Abstract
The identity of the endogenous epithelial cells in the adult lung that are responsible for normal turnover and repair after injury is still controversial. In part, this is due to a paucity of highly specific genetic lineage tools to follow efficiently the fate of the major epithelial cell populations: the basal, secretory, ciliated, neuroendocrine, and alveolar cells. As part of a program to address this problem we have used a 1-kb FOXJ1 promoter to drive CreER in the ciliated cells of the embryonic and adult lung. Analysis of FOXJ1-GFP transgenic lungs shows that labeled cells appear in a proximal-distal pattern during embryogenesis and that the promoter drives expression in all ciliated cells. Using FOXJ1CreER adult mice, we have followed the fate of ciliated cells after epithelial injury by naphthalene or sulfur dioxide. From quantitative analysis and confocal microscopy we conclude that ciliated cells transiently change their morphology in response to lung injury but do not proliferate or transdifferentiate as part of the repair process.
Keywords: ciliated cell, injury, lung stem cells
With each breath we take, air passes through the highly branched epithelial tubes of the respiratory tree into the terminal alveoli, where gas exchange takes place. Under normal conditions, cell turnover in the lung is relatively low, at least compared with tissues such as the intestine and skin. Nevertheless, if the epithelium is extensively damaged, the lung can effectively replace lost cells. Identifying the stem or progenitor cell populations responsible for this repair is an active area of research not lacking in controversy (reviewed in refs. 1 and 2). In addition, compared with organs such as the pancreas and nervous system, we have a rather elementary view about how the major epithelial cell types of the lung are generated in the embryo (3). Because the repair of adult tissues may involve, at least in part, the recapitulation of developmental mechanisms, discoveries in one area are likely to inform the other.
The lung develops during embryogenesis from two primary buds that arise in the ventral foregut. These buds undergo extensive branching morphogenesis, directed by reciprocal signaling between the epithelial endoderm and the surrounding mesoderm. During this branching stage it is thought that the terminal buds contain a population of multipotent epithelial progenitors (4). As the tubes extend, descendants of these cells give rise to the progenitors of the major cell types of the conducting airways—certainly to the ciliated and secretory (Clara) cells (5). The appearance of morphologically differentiated epithelial cells begins proximally and proceeds distally (6, 7). We need to know a great deal more about the steps involved in generating these differentiated cells: the identity of intermediate cell types, whether these intermediates self renew as transit amplifying populations, their lineage relationships, and the precise mechanism of their commitment to different fates. Once morphogenesis is complete, the lung continues to increase in size. During this phase, new epithelial cells are generated in the intralobular airways, alveoli, and trachea. There is evidence that during this phase, cells expressing Clara cell markers can give rise to ciliated cells (8, 9). Moreover, in the proximal airways, columnar epithelial cells give rise to basal cells (10). Once formed, basal cells can self-renew and contribute to other lineages, but differentiated ciliated cells do not appear to divide (10, 11).
A number of experimental systems are used for studying repair processes in the adult lung. In all cases, the response of the epithelium varies with the region being studied (proximal versus distal airways) and mouse strain and sex. One widely used injury model is naphthalene exposure, which destroys most of the secretory Clara cells of both the proximal and distal conducting airways (12, 13). Studies have identified two cell populations that proliferate in response to this injury. These are the basal cells in the trachea and primary bronchi, and naphthalene-resistant Clara cells in the more distal bronchi, bronchioles and the bronchoalveolar duct junction (BADJ) (14–18). The evidence that the naphthalene-resistant Clara cells give rise to other lineages after proliferation is indirect. First, repair does not occur if all Scgb1a1+ cells are ablated, including both the naphthalene-sensitive Clara cells and the naphthalene-resistant Clara (putative stem) cells (16). Second, cells isolated from the BADJ can give rise to several differentiated cell types when cultured in vitro (18). By contrast, the evidence that basal cells in the upper airways can both proliferate and function as stem cells after naphthalene injury is based on in vivo lineage-labeling (14, 15). In addition, indirect evidence that the population of basal cells in the upper airway and submucosal gland (SMG) ducts includes label-retaining, putative stem cells comes from an injury model using high doses of sulfur dioxide (SO2) gas that destroys most of the epithelial cell layer (19).
Recently, based on morphological and immunohistochemical data, it has been suggested that ciliated cells can proliferate and transdifferentiate into Clara cells in response to naphthalene injury or partial pneumonectomy (20). This result is surprising because ciliated cells are generally considered to be terminally differentiated and nonproliferative (for example, see refs. 21–23). However, there is morphological evidence for transdifferentiation of ciliated cells into mucus secreting cells in murine models of asthma and viral infection (24, 25).
One way to follow cell fate during lung development, homeostasis, and repair is to use genetic lineage-labeling techniques to indelibly mark the descendants of specific cell types. In this method, the daughter cells express the lineage marker whatever phenotype they acquire. Moreover, the genetic lineage-labeling tools can be designed to have other advantages; they can be used to isolate progenitor cells and their daughters for gene expression analysis to investigate the mechanisms of cell fate specification and differentiation, and they can be exploited for functional studies based on gene inactivation and overexpression. Such tools have so far only been applied in a very limited way to the lung. Moreover, they have sometimes been used in the absence of a completely cell type-specific promoter, making interpretation difficult (5, 8, 14, 15, 26). A long-term goal in the field is thus to engineer mice for efficient lineage-labeling of each of the different airway epithelial cell types.
Here, we use two transgenic mouse strains to investigate ciliated cells and their precursors during lung development and repair after injury. Both strains use a promoter region of FOXJ1, a gene that encodes a forkhead domain transcription factor required for late stages of ciliogenesis (27, 28). Our analysis of FOXJ1-GFP embryonic lungs provides a unique overview of the appearance of ciliated cells during development. Moreover, the FOXJ1-GFP transgene is ultimately expressed in virtually all of the ciliated cells of the adult lung. We have also made a new transgenic mouse strain in which the same promoter directs expression of a tamoxifen-inducible allele of the Cre DNA recombinase (CreER2T). This allele allows us to indelibly mark both ciliated cells and any of their descendents in vivo after two different kinds of injury: exposure to naphthalene and sulfur dioxide.
Results
Ciliated Cells in Lung Development.
