. 2001;25:195–203. doi: 10.1016/S0893-133X(01)00222-6. [DOI] [PubMed] [Google Scholar]
- 35.Broaddus WC, Bennett JP., Jr Postnatal development of striatal dopamine function. I. An explanation of D1 and D2 receptors, adenylate cyclase regulation and presynaptic markers. Brain Research Developmental Brain Research. 1990;52:275–271. doi: 10.1016/0165-3806(90)90244-s. [DOI] [PubMed] [Google Scholar]
- 36.Tarazi FI, Tomasini EC, Baldessarini RJ. Postnatal development of dopamine D1-like receptors in rat cortical and striatolimbic brain regions: An autoradiographic study. Developmental Neuroscience. 1999;21:43–49. doi: 10.1159/000017365. [DOI] [PubMed] [Google Scholar]
- 37.Teicher MH, Andersen SL, Hostetter JC., Jr Evidence for dopamine receptor pruning between adolescence and adulthood in striatum but not nucleus accumbens. Dev Brain Res. 1995;89:167–172. doi: 10.1016/0165-3806(95)00109-q. [DOI] [PubMed] [Google Scholar]
- 38.Moll GH, Mehnert C, Wicker M, Bock N, Rothenberger A, Ruther E, Huether G. Age-associated changes in the densities of presynaptic monoamine transporters in different regions of the rat brain from early juvenile life to late adulthood. Dev Brain Res. 2000;119:251–257. doi: 10.1016/s0165-3806(99)00182-0. [DOI] [PubMed] [Google Scholar]
- 39.McCarthy LE, Mannelli P, Niculescu M, Gingrich K, Unterwald EM, Ehrlich ME. The distribution of cocaine in mice differs by age and strain. Neurotoxicol Teratol. 2004;26:839–848. doi: 10.1016/j.ntt.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 40.Caster JM, Walker QD, Kuhn CM. Enhanced behavioral response to repeated-dose cocaine in adolescent rats. Psychopharmacol. 2005;183:218–225. doi: 10.1007/s00213-005-0159-4. [DOI] [PubMed] [Google Scholar]
- 41.Wiener HL, Reith MEA. Correlation between cocaine-induced locomotion and cocaine disposition in the brain among four inbred strains of mice. Pharmacol Biochem Behav. 1990;36:699–701. doi: 10.1016/0091-3057(90)90277-o. [DOI] [PubMed] [Google Scholar]
- 42.Reith MEA, Benuck M, Lajtha A. Cocaine disposition in the brain after continuous or intermittent treatment and locomotor stimulation in mice. J Pharmacol Exp Ther. 1987;243:281–287. [PubMed] [Google Scholar]
- 43.Benuck M, Lajtha A, Reith ME. Pharmacokinetics of systemically administered cocaine and locomotor stimulation in mice. J Pharmacol Exp Ther. 1987;243:144–149. [PubMed] [Google Scholar]
- 44.Kokoshka JM, Fleckenstein AE, Wilkins DG, Hanson GR. Age-dependent differential responses of monoaminergic systems to high doses of methamphetamine. J Neurochem. 2000;75:2095–2102. doi: 10.1046/j.1471-4159.2000.0752095.x. [DOI] [PubMed] [Google Scholar]
- 45.Pu C, Vorhees CV. Developmental dissociation of methamphetamine-induced depletion of dopaminergic terminals and astrocyte reaction in rat striatum. Brain Res Dev Brain Res. 1993;72:325–328. doi: 10.1016/0165-3806(93)90201-k. [DOI] [PubMed] [Google Scholar]
- 46.Cappon GD, Morford LL, Vorhees CV. Ontogeny of methamphetamine-induced neurotoxicity and associated hyperthermic response. Brain Res Dev Brain Res. 1997;103:155–162. doi: 10.1016/s0165-3806(97)81791-9. [DOI] [PubMed] [Google Scholar]
- 47.Andersen SL, LeBlanc CJ, Lyss PJ. Maturational increases in c-fos expression in the ascending dopamine systems. Synapse. 2001;41:345–350. doi: 10.1002/syn.1091. [DOI] [PubMed] [Google Scholar]
- 48.Ehrlich ME, Sommer J, Canas E, Unterwald EM. Periadolescent mice show enhanced DFosB upregulation in response to cocaine and amphetamine. J Neurosci. 2002;22:9155–9159. doi: 10.1523/JNEUROSCI.22-21-09155.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wallace TL, Gudelsky GA, Vorhees CV. Alterations in diurnal and nocturnal locomotor activity in rats treated with a monoamine-depleting regimen of methamphetamine or 3,4-methylenedioxymethamphetamine. Psychopharmacol. 2001;153:321–326. doi: 10.1007/s002130000578. [DOI] [PubMed] [Google Scholar]
- 50.Broening HW, Bacon LW, Slikker J. Age modulates the long-term but not the acute effects of the serotonergic neurotoxicant 3,4-methylenedioxymethamphetamine. J Pharmacol Exp Ther. 1994;271:285–293. [PubMed] [Google Scholar]
- 51.Aguirre N, Barrionuevo M, Lasheras B, Rio JD. The role of dopaminergic systems in the perinatal sensitivity to 3,4-methylenedioxymethamphetamine-induced neurotoxicity in rats. J Pharmacol Exp Ther. 1998;286:1159–1165. [PubMed] [Google Scholar]
- 52.Cannon DM, Keenan AK, Guiry PJ, Buon C, Baird AW, McBean GJ. In vitro neuronal and vascular responses to 5-HT in rats chronically exposed to MDMA. Brit J Pharmacol. 2001;134:1455–1460. doi: 10.1038/sj.bjp.0704402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Adriani W, Spijker S, Deroche-Gammonet V, Laviola G, Le Moal M, Smit AB, Piazza PV. Evidence for enhanced neurobehavioral vulnerability to nicotine during periadolescence in rats. J Neurosci. 2003;23:4712–4716. doi: 10.1523/JNEUROSCI.23-11-04712.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Collins SL, Izenwasser S. Chronic nicotine differentially alters cocaine-induced locomotor activity in adolescent vs. adult male and female rats. Neuropharmacol. 2004;46:349–362. doi: 10.1016/j.neuropharm.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 55.Collins SL, Montano R, Izenwasser S. Nicotine treatment produces persistent increases in amphetamine-stimulated locomotor activity in periadolescent male but not female or adult male rats. Dev Brain Res. 2004;153:175–187. doi: 10.1016/j.devbrainres.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 56.Cruz FC, Delucia R, Planeta CS. Differential behavioral and neuroendocrine effects of repeated nicotine in adolescent and adult rats. Pharmacol Biochem Behav. 2005;80:411–417. doi: 10.1016/j.pbb.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 57.Schochet TL, Kelley AE, Landry CF. Differential behavioral effects of nicotine exposure in adolescent and adult rats. Psychopharmacol. 2004;175:265–273. doi: 10.1007/s00213-004-1831-9. [DOI] [PubMed] [Google Scholar]
- 58.Faraday MM, Elliott BM, Phillips JM, Grunberg NE. Adolescent and adult male rats differ in sensitivity to nicotine’s activity effects. Pharmacol Biochem Behav. 2003;74:917–931. doi: 10.1016/s0091-3057(03)00024-8. [DOI] [PubMed] [Google Scholar]
- 59.Trauth JA, Seidler FJ, Slotkin TA. Persistent and delayed behavioral changes after nicotine treatment in adolescent rats. Brain Res. 2000;880:167–172. doi: 10.1016/s0006-8993(00)02823-7. [DOI] [PubMed] [Google Scholar]
- 60.Levin ED, Rezvani AH, Montoya D, Rose JE, Swartzwelder HS. Adolescent-onset nicotine self-administration modeled in female rats. Psychopharmacol. 2003;169:141–149. doi: 10.1007/s00213-003-1486-y. [DOI] [PubMed] [Google Scholar]
- 61.Vastola BJ, Douglas LA, Varlinskaya EI, Spear LP. Nicotine-induced conditioned place preference in adolescent and adult rats. Physiology & Behavior. 2002;77 doi: 10.1016/s0031-9384(02)00818-1. [DOI] [PubMed] [Google Scholar]
- 62.Belluzzi JD, Lee AG, Oliff HS, Leslie FM. Age-dependent effects of nicotine on locomotor activity and conditioned place preference in rats. Psychopharmacol. 2004;174:389–395. doi: 10.1007/s00213-003-1758-6. [DOI] [PubMed] [Google Scholar]
- 63.Cheeta S, Irvine EE, Tucci S, Sandhu J, File SE. In adolescence, female rats are more sensitive to the anxiolytic effect of nicotine than are male rats. Neuropsychopharmacol. 2001;25:601–607. doi: 10.1016/S0893-133X(01)00258-5. [DOI] [PubMed] [Google Scholar]