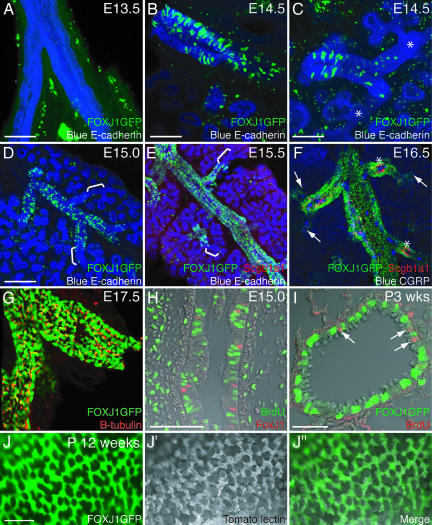
Previous studies have shown that a 1-kb fragment of the human FOXJ1 upstream promoter region is sufficient to direct expression of GFP in ciliated cells of the adult mouse, including the trachea and lungs (29). We used 100-μm vibratome sections and confocal microscopy of these FOXJ1-GFP transgenic mice to qualitatively describe the appearance of ciliated cells throughout the trachea and intralobular airways during development. At embryonic day (E) 13.5, there was no detectable GFP expression from the transgene (Fig. 1A). By E14.0, a small number of scattered GFP+ cells were visible in the trachea and primary bronchi (data not shown). The appearance of these GFP+ cells clearly proceeded in a proximal-distal sequence. Thus, at E14.5, the primary and most proximal intralobular bronchi had many GFP+ cells (Fig. 1B), but only a few GFP+ cells were seen at lower airway generations and none at all were seen in the most distal tubes undergoing branching (Fig. 1C). This gap between GFP+ proximal regions and the terminal tubules was still seen at E15.0 and E15.5 (Fig. 1 D and E). E15.5 was the first time at which we were able to detect the Clara cell marker, Scgb1a1 (secretoglobin 1a1, also known as CC10 and CCSP) by immunostaining. The Scgb1a1+ cells were interspersed with the GFP+ cells. At E16.5, the lung was still increasing in size, but GFP+ cells now extended to the BADJ (Fig. 1F). E16.5 was the first time that we detected, by immunohistochemistry, the neuroendocrine cell marker CGRP (calcitonin gene-related peptide). It was also the first time, in agreement with other studies, when surface cilia were detected, using antibodies to β-tubulin (6, 30). It was now possible to observe, as previously described in human embryonic lung samples, that some of the CGRP+ neuroendocrine bodies are surrounded by Scgb1a1+ Clara precursors, whereas others are ringed by a mixture of ciliated and Clara precursor cells (31). At E16.5, in contrast to the earlier stages, there was no significant population of epithelial cells that did not express one of the three markers: GFP, Scgb1a1, and CGRP. Taken together, these results confirm that morphological differentiation of the airway epithelium occurs in a proximal-distal sequence. It is important to note that by E17.5 and later, FOXJ1-GFP is apparently expressed in all of the ciliated cells in the lung and trachea (Fig. 1 G and J). Moreover, the presence of GFP+ cells before the appearance of surface cilia suggests that the 1-kb promoter region is able to drive transgene expression in precursors of ciliated cells that are not yet fully differentiated, as judged by the presence of surface cilia.
Fig. 1.
Ciliated cell patterning during development. (A–G) One hundred-micrometer vibratome sections of FOXJ1-GFP transgenic lungs. (A–E) Green, FOXJ1-GFP; red, anti-Scgb1a1; blue, anti-E-cadherin. (A) E13.5 mainstem-bronchi. FOXJ1-GFP is not yet expressed (green staining in the mesenchyme is nonspecific). (B) E14.5 mainstem-bronchus. FOXJ1-GFP is expressed in many cells. (C) E14.5 distal bronchiole from the same lung. FOXJ1-GFP-expressing cells are more scattered and are absent from distal tubules (∗). (D) E15.0 lung lobe. FOXJ1-GFP is expressed in scattered cells in the bronchioles but does not extend to the branching tips (brackets). (E) E15.5. FOXJ1-GFP is expressed in more cells but still does not extend to the branching tips (brackets). Scgb1a1 is first detectable at this stage by immunostaining. (F) E16.5. Green, FOXJ1-GFP; red, anti-Scgb1a1; blue, anti-CGRP. FOXJ1-GFP expression extends to the terminal bronchioles (arrows). Almost every epithelial cell expresses one of the three markers, but no cell expressing more than one marker was detected (the small amount of yellow labeling in the image is a result of boosting Scgb1a1 detection to visualize the more weakly expressing cells). Note that some CGRP-expressing cells are surrounded by clusters of Scgb1a1+ cells (∗). (G) E17.5. FOXJ1-GFP (green) colocalizes with anti-β-tubulin, a marker of surface cilia (red). (H) E15.0. Seven-micrometer paraffin section through an intralobular airway after 1 h of BrdU exposure. Green, anti-BrdU; red, anti-FoxJ1. The BrdU label does not colocalize with FoxJ1. (I) FOXJ1-GFP postnatal stage 3 weeks; 7-μm paraffin section through a bronchiole after 1 h of BrdU exposure. Green, anti-GFP; red, anti-BrdU. Proliferating cells (arrows) do not colocalize with FoxJ1. (J and J″) Whole-mount view of adult FOXJ1-GFP (green in J) bronchiole stained with tomato lectin (grayscale in J′) to visualize cell surfaces including cilia. The merged image (J″) shows that FOXJ1-GFP is expressed in all of the ciliated cells. (Scale bars: A–C, 100 μm; D–G, 200 μm; H, 20 μm; I and J, 40 μm.)
To assess whether the FOXJ1+ ciliated cell precursors can divide, we labeled cells in S-phase with BrdU at E15.0 when FOXJ1-GFP is expressed but surface cilia are not yet detectable. We used immunofluorescence of paraffin sections to detect GFP or FoxJ1 protein and BrdU, and then analyzed the lungs by confocal microscopy to accurately determine cell boundaries. After a 1-h BrdU pulse, we scored 1,991 BrdU+ cells and 499 FoxJ1+ cells and found only 1 (BrdU+, FoxJ1+) cell (n = 8 embryos) (Fig. 1H). Moreover, we were unable to detect FoxJ1+ cells that also express the G2/M-phase marker phosphorylated histone H3 (data not shown). We repeated the BrdU analysis at postnatal stages 2–3 weeks, when the rate of proliferation of lung cells is dramatically decreased. We counted 108 BrdU+ cells and 1,142 FoxJ1+ cells and found 0 (BrdU+, FoxJ1+) cells (n = 5 lungs) (Fig. 1I). Taken together, these findings suggest that once ciliated cell precursors express detectable levels of FoxJ1, they either do not proliferate or have a significantly longer cell cycle time than other dividing lung epithelial cells.
FOXJ1CreER2T Mouse Strain.
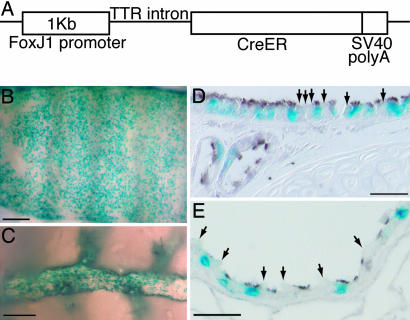
We made transgenic mice expressing CreER2T under the control of the same FOXJ1 promoter fragment (Fig. 2A). Crossing the FOXJ1CreER2T mouse line to the Rosa26R (Gt(ROSA)26Sortm1Sor) reporter strain and exposing the adult offspring to tamoxifen gave widespread activation of the heritable reporter gene in epithelial cells throughout the airways (Fig. 2 B and C). Sections were stained for β-tubulin, which strongly labels cilia, to confirm that recombination of the reporter gene was restricted to ciliated cells (Fig. 2 D and E). For each experiment, the proportion of ciliated cells that were lineage-labeled varied from 50% to 70%, depending on the dose of tamoxifen. This level of recombination compares favorably with that seen in similar studies using other CreER transgenes (see Discussion). Injection of the FOXJ1CreER2T; Rosa26R mice with corn oil vehicle did not result in detectable expression of the reporter gene (Fig. 7 B and G), demonstrating that Cre activation is not leaky.
Fig. 2.
FOXJ1CreER2T transgene efficiently lineage-labels ciliated cells after tamoxifen injection. (A) FOXJ1CreER2T transgene construct. (B–E) X-gal-stained (blue) FOXJ1-CreER2T; Rosa26R adult airways after tamoxifen injection. (B) Whole-mount ventral trachea. (C) Intralobular airway. (D and E) Sections of anti-β-tubulin-stained trachea (brown) (D) and distal bronchiole (E). Most ciliated cells but no Clara cells (arrows) are lineage-labeled. (Scale bars: B and D, 200 μm; C and E, 20 μm.)
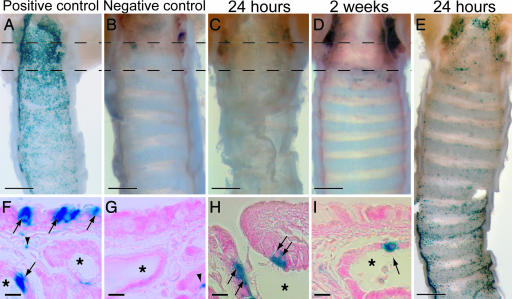
Fig. 7.
Ciliated cells after SO2 inhalation. Shown are FOXJ1-CreER2T; Rosa26R ventral trachea. Blue, X-gal. (A–E) Whole-mount images of ventral tracheal epithelial surface. (F–I) Seven-micrometer paraffin sections taken from the regions between the dotted lines. (A and F) Tamoxifen-treated positive control. X-gal+ cells (arrows) are in the luminal epithelium and SMG ducts (∗). (B and G) Negative control (no tamoxifen injections). Low level of endogenous X-gal staining in the SMG mucus cells (arrowhead). (C and H) Tamoxifen-treated, 24 h after SO2 inhalation. X-gal+ cells (arrows) are restricted to the SMG ducts. (D and I) Tamoxifen-treated, 2 weeks after SO2 inhalation. X-gal+ cells (arrows) are still restricted to the SMG ducts. (E) Tamoxifen-treated, 24 h after SO2 inhalation. In some animals, the surviving X-gal+ cells are scattered throughout the luminal epithelium, particularly in the distal region. (Scale bars: A–E, 0.5 mm; G–I, 10 μm.)
Behavior of Ciliated Cells in Response to Naphthalene Injury.
Previous reports have demonstrated that the severity of naphthalene injury depends on the strain and sex of the mice used (32, 33). As expected, a 250 mg/kg dose of naphthalene was sufficient to kill the majority, but not all, of the Clara cells in the bronchioles (Fig. 3A–D). Analysis of cell proliferation showed that in male mice, DNA replication begins throughout the airways at 52 h after the injury and continues for several more days (Fig. 3 E and F and data not shown). In female mice, the injury was more severe, and proliferation began slightly later (data not shown). We used both male and female mice in our experiments to elicit a range of repair responses and so increase the scope of our analysis. However, no significant difference was seen in the behavior of ciliated cells between the sexes. Importantly, at this dose of naphthalene, Clara cells were destroyed at all airway levels including the tracheobronchial region and the bronchiolar/BADJ level (data not shown). Our analysis included ciliated cells in all of these different airway regions. However, the lineage-labeled ciliated cells in different regions of the airways did not behave differently and are therefore described together.
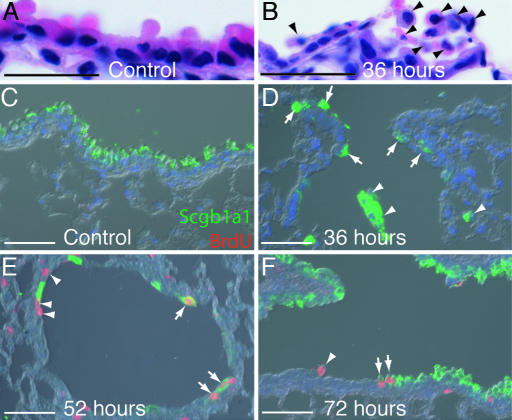
Fig. 3.