- 64.Slawecki CJ, Gilder A, Roth J, Ehlers CL. Increased anxiety-like behavior in adult rats exposed to nicotine as adolescents. Pharmacol Biochem Behav. 2003;75:355–361. doi: 10.1016/s0091-3057(03)00093-5. [DOI] [PubMed] [Google Scholar]
- 65.Elliott BM, Faraday MM, Phillips JM, Grunberg NE. Effects of nicotine on elevated plus maze and locomotor activity in male and female adolescent and adult rats. Pharmacol Biochem Behav. 2004;77:21–28. doi: 10.1016/j.pbb.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 66.McMillen BA, Davis BJ, Williams HL, Soderstrom K. Periadolescent nicotine exposure causes heterologous sensitization to cocaine reinforcement. Eur J Pharmacol. 2005;509:161–164. doi: 10.1016/j.ejphar.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 67.Klein LC. Effects of adolescent nicotine exposure on opioid consumption and neuroendocrine responses in adult male and female rats. Exp Clin Psychopharmacol. 2001;9:251–261. doi: 10.1037//1064-1297.9.3.251. [DOI] [PubMed] [Google Scholar]
- 68.Kelley BM, Middaugh LD. Periadolescent nicotine exposure reduces cocaine reward in adult mice. J Addictive Dis. 1999;18:27–39. doi: 10.1300/J069v18n03_04. [DOI] [PubMed] [Google Scholar]
- 69.Kelley BM, Rowan JD. Long-term, low-level adolescent nicotine exposure produces dose-dependent changes in cocaine sensitivity and reward in mice. Int J Dev Neurosci. 2004;22:339–348. doi: 10.1016/j.ijdevneu.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 70.Trauth JA, Seidler FJ, McCook EC, Slotkin TA. Adolescent nicotine exposure causes persistent upregulation of nicotinic cholinergic receptors in rat brain regions. Brain Res. 1999;851:9–19. doi: 10.1016/s0006-8993(99)01994-0. [DOI] [PubMed] [Google Scholar]
- 71.Abreu-Villaca Y, Seidler FJ, Qiao D, Tate CA, Cousins MM, Thillai I, Slotkin TA. Short-term adolescent nicotine exposure has immediate and persistent effects on cholinergic systems: critical periods, patterns of exposure, dose thresholds. Neuropsychopharmacol. 2003;28:1935–1949. doi: 10.1038/sj.npp.1300221. [DOI] [PubMed] [Google Scholar]
- 72.Abreu-Villaca Y, Seidler FJ, Tate CA, Cousins MM, Slotkin TA. Prenatal nicotine exposure alters the response to nicotine administration in adolescence: effects on cholinergic systems during exposure and withdrawal. Neuropsychopharmacol. 2004;29:879–890. doi: 10.1038/sj.npp.1300401. [DOI] [PubMed] [Google Scholar]
- 73.Trauth JA, McCook EC, Seidler FJ, Slotkin TA. Modeling adolescent nicotine exposure: effects on cholinergic systems in rat brain regions. Brain Res. 2000;873:18–25. doi: 10.1016/s0006-8993(00)02465-3. [DOI] [PubMed] [Google Scholar]
- 74.Trauth JA, Seidler FJ, Ali SF, Slotkin TA. Adolescent nicotine exposure produces immediate and long-term changes in CNS noradrenergic and dopaminergic function. Brain Res. 2001;892:269–280. doi: 10.1016/s0006-8993(00)03227-3. [DOI] [PubMed] [Google Scholar]
- 75.Badanich KA, Kirstein CL. Nicotine administration significantly alters accumbal dopamine in the adult but not in the adolescent rat. Annals NY Acad Sci. 2004;1021:410–417. doi: 10.1196/annals.1308.054. [DOI] [PubMed] [Google Scholar]
- 76.Schochet TL, Kelley AE, Landry CF. Differential expression of arc mRNA and other plasticity-related genes induced by nicotine in adolescent rat forebrain. Neurosci. 2005;135:285–297. doi: 10.1016/j.neuroscience.2005.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xu Z, Seidler FJ, Ali SF, Slikker WJ, Slotkin TA. Fetal and adolescent nicotine administration: effects on CNS serotonergic systems. Brain Res. 2001;914:166–178. doi: 10.1016/s0006-8993(01)02797-4. [DOI] [PubMed] [Google Scholar]