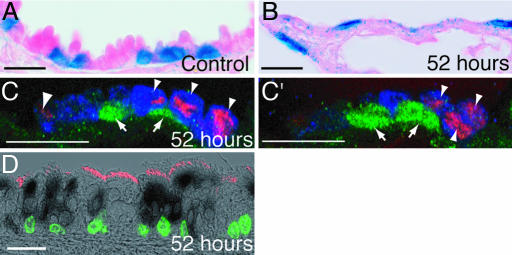
Characterization of the extent of the naphthalene injury and timing of proliferation. (A–F) Paraffin sections of bronchiole epithelium from male mice. (A and B) Hematoxylin and eosin staining. (A) Thirty-six hours after control corn oil injection. (B) Thirty-six hours after naphthalene. Arrowheads mark cells with no attachment to the basal lamina. (C–F) Anti-Scgb1a1 (green), anti-BrdU (red), and DAPI (blue). (C) Fifty-two hours after control injection. Clara cells predominate the airway epithelium and no dividing cells are present. (D) Thirty-six hours after naphthalene. Clara cells have detached from the basal lamina (arrowheads), but small numbers are retained at the BADJ (arrows) and at intervals throughout the bronchioles (data not shown). Cells have not yet started to divide. (E) Fifty-two hours after naphthalene injection. Dividing epithelial cells are observed; they are either Scgb1a1+ (arrows) or Scgb1a1− (arrowheads). (F) Seventy-two hours after naphthalene injection. Dividing Scgb1a1+ (arrows) and Scgb1a1− (arrowhead) cells are still observed, and many more Clara cells are also present. (Scale bars: A and B, 20 μm; C–F, 200 μm.)
To assess the fate of the ciliated cells after naphthalene injury, we exposed male and female FOXJ1CreER2T; Rosa26R mice to tamoxifen and then, at least 1 week later, to naphthalene. The overall extent of labeling of ciliated cells throughout the airways before injury in these experiments was 50% [4,047 of 8,103 ciliated cells (n = 5 mice in two independent experiments)]. Cilia could not be detected on 0.8% of lineage-labeled cells; these may be Foxj1-expressing precursors of ciliated cells generated during steady-state turnover. In addition, 1% of lineage-labeled cells coexpressed the Clara cell marker Scgb1a1. Morphologically, these cells did not have cilia [11 of 1,599 X-gal+ cells (n = 3 mice in two independent experiments); in contrast, 2,943 (X-gal−, Scgb1a1+) cells were also scored].
Lineage-labeled ciliated cells survived the naphthalene exposure but became flattened (Fig. 4A and B). Squamation of ciliated cells has previously been observed in response to naphthalene injury (12, 32).
Fig. 4.
Response of ciliated cells to naphthalene injury. (A–D) FOXJ1-CreER2T; Rosa26R 7-μm paraffin sections. (A and B) X-gal-stained (blue) and eosin-stained (pink) bronchioles. (A) Fifty-two hours after control corn oil injection. (B) Fifty-two hours after naphthalene. Domed Clara cells are absent, and X-gal+ cells are flattened. (C and C′) Fifty-two hours after naphthalene. Two confocal z-sections of the same BADJ. Green, anti-β-gal; red, anti-BrdU; blue, anti-Scgb1a1. Lineage-labeled cells (arrows) have not incorporated BrdU. Proliferating cells are Scgb1a1+ (arrowheads). (D) Proximal tracheal section 52 h after naphthalene injury. Black cytoplasm, X-gal; red cilia, anti-β-tubulin; green nuclei, anti-BrdU. Lineage-labeled cells have not incorporated BrdU. (Scale bars: 20 μm.)
We assessed proliferation at 52, 60, and 72 h after the injury when levels of BrdU incorporation are highest. Lineage-labeled cells were detected by X-gal staining or with anti-β-galactosidase, and antibody staining was performed to score proliferating (BrdU+) cells. Images were captured on a laser scanning confocal microscope, analyzed in more than one z-section plane to clearly determine cell boundaries, and the number of proliferating (BrdU+) lineage-labeled (β-gal+) cells was scored. Overall, we counted 11,270 cells from 14 animals in three independent experiments. We scored 2,081 BrdU+ cells and 9,188 β-gal+ cells and found only 1 cell that was both (BrdU+, β-gal+). Moreover, in experiments where surviving Scgb1a1+ Clara cells were detected, 93% of the BrdU+ cells were also Scgb1a1+ [85 of 91 BrdU+ cells (n = 7 mice); Fig. 4C] in the intralobular airways and terminal bronchioles. This is consistent with previous reports that identified the dividing cells in distal regions of naphthalene-injured lungs as naphthalene-resistant Clara cells and neuroendocrine cells (16, 21, 34). In the trachea, Scgb1a1 was not detectable on the proliferating cells, which instead had the morphology of basal cells (Fig. 4D and data not shown). This is consistent with previous reports which demonstrated that basal cells can act as multipotent progenitors after naphthalene injury (14, 15). Our comprehensive survey of BrdU+ cells during the peak period of proliferation leads us to conclude that although ciliated cells change their shape in response to naphthalene injury, they do not divide. Therefore, they are neither self-renewing nor functioning as progenitor cells in this injury model.
To test the hypothesis that ciliated cells can transdifferentiate to another epithelial cell type without undergoing cell division, we killed animals 2–3 weeks after the naphthalene injury. At this time, repair was essentially complete, based on normal epithelial morphology and the absence of BrdU labeling (Fig. 5B and C). The lineage-labeled cells were visualized by using X-gal staining and colabeled either with anti-β-tubulin (cilia) or Scgb1a1 (Clara cells) (Fig. 5 B–E). Throughout the airways, 98% of the lineage-labeled cells had visible cilia [4,548 of 4,642 X-gal+ cells (n = 7 mice in three independent experiments)]. By contrast, of 4,111 Scgb1a1+ cells scored, none were X-gal+, showing that there was not a large-scale transdifferentiation of lineage-labeled ciliated cells into Clara cells. Only 1% of the lineage-labeled cells strongly expressed Scgb1a1 [19 of 1,575 X-gal+ cells (n = 8 mice in two independent experiments)]. This corresponds very well with the 1% of Scgb1a1-expressing lineage-labeled cells observed in control animals (see above). We do not know whether the (Scgb1a1+, X-gal+) cells also express FoxJ1. However, in the uninjured FoxJ1-GFP transgenic animal, a few GFP+ cells are also Scgb1a1+ [4 of 1,162 GFP+ cells (n = 1 mouse)]. After naphthalene injury, the absence of dividing lineage-labeled cells or increased numbers of (Scgb1a1+, X-gal+) cells suggests that the dual-positive (Scgb1a1+, X-gal+) cells survived the injury but did not contribute to the repair process. Other studies have suggested that cells expressing Scgb1a1 and a marker of another differentiated lineage (type II alveolar or neuroendocrine cells) function as stem cells after naphthalene injury (18, 21). Our evidence does not support this possibility for the ciliated cells.
Fig. 5.
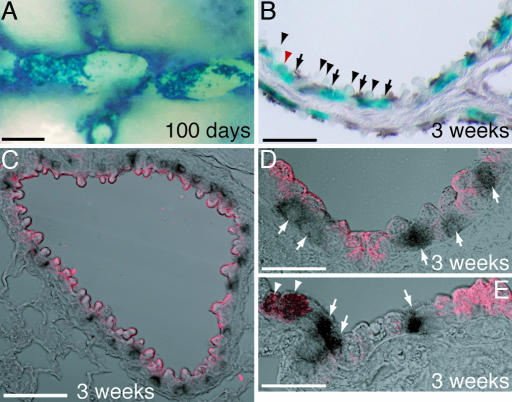
Ciliated cells do not transdifferentiate after naphthalene injury. FOXJ1-CreER2T; Rosa26R airways. (A) One hundred days after naphthalene exposure. Whole-mount image of X-gal-stained (blue), microdissected bronchiole. The X-gal+ cells are tightly clustered and separated by regions of unlabeled cells. Compare with Fig. 2C. (B–E) Seven-micrometer paraffin sections of bronchioles 3 weeks after naphthalene injury. (B) X-gal (blue) and anti-β-tubulin (brown). Ninety-eight percent of lineage-labeled cells are clearly β-tubulin+ (arrows). Domed Clara cells are not lineage-labeled (arrowheads). Two percent of lineage-labeled cells do not have visible cilia (red arrowhead). (C–E) Single confocal z-sections of lineage-labeled X-gal+ cells (black) and Scgb1a1+ cells (red). (C and D) Typical bronchioles. Lineage-labeled cells are Scgb1a1− (arrows). (E) One percent of lineage-labeled cells colabel with Scgb1a1 (arrowheads). (Scale bars: A, 200 μm; B, 40 μm; C, 60 μm; D and E, 20 μm.)
Whole-mount analysis of the patterns of X-gal staining in the smaller bronchi and bronchioles at 2–3 weeks (data not shown) and 100 days (compare Figs. 5A to 2C) after naphthalene injury revealed regions of largely X-gal+ or largely X-gal− epithelium. This indirect evidence is consistent with idea that an X-gal− progenitor cell population proliferates to repair the injury, and in the process the nondividing lineage-labeled cells are pushed together.
Behavior of Ciliated Cells in Response to SO2 Exposure.
Inhalation of high doses of SO2 has been used to induce cell proliferation in the trachea to identify label retaining cells (putative stem cells) (19). We exposed adult male mice to 500 ppm SO2 for 3 h. This caused sloughing of large sheets of tracheal epithelial cells (Fig. 6A–C). Repair was rapid, and the epithelium was virtually indistinguishable from wild type 2 weeks after the injury. This conclusion is based on normal morphology and the absence of BrdU incorporation (Fig. 8 A–D).
Fig. 6.
Characterization of the extent of SO2 injury and timing of proliferation. (A–C) Transverse tracheal sections. (A) Uninjured control. (B) Six hours after SO2 inhalation. Epithelial cells are sloughing off. (C) Twenty-four hours after SO2 inhalation. The luminal epithelium consists of a monolayer of low cuboidal cells. (D) Percentage of epithelial cells (in the most proximal one-third of the trachea) that incorporated BrdU in a 1-h pulse at different times after the injury. Bars are the standard error of the mean. Epithelial cell proliferation peaks 24 h after the injury. (Scale bars: 200 μm.)
Fig. 8.
Ciliated cells do not divide or transdifferentiate after SO2 exposure. Shown are FOXJ1-CreER2T; Rosa26R X-gal-stained 7-μm paraffin sections of the trachea. (A and B) Twenty-four hours after SO2 inhalation. (A) Proximal trachea with SMG duct. Anti-BrdU (black) and X-gal (blue; arrows) do not colocalize. (B) Single confocal z-section of distal trachea. Anti-BrdU (red) and X-gal (black) do not colocalize. (C and D) Two weeks after SO2 inhalation. Black, anti-β-tubulin; blue, X-gal. (C) Typical trachea section; ciliated cells have differentiated in the repaired epithelium but are not lineage-labeled. (D) Lineage-labeled cells have cilia (arrows). Domed Clara cells have not inherited the lineage label (arrowheads). (Scale bars: 20 μm.)
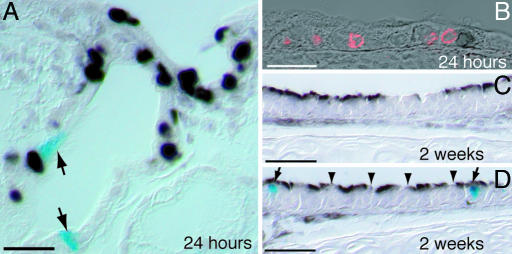
To follow the fate of the lineage-labeled ciliated cells after SO2 injury to the trachea, we injected FOXJ1CreER2T; Rosa26R adult males with tamoxifen and then exposed the animals to SO2 gas. The overall extent of lineage-labeling of ciliated cells in these experiments was 65% [3,884 of 6,012 ciliated cells (n = 6 mice in three independent experiments); Fig. 7A]. Cilia were not visible on 0.4% of lineage-labeled cells. Twenty-four hours after the injury, very few lineage-labeled cells remained in the proximal trachea (Fig. 7C), and those cells that did survive were located in the ducts of the SMGs (Fig. 7H). The number surviving in the distal half of the trachea was more variable; either there were no lineage-labeled cells in this region or labeled cells were scattered throughout the surface epithelium (Fig. 7E). In both cases, there was no apparent increase in the number of lineage-labeled cells when mice were killed 2 weeks after the injury (Fig. 7D and data not shown). Moreover, the lineage-labeled cells remained in the SMG ducts (Fig. 7 H and I) or scattered throughout the luminal epithelium (data not shown). They were not found in patches as might be expected if they had divided.
To confirm this lack of proliferation, we scored BrdU+ cells at 24 h after the injury, when proliferation is at its peak (Fig. 6D). We could not identify a lineage-labeled ciliated cell that had incorporated BrdU in either the SMG ducts or the tracheal epithelium [0 of 3,667 BrdU+ cells; 223 X-gal+ cells scored (n = 6 mice in two independent experiments); Fig. 8A and B]. This analysis included animals with many surviving lineage-labeled cells in the distal trachea as well as animals where almost all of the lineage-labeled cells were killed. The repaired tracheas all contained the normal complement of ciliated epithelial cells. These were not lineage-labeled (Fig. 8C) and must therefore have been derived from a different unlabeled epithelial cell type. These data strongly support the idea that after the SO2 injury, the ciliated cells do not function as either a self-renewing or a stem cell population.