- 78.Chow FA, Seidler FJ, McCook EC, Slotkin TA. Adolescent nicotine exposure alters cardiac autonomic responsiveness: b-adrenergic and m2-muscarinic receptors and their linkage to adenylyl cyclase. Brain Res. 2000;878:119–126. doi: 10.1016/s0006-8993(00)02697-4. [DOI] [PubMed] [Google Scholar]
- 79.Xu Z, Seidler FJ, Cousins MM, Slikker WJ, Slotkin TA. Adolescent nicotine administration alters serotonin receptors and cell signaling mediated through adenylyl cyclase. Brain Res. 2002;951:280–292. doi: 10.1016/s0006-8993(02)03174-8. [DOI] [PubMed] [Google Scholar]
- 80.Gonzalez S, Cascio MG, Fernandez-Ruiz J, Fezza F, Marzo VD, Ramos JA. Changes in endocannabinoid contents in the brain of rats chronically exposed to nicotine, ethanol or cocaine. Brain Res. 2002;954:73–81. doi: 10.1016/s0006-8993(02)03344-9. [DOI] [PubMed] [Google Scholar]
- 81.Arnold J, Topple A, Hunt G, McGregor I. Effects of pre-exposure and co-administration of the cannabinoid receptor agonist CP 55,940 on behavioral sensitization to cocaine. Eur J Pharmacol. 1998;354:9–16. doi: 10.1016/s0014-2999(98)00433-6. [DOI] [PubMed] [Google Scholar]
- 82.Higuera A, Biscaia M, Fernández B, Miguéns M, Olmo Nd, Torres I, García-Lecumberri C, Viveros MP and Ambrosio E, Pre-exposure to cannabinoid agonist CP 55,940 during rat early adolescence facilitates acquisition of cocaine self-administration behavior in the adulthood. CPDD abstract. 2005;
- 83.Biscaia M, Fernandez B, Marco EM, Rubio M, Guaza C, Ambrosio E, Viveros MP. Chronic treatment with CP55, 940 during the periadolescent period differentially affects the behavioural responses of male and female rats in the adulthood. Psychopharmacol. 2003;170:301–308. doi: 10.1007/s00213-003-1550-7. [DOI] [PubMed] [Google Scholar]
- 84.Breivogel CS, Scates SM, Beletskaya IO, Lowery OB, Aceto MD, Martin BR. The effects of D9-tetrahydrocannabinol physical dependence on brain cannabinoid receptors. Eur J Pharmacol. 2003;459:139–150. doi: 10.1016/s0014-2999(02)02854-6. [DOI] [PubMed] [Google Scholar]
- 85.de Fonseca FR, Gorriti MA, Fernandez-Ruiz JJ, Palomo T, Ramos JA. Downregulation of rat brain cannabinoid binding sites after chronic delta 9-tetrahydrocannabinol treatment. Pharmacol Biochem Behav. 1994;47:33–40. doi: 10.1016/0091-3057(94)90108-2. [DOI] [PubMed] [Google Scholar]
- 86.Romero J, Berrendero F, Manzanares J, Perez A, Corchero J, Fuentes JA, Fernandez-Ruiz JJ, Ramos JA. Time-course of the cannabinoid receptor down-regulation in the adult rat brain caused by repeated exposure to D9-tetrahydrocannabinol. Synapse. 1998;30:298–308. doi: 10.1002/(SICI)1098-2396(199811)30:3<298::AID-SYN7>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 87.Romero J, Garcia-Palomero E, Castro JG, Garcia-Gil L, Ramos JA, Fernandez-Ruiz JJ. Effects of chronic exposure to D9-tetrahydrocannabinol on cannabinoid receptor binding and mRNA levels in several rat brain regions. Mol Brain Res. 1997;46:100–108. doi: 10.1016/s0169-328x(96)00277-x. [DOI] [PubMed] [Google Scholar]
- 88.Pistis M, Perra S, Pillolla G, Melis M, Muntoni AL, Gessa GL. Adolescent exposure to cannabinoids induces long-lasting changes in the response to drugs of abuse of rat midbrain dopamine neurons. Biol Psychiat. 2004;56:86–94. doi: 10.1016/j.biopsych.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 89.Faraday MM, Elliott BM, Grunberg NE. Adult vs. adolescent rats differ in biobehavioral responses to chronic nicotine administration. Pharmacol Biochem Behav. 2001;70:475–489. doi: 10.1016/s0091-3057(01)00642-6. [DOI] [PubMed] [Google Scholar]
- 90.Wallace TL, Gudelsky GA, Vorhees CV. Neurotoxic regimen of methamphetamine producers evidence of behavioral sensitization in the rat. Synapse. 2001;39:1–7. doi: 10.1002/1098-2396(20010101)39:1<1::AID-SYN1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]