To test the hypothesis that the ciliated cells transdifferentiate in response to SO2 injury, we assessed which epithelial cell types carried the lineage-label 2 weeks after the SO2 exposure when the epithelium was largely repaired. Ninety-nine percent of the lineage-labeled cells had cilia [548 of 551 X-gal+ cells (n = 9 mice in three independent experiments); Fig. 8D]. We counted 3,609 (Scgb1a1+, X-gal−) cells after repair. Only 0.1% of the lineage-labeled cells expressed the Clara cell marker Scgb1a1 [1 of 870 X-gal+ cells (n = 7 mice in three independent experiments)]. This analysis does not provide any support for the hypothesis that ciliated cells can transdifferentiate into another cell lineage in response to SO2 injury.
Discussion
Our results confirm the usefulness of transgenic reporter and lineage tracing mouse strains for investigating basic questions related to lung epithelial cell differentiation and repair after injury. They also illustrate some of the limitations that have to be taken into consideration when interpreting the data.
Ciliated Cell Specification and Differentiation During Development.
We first detected FOXJ1-GFP expression at E14.0, which agrees with published data that Foxj1 protein expression begins in the airways around this time (35). Evidence suggests that Foxj1 functions in airway cells during late stages of ciliogenesis and is not sufficient to drive cilia formation in nonciliated adult epithelial cells (27, 28). Therefore, FOXJ1-GFP expression in cells without cilia most likely marks embryonic cells already committed to the ciliated lineage. Similarly, we find that Scgb1a1 is only reliably detected by immunohistochemistry in secretory cells as they begin to differentiate. Our results show that the appearance of epithelial differentiation markers clearly proceeds in a proximal-distal temporal and spatial sequence, as reported by others (6, 36). However, using 100-μm vibratome sections to visualize distal intralobular airways in three dimensions, we were able to see that morphogenesis does not involve a wave of differentiation that simultaneously enjoins all of the cells at a particular airway level. Rather, GFP+ cells initially appear in a scattered or mosaic pattern, and additional cells are specified as development progresses. This observation is consistent with potential models in which Notch signaling is involved in the specification of the ciliated lineage in the lung (37, 38). However, the field urgently needs earlier markers of Clara and ciliated cell fate to test this hypothesis and to determine exactly how the pattern is set up.
Ciliated Cells in Injury and Repair.
We were unable to detect proliferating FoxJ1-positive ciliated cell precursors during development. Similarly, we have found no evidence that lineage-labeled ciliated cells can self-renew, transdifferentiate, or function as stem cells in response to airway injury. This analysis included examination of the effects of naphthalene-mediated destruction of the Clara cells in all regions of the conducting airways from the trachea to the BADJ, and of SO2-mediated injury to all epithelial cells in the tracheobronchial epithelium. These results contradict the recent claims that ciliated cells can transdifferentiate into Clara cells in response to naphthalene injury in the bronchiolar regions (22). We observed the same cell shape changes in the ciliated cells in response to naphthalene injury as Park and colleagues (20). This suggests that the extent of the injury did not differ significantly between the two sets of experiments. One explanation for the different conclusions is that the method used by Park et al. (20) to trace the fate of ciliated cells after injury (immunohistochemistry for tubulin) does not provide a precise longitudinal analysis of heritably marked cells. One potential drawback of our approach is that we only label 50–70% of the ciliated cells in the adult lung, depending on the dose of tamoxifen. This failure to label all cells is an inherent limitation of using tamoxifen to stochastically induce translocation of Cre into the nucleus and has been seen in many other lineage tracing studies with adult mice (for example, those studies described in refs. 39–41). For this limitation to affect the conclusions of our study it would be necessary to argue that recombination only occurs, either by chance or for mechanistic reasons, in a specific subpopulation of ciliated cells that is unable to undergo transdifferentiation. We consider this argument unlikely for two reasons. First, we examined a total of 42 mice, which makes it very unlikely that failure to activate recombination occurred in a specific subpopulation by chance. Second, we show that the FOXJ1 promoter is able to drive transgene expression in all ciliated cells, as well as in precursors that do not have surface cilia and may therefore be more likely to transdifferentiate.
The most parsimonious explanation of our results is therefore that ciliated cells do not function as progenitors during development or in two commonly used models of airway injury and repair. However, our findings do not exclude the possibility that ciliated cells can function as progenitors in response to different injuries or in models of diseases such as acute asthma or viral infection where there is morphological evidence that ciliated cells transdifferentiate into mucous-secreting cells (24, 25). In the future, these possibilities can be tested by using our FOXJ1CreER2T mouse line.
Materials and Methods
Transgenic Mice.
A CreER2T cassette (39) was inserted into StuI/MluI restriction sites in the 1,008-bp human FOXJ1 promoter vector (29), just 3′ of the mouse transthyretin (TTR) intron (Fig. 2A). Transgenic mice were created by pronuclear injection into (C57BL/6 × DBA) fertilized eggs. Five independent FOXJ1CreER2T founder lines were established and tested by mating to Rosa26R (Gt(ROSA)26Sortm1Sor) reporter mice (42) and injecting the adult progeny with tamoxifen (Sigma, St. Louis, MO). Three of the lines gave indistinguishable and efficient recombination specifically in the ciliated cells of the adult lung.
Mouse Strains.
The FOXJ1-GFP mice were maintained by crossing to C57BL/6. Timed matings for embryonic studies were generated by crossing with the outbred ICR strain (Harlan Sprague Dawley, Indianapolis, IN). For injury/repair experiments, we used C57BL/6 (Charles River Laboratories, Wilmington, MA) as wild type and FOXJ1CreER2T and Rosa26R strains at the N2 and N3 C57BL/6 backcross generations. FOXJ1CreER2T; Rosa26R animals (8–12 weeks) were injected i.p. (intraperitoneally) up to five times (every 2–3 days) with 4 mg of tamoxifen in Mazola (Cordova, TN) corn oil. Control mice were injected with corn oil only. At least 1 week separated the final tamoxifen injection and airway injury.
Airway Injury and BrdU Exposure.
Adult (10–14 weeks) mice were injected i.p. with 250 mg/kg naphthalene (Sigma) dissolved in Mazola corn oil between 8 and 10 a.m. Adult males were placed in individual compartments of a chamber and exposed to 500 ppm SO2 in air for 3 h. To detect cell proliferation, mice were injected i.p. with 10 μl per g of body weight of a solution containing 3 mg/ml BrdU (Amersham Biosciences, Piscataway, NJ) 2 h (naphthalene-injured) or 1 h (SO2-injured and developmental stages) before sacrifice.
Fixation and X-gal/Lectin Staining.
Adult lungs were inflated with 1 ml of fixative. Trachea and lungs were placed into 4% paraformaldehyde or 10% formalin for anti-β-galactosidase (anti-β-gal) staining and fixed for 4–6 h at 4°C. The X-gal color reaction was as described (43). To label the surface of the airway epithelial cells, we incubated lungs for 20 min with biotin-labeled tomato lectin (Lycopersicon esculentum) (Vector Laboratories, Burlingame, CA) and detected the biotin label using a TSA kit and Cy3-tyramide (PerkinElmer, Wellesley, MA) (6). Embryonic lungs were fixed 1–2 h at 4°C in 4% paraformaldehyde. For vibratome sectioning, they were embedded in 4% agarose, and 100-μm sections were cut.
Immunohistochemistry.
Vibratome sections were stained by using a whole-mount protocol. Paraffin sections were immunostained by using standard protocols, including controls lacking the primary antibody. Mouse anti-β-tubulin (1:1,500; BioGenex, San Ramon, CA), rat anti-E-cadherin (1:2,000; Zymed, South San Francisco, CA), and rabbit anti-CGRP (1:1,000; Peninsula Laboratories) were used without antigen retrieval. Other antibodies required antigen retrieval: 0.05% trypsin digestion for 5 min for goat anti-secretoglobin 1a1 (Scgb1a1) (1:10,000) and rabbit anti-Scgb1a1 (1:5,000; both kindly provided by Barry Stripp, University of Pittsburgh, Pittsburgh, PA), 30 min at 37°C in 2 M HCl followed by trypsin digestion for mouse anti-BrdU (1:200; Sigma) and rat anti-BrdU (1:200; Accurate Chemical, Westbury, NY), boiling for 5 min in 100 mM sodium citrate buffer (pH 6) followed by trypsin digestion for rabbit anti-β-gal (1:5,000; Cappel), and 4 h heating at 60°C in 100 mM Tris (pH 10) for mouse anti-FoxJ1 (1:500; kindly provided by Steve Brody, Washington University, St. Louis, MO). Biotinylated secondary antibodies (1:500; Jackson ImmunoResearch, West Grove, PA) were amplified with the VECTASTAIN Elite ABC kit and detected with DAB (Vector Laboratories). Alexa Fluor-coupled secondary antibodies (Invitrogen, Carlsbad, CA) were used at 1:500 dilution.
Confocal Microscopy and Cell Counting.
Cells were counted manually. Approximately equal numbers of cells were counted for each experimental lung. Cells whose apical surface was not visible because of the plane of the section were not counted. All immunofluorescence images used for scoring cells consisted of a z-series of optical sections captured on a Zeiss LSM 510 Meta laser scanning confocal microscope. Multiple optical sections were examined to accurately determine cell boundaries.
Acknowledgments
We thank W. Michael Foster and Barry Stripp for advice on injury models and Rodney Gilmore, Lisa Jones, Wanda O'Neal, and Lindsey Becker for technical assistance. Work in the B.L.M.H. laboratory is supported by National Institutes of Health Grant NIH-080517, and SO2 exposures are supported by National Institutes of Health Grant ES011961.
Abbreviations
- BADJ
bronchoalveolar duct junction
- CGRP
calcitonin gene-related peptide
- En
embryonic day n
- SMG
submucosal gland.
Footnotes
The authors declare no conflict of interest.
References
- 1.Rawlins EL, Hogan BL. Development (Cambridge, UK) 2006;133:2455–2465. doi: 10.1242/dev.02407. [DOI] [PubMed] [Google Scholar]
- 2.Neuringer IP, Randell SH. Monaldi Arch Chest Dis. 2006;65:47–51. doi: 10.4081/monaldi.2006.587. [DOI] [PubMed] [Google Scholar]
- 3.Cardoso WV, Lu J. Development (Cambridge, UK) 2006;133:1611–1624. doi: 10.1242/dev.02310. [DOI] [PubMed] [Google Scholar]
- 4.Okubo T, Knoepfler PS, Eisenman RN, Hogan BL. Development (Cambridge, UK) 2005;132:1363–1374. doi: 10.1242/dev.01678. [DOI] [PubMed] [Google Scholar]
- 5.Perl AK, Wert SE, Nagy A, Lobe CG, Whitsett JA. Proc Natl Acad Sci USA. 2002;99:10482–10487. doi: 10.1073/pnas.152238499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Toskala E, Smiley-Jewell SM, Wong VJ, King D, Plopper CG. Am J Physiol. 2005;289:L454–L459. doi: 10.1152/ajplung.00036.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sarikas SN, Hoyt RF, Jr, Sorokin SP. Anat Rec. 1985;213:396–409. doi: 10.1002/ar.1092130306. [DOI] [PubMed] [Google Scholar]
- 8.Perl AK, Wert SE, Loudy DE, Shan Z, Blair PA, Whitsett JA. Am J Respir Cell Mol Biol. 2005;33:455–462. doi: 10.1165/rcmb.2005-0180OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Plopper CG, Nishio SJ, Alley JL, Kass P, Hyde DM. Am J Respir Cell Mol Biol. 1992;7:606–613. doi: 10.1165/ajrcmb/7.6.606. [DOI] [PubMed] [Google Scholar]
- 10.McDowell EM, Newkirk C, Coleman B. Anat Rec. 1985;213:448–456. doi: 10.1002/ar.1092130310. [DOI] [PubMed] [Google Scholar]
- 11.Otani EM, Newkirk C, McDowell EM. Anat Rec. 1986;214:183–192. doi: 10.1002/ar.1092140213. [DOI] [PubMed] [Google Scholar]
- 12.Van Winkle LS, Buckpitt AR, Nishio SJ, Isaac JM, Plopper CG. Am J Physiol. 1995;269:L800–L818. doi: 10.1152/ajplung.1995.269.6.L800. [DOI] [PubMed] [Google Scholar]
- 13.Plopper CG, Suverkropp C, Morin D, Nishio S, Buckpitt A. J Pharmacol Exp Ther. 1992;261:353–363. [PubMed] [Google Scholar]
- 14.Hong KU, Reynolds SD, Watkins S, Fuchs E, Stripp BR. Am J Pathol. 2004;164:577–588. doi: 10.1016/S0002-9440(10)63147-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hong KU, Reynolds SD, Watkins S, Fuchs E, Stripp BR. Am J Physiol. 2004;286:L643–L649. doi: 10.1152/ajplung.00155.2003. [DOI] [PubMed] [Google Scholar]
- 16.Hong KU, Reynolds SD, Giangreco A, Hurley CM, Stripp BR. Am J Respir Cell Mol Biol. 2001;24:671–681. doi: 10.1165/ajrcmb.24.6.4498. [DOI] [PubMed] [Google Scholar]
- 17.Giangreco A, Reynolds SD, Stripp BR. Am J Pathol. 2002;161:173–182. doi: 10.1016/S0002-9440(10)64169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, Crowley D, Bronson RT, Jacks T. Cell. 2005;121:823–835. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 19.Borthwick DW, Shahbazian M, Krantz QT, Dorin JR, Randell SH. Am J Respir Cell Mol Biol. 2001;24:662–670. doi: 10.1165/ajrcmb.24.6.4217. [DOI] [PubMed] [Google Scholar]
- 20.Park KS, Wells JM, Zorn AM, Wert SE, Laubach VE, Fernandez LG, Whitsett JA. Am J Respir Cell Mol Biol. 2005;34:151–157. doi: 10.1165/rcmb.2005-0332OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reynolds SD, Giangreco A, Power JH, Stripp BR. Am J Pathol. 2000;156:269–278. doi: 10.1016/S0002-9440(10)64727-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aso Y, Yoneda K, Kikkawa Y. Lab Invest. 1976;35:558–568. [PubMed] [Google Scholar]
- 23.Kauffman SL. Int Rev Exp Pathol. 1980;22:131–191. [PubMed] [Google Scholar]
- 24.Reader JR, Tepper JS, Schelegle ES, Aldrich MC, Putney LF, Pfeiffer JW, Hyde DM. Am J Pathol. 2003;162:2069–2078. doi: 10.1016/S0002-9440(10)64338-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tyner JW, Kim EY, Ide K, Pelletier MR, Roswit WT, Morton JD, Battaile JT, Patel AC, Patterson GA, Castro M, et al. J Clin Invest. 2006;116:309–321. doi: 10.1172/JCI25167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim KK, Kugler MC, Wolters PJ, Robillard L, Galvez MG, Brumwell AN, Sheppard D, Chapman HA. Proc Natl Acad Sci USA. 2006;103:13180–13185. doi: 10.1073/pnas.0605669103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.You Y, Huang T, Richer EJ, Schmidt JE, Zabner J, Borok Z, Brody SL. Am J Physiol. 2004;286:L650–L657. doi: 10.1152/ajplung.00170.2003. [DOI] [PubMed] [Google Scholar]
- 28.Gomperts BN, Gong-Cooper X, Hackett BP. J Cell Sci. 2004;117:1329–1337. doi: 10.1242/jcs.00978. [DOI] [PubMed] [Google Scholar]
- 29.Ostrowski LE, Hutchins JR, Zakel K, O'Neal WK. Mol Ther. 2003;8:637–645. doi: 10.1016/s1525-0016(03)00221-1. [DOI] [PubMed] [Google Scholar]
- 30.Blatt EN, Yan XH, Wuerffel MK, Hamilos DL, Brody SL. Am J Respir Cell Mol Biol. 1999;21:168–176. doi: 10.1165/ajrcmb.21.2.3691. [DOI] [PubMed] [Google Scholar]
- 31.Khoor A, Gray ME, Singh G, Stahlman MT. J Histochem Cytochem. 1996;44:1429–1438. doi: 10.1177/44.12.8985135. [DOI] [PubMed] [Google Scholar]
- 32.Lawson GW, Van Winkle LS, Toskala E, Senior RM, Parks WC, Plopper CG. Am J Pathol. 2002;160:315–327. doi: 10.1016/S0002-9440(10)64375-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Winkle LS, Gunderson AD, Shimizu JA, Baker GL, Brown CD. Am J Physiol. 2002;282:L1122–L1134. doi: 10.1152/ajplung.00309.2001. [DOI] [PubMed] [Google Scholar]
- 34.Reynolds SD, Hong KU, Giangreco A, Mango GW, Guron C, Morimoto Y, Stripp BR. Am J Physiol. 2000;278:L1256–L1263. doi: 10.1152/ajplung.2000.278.6.L1256. [DOI] [PubMed] [Google Scholar]
- 35.Tichelaar JW, Wert SE, Costa RH, Kimura S, Whitsett JA. J Histochem Cytochem. 1999;47:823–832. doi: 10.1177/002215549904700612. [DOI] [PubMed] [Google Scholar]
- 36.McDowell EM, Hoyt RF, Jr, Sorokin SP. Cell Tissue Res. 1994;275:157–167. doi: 10.1007/BF00305383. [DOI] [PubMed] [Google Scholar]
- 37.Ito T, Udaka N, Yazawa T, Okudela K, Hayashi H, Sudo T, Guillemot F, Kageyama R, Kitamura H. Development (Cambridge, UK) 2000;127:3913–3921. doi: 10.1242/dev.127.18.3913. [DOI] [PubMed] [Google Scholar]
- 38.Post LC, Ternet M, Hogan BL. Mech Dev. 2000;98:95–98. doi: 10.1016/s0925-4773(00)00432-9. [DOI] [PubMed] [Google Scholar]
- 39.Hayashi S, McMahon AP. Dev Biol. 2002;244:305–318. doi: 10.1006/dbio.2002.0597. [DOI] [PubMed] [Google Scholar]
- 40.Dor Y, Brown J, Martinez OI, Melton DA. Nature. 2004;429:41–46. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- 41.Zhang H, Fujitani Y, Wright CV, Gannon M. Genesis. 2005;42:210–217. doi: 10.1002/gene.20137. [DOI] [PubMed] [Google Scholar]
- 42.Soriano P. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- 43.Eblaghie MC, Reedy M, Oliver T, Mishina Y, Hogan BL. Dev Biol. 2006;291:67–82. doi: 10.1016/j.ydbio.2005.12.006. [DOI] [PubMed] [Google Scholar]